Abstract
Objective
To assess the value of annual serum neurofilament light (NfL) measures in predicting 10‐year clinical and MRI outcomes in multiple sclerosis (MS).
Methods
We identified patients in our center's Comprehensive Longitudinal Investigations in MS at Brigham and Women's Hospital (CLIMB) study enrolled within 5 years of disease onset, and with annual blood samples up to 10 years (n = 122). Serum NfL was measured using a single molecule array (SIMOA) assay. An automated pipeline quantified brain T2 hyperintense lesion volume (T2LV) and brain parenchymal fraction (BPF) from year 10 high‐resolution 3T MRI scans. Correlations between averaged annual NfL and 10‐year clinical/MRI outcomes were assessed using Spearman's correlation, univariate, and multivariate linear regression models.
Results
Averaged annual NfL values were negatively associated with year 10 BPF, which included averaged year 1–5 NfL values (unadjusted P < 0.01; adjusted analysis P < 0.01), and averaged values through year 10. Linear regression analyses of averaged annual NfL values showed multiple associations with T2LV, specifically averaged year 1–5 NfL (unadjusted P < 0.01; adjusted analysis P < 0.01). Approximately 15–20% of the BPF variance and T2LV could be predicted from early averaged annual NfL levels. Also, averaged annual NfL levels with fatigue score worsening between years 1 and 10 showed statistically significant associations. However, averaged NfL measurements were not associated with year 10 EDSS, SDMT or T25FW in this cohort.
Interpretation
Serum NfL measured during the first few years after the clinical onset of MS contributed to the prediction of 10‐year MRI brain lesion load and atrophy.
Introduction
Multiple sclerosis (MS) is a demyelinating and degenerative disease with a heterogeneous disease course.1 Patients experience periodic relapses and varying ranges of disability accrual over their lifetime.
Neurofilament light chain (NfL) is a major component of the neuronal cytoskeleton and is important for axonal growth, stability, and intracellular transport.2, 3 NfL are released upon axonal or neuronal damage or degeneration, and can be found as a consequence, in the CSF and blood. Prior studies have shown that NfL concentrations in cerebrospinal fluid (CSF) are associated with the occurrence of MRI lesions, relapses, neurological disability, and treatment status in MS.4, 5, 6, 7 Additional studies have demonstrated predictive value of CSF neurofilament light or heavy chain levels with clinical outcomes,8, 9, 10 and MRI measures.11 More recently, single molecule array (SIMOA) based assays, which offer improved sensitivity for detection of molecules, have been used to measure NfL in serum samples. SIMOA‐based assays of serum NfL have demonstrated high correlation with CSF values7, 12, 13 and potentially provide a more accessible means to monitor MS patients. Serum NfL measurements by SIMOA correlate with disease state as well as short‐term outcomes in MS,12, 13, 14, 15, 16, 17, 18 however, the associations of serum NfL levels in predicting longer term outcomes, have not been explored.
In this study, we assessed serum NfL levels collected annually for 10 years in a cohort of MS patients with first sample within the first 5 years of disease onset. We assessed correlation with clinical, cognitive, and MRI outcomes at 10 years.
Methods
Subjects
The MS subjects included in this study were patients enrolled in the Comprehensive Longitudinal Investigation of MS at the Brigham and Women's Hospital (CLIMB, www.climbstudy.org).19 This study has enrolled over 2100 patients since 2000, and patients are followed longitudinally with biannual standardized clinical exams, annualized MRI scans, and stored blood samples. Subjects in this analysis met additional specific inclusion criteria: (1) enrolled in the quality of life (QOL) subgroup of the CLIMB study; (2) met the diagnostic criteria of MS by the 2010 McDonald criteria at last visit20; (3) first blood drawn within 5 years of first symptom onset; (4) at least 8/10 annual blood draws from first collection to year 10; (4) provided consent for sample sharing. EDSS and T2FW are collected in all CLIMB subjects annually. Subjects in the QOL subgroup of CLIMB annually completed several patient reported outcomes (PROs) and this analysis included a fatigue measurement (modified fatigue impact scale, MFIS)21 and cognition (symbol digit modalities test, SDMT).22
Standard protocol approvals, registrations, and patient consents
Institutional Review Board approval was granted by the Partners Human Research Committee, and participants provided written informed consent for participation.
NfL measurements
Serum samples were collected at annual CLIMB visits and were stored at −80°C following standardized procedures. The NfL serum samples were shipped on dry ice from Boston to Basel in a temperature controlled container and were measured by SIMOA assay as previously described.13 Inter‐assay coefficients of variation (CV) for three native serum samples were 10.8%, 8.3%, and 5.7% for control samples with mean concentrations of 9.2 pg/mL, 24.4 pg/mL, and 101.4 pg/mL, respectively. The mean intra‐assay CV of duplicated determinations for concentration was 5.1%. Repeat measurements were performed for few samples with intra‐assay CV above 20%. 4 samples showed an NfL value below 1.3 pg/mL (i.e., the lower limit of quantification), these were extrapolated from the standard curve and 12 values were measured as zero.
Untransformed NfL levels were used in all analyses. Several subjects were missing NfL measurements at some timepoints, and these subjects were removed from analyses related to that specific timepoint. In some analyses, NfL values were averaged across multiple time points (e.g., averaged yearly 1–2 NfL was calculated by the sum of the year 1 and year 2 NfL, then were divided by (2). If subjects were missing one or more of the values for the interval, the average was calculated using the available measurements. In additional analyses not presented in this paper, log‐transformed NfL levels were also analyzed, and we converted all 0's to 1 prior to log transformation.
Clinical outcomes
The primary clinical outcome for our analyses was disability measured by the Expanded Disability Status Scale (EDSS) at year 10. Secondary outcomes at year 10 were SDMT, MFIS, and Timed 25‐Foot Walk (T25FW). The SDMT tested executive function and processing speed which was a sensitive early marker of longitudinal cognitive changes in MS. The MFIS was a commonly used measure of fatigue for MS patients, and has three subscale scores (physical, mental, and psychosocial) as well as a total fatigue score. For the T25FW, there were 16 (1.62%) individuals who had high values for T25FW or were unable to complete the walk. For these patients, a score of 25 was assigned to limit the impact of those extreme observations on the analysis. The SDMT, MFIS, and T25FW measurements closest to the 10‐year sample were used for analysis. Also, a calculation in the difference between year 10 and year 1 SDMT, T25FW, and MFIS were performed.
MRI acquisition and processing
Brain MRI acquisition protocol was performed on a 3T unit (Siemens Skyra) which used a 20‐channel head coil, comprised of 3 sagittal sequences, and covered the whole head with 1 mm3 isotropic voxel sizes. This included a 3D T1‐weighted gradient echo (TE/TR = 2.96/2300 msec, TI = 900 msec, flip angle = 9 deg), 3D T2 spin echo (TE/TR = 300/2500 msec, echo train length = 160), and 3D T2‐FLAIR (TE/TR = 389/5000 msec, TI = 1800 msec, echo train length = 248). The sequences were optimized in contrast for depicting brain‐cerebrospinal fluid (CSF) interfaces and white matter lesions. The main steps of the fully automated quantitative analysis pipeline were outlined in Meier et al.23 Key steps were co‐registration of the three MR sequences, anatomical parcellation with heuristic misclassification correction, and an expectation‐maximization algorithm. The output provided brain T2 hyperintense lesion volume (T2LV) and brain parenchymal fraction (BPF), a surrogate of whole brain atrophy. This pipeline showed high accuracy and reliability.23 Intraclass correlation coefficients of 0.95, 0.91, and 0.86 were obtained for T2LV, CSF, and BPF accuracy. A scan‐rescan reliability experiment showed coefficients of variation (COVs) of 8%, 2%, and 0.4% for T2LV, CSF volume, and BPF. For this study, BPF values were multiplied by 100 to yield interpretable estimates for our analyses, and T2LV was log transformed due to skewness.
Statistical analysis
MS patients: To assess the potential long‐term association between NfL and clinical/MRI outcomes, correlations between each NfL sample year and the 10‐year clinical/MRI outcomes were assessed using Spearman's correlation and linear regression. Beyond the individual NfL measurements, the association between averaged yearly NfL values from specific intervals, the 10‐year clinical/MRI outcomes, and the year 1 and year 10 differences were also assessed using linear regression models. In addition, multiple linear regression models adjusted for sex, age, and disease duration at baseline. Additionally, in order to quantify the additional variance explained by adding NfL levels to the multiple regression model, we reported the R‐squared from reduced (the absence of the averaged yearly NfL) and full models. To further investigate the relationship between NfL and clinical disability, logistic regression was used to compare the relationship of NfL values with year 10 EDSS measurement (±1.5 years). We additionally performed all analyses using log NfL values and the results were generally similar compared to the untransformed NfL values presented in this paper. Also, given we completed 21 comparisons for each outcome, the Bonferroni corrected alpha level was 0.0024. P‐values for all analyses will be compared to 0.05 as well as 0.0024 to account for multiple comparisons. All analyses were performed using the Statistical Analysis System (SAS) 9.4 (Cary, NC).
Results
Patients and NFL characteristics
The baseline demographic and clinical characteristics of our MS patient cohort are shown in Table 1. 66% of patients were treated with a DMT at year 1 NfL measurement and the proportion of treated patients increased in year 2 to 85%. The arithmetic mean of NfL values per year show the highest levels at years 1 and 5 (Fig. 1A), and a spaghetti plot of individual MS patient trajectories showed variability (Fig. 1B).
Table 1.
Participant demographics
| Characteristics | MS (N = 122) |
|---|---|
| Race, n(%) | |
| Black or African American | 2 (1.64%) |
| Missing | 1 (0.82%) |
| More than one race | 1 (0.82%) |
| Unknown or not reported | 1 (0.82%) |
| White | 117 (95.90%) |
| Sex, n(%) | |
| Female | 89 (72.95%) |
| Male | 33 (27.05%) |
| Age at first sample years (mean ± SD) | 37.95 ± 9.09 |
| Age at first symptom, years (mean ± SD) | 36.35 ± 9.01 |
| Disease duration at first visit, years (mean ± SD) | 1.61 ± 1.08 |
| EDSS at year 10, N = 117 (mean ± SD) | 1.61 ± 1.36 |
| T25FW at year 10, N = 117 (mean ± SD) | 4.87 ± 2.80 |
| SDMT at year 10, N = 99 (mean ± SD) | 59.16 ± 13.33 |
| MFIS at year 10, N = 79 (mean ± SD) | 21.68 ± 13.87 |
| 3T BPFx100, N = 91 (mean ± SD) | 78.32 ± 3.77 |
| 3T Log T2Lesion volume, N = 91 (mean ± SD) | 0.79 ± 1.31 |
| SDMT at year 1, N = 27 (mean ± SD) | 53.67 ± 10.71 |
| SDMT at year 10, N = 27 (mean ± SD) | 59.19 ± 14.40 |
| MFIS at year 1, N = 31 (mean ± SD) | 24.45 ± 17.04 |
| MFIS at year 10, N = 31 (mean ± SD) | 20.48 ± 14.99 |
| T25FW at year 1, N = 92 (mean ± SD) | 4.74 ± 1.09 |
| T25FW at year 10, N = 92 (mean ± SD) | 5.02 ± 3.13 |
EDSS, expanded disability status scale; T25FW, timed 25‐foot walk; SDMT, symbol digit modalities test; MFIS, modified fatigue impact scale.
Figure 1.
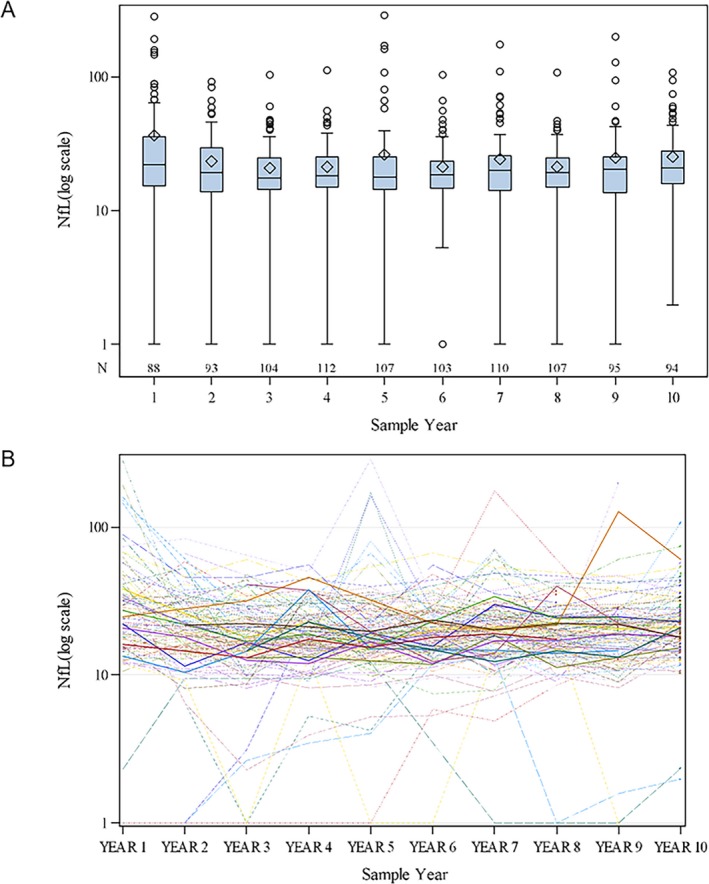
Boxplot of NfL distribution for each year. (A) Boxplot distributions of NfL during each sample year (N subjects=122), using the log scale. (B) Spaghetti plot of the arithmetic mean observed trajectories per each subject
Association of NfL levels with MS clinical outcomes
In the MS cohort, the median EDSS at year 10 was 1.5, and approximately 11% of patients had an EDSS of 3 or higher. We assessed the correlation of each yearly NfL measurement with year 10 EDSS, and only year 2 NfL showed an association (r s = 0.21, P = 0.04). When we assessed the association between yearly NfL measurements and averaged yearly measurements with univariate and multiple linear regression models, no statistically significant associations were observed (Table 2 for averaged yearly NfL and Table S1 for yearly NfL measurements). Further, we did not find any associations of yearly or averaged yearly NfL values with the status of benign (EDSS≤2) or nonbenign (EDSS>2) at year 10 (data not shown).
Table 2.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with Year 10 EDSS
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 104 | 0.075 | −0.002,0.017 | 0.1374 | 1.0000 | 104 | 0.076 | −0.002,0.018 | 0.1373 | 1.0000 | 0.074 | 0.053 |
| Average year 1–3 NfL | 114 | 0.103 | −0.004,0.024 | 0.1497 | 1.0000 | 114 | 0.101 | −0.004,0.024 | 0.1642 | 1.0000 | 0.073 | 0.056 |
| Average year 1–4 NfL | 117 | 0.121 | −0.005,0.029 | 0.1716 | 1.0000 | 117 | 0.117 | −0.006,0.029 | 0.1866 | 1.0000 | 0.074 | 0.060 |
| Average year 1–5 NfL | 117 | 0.078 | −0.008,0.024 | 0.3382 | 1.0000 | 117 | 0.066 | −0.010,0.023 | 0.4259 | 1.0000 | 0.065 | 0.060 |
| Average year 1–6 NfL | 117 | 0.096 | −0.009,0.028 | 0.2977 | 1.0000 | 117 | 0.076 | −0.011,0.026 | 0.4180 | 1.0000 | 0.065 | 0.060 |
| Average year 1–7 NfL | 117 | 0.103 | −0.010,0.031 | 0.3151 | 1.0000 | 117 | 0.083 | −0.012,0.029 | 0.4292 | 1.0000 | 0.065 | 0.060 |
| Average year 1–8 NfL | 117 | 0.103 | −0.012,0.032 | 0.3574 | 1.0000 | 117 | 0.074 | −0.015,0.030 | 0.5096 | 1.0000 | 0.063 | 0.060 |
| Average year 1–9 NfL | 117 | 0.087 | −0.012,0.030 | 0.4149 | 1.0000 | 117 | 0.059 | −0.015,0.027 | 0.5838 | 1.0000 | 0.062 | 0.060 |
| Average year 1–10 NfL | 117 | 0.092 | −0.012,0.030 | 0.3941 | 1.0000 | 117 | 0.062 | −0.015,0.028 | 0.5702 | 1.0000 | 0.062 | 0.060 |
| Average year 2–10 NfL | 117 | 0.037 | −0.019,0.026 | 0.7447 | 1.0000 | 117 | −0.001 | −0.022,0.022 | 0.9950 | 1.0000 | 0.060 | 0.060 |
| Average year 3–10 NfL | 117 | 0.023 | −0.020,0.024 | 0.8357 | 1.0000 | 117 | −0.015 | −0.023,0.020 | 0.8908 | 1.0000 | 0.060 | 0.060 |
| Average year 4–10 NfL | 117 | 0.029 | −0.019,0.024 | 0.7900 | 1.0000 | 117 | −0.009 | −0.022,0.021 | 0.9328 | 1.0000 | 0.060 | 0.060 |
| Average year 5–10 NfL | 117 | 0.020 | −0.018,0.021 | 0.8409 | 1.0000 | 117 | −0.018 | −0.021,0.018 | 0.8527 | 1.0000 | 0.060 | 0.060 |
| Average year 6–10 NfL | 117 | 0.047 | −0.017,0.027 | 0.6733 | 1.0000 | 117 | 0.008 | −0.021,0.023 | 0.9412 | 1.0000 | 0.060 | 0.060 |
| Average year 7–10 NfL | 117 | 0.029 | −0.016,0.022 | 0.7633 | 1.0000 | 117 | 0.001 | −0.019,0.019 | 0.9942 | 1.0000 | 0.060 | 0.060 |
| Average year 8–10 NfL | 117 | 0.064 | −0.013,0.026 | 0.5164 | 1.0000 | 117 | 0.021 | −0.017,0.022 | 0.8277 | 1.0000 | 0.060 | 0.060 |
| Average year 9–10 NfL | 110 | 0.037 | −0.008,0.016 | 0.5398 | 1.0000 | 110 | 0.024 | −0.010,0.014 | 0.6982 | 1.0000 | 0.052 | 0.050 |
| Average year 5–6 NfL | 116 | −0.001 | −0.015,0.014 | 0.9839 | 1.0000 | 116 | −0.030 | −0.018,0.012 | 0.6911 | 1.0000 | 0.066 | 0.065 |
| Average year 5–7 NfL | 117 | 0.001 | −0.018,0.018 | 0.9954 | 1.0000 | 117 | −0.028 | −0.021,0.015 | 0.7561 | 1.0000 | 0.061 | 0.060 |
| Average year 5–8 NfL | 117 | −0.014 | −0.022,0.019 | 0.8928 | 1.0000 | 117 | −0.055 | −0.026,0.015 | 0.5921 | 1.0000 | 0.062 | 0.060 |
| Average year 5–9 NfL | 117 | 0.001 | −0.020,0.020 | 0.9950 | 1.0000 | 117 | −0.039 | −0.024,0.016 | 0.6984 | 1.0000 | 0.061 | 0.060 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL.
Adjusted for multiple comparisons using Bonferroni correction.
We next examined the association of NfL with other clinical measures. Averaged NfL levels during years 1–3 were associated with the increase between baseline (year 1) and year 10 fatigue score measured by the MFIS21 (R² full = 0.207, Puncorrected = 0.04, PBonferroni = 0.87, n = 31) (Table 3). When we assessed the association between yearly NfL measurements and averaged yearly measurements with univariate and multiple linear regression models, no statistically significant associations were observed in the changes in year 1 and year 10 for SDMT (Table 4) or T25FW with averaged NfL levels (Table 5). We found no significant associations of either annual or averaged yearly NfL with year 10 SDMT, T25FW, and MFIS measures (data not shown) in univariate or multiple linear regression models.
Table 3.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with year 1 and year 10 MFIS difference
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 31 | 1.360 | −0.032,0.304 | 0.1083 | 1.0000 | 31 | 1.514 | −0.024,0.327 | 0.0878 | 1.0000 | 0.167 | 0.067 |
| Average year 1–3 NfL | 31 | 2.177 | −0.014,0.449 | 0.0640 | 1.0000 | 31 | 2.506 | 0.011,0.490 | 0.0413 | 0.8668 | 0.207 | 0.067 |
| Average year 1–4 NfL | 31 | 3.078 | 0.024,0.592 | 0.0346 | 0.7257 | 31 | 3.438 | 0.052,0.635 | 0.0227 | 0.4763 | 0.238 | 0.067 |
| Average year 1–5 NfL | 31 | 3.616 | 0.030,0.693 | 0.0334 | 0.7019 | 31 | 4.056 | 0.064,0.747 | 0.0219 | 0.4593 | 0.240 | 0.067 |
| Average year 1–6 NfL | 31 | 4.019 | 0.028,0.776 | 0.0361 | 0.7589 | 31 | 4.662 | 0.079,0.853 | 0.0201 | 0.4223 | 0.245 | 0.067 |
| Average year 1–7 NfL | 31 | 4.587 | 0.025,0.892 | 0.0389 | 0.8162 | 31 | 5.477 | 0.102,0.994 | 0.0180 | 0.3787 | 0.250 | 0.067 |
| Average year 1–8 NfL | 31 | 5.302 | 0.052,1.008 | 0.0308 | 0.6475 | 31 | 6.304 | 0.134,1.127 | 0.0149 | 0.3119 | 0.260 | 0.067 |
| Average year 1–9 NfL | 31 | 5.231 | 0.038,1.008 | 0.0355 | 0.7462 | 31 | 5.827 | 0.083,1.083 | 0.0241 | 0.5068 | 0.235 | 0.067 |
| Average year 1–10 NfL | 31 | 5.788 | 0.082,1.076 | 0.0240 | 0.5033 | 31 | 6.348 | 0.121,1.148 | 0.0174 | 0.3647 | 0.252 | 0.067 |
| Average year 2–10 NfL | 31 | 6.320 | 0.037,1.227 | 0.0380 | 0.7989 | 31 | 7.113 | 0.102,1.321 | 0.0239 | 0.5013 | 0.236 | 0.067 |
| Average year 3–10 NfL | 31 | 6.123 | −0.016,1.240 | 0.0556 | 1.0000 | 31 | 6.765 | 0.029,1.324 | 0.0411 | 0.8636 | 0.207 | 0.067 |
| Average year 4–10 NfL | 31 | 5.511 | −0.059,1.162 | 0.0750 | 1.0000 | 31 | 5.665 | −0.065,1.198 | 0.0766 | 1.0000 | 0.174 | 0.067 |
| Average year 5–10 NfL | 31 | 4.534 | −0.140,1.047 | 0.1293 | 1.0000 | 31 | 4.571 | −0.160,1.074 | 0.1397 | 1.0000 | 0.143 | 0.067 |
| Average year 6–10 NfL | 31 | 4.428 | −0.154,1.040 | 0.1400 | 1.0000 | 31 | 4.336 | −0.186,1.053 | 0.1619 | 1.0000 | 0.135 | 0.067 |
| Average year 7–10 NfL | 31 | 3.674 | −0.154,0.889 | 0.1603 | 1.0000 | 31 | 3.344 | −0.215,0.884 | 0.2218 | 1.0000 | 0.120 | 0.067 |
| Average year 8–10 NfL | 31 | 3.138 | −0.110,0.737 | 0.1406 | 1.0000 | 31 | 2.846 | −0.162,0.731 | 0.2016 | 1.0000 | 0.124 | 0.067 |
| Average year 9–10 NfL | 31 | 1.536 | −0.160,0.467 | 0.3245 | 1.0000 | 31 | 1.304 | −0.195,0.456 | 0.4179 | 1.0000 | 0.090 | 0.067 |
| Average year 5–6 NfL | 31 | 3.168 | −0.288,0.921 | 0.2927 | 1.0000 | 31 | 4.388 | −0.224,1.101 | 0.1850 | 1.0000 | 0.129 | 0.067 |
| Average year 5–7 NfL | 31 | 2.995 | −0.399,0.998 | 0.3879 | 1.0000 | 31 | 4.495 | −0.284,1.183 | 0.2192 | 1.0000 | 0.120 | 0.067 |
| Average year 5–8 NfL | 31 | 5.119 | −0.273,1.297 | 0.1927 | 1.0000 | 31 | 6.519 | −0.189,1.493 | 0.1231 | 1.0000 | 0.150 | 0.067 |
| Average year 5–9 NfL | 31 | 3.840 | −0.227,0.995 | 0.2089 | 1.0000 | 31 | 3.971 | −0.233,1.027 | 0.2067 | 1.0000 | 0.123 | 0.067 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL.
Adjusted for multiple comparisons using Bonferroni correction.
Table 4.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with year 1 and year 10 SDMT difference
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 27 | 0.163 | −0.147,0.179 | 0.8382 | 1.0000 | 27 | 0.109 | −0.164,0.186 | 0.8985 | 1.0000 | 0.056 | 0.055 |
| Average year 1–3 NfL | 27 | 0.252 | −0.264,0.314 | 0.8590 | 1.0000 | 27 | 0.189 | −0.287,0.325 | 0.8994 | 1.0000 | 0.056 | 0.055 |
| Average year 1–4 NfL | 27 | 0.735 | −0.322,0.469 | 0.7051 | 1.0000 | 27 | 0.656 | −0.351,0.482 | 0.7474 | 1.0000 | 0.060 | 0.055 |
| Average year 1–5 NfL | 27 | 0.152 | −0.448,0.478 | 0.9465 | 1.0000 | 27 | 0.057 | −0.481,0.492 | 0.9807 | 1.0000 | 0.055 | 0.055 |
| Average year 1–6 NfL | 27 | 0.047 | −0.528,0.537 | 0.9856 | 1.0000 | 27 | −0.017 | −0.561,0.558 | 0.9952 | 1.0000 | 0.055 | 0.055 |
| Average year 1–7 NfL | 27 | 0.108 | −0.507,0.529 | 0.9661 | 1.0000 | 27 | −0.075 | −0.549,0.534 | 0.9773 | 1.0000 | 0.055 | 0.055 |
| Average year 1–8 NfL | 27 | 0.097 | −0.516,0.536 | 0.9699 | 1.0000 | 27 | −0.157 | −0.567,0.536 | 0.9534 | 1.0000 | 0.056 | 0.055 |
| Average year 1–9 NfL | 27 | −0.492 | −0.615,0.517 | 0.8594 | 1.0000 | 27 | −0.819 | −0.676,0.512 | 0.7776 | 1.0000 | 0.059 | 0.055 |
| Average year 1–10 NfL | 27 | 0.490 | −0.547,0.645 | 0.8669 | 1.0000 | 27 | 0.114 | −0.618,0.640 | 0.9703 | 1.0000 | 0.056 | 0.055 |
| Average year 2–10 NfL | 27 | 0.158 | −0.588,0.620 | 0.9575 | 1.0000 | 27 | −0.306 | −0.677,0.616 | 0.9226 | 1.0000 | 0.056 | 0.055 |
| Average year 3–10 NfL | 27 | 0.034 | −0.604,0.611 | 0.9910 | 1.0000 | 27 | −0.447 | −0.696,0.606 | 0.8881 | 1.0000 | 0.056 | 0.055 |
| Average year 4–10 NfL | 27 | 0.296 | −0.531,0.590 | 0.9143 | 1.0000 | 27 | −0.123 | −0.611,0.586 | 0.9663 | 1.0000 | 0.056 | 0.055 |
| Average year 5–10 NfL | 27 | −0.127 | −0.495,0.470 | 0.9574 | 1.0000 | 27 | −0.449 | −0.557,0.467 | 0.8574 | 1.0000 | 0.057 | 0.055 |
| Average year 6–10 NfL | 27 | 0.394 | −0.352,0.431 | 0.8377 | 1.0000 | 27 | 0.152 | −0.399,0.429 | 0.9399 | 1.0000 | 0.056 | 0.055 |
| Average year 7–10 NfL | 27 | 0.562 | −0.235,0.347 | 0.6944 | 1.0000 | 27 | 0.373 | −0.271,0.345 | 0.8041 | 1.0000 | 0.058 | 0.055 |
| Average year 8–10 NfL | 27 | 1.126 | −0.505,0.730 | 0.7103 | 1.0000 | 27 | 0.855 | −0.617,0.788 | 0.8031 | 1.0000 | 0.058 | 0.055 |
| Average year 9–10 NfL | 27 | 0.934 | −0.521,0.708 | 0.7569 | 1.0000 | 27 | 0.600 | −0.620,0.740 | 0.8565 | 1.0000 | 0.057 | 0.055 |
| Average year 5–6 NfL | 27 | −4.267 | −1.066,0.213 | 0.1815 | 1.0000 | 27 | −4.421 | −1.129,0.245 | 0.1956 | 1.0000 | 0.126 | 0.055 |
| Average year 5–7 NfL | 27 | −0.948 | −0.474,0.285 | 0.6114 | 1.0000 | 27 | −1.093 | −0.507,0.288 | 0.5742 | 1.0000 | 0.069 | 0.055 |
| Average year 5–8 NfL | 27 | −0.591 | −0.445,0.327 | 0.7552 | 1.0000 | 27 | −0.781 | −0.483,0.327 | 0.6932 | 1.0000 | 0.062 | 0.055 |
| Average year 5–9 NfL | 27 | −1.418 | −0.614,0.331 | 0.5420 | 1.0000 | 27 | −1.727 | −0.669,0.324 | 0.4785 | 1.0000 | 0.077 | 0.055 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL.
Adjusted for multiple comparisons using Bonferroni correction.
Table 5.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with year 1 and year 10 T25FW difference
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 80 | −0.077 | −0.034,0.019 | 0.5691 | 1.0000 | 80 | −0.115 | −0.039,0.016 | 0.4123 | 1.0000 | 0.053 | 0.044 |
| Average year 1–3 NfL | 89 | −0.135 | −0.051,0.024 | 0.4734 | 1.0000 | 89 | −0.194 | −0.058,0.019 | 0.3221 | 1.0000 | 0.051 | 0.039 |
| Average year 1–4 NfL | 92 | −0.188 | −0.064,0.026 | 0.4061 | 1.0000 | 92 | −0.258 | −0.072,0.021 | 0.2749 | 1.0000 | 0.052 | 0.039 |
| Average year 1–5 NfL | 92 | −0.165 | −0.055,0.022 | 0.3979 | 1.0000 | 92 | −0.260 | −0.067,0.015 | 0.2085 | 1.0000 | 0.056 | 0.039 |
| Average year 1–6 NfL | 92 | −0.169 | −0.061,0.027 | 0.4455 | 1.0000 | 92 | −0.283 | −0.075,0.018 | 0.2267 | 1.0000 | 0.055 | 0.039 |
| Average year 1–7 NfL | 92 | −0.208 | −0.069,0.028 | 0.3974 | 1.0000 | 92 | −0.322 | −0.083,0.019 | 0.2113 | 1.0000 | 0.056 | 0.039 |
| Average year 1–8 NfL | 92 | −0.219 | −0.075,0.031 | 0.4112 | 1.0000 | 92 | −0.336 | −0.088,0.021 | 0.2251 | 1.0000 | 0.055 | 0.039 |
| Average year 1–9 NfL | 92 | −0.186 | −0.068,0.031 | 0.4597 | 1.0000 | 92 | −0.291 | −0.081,0.022 | 0.2647 | 1.0000 | 0.052 | 0.039 |
| Average year 1–10 NfL | 92 | −0.172 | −0.067,0.033 | 0.4954 | 1.0000 | 92 | −0.277 | −0.079,0.024 | 0.2875 | 1.0000 | 0.051 | 0.039 |
| Average year 2–10 NfL | 92 | −0.161 | −0.067,0.035 | 0.5327 | 1.0000 | 92 | −0.253 | −0.077,0.027 | 0.3371 | 1.0000 | 0.049 | 0.039 |
| Average year 3–10 NfL | 92 | −0.145 | −0.064,0.035 | 0.5647 | 1.0000 | 92 | −0.234 | −0.074,0.028 | 0.3629 | 1.0000 | 0.048 | 0.039 |
| Average year 4–10 NfL | 92 | −0.145 | −0.064,0.035 | 0.5586 | 1.0000 | 92 | −0.234 | −0.074,0.027 | 0.3563 | 1.0000 | 0.048 | 0.039 |
| Average year 5–10 NfL | 92 | −0.108 | −0.055,0.034 | 0.6312 | 1.0000 | 92 | −0.193 | −0.065,0.026 | 0.4017 | 1.0000 | 0.046 | 0.039 |
| Average year 6–10 NfL | 92 | −0.092 | −0.060,0.042 | 0.7204 | 1.0000 | 92 | −0.154 | −0.067,0.036 | 0.5541 | 1.0000 | 0.042 | 0.039 |
| Average year 7–10 NfL | 92 | −0.107 | −0.055,0.034 | 0.6343 | 1.0000 | 92 | −0.147 | −0.059,0.030 | 0.5141 | 1.0000 | 0.043 | 0.039 |
| Average year 8–10 NfL | 92 | −0.071 | −0.052,0.038 | 0.7531 | 1.0000 | 92 | −0.151 | −0.061,0.030 | 0.5114 | 1.0000 | 0.043 | 0.039 |
| Average year 9–10 NfL | 85 | −0.036 | −0.024,0.017 | 0.7313 | 1.0000 | 85 | −0.049 | −0.026,0.016 | 0.6466 | 1.0000 | 0.008 | 0.006 |
| Average year 5–6 NfL | 92 | −0.072 | −0.040,0.026 | 0.6638 | 1.0000 | 92 | −0.158 | −0.050,0.019 | 0.3656 | 1.0000 | 0.048 | 0.039 |
| Average year 5–7 NfL | 92 | −0.134 | −0.055,0.028 | 0.5202 | 1.0000 | 92 | −0.218 | −0.064,0.021 | 0.3102 | 1.0000 | 0.050 | 0.039 |
| Average year 5–8 NfL | 92 | −0.149 | −0.062,0.032 | 0.5285 | 1.0000 | 92 | −0.241 | −0.072,0.024 | 0.3187 | 1.0000 | 0.050 | 0.039 |
| Average year 5–9 NfL | 92 | −0.126 | −0.058,0.033 | 0.5801 | 1.0000 | 92 | −0.212 | −0.067,0.025 | 0.3619 | 1.0000 | 0.048 | 0.039 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL.
Adjusted for multiple comparisons using Bonferroni correction.
Association of NfL levels with MRI outcomes and variance
When the associations between NfL levels and year 10 BPF were assessed, we found a negative correlation between year 5 NfL levels with year 10 BPF (r s=−0.22, P = 0.0479). Linear regression analysis of yearly NfL values and averaged yearly NfL values were provided in Table S2 and Table 6, respectively. Several averaged yearly NfL values had a statistically significant association with year 10 BPF. In the univariate analysis present in Table 6, a 10 pg/mL increase in the average yearly 1–5 NfL was associated with a mean reduction of 0.849% in the BPF (Puncorrected < 0.01, PBonferroni = 0.0035, n = 91). In the multivariate analysis, adjusted for sex, baseline age and disease duration, a 10 pg/mL increase in the averaged yearly 1–5 NfL was associated with a mean reduction of 0.920% in the BPF (Puncorrected < 0.01, PBonferroni = 0.0027, n = 91) (Table 6). Overall, 5% of the variance in BPF was predicted from the variables sex, baseline age and disease duration. While 20% of the BPF variance was predicted from the variables averaged yearly 1–5 NfL, sex, baseline age, and disease duration, 15% variance is accounted for by averaged yearly 1–5 NfL.
Table 6.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with BPF
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 81 | −0.422 | −0.068,−0.017 | 0.0014 | 0.0291 | 81 | −0.440 | −0.070,−0.018 | 0.0013 | 0.0280 | 0.164 | 0.041 |
| Average year 1–3 NfL | 89 | −0.645 | −0.103,−0.026 | 0.0012 | 0.0262 | 89 | −0.683 | −0.108,−0.028 | 0.0011 | 0.0227 | 0.156 | 0.041 |
| Average year 1–4 NfL | 91 | −0.776 | −0.126,−0.029 | 0.0021 | 0.0447 | 91 | −0.823 | −0.133,−0.032 | 0.0017 | 0.0355 | 0.150 | 0.046 |
| Average year 1–5 NfL | 91 | −0.849 | −0.128,−0.042 | 0.0002 | 0.0035 | 91 | −0.920 | −0.137,−0.046 | 0.0001 | 0.0027 | 0.197 | 0.046 |
| Average year 1–6 NfL | 91 | −0.922 | −0.142,−0.043 | 0.0004 | 0.0081 | 91 | −0.985 | −0.151,−0.046 | 0.0004 | 0.0079 | 0.177 | 0.046 |
| Average year 1–7 NfL | 91 | −0.985 | −0.155,−0.041 | 0.0009 | 0.0192 | 91 | −1.058 | −0.167,−0.045 | 0.0009 | 0.0179 | 0.162 | 0.046 |
| Average year 1–8 NfL | 91 | −1.019 | −0.165,−0.039 | 0.0019 | 0.0402 | 91 | −1.064 | −0.174,−0.039 | 0.0023 | 0.0478 | 0.144 | 0.046 |
| Average year 1–9 NfL | 91 | −0.972 | −0.158,−0.037 | 0.0020 | 0.0413 | 91 | −1.004 | −0.165,−0.036 | 0.0026 | 0.0540 | 0.142 | 0.046 |
| Average year 1–10 NfL | 91 | −0.907 | −0.152,−0.029 | 0.0045 | 0.0938 | 91 | −0.911 | −0.156,−0.026 | 0.0069 | 0.1446 | 0.124 | 0.046 |
| Average year 2–10 NfL | 91 | −0.777 | −0.145,−0.011 | 0.0234 | 0.4911 | 91 | −0.738 | −0.144,−0.003 | 0.0399 | 0.8378 | 0.092 | 0.046 |
| Average year 3–10 NfL | 91 | −0.673 | −0.133,−0.002 | 0.0442 | 0.9275 | 91 | −0.624 | −0.131,0.006 | 0.0740 | 1.0000 | 0.081 | 0.046 |
| Average year 4–10 NfL | 91 | −0.627 | −0.128,0.003 | 0.0611 | 1.0000 | 91 | −0.560 | −0.124,0.012 | 0.1075 | 1.0000 | 0.074 | 0.046 |
| Average year 5–10 NfL | 91 | −0.568 | −0.116,0.003 | 0.0605 | 1.0000 | 91 | −0.496 | −0.112,0.012 | 0.1153 | 1.0000 | 0.073 | 0.046 |
| Average year 6–10 NfL | 91 | −0.183 | −0.095,0.059 | 0.6368 | 1.0000 | 91 | −0.054 | −0.084,0.073 | 0.8910 | 1.0000 | 0.046 | 0.046 |
| Average year 7–10 NfL | 91 | −0.183 | −0.090,0.054 | 0.6151 | 1.0000 | 91 | −0.060 | −0.079,0.067 | 0.8707 | 1.0000 | 0.046 | 0.046 |
| Average year 8–10 NfL | 91 | −0.331 | −0.093,0.027 | 0.2775 | 1.0000 | 91 | −0.213 | −0.083,0.040 | 0.4949 | 1.0000 | 0.051 | 0.046 |
| Average year 9–10 NfL | 86 | −0.350 | −0.072,0.002 | 0.0659 | 1.0000 | 86 | −0.303 | −0.069,0.008 | 0.1196 | 1.0000 | 0.074 | 0.046 |
| Average year 5–6 NfL | 90 | −0.564 | −0.096,−0.017 | 0.0057 | 0.1188 | 90 | −0.561 | −0.098,−0.014 | 0.0093 | 0.1952 | 0.120 | 0.047 |
| Average year 5–7 NfL | 91 | −0.614 | −0.115,−0.008 | 0.0253 | 0.5323 | 91 | −0.613 | −0.118,−0.004 | 0.0354 | 0.7442 | 0.094 | 0.046 |
| Average year 5–8 NfL | 91 | −0.630 | −0.127,0.001 | 0.0524 | 1.0000 | 91 | −0.582 | −0.125,0.009 | 0.0886 | 1.0000 | 0.078 | 0.046 |
| Average year 5–9 NfL | 91 | −0.651 | −0.125,−0.005 | 0.0327 | 0.6865 | 91 | −0.600 | −0.123,0.003 | 0.0607 | 1.0000 | 0.084 | 0.046 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL.
Adjusted for multiple comparisons using Bonferroni correction.
When the association between NfL values and year 10 T2LV were assessed, there were positive correlations between years 1 through 4 with T2LV (year 1 r s = 0.39, P < 0.01; year 2 r s = 0.38, P < 0.01; year 3 r s = 0.24, P = 0.04; year 4 r s = 0.32, P < 0.01), which suggested that higher NfL levels were associated with higher brain lesion load. Table S3 and Table 7 showed the linear regression analysis of yearly NfL values and averaged yearly NfL values. In the univariate analysis, a 10 pg/mL increase in the average yearly 1–5 NfL was associated with a mean log‐transformed T2LV increase of 0.307 (Puncorrected < 0.01, PBonferroni = 0.0017, n = 91) (Table 7). When adjusted for sex, baseline age and disease duration, there was a mean log‐transformed T2LV increase of 0.335 (Puncorrected < 0.01, PBonferroni = 0.0014, n = 91) (Table 7). Overall, 2% of the variance in log‐transformed T2LV was predicted from the variables sex, baseline age, and disease duration, whereas 18% of the variance in log T2LV was predicted from these variables, and including averaged yearly 1–5 NfL.
Table 7.
Linear regression (univariate & multivariate) models show associations of averaged yearly NfL with Log T2LV
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Estimate | 95% CI | P‐value | Correctiona | N | Estimate | 95% CI | P‐value | Correctiona | R² Full | R² Reduced |
| Average year 1–2 NfL | 81 | 0.148 | 0.006,0.024 | 0.0019 | 0.0393 | 81 | 0.153 | 0.006,0.025 | 0.0022 | 0.0455 | 0.135 | 0.020 |
| Average year 1–3 NfL | 89 | 0.247 | 0.012,0.038 | 0.0002 | 0.0051 | 89 | 0.261 | 0.012,0.040 | 0.0003 | 0.0056 | 0.160 | 0.016 |
| Average year 1–4 NfL | 91 | 0.326 | 0.016,0.049 | 0.0002 | 0.0033 | 91 | 0.339 | 0.017,0.051 | 0.0002 | 0.0041 | 0.165 | 0.017 |
| Average year 1–5 NfL | 91 | 0.307 | 0.016,0.045 | <.0001 | 0.0017 | 91 | 0.335 | 0.018,0.049 | <.0001 | 0.0014 | 0.184 | 0.017 |
| Average year 1–6 NfL | 91 | 0.340 | 0.017,0.051 | 0.0001 | 0.0030 | 91 | 0.372 | 0.019,0.056 | 0.0001 | 0.0025 | 0.174 | 0.017 |
| Average year 1–7 NfL | 91 | 0.405 | 0.021,0.060 | <.0001 | 0.0014 | 91 | 0.442 | 0.023,0.065 | <.0001 | 0.0012 | 0.187 | 0.017 |
| Average year 1–8 NfL | 91 | 0.438 | 0.023,0.065 | <.0001 | 0.0020 | 91 | 0.473 | 0.024,0.070 | <.0001 | 0.0019 | 0.179 | 0.017 |
| Average year 1–9 NfL | 91 | 0.396 | 0.019,0.060 | 0.0002 | 0.0049 | 91 | 0.425 | 0.020,0.065 | 0.0002 | 0.0050 | 0.161 | 0.017 |
| Average year 1–10 NfL | 91 | 0.390 | 0.018,0.060 | 0.0004 | 0.0074 | 91 | 0.416 | 0.019,0.064 | 0.0004 | 0.0077 | 0.153 | 0.017 |
| Average year 2–10 NfL | 91 | 0.355 | 0.013,0.058 | 0.0025 | 0.0531 | 91 | 0.365 | 0.012,0.061 | 0.0034 | 0.0724 | 0.111 | 0.017 |
| Average year 3–10 NfL | 91 | 0.320 | 0.010,0.054 | 0.0053 | 0.1116 | 91 | 0.326 | 0.009,0.056 | 0.0072 | 0.1518 | 0.097 | 0.017 |
| Average year 4–10 NfL | 91 | 0.299 | 0.008,0.052 | 0.0093 | 0.1954 | 91 | 0.303 | 0.007,0.054 | 0.0122 | 0.2557 | 0.087 | 0.017 |
| Average year 5–10 NfL | 91 | 0.250 | 0.005,0.045 | 0.0165 | 0.3461 | 91 | 0.257 | 0.004,0.047 | 0.0193 | 0.4051 | 0.078 | 0.017 |
| Average year 6–10 NfL | 91 | 0.220 | −0.004,0.048 | 0.1002 | 1.0000 | 91 | 0.218 | −0.005,0.049 | 0.1136 | 1.0000 | 0.046 | 0.017 |
| Average year 7–10 NfL | 91 | 0.199 | −0.005,0.045 | 0.1110 | 1.0000 | 91 | 0.202 | −0.005,0.045 | 0.1165 | 1.0000 | 0.045 | 0.017 |
| Average year 8–10 NfL | 91 | 0.149 | −0.006,0.036 | 0.1571 | 1.0000 | 91 | 0.156 | −0.006,0.037 | 0.1538 | 1.0000 | 0.041 | 0.017 |
| Average year 9–10 NfL | 86 | 0.100 | −0.003,0.023 | 0.1237 | 1.0000 | 86 | 0.100 | −0.003,0.023 | 0.1388 | 1.0000 | 0.038 | 0.012 |
| Average year 5–6 NfL | 90 | 0.184 | 0.005,0.032 | 0.0092 | 0.1931 | 90 | 0.192 | 0.004,0.034 | 0.0113 | 0.2379 | 0.092 | 0.020 |
| Average year 5–7 NfL | 91 | 0.273 | 0.009,0.046 | 0.0037 | 0.0786 | 91 | 0.284 | 0.009,0.048 | 0.0051 | 0.1071 | 0.104 | 0.017 |
| Average year 5–8 NfL | 91 | 0.302 | 0.009,0.052 | 0.0068 | 0.1436 | 91 | 0.313 | 0.008,0.054 | 0.0083 | 0.1750 | 0.094 | 0.017 |
| Average year 5–9 NfL | 91 | 0.260 | 0.005,0.046 | 0.0135 | 0.2840 | 91 | 0.269 | 0.005,0.049 | 0.0162 | 0.3393 | 0.082 | 0.017 |
The estimate corresponds to the change in the mean of the outcome for a 10 pg/mL increase in NfL
Adjusted for multiple comparisons using Bonferroni correction.
Discussion
In this study, we found that averaged annual serum NfL levels correlated with 10‐year MRI derived brain lesions (T2LV) and whole brain atrophy (BPF) in MS patients. We found an association with increased averaged annual NfL levels with fatigue score worsening between years 1 and 10. However, we did not find significant correlations with clinical measures including EDSS, benign status, SDMT, or T25FW.
Serum NfL levels have emerged as an important measurable biomarker for several neurological diseases which included MS,12, 13, 14, 15, 16, 17 Alzheimer's,24, 25, 26 ALS, and head and spinal cord trauma.24, 27, 28, 29 Serum NfL levels have shown associations with short‐term outcomes in MS, however, no published studies have explored long‐term outcomes. We examined the value of averaged annual NfL levels on disease course, since annual or periodic measurements may reflect what occurred in clinical practice.
Short‐term studies have found that in patients with high baseline NfL, brain volume decreased more rapidly (P = 0.05 at 12 months and P = 0.008 at 24 months).15 Our BPF measurements of brain atrophy at 10 years consistently correlated with averaged yearly NfL levels, however, limited strength of association was gained by measurements beyond years 1–5. In fact, point estimates for the averaged values in years 1–2 and 1–3 were similar to the years 1–5 associations, suggesting that early axonal damage has the greatest impact on 10‐year BPF. Further studies to understand the mechanisms and impact of early damage are needed.
T2LV can be considered a cumulative measure of total lesion formation, although it may not reflect the accumulation of all new Gd+ lesions along the disease course, since new lesions may undergo spontaneous regression/repair without leaving a permanent MRI change30. Shorter term studies have found correlations between new gadolinium‐enhancing lesions and serum NfL values.13, 15, 31 Patients with either brain, spinal, or both brain and spinal gadolinium‐enhancing lesions had higher serum NfL than those without.13 Our study found associations between averaged NfL values up to year 9 with T2LV measured at or close to year 10, however, the strongest associations was with averaged values from years 1 through 5 with little gain beyond that timepoint. The strongest associations of NfL with both BPF and T2LV were found with the inclusion of year 1 measures, which suggests that early axonal damage is an important contributor toward long‐term MRI outcomes.
Our results showed an association with increased average annual NfL levels with increased fatigue scores between year 1–10, measured by the MFIS scale. Fatigue was known as a debilitating symptom, which was frequently reported among MS patients.32, 33 Little is understood about the mechanisms and determinants of fatigue.34 Axonal damage as measured by N‐acetylaspartate‐creatinine ratio on proton magnetic resonance spectroscopy scanning have been associated with fatigue in MS patients.35 Our results supported these findings, and may provide serum NfL as a potential biomarker and predictor of fatigue, which may be utilized in clinical monitoring or for clinical trials.
Our study examined the associations of serum NfL in predicting longer term physical disability measured with EDSS. Year 10 EDSS was not associated with either individual year, or averaged serum NfL levels. We note that in our sample, only a minority of patients (11%) had an EDSS of 3 or higher, and <5% had an EDSS of 6 or greater, which may account for the challenges in distinguishing between patients with relatively low levels of disability. Several studies have shown short‐term correlations of serum NfL values and EDSS over periods of 2–3 years, which included more debilitated patients, than in our cohort. In a cohort of 42 MS patients followed in a trial of riluzole over 24 months which included MS patients with high disability scores, serum NfL levels were correlated with EDSS change (P = 0.009)15. In a study following subjects for a mean of 3.1 years, serum NfL levels were associated with EDSS assessments (beta = 1.105, P < 0.001).13 As discussed above, NfL increases are closely correlated with new T2 lesions, and thus the short‐term correlations may reflect the effect of relapses and new lesion formation, which has limited impact on longer term outcomes.
SDMT is a measure of processing speed, commonly used in MS. In our study, 10‐year SDMT or change from year 1 through 10 SDMT showed no significant associations with annual or averaged serum NfL values, which suggests that mechanisms other than axonal shedding could contribute to those clinical outcomes. We note that in our sample, SDMT baseline scores were relatively high, did not change significantly over time, and actually improved at year 10, likely reflecting the known practice effect of this test as well as the fact that the majority of patients in the cohort were on treatment. It has been recently shown that in a large SPMS cohort, with much lower SDMT scores at baseline and relatively higher NfL levels than in this study, SDMT scores were significantly correlated with NfL levels at baseline, indicating that higher degree of neuronal damage and more severe cognitive impairment may be needed for establishing a relationship between these measures (Kuhle et al., AAN 2018 S8.006).
The strengths of this study were the longitudinal study design, with annual serum NfL measured in a well characterized cohort, with year 10 clinical and MRI outcomes. The limitations of this study were that not all subjects had MRI scans meeting criteria for analysis, and only one MRI time point was used which did not allow for calculating change over time. Also, serum samples and/or clinical outcomes were not available for all subjects for each time point throughout the 10‐year period which resulted in lower participant counts for some analyses. This is a highly treated cohort of patients, with limited variability in 10‐year EDSS, which potentially limits the ability to detect effects of NfL on EDSS. The variability in NfL values was lower after the second year, especially after the initiation of treatment, thereby potentially limiting predictive ability. DMT can impact NfL levels, however, secondary analysis of our results including only patients on DMT yielded similar results (data not shown). We did not have concurrent CSF samples with our serum samples, and therefore cannot comment on additional associations of CSF NfL levels in our cohort.
Our study found a correlation of early annual and averaged yearly serum NfL levels with 10‐year MRI outcomes, and worsening fatigue measures. The association of early NfL levels with long‐term outcomes informs the development of predictive models, potentially identifying patients at risk for more severe disease, and more aggressive treatments. Further analyses will explore effects of specific treatments on NfL levels.12, 36, 37 Future studies should validate our findings and explore the additional predictors of long‐term disease course and MRI outcomes in multivariate and machine learning models38.
Conflicts of Interest
None declared.
Supporting information
Figure S1. Distribution of clinical outcomes at year 10.
Table S1. Linear regression (univariate & multivariate) models show associations of yearly NfL with Year 10 EDSS.
Table S2. Linear regression (univariate & multivariate) models show associations of yearly NfL with BPF at year 10.
Table S3. Linear regression (univariate & multivariate) models show associations of yearly NfL with T2LV at year 10.
Acknowledgments
Dr. Tanuja Chitnis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the following colleagues at the Brigham and Women's Hospital: Mariann Polgar‐ Turcsanyi, MS and Mark Anderson, MS for their role in managing the Partners MS Center research database, as well as Taylor Saraceno, BA for her assistance in manuscript preparation.
Funding
This study was funded in part by Novartis, the Swiss National Research Foundation (320030_160221) and the U.S. Department of Defense (MS170140). The National MS Society has provided funding for the CLIMB and SUMMIT cohort studies. We thank Merck Serono and the Nancy Davis Center Without Walls for their support of the CLIMB study.
Funding Statement
This work was funded by Novartis grant ; Swiss National Research Foundation grant 320030_160221; U.S. Department of Defense grant MS170140; CLIMB grant ; SUMMIT grant .
References
- 1. Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol 2009;65:239–248. [DOI] [PubMed] [Google Scholar]
- 2. Yabe JT, Chylinski T, Wang FS, et al. Neurofilaments consist of distinct populations that can be distinguished by C‐terminal phosphorylation, bundling, and axonal transport rate in growing axonal neurites. J Neurosci 2001;21:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacioglu M, Maia LF, Preische O, et al. Neurofilament light chain in blood and csf as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016;91:56–66. [DOI] [PubMed] [Google Scholar]
- 4. Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998;64:402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hakansson I, Tisell A, Cassel P, et al. Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing‐remitting multiple sclerosis. Eur J Neurol 2017;24:703–712. [DOI] [PubMed] [Google Scholar]
- 6. Teunissen CE, Iacobaeus E, Khademi M, et al. Combination of CSF N‐acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009;72:1322–1329. [DOI] [PubMed] [Google Scholar]
- 7. Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler 2016;23:62–71. [DOI] [PubMed] [Google Scholar]
- 8. Soelberg Sorensen P, Sellebjerg F. Neurofilament in CSF‐A biomarker of disease activity and long‐term prognosis in multiple sclerosis. Mult Scler 2016;22:1112–1113. [DOI] [PubMed] [Google Scholar]
- 9. Salzer J, Svenningsson A, Sundstrom P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 2010;16:287–292. [DOI] [PubMed] [Google Scholar]
- 10. Martinez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 2015;21:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petzold A. The prognostic value of CSF neurofilaments in multiple sclerosis at 15‐year follow‐up. J Neurol Neurosurg Psychiatry 2015;86:1388–1390. [DOI] [PubMed] [Google Scholar]
- 12. Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2018;24:1046–1054. [DOI] [PubMed] [Google Scholar]
- 13. Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Disanto G, Adiutori R, Dobson R, et al. Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. J Neurol Neurosurg Psychiatry 2016;87:126–129. [DOI] [PubMed] [Google Scholar]
- 15. Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017;88:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amor S, van der Star BJ, Bosca I, et al. Neurofilament light antibodies in serum reflect response to natalizumab treatment in multiple sclerosis. Mult Scler 2014;20:1355–1362. [DOI] [PubMed] [Google Scholar]
- 17. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 19. Gauthier SA, Glanz BI, Mandel M, Weiner HL. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev 2006;5:532–536. [DOI] [PubMed] [Google Scholar]
- 20. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care 2013;15:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meier DS, Guttmann CRG, Tummala S, et al. Dual‐sensitivity multiple sclerosis lesion and CSF segmentation for multichannel 3T brain MRI. J Neuroimaging 2017;28:36–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 2017;89:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattsson N, Andreasson U, Zetterberg H, Blennow K. Alzheimer's Disease neuroimaging I. association of plasma neurofilament light with neurodegeneration in patients with Alzheimer Disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Julien JP. A role for neurofilaments in the pathogenesis of amyotrophic lateral sclerosis. Biochem Cell Biol 1995;73:593–597. [DOI] [PubMed] [Google Scholar]
- 28. Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 2016;79:152–158. [DOI] [PubMed] [Google Scholar]
- 29. Kuhle J, Gaiottino J, Leppert D, et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 2015;86:273–279. [DOI] [PubMed] [Google Scholar]
- 30. Oommen VV, Tauhid S, Healy BC, et al. the effect of fingolimod on conversion of acute gadolinium‐enhancing lesions to chronic T1 hypointensities in multiple sclerosis. J Neuroimaging 2016;26:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing‐remitting MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krupp LB, Elkins LE. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology 2000;55:934–939. [DOI] [PubMed] [Google Scholar]
- 33. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12:367–368. [DOI] [PubMed] [Google Scholar]
- 34. Newland P, Starkweather A, Sorenson M. Central fatigue in multiple sclerosis: a review of the literature. J Spinal Cord Med 2016;39:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tartaglia MC, Narayanan S, Francis SJ, et al. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol 2004;61:201–207. [DOI] [PubMed] [Google Scholar]
- 36. Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing‐remitting multiple sclerosis. Neurology 2015;84:1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 2011;69:83–89. [DOI] [PubMed] [Google Scholar]
- 38. Zhao Y, Healy BC, Rotstein D, et al. Exploration of machine learning techniques in predicting multiple sclerosis disease course. PLoS ONE 2017;12:e0174866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of clinical outcomes at year 10.
Table S1. Linear regression (univariate & multivariate) models show associations of yearly NfL with Year 10 EDSS.
Table S2. Linear regression (univariate & multivariate) models show associations of yearly NfL with BPF at year 10.
Table S3. Linear regression (univariate & multivariate) models show associations of yearly NfL with T2LV at year 10.


