Abstract
Shared decision making (SDM) is not widely practiced in routine care due to a variety of organizational, provider, patient, and contextual factors. This article explores how implementation science—which encourages attention to the multilevel contextual factors that influence the adoption, implementation, and sustainment of health care practices—can provide useful insights for increasing SDM use in routine practice. We engaged with stakeholders representing different organizations and geographic locations over three phases: 1) multidisciplinary workgroup meeting comprising researchers and clinicians (n = 11); 2) survey among a purposive sample of 47 patient advocates, clinicians, health care system leaders, funders, policymakers, and researchers; and 3) working session among diverse stakeholders (n = 30). The workgroup meeting identified priorities for action and research, which included targeting multiple audiences and levels, shifting culture toward valuing and supporting SDM, and considering contextual factors influencing SDM implementation. Survey respondents provided recommendations for increasing adoption, implementation, and maintenance of SDM in practice including providing tools to support SDM, obtaining stakeholders’ involvement, and raising awareness of the importance of SDM. Stakeholders in the working session provided recommendations on the design of a guide for implementation of SDM in clinical settings, strategies to disseminate educational curricula on SDM, and strategies to influence policies to increase SDM use. These specific recommendations serve as a call to action to pursuing specific promising strategies aimed at increasing SDM use in practice and enhance understanding of the perspectives of diverse stakeholders at multiple levels from an implementation science perspective that appear fruitful for further study and application.
Keywords: shared decision making, implementation science
Shared decision making (SDM) is defined as a collaborative process that encourages patients and their providers to make health care decisions together, taking into account the best clinical evidence available to weigh the risks and benefits, as well as the patient’s values and preferences.1 SDM has been referred to as a pinnacle of patient-centered care.2 From the bioethical perspective, it is also viewed as the right thing to do.3 SDM is conceptualized as a complex set of behaviors that require engagement beyond the patient–provider dyad level, involving the health care team, health system, and health policy levels.4
Impetus for SDM Implementation
Recent trends in health care and health-related research have significant implications for implementing SDM in practice. First, there is growing emphasis on ensuring patient engagement and patient-centered care, development of SDM tools, training,4 policies, and incentives to promote SDM (e.g., Affordable Care Act provisions to encourage SDM).5,6 Second, there is increased recognition of the need for and investment in pragmatic research and comparative effectiveness research in patient-centered care as evidenced by the establishment of the Patient Centered Outcomes Research Institute in 2010.7 Third, the growing field of dissemination and implementation science8,9 has fueled a focus on population impact and the importance of translation of health care interventions by emphasizing factors that maximize reach, implementation, feasibility, and sustainability while minimizing cost and addressing disparities.
Evidence of Effects of SDM and Implementation Barriers and Facilitators of SDM
A growing body of evidence suggests SDM is associated with reduced decisional conflict and improved patient knowledge, participation, accurate risk perceptions, behaviors, treatment satisfaction, completion, and patient-reported outcomes in the short term.4,10–13 However, there is limited research on the potential consequences of SDM on patients at distal and distant time points or effects of SDM on clinicians, relationships with patients and within the care teams, the health care organization, and the health care system.14 Despite the availability of SDM interventions—defined as efforts, approaches, or tools applied at multiple levels to support the use of SDM in practice including decision support tools, training, policies, and incentives to promote collaborative deliberation between patients and their health care providers—SDM is not widely practiced in routine care.15 Research has documented extensively the key barriers and facilitators of using SDM at multiple levels. Examples of barriers include health care professionals’ indifference16; lack of consideration of outcomes of SDM at the team, organization, or health care system culture17; time constraints, patients’ interest and preparedness in perceived SDM; lack of applicability for clinical situations (e.g., when there is a medically preferable treatment decision); and funding models and incentives that conflict with SDM goals (e.g., fee-for-service or pay for performance).18 Facilitators include health professionals’ motivation, perceptions of positive outcomes of SDM on patient outcomes, clinical process, supportive leadership at all levels to integrate SDM into practice, constant evaluation and iterative improvement, and embedding SDM in physician training and culture.19–21 In this article, we seek to expand on prior literature that describes the multilevel factors of integrating SDM into practice by examining these issues through an implementation science perspective8,9 and obtaining concrete actionable recommendations from diverse stakeholders at each level (patient, provider and care team, organization, and policymakers) on how to promote the adoption, implementation, and maintenance of SDM in practice.
Leveraging Implementation Science to Translate SDM Into Routine Practice
Implementation science provides a framework to inform the integration of SDM into routine practice because it acknowledges that successful implementation of health care interventions into practice depends on addressing contextual and multilevel factors.8 Also, it addresses the balance between fidelity of an intervention (to evidence-based principles and key components of an intervention) and adaptation of the intervention to best suit each local setting, available resources, and patients.22 Implementation science can also help broaden conceptualization and measurement of outcomes to address population reach and impact, generalizability of results, and feasibility of applying different SDM interventions. Maybe most important, implementation science perspectives are increasingly being used to “design for dissemination and sustainability.”23
Our work was informed by an implementation science framework, the Practical Robust Implementation and Sustainability Model (PRISM),24 which helps researchers consider related concepts from diffusion of innovations, social ecology, the PRECEDE/PROCEED model, quality improvement, the Chronic Care Model, and the RE-AIM framework.25–31 PRISM considers how an intervention’s design, the external environment, implementation and sustainability infrastructure, and the characteristics of recipients of the intervention (i.e., the adopting organization, providers, and patients) interact to influence the intervention’s adoption, implementation, and maintenance. PRISM has been utilized in assessing the perspectives of stakeholders toward the implementation of a variety of health care services (e.g., population-based colorectal cancer screening and well-child care within large health care delivery systems).32,33 In this current work, we focused on the adoption, implementation, and maintenance dimensions of the PRISM.
This work was further guided by findings from SDM implementation projects that described the contextual factors, processes, barriers, and facilitators at multiple levels to successful integration of SDM interventions within health care practices. For instance, Lin and colleagues evaluated the dissemination of decision aids for facilitating patient engagement and SDM for two topics—colorectal cancer screening and low back pain—within five primary care practices in Northern California.34 The authors reported low distribution of decision aids; approximately 10% of eligible patients received these decision aids during the project period. They identified significant structural and cultural barriers to implementing the integration of decision aids distribution. These included lack of physician training, physicians’ reluctance to utilize SDM, time constraints, and the lack of a supportive clinic culture.34 In addition, King and Moulton described the lessons from the Group Health Shared Decision-Making Demonstration Project to distribute decision aids for 12 preference-sensitive surgical conditions (e.g., knee and hip osteoarthritis, benign prostatic hyperplasia, and early-stage breast cancer).20 The authors acknowledged the importance of the Washington state legislature’s role in catalyzing meaningful changes within health care systems to encourage SDM into clinical practice.20 They further identified important factors from Group Health’s experience that might inform other institutions interested in integration of SDM into routine clinical care. These factors include having substantial leadership committed to integrating SDM into practice at all levels of the organization, conducting constant evaluation and iterative refinement to the delivery of decision aids, and supporting physicians with training and efforts to change culture at the systemwide level.20 A recent scoping review by Scholl and colleagues identified several organizational characteristics that influence SDM implementation including leadership, culture, teamwork, resources, priorities, and workflows reported in implementation projects.17 The authors also described health care system–level characteristics including incentives, policies and guidelines, culture, and provider education and licensing that were described in the literature.17
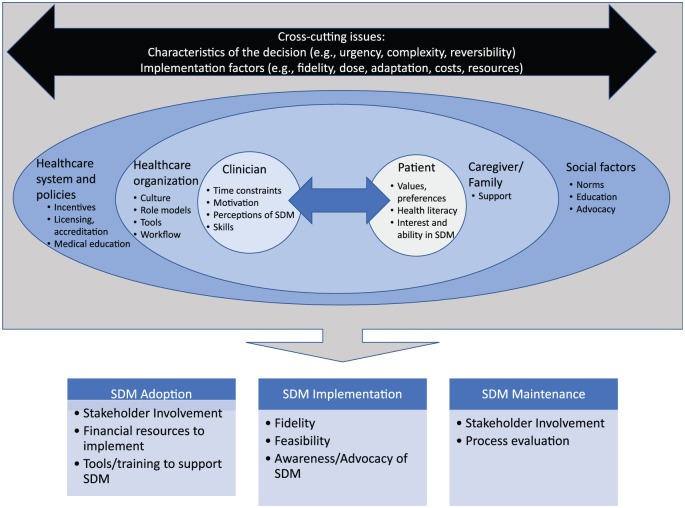
Figure 1 illustrates a proposed conceptual model integrating the key contextual factors and cross-cutting implementation issues that influence SDM adoption, implementation, and maintenance based on the PRISM framework and prior literature on SDM implementation. For instance, as described in PRISM, in considering implementation of an evidence-based SDM intervention into practice, it is critical to first understand the characteristics, values, resources, contingencies, and priorities of each of the target audiences at multiple contextual levels including health care organization leaders (and guidelines), clinicians and health care team, policy and financial issues, and patients and their families. It is also important to consider cross-cutting issues across these audiences including the characteristics of the health care decision and implementation factors associated with the intervention. In sum, these contextual factors influence the extent of successful adoption, implementation, and maintenance of the SDM intervention.
Figure 1.
Contextual factors influencing shared decision making adoption, implementation, and maintenance.
Population Health Impact of Designing SDM Interventions for Dissemination and Sustainment
The convergence of the growing emphasis on patient-centered care in health care, availability of evidence-based SDM interventions, and advances in implementation science present new opportunities for facilitating integration of SDM into routine care. At the same time, they provide an impetus to look closely at the current state of evidence and application of that knowledge, and to identify the key gaps in SDM reach, adoption, implementation, and sustainment within diverse practices. Evidence-based SDM, if consistently implemented with fidelity, has the potential to realize the benefits for individual patients and families, improve the quality of medical decision making, ensure respect for patient preferences, minimize adverse effects from under- or overutilization of care, and ultimately improve patient and population outcomes and reduce health inequalities.35 Implementation science has the potential to help realize the benefits of progress in each of these areas, to sustain effective SDM interventions (or discontinue unsuccessful ones) and to scale up to different settings and populations36 to improve population health outcomes, and reduce disparities in care. For instance, researchers proposed implementing SDM to reduce health care disparities through designing materials that will help people with lower literacy or socioeconomic status with medical decision making.37 A systematic review found that interventions to support SDM improved knowledge, informed choice, and participation in decision making among patients from disadvantaged populations more than patients with higher literacy, education, and socioeconomic status and suggested that supporting SDM would help reduce health inequalities.35
Objectives
The objectives of this work are threefold. First, we summarize input from experts in SDM and implementation science on priorities for actionable approaches for improving SDM use and a research agenda that would better integrate SDM and implementation science. Second, beyond describing facilitators and challenges to using SDM, we aimed to generate concrete and actionable recommendations from stakeholders at multiple levels to increase the adoption, implementation, and maintenance/sustainment of SDM into practice. Third, we aimed to obtain suggestions for strategies from various stakeholders on how to address specific action priorities.
Methods
Overview
We addressed the study objectives through engaging with diverse stakeholders across different organizations and geographic locations in implementing SDM interventions over three phases. In Phase 1, we convened a multidisciplinary workgroup meeting of researchers and clinicians to discuss illustrative cases of using SDM in cancer prevention and control, with the goal of applying an implementation science lens to the challenges and facilitators to SDM use. In Phase 2, we conducted a survey among a purposive sample of patient advocates, clinicians, health care system leaders, funders, policymakers, and researchers to obtain actionable recommendations to improve SDM adoption, implementation, and sustainment in a variety of cancer care scenarios. By actionable recommendations, we mean to encompass discrete implementation strategies for getting evidence-based practices into routine use as described in existing literature,38–41 as well as recommendations that address the “how” to do so, such as recommendations that are feasible, achievable, align with levels of resources, training, and time available in most settings from stakeholders’ perspectives. In Phase 3, we invited diverse stakeholders representing different roles and organizations to a working session to generate recommendations to apply implementation science to increase SDM use in practice through training, tools to support SDM use, and policy change. We synthesized the recommendations from all three phases as a call to action and research to increase the use of SDM in routine care.
Phase 1: Expert Panel Workgroup
Participants
We convened an expert panel composed of researchers and health care practitioners across different disciplines (including implementation science, health communication, decision science, health services, oncology, gerontology, chronic care, and patient-centered outcomes research; n = 11) for a workgroup meeting at the 2016 Annual Conference on the Science of Dissemination and Implementation in Health. The objective of the meeting was to generate input on priorities for actionable approaches for improving SDM use and a research agenda that would better integrate SDM and implementation science. During the meeting, participants considered three clinical decision scenarios where patients faced a decision—human papillomavirus (HPV) vaccination for cancer prevention, lung cancer screening with low-dose computed tomography (CT) scans for early detection, and advance care planning with patients diagnosed with advanced cancers. We selected these scenarios to highlight certain variations in the contextual factors related to different decisions. For instance, in the case of HPV vaccination, the decision is typically made in a nonurgent, outpatient setting. The vaccination is generally considered by most clinicians to be low in complexity, has minimal adverse effects, is nonreversible, and not preference-sensitive. In comparison, the scenario for CT scans for lung cancer screening is low-to-moderate complexity; findings may trigger additional invasive diagnostic tests such as biopsies and treatments, which could pose adverse effects. The patient may also require recurrent scans for up to 3 years. Advance care planning creates a third scenario where patients are tasked with forecasting about decisions related to the end of their lives. The workgroup members discussed what the priority areas are to focus, key challenges, and opportunities to integrate SDM and implementation science. Following the meeting, a core group from this workgroup (RG, DDM, KM, and AT) synthesized the input from the meeting on the key contextual factors and implementation issues in the use of SDM.
Summary of Findings
The workgroup identified three key priorities for action and research to increase SDM use from an implementation science perspective. First, members recommended targeting multiple audiences and levels in promoting wide SDM use. For instance, beyond targeting interventions at the main enactors of SDM (i.e., patients and clinicians), efforts should engage health care organizational leaders, policymakers, and funders and intervene at the individual, interpersonal, organizational, and policy levels. Second, generating the evidence base for SDM interventions is necessary but insufficient. Beyond generating evidence-based SDM interventions, greater attention is needed on shifting the culture toward valuing SDM and providing the necessary organizational infrastructure, incentives, and policies to support SDM. Third, there was consensus within the workgroup that one size does not fit all and that it is critical to consider contextual factors that would influence implementing SDM interventions in different settings and decision situations. For instance, members suggested that SDM interventions including decision aids and communication training will need to allow for adaptation to meet the needs of individual patients, clinicians, health care organizations, and for the specific medical decision being considered.
Phase 2: Stakeholder Survey
Participants and Data Collection
Based on the key findings from Phase 1, we designed a web-based open-ended survey and invited a purposeful sample of members from the aforementioned workgroup and additional patient advocates, clinicians, researchers, health systems leaders, and funders (n = 47) to complete the survey. The objective of the survey was to generate actionable recommendations from these diverse stakeholders of ways to increase consistent use of SDM in practice. A list of 86 potential participants was generated based on discussion among the core group with the goal of obtaining the perspectives from different stakeholders who are knowledgeable about SDM and represent different organizations and perspectives. Potential participants were invited via email and completed the survey through the REDCap platform (Online Appendix A). Those who agreed to participate were asked to identify their primary role (e.g., patient advocate [n = 4], clinician [n = 10], researcher [n = 21], health system leader [n = 4], policymaker [n = 2], or funder [n = 5]). One individual did not select a role. They were also asked to choose one decision scenario that they felt SDM was most important (e.g., HPV vaccine, prostate-specific antigen screening, screening mammography, genetic testing for cancer risk, adjuvant chemotherapy for early-stage breast cancer, making decisions about stopping or forgoing aggressive curative treatment, or other). Those who selected other scenarios wrote to specify that they thought SDM were equally important in all situations and not just one of the above-mentioned scenarios or specific alternative treatment scenarios (e.g., depression, chronic conditions, deciding on medications). Participants were then asked to provide their recommendations for ways to increase the adoption, implementation, and sustained use of SDM in practice based on their selected role and decision scenario (Online Appendix B).
Analysis
Responses were coded using the qualitative analysis software NVivo (version 11). Two coders (DM and SJL) reviewed the survey responses to identify potential themes for coding. The unit of analysis was at the level of individual participants, and the unit of coding was the response to each question within the survey. After reading through a subset of responses (n = 3), coders defined the following primary themes: 1) Evaluation, 2) Level of intervention, 3) Obtain stakeholders’ involvement, 4) Raise awareness, and 5) Support SDM use. Three of the primary themes were further coded into more specific subthemes (Evaluation, Level of intervention, and Support SDM use). For instance, the primary theme “Support SDM use” included subthemes for training, tools, funding or reimbursement, culture, and other support. We created a codebook (Online Appendix C) that defined each of the primary themes and subthemes to facilitate consistency across coders for the remaining responses. Coders met regularly to review and discuss disagreements for any theme or subtheme. Disagreements were resolved by consensus following discussion between the coders. The coders achieved an average kappa score above .75 for all codes. We analyzed the themes and subthemes by stage of implementation (adoption, implementation, and maintenance), role that the participant identified with, the selected decision scenario, and also by the level of intervention.
Results
Table 1 summarizes the most frequent themes based on participants’ recommendations for increasing adoption, implementation, and maintenance of SDM. For increasing adoption, the most frequent themes were providing tools to support SDM (n = 11) followed by obtaining stakeholders’ involvement (n = 9). For instance, participants recommended the use of decision aids, provider recommendations, and “better tools for clinicians to use to help describe to patients the benefits, risks, harms, and costs of various treatments.” For obtaining stakeholders’ involvement, suggestions included “having the providers at the table when the research project is being designed,” including family members early in the treatment conversation, and bringing the clinical leaders together to discuss the issues pertaining to SDM adoption in practice.
Table 1.
Recommendations for Increasing SDM Adoption, Implementation, and Maintenancea
| Adoption | Implementation | Maintenance | |
|---|---|---|---|
| Provide tools to support SDM | 11 | 12 | 6 |
| Obtain stakeholders’ involvement, discussion, and communication including patients, families, clinicians, and health systems leaders | 9 | 10 | 9 |
| Raise awareness and/or advocacy to promote SDM | 6 | 6 | 8 |
| Evaluate outcomes of SDM to make sure it is beneficial | 6 | 4 | 3 |
| Provide training for clinicians and patients to use SDM | 6 | 4 | 2 |
| Funding or reimbursement to implement SDM | 6 | 3 | 5 |
| Determine feasibility of SDM in practice | 3 | 4 | 0 |
| Evaluate process by following up to make sure SDM is happening | 2 | 4 | 5 |
| Culture change to make SDM the norm | 3 | 1 | 1 |
SDM = shared decision making.
Frequencies refer to number of respondents who indicated a recommendation related to the theme or subtheme. Individual responses may be coded as more than one theme.
Participants also recommended providing tools (n = 12) and obtaining stakeholders’ involvement, discussion, and communication (n = 10) as the most frequent ways to increase implementation. Recommendations related to these themes for the implementation of SDM include integration of SDM tools within the electronic health record and existing care workflow, convening patient and family councils to review the quality of clinical care, involving the patient’s voice, and working with community members on the development of SDM in practice. For instance, one participant recommended “integrat[ing] patient voice into practice management to assure that patient perspective is considered in design, implementation, assessment and maintenance of practice operations, including but not limited to shared decision making.”
To sustain maintenance of SDM use, participants most frequently recommended obtaining stakeholders’ involvement (n = 9) followed by raising awareness or advocacy (n = 8). An example of recommendations to obtain stakeholders’ involvement was to regularly reassess the patient’s values regarding their health and having multiple conversations so the patient is aware of their ongoing treatment. Suggestions for raising awareness included political advocacy for metrics to measure clinician effectiveness in SDM, developing learning networks to promote SDM models, and presenting the benefits of SDM at scientific meetings.
Overall, providing tools to support SDM, obtaining stakeholders’ involvement, and raising awareness were the most common recommendations across different self-identified roles of the participants, the decision scenario, and levels of intervention. Table 2 summarizes illustrative quotes from each theme/subtheme based on participants’ recommendations.
Table 2.
Illustrative Quotes From Stakeholder Survey
| Theme/Subtheme | Quote |
|---|---|
| Evaluation | |
| Outcomes | “Feedback loops regarding effectiveness of the SDM tools. Effectiveness could/should be measured on patient reported measures (e.g., satisfaction, QOL, etc.).” |
| Process | “Culture change, training in principles and techniques, better ways to document and measure SDM to know it is actually happening in practice (rather than something else, like just giving a decision aid).” |
| Feasibility | “Conduct well-designed stakeholder-engaged studies to show the feasibility and value for all stakeholders, and work closely with a full range of supportive stakeholders to facilitate adoption by identifying and working to overcome barriers.” |
| Other evidence | “Revisit decisions after they have been made and implications are clear. Revisit decision trees that led to current choices.” |
| Level | |
| Patient and/or family | “Give patients the time to think about the items in the SDM tool, but set a clear deadline for when you will return to discuss their thoughts and values (otherwise patients may avoid these questions because they are difficult issues and this will not be done). Offer support to the patient to use the SDM tool, that is, offer to have the social worker, chaplain, or palliative care or family members work with the patient to elicit their values and preferences.” |
| Provider and/or practice | “Develop simple methods to apply a shared decision-making tool in clinical practice—incorporate it into the electronic health record and include a simple training video with the tool to help providers recognize how to use it—and to provide links to additional free archives webinars that give more in-depth training on how to use it.” |
| System | “SDM has most chance of success if included as part of a larger system of coordinated care that supports a team-based, multidisciplinary approach with patients and caregivers.” |
| Other levels | “Again, working with community members on the development and delivery (how do they want to hear about it, from who, when, what media) and then taking answers to those questions into consideration in developing the stakeholder group that will champion delivery. Acknowledging the answers to these questions will vary by community and experience with historical distrust of medical and research professionals.” |
| Obtain stakeholders’ involvement, discussion, and communication | “External pressure from patients, families, and communities. Give them a voice and some real power.” |
| Raise awareness and/or advocacy | “Raise awareness! Give us a campaign (posters and short/simple YouTube) to spread among patient communities.” |
| Support | |
| Tools | “Better tools for clinicians to use to help describe to patients the benefits, risks, harms, and costs of various treatments.” |
| Training | “Training clinicians in what SDM means and how to have the conversations. Connecting with guidelines (which seem to drive adjuvant therapy decisions) to place more emphasis on variation in patient preferences, risk tolerance, etc.” |
| Culture | “Cultural change is key. In theory, many agree that shared decision making is important, but on the ground, clinical enterprises are still very much provider-centric. The voice and choice of the patient is not nearly valued as much as the voice/choice of the ‘expert’ provider. Until that power differential exists, it will be hard to have true ‘shared’ decision making. This power differential is particularly real for patients that come from diverse ethnic and cultural background and/or come from lower SES.” |
| Funding/reimbursement | “Make it a normal, expected, quality, reimbursed part of care.” |
| Other support | “Develop system for providing patients with information (e.g., written); develop mechanisms for standardized time/workflow; have some follow-up or check-in for completion” |
QOL, quality of life; SDM, shared decision making; SES, socioeconomic status.
Phase 3: Multistakeholder Working Session
Participants
Drawing from the findings obtained in Phase 1 and Phase 2, the core group identified three promising action priorities for increasing SDM use in routine care across multiple levels: 1) design a guide for implementation of SDM in clinical settings, 2) disseminate educational curricula on SDM, and 3) influence policies to increase SDM use. We convened a working session at the 2017 Annual Conference on the Science of Dissemination and Implementation in Health. The working session comprised 30 stakeholders including some of the participants from Phases 1 and 2 and additional stakeholders identified by the core group to have relevant expertise in SDM and/or implementation science based on their current and prior work. Patient advocates, clinicians, researchers, health system leaders, policymakers, and funders were represented at the working session. The working session was not limited to conference attendees and included stakeholders who were invited specifically for the session. The objective of the working session was to obtain specific strategies from various stakeholders who self-selected into one of three breakout group discussions addressing each of the three action priorities. For the group addressing characteristics of a guide for implementation of SDM, participants were asked to recommend key components of such a guide, adaptations and customization of SDM for different settings, tracking the progress of SDM implementation, and research that would be needed to develop and evaluate the guide. The implementation guide is intended to include key components to be consistently implemented and also to consider the contextual factors unique to each health care environment and to facilitate the implementation of SDM. This balance between fidelity to the core components of SDM and appropriate adaptation to local context is another contribution of implementation science to this work as both fidelity and appropriate adaptation to local contexts are needed.23,42 Participants in the second group addressing educational curricula were asked to recommend strategies to facilitate adoption of SDM training curricula in medical, nursing and allied health schools, fellowship programs, and among practicing clinicians. Finally, participants in the third group addressing policies to increase SDM use were asked to recommend target audiences for policy intervention, strategies to engage these audiences for policy change, and essential questions to answer to influence policy. This group included experts in health care communication, health care professional training, SDM training curricula development, or were patient advocates who have advocated for SDM training. Participants were asked to complete a brief survey providing their recommendations for their breakout group topic.
Results
Table 3 summarizes key recommendations from each breakout group discussion, and Table 4 presents illustrative quotes from participants’ responses to the brief survey.
Table 3.
Summary of Specific Recommendations From Breakout Group Discussions in Phase 3
| Group 1: Designing a guide for implementation of SDM in clinical settings. | |
| Key components of adaptation guides | •Have a specific and measurable definition of SDM •Include the ability to identify eligible population •Incorporate a tracking and monitoring system •Elicit patient preferences •Allow provider feedback •Ensure the guide is integrated with the clinics’ workflow •Show evidence-based visual representations of risk and benefits •Build in prompts/reminders within the system |
| SDM adaptations and customizations | •Consider workflow and how to integrate SDM into workflow •Allow customization to different practice settings •Allow adaptation to fit system, setting, workflow, provider input and tracking and patient preference •Create a framework with primary stakeholder (end users), with interchangeable details within model (interchangeable parts theory). •Track fidelity of adaptation |
| SDM implementation progress tracking | •Track implementation results and adaptations made •Specify what will be tracked, how, and when to report or provide feedback to decision makers •Train staff on what will be tracked and account for patient preferences •Design “EHR/certification” that SDM has occurred that is not easy to “game” |
| Needed research for guide development and application | •Determine the minimum eligibility criterion for when to implement SDM •Isolate the most parsimonious set of strategies to support SDM •Determine when is the optimal time to “trigger” engagement with patients in SDM |
| Group 2: Disseminate educational curricula on SDM | |
| Strategies to ensure wide adoption of SDM curricula among medical, nursing, and allied health professional schools | •Work with school leadership to create culture for SDM •Provide adequate role models among faculty and clinicians for students •Timing of SDM training—Expose students early to increase awareness and later to get hands-on experience •Incorporate SDM competency as part of National Board of Medical Examiners (NBME) clinical skills exams |
| Strategies to ensure wide adoption of SDM curricula among residency/fellowship programs | •Provide role models and direct observation of senior clinicians for trainees •Build SDM into competency for graduating or completing course/rotation |
| Strategies to ensure wide adoption of SDM curricula for continuing education among practicing health professionals | •Institute health system policies to require annual SDM training of all clinicians •Build the organizational and professional culture to support SDM •Tie SDM training to existing metrics such as patient experience, physician/employee benefit •Use the intrinsic lever of providing best care •Develop good SDM metrics |
| Group 3: Influencing policies to increase SDM use | |
| Targets for policy interventions | •Target national agencies—Centers for Medicare and Medicaid Services (CMS), National Quality Forum (NQF), National Committee for Quality Assurance (NCQA) •Engage with states (e.g., Washington state), health lawyers, and health systems (e.g., Kaiser Permanente) •Convene patient advocacy groups (Partnership to Improve Patient Care [PIPC], Society for Participatory Medicine [S4PM], Faster Cures, etc.) |
| Strategies to engage these groups | •Support the process of regulation of decision aids, for example, NQF •Work with government relations and reimbursement experts •Determine the return on investment, business case or value proposition for all stakeholders using decision tools, for example, health care system, patients, doctors, and policymakers •“Pick your battles” and focus on SDM for high-impact situations, for example, costly and preference-sensitive decisions/domains |
| Essential questions to answer to influence policy | •Create certified, high-impact SDM tools and aids before beginning implementation •Conduct case studies of states that created a favorable policy landscape for SDM, for example, Washington |
SDM, shared decision making.
Table 4.
Illustrative Quotes From Working Session Breakout Group Discussions
| Group 1: Designing a guide for implementation of SDM in clinical settings | |
| Key components of adaptation guides | “Way to identify eligible population. Core and optional components of SDM. Ways to track and monitor progress. Roles-definition and process tools. Itemize and document.” |
| SDM adaptations and customizations | “Using the interchangeable parts theory, creating a framework with primary stakeholder (end users), with interchangeable details within model. Each discipline may have differing specifics, but the process framework seems universal.” |
| SDM implementation progress tracking | “Incorporate into workflow. Engage and train staff. Engage stakeholders and build consensus about timing and tracking procedures. Account for patient preferences.” |
| Needed research for toolkit development and application | “Need to isolate/test the most parsimonious set of strategies to support SDM delivery with fidelity.” |
| Group 2: Disseminate educational curricula on SDM | |
| Strategies to ensure wide adoption of SDM curricula among medical, nursing, and allied health professional schools | “Work with student organizations like American Medical Student Association to create the ground swell for culture of SDM.” |
| Strategies to ensure wide adoption of SDM curricula among residency/fellowship programs | “Need for role models and direct observation. Build into competency for graduating or completing course/rotation.” |
| Strategies to ensure wide adoption of SDM curricula for continuing education among practicing health professionals | “Provide CE across multiple disciplines to support team based implementation.” |
| Other strategies | “By the way we need to train the public in this starting in grade school! Every patient should have a clear sense of the question ‘What’s important to you?’ before each visit. This must be taught.” |
| Group 3: Influencing policies to increase SDM use | |
| Targets for policy interventions | “Focus on CMS. Their decision to pay for SDM is huge. We should push them to incentivize SDM for more treatment decisions, and help them by advocating for a certifying body to ensure SDM is done well.” |
| Strategies to engage these groups | “Know your context (ecosystem)-where is the perceived need greatest? Know your competition-competing messaging and behaviors. Focus the message on providing a solution for ‘jobs to be done’ of your target audience adopter, for example, meeting accreditation, saving money, meeting patient demand or satisfaction” |
| Essential questions to answer to influence policy | “Do a case study of Washington State to examine how policy levers, health systems, patient voice, regulation/certification, and payment approaches combined to create a favorable landscape for SDM.” |
CE, continuing education; CMS, Centers for Medicare and Medicaid Services; SDM, shared decision making.
Designing a guide for implementation of SDM in clinical settings
Participants identified key components of an implementation guide including defining SDM and specific measures for SDM, built-in prompts or reminders to trigger SDM within the existing workflow, incorporating patient preferences, clinician feedback, and a tracking system. Participants recommended allowing for adaptation and customization to different health systems, setting, workflow, provider, and patient preferences, and tracking fidelity of the adaptation. Addressing this recommendation, one participant suggested using “the interchangeable parts theory, creating a framework with primary stakeholders (end users), with interchangeable details within [the] model.”
Disseminate educational curricula on SDM
Key recommendations related to education included early exposure to SDM and repeated training over the arc of health professionals’ education and career to ensure adoption. Another recommendation was to ensure adequate role modeling by practicing clinicians for trainees and junior clinicians such that SDM skills learned through educational curricula are consistently practiced and not discouraged. Participants further described incentives and levers to promote SDM as a required skill, such as providing continuing education credits to support team-based implementation of SDM, including SDM within clinical certification examinations, employment policies for clinicians requiring annual SDM training, and connecting SDM training to existing metrics such as patient experience and benefits to physicians and employees. One participant recommended training the public beginning in grade school to be able to enact SDM conversations at each visit with their health care provider.
Influencing policies to increase SDM use
Participants identified several key target audiences for influencing policy interventions to promote SDM. These included national organizations (e.g., Centers for Medicare and Medicaid Services, National Quality Forum, and National Committee for Quality Assurance), patient advocacy groups (e.g., Partnership to Improve Patient Care, Society for Participatory Medicine, and Faster Cures), states, health lawyers, and health systems. The group recommended specific strategies to best engage with these groups that included identifying the value proposition for various stakeholders involved in implementing SDM and focusing on SDM for high-priority decision situations (e.g., costly and preference-sensitive treatment decisions). For example, one participant recommended “choos[ing] the decision context carefully based upon disease burden and variability that leads to overtreatment and undertreatment.”
Discussion
Our findings are consistent with current literature describing multilevel factors as key influences on SDM implementation including organizational and health care system characteristics.17,43,44 For instance, we obtained recommendations from diverse stakeholders obtained in Phases 2 and 3 of our work to conduct SDM and communication skills training for health care professionals, provide tools to support SDM, and raise awareness of the importance of SDM. Similarly, Scholl et al. summarized strategies to address organizational- and system-level characteristics, which included supporting health care professionals in learning SDM skills, making decision aids and tools more accessible in exam rooms and workplaces, and promoting a strong consistent message about the importance of SDM.17
In addition, this research adds to the literature in the following ways. First, we obtained more detailed input on specific ways to achieve these recommendations for improving SDM use compared with those described in the Scholl et al. review, which the authors deemed as vague and needing further specification and tailoring to a specific context.17 Second, the recommendations we obtained from diverse stakeholders were also more comprehensive and spanned multiple levels influencing SDM implementation compared with prior reviews that focused on either individual factors or organizational/system-level factors but not across these levels of influences.17,18,43,45 In sum, this study informs future research and applications to focus on promising strategies at multiple levels including to enhance SDM use. The current work provides an example for integrating principles of implementation science with SDM research to inform promising and novel strategies for SDM adoption, implementation, and sustainment in routine practice using an implementation science model and approaches (PRISM).
There is growing interest from clinicians, health systems, and funding agencies in utilizing an implementation science framework for advancing evidence-based SDM interventions in health care settings.14,46 There are several implementation science frameworks that could be used to approach this issue, including the Consolidated Framework for Implementation Research that has described characteristics of inner and outer settings to influence implementation.47 We elected to use the PRISM (and its component RE-AIM dimensions) to inform our work because of the objective of obtaining recommendations that were specific to the phases of adoption, implementation, and maintenance. Funding agencies including the Patient-Centered Outcomes Research Institute have also initiated opportunities to target implementation science approaches in SDM delivery.48
One of the key findings that emerged from the work reported here is that translating SDM into practice is complex and requires an in-depth understanding of and attention to multilevel contextual factors. Contextual factors include not only characteristics of the patient, clinician, or the health care organization but also the specific health care decision under consideration. Each of these factors has the potential to influence whether and how SDM occurs. Both the design of interventions to support SDM and the strategies used to implement those interventions in practice will need to differ to fit diverse settings. For example, implementing a decision support tool in an outpatient setting around prostate cancer screening will require a decision support strategy that considers the time pressures and competing demands of the outpatient clinicians, the availability of other staff to do SDM, and the desire of the patient for SDM. In contrast, a patient faced with a decision of whether to pursue additional chemotherapy or transition to hospice care will have very different needs, and the optimal decision support strategy may be having the oncologist respond effectively to emotions and present prognosis information empathetically.
Another key lesson from this work is that a coordinated approach to engage all stakeholders is critical to ensuring success in efforts to plan, deliver, implement, and sustain SDM in routine practice. This message emerged consistently as one of the top recommendations from our stakeholder survey in Phase 2. Effective stakeholder engagement will require understanding their priorities, obtaining their buy-in and commitment, building strong working relationships at each phase of the SDM intervention, identifying contribution and resources from each stakeholder, sharing data on whether SDM is occurring as planned, and demonstrating the value and impact of SDM for each stakeholder from their perspectives. For instance, access to tools and training for health care professionals throughout their careers and to patients was a key priority that would help them engage in SDM effectively with one another. Clinicians recommended that tools to support SDM should be integrated within their workflow to ensure that they would be utilized. In addition, patient advocates wanted greater representation of the patients’ voice in developing SDM interventions. From health care system leaders’ and funders’ perspective, it would be important to measure and highlight the business case and value proposition for SDM use. Ultimately, ongoing and sustained engagement with all relevant stakeholders is needed to motivate sustainable organizational and cultural change to value SDM as an integral part of high-quality health care.
The broader policy and resource challenges to support SDM use in practice should be acknowledged in prioritizing the recommendations obtained in this work. For example, the Center for Medicare and Medicaid Innovation (CMMI) announced in 2016 an incentive model to provide financial support for accountable care organizations (ACOs) to invest in a structured process of integrating SDM into clinical practice and to reimburse ACOs for SDM services (e.g., for lung cancer screening). However, the model was discontinued in 2017 following low participation among the ACOs. The Agency for Healthcare Research and Quality (AHRQ) has the SHARE Approach and published the training curriculum and implementation tools although it is unclear if the SHARE Approach is being supported. In 2016, AHRQ had issued a funding announcement focused on development of SDM measures. To our knowledge, no study has been funded under this announcement. Although research support to develop evidence-based SDM tools is available, the field faces challenges in supporting the dissemination and updating of these tools once they are developed. For example, evidence-based, certified, and well-designed decision aids may not be available for certain conditions, and more important, even when they are available, they may be hard to integrate into the clinical workflow. Given the earlier interest from CMS/CMMI to reimburse providers for delivery of SDM services, efforts should be targeted at CMS to incentivize SDM for more medical decisions and to support CMS through certification of evidence-based SDM tools, monitoring and reporting of performance of SDM, and measuring health outcomes arising from SDM.
The work reported here has both strengths and limitations. Strengths include the use of a sequence of meetings and different data collection methods and the diverse perspectives involved. The descriptive quantitative survey data are clarified through qualitative coding and illustrative quotes. One limitation is despite considerable recruitment efforts, there were few participants for some roles (e.g., policymakers, patient advocates) to investigate possible differences among stakeholders from these different roles. Second, real-world patients’ and clinicians’ voices may be underrepresented within the purposive samples of academic clinicians and professional patient advocates. Future research should involve diverse stakeholders including patients and clinicians to provide their perspectives on practicing SDM in real-world settings.
In conclusion, although preliminary, this report identified several key recommendations from an implementation science perspective that appear fruitful for further study and application. We summarized specific recommendations from stakeholders to develop adaptation guides for implementing SDM in practice, enhance the adoption of educational curricula in SDM, and influence policy actions to support SDM. This effort both revealed the complexity involved in getting SDM approaches consistently into practice and also identified several promising ways to address these challenges.
Supplemental Material
Supplemental material, DS_10.1177_2381468318808503 for Designing Shared Decision-Making Interventions for Dissemination and Sustainment: Can Implementation Science Help Translate Shared Decision Making Into Routine Practice? by Andy S. L. Tan, Kathleen M. Mazor, Daniel McDonald, Stella J. Lee, Demetria McNeal, Daniel D. Matlock and Russell E. Glasgow in MDM Policy & Practice
Acknowledgments
We acknowledge all the meeting participants who contributed to this work.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andy S. L. Tan  https://orcid.org/0000-0001-6459-6171
https://orcid.org/0000-0001-6459-6171
Stella J. Lee  https://orcid.org/0000-0002-6716-1998
https://orcid.org/0000-0002-6716-1998
Supplemental Material: The online supplementary appendixes for this article are available on the Medical Decision Making Policy & Practice website at http://journals.sagepub.com/home/mpp.
Contributor Information
Andy S. L. Tan, Dana-Farber Cancer Institute, Population Sciences Division, Center for Community-Based Research, Boston, MA; Harvard University, T.H. Chan School of Public Health, Boston, MA.
Kathleen M. Mazor, Meyers Primary Care Institute, a joint Endeavor of the University of Massachusetts Medical School, Reliant Medical Group, and Fallon Health, Worcester, NA
Daniel McDonald, Dana-Farber Cancer Institute, Population Sciences Division, Center for Community-Based Research, Boston, MA.
Stella J. Lee, Dana-Farber Cancer Institute, Population Sciences Division, Center for Community-Based Research, Boston, MA Harvard University, T.H. Chan School of Public Health, Boston, MA.
Demetria McNeal, Dissemination and Implementation Science Program, Adult and Child Center for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO.
Daniel D. Matlock, Division of Geriatric Medicine, Department of Medicine, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO VA Eastern Colorado Geriatric Research Education and Clinical Center, Denver, CO; Dissemination and Implementation Science Program, Adult and Child Center for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO.
Russell E. Glasgow, VA Eastern Colorado Geriatric Research Education and Clinical Center, Denver, CO Dissemination and Implementation Science Program, Adult and Child Center for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO.
References
- 1. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: A model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barry MJ, Edgman-Levitan S. Shared decision making—the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–1. doi: 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- 3. King JS, Moulton BW. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32(4):429–501. [DOI] [PubMed] [Google Scholar]
- 4. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi: 10.1002/14651858.CD006732.pub3 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) [cited January 3, 2018]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 6. Lee EO, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(1):6–8. doi: 10.1056/NEJMp1209500 [DOI] [PubMed] [Google Scholar]
- 7. Clancy C, Collins FS. Patient-Centered Outcomes Research Institute: the intersection of science and health care. Sci Transl Med. 2010;2(37):37cm18. doi:10.1126/scitr anslmed.3001235 [DOI] [PubMed] [Google Scholar]
- 8. Brownson RC, Colditz GA, Proctor EK, eds. Dissemination and Implementation Research in Health: Translating Science to Practice. 2nd ed. Oxford: Oxford University Press; 2017. [Google Scholar]
- 9. Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274–81. doi: 10.2105/AJPH.2012.300755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–31. doi:10.1177/027 2989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. doi: 10.1002/14651858.CD001431.pub4 [DOI] [PubMed] [Google Scholar]
- 12. Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–17. doi: 10.1016/j.cpr.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes TM, Merath K, Chen Q, et al. Association of shared decision-making on patient-reported health outcomes and healthcare utilization. Am J Surg. 2018;216(1):7–12. doi: 10.1016/j.amjsurg.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 14. Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11:114. doi: 10.1186/s13012-016-0480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18(4):542–61. doi: 10.1111/hex.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go . . .”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(Suppl. 2):S14. doi: 10.1186/1472-6947-13-S2-S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them—a scoping review. Implement Sci. 2018;13(1):40. doi: 10.1186/s13012-018-0731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32(2):276–84. doi: 10.1377/hlthaff.2012.1078 [DOI] [PubMed] [Google Scholar]
- 19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–35. doi: 10.1016/j.pec.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 20. King J, Moulton B. Group health’s participation in a shared decision-making demonstration yielded lessons, such as role of culture change. Health Aff (Millwood). 2013;32(2):294–302. doi: 10.1377/hlthaff.2012.1067 [DOI] [PubMed] [Google Scholar]
- 21. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at group health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31(9):2094–104. doi: 10.1377/hlthaff.2011.0686 [DOI] [PubMed] [Google Scholar]
- 22. Cohen DJ, Crabtree BF, Etz RS, et al. Fidelity versus flexibility: translating evidence-based research into practice. Am J Prev Med. 2008;35(5 Suppl.):S381–S389. doi: 10.1016/j.amepre.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 23. Stirman SW, Miller CJ, Toder K, Calloway A. Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implement Sci. 2013;8:65. doi: 10.1186/1748-5908-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228–43. [DOI] [PubMed] [Google Scholar]
- 25. Rogers EM. Diffusion of Innovations. 5th ed. New York: Free Press; 2003. [Google Scholar]
- 26. Green LW, Kreuter MW. Health Promotion Planning: An Educational and Ecological Approach. 3rd ed. Mountain View: Mayfield Publishing; 1999. [Google Scholar]
- 27. Green LW, Richard L, Potvin L. Ecological foundations of health promotion. Am J Health Promot. 1996;10(4):270–81. doi: 10.4278/0890-1171-10.4.270 [DOI] [PubMed] [Google Scholar]
- 28. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass; 2009. [Google Scholar]
- 29. Nolan K, Schall MW, Erb F, Nolan T. Using a framework for spread: the case of patient access in the Veterans Health Administration. Jt Comm J Qual Patient Saf. 2005;31(6):339–47. [DOI] [PubMed] [Google Scholar]
- 30. Improving Chronic Illness Care. The chronic care model [cited June 5, 2018]. Available from: http://www.improvingchroniccare.org/index.php?p=the_chronic_caremodel&s=2
- 31. Glasgow RE. Evaluation of theory-based interventions: the REAIM model. In: Glanz K, Rimer BK, Lewis FM, eds. Health Behavior and Health Education. Theory, Research, and Practice. 3rd ed. San Francisco: Jossey-Bass; 2002. p 119–27. [Google Scholar]
- 32. Liles EG, Schneider JL, Feldstein AC, et al. Implementation challenges and successes of a population-based colorectal cancer screening program: a qualitative study of stakeholder perspectives. Implement Sci. 2015;10:41. doi: 10.1186/s13012-015-0227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beck A, Bergman DA, Rahm AK, Dearing JW, Glasgow RE. Using implementation and dissemination concepts to spread 21st-century well-child care at a health maintenance organization. Perm J. 2009;13(3):10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin GA, Halley M, Rendle KAS, et al. An effort to spread decision aids in five California primary care practices yielded low distribution, highlighting hurdles. Health Aff (Millwood). 2013;32(2):311–20. doi:10.1377/hlthaff.2012 .1070 [DOI] [PubMed] [Google Scholar]
- 35. Durand MA, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9(4):e94670. doi:10.1371/journal.pone .0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aarons GA, Sklar M, Mustanski B, Benbow N, Brown CH. “Scaling-out” evidence-based interventions to new populations or new health care delivery systems. Implement Sci. 2017;12(1):111. doi: 10.1186/s13012-017-0640-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Volandes AE, Ariza M, Abbo ED, Paasche-Orlow M. Overcoming educational barriers for advance care planning in Latinos with video images. J Palliat Med. 2008;11(5):700–6. doi: 10.1089/jpm.2007.0172 [DOI] [PubMed] [Google Scholar]
- 38. Waltz TJ, Powell BJ, Matthieu MM, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10:109. doi: 10.1186/s13012-015-0295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. doi: 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi:10.1186/s13012- 015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirchner JE, Waltz TJ, Powell BJ, Smith JL, Proctor EK. Implementation strategies. In: Brownson RC, Colditz GA, Proctor EK, eds. Dissemination and Implementation Research in Health: Translating Science to Practice. 2nd ed. Oxford: Oxford University Press; 2017. [Google Scholar]
- 42. Rabin BA, McCreight M, Battaglia C, et al. Systematic, multimethod assessment of adaptations across four diverse health systems interventions. Front Public Health. 2018;6:102. doi: 10.3389/fpubh.2018.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi: 10.1016/j.pec.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 44. Joseph-Williams N, Lloyd A, Edwards A, et al. Implementing shared decision making in the NHS: lessons from the MAGIC programme. BMJ. 2017;357:j1744. doi: 10.1136/bmj.j1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gravel K, Légaré F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. doi: 10.1186/1748-5908-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanzaria HK, Booker-Vaughns J, Itakura K, et al. Dissemination and implementation of shared decision making into clinical practice: a research agenda. Acad Emerg Med. 2016;23(12):1368–79. doi: 10.1111/acem.13075 [DOI] [PubMed] [Google Scholar]
- 47. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patient-Centered Outcomes Research Institute (PCORI). Implementing shared decision making: a new PCORI initiative [cited February 8, 2018]. Available from: https://www.pcori.org/blog/implementing-shared-decision-making-new-pcori-initiative
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_2381468318808503 for Designing Shared Decision-Making Interventions for Dissemination and Sustainment: Can Implementation Science Help Translate Shared Decision Making Into Routine Practice? by Andy S. L. Tan, Kathleen M. Mazor, Daniel McDonald, Stella J. Lee, Demetria McNeal, Daniel D. Matlock and Russell E. Glasgow in MDM Policy & Practice