Abstract
The endoplasmic reticulum (ER) is the entry portal of the conventional secretory pathway where the newly synthesized polypeptides fold, modify, and assemble. The ER responses to the unfolded proteins in its lumen (ER stress) by triggering intracellular signal transduction pathways include the ER-associated degradation (ERAD) pathway and the unfolded protein response (UPR) pathway. In yeast and mammals, the ubiquitin ligase Hrd1 is indispensable for the ERAD pathway, and also Hrd1-mediated ERAD pathway plays a crucial role in maintaining homeostasis and metabolism of human beings. However, the underlying physiological roles and regulatory mechanism of the Hrd1-involved ERAD pathway in the plant pathogenic fungi are still unclear. Here, we identified the Hrd1 orthologous proteins from 9 different fungi and noticed that these Hrd1 orthologs are conserved. Through identification of MoHrd1 putative interacting proteins by co-immunoprecipitation assays and enrichment analysis, we found that MoHrd1 is involved in the secretory pathway, energy synthesis, and metabolism. Taken together, our results suggest that MoHrd1 is conserved among fungi and play an important role in cellular metabolism and infection-related development. Our finding helps uncover the mechanism of Hrd1-involved ERAD pathway in fungi and sheds a new light to understand the pathogenic mechanism of Magnaporthe oryzae.
Keywords: endoplasmic reticulum–associated degradation (ERAD), the ubiquitin ligase Hrd1, mass spectrometry, Magnaporthe oryzae
Introduction
Approximately one-third of the cellular proteomes is destined for the plasma membrane, extracellular space, or secretory compartments via targeting to the entry portal of the secretory pathway-endoplasmic reticulum (ER).1 The newly synthesized polypeptides enter into the ER through the Sec61 translocon complex for folding and N-linked glycosylation. Then, a diverse set of chaperones and accessory proteins assist immature polypeptides in folding to the native conformation, whereas chaperone interactions keep these proteins soluble to prevent aggregation in the ER lumen.2–5 The ER is a checkpoint of the secretory pathway, with hosting a stringent and specialized protein quality control (PQC) system, which delivers the correctly folded proteins to the Golgi complex while retaining the abnormal folding proteins in the ER.1,6,7 The ER PQC machinery regulates both the ER-associated degradation (ERAD) pathway for eliminating the ultimately unfolded proteins and unfolded protein response (UPR) pathway, a signaling cascade pathway for reducing protein biosynthesis and facilitating ER folding and degradation ability.7–9
ERAD pathway removed the aberrant proteins via a series of tightly coupled stages consisting of substrate recognition, retrotranslocation, and ubiquitin proteasome system (UPS)-dependent degradation4,8,10,11 In the budding yeast, ER luminal substrates are recognized by the molecular chaperones such as Kar2, Scj1, Jem1, and Pdi1, retrotranslocated, and ubiquitinated by the Hrd1 complex and are finally delivered to the UPS for degradation.4,12–16 The multitransmembrane protein Hrd1 is the E3 ubiquitin ligase for the substrate ubiquitination. Moreover, cryo-electron microscopic structure suggested that the retrotranslocation channel for the misfolded proteins crossing the ER membrane is formed by the membrane protein Hrd1.17–20 In the mammals, Hrd1 is indispensable for the misfolded proteins which retrotranslocated from the ER lumen to the cytosolic UPS for degradation.6,21–23 Moreover, Hrd1-mediated ERAD pathway is essential for maintaining metabolic homeostasis and provides a breathtaking new challenge of disease control such as Alzheimer disease and Parkinson disease.24,25 However, the physiological roles of the Hrd1-involved ERAD pathway in the plant pathogenic fungi are still unclear.
The hemibiotrophic filamentous fungus Magnaporthe oryzae (Pyricularia oryzae) is the causal agent of rice blast disease which is one of the most destructive diseases of cultivated rice worldwide and threats rice production and global food security seriously. Magnaporthe oryzae has been used as a primary model organism for studying plant-fungi interaction.26–29 Similar to other fungi and oomycetes, rice blast fungus secretes some protein termed effectors during infection development for suppressing plant immunity and establishing plant disease. Sequentially, M. oryzae has evolved two distinct secretion systems: on one hand, apoplastic effectors such as Bas4 and Slp1 are delivered into the space between the fungal cell wall and extra-invasive hyphal membrane (EIHM) by conventional ER-Golgi secretion pathway; on the other hand, cytoplasmic effectors (such as Pwl2, AvrPiz-t, and AvrPi9) are secreted from invasive hyphae into the extracellular compartment and the biotrophic interfacial complex (BIC).30–36 The ER-associated secretory pathway is important for rice blast disease development. Previous studies elaborated that MoHac1, MoLhs1, and MoKar2 involved in UPR pathway are essential for asexual development and infection-related morphogenesis,37,38 but little is known about another cascade pathway of the ER PQC system in M. oryzae.
Protein-protein interactions play a vital role in many cellular processes, such as translation and signal transduction.39–41 Immunoprecipitation (IP) is a proven technique for the enrichment and isolation of interacting proteins via an antigen binding to a specific antibody.42 Tandem mass spectrometry (MS) could identify the proteins which directly or indirectly bind each other.39,43 Thus, IP-MS is a feasible and complementary approach to screen interaction proteins and investigate the potential biological function of the target protein. For example, Li et al. screened out MoArk1 interaction proteins MoCapA and MoCapB using IP-MS. Further study showed that MoArk1 regulated MoCapA and MoCapB to involve in control of growth, conidiation, and pathogenesis in M. oryzae.44
In this study, we identified the Hrd1 orthologous proteins in different fungi and noticed that these proteins are conserved. Moreover, we demonstrated that MoHrd1 could be crucial for the secretory pathway, energy synthesis, and metabolism in M. oryzae both by bioinformatics analysis and by the co-immunoprecipitation (Co-IP) assays.
Materials and Methods
Strains and culture conditions
The M. oryzae Guy11 strain was used as wild type (WT) in this study. All strains were cultured on complete medium (CM) agar plates at 28°C (CM: 10 g D-glucose, 2 g peptone, 1 g yeast extract, 1 g casamino acid, 50 mL 20× nitrate salts, 1 mL trace elements, 1 mL vitamin solution, 15 g agar, and 1 L distilled water). Liquid CM medium was used to prepare the mycelia for DNA and protein extraction.
Construction of MoHRD1::GFP
To establish MoHRD1::GFP construct, full-length genomic DNA of MoHRD1 with the native promoter (1.5 kb upstream fragment) was cloned from Guy11 using primer pair HRD1 Com-F and HRD1 Com-R which was then co-introduced with Xho I (Takara, Shiga, Japan)-digested pYF11 into the XK125 yeast competent cell. Plasmids of MoHRD1::GFP were recovered from generating Trp+ yeast transformants and verified by performing polymerase chain reaction (PCR) assays with primer pair HRD1 Con-F and GFP-R.
IP assays
The MoHRD1-GFP fusion construct was introduced by transformation into protoplasts of the Mohrd1 null mutant. Mycelia were ground into fine powder in liquid nitrogen and resuspended in 20 mL lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40) with freshly added 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 μL of protease inhibitor cocktail (Sigma Aldrich , Shanghai, China). Then, total proteins were incubated with anti-GFP (green fluorescent protein) agarose beads (ChromoTek, Planegg-Martinsried, Germany). Proteins bound to GFP agarose beads were eluted after a series of washing steps according to the manufacturer’s protocol. Analysis of bound proteins was performed by the Beijing Genomics Institute using MS.
Pathway enrichment analysis and Gene Ontology enrichment analysis of MoHrd1 putative interaction proteins
KEGG (Kyoto Encyclopedia of Genes and Genome; http://www.genome.jp/kegg/) is a primary biological database of the pathway and an efficient instrument for characterization of metabolism and metabolic network.45 The most important metabolic pathways and signal transduction pathways that putative interacting proteins are involved could be identified via pathway enrichment analysis. Providing functional information of gene product, GO (Gene Ontology; http://www.geneontology.org/) is a published bioinformatics database that classifies functions along molecular function, biological process, and cellular component in 3 aspects. The enrichment analysis of MoHrd1 putative interaction proteins was performed using ClueGO46 which is a Cytoscape plugin to improve the biological interpretation of gene lists and a functional organization network was constructed.
Results
Identification of Hrd1p orthologous proteins in different fungi
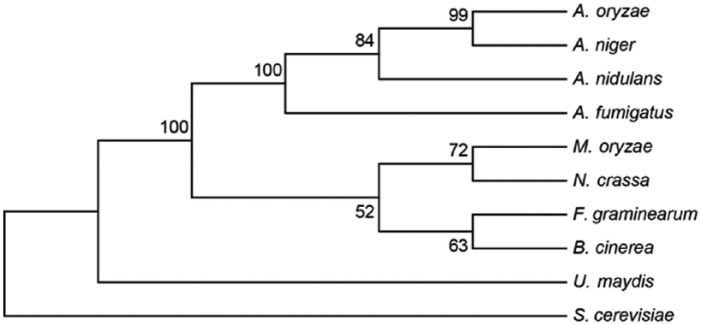
Using the amino acid sequence of Hrd1p from Saccharomyces cerevisiae as a query, we performed a homology search in 9 fungal species including M. oryzae, Ustilago maydis, Fusarium graminearum, Botrytis cinerea, Neurospora crassa, Aspergillus fumigatus, Aspergillus nidulans, Aspergillus oryzae, and Aspergillus niger in the NCBI (National Center for Biotechnology Information) database (http://blast.ncbi.nlm.nih.gov/). Nine potential candidates including the orthologous protein of M. oryzae encoded by MGG_09205 were found. Based on the sequences of these 9 Hrd1 orthologous proteins, a phylogenetic tree was constructed using the ClustalX and the MEGA version 6 software47,48 (Figure 1). The phylogenetic dendrogram shows that the 4 potential Hrd1 orthologs in the Aspergillus species are most closely related, whereas the Hrd1 orthologous protein in M. oryzae is closely related to orthologs in N. crassa, F. graminearum, and B. cinerea.
Figure 1.
Phylogenetic tree of the Hrd1 orthologous proteins in different fungi. The Hrd1 protein sequences from different fungi were aligned using the ClustalX software. Phylogenetic analysis was performed using MEGA version 6.
Hrd1 is highly conserved in different fungi
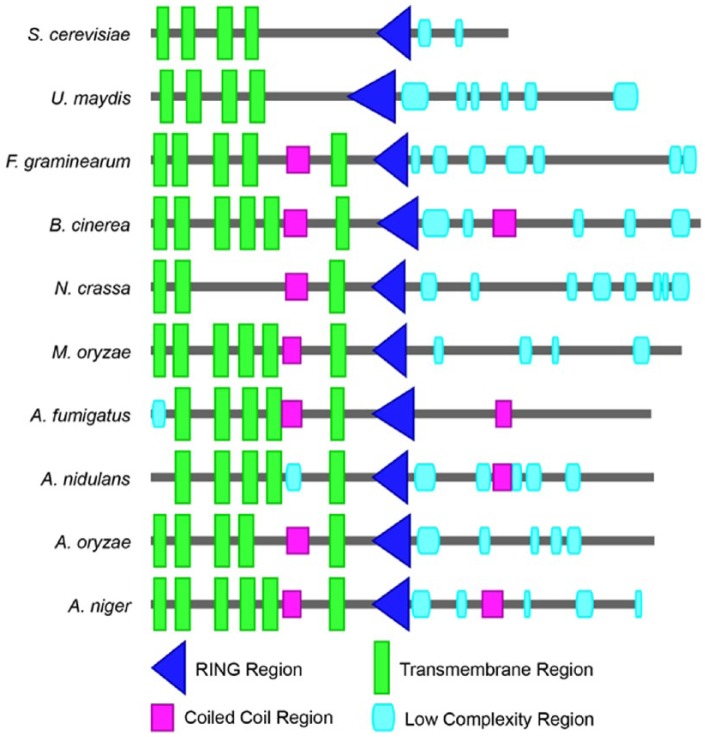
To investigate and compare the function of these Hrd1 orthologous proteins, functional domains were identified using the SMART software49,50 (http://smart.embl-heidelberg.de/; Figure 2). Identification of domains from protein sequences demonstrated that Hrd1 proteins are highly conserved among selected fungi and all contain a RING domain and several transmembrane regions. Moreover, 4 to 6 transmembrane regions and a RING domain localize to the similar loci from N-terminus, whereas most of them hold a coiled-coil domain between the last 2 transmembrane regions. These observations suggest that the function of these Hrd1 orthologous proteins could be highly conserved.
Figure 2.
Domain architecture of Hrd1 orthologous proteins in different fungi. The protein functional domains were identified using the SMART software (http://smart.embl-heidelberg.de/).
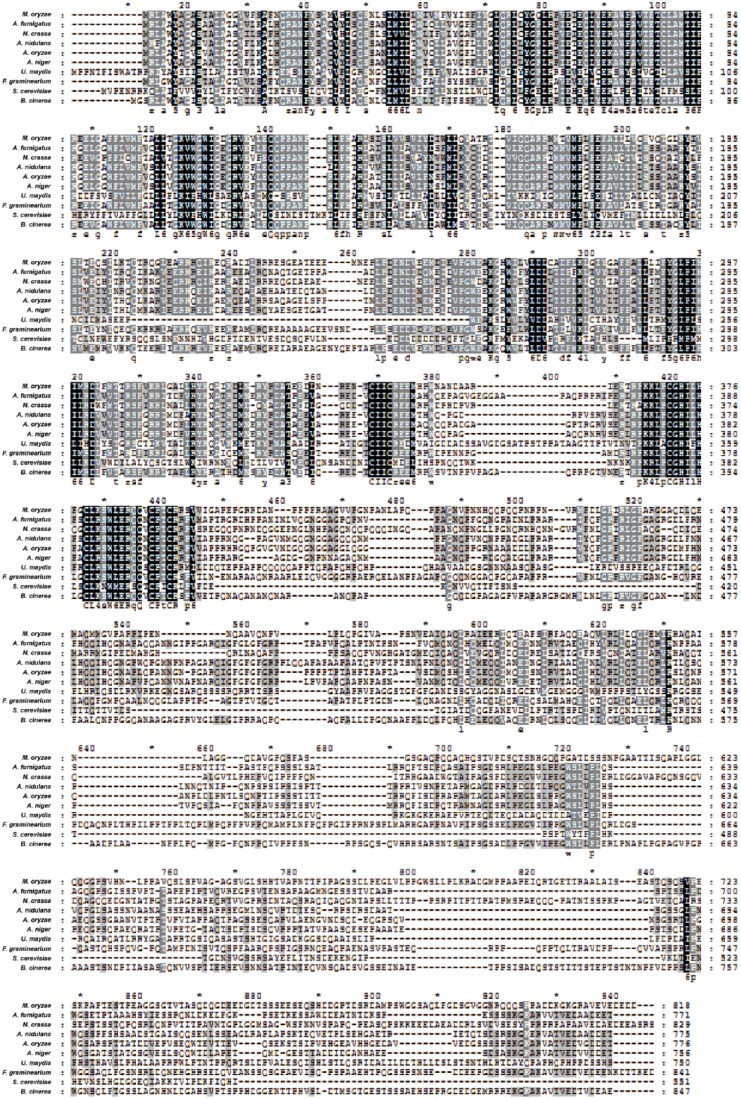
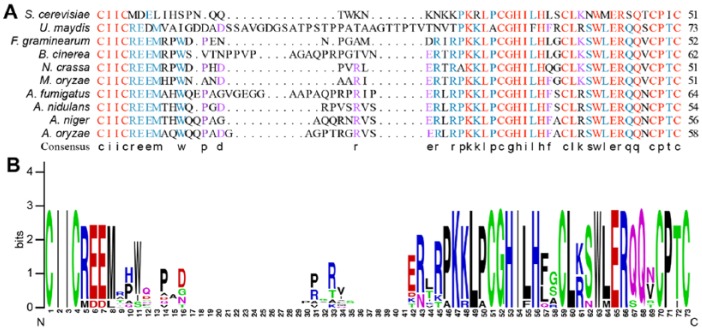
We further performed a sequence alignment using the ClustalX software.47 Amino acid alignments of Hrd1 orthologs display high conservation. Almost one-half of the amino acids are highly conserved among these 10 fungi, especially in the N-terminus transmembrane regions and RING region (Figure 3). In the RING region, almost one-third of the amino acids are identical among all selected fungi; meanwhile, more than one-half of the amino acids are uniform among F. graminearum, B. cinerea, N. crassa, M. oryzae, A. fumigatus, A. nidulans, A. oryzae, and A. niger (Figure 4A). For visually displaying the conservation of the RING domain, we built a sequence logo diagram using the WebLogo software (http://weblogo.berkeley.edu/logo.cgi).51 Simultaneously, the amino acids logo diagram showed that Hrd1 RING domain is highly conserved in these selected fungi (Figure 4B).
Figure 3.
Hrd1 is conserved in different fungi. Sequence alignment of Hrd1 in different fungi. Sequence identity is shaded in black; shading in dark gray shows that amino acid sequence are conserved over 75% species, whereas shading in light gray shows only over 50% species. The alignment was performed using ClustalX software.
Figure 4.
Hrd1 RING domain is conserved in different fungi. (A) Sequence alignment of the Hrd1 RING domains in different fungi. Red shows sequence identity, blue shows sequence is conserved over 75% species, whereas purple displays only over a half, and the consensus exhibited the conserved amino acids. (B) Sequence logo diagram shows Hrd1 RING domain ortholog in different fungi. The alignment was performed using the ClustalX software. The logo was built using the WebLogo software (http://weblogo.berkeley.edu/logo.cgi).
In the human pathogenic fungi A. fumigatus, loss of HrdA (the Hrd1 ortholog in A. fumigatus) exhibited defects in degradation of a folding-defective ERAD substrate, as well as activation of the UPR.52 In the filamentous fungus A. oryzae, HrdA (the Hrd1 ortholog in A. oryzae) mediated ERAD pathway is required for the degradation of the moderate-level MsdS mutant.53 We found that approximately 80% of the MoHrd1 RING domain amino acid sequences are identical to those in A. fumigatus and A. oryzae (Figure 4A). Meanwhile, Hrd1 orthologs in M. oryzae, A. fumigatus, and A. oryzae contain similar functional domain combination (Figure 2). In brief, these results suggest that MoHrd1 may play an important role in the ER PQC system of M oryzae.
Putative interaction proteins of MoHrd1 were identified by IP-MS
To identify MoHrd1 putative interaction proteins, Co-IP assays were performed. A GFP-encoding gene was fused at the C-terminus of the MoHrd1-coding sequence using the native promoter and was transformed into the ΔMohrd1 mutant (unpublished data). Wild type expressing GFP gene driven by a constitutive promoter was used as a negative control. Western blots were used to detect Co-IP assays with GFP-tag proteins extracted from MoHrd1::GFP and the GFP control strain of the WT. As shown in Figure 5, the anti-GFP antibody detected the 117-kDa MoHrd1::GFP-fusing protein and the GFP, 26-kDa bands, respectively, from total proteins isolated from the corresponding strains and proteins eluted from anti-GFP beads, indicating that GFP-tag proteins were successfully co-immunoprecipitated in the elution buffer.
Figure 5.
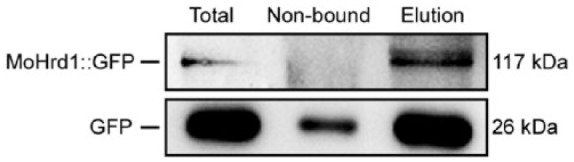
Western blots of total proteins (total) and proteins eluted (elution) from the anti-GFP agarose beads from the wild type constitutively expressing GFP and transformant expressing MoHrd1::GFP were probed with the anti-GFP (GFP-Tag 7G9 Mouse mAb; Abmart, Shanghai, China) antibody. GFP indicates green fluorescent protein.
Meanwhile, tandem MS was employed to identify MoHrd1 putative interacting proteins in the immunoprecipitates. The candidate interaction proteins present in the different replicates but not present in the negative control were regarded as potential interaction proteins; in total, 161 putative interaction proteins were identified (Supplementary Table S2).
In S. cerevisiae, 60 Hrd1p interaction proteins were identified using matrix-assisted laser desorption/ionization-time of flight MS and liquid chromatography-tandem MS.54 Using S cerevisiae Hrd1 interaction proteins as a query, we further performed a homologous search in M. oryzae; in total, 60 homologous proteins were found (Supplementary Table S3). Moreover, 5 of the MoHrd1 putative interaction proteins consisting of MGG_05193, MGG_13508, MGG_01790, MGG_04400, and MGG_02297 were present in these 60 homologous proteins. MGG_13508, MGG_02297, and MGG_05193 were the homologous proteins of S. cerevisiae Hrd3, Yos9, and Cdc48, respectively. In yeast, Hrd3 was required for ERAD pathway and was indispensable for Hrd1 stability55; Yos9 was essential for ERAD substrate recognition and took part in delivering substrates into Hrd1 complex56–58; Cdc48 formed a heterotrimeric complex with Npl4 and Ufd1 to facilitate substrate retrotranslocation.59 In short, S. cerevisiae Hrd3, Yos9, and Cdc48 were required for Hrd1-involved ERAD pathway. Taken together, this may suggest that MoHrd1 synergizes with MGG_13508, MGG_02297, and MGG_05193 to play a similar role in the ERAD pathway of M. oryzae.
Pathway enrichment analysis and GO enrichment analysis of the MoHrd1 putative interaction proteins
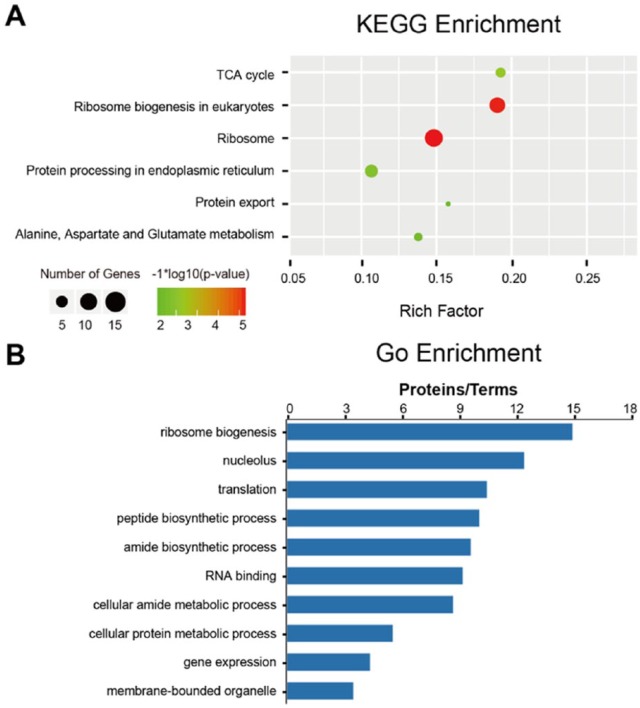
Pathway enrichment analysis (KEGG) and functional enrichment analysis (GO) were applied to elucidating the biological functions of the putative interaction proteins related to MoHrd1. Enriched results were subjected to multiple testing adjustment with a threshold value (adjusted P value with Bonferroni correction) less than 0.05. In total, 5 significantly enriched pathways were annotated, and their descriptions suggest that they are highly correlated with energy metabolisms such as tricarboxylic acid (TCA) cycle, ribosome biogenesis, and secretory pathways such as protein export and protein processing in ER in eukaryotes (Figure 6A). To better exhibit functional consequence, only the top 10 significant enriched GO terms are shown in Figure 6B. Consistent with pathway enrichment results, several GO terms such as gene expression, translation, ribosome biogenesis, metabolic process, and biosynthetic process indicate that these protein data sets belong to the secretory pathway, energy synthesis, and metabolism.
Figure 6.
Pathway enrichment analysis and GO (Gene Ontology) enrichment analysis of MoHrd1 putative interaction proteins. (A) Advanced bubble chart shows enrichment of MoHrd1 putative interaction proteins in different signaling pathways. Y-axis label represents pathway, and X-axis label represents rich factor (rich factor = amount of putative interaction proteins enriched in the pathway/amount of all proteins in background proteins set). Size and color of the bubble represent the amount of putative interaction proteins enriched in pathway and enrichment significance, respectively. (B) Column diagram shows enrichment of MoHrd1 putative interaction proteins in different metabolic pathways. Y-axis label represents pathway, and X-axis label represents the ratio of putative interaction proteins.
Discussion
In this study, we identified the Hrd1 orthologous proteins in 9 different fungi and found that these orthologs are conserved. Further enrichment analysis suggested that the Hrd1 ortholog in M. oryzae should be essential for the secretory pathway, energy synthesis, and metabolism.
Previous studies on the ubiquitin ligase Hrd1 revealed that the multispanning membrane Hrd1 is required for the ER luminal substrates retrotranslocation and ubiquitination, and its autoubiquitination triggered protein retrotranslocation. Moreover, this membrane-anchored protein is involved in the constitution of the retrotranslocation channel for the movement of the abnormal polypeptides through the ER membrane.4,17,18,20,60 And, the Hrd1 RING domain is indispensable for the function of Hrd1 in the ERAD pathway; the site-specific mutant (C399S) is not functional.61 More than that recent studies revealed that the Hrd1 RING domain plays an important role in the activation of its cognate E2 ubiquitin-conjugating enzyme.62 Our results showed that all 10 Hrd1 orthologs contain similar functional domain combination (Figure 2) and the RING domain is highly conserved (Figure 4). This may suggest that the Hrd1 orthologs including MoHrd1 play a similar role in the ERAD pathway among different fungi.
Recent studies have shown that M. oryzae secreted effectors to suppress plant immunity and support rice blast development.30,33 For instance, M. oryzae secreted Slp1 (secreted LysM protein 1) to overcome PTI (pathogen-associated molecular patterns–triggered immunity) via suppressing chitin-induced plant immune responses.63 Hence, correct secretion of effectors is necessary for the M oryzae infection–related development and abnormal secretory proteins should be eliminated in time. Consequently, MoHrd1-mediated ERAD pathway may be important for the infection-related morphogenesis. Similarly, our results showed that MoHrd1 is related to the conventional secretory pathway (translation, protein processing in ER, protein export; Figure 6). This may suggest that MoHrd1 is required for the pathogenesis of M. oryzae via mediating secretion of effectors.
Misfolded proteins undergo 2 sequential steps after retrotranslocation and ubiquitination in the cytosol, N-linked glycans are removed from misfolded proteins, and remaining proteins delivered to the proteasome for degradation into small peptides; meanwhile, free oligosaccharides are generated with the Png1 catalyzation.4 Our results showed that MoHrd1 putative interaction proteins mainly enriched the energy synthesis and metabolism (Figure 6). This may reveal that the product of the ERAD pathway such as small peptides and free oligosaccharides is recycled into the cellular metabolism such as the TCA cycle in M. oryzae. Hrd1 (HMG-CoA reductase degradation) was discovered in the genetic analysis of Hmg2 degradation. Hmg2 is one of the HMG-CoA (3-hydroxy-3-methyl-glutaryl-CoA) reductase isozymes which is considered as a rate-controlling enzyme of sterol synthesis (mevalonate pathway) in eukaryotes. Hrd1 contributes to the regulation of sterol synthesis via degradation of Hmg2.64,65 Similar to these studies, KEGG enrichment analysis and GO enrichment analysis results supported that Hrd1 contributes to the energy synthesis and metabolism (Figure 6). In summary, we found that MoHrd1 is conserved with other fungi and may play an important role in cellular metabolism and infection-related development. This study helps unveil the physiological roles and regulatory mechanism of Hrd1-involved ERAD pathway in fungi and provides a new idea to understand the pathogenic mechanism of M. oryzae.
Supplementary Material
Acknowledgments
The authors thank Dr Mingxiong Pang (Texas A&M AgriLife Dallas Center, Dallas, TX, USA) for English language editing and improving the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Natural Science Foundation of China (31601584 and 31701733), the Natural Science Foundation of Fujian Province (2016J05070), Science Fund for Distinguished Young Scholars of Fujian Agriculture and Forestry University to W.T. (KXJQ17020), and China Scholarship Council.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HJ, WT, and ZW conceived and designed the experiments and contributed reagents/materials/analysis tools. HJ, XC, QZ, WT, LS, TY, YD and BW performed the experiments. HJ, WT, and LL analyzed the experiment data. HJ, WT, JH, and ZW wrote the paper. All authors have read and approved the final manuscript.
References
- 1. McCaffrey K, Braakman I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016;60:227–235. [DOI] [PubMed] [Google Scholar]
- 2. Rothman JE, Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355:409–415. [DOI] [PubMed] [Google Scholar]
- 3. Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. [DOI] [PubMed] [Google Scholar]
- 4. Thibault G, Ng DT. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harb Perspect Biol. 2012;4:a013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koenig PA, Ploegh HL. Protein quality control in the endoplasmic reticulum. F1000Prime Rep. 2014;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb Perspect Biol. 2013;5:a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. [DOI] [PubMed] [Google Scholar]
- 8. Stevenson J, Huang EY, Olzmann JA. Endoplasmic reticulum-associated degradation and lipid homeostasis. Annu Rev Nutr. 2016;36:511–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. [DOI] [PubMed] [Google Scholar]
- 10. Christianson JC, Ye Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol. 2014;21:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. [DOI] [PubMed] [Google Scholar]
- 12. Gillece P, Luz JM, Lennarz WJ, de la Cruz FJ, Römisch K. Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol. 1999;147:1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakatsukasa K, Nishikawa S, Hosokawa N, Nagata K, Endo T. Mnl1p, an alpha-mannosidase-like protein in yeast saccharomyces cerevisiae, is required for endoplasmic reticulum-associated degradation of glycoproteins. J Biol Chem. 2001;276:8635–8638. [DOI] [PubMed] [Google Scholar]
- 14. Grubb S, Guo L, Fisher EA, Brodsky JL. Protein disulfide isomerases contribute differentially to the endoplasmic reticulum-associated degradation of apolipoprotein B and other substrates. Mol Biol Cell. 2012;23:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thibault G, Ismail N, Ng DT. The unfolded protein response supports cellular robustness as a broad-spectrum compensatory pathway. Proc Natl Acad Sci U S A. 2011;108:20597–20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silberstein S, Schlenstedt G, Silver PA, Gilmore R. A role for the DnaJ homologue Scj1p in protein folding in the yeast endoplasmic reticulum. J Cell Biol. 1998;143:921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bordallo J, Plemper RK, Finger A, Wolf DH. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell. 1998;9:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldridge RD, Rapoport TA. Autoubiquitination of the Hrd1 ligase triggers protein retrotranslocation in ERAD. Cell. 2016;166:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stein A, Ruggiano A, Carvalho P, Rapoport TA. Key steps in ERAD of luminal ER proteins reconstituted with purified components. Cell. 2014;158:1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoebel S, Mi W, Stein A, et al. Cryo-EM structure of the protein-conducting ERAD channel Hrd1 in complex with Hrd3. Nature. 2017;548:352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kikkert M, Doolman R, Dai M, et al. Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem. 2004;279:3525–3534. [DOI] [PubMed] [Google Scholar]
- 22. Maeda T, Marutani T, Zou K, et al. An E3 ubiquitin ligase, synoviolin, is involved in the degradation of immature nicastrin and regulates the production of amyloid beta-protein. FEBS J. 2009;276:5832–5840. [DOI] [PubMed] [Google Scholar]
- 23. Burr ML, Cano F, Svobodova S, Boyle LH, Boname JM, Lehner PJ. HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A. 2011;108:2034–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qi L, Tsai B, Arvan P. New insights into the physiological role of endoplasmic reticulum-associated degradation. Trends Cell Biol. 2017;27:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaneko M. Physiological roles of ubiquitin ligases related to the endoplasmic reticulum. Yakugaku Zasshi. 2016;136:805–809. [DOI] [PubMed] [Google Scholar]
- 26. Wilson RA, Talbot NJ. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol. 2009;7:185–195. [DOI] [PubMed] [Google Scholar]
- 27. Talbot NJ. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu Rev Microbiol. 2003;57:177–202. [DOI] [PubMed] [Google Scholar]
- 28. Ebbole DJ. Magnaporthe as a model for understanding host-pathogen interactions. Annu Rev Phytopathol. 2007;45:437–456. [DOI] [PubMed] [Google Scholar]
- 29. Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol. 2016;34:147–153. [DOI] [PubMed] [Google Scholar]
- 30. Valent B, Khang CH. Recent advances in rice blast effector research. Curr Opin Plant Biol. 2010;13:434–441. [DOI] [PubMed] [Google Scholar]
- 31. Park CH, Chen S, Shirsekar G, et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell. 2012;24:4748–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hogenhout SA, Van der Hoorn RA, Terauchi R, Kamoun S. Emerging concepts in effector biology of plant-associated organisms. Mol Plant Microbe Interact. 2009;22:115–122. [DOI] [PubMed] [Google Scholar]
- 33. Giraldo MC, Dagdas YF, Gupta YK, et al. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat Commun. 2013;4:1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khang CH, Berruyer R, Giraldo MC, et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell. 2010;22:1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J, Kou Y, Bao J, et al. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers pi9-mediated blast resistance in rice. New Phytol. 2015;206:1463–1475. [DOI] [PubMed] [Google Scholar]
- 36. Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B. Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell. 1995;7:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montenegro-Montero A, Goity A, Larrondo LF. The bZIP transcription factor HAC-1 is involved in the unfolded protein response and is necessary for growth on cellulose in Neurospora crassa. PLoS ONE. 2015;10:e0131415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yi M, Chi MH, Khang CH, et al. The ER chaperone LHS1 is involved in asexual development and rice infection by the blast fungus Magnaporthe oryzae. Plant Cell. 2009;21:681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin Z, Crockett DK, Lim MS, Elenitoba-Johnson KS. High-throughput analysis of protein/peptide complexes by immunoprecipitation and automated LC-MS/MS. J Biomol Tech. 2003;14:149–155. [PMC free article] [PubMed] [Google Scholar]
- 40. Lewis TS, Hunt JB, Aveline LD, et al. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol Cell. 2000;6:1343–1354. [DOI] [PubMed] [Google Scholar]
- 41. Gallie DR. Protein-protein interactions required during translation. Plant Mol Biol. 2002;50:949–970. [DOI] [PubMed] [Google Scholar]
- 42. Bonifacino JS, Dell’Angelica EC, Springer TA. Immunoprecipitation. Curr Protoc Immunol. 2001;41:8.3.1–8.3.28. [DOI] [PubMed] [Google Scholar]
- 43. Edmondson RD, Vondriska TM, Biederman KJ, et al. Protein kinase C epsilon signaling complexes include metabolism- and transcription/translation-related proteins: complimentary separation techniques with LC/MS/MS. Mol Cell Proteomics 2002;1:421–433. [DOI] [PubMed] [Google Scholar]
- 44. Li L, Chen X, Zhang S, et al. MoCAP proteins regulated by MoArk1-mediated phosphorylation coordinate endocytosis and actin dynamics to govern development and virulence of Magnaporthe oryzae. PLoS Genet. 2017;13:e1006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanehisa M, Araki M, Goto S, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. [DOI] [PubMed] [Google Scholar]
- 48. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43:D257–D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46:D493–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krishnan K, Feng X, Powers-Fletcher MV, et al. Effects of a defective endoplasmic reticulum-associated degradation pathway on the stress response, virulence, and antifungal drug susceptibility of the mold pathogen Aspergillus fumigatus. Eukaryot Cell. 2013;12:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yokota JI, Shiro D, Tanaka M, et al. Cellular responses to the expression of unstable secretory proteins in the filamentous fungus Aspergillus oryzae. Appl Microbiol Biotechnol. 2017;101:2437–2446. [DOI] [PubMed] [Google Scholar]
- 54. Krogan NJ, Cagney G, Yu H, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. [DOI] [PubMed] [Google Scholar]
- 55. Gardner RG, Swarbrick GM, Bays NW, et al. Endoplasmic reticulum degradation requires lumen to cytosol signaling: transmembrane control of Hrd1p by Hrd3p. J Cell Biol. 2000;151:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhamidipati A, Denic V, Quan EM, Weissman JS. Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol Cell. 2005;19:741–751. [DOI] [PubMed] [Google Scholar]
- 57. Kim W, Spear ED, Ng DT. Yos9p detects and targets misfolded glycoproteins for ER-associated degradation. Mol Cell. 2005;19:753–764. [DOI] [PubMed] [Google Scholar]
- 58. Izawa T, Nagai H, Endo T, Nishikawa S. Yos9p and Hrd1p mediate ER retention of misfolded proteins for ER-associated degradation. Mol Biol Cell. 2012;23:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol. 2000;3:24–29. [DOI] [PubMed] [Google Scholar]
- 61. Bordallo J, Wolf DH. A RING-H2 finger motif is essential for the function of Der3/Hrd1 in endoplasmic reticulum associated protein degradation in the yeast Saccharomyces cerevisiae. FEBS Lett. 1999;448:244–248. [DOI] [PubMed] [Google Scholar]
- 62. Cohen I, Wiener R, Reiss Y, Ravid T. Distinct activation of an E2 ubiquitin-conjugating enzyme by its cognate E3 ligases. Proc Natl Acad Sci U S A. 2015;112:E625–E632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mentlak TA, Kombrink A, Shinya T, et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geelen MJ, Gibson DM, Rodwell VW. Hydroxymethylglutaryl-CoA reductase—the rate-limiting enzyme of cholesterol biosynthesis. A report of a meeting held at Nijenrode Castle, Breukelen, the Netherlands, August 24, 1985. FEBS Lett. 1986;201:183–186. [DOI] [PubMed] [Google Scholar]
- 65. Wangeline MA, Vashistha N, Hampton RY. Proteostatic tactics in the strategy of sterol regulation. Annu Rev Cell Dev Biol. 2017;33:467–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.