Abstract
Objective
There is currently an urgent need for reliable clinical biomarkers of remyelination to be used in Phase 2 and Phase 3 clinical trials. Low contrast visual acuity (LCVA) has been suggested as a functional measure of the integrity of the visual pathway. Therefore, the aim of this study was to elucidate the potential contribution of axonal loss and demyelination to LCVA loss in MS patients.
Method
In this study, 50 consecutive relapsing remitting MS patients with a previous history of unilateral optic neuritis were enrolled. Using the linear regression model, we assessed the relative contribution of multifocal Visual Evoked Potentials (mfVEP) latency and Retinal Nerve Fiber Layer (RNFL) thickness to LCVA deficit.
Results
Intereye asymmetry of mfVEP latency and RNFL thickness correlated significantly with intereye asymmetry of LCVA (P < 0.001). A linear regression model demonstrated increased predictive power of LCVA when mfVEP latency and RNFL thinning were combined (reaching R 2 = 0.67) and confirmed a higher predictive value of RNFL thinning compared to mfVEP latency delay for both contrast levels. However, elimination of subjects with severe axonal loss dramatically increased the relative contribution of mfVEP latency, with contribution of RNFL thickness losing significance for both 1.25% and 2.5% LCVA.
Interpretation
While retinal ganglion cell axonal loss is a superior predictor of LCVA, the degree of demyelination contributes significantly to worsening of LCVA, particularly when patients with severe axonal loss are excluded. These results support the feasibility of using LCVA as a functional biomarker in remyelination therapy trials, providing appropriate patient's selection criteria are implemented.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the central nervous system (CNS) characterized by multi‐focal demyelination. Episodes of acute focal demyelination result in significant axonal injury and loss.1 While the majority of axons within lesions survive the initial insult and undergo some remyelination, they remain largely devoid of myelin, forming the so‐called chronic lesions.2 Chronically demyelinated axons within established MS lesions may undergo further tissue damage as they are exposed to toxic effects of proinflammatory cytokines, reactive oxygen and nitrogen species, increased Ca2+ concentration and activated astrocytes.3 Additionally, loss of myelin trophic support coupled with energy deficiency produced by mitochondrial dysfunction may exacerbate the situation.4
Recently, various remyelinating strategies have been suggested5 and several potential remyelinating agents are currently under investigation.6, 7, 8, 9 Therefore, there is an urgent need for reliable in vivo biomarkers of remyelination.
The visual system presents an ideal opportunity to measure the efficacy of remyelinating therapies. Firstly, the optic nerve and visual pathways are commonly affected in MS, often resulting in chronic visual impairment. Secondly, the visual pathways represent the part of the CNS where de/remyelination and axonal loss can be accurately measured. The latency of the visual evoked potential (VEP), which is a measure of conduction speed along the visual pathway, has been suggested as a marker of myelination. Several recent clinical trials based on the acute optic neuritis model demonstrated a positive effect of remyelinating treatment on VEP latency improvement (recovery).6, 9 Multifocal visual evoked potentials (mfVEP) were demonstrated to be even more sensitive in measuring optic nerve de/remyelination caused by acute optic neuritis, particularly when intereye asymmetry analysis is used.10, 11, 12, 13
OCT measurement of inner retinal layers (including the retinal nerve fiber layer (RNFL) has been validated as an accurate measure on axonal loss in anterior visual pathway and has been extensively applied to the investigation of MS.14, 15, 16, 17, 18 Thinning of the RNFL identifies retrograde axonal degeneration following damage to the optic nerve or the optic tract, providing an opportunity to quantitatively assess axonal damage associated with ON. RNFL thinning in ON affected eyes correlates with visual acuity, LCVA, visual field, color vision, and VEPs.19, 20
Though mfVEP latency and RNFL thickness give information on the physiological and morphological status of the visual pathway, future phase 3 clinical trials of remyelinating therapies will require a functional, clinically meaningful measure of the integrity of the visual pathway. Low contrast visual acuity (LCVA), also known as low contrast letter acuity (LCLA), has been suggested as such a measure.
LCVA was shown to correlate with unique aspects of neurological dysfunction and vision‐specific quality of life measures.21, 22, 23 LCVA can differentiate MS patients from healthy controls, even in patients with normal Snellen VA scores (Balcer 2003). The relationship between contrast sensitivity and injury along the visual pathway is supported by the correlation of LCVA with retinal structure by OCT14, 15, 24 and lesions in the posterior visual pathway by magnetic resonance imaging (MRI).25, 26 However, the extent to which abnormalities in LCVA are related to the degree of demyelination remains uncertain. Currently, there is conflicting evidence regarding the correlation between full‐field VEP latency and LCVA severity.27, 28, 29 The correlation between LCVA and mfVEP has not been previously investigated.
In this study, the contribution of demyelination, as measured by mfVEP latency, and axonal loss, as measured by RNFL thickness, to LCVA deficits was investigated to elucidate the potential role of LCVA as a marker of remyelination. A unilateral optic neuritis model and intereye asymmetry analysis was used to maximize the sensitivity of the analysis.
Methods
Here, 50 patients with relapsing‐remitting multiple sclerosis (RRMS) and a previous history of unilateral optic neuritis at least 6 months prior to the study were enrolled. RRMS was defined according to standard criteria.30 Patients with any other systemic or ocular disease that could confound results, such as diabetes, retinal lesions or glaucoma, were excluded.
The study was approved by the University of Sydney and Macquarie University Human Research Ethics Committees. All procedures followed the tenets of the Declaration of Helsinki and written informed consent was obtained from all participants.
mfVEP recording and analysis
Multifocal VEP testing was performed, using the VisionSearch1 (VisionSearch Pty. Ltd., Sydney, Australia) employing standard stimulus conditions that entailed recordings from 56 segments of the visual field. Patients were refracted for near vision, using the trial frame. Monocular recordings were completed typically within 8–10 min until a sufficient signal‐to‐noise ratio was reached. Four gold cup electrodes were placed around the inion and used for bipolar recording from two channels: superior and inferior electrodes for vertical channel and left and right electrodes for horizontal channel. The channel with the largest amplitude (difference between min and max within the interval of 70–200 msec) was selected for each segment of the visual field. The second peak of the wave of maximum amplitude for the selected channel of each segment in the visual field was used for latency analysis of the baseline test, as previously described.31 Figure 1 demonstrates examples of mfVEP recording. Average latency from all segments with identifiable waveform (as determined by the software) was calculated for each eye.
Figure 1.
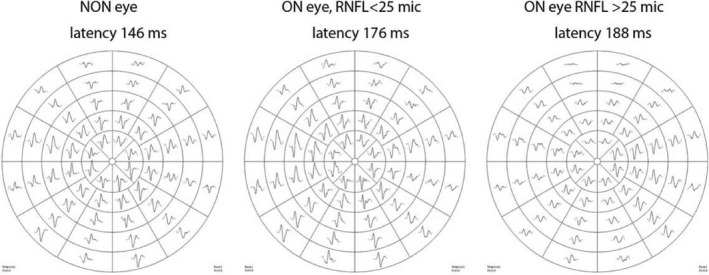
Examples of mfVEP recorded from non‐ON eye, ON eye with RNFL asymmetry <25 mic and ON eye with RNFL asymmetry >25 mic.
LCVA recording and analysis
LCVA was tested unilaterally, using Sloan letter logarithmic translucent contrast (SLLTC) charts at 2.5%, and 1.25% contrast levels (Precision Vision, La Salle, USA). Testing was performed on a retroilluminated background at the distance of 4 m. The charts were scored based on the number of letters identified correctly (maximum of 70 letters per chart). The difference between number of letters identified using ON and NON eyes was used for analysis.
OCT testing and analysis
OCT was performed using a Spectralis imaging platform (Heidelberg Engineering, Carlsbad,CA) as recommended by The APOSTEL and as described previously.32, 33 A peripapillary circular scan (axonal protocol) was used to measure Global RNFL (gRNFL) thickness (Fig. 2). OSCAR‐IB criteria were used for quality control of images.34
Figure 2.
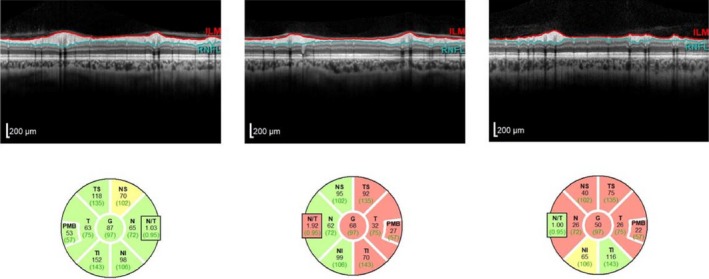
Examples of OCT examination from non‐ON eye, ON eye with RNFL asymmetry <25 mic and ON eye with RNFL asymmetry >25 mic.
Intereye asymmetry analysis
Effect of cortical convolution on mfVEP is well documented.35 In addition, both mfVEP and RNFL measures suffer from inherently high intersubject variability, which in MS patients is exacerbated by potential presence of retrochiasmal (optic radiation) lesions. Therefore, in order to reduce intersubject variability and the potential effects of cortical convolution and retrochiasmal lesions (which affect both eyes equally), intereye asymmetry values were used for analysis. Intereye asymmetry was calculated for mfVEP latency, RNFL thickness and LCVA by subtracting values obtained from the ON eye from values recorded from the NON eye.
Statistical analyses
Statistical analyses were performed using IBM SPSS 22.
Student's t‐test was used to compare means. Significance was determined at 0.05 level. Normality of data was assessed using Shapiro–Wilk test. All correlations were adjusted for age, gender, and disease duration.
A linear regression model was used to assess the relative contribution of various factors on LCVA. Since standardized beta coefficients of the linear regression model estimates the strength of the effect of each individual independent variable to the dependent variable, the coefficient's ratio was used to compare the relative contribution of mfVEP latency and RNFL thinning to reduction in LCVA. In order to identify relationship between contribution of axonal loss and demyelination to the LCVA deficit, the patients with severe retinal atrophy were gradually excluded from analysis up to the point where curves representing standardized beta coefficients for RNFL thickness and latency delay crossed. Age, disease duration, and gender were used as covariates in regression models.
Results
Here, 50 consecutive patients with RRMS (11M/39F, age 40.4 ± 9.2 years, disease duration 6.8 ± 4.5 years, EDSS 1.24 ± 1.1) and a previous history of optic neuritis were enrolled. All patients had a single episode of unilateral optic neuritis at least 6 months prior to the study and demonstrated various degree of visual recovery with best corrected visual acuity of 6/18 or better. Average time since ON diagnosis was 2.8 ± 1.8 years. During acute stage of ON, all patients had completed a 3‐day course of 1 g per day intravenous methylprednisolone and a 2‐week oral taper of steroids. Forty‐five patients were receiving disease‐modifying therapy (13‐Gilenia, 10‐Copaxon, 6‐Tysabril, 4‐Rebif, 4‐Aubagio, 4‐Tysabril, 2‐Lemtrada).
There was a significant increase in the mfVEP latency and reduction in RNFL thickness in ON eyes compared to fellow (NON) eyes (Table 1). The two measures, however, did not correlate with each other (r = 0.28, P = 0.07). There was also a significant difference between number of letters identified by ON and fellow eyes for both levels of low luminance contrast (P < 0.001).
Table 1.
Values of mfVEP latency, RNFL thickness, and LCVA in ON and fellow eyes
| ON eye | Fellow eye | P | |
|---|---|---|---|
| mfVEP latency (msec) | 166.7 ± 12.5 | 151.8 ± 7.7 | <0.001 |
| gRNFL (mic) | 76.7 ± 10.9 | 90.3 ± 9.6 | <0.001 |
| 1.25% LCVA (letters) | 28.4 ± 13.0 | 40.8 ± 11.9 | <0.001 |
| 2.5% LCVA (letters) | 42.4 ± 13.2 | 53.5 ± 9.6 | <0.001 |
Intereye asymmetry of mfVEP latency and RNFL thickness correlated significantly with intereye asymmetry of 1.25% and 2.5% LCVA (Table 2). The correlation, however, was considerably stronger for gRNFL thinning.
Table 2.
Partial correlation of RNFL thickness and mfVEP latency with LCVA. Correlation was adjusted for age, gender, and disease duration)
| Partial correlation | (r 2) | P | |
|---|---|---|---|
| gRNFL vs. 1.25% LCVA | 0.60 | (0.36) | <0.001 |
| gRNFL vs. 2.5% LCVA | 0.73 | (0.53) | <0.001 |
| Latency vs. 1.25% LCVA | 0.52 | (0.27) | <0.001 |
| Latency vs. 2.5% LCVA | 0.59 | (0.36) | <0.001 |
Use of a linear regression model demonstrated increased predictive power of LCVA when both latency and gRNFL thinning were combined. The best model explained 67% of LCVA variability. The linear regression model also confirmed a higher association of LCVA with RNFL thinning compared to mfVEP latency delay (Table 3, Fig. 3). Interestingly, gender was found to have a significant effect on LCVA values with males demonstrating lower LCVA, a finding that was consistent across all models.
Table 3.
Result of linear regression analysis. Adjusted R 2 is calculated for each model
| Partial correlation | Standardized coefficient of beta | P | |
|---|---|---|---|
| 1.25% LCVA | |||
| Latency | 0.40 | 0.30 | 0.004 |
| gRNFL | 0.65 | 0.60 | <0.001 |
| Adjusted R 2 = 0.54 | |||
| 2.5% LCVA | |||
| Latency | 0.58 | 0.42 | <0.001 |
| gRNFL | 0.71 | 0.60 | <0.001 |
| Adjusted R 2 = 0.67 | |||
Figure 3.
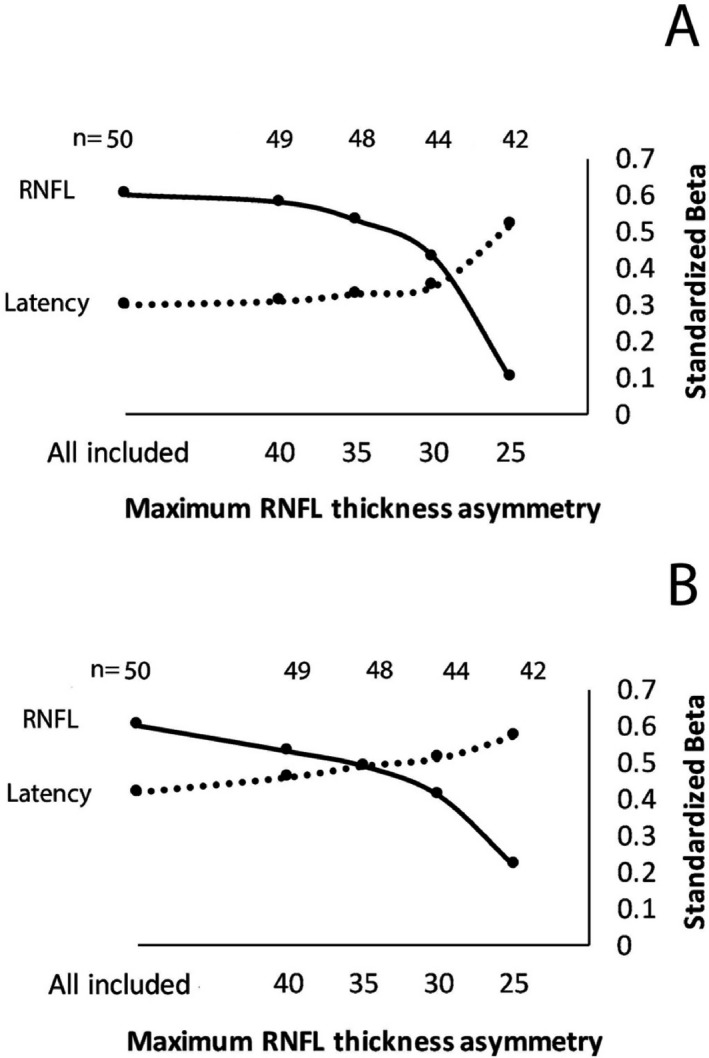
Contribution of RNFL thickness and mfVEP latency to LCVA (A‐1.25% LCVA, B‐2.5% LCVA) expressed as Standardized (Beta) coefficients at different degrees of elimination of subjects with severe axonal loss.
To estimate the relative contribution of latency delay to the model at different levels of LCVA contrast, the ratio of Standardized (Beta) coefficients for RNFL and latency at 1.25 and 2.5% LCVA was calculated. It demonstrated that, while the association of LCVA with mfVEP latency delay was lower than with RNFL thinning for both levels of contrast, the effect of latency on the prediction of 2.5% LCVA was considerably higher compared to 1.25% LCVA (Ratio 0.40 vs. 0.58 for 1.25 and 2.5% LCVA, respectively).
Elimination of subjects with severe axonal loss further increased the relative contribution of mfVEP latency to the model. Thus, gradual exclusion of patients with RNFL asymmetry up to the level of 25 microns resulted in reduction in contribution from RNFL thickness to the model, while effect of mfVEP latency delay on LCVA became predominant (Fig. 3). This trend was again particularly apparent for 2.5% LCVA. When all patients with gRNFL thickness asymmetry of larger than 25 microns were excluded from the linear regression analysis (16% or 8 patients), the contribution of RNFL thickness lost significance for both 1.25 and 2.5% LCVA (P = 0.6 and 0.2, respectively), while predictive power of mfVEP latency (standardized (Beta) coefficient) considerably increased. Table 4 shows clinical, OCT and mfVEP variables between patients with moderate and severe axonal loss.
Table 4.
Clinical, OCT and mfVEP variables between patients with moderate and severe axonal loss (data are mean ± SD)
| Moderate axonal loss group | Severe axonal loss group | |||
|---|---|---|---|---|
| ON eye | Fellow eye | ON eye | Fellow eye | |
| Age | 40.4 ± 9.7 | 40.5 ± 6.2 | ||
| Gender | 8M/36F | 2M/6F | ||
| Disease duration | 6.8 ± 4.8 | 6.8 ± 2.7 | ||
| EDSS | 1.2 ± 1.3 | 1.0 ± 0.7 | ||
| mfVEP latency (msec) | 166.2 ± 12.5 | 151.9 ± 7.8 | 169.3 ± 10.7 | 147.4 ± 23.3 |
| gRNFL (mic) | 80.1 ± 7.8 | 89.5 ± 9.4 | 60.1 ± 7.5 | 95.0 ± 8.2 |
| 1.25% LCVA (letters) | 30.7 ± 13.1 | 39.5 ± 12.3 | 17.5 ± 2.7 | 46.9 ± 7.9 |
| 2.5% LCVA (letters) | 43.9 ± 12.7 | 51.7 ± 9.2 | 35.0 ± 14.1 | 62.4 ± 5.8 |
In addition, a power calculation was performed to estimate a sample size required to measure significant change of the 2.5% LCVA (defined as 7 letters, that is, minimal clinically important difference (MCID)) in a group of patients with moderate axonal loss (a group where mfVEP was a main predictor of visual acuity). It resulted in 35 patients per group (P = 0.05, power = 90%).
Discussion
In this study, intereye asymmetry analysis was used to examine the independent contribution of demyelination and axonal loss to the reduction in LCVA in optic neuritis eyes of patients with MS. RNFL thickness was employed to measure optic nerve axonal loss, while latency of mfVEP was used as a marker of demyelination.
The VEP represents a response of the brain following visual stimulation and is generated at the level of the primary visual cortex. Thus, the latency of the VEP reflects the speed of conduction, and consequently the degree of demyelination along the entire visual pathway. However, the use of intereye asymmetry analysis effectively eliminates the contribution of retrochiasmal demyelination. RNFL thickness reflects axonal density and can also be affected by retrochiasmal MS lesions via the mechanism of transsynaptic degeneration,36, 37 however, by using intereye asymmetry analysis, the effect of retrochiasmatic demyelination is effectively negated. Similar reasoning applies to the measurement of LCVA. Therefore, the use of intereye asymmetry testing for all measures allowed a high degree of confidence that differences seen were solely due to damage related to ON.
The individual relationship between RGC neuroaxonal loss or VEP latency delay with worsening LCVA has been demonstrated previously.14, 15, 24, 27, 38, 39 However, the associations found in our study were generally stronger, possibly due to the use of asymmetry analysis, which eliminates the effect of intersubject variability. Furthermore, a comparison of the two techniques with LCVA has not been performed previously.
While our study demonstrated a correlation between LCVA and both the level of axonal loss and the degree of demyelination, individual correlations revealed considerably tighter relationship of LCVA with RNFL thinning. Thus, mfVEP latency delay by itself can explain at best only about one‐third of LCVA variability, while axonal loss is responsible for more than half of LCVA reduction. This stronger association between LCVA and RNFL thickness compared to mfVEP latency delay is likely to be related to the fact that while RNFL thinning reflects the degree of axonal loss in the optic nerve (and, therefore, proportional deafferentation of the visual system), latency delay measures the degree of conduction in partially demyelinated and actively conducting axons. In other words, axonal loss acts a limiting factor in the functioning of the afferent visual system while the degree of demyelination acts as a modifying factor.
Univariate regression analysis, which represents a novel aspect of this study, demonstrated that both factors contribute significantly and independently to the worsening of LCVA. Due to this synergistic effect, the best model explained 67% of LCVA variability. However, combined analysis of both measures also confirmed that the bulk of LCVA variability is attributable to axon loss, while the myelination status of surviving axons plays a lesser role.
The results of the regression analysis also indicated that the relative contribution of mfVEP latency to the model is highest at 2.5% LCVA, suggesting that the 2.5% contrast chart is likely to be a more sensitive marker for the assessment of optic nerve myelination. More importantly, gradual exclusion of patients with severe axonal loss increased the contribution of mfVEP latency to the model. Thus, excluding patients with RNFL thickness asymmetry larger than 25 microns (which constituted only 16% of the tested population) practically eliminated the effect of RNFL thinning on LCVA modeling, rendering demyelination a better predictor of LCVA than axonal loss in this population.
Due to OCT acquisition protocol used in this study, the analysis of the relationship between LCVA and the thickness of other retinal layers (RGC layer in particular, which is known to be a good predictor of LCVA23) has not been performed. This limitation will be addressed in future studies.
Conclusion
While RGC axonal loss, as measured by relative RNFL thinning was shown to be a better predictor of LCVA, demyelination measured by mfVEP latency delay contributed significantly to worsening of LCVA. Furthermore, the contribution of mfVEP latency to the LCVA model dramatically improved after cases with severe axonal loss were excluded. Therefore, the application of LCVA as a clinical outcome measure in trials of novel remyelinating therapies is feasible, but may require selecting patients with less severe axonal loss.
Conflict of Interest
Dr. Klistorner and Dr. Graham each has two patents licensed to Sydney University. Dr. Graham reports grants and personal fees from Novartis, outside the submitted work. Dr. Barnett reports grants from Biogen, Genzyme, Teva, and Merck outside the submitted work. Dr. Yiannikas, Dr. Barton, Dr. You, and Dr. Triplett have no disclosures.
Funding Information
National Multiple Sclerosis Society (NMSS), Novartis Save Neuron Grant, Sydney Eye Hospital foundation grant and Sydney Medical School Foundation.
Funding Statement
This work was funded by National Multiple Sclerosis Society (NMSS) grant ; Novartis Save Neuron Grant grant ; Sydney Eye Hospital foundation grant grant ; Sydney Medical School Foundation grant .
References
- 1. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285. [DOI] [PubMed] [Google Scholar]
- 2. Barkhof F, Brück W, De Groot CJ, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol 2003;60:1073–1081. [DOI] [PubMed] [Google Scholar]
- 3. Correale J, Gaitán MI, Isrraelit MC, Fiol M. Progressive multiple sclerosis: from pathogenic mechanisms to treatment multiple sclerosis. Brain 2017;140:527–546. [DOI] [PubMed] [Google Scholar]
- 4. Evangelou N, Paine SML, Tallantyre EC. The neurophatology of progressive multiple sclerosis In: Wilkins A., ed. Progressive multiple sclerosis pp. 49–66. NY: Springer, 2018. [Google Scholar]
- 5. Olsen JA, Akirav EM. Remyelination in multiple sclerosis: cellular mechanisms and novel therapeutic approaches. J Neurosci Res 2015;93:687–696. [DOI] [PubMed] [Google Scholar]
- 6. Cadavid D, Balcer L, Galetta S, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo‐controlled, phase 2 trial. Lancet Neurol 2017;16:189–199. [DOI] [PubMed] [Google Scholar]
- 7. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double‐blind, crossover trial. Lancet 2017;390:2481–2489. [DOI] [PubMed] [Google Scholar]
- 8. Cadavid D, Klistorner A, Ampapa R, et al. Correlation of physical, cognitive and MRI measures with multifocal visual evoked potential using baseline data from the Anti‐LINGO‐1 SYNERGY Trial in multiple sclerosis. Nerology 2016;86(16 Supplement):P3‐041. [Google Scholar]
- 9. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double‐blind, crossover trial. Lancet 2017;390(10111):2481–2489. [DOI] [PubMed] [Google Scholar]
- 10. Hood DC, Odel JG, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci 2000;41:4032–4038. [PubMed] [Google Scholar]
- 11. Niklas A, Sebraoui H, Heb E, et al. Outcome measures for trials of remyelinating agents in multiple sclerosis: retrospective longitudinal analysis of visual evoked potential latency. Mult Scler 2009;15:68–74. [DOI] [PubMed] [Google Scholar]
- 12. Yang EB, Hood DC, Rodarte C, et al. Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci 2007;48:692–698. [DOI] [PubMed] [Google Scholar]
- 13. Klistorner A, Arvind H, Garrick R, et al. Remyelination of optic nerve lesions: spatial and temporal factors. Mult Scler J 2010;16:786–795. [DOI] [PubMed] [Google Scholar]
- 14. Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006;113:324–332. [DOI] [PubMed] [Google Scholar]
- 15. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010;67:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frohman EM, Costello F, Stüve O, et al. Modeling axonal degeneration within the anterior visual system: implications for demonstrating neuroprotection in multiple sclerosis. Arch Neurol 2008;65:26–35. [DOI] [PubMed] [Google Scholar]
- 17. Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler J 2011;17:1449–1463. [DOI] [PubMed] [Google Scholar]
- 18. Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013;80:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005;58:383–391. [DOI] [PubMed] [Google Scholar]
- 20. Henderson AP, Altmann DR, Trip SA, et al. Early factors associated with axonal loss after optic neuritis. Ann Neurol 2011;70:955–963. [DOI] [PubMed] [Google Scholar]
- 21. Baier ML, Cutter GR, Rudick RA, et al. Low‐contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology 2005;64:992–995. [DOI] [PubMed] [Google Scholar]
- 22. Mowry EM, Loguidice MJ, Daniels AB, et al. Vision‐related quality of life in multiple sclerosis: correlation with new measures of low‐and high‐contrast letter acuity. J Neurol Neurosurg Psychiat 2009;80:767–772. 10.1136/jnnp.2008.165449 [DOI] [PubMed] [Google Scholar]
- 23. Balcer LJ, Raynowska J, Nolan R, et al., Multiple Sclerosis Outcome Assessments Consortium . Validity of low‐contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler J 2017;23:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012;119:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Z, Vazeen M, Varma R, et al. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology 2007;114:1505–1512. [DOI] [PubMed] [Google Scholar]
- 26. Reich DS, Smith SA, Gordon‐Lipkin EM, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol 2009;66:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schinzel J, Zimmermann H, Paul F, et al. Relations of low contrast visual acuity, quality of life and multiple sclerosis functional composite: a cross‐sectional analysis. BMC Neurol 2014;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinstock‐Guttman B, Baier M, Stockton R, et al. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult Scler J 2003;9:529–534. [DOI] [PubMed] [Google Scholar]
- 29. Shandiz JH, Nourian A, Hossaini MB, Moghaddam HO. Contrast sensitivity versus visual evoked potentials in multiple sclerosis. J Ophthal Vis Res 2010;5:175. [PMC free article] [PubMed] [Google Scholar]
- 30. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sriram P, Wang C, Yiannikas C, et al. Relationship between optical coherence tomography and electrophysiology of the visual pathway in non‐optic neuritis eyes of multiple sclerosis patients. PLoS ONE 2014;9:e102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cruz‐Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016;86:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sriram P, Graham SL, Wang C, et al. Transsynaptic retinal degeneration in optic neuropathies: optical coherence tomography study. Invest Ophthalmol Vis Sci 2012;53:1271–1275. [DOI] [PubMed] [Google Scholar]
- 34. Tewarie P, Balk L, Costello F, et al. The OSCAR‐IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012;7:e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graham SL, Klistorner AI, Grigg JR, Billson FA. Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma 2000;9:10–19. [DOI] [PubMed] [Google Scholar]
- 36. Balk LJ. Bi‐directional trans‐synaptic degeneration in the visual pathway in Multiple Sclerosis. J Neurol Neurosur Psych 2014;86:419–424. [DOI] [PubMed] [Google Scholar]
- 37. Gabilondo I, Martínez‐Lapiscina EH, Martínez‐Heras E, et al. Trans‐synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 2014;75:98–107. [DOI] [PubMed] [Google Scholar]
- 38. Taddei F, Viggiano MP, Mecacci L. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Int J Psychophysiol 1991;11:257–260. [DOI] [PubMed] [Google Scholar]
- 39. Sakai RE, Feller DJ, Galetta KM, et al. Vision in multiple sclerosis. J Neuro‐Ophthalmol 2012;31:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]


