Abstract
Methamphetamine (METH) is an illicit psychostimulant that is subject to abuse worldwide. While the modulatory effects of METH on dopamine neurotransmission and its neurotoxicity in the central nervous system are well studied, METH’s effects on modulating microglial neuroimmune functions and on eliciting neuroinflammation to affect dopaminergic neurotoxicity has attracted considerable attention in recent years. The current review illuminates METH-induced neurotoxicity from a neuropathological perspective by summarizing studies reporting microglial activation after METH administration in rodents. Assessing microglial reactivity in terms of the cells’ morphology and immunophenotype offers an opportunity for comprehensive and objective assessment of the severity and nature of METH-induced neuronal perturbations in the CNS and can thus contribute to a better understanding of the nature of METH toxicity. We reach the conclusion here that the intensity of microglial activation reported in the majority of animal models after METH administration is quite modest, indicating that the extent of dopaminergic neuron damage directly caused by this neurotoxicant is relatively minor. Our conclusion stands in contrast to claims of excessive and detrimental neuroinflammation believed to contribute and exacerbate METH neurotoxicity. Thus, our analysis of published studies does not support the idea that suppression of microglial activity with anti-inflammatory agents could yield beneficial effects in terms of treating addiction disorders.
Keywords: Microglia, Methamphetamine, Dopamine, Neuroimmune
Background
The effects of METH in the central nervous system (CNS) are well studied, and for some time now, METH has been reported to increase dopamine (DA) neurotransmission through regulation of dopamine transporter activity [1–3], and to cause neurotoxic effects, notably the apparent degeneration of dopaminergic terminals in the striatum [4]. The responses of microglia, as well as those of astrocytes, have been studied following METH administration to laboratory animals, and our objective here is to summarize and critically evaluate methamphetamine-induced, histopathologically evident microglial responses that occur following METH administration to rodents. Neuroimmune activity involving microglia has been implicated in METH toxicity in the sense that microglial activation is thought to contribute to neurotoxicity [5, 6], but this role of microglia remains mostly speculative for a variety of reasons discussed in the paragraphs following.
We view microglia as “sensors of pathology,” cells that continuously monitor neuronal well-being and become alerted (activated) when neuronal activity is disturbed or compromised by injury, disease, or neurotoxic agents. Microglia are thus the first line of defense against toxic insults and most sensitive sentinels of neurotoxic effects and neuronal damage in general. Their responses to disturbed neuronal homeostasis likely result in neuroprotective and/or reparative mechanisms in order to minimize neuronal damage. As we discuss here, the experimental evidence available does indeed support this notion by showing relatively mild activation of microglia after METH and little, if any, evidence of frank degeneration of DA neurons.
METH neurotoxicity and reactive gliosis
For a variety of reasons methamphetamine’s neurotoxic effects, although well-studied, are not well-defined [6]. In experimental studies, variations of METH doses, routes of administration, duration of METH exposure, and species specificity have collectively confounded the interpretation of the neurotoxic effects of METH and therefore their pathophysiological significance for humans (Fig. 1). Multiple mechanisms are thought to mediate METH-induced neurotoxicity: increase in neuronal firing rate, increased concentrations of intracellular Ca+ 2 and Na+ ions, dysregulation of mitochondrial function, neuronal energetic imbalance, and overproduction of reactive oxygen species. The primary goal of the current review is to re-evaluate this neurotoxicity from the perspective of reactive microglial cell changes, as neuroinflammatory reactions have been reported to occur following METH administration and are believed to causally contribute to METH-induced neurotoxicity [5–7].
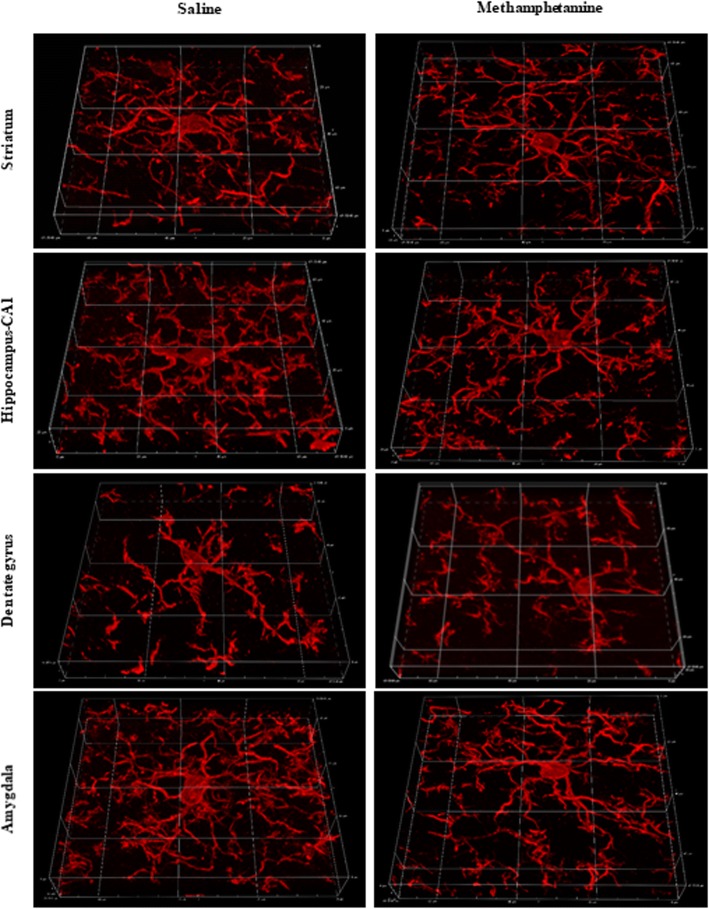
Fig. 1.
Immunohistochemical staining of Iba1 antigen for visualizing mouse microglial cells after METH administration. 3D reconstructions of 60× stacked images of microglia from striatum, CA1 and dentate gyrus of hippocampus, and from amygdala after 7 days of single, daily i.p. injections of either saline or METH (4 mg/kg). Note the absence of microglial hypertrophy (activation) after METH suggesting minimal neurotoxic damage in this particular injection paradigm. Graticule size, 20 μm
Rather than viewing microglia as potentially harmful cells that attack neurons, we view microglia as sensitive biological indicators of neuronal perturbations and perhaps the most reliable cellular sentinels of neurotoxic effects and neuronal damage in general. Microglia have long been recognized as “sensors of pathology” [8] because they are continuously monitoring neuronal well-being and become alerted (activated) when neuronal activity is disturbed or compromised by injury, disease, or neurotoxic agents. There is a regular and bidirectional crosstalk between microglia and dopamine neurons involving diverse receptors and ligands, including both D1-like and D2-like dopamine receptors [9]. Therefore, increased or decreased extracellular dopamine levels are predicted to affect the biology of microglial cells and dopamine neurons in a bidirectional manner. As the brain’s immune system, one of the prime functions of microglia is to be the first line of defense and engage in neuroprotection whenever necessary [10, 11]. To this end, microglia need to sense which neuronal perturbations require their attention and intervention.
We understand the term “neurotoxicity” to mean that METH exposure is directly toxic to neurons, but not necessarily in the sense that METH kills neurons. Dopaminergic neurons are of particular interest because METH is known to directly target dopamine neurons; it competes with the uptake of released dopamine via the dopamine transporter (DAT); it disrupts vesicular storage of dopamine; and induces reverse transport of dopamine through the transporter termed “dopamine efflux” [12–15]. These actions of METH result in extracellular dopamine concentrations remaining elevated while METH is present, but it is still being debated whether or not elevated dopamine itself is neurotoxic. Neurotoxicity is indicated by the sequelae of METH exposure, which include degeneration of axon terminals in the striatum, as shown by silver cupric impregnation [16, 17], neurochemical changes, such as decreased tyrosine hydroxylase (TH) and DAT levels, depletion of dopamine and its metabolites [16, 18–20], as well as reactive astrogliosis [16] and microgliosis (next paragraph). Reactive astrogliosis (astrocytic scarring) typically follows an initial response by microglial cells. Robust and persistent increases in the astroglial intermediate filament protein, GFAP, have been measured [21], but increased GFAP levels allow no more than a general conclusion that sustained astroglial scarring has occurred due to some (unspecified) neuronal damage. Measurements of GFAP expression do not allow conclusions about whether or not neuronal death has occurred acutely. Neuronal death is the most severe consequence of neurotoxicity and the primary cause for neuronal loss.
Microglial reactions visualized histopathologically are more telling than biochemical measures of GFAP in that the microscopic observation of microglial responses better inform about the regional specificity, nature, and severity of METH-induced neurotoxicity. Not only do microglia display different morphological forms after a damaging event, their surface immunophenotype is marked by presence of multiple membrane-bound receptors that undergo characteristic changes during activation, which are detectable with antibodies directed against a variety of immunological important molecules, such as major histocompatibility (MHC) antigens, complement and Fc receptors, integrins, surface immunoglobulins, and toll-like receptors, in addition to presence of intracellular microglial antigens, like the ionized calcium-binding adapter molecule 1 recognized by the Iba1 antibody [22]. Nevertheless, these immunophenotypical and microanatomical changes are neither global nor universal amongst different species and can vary substantially in accordance with lesion severity thus allowing refined assessments of pathology.
Regional sensitivity and species specificity of METH modulation of microglial activity
In mice exposed to METH, there are noticeable differences in the nature and intensity of microglial activation that occurs in the nigrostriatal dopaminergic system versus how microglia respond in limbic structures, such as hippocampus and amygdala. While the effects of METH on microglial biology in the ventral tegmental area (VTA) are unclear and there are no reports of microglial activation occurring around VTA dopaminergic neurons, in the striatum where dopaminergic terminal degeneration has been suggested by positive silver staining and decreased levels of dopamine and TH [16, 23, 24], microglia show only mild hypertrophy (hyperramification). Microglial hypertrophy is the critical morphological feature for identifying activated microglia. Studies show that in the striatum microglia remain ramified, but upregulate isolectin B4 binding relative to controls where microglia are unstained [25–27]. In addition, the loss of TH immunoreactivity in the striatum appears to be reversible, is affected by hyperthermia, and its association with neurodegeneration is asymmetrical in that Fluoro-Jade C staining is reported in one hemisphere only [24]. The situation is similar in the substantia nigra where a rather mild increase in Mac-1 immunoreactivity has been shown to occur on ramified microglia [23] but no upregulation of isolectin B4 binding [25]. These observations regarding microglia in the dopaminergic system point towards minimal damage of DA neurons following METH exposure. They stand in stark contrast to what is described in the hippocampus and amygdala where microglial hypertrophy is pronounced and indicative of phagocytosis [28]. Together with a positive signal for Fluoro-Jade C indicating neurodegeneration, it is likely that significant neuronal death occurs in these limbic structures. In contrast, METH exposure in rats reveals little or no evidence for neuronal damage in the hippocampus, but instead prominent neurodegeneration in the thalamus in parallel with robust microglial activation [29]. Interestingly, many activated microglia in the thalamus and parietal cortex are seen in close association with the vasculature pointing towards methamphetamine-induced vascular damage and BBB compromise. Both vascular toxicity and neurotoxicity are exacerbated by high blood corticosterone pretreatment [30].
Vascular toxicity of METH and its effects on reactive microgliosis
The direct effects of METH are not limited to neurons; there is also vascular toxicity and blood-brain barrier (BBB) damage, as well as hyperthermia and seizures that contribute to neurotoxicity and to microglial activation. Not surprisingly, hyperthermia and neuroinflammation are much exacerbated if animals receive bacterial lipopolysaccharide after METH [31]. While there are differences between mice and rats [32], generally METH-induced hyperthermia appears to exacerbate neurotoxicity via disruption of ion channel functions and reactive oxygen species overproduction, as well as through vascular leakage [33]. It has been shown that BBB breakdown occurs in the septum, hippocampus, and amygdala of mice and rats after exposure to acute, very high doses of METH [28, 32]. The high-dose regimen of METH can disrupt integrity and function of the BBB particularly when accompanied by hyperthermia and increased brain temperature. Focal areas of vascular leakage and BBB permeability have been reported in specific brain regions in hyperthermic rats when body temperatures are ≥ 41.7 °C [34]. Bowyer and colleagues showed that following METH injection, activated microglia dramatically increased around the vasculature with or without minimal neurodegeneration. There was a positive correlation between number of activated microglia in septum, hippocampus, and intralaminar, ventromedial, and ventrolateral thalamus nuclei with the number of the episodes of peak body temperatures ≥ 41.7 °C in animals sacrificed 3 days after METH exposure [29]. It appears that activation of microglia surrounding regions of vascular damage is influenced by microglia interacting with blood-borne factors leaking into the CNS rather than as a direct impact of METH on microglia. These results suggest that microglia are not involved in either the initiation or the progression of METH-induced neurodegeneration. Instead this is more likely to occur as a consequence of direct METH-induced vascular toxicity.
Effects of METH on the immune system
METH is reported to affect immune function in multiple and generally suppressive ways, which were reviewed recently [6]. In terms of how METH toxicity may directly affect microglia, there has been much speculation about excessive microglial activation contributing to or exacerbating brain damage, but it is not clear what evidence there is to support this idea, e.g., at what point does microglial activation become excessive? Proinflammatory cytokines and chemokines are frequently cited as mediators of brain damage, but these substances are produced all the time in the brain, where they serve as intercellular signaling molecules as well as potential modulators of classical neurotransmission [35]. The idea of microglia producing neurotoxins and proinflammatory cytokines that exacerbate brain damage has had a longstanding history and has been implicated in other scenarios, such as CNS trauma and neurodegenerative diseases despite the lack of compelling in vivo evidence showing microglia-mediated neurodegeneration. The notion that activated microglia are harmful is rooted primarily in cell culture studies [11].
The claim that METH-induced neuroinflammation perpetuates neurodegeneration is speculative and unproven, and it is unclear whether it contributes to METH-induced neurotoxicity [36]. We are not promoting this hypothesis because it has not been shown that microglial activation causes neurodegeneration or is toxic in vivo. Rather, microglial activation is a consequence of neurotoxicity exerted by METH and other agents; it represents the cellular response to neuronal damage and is most likely aimed at restoring homeostasis. From the discussion in the paragraphs above, it seems clear that microglial activation observed after METH is the result of both neuronal and vascular injury induced by METH. Recent work has identified a potential mechanism by which damaged neurons release danger-associated molecular patterns, such as high-mobility group box-1 (HMGB1), and/or through purinoceptors (P2X7R) that could mediate signaling between neurons damaged by METH and surrounding microglial cells [37, 38]. There is also pharmacological evidence to support a lack of involvement of neuroinflammation in METH neurotoxicity, for example, lowering core temperature with ethanol can block neuroinflammation, which means that it is the tissue damage that initiates neuroinflammation, not the other way around [39].
Additional evidence against the idea that neuroinflammation causes neurodegeneration stem from observations that the microglial response in the striatum after METH administration is quite mild and can be characterized as minimal microglial activation, if it occurs at all (Fig. 1). This slight microglial activation is unlikely to be causing degeneration of axon terminals in the striatum. In fact, electron microscopy studies, which represent the gold standard for unequivocally demonstrating degeneration, have produced no evidence of axon terminal degeneration in this region [40]. We agree with these authors that the lack of ultrastructural evidence together with rapid recovery of TH immunoreactivity in the striatum is indicative merely of disturbed dopamine biosynthesis rather than some form of severe or even lethal neurotoxic injury. Moreover, the microgliosis that occurs appears acutely after binge-dosed METH administration but then becomes attenuated over time in parallel with recovery of TH immunoreactivity while astroglial scarring persists [21, 36], which is typical for other neurotoxicants that produce reactive gliosis [41]. Microglial activation also decreases rapidly after chronic METH exposure suggesting dissipation of the acute neurotoxic insult [42].
Conclusions
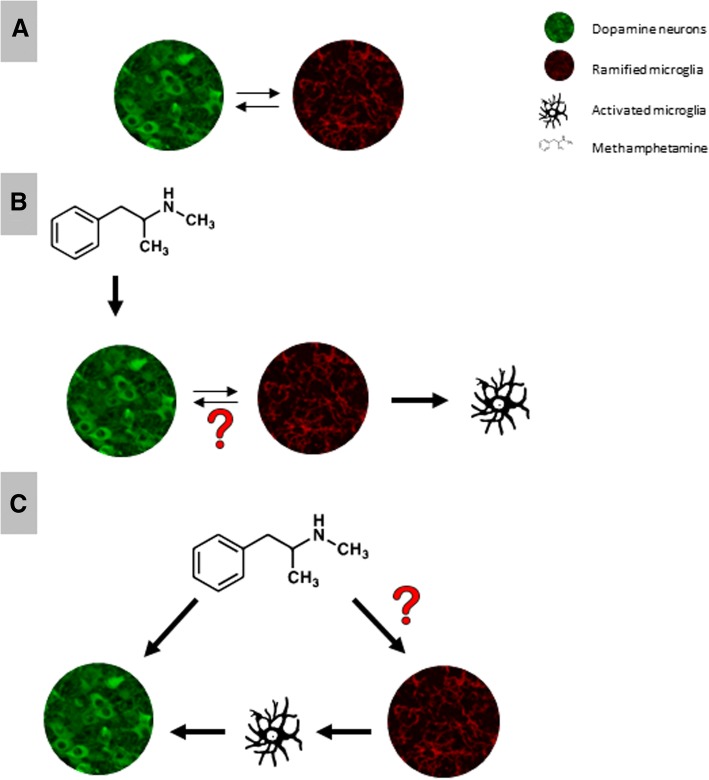
In summary, immune responses are not required to elicit METH neurotoxicity, rather the immune reaction is a response to the damaging effects of this toxic substance on neurons. Whether or not microglia play an important role in cellular mechanisms of addiction remains an interesting question at this time that will require further investigation (Fig. 2).
Fig. 2.
a Under normal condition (absence of METH), there are reciprocal interactions occurring between DA neurons and microglia. b METH-mediated dysregulation of DA neurons alters neuronal-microglial interactions leading to mild microglial activation; however, the intercellular signaling is unknown. c It is unknown whether or not METH can directly induce microglial activation or otherwise alter microglial function to influence its neurotoxicity
Acknowledgements
Not applicable
Funding
DA026947, DA043895, NS103108, S10OD020026
Availability of data and materials
The datasets generated and/or analyzed during the current study (Fig. 1) are not publicly available due to their preliminary nature but are available from the corresponding author on reasonable request.
Abbreviations
- BBB
Blood-brain barrier
- CNS
Central nervous system
- DA
Dopamine
- DAT
Dopamine transporter
- GFAP
Glial fibrillary acidic protein
- METH
Methamphetamine
- TH
Tyrosine hydroxylase
- VTA
Ventral tegmental area
Authors’ contributions
FS and SH wrote the first draft of this manuscript, which was subsequently edited by WJS and HK. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Shaerzadeh, Email: f.shaerzadeh@ufl.edu.
Wolfgang J. Streit, Phone: 352-273-5096, Email: streit@mbi.ufl.edu
Soomaayeh Heysieattalab, Email: heysieattalab@gmail.com.
Habibeh Khoshbouei, Email: habibeh@ufl.edu.
References
- 1.Sambo DO, Lin M, Owens A, Lebowitz JJ, Richardson B, Jagnarine DA, et al. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat Commun. 2017;8(1):2228. doi: 10.1038/s41467-017-02087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin M, Sambo D, Khoshbouei H. Methamphetamine regulation of firing activity of dopamine neurons. J Neurosci. 2016;36(40):10376–10391. doi: 10.1523/JNEUROSCI.1392-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha K, Sambo D, Richardson BD, Lin LM, Butler B, Villarroel L, et al. Intracellular methamphetamine prevents the dopamine-induced enhancement of neuronal firing. J Biol Chem. 2014;289(32):22246–22257. doi: 10.1074/jbc.M114.563056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, et al. Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse. 2013;67(5):245–257. doi: 10.1002/syn.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187(1):47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. Int Rev Neurobiol. 2014;118:165–197. doi: 10.1016/B978-0-12-801284-0.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu E, Liu J, Liu H, Wang X, Xiong H. Role of microglia in methamphetamine-induced neurotoxicity. Int J Physiol Pathophysiol Pharmacol. 2017;9(3):84–100. [PMC free article] [PubMed] [Google Scholar]
- 8.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 9.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 10.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 11.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21(16):5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284(5):2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69(4):628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270(2):741–751. [PubMed] [Google Scholar]
- 17.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235(1):93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 18.Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151(2):533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5(1):e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buening MK, Gibb JW. Influence of methamphetamine and neuroleptic drugs on tyrosine hydroxylase activity. Eur J Pharmacol. 1974;26(1):30–34. doi: 10.1016/0014-2999(74)90070-3. [DOI] [PubMed] [Google Scholar]
- 21.Friend DM, Keefe KA. Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J Neurochem. 2013;125(4):566–574. doi: 10.1111/jnc.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 23.Ares-Santos S, Granado N, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012;45(2):810–820. doi: 10.1016/j.nbd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Bowyer JF, Robinson B, Ali S, Schmued LC. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62(3):193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311(1):1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 26.Kelly KA, Miller DB, Bowyer JF, O'Callaghan JP. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. J Neurochem. 2012;122(5):995–1009. doi: 10.1111/j.1471-4159.2012.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J Neurochem. 2008;106(2):696–705. doi: 10.1111/j.1471-4159.2008.05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60(7):521–532. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- 29.Bowyer JF, Sarkar S, Tranter KM, Hanig JP, Miller DB, O'Callaghan JP. Vascular-directed responses of microglia produced by methamphetamine exposure: indirect evidence that microglia are involved in vascular repair? J Neuroinflammation. 2016;13(1):64. doi: 10.1186/s12974-016-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowyer JF, Tranter KM, Sarkar S, George NI, Hanig JP, Kelly KA, et al. Corticosterone and exogenous glucose alter blood glucose levels, neurotoxicity, and vascular toxicity produced by methamphetamine. J Neurochem. 2017;143(2):198–213. doi: 10.1111/jnc.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchanan JB, Sparkman NL, Johnson RW. A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J Neuroinflammation. 2010;7:82. doi: 10.1186/1742-2094-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowyer JF, Thomas M, Schmued LC, Ali SF. Brain region-specific neurodegenerative profiles showing the relative importance of amphetamine dose, hyperthermia, seizures, and the blood-brain barrier. Ann N Y Acad Sci. 2008;1139:127–139. doi: 10.1196/annals.1432.005. [DOI] [PubMed] [Google Scholar]
- 33.Miller DB, O'Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 2003;92(1):48–53. doi: 10.1016/s0013-9351(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 34.Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci. 2007;26(5):1242–1253. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- 35.Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8(11):895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- 36.McConnell SE, O'Banion MK, Cory-Slechta DA, Olschowka JA, Opanashuk LA. Characterization of binge-dosed methamphetamine-induced neurotoxicity and neuroinflammation. Neurotoxicology. 2015;50:131–141. doi: 10.1016/j.neuro.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108. doi: 10.1016/j.bbi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes NC, Sriram U, Gofman L, Cenna JM, Ramirez SH, Potula R. Methamphetamine alters microglial immune function through P2X7R signaling. J Neuroinflammation. 2016;13(1):91. doi: 10.1186/s12974-016-0553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS One. 2014;9(7):e102003. doi: 10.1371/journal.pone.0102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burrows KB, Meshul CK. Methamphetamine alters presynaptic glutamate immunoreactivity in the caudate nucleus and motor cortex. Synapse. 1997;27(2):133–144. doi: 10.1002/(SICI)1098-2396(199710)27:2<133::AID-SYN4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 41.McCann MJ, O'Callaghan JP, Martin PM, Bertram T, Streit WJ. Differential activation of microglia and astrocytes following trimethyl tin-induced neurodegeneration. Neuroscience. 1996;72(1):273–281. doi: 10.1016/0306-4522(95)00526-9. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd SA, Corkill B, Bruster MC, Roberts RL, Shanks RA. Chronic methamphetamine exposure significantly decreases microglia activation in the arcuate nucleus. J Chem Neuroanat. 2017;82:5–11. doi: 10.1016/j.jchemneu.2017.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study (Fig. 1) are not publicly available due to their preliminary nature but are available from the corresponding author on reasonable request.