Abstract
Background:
No systematic review has focused on conceptual models underpinning advance care planning for patients with advanced cancer, and the mechanisms of action in relation to the intended outcomes.
Aim:
To appraise conceptual models and develop a logic model of advance care planning for advanced cancer patients, examining the components, processes, theoretical underpinning, mechanisms of action and linkage with intended outcomes.
Design:
A systematic review of randomised controlled trials was conducted, and was prospectively registered on PROSPERO. Narrative synthesis was used for data analysis.
Data sources:
The data sources were MEDLINE, CINAHL, PsycINFO, EMBASE, CENTRAL, PROSPERO, CareSearch, and OpenGrey with reference chaining and hand-searching from inception to 31 March 2017, including all randomised controlled trials with advance care planning for cancer patients in the last 12 months of life. Cochrane quality assessment tool was used for quality appraisal.
Results:
Nine randomised controlled trials were included, with only four articulated conceptual models. Mechanisms through which advance care planning improved outcomes comprised (1) increasing patients’ knowledge of end-of-life care, (2) strengthening patients’ autonomous motivation, (3) building patients’ competence to undertake end-of-life discussions and (4) enhancing shared decision-making in a trustful relationship. Samples were largely highly educated Caucasian.
Conclusion:
The use of conceptual models underpinning the development of advance care planning is uncommon. When used, they identify the individual behavioural change. Strengthening patients’ motivation and competence in participating advance care planning discussions are key mechanisms of change. Understanding cultural feasibility of the logic model for different educational levels and ethnicities in non-Western countries should be a research priority.
Keywords: Systematic review, advance care planning, cancer, conceptual models, mechanisms of action
What is already known about the topic?
Advance care planning (ACP) has proved its effectiveness in trials and has been widely used and promoted in Western countries.
No systematic reviews focusing on the conceptual model and mechanisms of action of ACP have been critically appraised.
What this paper adds?
A novel logic model of ACP for advanced cancer patients drawn from Western studies was constructed. No non-Western studies and models were discovered.
It is uncommon to apply conceptual models to underpin the development of ACP, while anticipated individual behaviour change was mainly identified in trials.
Key mechanisms of action focused on facilitating patient’s knowledge and building up motivation and competence in participating ACP, leading to making a decision within a trustful clinician–patient relationship.
Implications for practice, theory or policy
This logic model cannot be currently transferred as theory as it is mainly Western oriented. This presents an obstacle to good care globally.
There is an urgent need to explore the applicability of this Western-oriented logic model for different educational levels and diverse ethnicities in non-Western countries to meet the Universal Health Coverage palliative care goal.
Background
There has been increasing awareness of the importance of enabling a person’s autonomous decision-making at the end of life. Attainment of preferences, such as place of death, is considered an indicator of high-quality end-of-life care.1 However, patients’ end-of-life preferences such as preferences for life-sustaining treatments, artificial nutrition and hydration, and place of death are not routinely discussed prior to patients’ loss of capacity to make the decision for themselves.2 This may negatively impact quality of life as the individual might not be cared for in a way they would have chosen, which in turn may increase levels of stress, anxiety and depression for their families.3,4
Several written documents were introduced and promoted in the late 1960s as a tool to maintain a person’s autonomy about their end-of-life care (e.g. Advance Directives, Living Wills).5 However, evaluations of these focused largely on the completion rate of these written documents, thus failing to understand the achievement of a person’s values, goals and preferences for end-of-life care and the impact on their quality of life.5–9 The documentation also poorly specified a person’s preferences and did not recognise that availability of health resources may differ from the hypothetical situation detailed in an advance directive.10 These challenges led in the 1990s to the development of a conceptual alternative – Advance Care Planning (ACP).11 ACP was considered more appropriate in ensuring patients’ access to preferred care, by conducting a mutual communication between patients, families and healthcare professionals to achieve consensus on future care. Evidence suggests that ACP benefit patients (e.g. quality of life, compliance with wishes), their family (e.g. satisfaction with care, emotional distress, bereavement), the healthcare system (e.g. cost, hospitalisation rate) and increases completion rates.4,8,10,12,13 At present, the practice of ACP is more common in mainly the Western countries such as Australia,14 United Kingdom15 and North America.16
Careful consideration of cultural appropriateness, context and adaptation is absolutely critical to enhance uptake in other parts of the world.17,18 A systematic review highlighted that the standard ACP failed to capture patient’s preferences across cultures.19 This emphasises the importance of investigating the intervention’s suitability and developing a culturally sensitive ACP before adopting it.20 Moreover, a better understanding of the underpinning conceptual models (defined as a guide to understand the interactions between implementation processes and the systems in which the intervention is implemented21) and mechanisms of action (defined as a comprehensive description of how and why a desire change is expected to happen in a certain context21) is required to ensure the cultural appropriateness and potential effectiveness of ACP. This is imperative prior to it being tested and implemented.22,21 No review to our knowledge has specifically focused on conceptual models that underpin ACP for advanced cancer patients, nor critically considered the process of delivery and mechanisms of action in relation to the intended outcomes.
To address this, we conducted a systematic review that aimed to identify and appraise the conceptual models underpinning ACP interventions and the components of implementing ACP, identifying the outcomes measured and tools used, and how the outcomes are achieved (mechanisms of action) in order to develop an evidence-based logic model (defined as a diagram showing how a programme influences its participants to achieve intended outcomes or sustainable change21). A logic model can be used to describe the resources needed to operate the programme, and to communicate the programme design to potential stakeholders for developing theoretically plausible and acceptable ACP interventions for advanced cancer patients.21
Methods
Study design
A systematic literature review drew on Cochrane guidance23, Guidance on the Conduct of Narrative Synthesis in Systematic Reviews,24 and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidance25 for reporting systematic reviews. Previous systematic reviews on ACP 4,7,9,10,12,13 were used to inform the search strategy.
Protocol and registration
The systematic review protocol was prospectively registered on PROSPERO26 (CRD42017067628; http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID = CRD42017067628).
Databases and search strategy
We searched eight electronic databases (MEDLINE, CINAHL, PsycINFO, EMBASE, Cochrane Register of Controlled Trials (CENTRAL), York Centre (PROSPERO), CareSearch, OpenGrey from inception to 31 March 2017. The PICOS framework27 was applied to the study aim to inform the search terms, drawing on previous systematic reviews on ACP4,9,10 to refine the search strategy (see Table S1). Medical subject headings were used for exploring synonyms and Boolean operators ‘AND’ and ‘OR’ were applied (see Figure S1). In addition, we hand-searched key journals (Journal of Palliative Care, Journal of Palliative Medicine, Psycho-Oncology, BMJ Supportive and Palliative Care, BMC Palliative Care, Journal of Clinical Oncology) to perform a comprehensive search.28
Inclusion and exclusion criteria
ACP is defined as a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals and preferences regarding future medical care.29 It includes written documents or any type of record to reflect a patient’s values, goals, preferences and aspirations (e.g. Advance Statement) and/or a decision-making for specific medical treatments or care (e.g. Advance Directive) regarding end of life.30 All types of randomised controlled trials testing an ACP intervention for advanced cancer patients in any setting were included. No publication date was imposed. The target population was defined as adults (⩾18 years old) with any type of cancer who were in the last 12 months of their life. Studies were included if cancer patients formed the majority (⩾50%) of participants.4 Studies were excluded that focused exclusively on interventions for promoting ACP completion rates or reported non-primary data.
Study selection
C.L. scanned all articles by title and abstract, and any with ambiguity as to whether with respect to inclusion/exclusion criteria were retained for full-text review. Discussion to establish consensus with three other authors (R.H., C.E. and J.K.) resolved any disagreements. Endnote31 bibliographic software version X8 was used to manage references and remove duplicates.
Data extraction and management
Two data extraction sheets were developed based on Template for Intervention Description and Replication (TIDieR)22 and the Cochrane Consumers and Communi-cation Review Group’s Data Extraction Template.32 Data were extracted by C.L. and checked by J.K. Two authors of included randomised controlled trials were contacted for further information. Extracted data items included setting (country, study setting), study design (pilot randomised controlled trials, parallel-group randomised controlled trials, cluster randomised controlled trials and number of participants), participant characteristics (age, sex, ethnicity, cancer type and education level), interventions (components and processes, underpinning conceptual models and mechanisms of action) and outcomes and measurement tools used. If information regarding underpinning conceptual models and mechanisms of action were not detailed in the included studies, then we extracted data from the supporting references cited in the included studies for further information. In the case of disagreement, discussion with R.H. and C.E. aimed to achieve consensus.
Assessment of risk of bias
Randomised controlled trials were critically graded by applying the tool for assessing risk of bias for randomised controlled trials proposed by Cochrane, which includes assessment of random sequence generation, allocation concealment, blinding (of patients, healthcare providers and outcome assessors), incomplete outcome data and selective reporting.23 C.L. assessed all studies and 50% was randomly selected and checked independently by J.K. Any disagreement was resolved by discussion and consensus with R.H. and C.E. Review Manager (RevMan) Version 5.3 software33 was used to manage and summarise the risk of bias assessment for all included randomised controlled trials.
Synthesis of results
Data synthesis consisted of two parts: (1) narrative analysis24 and (2) intervention synthesis.34
First, a narrative synthesis was conducted following Guidance on the Conduct of Narrative Synthesis in Systematic Review.24 A preliminary synthesis of extracted data was performed by textual description, tabulation, grouping and clustering in order to demonstrate the characteristics of each included paper. Variability of context, study design, population, conceptual models, mechanisms of action, outcomes and outcome measures were examined to explore the relationships within and between randomised controlled trials. An assessment of robustness and risk of bias was conducted to appraise the quality of the evidence (see Assessment of risk of bias for detail). Meta-analysis was considered to examine intervention sub-groups and linkage with the respective outcomes if appropriate.
Second, an intervention synthesis was undertaken by summarising the processes of implementing ACP, the components, underpinning conceptual models and mechanisms of action, by applying the TIDieR checklist.22 The Common Components Hybrid method was used to categorise selected randomised controlled trials into different sub-groups according to the key processes, components and their characteristics.34,35
Finally, a logic model was developed following the Medical Research Council guidance on Process Evaluation of Complex Intervention21 and the Theory of Change, which is considered to enhance the Medical Research Council framework.36 The Theory of Change was used to describe how and why a desire behaviour change is expected to happen in a certain context. This aimed to depict how the ACP intervention was delivered, and mechanisms of action attributable to the intervention components, underpinning conceptual models and their linkage to intended outcomes.
Results
Study retrieval and characteristics
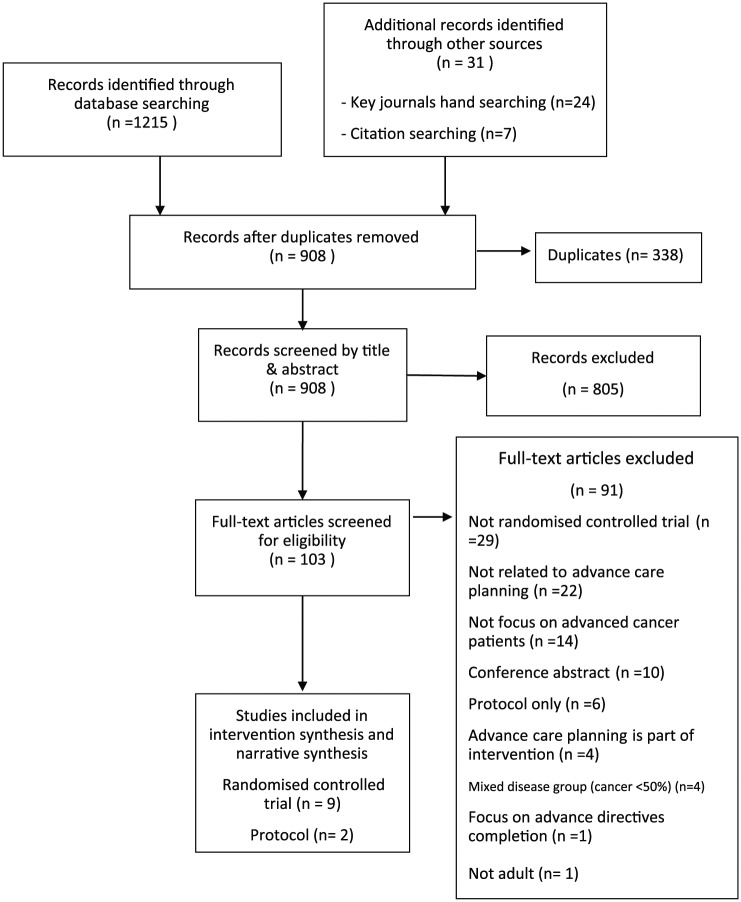
The search strategy retrieved 1246 records. After duplicates were removed, 908 were reviewed by title and abstract, and 103 for full-text screening at eligibility stage. A total of 91 papers were excluded. The most frequent reasons for exclusion were ineligible study design, research topic and target population (see Figure 1). In all, 11 papers met the eligibility criteria detailing 9 randomised controlled trials including 1172 patients with progressive, incurable, recurrent and life-limiting advanced cancer (606 males and 566 females). Of two authors37,38 contacted for further information, one37 provided supplementary data. All included studies were written in English and were from Western countries. The majority of randomised controlled trials were conducted in North America (n = 5),38–42 seven37,39–44 were definitive randomised controlled trials and two of these applied a cluster randomised controlled trials design (see Table 1). More than half of the participants (628 patients) were highly educated (university or postgraduate) and Caucasian.
Figure 1.
PRISMA flow chart of study selection.
Table 1.
Description of the included randomised controlled trials.
| Study | Settinga | Study design | Intervention versus control | Participants characteristics | Outcomesb (measurement tools) |
|---|---|---|---|---|---|
| El-Jawahri et al.39
USA |
OC | Parallel-group randomised controlled trial | 6-min video with verbal narrative of goals-of-care versus verbal narrative of goals-of-care | 50 patients with malignant glioma Cancer type: Malignant giloma Ethnicity: White: 92% Education: Higher education (university and postgraduate): 66% |
Patient-reported:
Patients’ preference for cardiopulmonary resuscitation (intervention 8.7% vs control 40.7%; p = 0.02, SDQ), patients’ knowledge of cardiopulmonary resuscitation (SDQ), patients’ uncertainty of decision-making (DCS), patients’ comfort, helpful and recommendation level with the video (SDQ) Process: None applicable |
| Epstein et al.38
USA |
OC | Pilot randomised controlled trial | 3-min video decision aids with image of cardiopulmonary resuscitation and mechanical ventilation versus verbal narrative about cardiopulmonary resuscitation and mechanical ventilation | 56 patients with progressive pancreas or hepatobiliary
cancer Cancer type (top 3): 1. Exocrine pancreas Carcinoma (78.6%) 2. Cholangiocarcinoma (8.9%) 3. Hepatocellular carcinoma (5.4%) Ethnicity: White: 62.5% Education: Higher education (university and postgraduate): 80.4% |
Patient-reported:
Study impressions (SDQ), patients’ preference for cardiopulmonary resuscitation (SDQ), patients’ knowledge of cardiopulmonary resuscitation (SDQ) Process: Completion of advance care planning (intervention 40% vs control 15%; OR = 3.6, 95% CI 0.9–18.0; p = 0.07, MR), deaths occurred (MR), the number of patients died in hospice setting (MR) |
| Volandes et al.42
USA |
OC | Parallel-group randomised controlled trial | 3-min video depicting a patient on a ventilator and cardiopulmonary resuscitation being performed on a stimulated patient versus verbal narrative describing cardiopulmonary resuscitation | 150 patients with advanced cancer Cancer type (top 3): 1. Lung (23.3%) 1. Colon (23.3%) 2. Breast (11.3%) Ethnicity: White: 47.3% Education: Higher education (university and postgraduate): 44.6% |
Patient-reported:
Patients’ preference for cardiopulmonary resuscitation (intervention 20% vs control 48%, OR = 3.5, 95% CI 1.7–7.2; p < 0.001, SDQ), patients’ knowledge of cardiopulmonary resuscitation (SDQ), patient’s perception of watching video (SDQ) Process: None applicable |
| Jones et al.45
UK |
OC, HOC | Feasibility randomised controlled trial | Meeting with a trained medical staff using a checklist of topic domains versus usual care | 77 patients with recurrent advanced
cancer Cancer type (top 3): 1. Bowel (14.3%) 2. Prostate (13%) 3. Gynaecological (10.4%) Ethnicity: White: 92.1% Education: Higher education (university and postgraduate): 62% |
Patient-reported:
Willingness to discuss about future (coefficient 0.7, 95%CI -1.9-3.2; p = 0.611, VAS), happiness with communication (VAS), degree of satisfaction of care (VAS), anxiety and depression (HADS) Process: None applicable |
| Stein et al.43
Australia |
HOT | Parallel-group randomised controlled trial | A semi-structured discussion with a psychologist using a pamphlet called ‘Living with Advanced Cancer’ versus usual care | 120 patients with metastatic cancer who were no longer being
treated with curative intent Cancer type (top 3): 1. Colorectal (26.7%) 2. Other (25%) 3. Lung (16.7%) Ethnicity: None stated Education: Higher education (university and postgraduate): 76.6% |
Patient-reported:
Depression and anxiety (HADS), patients’ knowledge of cardiopulmonary resuscitation (SDQ) Process: Hospital death (intervention 19% vs control 50%, 95% CI 11%–50%; p = 0.004, MR), whether a patient had a do not resuscitation order (MR), the number of days between the earliest do not resuscitation order documentation and death (MR), caregiver burden/(CRA) |
| Clayton et al.37
Australia |
PCC | Parallel-group randomised controlled trial | Provision of a question prompt list to patients before consultation with physicians versus standard consultation | 174 patients with an advanced progressive life limiting
illness Cancer type (top 3): 1. Gastrointestinal (37.9%) 2. Other (22.4%) 3. Lung (20.1%) Ethnicity: None stated Education: Higher education (university and postgraduate): 39% |
Patient-reported:
Discussion about prognosis and end-of-life care (ratio 2.3; 95% CI, 1.7–3.2; p < 0.0001, coding), achievement of patient information needs (CISQ), satisfaction with the consultation (SDQ), anxiety (SSAI) Process: Physician satisfaction with communication during the consultation (SDQ), consultation duration (coding) |
| Rodenbach et al.41
USA |
OC | Cluster randomised controlled trial | A communication coaching with a question prompt list for patients before the consultation with oncologist versus usual care | 180 patients who had advanced non-hematologic
cancer Cancer type: None stated Ethnicity: White: 83.9% Education: Higher education (university and postgraduate): 61.1% |
Patient-reported:
Discussion about prognosis and end-of-life care (intervention 70.2% vs control 32.6%; p < 0.001, coding) Process: None applicable |
| Epstein et al.40
USA |
OC, CC, HOT | Cluster randomised controlled trial | Values and options in cancer care (VOICE) versus usual care | 265 Patients had either stage IV non-hematologic cancer or
stage III cancer and whose physician ‘would not be
surprised’ if the patient were to die within
12 months. Cancer type: 1. Aggressive cancer 2. Less aggressive Ethnicity: White: 88.7% Education: Higher education (university and postgraduate): 72.5% |
Patient-reported:
Physician-patient communication (intervention effect,0.34; 95% CI,0.06–0.62; p = 0.02, APPC, VR-CoDES, PTCC and FPI), quality of life (FACT-G, McGill QOL) Process: Utilisation of aggressive treatment in the last 30 days of life (MR), hospice utilisation (MR), intervention fidelity (SDQ) |
| Walczak et al.44
Australia |
CC | Parallel-group randomised controlled trial | Communication support programme versus usual care | 110 patients with advanced, incurable
cancer Cancer type (top 3): 1. Lung (16.4%) 2. Prostate (15.5%) 3. Bowel/anus (11.8%) Ethnicity: None stated Education: Higher education (university and postgraduate): 62% |
Patient-reported:
Discussion about future (intervention 1.0 vs control 0.6; p = 0.028, coding), communication self-efficacy (PEPPI), preference for information and decision-making (CISQ), quality-of-life (FACT-G, McGill QOL scale), satisfaction of intervention (SDQ) Process: Consultation length (SDQ), intervention fidelity (SDQ) |
Setting: OC: oncology clinic; HOC: hospice; CC: cancer centre; PCC: palliative care centre; HOT: hospital; HC: home care organization; MC: managed care organisation.
Outcomes (measurement tools): SDQ: self-developed questionnaire; MR: medical records; VAS: Visual Analogue Scale; HADS: Hospital Anxiety and Depression Survey; DCS: Decisional Conflict Scale; PEPPI: Perceived Efficacy in Physician/Patient Interactions Scale; CISQ: Cassileth Information Styles Questionnaire; FACT-G: Function Assessment of Cancer Therapy: General; McGill QOL: McGill Quality of Life Questionnaire; SSAI: Spielberger State Anxiety Inventory; CRA: Caregivers Reaction Assessment; APPC: The Active Patient Participation Coding; VR-CoDES: The Verona VR-CoDES system; PTCC: the prognostic and treatment choices; FPI: The Framing of Prognostic Information Scale.
Italics indicate primary outcome if multiple outcomes were evaluated.
Key to colour coding.
 Single-element intervention – video decision aids.
Single-element intervention – video decision aids.
 Single-element intervention – written information materials.
Single-element intervention – written information materials.
 Multiple-elements intervention – written information
materials + communication coaching.
Multiple-elements intervention – written information
materials + communication coaching.
 Multiple-elements intervention – video decision aids + written
information materials + communication coaching.
Multiple-elements intervention – video decision aids + written
information materials + communication coaching.
Quality appraisal
All included randomised controlled trials adhered to a rigorous randomisation process by using either a computer-generated random number table or a permuted block. Five randomised controlled trials adequately concealed allocation by using sealed envelopes,37,39,42,44,45 three randomised controlled trials did not report the concealment method although participants blinding to the assignment was articulated,40,41,43 the remaining one randomised controlled trial did not provide information regarding allocation concealment.38 None of the randomised controlled trials were able to blind intervention providers and participants, however, five randomised controlled trials blinded the assessors to minimise detection bias.37,40,43–45 Most randomised controlled trials (n = 5)38,42–45 had high attrition bias due to the high attrition rate (12%–55.3%). The common reasons for attrition were participants’ death,40–43,45 poor health,43–45 withdrawal40,41 or unable to contact the participants42,43 (see Figure 2, Table S2).
Figure 2.
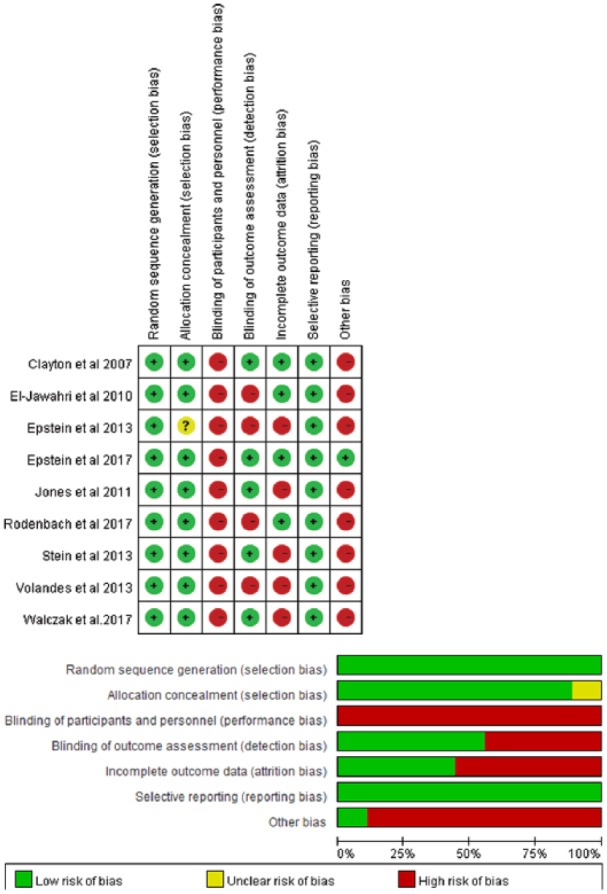
Risk of bias assessment of randomised controlled trials within and across studies.
Synthesis of results
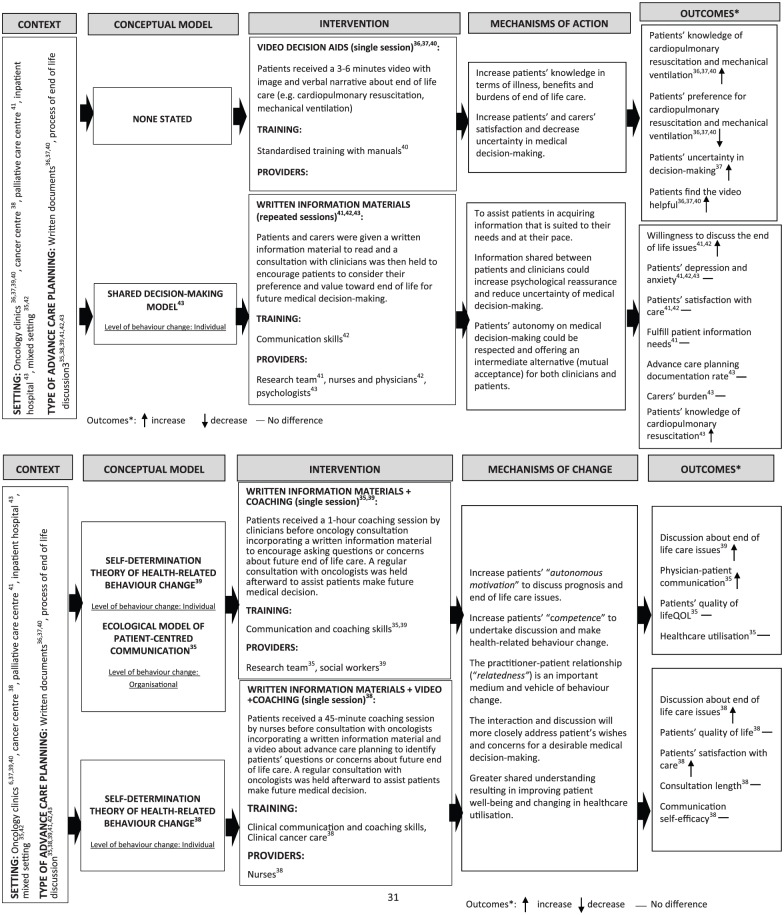
We present a logic model of ACP for people with advanced cancer based on the review findings (Figure 3). These should be read in conjunction with Tables 1 and 2.
Figure3.
Logic model of advance care planning for people with advanced cancer.
Table 2.
The conceptual model underpinning ACP intervention implementation, training and the mechanisms of action.
| Study | Conceptual model | Intervention implementation | Intervention providers/training | Mechanisms of action |
|---|---|---|---|---|
| El-Jawahri et al.39
USA |
None stated46 | Sessions: single 6-min session Patients received a 6-min video presenting the three levels (life-prolonging care, basic care and comfort care) of medical care, shown on the portable computer |
Research members/none stated | 1. Help patients and carers to imagine the disease state and
reify suffering. 2. Increase patients’ knowledge in terms of illness, benefits and burdens of medical intervention. 3. Patients can receive information necessary for informed decision-making in an unbiased and structured format. 4. Increase patients’ and carers’ satisfaction and decrease uncertainty in medical decision-making. 5. Complex information about illness and medical interventions is more easily transmitted by videos than print materials for people with limited health literacy or for whom English is a second language. 6. Voice-over narratives can reinforce the information content of the visual image to help viewers absorb information |
| Epstein et al.38
USA |
None stated46 | Sessions: single 3-min session Patients received a 3-min video with image and verbal narrative about cardiopulmonary resuscitation and mechanical ventilation |
Medical staff (physicians)/none stated | 1. Help patients and carers to imagine the disease state and
reify suffering. 2. Increase patients’ knowledge in terms of illness, benefits and burdens of medical intervention. 3. Patients can receive information necessary for informed decision-making in an unbiased and structured format. 4. Increase patients’ and carers’ satisfaction and decrease uncertainty in medical decision-making. 5. Complex information about illness and medical interventions is more easily transmitted by videos than print materials for people with limited health literacy or for whom English is a second language. 6. Voice-over narratives can reinforce the information content of the visual image to help viewers absorb information |
| Volandes et al.42
USA |
None stated46 | Sessions: single 3-min session. Patients received a 3-min video with image and verbal narrative about cardiopulmonary resuscitation with the research assistant present and anyone accompanying the patient was invited to view the video |
Research members / Standardised training with manuals of intervention |
1. Help patients and carers to imagine the disease state and
reify suffering. 2. Increase patients’ knowledge in terms of illness, benefits and burdens of medical intervention. 3. Patients can receive information necessary for informed decision-making in an unbiased and structured format. 4. Increase patients’ and carers’ satisfaction and decrease uncertainty in medical decision-making. 5. Complex information about illness and medical interventions is more easily transmitted by videos than print materials for people with limited health literacy or for whom English is a second language. 6. Voice-over narratives can reinforce the information content of the visual image to help viewers absorb information |
| Jones et al.45
UK |
None stated47,48 | Sessions: three 1-h sessions. Discussions explored patient’s perceptions of their current situation, their communication with health professionals and significant others, and their hopes and fears for the future and about making future healthcare decision. For those who wished to document future decisions, living wills documents were used |
Medical staff (nurses and physicians)/advanced communication skill training (role-play model) | 1. Prompt and offer an opportunity for patients to think
about end-of-life issues in advance. 2. Healthcare professionals could sense patients’ cue of the need of end-of-life issues discussion and initiate the discussion. 3. Healthcare professionals could recognise the individuality. 4.Patients might lose a sense of hope or maintain a sense of control. 5. Initiating end-of-life issues discussion between patients and healthcare professionals could have wider benefits in enabling patients to communicate with family |
| Stein et al.43
Australia |
Shared decision-making model49
A model focuses on the process through which an intermediate medical decision will be made by sharing information from both physician and patient. |
Sessions: none stated. The patients received a pamphlet and a discussion with a psychologist based on a shared decision-making model in order to encourage patients to consider their preferences and values towards end of life |
Medical staff (psychologists)/none stated | 1. Shared decision-making involves at least two participants
(patients and clinicians). 2. Both parties take steps to participate in the process of shared decision-making (mutual interaction). 3. Information shared between patients and clinicians could increase psychological reassurance and reduce uncertainty of medical decision-making. 4. Patients’ autonomy on medical decision-making could be respected and offering an intermediate alternative (mutual acceptance) for both clinicians and patients. 5. Increase the trusting clinician–patient relationship |
| Clayton et al.37
Australia |
None stated50 | Sessions: single 30-min session Patients/caregivers given Question Prompt List about 20–30 min before the consultation with palliative care physicians to elicit their questions and concerns about end-of-life care |
Research members/none stated | 1. To encourage patients’ participation by actively asking
questions during a medical consultation and make an informed
decision. 2. To assist patients in acquiring information that is suited to their needs and at their pace |
| Rodenbach et al.41
USA |
Self-determination theory of health-related behaviour
change51,52
A model focuses on the process through which a person acquires the motivation and competence for initiating new health-related behaviour and maintaining them over time |
Sessions: single 1-h session Patients/caregivers met face to face with a social worker during a 1-h session, during which time they were each given a Question Prompt List booklet for boosting asking questions, identifying, prioritising or expressing concerns for the upcoming consultation |
Medical staff (social workers)/standardised coaching training (3 days) |
1. Increase patients’ ‘autonomous motivation’ to discuss
prognosis and end-of-life care issues. 2. Increase self-perceived sense of ‘competence’ to undertake discussion and make health-related behaviour change. 3. The practitioner–patient relationship ( ‘relatedness’) is an important medium and vehicle of behaviour change. 4. Patients can experience more volitional engagement in medical decision-making and maintain the behaviour change over time |
| Epstein et al.40
USA |
Ecological Model of Patient-Centred Communication53
A system-oriented theory in terms of clinical communication involving mutual interactions between physicians and patients as well as the social and clinical contexts, rather than targeting the individual’s communication behaviour |
Sessions: single 1-h session A patient and caregiver coaching session incorporating a Question Prompt List to help patients bring their most important concerns to their oncologist at an upcoming consultation |
Research members and medical staff (physicians)/research
members: standardised coaching training
(3 days). Medical staff: brief video and communication skill training with standardised patients (role-play model) |
1. The interaction between patients’ and caregivers’
assertive behaviours, and physicians’ facilitative behaviour
can reinforce patients’ active participation in end-of-life
issues discussion. 2. The end-of-life issues discussion will help patients receive more useful information, support and empathy. 3. The interaction and discussion will more closely address patient’s wishes and concerns for a desirable medical decision-making. 4. Greater shared understanding resulting in improving patient well-being and changing in healthcare utilisation |
| Walczak et al.44
Australia |
Self-determination theory of health-related behaviour
change51,52
A model focused on the process through which a person acquires the motivation and competence for initiating new health-related behaviour and maintaining them over time |
Sessions: single 45-min session Patients attended face-to-face meetings 1 week before a regular oncology consultation and a Question Prompt List was introduced to cancer patients by nurses to identify questions that were relevant to them (prognosis, treatment options and decision, palliative care, lifestyle and so forth). Patients were given a DVD with information about advance care planning. Patients were prompt to select 1–3 questions for discussion with oncologist at the next oncology consultation using Question Prompt List |
Medical staff (nurses)/clinical communication skill and cancer care training (40 h) | 1. Increase patients’ ‘autonomous motivation’ to discuss
prognosis and end-of-life care issues. 2. Increase self-perceived sense of ‘competence’ to undertake discussion and make health-related behaviour change. 3. The practitioner–patient relationship ( ‘relatedness’) is an important medium and vehicle of behaviour change. 4. Patients can experience more volitional engagement in medical decision-making and maintain the behaviour change over time |
Context
Setting
Most study sites were oncology clinics (n = 4).38,39,41,42 Only n = 2 randomised controlled trials40,45 were conducted across care settings including a cancer centre, oncology clinic, inpatient hospital, managed care organisation, hospice facility and home care organisation (see Table 1, Figure 3).
Type of ACP
N = 3 randomised controlled trials38,39,42 applied ACP as a written document to record patient’s preference for life-sustaining treatments (cardiopulmonary resuscitation and mechanical ventilation); the other n = 6 randomised controlled trials37,40,41,43–45 considered ACP to be a dynamic process of discussion and decision-making about the patients’ prognosis and end-of-life care15,30 (see Figure 3).
Intervention
Tools to facilitate ACP for patients and family members
To understand the effect of different interventions, we categorised them as either single- or multiple-element interventions. Of the nine included randomised controlled trials, n = 6 used single-element interventions. Of these, n = 338,39,42 adopted video decision aids simulating cardiopulmonary resuscitation and mechanical ventilation in a clinical setting to explore a patient’s preference for life-sustaining treatments; the remaining n = 337,43,45 used written information materials including question prompt lists, topic checklists, living wills or pamphlets on ACP. The question prompt list was the most commonly used tool in these n = 3 randomised controlled trials. In contrast, only n = 3 randomised controlled trials40,41,44 adopted multiple-element interventions using at least two interacting tools/methods prior to the physician-patient consultation. Of these, n = 240,41 combined written information materials and communication coaching as tools for facilitating end-of-life care discussion. The remaining n = 144 included all the tools/methods (video decision aids, written information materials and communication coaching) to boost end-of-life care discussions (see Figure 3, Table 1).
Sessions and duration of intervention
Most interventions (n = 6) comprised only one session.37–42 For single-element interventions (video decision aids or written information materials), the average duration of video decision aids was 4 min (range 3–6 min),38,39,42 and the mean time for using written information materials was 45 min (range 30–60 min);37,43,45 In contrast, the mean duration of multiple-element interventions was 55 min (range = 45–60 min).40,41,44
Intervention providers and training
The main providers of the ACP interventions were physicians (n = 3)38,40,45 and nurses (n = 2).44,45 Only n = 1 randomised controlled trial45 involved members of the multidisciplinary team (e.g. physicians and nurses) to approach patients and family members. Most randomised controlled trials (n = 5)40–42,44,45 trained intervention providers using standardised training with intervention manuals,42 standardised communication coaching,40,41 clinical cancer care training,44 videos on physician-patient communication40 and communication skills training using, for example, role-play modelling45 or trained actors40 (see Figure 3, Table 2).
Intervention effect on intended outcomes
Video decision aids
N = 3 randomised controlled trials38,39,42 reported an increase in patients’ knowledge of cardiopulmonary resuscitation and mechanical ventilation and a decrease in preference for these. However, n = 1 randomised controlled trial39 identified that patients’ uncertainty in decision-making increased after receiving the intervention (see Figure 3).
Written information materials
N = 2 randomised controlled trials37,45 reported an increase in patients’ willingness to discuss end-of-life care issues with physicians. Only n = 1 randomised controlled trial43 showed an intervention effect of increased patients’ knowledge of cardiopulmonary resuscitation. There was no difference in patients’ depression and anxiety, satisfaction with care, achievement of patient information need, ACP documentation rate or caregiver burden (see Figure 3).
Written information materials and communication coaching
N = 1 randomised controlled trial41 reported an increase in the discussion of end-of-life issues with physicians. N = 140 identified an increase in physician–patient communication. However, there was no difference in patients’ quality of life or healthcare utilisation (see Figure 3).
Video decision aids, written information materials and communication coaching
Only n = 1 randomised controlled trial44 used all three tools, reporting increases in discussions about end-of-life care and patients’ satisfaction with care, but no difference in patients’ quality of life, communication self-efficacy or consultation length (see Figure 3).
The linkage between single/multiple-element intervention and outcomes
Single-element interventions37–39,42,43,45 tended to increase patients’ knowledge about life-sustaining treatment, and decrease preference for these. However, there was no significant intervention effect on other patient-reported outcomes (e.g. emotional distress, satisfaction with care) or process outcomes (e.g. ACP documentation rate); while multiple-element interventions40,41,44 were often used to facilitate end-of-life care discussions between physicians and patients, and increase patients’ satisfaction with care. But, no significant intervention effect was found to increase patient’s quality of life and improve healthcare utilisation (see Figure 3).
Outcomes measured and measurement tools
Heterogeneous outcomes and tools were utilised. The main outcomes measured were patient-reported outcomes including preference for cardiopulmonary resuscitation,38,39,42 knowledge of life-sustaining treatment (cardiopulmonary resuscitation and mechanical ventilation),38,39,42,43 anxiety,37,43,45 quality of life,40,44 satisfaction with care45 and physician–patient communication.40,44 The majority of outcomes were measured by self-developed tools which lacked validation, except for anxiety (Visual Analogue Scale, VAS;45 Hospital Anxiety and Depression Scale, HADS;43 Spielberger State Anxiety Inventory, SSAI37), quality of life (Function Assessment of Cancer Therapy: General, FACT-G;40,44 McGill Quality of Life Questionnaire, McGill QOL scale40,44) and physician–patient communication (Perceived Efficacy in Physician/Patient interaction Scale, PEPPI44). Completion of ACP,38,43 consultation length,37,44 place of death38,43 and intervention fidelity40,44 were the most frequently reported process outcomes (see Table1).
Unintended consequences and patient safety
Only n = 1 randomised controlled trial45 reported adverse effects caused by the ACP intervention: one patient stated it was too morbid to continue the study, and two withdrew for unknown reasons. N = 4 randomised controlled trials37–39,42 reported no adverse effects (e.g. patients’ emotional distress) after intervention implementation. However, no relevant information about adverse effects or patient safety was reported in the other n = 4 randomised controlled trials.40,41,43,44
Underpinning conceptual models, mechanisms of action and the linkage to outcomes
Underpinning conceptual models
N = 4 randomised controlled trials40,41,43,44 applied conceptual models to underpin the intervention (n = 3);41,43,44 study aims, intervention and outcome measures (n = 1)40. N = 241,44 used the Self-Determination Theory of Health-Related Behaviour Change,51,52 n = 143 used the Shared Decision-Making Model49 and n = 140 used the Ecological Model of Patient-Centred Communication.53 N = 3 models41,43,44 only considered individual behaviour change and the majority of underpinning models (n = 3)40,41,44 were identified in randomised controlled trials published during 2017 (see Figure 3).
Mechanisms of action
The mechanisms through which video decision aids improved outcomes were by increasing patients’ knowledge in terms of illness and the benefits and burdens of specific medical treatments. This assisted patients to imagine the disease state and its treatment. In addition, for people with limited health literacy or for whom English was an additional language, complex information about illness and medical treatment options could be transmitted more easily by videos than written materials.46 The mechanisms of action for written information materials aimed at facilitating end-of-life care discussion were via an opportunity for patients to learn about end-of-life issues prior to their consultations with physicians.47,48 This assisted them in thinking ahead and acquiring information that was suited to their needs and at their own pace.50 A consistent end-of-life care decision was made by the shared decision-making process between physicians and patients,49 and patients could maintain a sense of control through this process.47,48 The mechanisms through which communication coaching changed behaviour were by increasing patients’ ‘autonomous motivation’ to ask questions or discuss issues about their end of life, improving their self-perceived sense of ‘competence’ to participate in the discussion and make health-related behavioural change. These interventions were intended to be conducted in a trustful clinician–patient relationship when patients are ready (relatedness), so they can maintain behaviour change over time51–53 (see Figure 3, Table 2).
The linkage between underpinning conceptual models and outcomes
Patients’ knowledge of cardiopulmonary resuscitation was improved by the process of information sharing from physician and patient, which was stressed in the Shared Decision-Making Model.43 According to the Self-Determination Theory of Health-Related Behaviour Change,41,44 building up the patients’ motivation and competence for initiating new health-related change was the key factor for achieving an increase in end-of-life care discussions and patients’ satisfaction with care. Better physician–patient communication was found in the intervention underpinned by the Ecological Model of Patient-Centred Communication,40 which emphasised the mutual interaction between medical staff, patients, and clinical and social context for better outcomes. Regardless of whether underpinning theory was used or not, there was no significant intervention effect on some patient-reported outcomes (e.g. emotional distress and quality of life) and process outcomes (e.g. ACP documentation rate and consultation length).
Discussion
This is the first study to systematically appraise the evidence to investigate underpinning conceptual models, ACP interventions, mechanisms of action and intended outcomes of ACP for patients with cancer. A novel logic model was developed to better understand the context and problem, and linkage between active ingredients and the intended outcomes. Moreover, we found that the development of the ACP interventions was poorly reported, and the literature is heavily focused on Western highly educated Caucasian patients. This limits generalisability to other cultures. Conceptual models were used to inform the trial designs, but their use was uncommon. When used, they focused mainly on individual behaviour change. Only one trial used an organisational approach. This type of organisational approach is important to implement and realise the benefit of a complex intervention as it works on multiple interacting levels.21 If we little consider the organisational level of change, and only consider the individual, then it is unlikely to sustain the change. Therefore, an approach targeting multiple levels in a whole-systems approach should be encouraged for continuous change and impact on patients, families and healthcare system.17 Finally, the logic model is based on current best available evidence, which we have demonstrated to have a Western basis. Therefore, the cultural appropriateness of this newly developed logic model of ACP interventions should be assessed prior to implementation in non-Western cultures to enhance intervention uptake and effectiveness.
The logic model
This novel logic model presents key elements for ACP implementation. A noteworthy effect was increasing patients’ knowledge of life-sustaining treatments and decreasing their preference for these by using video decision aids was found.54 But there was no significant intervention effect on several patient-reported outcomes (e.g. anxiety, depression and quality of life) and process outcomes (e.g. ACP completion rate, consultation length and healthcare utilisation) by using written information materials and communication coaching, they did facilitate early end-of-life care discussion. These findings are dissimilar to findings from previous reviews on the effectiveness of ACP (e.g. ACP could potentially reduce patients’ and relatives’ emotional distress, increase the ACP documentation and palliative care utilisation8,10,55). This appears to relate to factors of heterogeneity in the target population (cancer and non-cancer), limitation of theory used and bias in trial design (e.g. underpowered). Only four trials were identified that explicitly used conceptual models to underpin the intervention development and only two cited separate publications articulating the development work that informed the ACP intervention. This indicated the lack of conceptual models usage when developing complex interventions, which might compromise the quality of research work as the effectiveness could not be promised.56 It is notable that a trend for applying conceptual models for study design was found over time (three trials applying theoretical underpinning were published during 2017). This shows a recent increase in incorporating theoretical underpinnings into interventions to maximise the effectiveness aligned to methodological guidance.56 However, use of theoretical underpinning in trials on ACP for advanced cancer patients and demonstration of effectiveness on the main outcome is equivocal. This might be explained by the complex nature of the components and mechanisms of action of the ACP process that came across strongly in our synthesis.
Active ingredients in mechanisms of action
It may be hard to judge the superiority of one ACP intervention over another due to the heterogeneity of interventions and outcome measures used. But it is potentially possible to identify the active ingredients of ACP mechanisms to inform an implementation model. Clinicians should focus on these active ingredients to support the delivery of ACP in practice. The mechanisms of action we identified in this review extended existing understanding. Increasing death literacy among patients and family members is deemed to facilitate ACP discussion.57 In this review, an increase in patient’s understanding of life-sustaining treatment, and willingness of participating in end-of-life care discussion were found by providing informative materials such as video decision aids and information sheets. However, there was scant evidence reporting the subsequent increase of ACP documentation to guide clinical practice in accordance with patient’s wishes, leading to better healthcare outcomes.10 Therefore, measuring ACP documentation use is suggested in further study on people with advanced cancer to examine the association with end-of-life discussion. ‘Autonomous motivation’, ‘competence’ and ‘relatedness’ were highlighted as mechanisms for individual (patient) behaviour change in Self-Determination Theory of Health-Related Behaviour Change,51 and found to improve the patients’ satisfaction with care at the end of life. A communication coaching programme for patients was recognised as the key intervention along with the informative materials to improve patients’ satisfaction with care. This highlighted the importance of actively educating patients prior to the regular oncology consultation to enhance their ‘motivation’ and ‘competence’ to take part in an ACP discussion rather than just providing information on ACP to them. This should occur in a trustful relationship between them and clinicians at patients’ pace (‘relatedness’). Most importantly, a supportive contextual environment (e.g. availability of administrative system, sufficient resources, policy convictions and cultural acceptance) should be in place to support the implementation.17 In addition, communication and coaching skills training for medical staff were identified as essential requirements for successful ACP implementation. This echoed the importance of adopting an organisational level theory such as Ecological Model of Patient-Centred Communication53 to develop an ACP intervention with optimal effectiveness. Our logic model therefore highlights the importance of applying a broader theory focusing on organisational change, addressing mutual interactions between patients, families and clinicians, as well as the clinical and social context. This enables patients’ ongoing participation in end-of-life care discussions and assists them to participate in decisions to improve outcomes over time.53
Details of ACP intervention
Details on intervention development were poorly reported and often lacking, making it difficult to understand the thoroughness of the components, conceptual frameworks, the mechanism of action and even patient safety information, which are all crucial for developing a feasible and effective intervention.34,58 A previous review investigating the descriptions of treatment in trials and reviews showed that only 50% of included studies could be replicated by healthcare professionals or researchers.59 The limited detail on the interventions and their underpinning conceptual models might hinder replication and translation by clinicians or other researchers both in clinical practice and research, and compromise the effectiveness of interventions. In line with the Medical Research Council guidance on Process Evaluation of Complex Interventions21 and TIDieR guideline,22 we suggest authors provide greater detailed information about the development and evaluation of interventions. In particular, it is important to identify causality and the mechanisms of action between each component and process of the intervention, so it can be translated to different cultures.
Cultural acceptability and transferability
Studies reporting the components, processes and underpinning conceptual frameworks of ACP are limited to Western countries. We identified that all the included randomised controlled trials were conducted in Western countries and mainly in North America, Australia and the United Kingdom. This reflects established legislation on facilitating patients’ right to self-determination in medical care decisions in these countries (e.g. Self-Determination Act in North America,60 Mental Capacity Act 2005 in England and Wales61 and Statute Law and Common Law in Australia62). In Eastern countries such as Taiwan, legislation to facilitate patient’s autonomy (Patient Autonomy Act63) was recently passed and, consequently, ACP is a relatively new concept. The cultural acceptability and transferability of this Western-oriented ACP logic model in different cultures are unknown. Studies evaluating ACP in non-Western countries have recruited from the non-cancer population (e.g. Chan and colleague’s work on nursing-home residents in Hong Kong64 and Stanford et al.’s20 work on professional groups including teachers, hospice staff and pastors in South Africa). This indicates the development and evaluation of ACP in other cultures, focused on older people with non-malignant conditions and healthy public. Subsequent experimental empirical studies are required among these populations.
Strengths and limitations
The strength of this review is that it is the first to clarify the components, processes, conceptual models and mechanisms of action underpinning ACP. From this, a novel logic model has been developed to inform the key information and characteristics for practice, further research and most importantly, translating ACP into other cultures. By analysing the components and conceptual models separately, we are able to determine the dynamic mechanism between interventions and outcomes. The findings are also strengthened by a comprehensive literature search including electronic databases and hand-searching, and adherence to many guidelines and methods. However, our review has several limitations. A meta-analysis was not appropriate to examine the pooled intervention effect due to the small number of participants in each sub-group (single or multiple-element interventions) and the heterogeneous nature of included interventions.65 Furthermore, the high attrition rate in the selected studies reduced statistical power, although this is expected in palliative care trials and research with advanced cancer patients.66 Finally, this logic model may not be generalised to other disease conditions, and it might be worth looking at how ACP for other patient groups might be adaptable to the cancer population (e.g. Respecting Choices67 or Physician Orders for Life-Sustaining Treatment68).
Conclusion
A novel logic model, illustrating how the components and processes of ACP operate, has been constructed, using robust trial evidence. The use of conceptual frameworks to underpin ACP is uncommon. When used, the frameworks mainly focus on individual behavioural change, rather than considering a wider organisational approach. Key mechanisms of action were focused on facilitating patient’s knowledge, and building up motivation and competence in participating ACP, then making a decision in a trustful clinician–patient relationship. Single-element intervention improved patients’ understanding of life-sustaining treatments and reduced their preference for these at the end of life. Multiple-element intervention facilitated end-of-life discussions in subsequent consultations. This logic model cannot be currently transferred as the theory is not underpinned enough and it is mainly Western oriented, leading to a major obstacle to good care globally. There is also an urgent requirement to explore applicability of this Western-oriented logic model for different educational levels and ethnicities in non-Western countries, aiming to improve the access of palliative care worldwide so as to meet the Universal Health Coverage goals.69
Supplemental Material
Supplemental material, Supplementary_materials_Figure_S1_Example_search_strategy_(MEDLINE)_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine
Supplemental Material
Supplemental material, Supplementary_materials_Table_S1._Search_strategy_developed_using_PICOS_framework_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine
Supplemental Material
Supplemental material, Supplementary_materials_Table_S2_Description_of_risk_of_bias_assessment_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine
Acknowledgments
Conception and design: C.L., C.J.E., J.K. and R.H.
Collection and assembly of data: C.L., J.K., C.J.E. and R.H.
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Footnotes
Data management and sharing: All the relevant data are available. The method of data collection and screening process was reported in the main text (Figure 1). The search strategy can be found in the Supplementary File (Figure S1). The data used from the included study are clearly described in the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval was not required due to the type of review (narrative review of literature).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was not supported by any funding body. C.J.E. is funded by a HEE/NIHR Senior Clinical Lectureship. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
ORCID iDs: Cheng-Pei Lin  https://orcid.org/0000-0001-5810-8776
https://orcid.org/0000-0001-5810-8776
Richard Harding  https://orcid.org/0000-0001-9653-8689
https://orcid.org/0000-0001-9653-8689
References
- 1. Dy SM, Kiley KB, Ast K, et al. Measuring what matters: top-ranked quality indicators for hospice and palliative care from the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association. J Pain Symptom Manage 2015; 49: 773–781. [DOI] [PubMed] [Google Scholar]
- 2. Winzelberg GS, Hanson LC, Tulsky JA. Beyond autonomy: diversifying end-of-life decision-making approaches to serve patients and families. J Am Geriatr Soc 2005; 53: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 3. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010; 340: c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson S, Butow P, Kerridge I, et al. Advance care planning for cancer patients: a systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology 2016; 25: 362–386. [DOI] [PubMed] [Google Scholar]
- 5. Tamayo-Velazquez MI, Simon-Lorda P, Villegas-Portero R, et al. Interventions to promote the use of advance directives: an overview of systematic reviews. Patient Educ Couns 2010; 80: 10–20. [DOI] [PubMed] [Google Scholar]
- 6. Mullick A, Martin J, Sallnow L. An introduction to advance care planning in practice. BMJ 2013; 347: f6064. [DOI] [PubMed] [Google Scholar]
- 7. Simón-Lorda P, Barrio-Cantalejo IM, Garcia-Gutierrez JF, et al. Interventions for promoting the use of advance directives for end-of-life decisions in adults. Cochrane Db Syst Rev 2012; 4: CD007460. [Google Scholar]
- 8. Ke LS, Wang SP. The effectiveness of advance care planning for hospitalized elderly patients: a systematic review. Taiwan J Hospice Palliat Care 2012; 17: 48–61. [Google Scholar]
- 9. Weathers E, O’Caoimh R, Cornally N, et al. Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas 2016; 91: 101–109. [DOI] [PubMed] [Google Scholar]
- 10. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014; 28: 1000–1025. [DOI] [PubMed] [Google Scholar]
- 11. In der Schmitten J, Lex K, Fau-Mellert C, et al. Implementing an advance care planning program in German nursing homes: results of an inter-regionally controlled intervention trial. Dtsch Arztebl Int 2014; 111: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin RS, Hayes B, Gregorevic K, et al. The effects of advance care planning interventions on nursing home residents: a systematic review. J Am Med Dir Assoc 2016; 17: 284–293. [DOI] [PubMed] [Google Scholar]
- 13. Robinson L, Dickinson C, Rousseau N, et al. A systematic review of the effectiveness of advance care planning interventions for people with cognitive impairment and dementia. Age Ageing 2012; 41: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cartwright CM, Parker MH. Advance care planning and end of life decision making. Aust Fam Physician 2004; 33: 815–819. [PubMed] [Google Scholar]
- 15. Henry C, Seymour J, Ryder S. Advance care planning: a guide for health and social care staff. Leicester: The National Council of Palliative Care, 2008. [Google Scholar]
- 16. Emanuel LL, Barry MJ, Stoeckle JD, et al. Advance directives for medical care—a case for greater use. N Eng J Med 1991; 324: 889–895. [DOI] [PubMed] [Google Scholar]
- 17. Gilissen J, Pivodic L, Smets T, et al. Preconditions for successful advance care planning in nursing homes: a systematic review. Int J Nurs Stud 2017; 66: 47–59. [DOI] [PubMed] [Google Scholar]
- 18. Lovell A, Yates P. Advance Care Planning in palliative care: a systematic literature review of the contextual factors influencing its uptake 2008–2012. Palliat Med 2014; 28: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 19. Zager BS, Yancy M. A call to improve practice concerning cultural sensitivity in advance directives: a review of the literature. Worldviews Evid Based Nurs 2011; 8: 202–211. [DOI] [PubMed] [Google Scholar]
- 20. Stanford J, Sandberg DM, Gwyther L, et al. Conversations worth having: the perceived relevance of advance care planning among teachers, hospice staff, and pastors in Knysna, South Africa. J Palliat Med 2013; 16: 762–767. [DOI] [PubMed] [Google Scholar]
- 21. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015; 350: h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 23. Higgins J, Green S. Cochrane handbook for systematic reviews of intervention version 5.1.0. London: The Cochrane Collaboration, 2011. [Google Scholar]
- 24. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews, version 1. 2006, pp. 1–92. [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 26. Lin CP, Evans CJ, Koffman J, et al. The conceptual models that underpin advance care planning for advanced cancer patients and their mechanisms of action: a systematic review of randomised controlled trials. PROSPERO 2017: CRD42017067628, http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067628 [DOI] [PMC free article] [PubMed]
- 27. O’Connor D, Green S, Higgins JP. Defining the review question and developing criteria for including studies. In: Higgins JP, Green S. (eds) Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration, 2011, pp. 81–94. [Google Scholar]
- 28. Greenhalgh T. How to read a paper. the Medline database. BMJ 1997; 315: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017; 53: 821.e1–832.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas K, Lobo B. 2011. Advance care planning in end of life care. Oxford: Oxford University Press. [Google Scholar]
- 31. Endnote. EndNote X8 for Windows. Philadelphia, PA: Clarivate Analytics, 2016. [Google Scholar]
- 32. Cochrane Consumers Communication Review Group. Data extraction template for included studies Australia. London: The Cochrane Collaboration, 2016. [Google Scholar]
- 33. Review Manager. Review manager (RevMan) version 5.3. Copenhagen: The Nordic Cochrane Centre and The Cochrane Collaboration, 2014. [Google Scholar]
- 34. Glasziou PP, Chalmers I, Green S, et al. Intervention synthesis: a missing link between a systematic review and practical treatment(s). PLoS Med 2014; 11: e1001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langhorne P, Pollock A. What are the components of effective stroke unit care? Age Ageing 2002; 31: 365–371. [DOI] [PubMed] [Google Scholar]
- 36. Silva MJ, Breuer E, Lee L, et al. Theory of change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014; 15: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clayton JM, Butow PN, Tattersall MH, et al. Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 2007; 25: 715–723. [DOI] [PubMed] [Google Scholar]
- 38. Epstein AS, Volandes AE, Chen LY, et al. A randomized controlled trial of a cardiopulmonary resuscitation video in advance care planning for progressive pancreas and hepatobiliary cancer patients. J Palliat Med 2013; 16: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El-Jawahri A, Podgurski LM, Eichler AF, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol 2010; 28: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: the VOICE randomized clinical trial. JAMA Oncol 2017; 3: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodenbach RA, Brandes K, Fiscella K, et al. Promoting end-of-life discussions in advanced cancer: effects of patient coaching and question prompt lists. J Clin Oncol 2017; 35: 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Volandes AE, Paasche-Orlow MK, Mitchell SL, et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. J Clin Oncol 2013; 31: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stein RA, Sharpe L, Bell ML, et al. Randomized controlled trial of a structured intervention to facilitate end-of-life decision making in patients with advanced cancer. J Clin Oncol 2013; 31: 3403–3410. [DOI] [PubMed] [Google Scholar]
- 44. Walczak A, Butow PN, Tattersall MH, et al. Encouraging early discussion of life expectancy and end-of-life care: a randomised controlled trial of a nurse-led communication support program for patients and caregivers. Int J Nurs Stud 2017; 67: 31–40. [DOI] [PubMed] [Google Scholar]
- 45. Jones L, Harrington J, Barlow CA, et al. Advance care planning in advanced cancer: can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Palliat Support Care 2011; 9: 3–13. [DOI] [PubMed] [Google Scholar]
- 46. Gillick MR, Volandes AE. The psychology of using and creating video decision aids for advance care planning. In: Lynch TE. (ed.) Psychology of decision making in medicine and health care. New York: Nova Science Publishers, 2007, pp. 193–206. [Google Scholar]
- 47. Barnes KA, Jones L, Tookman A, et al. Acceptability of an advance care planning interview schedule: a focus group study. Palliat Med 2007; 21: 23–28. [DOI] [PubMed] [Google Scholar]
- 48. Barnes KA, Barlow CA, Harrington J, et al. Advance care planning discussions in advanced cancer: analysis of dialogues between patients and care planning mediators. Palliat Support Care 2011; 9: 73–79. [DOI] [PubMed] [Google Scholar]
- 49. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 50. Clayton J, Butow P, Tattersall M, et al. Asking questions can help: development and preliminary evaluation of a question prompt list for palliative care patients. Br J Cancer 2003; 89: 2069–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000; 55: 68–78. [DOI] [PubMed] [Google Scholar]
- 52. Ryan RM, Patrick H, Deci EL, et al. Facilitating health behaviour change and its maintenance: interventions based on Self-Determination Theory. Euro Health Psycol 2008; 10: 2–5. [Google Scholar]
- 53. Street RL, Jr, Makoul G, Arora NK, et al. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 2009; 74: 295–301. [DOI] [PubMed] [Google Scholar]
- 54. Jain A, Corriveau S, Quinn K, et al. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open 2015; 5: e007491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Houben CH, Spruit MA, Groenen MT, et al. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc 2014; 15: 477–489. [DOI] [PubMed] [Google Scholar]
- 56. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prince-Paul M, DiFranco E. Upstreaming and normalizing advance care planning conversations-a public health approach. Behav Sci 2017; 7: E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Breuer E, Lee L, De Silva M, et al. Using theory of change to design and evaluate public health interventions: a systematic review. Implement Sci 2016; 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Glasziou P, Meats E, Heneghan C, et al. What is missing from descriptions of treatment in trials and reviews? BMJ 2008; 336: 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Danis M, Southerland LI, Garrett JM, et al. A prospective study of advance directives for life-sustaining care. New Engl J Med 1991; 324: 882–888. [DOI] [PubMed] [Google Scholar]
- 61. Lord Chancellor. Mental Capacity Act 2005: code of practice. London: TSO, 2007. [Google Scholar]
- 62. Carter RZ, Detering KM, Silvester W, et al. Advance care planning in Australia: what does the law say? Aust Health Rev 2016; 40: 405–414. [DOI] [PubMed] [Google Scholar]
- 63. Laws and Regulations Databases of The Republic of China. Patient autonomy act. Taipei, Taiwan: Ministry of Health and Welfare, 2016. [Google Scholar]
- 64. Chan HY, Pang SM. Let me talk-an advance care planning programme for frail nursing home residents. J Clin Nurs 2010; 19: 3073–3084. [DOI] [PubMed] [Google Scholar]
- 65. Haidich AB. Meta-analysis in medical research. Hippokratia 2010; 14: 29–37. [PMC free article] [PubMed] [Google Scholar]
- 66. Grande GE, Todd CJ. Why are trials in palliative care so difficult? Palliat Med 2000; 14: 69–74. [DOI] [PubMed] [Google Scholar]
- 67. Pecanac KE, Repenshek MF, Tennenbaum D, et al. Respecting Choices® and advance directives in a diverse community. J Palliat Med 2014; 17: 282–287. [DOI] [PubMed] [Google Scholar]
- 68. Hammes BJ, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliat Med 2012; 15: 77–85. [DOI] [PubMed] [Google Scholar]
- 69. World Health Organization. Universal health coverage (UHC). Geneva: World Health Organization, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_materials_Figure_S1_Example_search_strategy_(MEDLINE)_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine
Supplemental material, Supplementary_materials_Table_S1._Search_strategy_developed_using_PICOS_framework_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine
Supplemental material, Supplementary_materials_Table_S2_Description_of_risk_of_bias_assessment_by_CL_in_Palliative_Medicine for The conceptual models and mechanisms of action that underpin advance care planning for cancer patients: A systematic review of randomised controlled trials by Cheng-Pei Lin, Catherine J Evans, Jonathan Koffman, Jo Armes, Fliss E M Murtagh and Richard Harding in Palliative Medicine