Abstract
Objective
Mild traumatic brain injury (mTBI) is a serious health concern in the adolescent population. Repeated mTBI may result in more pronounced deficits, and has been associated with long‐term neurological consequences including neurodegeneration. As such, there is a critical need for the development of objective mTBI biomarkers to help guide medical management. Diffusion‐weighted imaging (DWI) is an advanced magnetic resonance imaging (MRI) technique that may detect brain abnormalities after mTBI. Diffusion tensor imaging (DTI) is the most commonly applied DWI method, and initial studies have reported DTI changes in mTBI patients. Furthermore, new DWI methods (e.g., track‐weighted imaging; TWI) are being developed that may also be sensitive to mTBIs, but remain to be comprehensively studied.
Methods
This study utilized the Awake Closed Head Injury (ACHI) model of mTBI to investigate changes in DTI and TWI following repeated mTBI in adolescent male and female rats. A total of four ACHI impacts, two/day over two consecutive days, were delivered beginning on postnatal day 25. At 1 day and 7 days postinjury, rats were euthanized and brains were collected for DWI analyses.
Results
Rats given repeated mTBI displayed changes in fractional anisotropy and radial diffusivity (i.e., DTI measures), as well as track density (i.e., TWI).
Interpretation
These findings are consistent with initial DTI findings in mTBI patients, suggest that TWI may complement DTI, support the utility of DWI measures as biomarkers in mTBI, and further validate the ACHI rat model of mTBI.
Introduction
Mild traumatic brain injury (mTBI), including concussion, is a widespread public health concern affecting millions of people annually.1 Children and adolescents are of particular interest as they are more likely to sustain a head injury than adults,2 and such injuries represent a leading cause of disability within this population.3, 4 An mTBI is induced by biomechanical forces, and a single mTBI typically results in transient neurological impairment.5 Sustaining repeated mTBI, however, may result in cumulative and persisting neurological deficits,6, 7, 8, 9, 10 and has been associated with increased risk of developing neurodegenerative disease such as chronic traumatic encephalopathy (CTE).11, 12 Considering the difficulty of mTBI diagnosis and prognosis, and the potential for long‐term sequalae after repeated mTBI, there have been increased research efforts to develop sensitive and objective biomarkers to help guide the clinical management of these injuries.10, 13, 14
Magnetic resonance imaging (MRI) is a noninvasive and readily available clinical tool. Although conventional structural imaging often fails to detect mTBI pathophysiology due to the absence of macroscopic changes,15 initial studies investigating diffusion‐weighted imaging (DWI) suggest that DWI measures may detect changes after mTBI.16, 17, 18 DWI is an MRI technique that is sensitive to the diffusion of water molecules as they move and interact within the brain. Diffusion tensor imaging (DTI) is the most commonly applied DWI method, and initial studies have reported changes on DTI measures (e.g., fractional anisotropy, FA) in the clinical mTBI setting.19, 20, 21, 22 Furthermore, new DWI acquisition and analysis methods (e.g., track‐weighted imaging, TWI) are being developed that may also be sensitive to mTBIs,23, 24, 25 but remain to be comprehensively studied in this context.
There are major limitations and confounding factors involved in studying mTBI and biomarkers in humans. For example, previous DWI studies in mTBI patients have been limited by the use of a single broad postinjury time point, lack of appropriate control groups, and may be confounded by other factors (e.g., lifestyle choices such as drug and alcohol use, genetics, selection bias).10 Animal models allow for the control of experimental parameters, as well as the rigorous characterization and validation of biomarkers in a relatively short time period. Due to limitations with previous rat models of mTBI (e.g., anesthesia, surgical procedures),26, 27 we recently developed the Awake Closed Head Injury (ACHI) rat model of mTBI that does not require anesthesia or surgery. Consistent with studies of patients that have sustained mTBIs, our initial results with this novel model identified acute neurological deficits and impaired spatial memory after repeated mTBIs without evidence of overt brain damage or volumetric loss on structural MRI.28 These findings support the use of this model to study mTBI; however further studies are needed to fully characterize the nature of changes after ACHI and whether they reflect the human condition. Therefore, the present study investigated whether DTI abnormalities similar to those reported in humans with repeated mTBI occurs using this model. Furthermore, we also examined whether more novel DWI measures, such as TWI, were affected by repeated mTBI. It was hypothesized that repeated ACHIs would induce DWI abnormalities in a manner consistent with initial human mTBI studies.
Materials and Methods
Subjects
Long‐Evans rats (n = 37; 16 male, 21 female) derived from Charles River Laboratories (St. Constant, PQ), were housed in standard cages and maintained on a 12 h light/dark cycle with ad libitum access to standard food and water. On postnatal day 21 (PND), 21 rats were weaned and housed in same‐sex groups of 2–3. All procedures were carried out in accordance with protocols approved by the Animal Care Committee at the University of Victoria and standards set by the Canadian Council for Animal Care. At PND 25–28, rats were randomly assigned to one of four experimental groups: sham‐injury controls at Day 1 (n = 10; 4 male, 6 female) and Day 7 postinjury (n = 8; 3 male, 5 female); or repeated mTBI at Day 1 (n = 11; 5 male, 6 female) and Day 7 postinjury (n = 8; 4 male, 4 female). It should be noted that these rats are a subgroup used for ex vivo MRI analysis and were included in our initial paper of the ACHI model.28 However, none of the DWI results included in this paper have been previously published.
Awake closed head injury model of mTBI
The ACHI model has previously been described in detail.28 Briefly, fully conscious juvenile rats are immobilized in a restraint cone and fitted with a 3D printed helmet. The back of the helmet is aligned with the interaural line, and the top includes a flat circular surface that aids in targeting of the impactor over the left parietal cortex. A modified controlled cortical impact (CCI) device (Impact One, Leica Biosystems Inc., ON) is used to deliver an impact at a velocity of 6 m/sec. On consecutive days, rats in the repeated mTBI group were given two impacts/day that were separated by 2 h for a total of four injuries. This injury protocol was based on prior work in mice model and found that repeated mTBI had cumulative effects.29 Sham‐injured rats followed the identical protocol with the omission of the impacts.
Acute neurological assessment
Immediately following each impact or sham procedure, rats underwent acute neurological testing as previously described.28 Briefly, acoustic startle is first evaluated in response to a loud hand clap directly above the rat. The rat is then grasped by the base of the tail and raised into the air to examine limb extension. Next, the rat is placed onto a narrow balance beam to test their ability to balance and traverse the beam (100 cm long × 2 cm wide × 0.75 cm thick). Lastly, the animal is placed back onto the beam, which is then elevated and rotated once per second for four rotations to test motor dexterity. Appropriate responses and ability to complete tasks constitute a pass (score of 1), while null or inappropriate response, immobility, or falling is scored as a fail (score of 0). Note that as the rats in this study are a subgroup from our initial study, this data also contributed to the data previously reported in that paper.28 The primary purpose for its inclusion in this paper is to demonstrate that the ACHIs resulted in acute neurological impairment (see Fig. 1).
Figure 1.
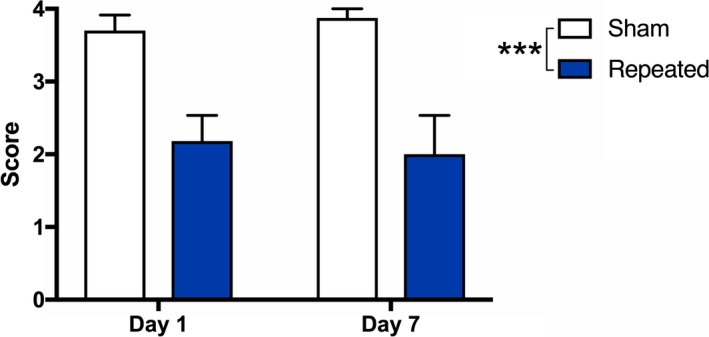
Repeated mTBI induces acute neurological impairment in rats. (A) There were acute neurological deficits in repeated mTBI rats in both the Day 1 and Day 7 cohorts compared to sham controls. ***P < 0.001. See Methods for further details.
Ex vivo DWI acquisition
At their specified postinjury time point, Day 1 or Day 7, after the final ACHI or sham procedure, rats were anaesthetized with isoflurane, transcardially perfused with 0.9% NaCl, and fixated with 4% paraformaldehyde. The excised whole fixated brains were then washed with PBS and embedded in 2–3% agar for MRI scanning. Images were acquired with a 4.7 T Bruker MRI (Bruker™ BioSpin®, USA) and actively decoupled transmit and four‐channel surface receive coils. DWI parameters were chosen to give sufficient diffusion weighting for estimating the fiber orientation distributions (FODs) in the fixed tissue, while also keeping the echo time to a minimum to maximize the available signal. Diffusion weighting was performed in 81 directions with diffusion duration (δ) = 6 msec, diffusion separation (Δ) = 17 msec and b‐value = 5000 s/mm2. Two nondiffusion (b 0) volumes were also acquired. Other image parameters were: Repetition time = 10 sec; echo time = 35 msec; field of view = 28.8 × 28.8 mm2; number of slices = 38; slice thickness = 300 μm, and matrix size = 96 × 96 giving an isotropic spatial resolution = 300 × 300 × 300 μm3.
DWI processing and analyses
Image processing was performed using MRtrix (www.mrtrix.org). Images were corrected for noise using MP‐PCA denoising and normalized using the median b 0 white matter value. Fiber orientation distributions (FODs) were computed using constrained spherical deconvolution with an average response function and a study template constructed using symmetric diffeomorphic registration.30 To avoid biasing, the study template to any one group, templates were first constructed for each group (i.e., sham Day 1 postinjury, sham Day 7 postinjury, repeat mTBI Day 1 postinjury, and repeat mTBI Day 7 postinjury) and then combined to create the study template.23, 24, 31
DTI metrics including FA, radial diffusivity (RD), and axial diffusivity (AD) were generated for each subject and transformed into study‐specific template space using MRtrix. For TWI, tractograms were generated for each rat using the iFOD2 algorithm and registered to the study‐specific FOD template. Three track‐weighted images were then generated using properties of the tractogram streamlines: track density imaging (TDI), which sums the number of streamlines passing through each voxel; average pathlength mapping (APM), which maps the mean length of each streamline traversing the voxel;32 and mean curvature, which maps the mean curvature of each streamline traversing the voxel.
For the analysis, ROIs were outlined in each hemisphere (ipsilateral and contralateral) of the mean FA image and included anterior, middle, and posterior regions of the corpus callosum, external and internal capsule, and fimbria.33 The mean value of each diffusion metric within each ROI was then calculated.
Statistical analysis
All imaging measures were analyzed with a two‐way analysis of variance (ANOVA), with injury and time as between‐subject factors. Bonferroni post hoc comparisons were completed when appropriate. Acute neurological deficit scores were analyzed with the Independent Samples Mann–Whitney U Test. Statistical significance was defined as P ≤ 0.05.
Results
DTI measures detect white matter abnormalities after repeated mTBI
For FA, two‐way ANOVA detected a significant effect of injury (F 1,33 = 4.98, P = 0.033) in the contralateral middle corpus callosum, with repeated mTBI rats having reduced FA regardless of time postinjury (Fig. 2).
Figure 2.
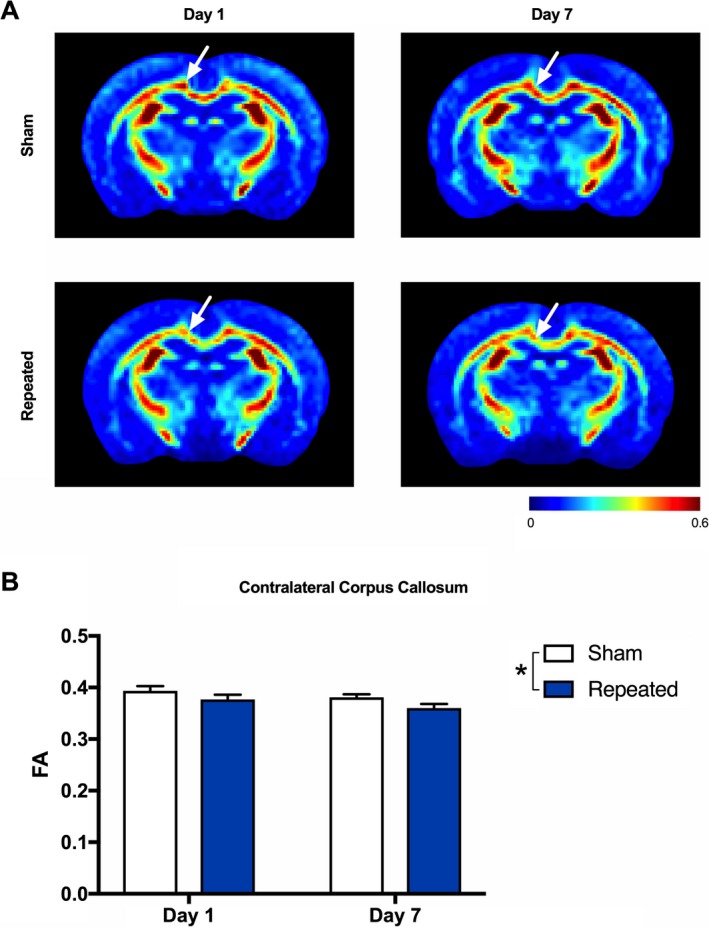
Reduced fractional anisotropy (FA) after repeated mTBI. (A) Representative FA images for sham (top row) and repeated mTBI (bottom row) rats at Day 1 and Day 7 recovery. (B) Repeated mTBI resulted in significantly decreased FA in the contralateral corpus callosum as compared to sham rats at both Day 1 and Day 7 recovery (see white arrows). Mean ± SEM, *P < 0.05. See Results for further details.
For RD, two‐way ANOVA detected a significant injury*time interaction in the ipsilateral posterior external capsule (F 1,33 = 5.19, P = 0.029) and in the contralateral posterior internal capsule (F 1,33 = 4.83, P = 0.035). Post hoc analyses indicated that the repeated mTBI group displayed significantly lower RD values in the ipsilateral posterior external capsule (P = 0.002, Fig. 3A, B) and contralateral posterior internal capsule (P < 0.001, Fig. 3A, C) at Day 7 as compared to Day 1 postinjury.
Figure 3.
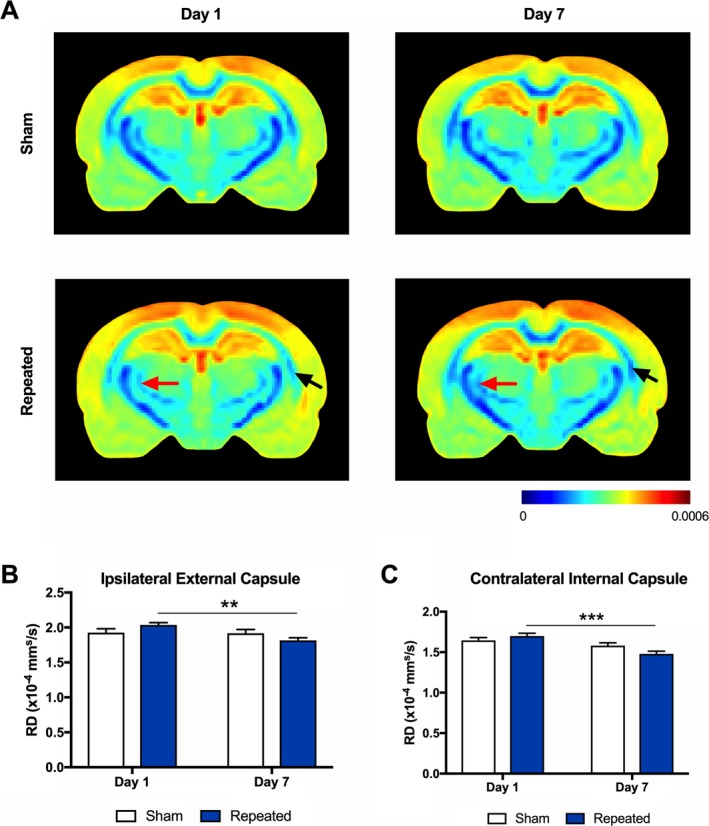
Radial diffusivity (RD) is altered in response to repeated mTBI. (A) Representative RD images in sham (top row) and repeated mTBI (bottom row) rats at Day 1 and Day 7. The repeated mTBI group displayed significantly lower RD values in the (B) ipsilateral external capsule (see black arrows) and (C) contralateral internal capsule (see red arrows) at Day 7 as compared to Day 1 postinjury. Mean ± SEM., **P < 0.01; ***P < 0.001. See Results for further details.
There were no significant findings between the injury groups on any of the other DTI measures.
TWI detects white matter abnormalities after repeated mTBI
For TDI, two‐way ANOVA identified a significant injury*time interaction in the ipsilateral middle fimbria (F 1,33 = 6.55, P = 0.015), the ipsilateral posterior external capsule (F 1,33 = 4.91, P = 0.034), the contralateral middle fimbria (F 1,33 = 9.24, P = 0.005), and the contralateral anterior external capsule (F 1,33 = 4.56, P = 0.040). Post hoc analysis revealed that the mean number of streamlines were significantly increased in repeat mTBI rats compared to shams at Day 1 postinjury in the ipsilateral middle fimbria (P = 0.011; Fig. 4A, B), the contralateral middle fimbria (P = 0.001; Fig. 4A, D), and the contralateral anterior external capsule (P = 0.041; Fig. 4E). Furthermore, the repeat mTBI group displayed significantly lower values at Day 7 as compared to Day 1 postinjury in the ipsilateral middle fimbria (P = 0.005; Fig. 4A, B), the contralateral middle fimbria (P < 0.001; Fig. 4A, D) and the contralateral anterior external capsule (P = 0.025; Fig. 4E). In the ipsilateral posterior external capsule, the mean number of streamlines were significantly decreased in the repeat mTBI group compared to the sham at Day 1 (P = 0.035; Fig. 4C), and the repeat mTBI values increased from Day 1 to Day 7 postinjury (P = 0.011; Fig. 4C). There were no significant findings between the injury groups on any of the other TWI measures.
Figure 4.
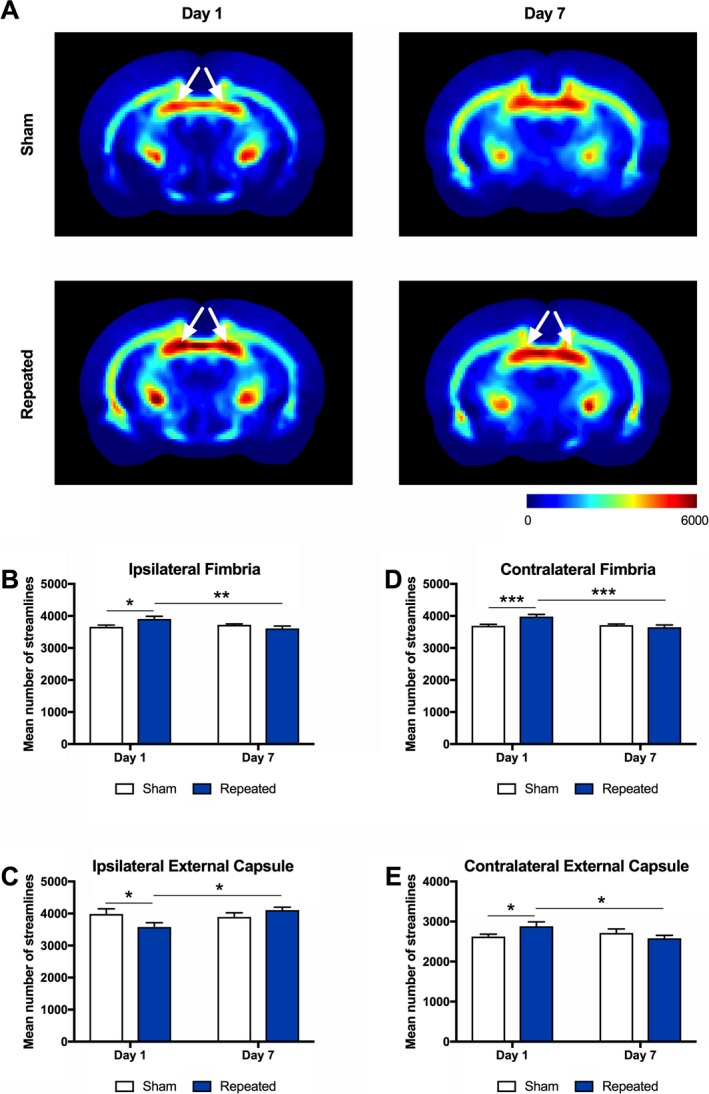
Track density imaging (TDI) detects abnormalities after repeated mTBI. (A) Representative images of the mean number of streamlines (i.e., TDI) in sham (top row) and repeated mTBI (bottom row) rats at Day 1 and Day 7. Repeated mTBI rats displayed a significant increase in streamlines at Day 1 as compared to sham in the (B) ipsilateral middle fimbria and (D) contralateral middle fimbria (see white arrows), and the repeated mTBI values decreased from Day 1 to Day 7 postinjury. (C) In the ipsilateral posterior external capsule, streamlines were decreased in the repeated mTBI group compared to the sham at Day 1, and the repeated mTBI values increased from Day 1 to Day 7 postinjury (note that posterior representative images are not shown). (E) In the contralateral anterior external capsule, streamlines were increased in the repeated mTBI group compared to the sham at Day 1, and the repeated mTBI values decreased from Day 1 to Day 7 postinjury (note that anterior representative images are not shown). Mean ± SEM, *P < 0.05; **P < 0.01; ***P < 0.001. See Results for further details.
Discussion
This study is the first to examine DWI measures in an awake rodent model of repeated mTBI. We previously reported that repeated ACHIs result in acute neurological deficits and impaired spatial memory. This altered behavior was in the absence of overt damage or volumetric loss as measured with structural MRI.28 However, it is well established that structural imaging does not typically detect abnormalities acutely following mTBI.34 On the other hand, DWI has greater sensitivity for identifying microscopic abnormalities as compared to conventional MRI. DWI measures the motion of water molecules within the brain. As this diffusion is restricted by biological barriers and other molecules, abnormalities in DWI may reflect microscopic alterations to tissue organization due to factors such as white matter injury,35, 36 edema,37 and gliosis.38 Indeed, preliminary preclinical studies suggest that DWI may be a useful biomarker of mTBI23 and repeated mTBI.39, 40 As such, DWI analysis post‐ACHI was warranted, despite the lack of structural MRI findings previously presented.28
DTI, the most commonly used DWI method, identified a significant effect of injury in the corpus callosum where FA values were decreased in the repeated mTBI group as compared to shams. FA in white matter regions has been shown to be affected by a variety of factors such as injury, axonal caliber, myelin sheath thickness, and the density and distribution of white matter tracts20. Our findings are consistent with those seen in clinical populations where FA is regularly shown to be reduced following mTBI.41, 42, 43, 44, 45, 46 Decreased FA values have been reported acutely,35 as well as chronically47, in the corpus callosum of patients with mTBI. In some cases, these white matter FA values were predictive of impaired executive functioning48 and were correlated with decreased behavioral outcomes.44, 49 The corpus callosum is among the most common locations of abnormal FA50, 51 and is thought to be particularly vulnerable to axonal injury resulting from TBI.52 As such, the reduced FA abnormalities in the corpus callosum found in this study would be expected and further support the clinical relevance of the ACHI model.
Further DTI analysis revealed a significant interaction between injury and time in the ipsilateral external capsule and the contralateral internal capsule on the measure of RD. Specifically, the repeated mTBI group displayed significantly lower RD values in these regions at Day 7 as compared to Day 1 postinjury. RD is often associated with myelin pathology and edema following trauma to the brain.53, 54 Although no differences were observed between sham and injured animals following repeat ACHI, decreasing RD values in a time dependent fashion may be indicative of injury progression. Few other studies have investigated RD at different mTBI recovery times, though our results are similar to prior findings in the clinical setting that report reduced RD subacutely after mTBI.19, 20
TWI is a recently developed DWI technique that uses properties of tractography streamlines, such as density, curvature, and length, to provide additional measures that may be sensitive to white matter pathology.55, 56 TWI measures have previously been shown to be altered in preclinical mTBI23, 24 and repeated mTBI25 models that involved the use of anesthetic and/or craniotomy. Compared to the sham group, repeated ACHI resulted in a significant increase in track density (i.e., TDI) in both the ipsilateral and contralateral fimbria, as well as the contralateral external capsule at Day 1. Moreover, rats given repeated mTBI displayed lower TDI values at Day 7 as compared to Day 1 in these regions. Interestingly, the contralateral external capsule displayed an opposite effect, where mean number of streamlines were reduced compared to sham at Day 1. Why the opposite effect was found in the contralateral external capsule remains to be determined, as do the pathophysiological mechanisms that underlie changes in TDI after mTBI. It should be noted that of the significant DWI findings in this study, only TDI distinguished between the sham versus repeated mTBI groups at Day 1, suggesting that TWI methods may complement traditional DTI measures.
Conclusions and Future Directions
This study investigated the utility of DWI (i.e., DTI and TWI) biomarkers in the ACHI rat model of repeated mTBI. Both DTI and TWI metrics identified white matter abnormalities after repeated impacts with the ACHI model. This work represents, to our knowledge, the first time DWI was performed in an awake animal model of mTBI. Our initial findings are consistent with DTI findings reported in mTBI patients, support the utility of DWI measures as biomarkers in the mTBI setting, and further validate the novel ACHI rat model.
With that said, there are some limitations with this study and further research on the topic of DWI in the context of mTBI is certainly justified. For example, further research is still required to better characterize the temporal complexities of these changes, and to determine the exact pathophysiological mechanisms underlying the DWI abnormalities. Of particular relevance would be the inclusion of more chronic postinjury recovery times considering evidence that repeated mTBI can result in chronic neurological deficits and neurodegenerative disease. Another topic of future study should be the investigation of how biological sex influences mTBI outcomes and biomarkers. Although our study incorporated both male and female rats, we were unable to investigate potential sex differences due to low group sizes. It should also be noted that our MRI study was conducted on ex vivo brain samples, which somewhat limits the clinical relevance of the findings. However, both the scan time (i.e., 2 h) and the DWI sequences that were used are appropriate for future in vivo studies.23, 31, 57 As our current study only investigated rats given repeated mTBI, future in vivo studies could investigate the effects of a single mTBI using serial MRI at different postinjury times. Such investigations would provide important information related to diagnosis and recovery/cerebral vulnerability after a single mTBI. Related to this point, while our DTI findings are largely consistent with those from initial clinical studies, further studies are still needed to better characterize these DTI abnormalities and also examine the utility of novel DWI methods, such as TWI, in single and repeated mTBI patients. Last, it is worth considering the clinical relevance of the repeated injury schedule used in this study (i.e., 2 injuries/day over two consecutive days). Our repeated ACHI model was adapted from a previous mouse study that found repeated ACHI had cumulative neurological effects.28, 29 As such, to facilitate the transition of the model into rats, we used a similar injury schedule in this initial study. With regards to clinical relevance, it is not unreasonable that an adolescent athlete could sustain multiple mild brain traumas in short succession (i.e., similar to the schedule used in this study). Though we do acknowledge that with the growing public awareness of the potential consequences of mTBIs and more conservative medical management of these injuries it is becoming increasingly unlikely for repeated mTBIs to occur in such a rapid manner. Now that this initial rat study has been done, future studies can modify the inter‐injury time accordingly.
In closing, although further studies are clearly needed, advanced diffusion‐based MRI methods are promising objective and noninvasive biomarkers for mTBI that that could 1 day be influential in the medical management of these injuries.
Author's Contribution
Study design: RCW, AM, BRC, and SRS; Data collection: RCW, AM, KJN, RDB, DKW; Data analysis: RCW, AM, KJN, SJM, DKW, and SRS; Writing and editing: RCW, AM, KJN, SJM, RDB, DKW, BRC, and SRS.
Conflict of Interest
Nothing to report.
Acknowledgments
RCW and AM were supported by CIHR graduate fellowships. The studies were funded by grants from NSERC and CFI to BRC, and the Australian NHMRC to SRS. The authors acknowledge the facilities, and the scientific and technical assistance, of the National Imaging Facility at the Florey Institute of Neuroscience and Mental Health (Parkville, VIC, Australia).
Funding Information
RCW and AM were supported by Canadian Institutes of Health Research graduate fellowships. The studies were funded by grants from NSERC and CFI to BRC, and the Australian NHMRC to SRS.
Funding Statement
This work was funded by Natural Sciences and Engineering Research Council of Canada grant ; National Health and Medical Research Council grant ; Canadian Institutes of Health Research grant ; NSERC grant ; CFI grant ; Australian NHMRC grant .
References
- 1. Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med 2004;(43 Suppl):28–60. [DOI] [PubMed] [Google Scholar]
- 2. Rutland‐Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil 2006;21:544–548. [DOI] [PubMed] [Google Scholar]
- 3. Kraus JF, Anderson C. Determinants of head injury mortality. Neurosurgery 1990;27:334–335. [DOI] [PubMed] [Google Scholar]
- 4. Levin HS, Aldrich EF, Saydjari C, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery 1992;31:434–435. [DOI] [PubMed] [Google Scholar]
- 5. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in Sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19:185–200. [DOI] [PubMed] [Google Scholar]
- 6. De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long‐term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 2007;61:329–367. [DOI] [PubMed] [Google Scholar]
- 7. Guskiewicz KM. Balance assessment in the management of sport‐related concussion. Clin Sports Med 2011;30:89–102. [DOI] [PubMed] [Google Scholar]
- 8. Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late‐life cognitive impairment in retired professional football players. Neurosurgery 2005;57:719–726. [DOI] [PubMed] [Google Scholar]
- 9. Jordan BD. The clinical spectrum of sport‐related traumatic brain injury. Nat Rev Neurol 2013;9:222–230. [DOI] [PubMed] [Google Scholar]
- 10. Shultz SR, McDonald SJ, Vonder Haar C, et al. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci Biobehav Rev 2017;76:396–414. [DOI] [PubMed] [Google Scholar]
- 11. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeter CB, Hergenroeder GW, Hylin MJ, et al. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 2013;30:657–670. [DOI] [PubMed] [Google Scholar]
- 14. Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 2013;9:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011;30:33–48. [DOI] [PubMed] [Google Scholar]
- 16. Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports‐related concussion. J Neurotrauma 2011;28:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiong K, Zhu Y, Zhang W. Diffusion tensor imaging and magnetic resonance spectroscopy in traumatic brain injury: a review of recent literature. [Internet]. Brain Imaging Behav 2014;8:487–496. [DOI] [PubMed] [Google Scholar]
- 19. Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955. [DOI] [PubMed] [Google Scholar]
- 21. Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130:2508–2519. [DOI] [PubMed] [Google Scholar]
- 22. Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports‐related concussion. J Neurotrauma 2011;28:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright DK, Trezise J, Kamnaksh A, et al. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Sci Rep 2016;6:28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright DK, O'Brien TJ, Mychasiuk R, Shultz SR. Telomere length and advanced diffusion MRI as biomarkers for repetitive mild traumatic brain injury in adolescent rats. Neuroimage Clin 2018;18:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright DK, O'Brien TJ, Shultz SR, Mychasiuk R. Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann Clin Transl Neurol 2017;4:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Statler KD, Alexander H, Vagni V, et al. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma 2006;23:97–108. [DOI] [PubMed] [Google Scholar]
- 27. Cole JT, Yarnell A, Kean WS, et al. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma 2011;28:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meconi A, Wortman RC, Wright DK, et al. Repeated mild traumatic brain injury can cause acute neurologic impairment without overt structural damage in juvenile rats. PLoS ONE 2018;13:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petraglia AL, Plog BA, Dayawansa S, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma 2014;31:1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raffelt D, Tournier J‐D, Fripp J, et al. Symmetric diffeomorphic registration of fibre orientation distributions. NeuroImage 2011;56:1171–1180. [DOI] [PubMed] [Google Scholar]
- 31. Wright DK, Liu S, van der Poel C, et al. Traumatic brain injury results in cellular, structural, and functional changes resembling motor neuron disease. Cereb Cortex 2017;27:4503–4515. [DOI] [PubMed] [Google Scholar]
- 32. Pannek K, Mathias JL, Bigler ED, et al. The average pathlength map: a diffusion MRI tractography‐derived index for studying brain pathology. NeuroImage 2011;55:133–141. [DOI] [PubMed] [Google Scholar]
- 33. Wright DK, Johnston LA, Kershaw J, et al. Changes in apparent fiber density and track‐weighted imaging metrics in white matter following experimental traumatic brain injury. J Neurotrauma 2017;34:2109–2118. [DOI] [PubMed] [Google Scholar]
- 34. Hughes D, Jackson A, Mason D, et al. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004;46:550–558. [DOI] [PubMed] [Google Scholar]
- 35. Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Rasmussen I‐A, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high‐resolution diffusion tensor imaging. J Neurotrauma 2007;24:753–765. [DOI] [PubMed] [Google Scholar]
- 37. Shultz SR, Wright DK, Zheng P, et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 2015;138:1297–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol 1992;159:591–599. [DOI] [PubMed] [Google Scholar]
- 39. Qin Y, Li G‐L, Xu X‐H, et al. Brain structure alterations and cognitive impairment following repetitive mild head impact: an in vivo MRI and behavioral study in rat. Behav Brain Res 2018;340:41–48. [DOI] [PubMed] [Google Scholar]
- 40. Yu F, Shukla DK, Armstrong RC, et al. Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J Neurotrauma 2017;34:1364–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008;29:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smits M, Houston GC, Dippel DWJ, et al. Microstructural brain injury in post‐concussion syndrome after minor head injury. Neuroradiology 2011;53:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 44. Yuh EL, Cooper SR, Mukherjee P, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK‐TBI study. J Neurotrauma 2014;31:1457–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adam O, Mac Donald CL, Rivet D, et al. Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology 2015;85:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kumar A, Rinwa P, Dhar H. Microglial inhibitory effect of ginseng ameliorates cognitive deficits and neuroinflammation following traumatic head injury in rats. Inflammopharmacology 2014;22:155–167. [DOI] [PubMed] [Google Scholar]
- 47. Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005;103:298–303. [DOI] [PubMed] [Google Scholar]
- 48. Miles L, Grossman RI, Johnson G, et al. Short‐term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj 2008;22:115–122. [DOI] [PubMed] [Google Scholar]
- 49. Wada T, Asano Y, Shinoda J. Decreased fractional anisotropy evaluated using tract‐based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. Am J Neuroradiol 2012;33:2117–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 2006;77:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rutgers DR, Fillard P, Paradot G, et al. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol 2008;29:1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 2005;196:126–137. [DOI] [PubMed] [Google Scholar]
- 53. Song S‐K, Sun S‐W, Ju W‐K, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 2003;20:1714–1722. [DOI] [PubMed] [Google Scholar]
- 54. Mac Donald CL, Dikranian K, Bayly P, et al. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007;27:11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pannek K, Mathias JL, Bigler ED, et al. The average pathlength map: a diffusion MRI tractography‐derived index for studying brain pathology. NeuroImage 2011;55:133–141. [DOI] [PubMed] [Google Scholar]
- 56. Calamante F, Tournier J‐D, Smith RE, Connelly A. A generalised framework for super‐resolution track‐weighted imaging. NeuroImage 2012;59:2494–2503. [DOI] [PubMed] [Google Scholar]
- 57. Clough M, Mutimer S, Wright DK, et al. Oculomotor cognitive control abnormalities in Australian rules football players with a history of concussion. J Neurotrauma 2018;35:730–738. [DOI] [PubMed] [Google Scholar]


