Abstract
Background
Drug resistance mutations (DRMs) increasingly jeopardize paediatric HIV programmes in sub-Saharan Africa. As individual monitoring of DRMs and viral loads has limited availability, population data on DRMs are essential to determine first-line susceptibility. Paediatric data from sub-Saharan Africa are scarce and unavailable for Malawi.
Objectives
To determine the prevalence of virological failure (VF) and DRMs among ART-naive HIV-infected Malawian children during the first year of first-line ART.
Methods
In a prospective cohort of HIV-infected Malawian children, on first-line treatment, children were followed monthly; blood was collected for viral load testing (6 and 12 months) and genotypic resistance testing (12 months). VF was defined as at least one viral load >1000 copies/mL or death after 6 months of ART. DRMs were identified and susceptibility to NRTIs and NNRTIs was scored using the Stanford algorithm and by calculating genotypic susceptibility scores (GSSs).
Results
VF occurred in 66% (23/35) of the children during 12 months of follow-up. DRMs were detected in 44% (15/34); all had NNRTI resistance and 12% (4/34) had dual-class NNRTI/NRTI resistance. Reduced susceptibility (DRMs and GSS <3) was seen in 41% (14/34) to their current first-line regimen. High-level resistance was most common for nevirapine [26% (9/34)].
Conclusions
In this first report on VF and DRMs in children on first-line ART in Malawi, the rates of VF and DRMs were alarmingly high. Paediatric HIV programmes in sub-Saharan Africa should emphasize programmatic evaluation of VF and include detection of DRMs to adjust and design adequate first- and second-line regimens and prevent widespread resistance in children.
Introduction
The development of drug resistance mutations (DRMs) among HIV-infected children in sub-Saharan African is increasing, which jeopardizes the outcomes of paediatric HIV programmes. Regular monitoring of viral load (VL) and sequencing data to detect DRMs are limited or not available for individual patients. Therefore, monitoring first-line susceptibility and design of appropriate first- and second line-strategies should be based on population data on DRM prevalence to prevent further accumulation of mutations in those who will require treatment for the rest of their lives.1 The data on DRMs in children on ART in sub-Saharan Africa are very scare.2 Although Malawi is heavily affected, as 10% of the population is HIV infected, data for children regarding DRMs are limited and no data on DRMs in Malawian children during ART are available.3,4
This study was conducted to document the prevalence of virological failure (VF) and DRMs in HIV-infected Malawian children during the first year of first-line ART.
Methods
This study is a sub-study of a larger prospective cohort of HIV-infected children commenced on first-line ART in the paediatric ART clinic of the Queen Elizabeth Central Hospital, Blantyre, Malawi. The study includes ART-naive HIV-infected children, aged 18 months to 18 years, who were eligible to commence ART according to the Malawi National ART Programme guidelines (2008–10). All children were started on a first-line regimen consisting of stavudine, lamivudine and nevirapine. Children were eligible for this sub-study if one or more samples were available for VL testing at either 6 or 12 months from recruitment. Virological outcome was evaluated by VF and viral suppression. VF was defined as either two consecutive detectable VLs >1000 copies/mL taken at least 6 months after treatment initiation, or a VL >1000 copies/mL at the last available measurement, or death after at least 6 months of treatment.5 Viral suppression was defined as VL <1000 copies/mL. Severe immunocompromise was defined according to the WHO criteria: age <59 months, CD4% <10% or CD4 count <200 cells/mm3; and age >59 months, CD4 count <100 cells/mm3.6 At enrolment sociodemographic data, TB status and anthropometry details were recorded and HIV diagnosis was confirmed (Abbott Determine HIV-1/2 Test). Venous blood samples were collected at 0, 6 and 12 months for CD4 count (flow cytometry; Becton Dickinson, CA, USA). VL testing was done at 6 and 12 months (Roche Amplicor; Roche, Basel, Switzerland; detection level 400 copies/mL). To evaluate DRMs after 1 year of ART genotypic resistance testing was performed, using dried blood spots collected at 12 months, which were available for 34 of 35 children. Outcome was not available during the time of study and therefore clinical regimens were not adjusted regarding this study outcome. Dried blood spots consisted of 50 μL of whole blood spotted on five circles of a Whatman 903 Protein Saver 903 Card filter paper.7 Elution of the nucleic acids was conducted and cDNA synthesis was performed using the Invitrogen Superscript One-Step RT-PCR System with Platinum Taq High Fidelity. DNA and cDNA were subjected to a nested PCR amplifying a conserved region in the HIV-1 polymerase gene, followed by direct sequencing.7,8 Major DRMs were identified for NRTIs (abacavir, zidovudine, stavudine, didanosine, emtricitabine, lamivudine and tenofovir disoproxil fumarate) and NNRTIs (efavirenz, etravirine, nevirapine and rilpivirine). Susceptibility to the prescribed regimen was determined using the genotypic susceptibility score (GSS) using the Stanford University HIV drug resistance database, version 8.3.9 Reduced susceptibility to the prescribed regimen was defined as GSS <3 (three fully susceptible drugs).
Ethics
The study was approved by the Research Ethics Committee of the University of Malawi College of Medicine and informed consent of the guardians was obtained.
Results
A total of 35 children with a mean±SD age of 7.1 ± 4.6 years were included. The baseline characteristics are shown in Table 1. At 6 and 12 months, respectively, 1/35 (2.9%) and 2/35 (5.7%) of the children were severely immunocompromised. For 35 children a VL sample was available at either 6 months (n = 24) or 12 months (n = 23), including 12 children who had samples taken at both timepoints (Table S1, available as Supplementary data at JAC Online). VF occurred in 23/35 (65.7%) of the children during the 12 months of follow-up (Table S1). Viral suppression was achieved in 12/24 (50.0%) and 3/23 (13.0%) at 6 and 12 months, respectively.
Table 1.
Baseline characteristics
| General | |
| male, n/N (%) | 17/35 (48.6) |
| age <5 years, n/N (%) | 13/35 (37.1) |
| mother or child had received drugs for PMTCT of HIV, n/N (%) | |
| yes | 1/35 (2.9) |
| no | 17/35 (48.6) |
| unknown | 17/35 (48.6) |
| clinically suspected TB infection at baselinea, n/N (%) | 3/30 (10.0) |
| Immune status and VL | |
| WHO stage, n/N (%) | |
| I/II | 15/35 (42.9) |
| III/IV | 20/35 (57.1) |
| CD4%, median (IQR) | 17.5 (1.1–28.5) |
| CD4 count (cells/mm3), median (IQR) | 266.5 (19.0–654.0) |
| severely immunocompromisedb, n/N (%) | 11/31 (35.5) |
| VL (log)c, median (IQR) | 5.4 (2.8–6.2) |
| anaemiad, n/N (%) | 20/33 (60.6) |
| Anthropometry | |
| underweighte, n/N (%) | 6/33 (18.2) |
Clinical TB infection was defined as being on TB medication at enrolment or having a clinical diagnosis at enrolment based on X-ray.
Age <59 months and CD4% <10% or CD4 count <200 cells/mm3, and age >59 months and CD4 count <100 cells/mm3.6
VL was measured for 22 children.
Haemoglobin (Hb) level: age 18–59 months and Hb <11.0 g/dL, age 5–11.9 years and Hb <11.5 g/dL, boys aged 12–15 years and Hb <12.0 g/dL, girls aged >12 years and Hb <12.0 g/dL, and boys aged >15 years and Hb <13.0 g/dL.25
A BMI z-score <−2 SD for all ages was used.
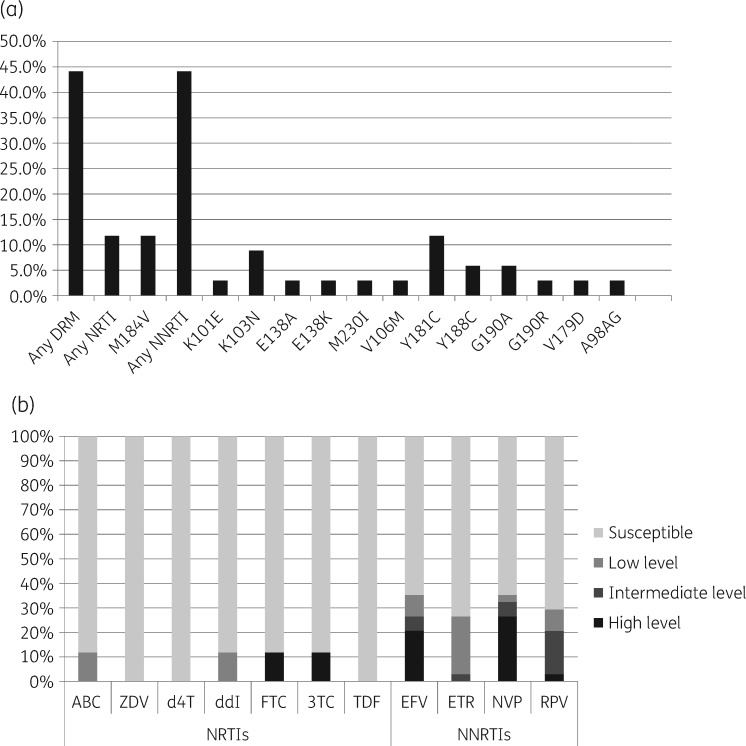
Genotypic resistance testing was available for 34/35 (97.1%) children; one patient did not have a dried blood spot available. DRMs were detected in 15/34 (44.1%) children, with a range of one to three major mutations. All 15 children with DRMs had mutations associated with NNRTI resistance, including 4/34 (11.8%) with dual-class NNRTI/NRTI resistance. DRMs are shown in Figure 1(a) and Table S1. At the end of follow-up a total of 14/34 (41.2%) children had resistance (DRMs and GSS <3) to at least one drug of their first-line regimen. Drug susceptibility is shown in Figure 1(b). High-level resistance (GSS = 0) to NRTIs and NNRTIs was seen for lamivudine in 4/34 (11.8%) and for nevirapine in 9/34 (26.5%) children.
Figure 1.
(a) Percentage of children with major HIV DRMs found at 12 months of follow-up in this cohort.9 (b) Percentage of children with low-level resistance (GSS = 0.5–0.75), intermediate-level resistance (GSS = 0.25), high-level resistance (GSS = 0) or susceptible (GSS = 1). NRTIs: abacavir (ABC), zidovudine (ZDV), stavudine (d4T), didanosine (ddI), emtricitabine (FTC), lamivudine (3TC) and tenofovir disoproxil fumarate (TDF). NNRTIs: efavirenz (EFV), etravirine (ETR), nevirapine (NVP) and rilpivirine (RPV).9
Discussion
This is the first report on VF and DRMs among children on first-line ART in Malawi and we identified that an alarming two-thirds of ART-naive children showed VF in the first year of treatment. In addition, nearly half of them showed an NNRTI mutation and >10% had dual-class NNRTI/NRTI resistance at the end of year 1.
The high prevalence of VF (65.7%) and the low viral suppression rates of 50% and 13% at 6 and 12 months are worse than previous reports from other sub-Saharan African countries. Previous reports show VF rates up to 50% and suppression rates of 74% (95% CI 70.2%–78.2%) during 12–24 months of ART.10–13 No data on HIV-infected children in Malawi are available. The only comparable data from Malawi are from a cohort of adolescents with a suppression rate of 70% after 12–15 months of ART.13 Several potential causes may explain the poor outcome of our cohort. VF in children may be caused by pretreatment DRMs and poor adherence.14–19 Pretreatment data were not available for our cohort (48.5% unknown); however, a study among Malawian children (age <15 years) at the start of ART treatment does report a 15% prevalence of pretreatment DRMs (NNRTI resistance, 14%; NRTI resistance, 5%; dual-class NNRTI/NRTI resistance, 4%).3 The 15% prevalence of DRMs is similar to the 16% reported in Ugandan and Nigerian children.16,20 The existence of pretreatment DRMs is therefore unlikely to be the only explanation of the poor performance of our Malawian cohort.
The pattern of DRMs found is comparable to previous reports on children and adults in sub-Saharan Africa.11,12,15,19,21–24 Like our study, Ugandan and Nigerian children most commonly showed NNRTI mutations (44%) and dual-class NNRTI/NRTI resistance was present in 27%.15,19 This is not surprising as NNRTI resistance develops rapidly due to failed prevention of mother-to-child transmission (PMTCT).2,12 As nevirapine is part of the PMTCT regimen in Malawi and we found that high-level resistance was most common for nevirapine, this likely applies to our Malawian cohort. We did not find any thymidine analogue mutations (TAMs) among the tested children. This outcome is surprising, as children were exposed to ART for 12 months and TAMs mostly occur after several months of ART. The combination of a high percentage of VF but no TAMs after 12 months of treatment may suggest a very poor adherence. To address the high rates of DRMs and VF, the WHO introduced several key actions, including a PI-based ART regimen for all HIV-infected infants (<3 years) in resource-limited settings.1 PI regimens are a promising next step among Malawian HIV-infected children as they will address the high rates of NNRTI resistance pretreatment among these infants; however, several logistic and financial barriers still delay implementation.2 Although resistance is less likely to develop in PI regimens, programmatic monitoring of DRMs remains important to prevent a new resistance problem from occurring in Malawi and other countries.1,15
The present study has several shortcomings. Firstly, it was relatively small and lacked pretreatment resistance and adherence data. Adherence was monitored by pill count and self-report, but documentation about clinicians’ suspicions of poor adherence was not sufficiently standardized to allow these concerns to be evaluated in this study. Secondly, we applied the WHO definition of VF, which restricts the diagnosis of failure to those with a detectable load or those who died after 6 months of ART.5 A total of five children died during the first 6 months of ART and were therefore excluded from this sub-study. The definition of VF includes two consecutive detectable VLs of >1000 copies/mL taken at least 6 months after treatment initiation; however, if not available only one VL >1000 copies/mL at the last available measurement will do. Because in 13/23 (56.5%) children VF diagnoses were based on one VL sample >1000 copies/mL, the outcome could be theoretically overrated. Baseline characteristics of the 35 included children did not differ from those of the 70 excluded children with missing VL samples (data not displayed). Additionally, the study was performed in 2010 and local guidelines regarding starting ART were used. Current WHO guidelines recommend an early start of ART in the disease course and embody the ‘treat all’ policy with regard to HIV disease. The study cohort might therefore be more severely immunocompromised in comparison with current HIV cohorts of children starting ART.
Despite these flaws, our findings are important; moreover, they are alarming, as we know that ART resistance has gradually increased during recent years and treatment options have not changed over recent years in Malawi.2,4,6 When DRM data are not available during ART, which was the case in our study and still the current practice in a lot of clinics in Malawi, children continue treatment on a partially active ART regimen, which increases the risk of further acquiring other DRMs.16 To improve the future outcome of paediatric HIV programmes, implementation of PI-based regimens and closer programmatic evaluation of VLs and DRMs are essential. Both actions should be given priority to prevent widespread resistance and further reduction of already limited treatment options in HIV-infected children in sub-Saharan Africa.
Supplementary Material
Acknowledgements
We thank all the children, the parents and guardians of the children and the staff of the Queen Elisabeth Central Hospital for their participation and cooperation.
Funding
The study was supported by a grant from NWO-NACCAP, the Emma Foundation of the Amsterdam Medical Centre and the Wellcome Trust. NWO-NACCAP supported the main study, financing the study staff, data collection and laboratory assays. The Emma Foundation of the Amsterdam Medical Centre and the Wellcome Trust financed additional laboratory assays. Authors were supported through their respective institutional contracts: M. H. W. H., J. C. J. C. and M. B. v. H., Global Child Health Group, Emma Children’s Hospital, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands; P. M., N. M. and M. B. G., Department of Paediatrics, University of Malawi College of Medicine, Blantyre, Malawi; and M. C. and F. Z., Laboratory of Experimental Virology, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands. O. H. I. received support through the Wellcome Trust. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Transparency declarations
None to declare.
Author contributions
All authors made substantial contributions to the design of this study, to the data collection and to the acquisition and synthesis of data. M. B. v. H., J. C. J. C. and P. M. conceived the design of the study. P. M., N. M., M. B. G. and O. H. I. collected the data. M. C. and F. Z. were responsible for the design, performance and interpretation of sequence results. M. H. W. H. and J. C. J. C. collected the data in a complete set and conducted statistical analysis. M. H. W. H. drafted the manuscript. All authors critically revised the manuscript for intellectual content and read and approved the final version. M. H. W. H. and J. C. J. C. are the guarantors for the paper.
References
- 1. WHO. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection 2017. http://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en.
- 2. WHO. Global Action Plan on HIV Drug Resistance 2017-2021 2017. http://www.who.int/hiv/pub/drugresistance/hivdr-action-plan-2017-2021/en/.
- 3. Weigel R, Buck WC, Kang'ombe AR. et al. HIV drug resistance in children starting ART: baseline surveillance results from Malawi. In: Abstracts of the Twentieth International AIDS Conference, Melbourne, Australia, 2014. WEPE128.
- 4. Kabue MM, Chitsulo C, Kazembe PN. et al. A paediatric HIV care and treatment programme in Malawi. Malawi Med J 2008; 20: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach—Second Edition 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/. [PubMed]
- 6. Gilks CF, Crowley S, Ekpini R. et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 2006; 368: 505–10. [DOI] [PubMed] [Google Scholar]
- 7. Boom R, Sol CJ, Salimans MM. et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990; 28: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoornenborg E, Prins M, Achterbergh RCA. et al. Acquisition of wild-type HIV-1 infection in a patient on pre-exposure prophylaxis with high intracellular concentrations of tenofovir diphosphate: a case report. Lancet HIV 2017; 4: e522–8. [DOI] [PubMed] [Google Scholar]
- 9. Wensing AM, Calvez V, Gunthard HF. et al. Update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24: 132–3. [PMC free article] [PubMed] [Google Scholar]
- 10. Boerma RS, Boender TS, Bussink AP. et al. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63: 1645–54. [DOI] [PubMed] [Google Scholar]
- 11. Pillay S, Bland RM, Lessells RJ.. Drug resistance in children at virological failure in a rural KwaZulu-Natal, South Africa, cohort. AIDS Res Ther 2014; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muri L, Gamell A, Ntamatungiro AJ. et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS 2017; 31: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadonda-Kabondo N, Bennett D, van Oosterhout JJ. et al. Prevalence of HIV drug resistance before and 1 year after treatment initiation in 4 sites in the Malawi antiretroviral treatment program. Clin Infect Dis 2012; 54 Suppl 4: S362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boerma RS, Bunupuradah T, Dow D. et al. Multicentre analysis of second-line antiretroviral treatment in HIV-infected children: adolescents at high risk of failure. J Int AIDS Soc 2017; 20: 21930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boerma RS, Sigaloff KC, Akanmu AS. et al. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: a systematic review and meta-analysis. J Antimicrob Chemother 2017; 72: 365–71. [DOI] [PubMed] [Google Scholar]
- 16. Kityo C, Boerma RS, Sigaloff KCE. et al. Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J Antimicrob Chemother 2017; 72: 2587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boerma RS, Kityo C, Boender TS. et al. Second-line HIV treatment in Ugandan children: favorable outcomes and no protease inhibitor resistance. J Trop Pediatr 2017; 63: 135–43. [DOI] [PubMed] [Google Scholar]
- 18. Zoufaly A, Fillekes Q, Hammerl R. et al. Prevalence and determinants of virological failure in HIV-infected children on antiretroviral therapy in rural Cameroon: a cross-sectional study. Antivir Ther 2013; 18: 681–90. [DOI] [PubMed] [Google Scholar]
- 19. Dow DE, Shayo AM, Cunningham CK. et al. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV-infected children in Tanzania: a four-year follow-up. BMC Infect Dis 2014; 14: 567.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boerma RS, Boender TS, Sigaloff KC. et al. High levels of pre-treatment HIV drug resistance and treatment failure in Nigerian children. J Int AIDS Soc 2016; 19: 21140.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kebe K, Thiam M, Diagne Gueye NR. et al. High rate of antiretroviral drug resistance mutations in HIV type 1-infected Senegalese children in virological failure on first-line treatment according to the World Health Organization guidelines. AIDS Res Hum Retroviruses 2013; 29: 242–9. [DOI] [PubMed] [Google Scholar]
- 22. Gody JC, Charpentier C, Mbitikon O. et al. High prevalence of antiretroviral drug resistance mutations in HIV-1 non-B subtype strains from African children receiving antiretroviral therapy regimen according to the 2006 revised WHO recommendations. J Acquir Immune Defic Syndr 2008; 49: 566–9. [DOI] [PubMed] [Google Scholar]
- 23. Chaix ML, Rouet F, Kouakoussui KA. et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Côte d'Ivoire. Pediatr Infect Dis J 2005; 24: 1072–6. [DOI] [PubMed] [Google Scholar]
- 24. Pere H, Carpentier C, Mbelesso P. et al. Virological response and resistance profiles after 18 to 30 months of first- or second-/third-line antiretroviral treatment: a cross-sectional evaluation in HIV type 1-infected children living in the Central African Republic. AIDS Res Hum Retroviruses 2012; 28: 87–94. [DOI] [PubMed] [Google Scholar]
- 25. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity 2011. http://www.who.int/vmnis/indicators/haemoglobin/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.