Abstract
Background:
End-stage liver disease is a common cause of morbidity and mortality worldwide, yet little is known about its symptomatology and impact on health-related quality of life.
Aim:
To describe symptom prevalence and health-related quality of life of patients with end-stage liver disease to improve care.
Design:
Systematic review.
Data sources:
We searched eight electronic databases from January 1980 to June 2018 for studies investigating symptom prevalence or health-related quality of life of adult patients with end-stage liver disease. No language restrictions were applied. Meta-analyses were performed where appropriate.
Results:
We included 80 studies: 35 assessing symptom prevalence, 41 assessing health-related quality of life, and 4 both. The instruments assessing symptoms varied across studies. The most frequently reported symptoms were as follows: pain (prevalence range 30%–79%), breathlessness (20%–88%), muscle cramps (56%–68%), sleep disturbance (insomnia 26%–77%, daytime sleepiness 29.5%–71%), and psychological symptoms (depression 4.5%–64%, anxiety 14%–45%). Erectile dysfunction was prevalent (53%–93%) in men. The health-related quality of life of patients with end-stage liver disease was significantly impaired when compared to healthy controls or patients with chronic liver disease. Compared with compensated cirrhosis, decompensation led to significant worsening of both components of the 36-Item Short Form Survey although to a larger degree for the Physical Component Summary score (decrease from average 6.4 (95% confidence interval: 4.0–8.8); p < 0.001) than for the Mental Component Summary score (4.5 (95% confidence interval: 2.4–6.6); p < 0.001).
Conclusion:
The symptom prevalence of patients with end-stage liver disease resembled that of patients with other advanced conditions. Given the diversity of symptoms and significantly impaired health-related quality of life, multidisciplinary approach and timely intervention are crucial.
Keywords: End-stage liver disease, liver cirrhosis, hepatocellular carcinoma, symptom assessment, prevalence, quality of life, palliative care, meta-analysis
What is already known about the topic?
End-stage liver disease is a major cause of morbidity and mortality worldwide, causing symptoms and reducing health-related quality of life.
There are no systematic reviews of symptom prevalence or health-related quality of life of patients with end-stage liver disease.
What this paper adds?
The symptom prevalence of patients with end-stage liver disease resembled that of patients with other advanced conditions.
The most frequently reported symptoms were pain, breathlessness, muscle cramps, sleep disturbance, depression, anxiety, and erectile dysfunction.
Decompensation led to significant worsening of health-related quality of life.
Implications for practice, theory, or policy
Given the diversity of symptoms and significantly impaired health-related quality of life, multidisciplinary approach and timely intervention are crucial in the care of patients with end-stage liver disease.
Introduction
End-stage liver disease is the final stage of a liver disease when liver failure is usually irreversible and liver transplantation is the only curative treatment. Worldwide, end-stage liver disease is a common cause of morbidity and mortality.1–6 A global estimate of its mortality in 2010 reported more than 1 million patients died from end-stage liver disease equating to about 2% of all deaths.4,5 In Europe, liver disease is the seventh leading cause of death.6 In the United Kingdom, liver disease is currently the fifth most common cause of death in those under 65 years of age, and the number of people who die from end-stage liver disease is still increasing.1 In the United States, end-stage liver disease is the 12th leading cause of death overall, and the 7th leading cause of death in people aged 25–64 years.2 Compared to other end-stage organ failure or terminal illness, end-stage liver disease disproportionately affects younger age groups, and the years of life lost is estimated to be around 20 years.7,8
Cirrhosis, which is defined as the histological development of advanced liver fibrosis, is a final pathway in patients with chronic liver injury from a variety of etiologies.9,10 Alcoholic liver disease and hepatitis C are the main causes in developed countries, while hepatitis B is the most common cause in most parts of Asia and sub-Saharan Africa.10 Patients with cirrhosis who have not developed major complications are classified as having compensated cirrhosis. As progressive liver damage occurs, the disease may proceed to decompensated cirrhosis, that is, end-stage liver disease. The major complications of decompensation include variceal bleeding, ascites, encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatopulmonary syndrome. Once decompensation has occurred, the 5-year mortality without transplantation is as high as 85%.10 Patients with end-stage liver disease face physical, psychological, and complex social and financial problems because the majority of them are in their working age.7,11
Although patients with end-stage liver disease are vulnerable and at risk of death, it is surprising that such little attention has been paid to describing their symptom prevalence and health-related quality of life. Another problem comes from the ambiguous study population representing patients with end-stage liver disease in previous research, such as patients with chronic liver disease, advanced liver disease, or liver cirrhosis without mentioning decompensation or not. Furthermore, studies looking into health-related quality of life of patients with end-stage liver disease have predominantly focused on the health-related quality of life of liver transplant recipients, not of candidates experiencing end-stage liver disease.12
This study aimed to describe symptom prevalence and health-related quality of life of patients with end-stage liver disease in order to understand their needs and to provide a good reference for developing tailored care services for them.
Materials and methods
Study design
We undertook a systematic review and meta-analysis, which was conducted and reported following the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement.13
Search strategy and selection criteria
We searched eight electronic databases including Cochrane Library (Wiley), MEDLINE (Ovid), EMBASE (Ovid), PsycINFO (Ovid), CINAHL (EBSCO Host), British Nursing Index (ProQuest), SCOPUS (Elsevier), and Web of Science (Thomson Reuters), between 1 January 1980, and 30 June 2018, using a combination of medical subject headings, title/abstract keywords, and free text (see Supplementary Material). In addition to database searching, hand searching (from reference lists of all of the eligible studies, key studies, and key journals) was performed. No language restrictions were applied, and the full eligibility criteria are presented in Table 1.
Table 1.
Eligibility criteria for the systematic review.
| Component | Criteria |
|---|---|
| Study population |
Inclusion criteria
• Adults (⩾18 years old) • End-stage liver disease (ESLD), with evidence of at least one of the following: (1) Liver cirrhosis, with at least an index clinical complication of decompensation (2) Liver cirrhosis, being referred for liver transplantation evaluation (3) Liver cirrhosis, with Child–Turcotte–Pugh (CPT) classification predominantly B or C of the study population (Child A ⩽ 40%, or mean/median CPT score > 7) (4) Patients being referred for liver transplantation evaluation, with clear description of the etiology from chronic/advanced liver disease (5) Liver transplantation candidates, liver cirrhosis related (or with clear description of the etiology from chronic or advanced liver disease) Exclusion criteria • Under 18 years old • Acute hepatic failure • Metabolic liver diseases (e.g. familial amyloid polyneuropathy) • Having other terminal diseases (except hepatocellular carcinoma) • Liver transplantation candidates waiting for combined liver and kidney transplantation • Liver transplant recipients only |
| Study design |
Inclusion criteria
Peer-reviewed clinical controlled trials or observational studies were eligible Exclusion criteria Review articles, discussions, letters, editorials, comments, case reports, case series, case studies, and qualitative studies were not considered. Before and after studies focusing on the change in quality of life by liver transplantation were excluded |
| Outcome | Any outcome related to symptom prevalence or health-related quality of life was eligible |
| Language | No language restrictions |
Following the search, two authors (J.-K.P. and N.H.) screened all titles and abstracts and excluded irrelevant papers independently. Then J.-K.P. assessed the full texts of all the remaining studies and discussed with N.H. as needed. Disagreement was resolved via discussion.
Data analysis
Data were extracted from all included studies (by J.-K.P.) using a data extraction spreadsheet. For each study, author/year, place of study, study design, study population, patient number, age, sex, etiology, Child–Turcotte–Pugh classification (or score),14 percentage of patients with hepatocellular carcinoma, Model for End-Stage Liver Disease score,15–17 symptom prevalence or health-related quality of life and their measurement tools, and comparison groups (if applicable) were recorded.
For quality assessment, J.-K.P. assessed all studies for risk of bias by using the validated review tool (QualSyst, score 0–1).18 The articles scoring greater or equal to 0·8 were regarded as high quality, 0·6–0·79 as medium, while less than 0·6 as poor quality. A random 10% of the assessments were independently checked for accuracy and completeness by N.H. One review author (W.G.) resolved disagreement. Articles graded as poor quality (score < 0·6) were not considered for final data synthesis.
For symptom prevalence of patients with end-stage liver disease, data were summarized by each symptom. As for health-related quality of life, a descriptive summary was given, and the results were combined for meta-analysis if appropriate, using a random effect model due to the degree of heterogeneity (defined if the p value of chi-square test was less than 0.1 or the I2 statistic was more than 40%).19 Forest plots were used to display the results. Stata/SE 14 (STATA, College Station, TX) was used for all statistical analyses.
Results
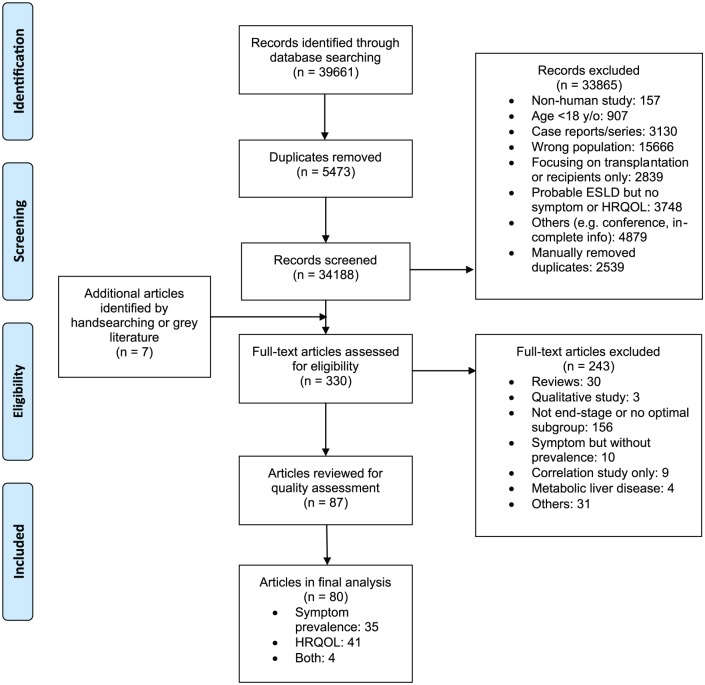
A total of 80 studies were included in the final review (Figure 1). Electronic database searching identified 39,661 references. A total of 5473 duplicate references were removed, and a further 33,865 references were excluded after title/abstract screening and further deduplication. Seven additional studies were identified from hand searching. The full-text of 330 articles were assessed, and 87 articles were kept for quality assessment. Seven papers were graded as low quality and therefore excluded. Among the remaining, 35 described symptom prevalence and 41 provided information on health-related quality of life, while four assessed both outcomes.
Figure 1.
PRISMA flow diagram.
Symptom prevalence of patients with end-stage liver disease
A total of 39 studies assessing symptom prevalence were included (see Supplementary Material).20–58 The majority reported data from North America (16) and Europe (14), although Asia (8) and Africa (1) were also covered. Most were cross-sectional studies conducted while patients were outpatients or stable after hospitalization. The patients were middle aged and predominantly male. For disease severity, 26 studies (67%) reported the percentage of patients with Child–Turcotte–Pugh class A or overall Child–Turcotte–Pugh score and 17 of 33 studies (52%) which were published between 2002 and 2018 provided Model for End-Stage Liver Disease scores. The percentage of comorbidity with hepatocellular carcinoma was reported in 18 studies (46%), ranging from 0% to 46·9%.
The instruments for symptom measurement varied across studies. For physical symptoms, most studies focused on one or two symptoms, and only three studies evaluated more than three symptoms at the same time.24,40,46 The only study systematically reporting the average number of symptoms in patients with end-stage liver disease was conducted by Baumann et al., using liver-specific Edmonton Symptom Assessment System to evaluate 50 liver transplantation candidates. Among them, 80% had at least one moderate to severe symptom, and the average number of moderate to severe symptoms was 4·1.24
The summary of symptom prevalence of patients with end-stage liver disease is shown in Table 2. Pain was one of the most frequently reported symptoms across the studies with a prevalence ranging between 30% and 79%.36,40,43,45,46 More specifically, Rogal et al. surveyed 193 advanced liver cirrhosis patients with a mean Child–Turcotte–Pugh score of 7.6. The authors found that 56% of patients’ pain occurred at least daily, and pain-related disability was noted in 75% of the whole population. The most frequent locations of their pain were the abdomen and lower back, which was similar in another study.36 Unfortunately, the pain of patients with end-stage liver disease was not well relieved. In the retrospective study conducted by Madan et al., 90% of 108 patients with end-stage liver disease were prescribed medication for pain, but only 33% of them received favorable pain relief. Furthermore, 74% of patients had already been given at least three medications for pain.36 Muscle cramps were also reported in several studies, occurring in 56%–68% of patients, but the given time period of the definition of prevalence varied across studies.21–23,25,26 Other most frequently reported physical symptoms were insomnia (26%–77%),28,29,38,39,42,58 erectile dysfunction (53%–93%),27,32,35,48,51 breathlessness (20%–88%),20,34,40,46 and daytime sleepiness (29.5%–71%).28,38,39,53,55 Fatigue and pruritus, which are regarded as disease-specific symptoms, were only reported in two articles for each, with a prevalence of 52%–86% and 47%–64%, respectively.25,42,56,57 Other symptoms reported in only one article were as follows: dyspepsia (85%),30 lower urinary tract symptoms (in men, 38%),37 nausea (58%),40 and poor appetite (49%).40 The prevalence of abdominal symptoms (e.g. distension, bloating) were not reported in these studies.
Table 2.
Symptom prevalence of patients with end-stage liver disease.
| Symptom | Outcome measure | Papers using outcome measure | Prevalence (%) |
|---|---|---|---|
| Pain | BPI | Madan et al.36 | 77 |
| ESAS | Poonja et al.40 | 65 | |
| MPQ | Rogal et al.43 | 79 | |
| Chart review | Rogal et al.45 | 47 | |
| Interview | Roth et al.46 | About 30–40 (from 6 month before death) | |
| Breathlessness | mMRC | Abdel-Bary et al.,20 Kaltsakas et al.34 | 80–88 |
| ESAS | Poonja et al.40 | 48 | |
| Interview | Roth et al.46 | About 20–45 (from 6 month before death) | |
| Muscle cramps | ⩾1/month for 1 year | Abrams et al.,21 Baskol et al.23 | 62–68 |
| ⩾3/month | Angeli et al.22 | 56–57 | |
| ⩾1 in last month | Bianchi et al.25 | 58 | |
| ⩾1 in last 12 weeks | Chatrath et al.26 | 67 | |
| Erectile dysfunction | IIEF-5 | Chien et al.,27 Huyghe et al.,32 Wang et al.51 | 74–93 (moderate–severe 17–59) |
| IIEF | Klein et al.35 | 53 (moderate–severe) | |
| Psychiatric interview | Sorrell and Brown48 | 54 | |
| Insomnia | STSQS | De Rui et al.28 | 36 |
| PSQI | Gencdal et al.,29 Montagnese et al.,38 Xiao et al.58 | 63–77 | |
| BNSQ | Mostacci et al.39 | 26 | |
| MFSI-SF | Rodrigue et al.42 | 73 | |
| Daytime sleepiness | ESS | Abdullah et al.,53 Ghabril et al.,55 De Rui et al.,28 Montagnese et al.,38 Mostacci et al.39 | 29.5–71 |
| Fatigue | FSI | Rodrigue et al.42 | 86 |
| Self-reported questions | Lai et al.56 | 52 | |
| Pruritus | Baseline clinical data | Bianchi et al.25 | 47 |
| Japanese guidance | Sumi et al.57 | 64 | |
| Anxiety | STAI | Annema,54 Gutteling31 | 25–45 |
| HADS | Kalaitzakis et al.33 | 16 | |
| ESAS | Poonja et al.40 | 36 | |
| Millon behavioral medicine diagnostic | Stewart et al.49 | 14 | |
| Depression | CES-D | Annema et al.,54 Baumann et al.24 | 35–36 |
| BDI | Bianchi et al.,25 Gutteling et al.,31 Singh et al.47 | 57–64 (moderate-severe 16–28) | |
| HADS | Kalaitzakis et al.23 | 14 | |
| HAM-D | Popovic et al.41 | 55 (moderate–severe 25) | |
| ESAS | Poonja et al.40 | 10 | |
| Chart review | Rogal et al.44 | 36 | |
| Millon behavioral medicine diagnostic | Stewart et al.49 | 23 | |
| BEF + DSM III | Trzepacz et al.50 | 4.5 (major depression) 12.6 (adjustment disorder + depressive mood) |
BDI: Beck Depression Inventory; BEF: brief evaluation form; BNSQ: Basic Nordic Sleep Questionnaire; BPI: Brief Pain Inventory; CES-D: Center for Epidemiological Studies–Depression; ESAS: Edmonton Symptom Assessment System; DSM: Diagnostic and Statistical Manual of Mental Disorders; ESS: Epworth Sleepiness Scale; FSI: Fatigue Symptom Inventory; HADS: Hospital Anxiety and Depression Scale; HAM-D: Hamilton Depression Rating Scale; IIEF: International Index of Erectile Function; MFSI-SF: Multidimensional Fatigue Symptom Inventory–Short Form; mMRC scale: modified Medical Research Council scale; MPQ: McGill Pain Questionnaire; PSQI: Pittsburgh Sleep Quality Index; STAI: State-Trait Anxiety Inventory; STSQS: Sleep Timing and Sleep Quality Screening questionnaire.
Pain: 30%–79%; breathlessness: 20%–88%; muscle cramps: 56%–68%; erectile dysfunction: 53%–93% (moderate–severe 17%–59%); insomnia: 26%–77%; daytime sleepiness: 29.5%–71%; fatigue: 52%–86%; pruritus: 47%–64%; anxiety: 14%–45%; depression: 4.5%–64% (moderate–severe 16%–28%).
Many of the studies also assessed psychological symptoms with a bigger focus on depression, which was reported by 11 articles. The prevalence of depression in patients with end-stage liver disease varied between 4.5% and 64%.24,25,31,33,40,41,44,47,49,50,54 This may in part be related to the measurement tools used as the Beck Depression Inventory (BDI) was applied in three studies, the Center for Epidemiological Studies–Depression (CES-D) was applied in two studies, while the other six studies used six different ways to identify depressive symptoms or major depression. The prevalence of anxiety was 14%–45%,31,33,40,49,54 and similarly, the five studies which assessed anxiety administered four different measurement tools.
Health-related quality of life of patients with end-stage liver disease
A total of 45 studies for health-related quality of life were included (see Supplementary Material).26,31,38,49,59–99 Except for two international studies, the majority of studies reported data from Europe (21) and North America (11), and there were five from China. Most of the studies were cross-sectional and outpatient-based. The patients were mostly in their 50s and predominantly male. A total of 28 studies (62%) reported the percentage of patients with Child–Turcotte–Pugh class A or overall Child–Turcotte–Pugh score; 21 of 42 (50%) studies published between 2002 and 2018 provided Model for End-Stage Liver Disease scores. The prevalence of hepatocellular carcinoma was provided in 18 studies, ranging from 0% to 41·1%. In general, the reporting quality of articles for health-related quality of life was better than those for symptoms.
The results of the included studies for health-related quality of life of patients with end-stage liver disease are shown in the Supplementary Material. The comparisons in these articles could be categorized into four themes: disease severity, clinical status (inclusive of decompensation, lab finding, or symptoms), etiology, and other factors (e.g. demographic characteristics, social economic status). Generic health-related quality of life tools were more frequently administered than disease-specific health-related quality of life tools. The Medical Outcome Study Questionnaire 36-Item Short Form Survey (SF-36)100,101 was applied in 36 studies (80%). As for disease-specific health-related quality of life tools, the Chronic Liver Disease Questionnaire (CLDQ)102 was applied in 14 studies, followed by the Liver Disease Quality Of Life 1.0103 (twice) and the Hepatitis Quality Of Life Questionnaire104 (once).
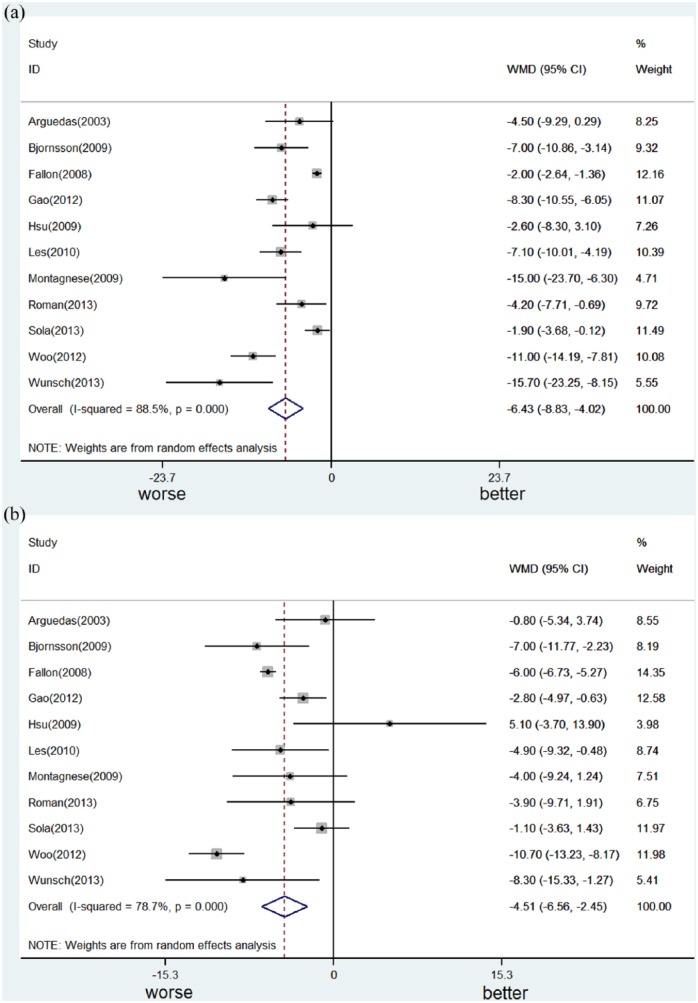
The health-related quality of life of patients with end-stage liver disease was significantly worse, compared to normal controls or patients with chronic hepatitis. We conducted a meta-analysis of the effect of Child–Turcotte–Pugh class (Child B or C vs Child A)61,70,84,93 or decompensation (decompensated vs compensated liver cirrhosis)38,63,67,73,79,87,92 on the Physical Component Summary score (Figure 2a) and the Mental Component Summary score (Figure 2b) of the 36-Item Short Form Survey as well as CLDQ (see Supplementary Material).38,62,64,74,79,93,95 Due to heterogeneity (chi-square p < 0.1, I2 statistic > 40%), a random-effect model was applied. We found that disease progression led to significant worsening of both components of the 36-Item Short Form Survey; however, this was to a larger degree for Physical Component Summary score than for Mental Component Summary score. The decrease from average Physical Component Summary score was 6.4 (95% confidence interval (CI): 4.0–8.8; p < 0.001) and that of average Mental Component Summary score was 4.5 (95% CI: 2.4–6.6; p < 0.001). As to CLDQ (global score, ranging from 1 to 7), disease progression significantly worsened health-related quality of life, and the difference between each group was 0·66 (95% CI: 0.34–0.98; p < 0.001).
Figure 2.
Meta-analysis of the effect of Child–Turcotte–Pugh class (Child B or C vs Child A) or decompensation (decompensated vs compensated liver cirrhosis) on the Physical Component Summary score (Figure 2a) and the Mental Component Summary score (Figure 2b) of the 36-Item Short Form Survey.
Discussion
Main findings of the study
This review described symptom prevalence and health-related quality of life of patients with end-stage liver disease. Pain, breathlessness, muscle cramps, sleep disturbances, and psychological symptoms are common and need to be managed carefully. Erectile dysfunction is prevalent in men with end-stage liver disease, who should be provided an opportunity for treatment. The health-related quality of life of patients with end-stage liver disease is significantly impaired when compared to healthy controls, patients with chronic liver disease or those with compensated liver cirrhosis.
This review highlighted the high degree of variability in symptom prevalence reported by different studies. This is probably owing to the within-patient variability and the differences between measurement tools. We have compared our results with that of a systematic review of symptom prevalence in advanced cancer, chronic obstructive pulmonary disease, congestive heart failure, end-stage renal disease, dementia, motor neuron disease and multiple sclerosis.105 (Table 3) The symptom prevalence of patients with end-stage liver disease resembles that of patients with these other advanced and chronic conditions. The wide range of symptoms experienced by these patients reminds healthcare professionals the importance of comprehensive and consecutive assessments for them.
Table 3.
Comparison of common symptom prevalence (range min to max) in end-stage liver disease and other advanced conditions.a
| Symptom | ESLD | Cancera | COPDa | CHFa | ESRDa | Dementiaa | MNDa | MSa |
|---|---|---|---|---|---|---|---|---|
| Pain | 30–79 | 30–97 | 21–77 | 14–78 | 11–83 | 14–63 | 52–76 | 68 |
| Breathlessness | 20–88 | 16–77 | 56–98 | 18–88 | 11–82 | 12–52 | 81–88 | 26 |
| Insomnia | 26–77 | 3–67 | 15–77 | 36–48 | 1–83 | 14 | 24–33 | |
| Fatigue | 52–86 | 23–100 | 32–96 | 42–82 | 13–100 | 22 | 80 | |
| Anorexia | 49 | 76–95 | 64–67 | 38–64 | ||||
| Nausea or vomiting | 58 | 2–78 | 4 | 2–48 | 8–52 | 8 | 26 | |
| Depression | 4·5–64 | 4–80 | 17–77 | 6–59 | 2–61 | 46 | 23 | 15 |
| Anxiety | 14–45 | 3–74 | 23–53 | 2–49 | 7–52 | 8–72 | 19 | 24 |
ESLD: end-stage liver disease; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; ESRD: end-stage renal disease; MND: motor neuron disease; MS: multiple sclerosis.
Adapted from Moens et al.105
Data on prevalence of symptoms.
Given that end-stage liver disease affects younger age groups compared to other end-stage organ failure or terminal illness, their needs for symptom treatment and support can be different. Sexual dysfunction is a good example of this. In men with end-stage liver disease, they may have hypogonadism resulting in impotence, infertility, loss of sexual drive, or testicular atrophy. In our review, the prevalence of erectile dysfunction is 53%–93% (moderate–severe: 17%–59%).27,32,35,48,51 For women with end-stage liver disease, chronic anovulation is common, which may manifest as irregular menstrual bleeding or amenorrhea,106 and the prevalence of sexual dysfunction is 77.8%.35 For both men and women, Sorrell and Brown.48 identified that frequency of and interest in sex decreased markedly (65.3% and 40.5% each). Hence, patients with end-stage liver disease should be provided an opportunity for assessment and treatment of sexual dysfunction.
Strengths and weakness of the study
One strength of this review is that we carefully considered our eligibility criteria to identify patients with end-stage liver disease and we provide clear descriptions of important clinical characteristics of the study population (e.g. etiology, percentage of Child A according to Child–Turcotte–Pugh classification, Model for End-Stage Liver Disease score, and the percentage of hepatocellular carcinoma). In addition, this review summarized the best available evidence by searching eight electronic databases. Moreover, we were also able to perform a meta-analysis to compare the health-related quality of life between compensated and decompensated liver cirrhosis, which is of value.
Our review has several limitations. First, this is a systematic review of observational studies. We were not able to control for all potential confounders, and not all the included studies had comparison groups. However, we conducted a quality assessment and presented good-quality studies clearly to allow readers to draw their own conclusions. Second, most studies were conducted while patients were outpatients or stable after hospitalization; hence, some acute symptoms related to decompensation were not captured. Third, due to limited articles for symptom prevalence and heterogeneity of the populations, meta-analysis could not be performed in many aspects. Finally, although there was no limitation of language in the process of study identification and selection, the majority of the included studies were from Europe and North America, where the cause of end-stage liver disease, the patterns of comorbidity (e.g. hepatocellular carcinoma), and the factors associated with health-related quality of life might be different from Asian or African countries.
What this study adds
Currently, the priority of the medical care for patients with end-stage liver disease is usually curative treatment (e.g. liver transplantation) rather than best supportive care because these patients are younger and not seen to be at the end of life by themselves or by their physicians, regardless of the risk of shortage of organs, delisting from transplantation waiting list due to contraindication, or facing life-threatening conditions.107,108 The lack of holistic approach, the high prevalence of depression and anxiety, and the dearth of social support all together bring about the complexity and difficulty of caring for patients with end-stage liver disease.7,107–111 Given the wide range of symptoms experienced by these patients and the significant impairment in health-related quality of life revealed by our systematic review, collaboration between primary care physicians, specialist hepatologists, liver transplantation providers, and specialist palliative care is needed.107–109 Early detection, consecutive monitoring, and timely management of symptoms may result in better health-related quality of life in these patients. Further studies focusing on comprehensive symptom evaluation, factors related to the variation of symptomatology, and developing consensus in symptom measurement or management would be helpful.
As for the symptomatology of patients with end-stage liver disease, several disease-specific symptoms were less reported, such as abdominal symptoms, fatigue, or pruritus. Abdominal symptoms, including abdominal distension, bloating, pain, or any kind of abdominal discomfort, may worsen alongside the development of refractory ascites and induce patient distress.112 On the contrary, fatigue and pruritus are common in patients with primary biliary cirrhosis or primary sclerosing cholangitis,113,114 yet we found no report for either of these symptoms after decompensation. A practical way to enrich our understanding of these symptoms is to provide the raw data when measuring disease-specific health-related quality of life in future research. For instance, these symptoms are embedded in CLDQ,102 Liver Disease Quality Of Life 1.0,103 as well as PBC-40115 for patients with primary biliary cirrhosis. Symptom prevalence can be identified from these tools. Furthermore, some other symptoms were not identified in this review and are worth exploring, including symptoms related to gastrointestinal disorder or bleeding (hematemesis, melena, hematochezia), jaundice, easy bruisability, lower extremity edema, and weight loss.
Finally, for better clarity and reporting quality of future research in patients with end-stage liver disease, we suggest that Child–Turcotte–Pugh classification (or score), Model for End-Stage Liver Disease score, and the information of comorbidity (especially hepatocellular carcinoma) should be reported. As for hepatocellular carcinoma, it is better presented considering the Milan criteria, which has been widely applied as a basis for selecting patients with hepatocellular carcinoma for liver transplantation.116
Conclusion
End-stage liver disease, a common cause of morbidity and mortality, disproportionally affects younger age groups and causes premature death. The symptom prevalence of patients with end-stage liver disease resembles that of patients with other advanced conditions. Given the diversity of symptoms and significantly impaired health-related quality of life, multidisciplinary approach and timely intervention are crucial. Palliative care should be integrated in the care of patients with end-stage liver disease.
Supplemental Material
Supplemental material, Supp_Database_searching_syntax_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental Material
Supplemental material, Supp_Meta-analysis_supplementary_materials_no_change for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental Material
Supplemental material, Supp_table_1_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental Material
Supplemental material, Supp_table_2_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental Material
Supplemental material, Supp_table_3_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Acknowledgments
J.-K.P. and W.G. designed the study; I.J.H. gave clinical and scientific input; J.K.P and N.H. completed searches and study selection; J.-K.P. completed data extraction; J.-K.P. and N.H. undertook the quality assessment, and W.G. resolved disagreement; and J.-K.P. performed analysis and W.G. provided statistic supervision. All authors reviewed the findings, agreed the interpretation, contributed to writing the paper, had full access to all data in the study, and read and approved the final version. The corresponding author (J.-K.P.) had final responsibility for the decision to submit for publication. The authors thank Ministry of Education of Taiwan for educational grant for J.-K.P.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was partly supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London at King’s College Hospital NHS Foundation Trust. The funders of the study had no role in study design, data collection, data analysis, data interpretation, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
ORCID iD: Jen-Kuei Peng  https://orcid.org/0000-0001-9997-9842
https://orcid.org/0000-0001-9997-9842
References
- 1. Bhala N, Aithal G, Ferguson J. How to tackle rising rates of liver disease in the UK. BMJ 2013; 346: f807. [DOI] [PubMed] [Google Scholar]
- 2. Cox-North P, Doorenbos A, Shannon SE, et al. The transition to end-of-life care in end-stage liver disease. J Hosp Palliat Nurs 2013; 15: 209–215. [Google Scholar]
- 3. da Rocha MC, Marinho RT, Rodrigues T. Mortality associated with hepatobiliary disease in Portugal between 2006 and 2012. GE Port J Gastroenterol 2018; 25: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between1980 and 2010: a systematic analysis. BMC Med 2014; 12: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO). Age-standardized death rates liver cirrhosis (15+ years) per 100000 population, 2012. Geneva: WHO, 2014. [Google Scholar]
- 6. Kotzeva M. (ed.). European social statistics (Eurostat Pocketbooks). 2013. ed. Luxembourg: European Union, 2013, https://annazavaritt.blog.ilsole24ore.com/wp-content/uploads/sites/54/files/eurostat-social-stats-2013-complete-edition.pdf [Google Scholar]
- 7. Effiong K, Osinowo A, Pring A. Deaths from liver disease: implications for end of life care in England. National End of Life Care Intelligence Network, 2012, http://www.endoflifecare-intelligence.org.uk/resources/publications/deaths_from_liver_disease
- 8. Marinho RT, Duarte H, Giria J, et al. The burden of alcoholism in fifteen years of cirrhosis hospital admissions in Portugal. Liver Int 2015; 35: 746–755. [DOI] [PubMed] [Google Scholar]
- 9. Rosselli M, MacNaughtan J, Jalan R, et al. Beyond scoring: a modern interpretation of disease progression in chronic liver disease. Gut 2013; 62: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 10. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008; 371: 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carvalho JR, Vasconcelos M, Marques da, Costa P, et al. Identifying palliative care needs in a Portuguese liver unit. Liver Int 2018. Epub ahead of print 23 April 2018. DOI: 10.1111/liv.13865. [DOI] [PubMed] [Google Scholar]
- 12. Yang LS, Shan LL, Saxena A, et al. Liver transplantation: a systematic review of long-term quality of life. Liver Int 2014; 34: 1298–1313. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 14. Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 15. Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31: 864–871. [DOI] [PubMed] [Google Scholar]
- 16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003; 124: 91–96. [DOI] [PubMed] [Google Scholar]
- 17. Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl 2001; 7: 567–580. [DOI] [PubMed] [Google Scholar]
- 18. Kmet LM, Lee RC, Cool LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Edmonton, AB, Canada: Alberta Heritage Foundation for Medical Research, 2004. [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdel-Bary SA, Yousif M, Hussein HA. Respiratory muscle strength, hypoxemia and dyspnea in liver cirrhosis patients. Egypt J Chest Dis Tuberculosis 2014; 63: 1059–1064. [Google Scholar]
- 21. Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol 1996; 91: 1363–1366. [PubMed] [Google Scholar]
- 22. Angeli P, Albino G, Carraro P, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology 1996; 23: 264–273. [DOI] [PubMed] [Google Scholar]
- 23. Baskol M, Ozbakir O, Coskun R, et al. The role of serum zinc and other factors on the prevalence of muscle cramps in non-alcoholic cirrhotic patients. J Clin Gastroenterol 2004; 38: 524–529. [DOI] [PubMed] [Google Scholar]
- 24. Baumann AJ, Wheeler DS, James M, et al. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015; 50: 882.e2–886.e2. [DOI] [PubMed] [Google Scholar]
- 25. Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis 2005; 37: 593–600. [DOI] [PubMed] [Google Scholar]
- 26. Chatrath H, Liangpunsakul S, Ghabril M, et al. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med 2012; 125: 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chien YC, Chiang HC, Lin PY, et al. Erectile function in men with end-stage liver disease improves after living donor liver transplantation. BMC Urol 2015; 15: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Rui M, Schiff S, Aprile D, et al. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis 2013; 28: 245–248. [DOI] [PubMed] [Google Scholar]
- 29. Gencdal G, Gunsar F, Meral CE, et al. Sleep disorders in cirrhotics; how can we detect? Liver Int 2014; 34: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 30. Grassi M, Albiani B, De Matteis A, et al. Prevalence of dyspepsia in liver cirrhosis: a clinical and epidemiological investigation. Minerva Med 2001; 92: 7–12. [PubMed] [Google Scholar]
- 31. Gutteling JJ, de Man RA, Busschbach JJ, et al. Health-related quality of life and psychological correlates in patients listed for liver transplantation. Hepatol Int 2007; 1: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huyghe E, Kamar N, Wagner F, et al. Erectile dysfunction in end-stage liver disease men. J Sex Med 2009; 6: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 33. Kalaitzakis E, Josefsson A, Castedal M, et al. Gastrointestinal symptoms in patients with cirrhosis: a longitudinal study before and after liver transplantation. Scand J Gastroenterol 2013; 48: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 34. Kaltsakas G, Antoniou E, Palamidas AF, et al. Dyspnea and respiratory muscle strength in end-stage liver disease. World J Hepatol 2013; 5: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein J, Tran SN, Mentha-Dugerdil A, et al. Assessment of sexual function and conjugal satisfaction prior to and after liver transplantation. Ann Transplant 2013; 18: 136–145. [DOI] [PubMed] [Google Scholar]
- 36. Madan A, Barth KS, Balliet WE, et al. Chronic pain among liver transplant candidates. Prog Transplant 2012; 22: 379–384. [DOI] [PubMed] [Google Scholar]
- 37. Margreiter M, Heinisch BB, Schwarzer R, et al. Lower urinary tract symptoms in patients with liver cirrhosis. World J Urol 2015; 33: 315–321. [DOI] [PubMed] [Google Scholar]
- 38. Montagnese S, Middleton B, Skene DJ, et al. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29: 1372–1382. [DOI] [PubMed] [Google Scholar]
- 39. Mostacci B, Ferlisi M, Baldi Antognini A, et al. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci 2008; 29: 237–240. [DOI] [PubMed] [Google Scholar]
- 40. Poonja Z, Brisebois A, van Zanten SV, et al. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014; 12: 692–698. [DOI] [PubMed] [Google Scholar]
- 41. Popovic D, Culafic DM, Tepavcevic DB, et al. Assessment of depression and anxiety in patients with chronic liver disease. Vojnosanit Pregl 2015; 72: 414–420. [DOI] [PubMed] [Google Scholar]
- 42. Rodrigue JR, Nelson DR, Reed AI, et al. Fatigue and sleep quality before and after liver transplantation. Prog Transplant 2010; 20: 221–233. [DOI] [PubMed] [Google Scholar]
- 43. Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol 2015; 13: 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogal SS, Landsittel D, Surman O, et al. Pretransplant depression, antidepressant use, and outcomes of orthotopic liver transplantation. Liver Transpl 2011; 17: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogal SS, Winger D, Bielefeldt K, et al. Pain and opioid use in chronic liver disease. Dig Dis Sci 2013; 58: 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roth K, Lynn J, Zhong Z, et al. Dying with end stage liver disease with cirrhosis: insights from SUPPORT: study to understand prognoses and preferences for outcomes and risks of treatment. J Am Geriatr Soc 2000; 48: S122–S130. [PubMed] [Google Scholar]
- 47. Singh N, Gayowski T, Wagener MM, et al. Depression in patients with cirrhosis: impact on outcome. Dig Dis Sci 1997; 42: 1421–1427. [DOI] [PubMed] [Google Scholar]
- 48. Sorrell JH, Brown JR. Sexual functioning in patients with end-stage liver disease before and after transplantation. Liver Transpl 2006; 12: 1473–1477. [DOI] [PubMed] [Google Scholar]
- 49. Stewart KE, Hart RP, Gibson DP, et al. Illness apprehension, depression, anxiety, and quality of life in liver transplant candidates: implications for psychosocial interventions. Psychosomatics 2014; 55: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trzepacz PT, Brenner R, Van Thiel DH. A psychiatric study of 247 liver transplantation candidates. Psychosomatics 1989; 30: 147–153. [DOI] [PubMed] [Google Scholar]
- 51. Wang GS, Yang JX, Li MR, et al. Liver transplant may improve erectile function in patients with benign end-stage liver disease: single-center Chinese experience. Exp Clin Transplant 2013; 11: 332–338. [DOI] [PubMed] [Google Scholar]
- 52. Zhu HP, Gu YR, Zhang GL, et al. Depression in patients with chronic hepatitis B and cirrhosis is closely associated with the severity of liver cirrhosis. Exp Ther Med 2016; 12: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abdullah AE, Al-Jahdali F, Ahmed AE, et al. Symptoms of daytime sleepiness and sleep apnea in liver cirrhosis patients. Ann Hepatol 2017; 16: 591–598. [DOI] [PubMed] [Google Scholar]
- 54. Annema C, Roodbol PF, Van den Heuvel ER, et al. Trajectories of anxiety and depression in liver transplant candidates during the waiting-list period. Br J Health Psychol 2017; 22: 481–501. [DOI] [PubMed] [Google Scholar]
- 55. Ghabril M, Jackson M, Gotur R, et al. Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatol 2017; 15: 1271–128e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017; 66: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sumi R, Fukuda K, Irishio K, et al. Current status of pruritus in chronic liver diseases and efficacy of nalfurafine hydrochloride. Acta Hepatologica Japonica 2017; 58: 486–493. [Google Scholar]
- 58. Xiao G, Ye Q, Han T, et al. Study of the sleep quality and psychological state of patients with hepatitis B liver cirrhosis. Hepatol Res 2018; 48: E275–E282. [DOI] [PubMed] [Google Scholar]
- 59. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther 2009; 30: 469–476. [DOI] [PubMed] [Google Scholar]
- 60. Ahluwalia V, Wade JB, Thacker L, et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol 2013; 59: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003; 48: 1622–1626. [DOI] [PubMed] [Google Scholar]
- 62. Bao ZJ, Qiu DK, Ma X, et al. Assessment of health-related quality of life in Chinese patients with minimal hepatic encephalopathy. World J Gastroenterol 2007; 13: 3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bjrnsson E, Verbaan H, Oksanen A, et al. Health-related quality of life in patients with different stages of liver disease induced by hepatitis C. Scandinavian Journal of Gastroenterology 2009; 44: 878–887. [DOI] [PubMed] [Google Scholar]
- 64. Che YH, You J, Chongsuvivatwong V, et al. Dynamics and liver disease specific aspects of quality of life among patients with chronic liver disease in Yunnan, China. Asian Pac J Cancer Prev 2014; 15: 4765–4771. [DOI] [PubMed] [Google Scholar]
- 65. Cordoba J, Flavia M, Jacas C, et al. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol 2003; 39: 231–238. [DOI] [PubMed] [Google Scholar]
- 66. Derck JE, Thelen AE, Cron DC, et al. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation 2015; 99: 340–344. [DOI] [PubMed] [Google Scholar]
- 67. Fallon MB, Krowka MJ, Brown RS, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008; 135: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fritz E, Hammer J. Gastrointestinal symptoms in patients with liver cirrhosis are linked to impaired quality of life and psychological distress. Eur J Gastroenterol Hepatol 2009; 21: 370–375. [DOI] [PubMed] [Google Scholar]
- 69. Galant LH, Forgiarini LA, Jr, Dias AS, et al. Functional status, respiratory muscle strength, and quality of life in patients with cirrhosis. Rev Bras Fisioter 2012; 16: 30–34. [PubMed] [Google Scholar]
- 70. Gao R, Gao F, Li G, et al. Health-related quality of life in Chinese patients with chronic liver disease. Gastroenterol Res Pract 2012; 2012: 516140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goetzmann L, Wagner-Huber R, Klaghofer R, et al. Waiting for a liver transplant: psychosocial well-being, spirituality, and need for counselling. Transplant Proc 2006; 38: 2931–2936. [DOI] [PubMed] [Google Scholar]
- 72. Groeneweg M, Quero JC, De Bruijn I, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 1998; 28: 45–49. [DOI] [PubMed] [Google Scholar]
- 73. Hsu PC, Krajden M, Yoshida EM, et al. Does cirrhosis affect quality of life in hepatitis C virus-infected patients? Liver Int 2009; 29: 449–458. [DOI] [PubMed] [Google Scholar]
- 74. Jara M, Bednarsch J, Malinowski M, et al. Predictors of quality of life in patients evaluated for liver transplantation. Clin Transplant 2014; 28: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 75. Jurado R, Morales I, Taboada D, et al. Coping strategies and quality of life among liver transplantation candidates. Psicothema 2011; 23: 74–79. [PubMed] [Google Scholar]
- 76. Kalaitzakis E, Josefsson A, Bjornsson E. Type and etiology of liver cirrhosis are not related to the presence of hepatic encephalopathy or health-related quality of life: a cross-sectional study. BMC Gastroenterol 2008; 8: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kanwal F, Hays RD, Kilbourne AM, et al. Are physician-derived disease severity indices associated with health-related quality of life in patients with end-stage liver disease? Am J Gastroenterol 2004; 99: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 78. Kim SH, Oh EG, Lee WH. Symptom experience, psychological distress, and quality of life in Korean patients with liver cirrhosis: a cross-sectional survey. Int J Nurs Stud 2006; 43: 1047–1056. [DOI] [PubMed] [Google Scholar]
- 79. Les I, Doval E, Flavia M, et al. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol 2010; 22: 221–227. [DOI] [PubMed] [Google Scholar]
- 80. Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001; 120: 170–178. [DOI] [PubMed] [Google Scholar]
- 81. Mina A, Moran S, Ortiz-Olvera N, et al. Prevalence of minimal hepatic encephalopathy and quality of life in patients with decompensated cirrhosis. Hepatol Res 2014; 44: E92–E99. [DOI] [PubMed] [Google Scholar]
- 82. Moscucci F, Nardelli S, Pentassuglio I, et al. Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health-related quality of life in cirrhotic patients. Liver Int 2011; 31: 1505–1510. [DOI] [PubMed] [Google Scholar]
- 83. Parkash O, Iqbal R, Jafri F, et al. Frequency of poor quality of life and predictors of health related quality of life in cirrhosis at a tertiary care hospital Pakistan. BMC Res Notes 2012; 5: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roman E, Cordoba J, Torrens M, et al. Falls and cognitive dysfunction impair health-related quality of life in patients with cirrhosis. Eur J Gastroenterol Hepatol 2013; 25: 77–84. [DOI] [PubMed] [Google Scholar]
- 85. Saab S, Ibrahim AB, Shpaner A, et al. MELD fails to measure quality of life in liver transplant candidates. Liver Transpl 2005; 11: 218–223. [DOI] [PubMed] [Google Scholar]
- 86. Siew CO, Mak B, Myat OA, et al. Health-related quality of life in chronic hepatitis B patients. Hepatology 2008; 47: 1108–1117. [DOI] [PubMed] [Google Scholar]
- 87. Sola E, Watson H, Graupera I, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol 2012; 57: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 88. Sumskiene J, Sumskas L, Petrauskas D, et al. Disease-specific health-related quality of life and its determinants in liver cirrhosis patients in Lithuania. World J Gastroenterol 2006; 12: 7792–7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van der Plas SM, Hansen BE, de Boer JB, et al. Generic and disease-specific health related quality of life in non-cirrhotic, cirrhotic and transplanted liver patients: a cross-sectional study. BMC Gastroenterol 2003; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang JY, Zhang NP, Chi BR, et al. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. World J Gastroenterol 2013; 19: 4984–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wiesinger GF, Quittan M, Zimmermann K, et al. Physical performance and health-related quality of life in men on a liver transplantation waiting list. J Rehabil Med 2001; 33: 260–265. [DOI] [PubMed] [Google Scholar]
- 92. Woo G, Tomlinson G, Yim C, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol 2012; 26: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wunsch E, Naprawa G, Koziarska D, et al. Serum natremia affects health-related quality of life in patients with liver cirrhosis: a prospective, single centre study. Ann Hepatol 2013; 12: 448–455. [PubMed] [Google Scholar]
- 94. Wunsch E, Szymanik B, Post M, et al. Minimal hepatic encephalopathy does not impair health-related quality of life in patients with cirrhosis: a prospective study. Liver Int 2011; 31: 980–984. [DOI] [PubMed] [Google Scholar]
- 95. Zhuang G, Zhang M, Liu Y, et al. Significant impairment of health-related quality of life in mainland Chinese patients with chronic hepatitis B: a cross-sectional survey with pair-matched healthy controls. Health Qual Life Outcomes 2014; 12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Benzing C, Krezdorn N, Forster J, et al. Health-related quality of life and affective status in liver transplant recipients and patients on the waiting list with low MELD scores. HPB 2016; 18: 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Janani K, Varghese J, Jain M, et al. HRQOL using SF36 (generic specific) in liver cirrhosis. Indian J Gastroenterol 2017; 36: 313–317. [DOI] [PubMed] [Google Scholar]
- 98. Lins L, Aguiar I, Carvalho FM, et al. Oral health and quality of life in candidates for liver transplantation. Transplant Proc 2017; 49: 836–840. [DOI] [PubMed] [Google Scholar]
- 99. Stepanova M, Nader F, Bureau C, et al. Patients with refractory ascites treated with alfapump(R) system have better health-related quality of life as compared to those treated with large volume paracentesis: the results of a multicenter randomized controlled study. Qual Life Res 2018; 27: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 100. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 101. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 102. Younossi ZM, Guyatt G, Kiwi M, et al. Development of a Disease Specific Questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gralnek IM, Hays RD, Kilbourne A, et al. Development and evaluation of the liver disease quality of life instrument in persons with advanced, chronic liver disease–the LDQOL 1.0. Am J Gastroenterol 2000; 95: 3552–3565. [DOI] [PubMed] [Google Scholar]
- 104. Younossi ZM, Boparai N, Price LL, et al. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol 2001; 96: 2199–2205. [DOI] [PubMed] [Google Scholar]
- 105. Moens K, Higginson IJ, Harding R, et al. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014; 48: 660–677. [DOI] [PubMed] [Google Scholar]
- 106. Burra P, Germani G, Masier A, et al. Sexual dysfunction in chronic liver disease: is liver transplantation an effective cure? Transplantation 2010; 89: 1425–1429. [DOI] [PubMed] [Google Scholar]
- 107. Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: “too well for transplant, too sick for life.” JAMA 2006; 295: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 108. Rakoski MO, Volk ML. Palliative care for patients with end-stage liver disease: an overview. Clin Liver Dis 2015; 6: 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Potosek J, Curry M, Buss M, et al. Integration of palliative care in end-stage liver disease and liver transplantation. J Palliat Med 2014; 17: 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Smith AK, Ladner D, MaCarthy EP. Racial/ethnic disparities in liver transplant surgery and hospice use: parallels, differences, and unanswered questions. Am J Hosp Palliat Med 2008; 25: 285–291. [DOI] [PubMed] [Google Scholar]
- 111. Sogolow ED, Lasker JN, Sharim RR, et al. Stigma and liver disease. Illn Crisis Loss 2010; 18: 229–255. [Google Scholar]
- 112. Tsai LH, Lin CM, Chiang SC, et al. Symptoms and distress among patients with liver cirrhosis but without hepatocellular carcinoma in Taiwan. Gastroenterol Nurs 2014; 37: 49–59. [DOI] [PubMed] [Google Scholar]
- 113. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261–1273. [DOI] [PubMed] [Google Scholar]
- 114. Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016; 375: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jacoby A, Rannard A, Buck D, et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut 2005; 54: 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp_Database_searching_syntax_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental material, Supp_Meta-analysis_supplementary_materials_no_change for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental material, Supp_table_1_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental material, Supp_table_2_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine
Supplemental material, Supp_table_3_revised for Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis by Jen-Kuei Peng, Nilay Hepgul, Irene J Higginson and Wei Gao in Palliative Medicine