Abstract
Background
There is limited information about the potential associations of multiple sclerosis (MS) and commonly used household chemicals.
Methods
We performed a case‐control study of exposures to common household chemicals during childhood in children with MS and healthy pediatric controls. Exposures to household products were collected from a comprehensive questionnaire (http://www.usnpmsc.org/Documents/EnvironmentalAssessment.pdf) completed by parents at the time of enrollment in the study. Cases included children diagnosed with MS or clinically isolated syndrome with at least two silent T2 bright lesions on MRI, recruited within 4 years of disease onset from 16 pediatric MS clinics in the USA. Multivariate analyses using logistic regression were adjusted for possible confounders including age, sex, race, ethnicity, mother's highest level of education, and urban versus rural living.
Results
Questionnaire responses to household chemicals were available for 312 eligible cases (median age 15.7 years, 63% girls) and 490 healthy controls (median age 15.0, 57% girls). Exposure to rodenticides (odds ratio [OR] 2.10, 95% confidence interval [CI] 1.35–3.26, P ≤ 0.001), weed control agents (OR 1.99, 95% CI 1.36–2.92, P ≤ 0.001) and products for plant/tree disease control (OR 2.72, 95% CI 1.54–4.82, P ≤ 0.001) anytime during childhood were associated with an increased risk for pediatric‐onset MS in adjusted and multiple comparisons analyses.
Conclusions
Our findings suggest that exposure to specific household chemicals during early childhood is associated with the risk of developing pediatric‐onset MS. Future studies are needed to elucidate a causal relationship and the exact agents involved.
Introduction
Multiple sclerosis (MS) is an immune‐mediated disease that causes demyelination of the central nervous system. Of the estimated 2.3 million people living with MS worldwide, ~5% had a pediatric onset.1
Autoimmune diseases, including MS, occur as a result of complex interactions between genetic and environmental factors. While some environmental factors such as remote exposure to Epstein‐Barr virus infection, vitamin D deficiency, and exposure to cigarette smoking have been associated with MS, these factors only partially explain the risk.2, 3, 4, 5 Studies also identified toxic exposures including organic solvents to be associated with MS in adults.6 Organic solvents, which are chemical compounds used routinely in commercial industries, can alter cellular proliferation, apoptosis and tissue‐specific functions, and chronic exposure may lead to deposits in an organ.7, 8, 9 Self‐proteins that are modified by organic solvents may become immunogenic, and trigger an inflammatory cascade with resulting tissue injury.10 However, exposure to a chemical toxin alone may be insufficient to cause a disease such as MS, and more likely the environmental exposure become relevant in a genetically‐susceptible individual.
We have previously reported that pesticide exposures in the household in the perinatal period were associated with increased risk of pediatric‐onset MS.11 Our goal was to identify associations between exposures to household chemicals during childhood and the risk of pediatric‐onset MS, and annualized relapse rate in those with the disease.
Methods
Study design
We performed a cross‐sectional analysis of data collected from a large on‐going case control study of risk factors in pediatric MS (1R01NS071463, PI Waubant) to evaluate potential associations between MS and history of exposures to common household chemicals. An IRB or regional review board has approved the use of human subjects for this study and written informed consent was obtained from guardians of patients participating in the study at all study sites.
Participants
Pediatric‐onset MS or clinically isolated syndrome (CIS) cases with at least two silent MRI demyelinating lesions, with onset before 18 years, seen within 4 years of disease onset were enrolled (between November 1 2011 and July 1 2016) from 16 pediatric MS centers in the United States. These centers are tertiary referral centers, but also serve regional patients from all socioeconomic groups. Diagnosis for cases was ascertained by a panel of at least two experts in pediatric MS. Frequency matched healthy controls younger than 20 years were recruited from the pediatric general and subspecialty clinics at participating institutions during the same period of time as the cases. Controls were matched with patients in windows for age at enrollment: 3–10, 11–14, and 15–19 years. Pairwise matching was not used as for our cohort it would have resulted in a substantial loss of the sample.
Inclusion criteria for controls included absence of any autoimmune disease, except asthma and eczema, and no parent with MS. Controls with family history of MS were excluded to allow evaluation of genetic risk factors of pediatric MS as part of a larger study. Data were collected via an environmental questionnaire completed by a parent or legal guardian.
Questionnaire
Parents completed a comprehensive environmental questionnaire (http://www.usnpmsc.org/Documents/EnvironmentalAssessment.pdf). Questions included prior exposures and the timing of exposures to:
mothballs, crystals, bars, or synthetic closet fresheners,
antibacterial soap or other antibacterial hand products,
professional pest control or extermination service products,
ant, fly, or cockroach control products,
rat, mouse, gopher, or mole control products,
insect repellent for ticks or mosquitos,
slug or snail bait,
weed control products,
plant/tree insect or disease control products,
paint, stains, or lacquers,
adhesives or petroleum products,
indoor foggers for insect control,
sprays, dusts, powders, or skin applications for fleas or ticks, or flea or tick collars.
The questions were specific of various times of exposure: 3 months before pregnancy, during pregnancy, while breastfeeding, first year of life, age 1–5 years, 6–10 years, 11–15 years, and greater than 16 years. The questionnaires asked about the indirect or passive exposure, ever been exposed, frequency, duration, and timing of the exposure. Example of the indirect exposure questionnaire included “Did someone in the household ever use a professional pest control or extermination service? If yes, please provide the names of the products with the time frame the products were used. Please choose one of the following options for how often and who used the product and place the letters in the corresponding boxes”. An example question for passive exposure included “Did the biological mother or main mother figure/guardian smoke from the time the child was 1–5 years of age?”.
As we have previously reported associations related to before and during pregnancy, we have excluded exposures occurring 3 months before or during the pregnancy.11 For cases, only toxic exposures that took place before disease onset were included. Otherwise, the case was considered unexposed.
Statistical analysis
Demographics characteristics were compared with the Kruskal–Wallis test and chi‐squared test as appropriate. Household chemical exposures were compared between the cases and controls using a chi‐squared test. Multivariable logistic regression models adjusted for age, sex, race, ethnicity, mother's education (as a proxy for socioeconomic), and urban versus rural living were used to test for significant differences between cases and controls. P‐values of less than 0.05 were considered significant. Odds ratios were calculated with 95% confidence intervals. The household chemicals were put into both adjusted and unadjusted models.Annualized relapse rate (AAR) was modeled as the number of events over the total follow‐up time period in years. A negative binomial regression model was used for AAR and was adjusted for age at disease onset, sex, race, and ethnicity. Significant differences in the relapse rates were assessed using likelihood ratio tests. Analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Completion of questionnaires
Of 561 cases and 644 controls screened up to 1 July 2016, 326 eligible cases and 506 healthy controls completed the full questionnaires. Among them, 312 cases (95%, median age 15.7 years, 63% girls) and 490 controls (96%, median age 15.0 years, 57% girls) completed the household chemicals exposure questionnaire section. (Fig. 1).
Figure 1.
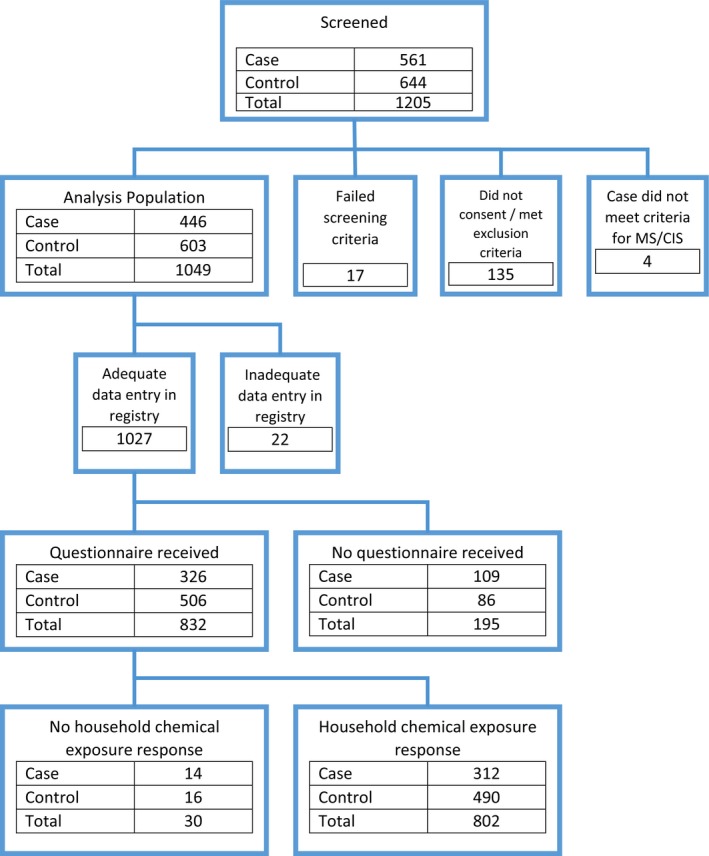
Case and control enrollment and inclusion.
Baseline characteristics
As shown in Table 1 individuals of Hispanic ethnicity were more frequent in cases than in controls (30% vs. 19%, P < 0.001). Controls relative to cases had a higher proportion of mothers with higher level of education (Bachelor's or graduate degree 48% vs. 32%, P < 0.001). A higher percentage of controls had a residence only in an urban ZIP code compared to cases (44% vs. 35%, P = 0.010). Other baseline characteristics were similar in both groups.
Table 1.
Baseline characteristics between cases and controls
| Subject status as case or control | P‐value | ||
|---|---|---|---|
| Control (N = 490) | Case (N = 312) | ||
| Childs age at disease onset (median [IQR]) | ‐ [‐, ‐] | 15.0 [12.6, 16.4] | |
| Childs age at consent (median [IQR]) | 15.0 [12.1, 17.4] | 15.7 [13.6, 17.3] | 0.06a |
| Sex | |||
| Male | 213 (43%) | 117 (38%) | 0.09b |
| Female | 277 (57%) | 195 (63%) | |
| Race | |||
| White | 323 (68%) | 206 (71%) | 0.64b |
| Black | 81 (17%) | 45 (15%) | |
| Asian | 28 (6%) | 12 (4%) | |
| Other | 42 (9%) | 28 (10%) | |
| Ethnicity | |||
| Hispanic or Latino | 92 (19%) | 93 (30%) | <0.001b |
| Not Hispanic or Latino | 387 (79%) | 207 (66%) | |
| Unknown or Not Reported | 11 (2%) | 12 (4%) | |
| Biological mothers highest level of education | |||
| None | 27 (6%) | 36 (12%) | <0.001b |
| High School or Associate's | 215 (47%) | 163 (56%) | |
| Bachelor's or Graduate | 220 (48%) | 94 (32%) | |
| Residence ZIP code only in an urban area | |||
| No | 260 (56%) | 190 (65%) | 0.010b |
| Yes | 207 (44%) | 102 (35%) | |
Kruskal–Wallis test.
Chi‐squared test of no association.
Household chemicals and risk of pediatric MS
As shown in Table 2, based on the unadjusted analysis, MS cases relative to Controls had a higher exposure to rodenticides (rat, mouse, gopher, or mole control products) (P = 0.001), weed control products (P = 0.012), plant/tree insect or disease control products (P = 0.031) and indoor foggers for insect control (P = 0.030). In addition, a multiple regression analysis adjusting for the covariants (age, sex, race, ethnicity, mother's highest level of education, urban versus rural living) showed among MS cases versus controls a higher frequency of exposure to rodenticides (odds ratio [OR] 2.10, 95% confidence interval [CI] 1.35–3.26, P ≤ 0.001), insect repellent (OR 1.47, 95% CI 1.05–2.06, P = 0.025), weed control agents (OR 1.99, 95% CI 1.36–2.92, P ≤ 0.001), products for plant/tree disease control (OR 2.72, 95% CI 1.54–4.82, P ≤ 0.001), adhesive and petroleum products (OR 1.45, 95% CI 1.01–2.08, P = 0.044) and indoor foggers for insect control (OR 1.70, 95% CI 1.04–2.79, P = 0.035) anytime during childhood. On multiple comparisons, insect repellent, adhesives, and indoor foggers were no longer significant based on their adjusted P P ‐values using the false discovery rate method. The rodenticides, weed control agents, and plant/tree disease control products were still significantly associated with risk of developing pediatric–onset MS. To rule out any potential heterogeneity in associations of household chemical exposures between the subjects who met MS criteria (McDonald 2010) and those who were CIS, we performed sensitivity analysis for the adjusted models with only MS patients by excluding those with most recent diagnosis with CIS at the time of last visit and found the results were unchanged. Given the wide recruitment window, we have also looked into the possibility of heterogeneity in cas/control characteristics over time and we have not seen any evidence of that. In addition, we did not see any meaningful change in results, when BMI was added in the model.
Table 2.
Toxic exposure analyses
| Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|
| Control (N = 490) | Case (N = 312) | P‐value | Odds ratio (95% CI) | P‐value | |
| Mothballs, crystals, bars, or synthetic closet fresheners | 31 (6%) | 26 (9%) | 0.18a | 1.70 (0.90, 3.18) | 0.10 |
| Antibacterial soap or other antibacterial hand products | 266 (55%) | 152 (52%) | 0.40a | 1.02 (0.73, 1.43) | 0.91 |
| Professional pest control or extermination service | 97 (20%) | 66 (22%) | 0.42a | 1.31 (0.87, 1.96) | 0.19 |
| Ant, fly, or cockroach control products | 192 (40%) | 126 (43%) | 0.34a | 1.33 (0.95, 1.86) | 0.10 |
| Rat, mouse, gopher, or mole control products | 60 (12%) | 62 (21%) | 0.001a | 2.10 (1.35, 3.26) | <0.001 |
| Insect repellent for ticks or mosquitos | 228 (47%) | 149 (50%) | 0.37a | 1.47 (1.05, 2.06) | 0.025 |
| Slug or snail bait | 17 (3%) | 10 (3%) | 0.93a | 0.94 (0.39, 2.14) | 0.89 |
| Weed control products | 123 (25%) | 99 (33%) | 0.012a | 1.99 (1.36, 2.92) | <0.001 |
| Plant/tree insect or disease control products | 34 (7%) | 34 (11%) | 0.031a | 2.72 (1.54, 4.82) | <0.001 |
| Paint, stains, or lacquers | 229 (48%) | 146 (50%) | 0.55a | 1.29 (0.91, 1.83) | 0.16 |
| Adhesives or petroleum products | 165 (34%) | 109 (37%) | 0.30a | 1.45 (1.01, 2.08) | 0.044 |
| Indoor foggers for insect control | 42 (9%) | 40 (13%) | 0.030a | 1.70 (1.04, 2.79) | 0.035 |
| Sprays, dusts, powders, or skin applications for fleas or ticks, or flea or tick collars | 130 (27%) | 79 (27%) | 0.96a | 1.25 (0.86, 1.81) | 0.25 |
Chi‐squared test of no association.
Adjusted for age, sex, race, ethnicity, mother's highest level of education, and urban only.
Exposure to other household chemicals such as mothballs, crystals, bars, or synthetic closet fresheners, antibacterial soap or other antibacterial hand products, professional pest control or extermination service products, ant, fly, or cockroach control products, slug or snail bait, dusts, powders, or skin applications for fleas or ticks, or flea or tick collars, were not associated with risk of MS in children. Specifically, unlike in adult onset‐MS, exposures to organic solvents (paint, stains, or lacquers) were not associated with an increased risk for pediatric‐ onset MS.
Household chemicals and ARR in children with MS
To determine the association between exposure to household chemicals and ARR, and to maximize the likelihood of seeing an effect, we performed a subgroup analysis of only cases who had neurological evaluation at a pediatric MS center within 3 years of their first demyelinating event and in whom prospective relapse documentation was complete at the time of last visit (n = 271). After adjusting for age at onset, sex, race and ethnicity, exposure to mothballs, crystals, bars, or synthetic closet fresheners were associated with increased ARR (P = 0.008). No other domestic exposures were associated with relapse rate (Table 3).
Table 3.
Annualized relapse rates
| Response | Subjects | Relapse rate (95% CI) | P‐value | |
|---|---|---|---|---|
| Mothballs, crystals, bars, or synthetic closet fresheners | Yes | 25 | 0.99 (0.57, 1.74) | 0.008 |
| No | 240 | 0.55 (0.37, 0.82) | ||
| Antibacterial soap or other antibacterial hand products | Yes | 142 | 0.67 (0.43, 1.03) | 0.08 |
| No | 127 | 0.52 (0.34, 0.79) | ||
| Professional pest control or extermination service | Yes | 62 | 0.72 (0.46, 1.14) | 0.06 |
| No | 206 | 0.53 (0.36, 0.79) | ||
| Ant, fly, or cockroach control products | Yes | 117 | 0.59 (0.38, 0.92) | 0.61 |
| No | 149 | 0.55 (0.36, 0.82) | ||
| Rat, mouse, gopher, or mole control products | Yes | 60 | 0.65 (0.40, 1.06) | 0.37 |
| No | 209 | 0.56 (0.37, 0.85) | ||
| Insect repellent for ticks or mosquitos | Yes | 135 | 0.58 (0.37, 0.89) | 0.99 |
| No | 136 | 0.58 (0.38, 0.88) | ||
| Slug or snail bait | Yes | 10 | 0.32 (0.14, 0.76) | 0.12 |
| No | 261 | 0.59 (0.40, 0.88) | ||
| Weed control products | Yes | 95 | 0.56 (0.35, 0.91) | 0.89 |
| No | 174 | 0.58 (0.38, 0.86) | ||
| Plant/tree insect or disease control products | Yes | 34 | 0.57 (0.33, 1.00) | 0.99 |
| No | 237 | 0.58 (0.38, 0.86) | ||
| Paint, stains, or lacquers | Yes | 134 | 0.60 (0.38, 0.96) | 0.58 |
| No | 132 | 0.55 (0.36, 0.83) | ||
| Adhesives or petroleum products | Yes | 100 | 0.55 (0.34, 0.90) | 0.88 |
| No | 164 | 0.57 (0.38, 0.85) | ||
| Indoor foggers for insect control | Yes | 39 | 0.61 (0.36, 1.02) | 0.76 |
| No | 231 | 0.57 (0.38, 0.86) | ||
| Sprays, dusts, powders, or skin applications for fleas or ticks, or flea or tick collars | Yes | 73 | 0.57 (0.36, 0.91) | 0.88 |
| No | 196 | 0.56 (0.37, 0.85) |
Adjusted for age at onset, sex, race, ethnicity.
Discussion
Our findings suggest that exposures to rodenticides, weed control products, plant/tree insect or disease control products during childhood are associated with pediatric‐onset MS. These results are consistent with our prior observations linking pediatric MS onset to certain environmental exposures just prior to pregnancy and through the first year of life.11
An ecological study investigating the potential link between environmental pesticide exposures and various neurologic and psychiatric conditions in southern Spain, reported an increased prevalence of adult MS in areas of high, compared to low, agricultural pesticide exposure (OR 1.23, 95% CI 1.05–1.43).12 Other environmental exposures, such as heavy metals and solvents, exhibited a stronger association with MS.12
Pesticides and other environmental toxic agents have been associated with neurodegeneration possibly due to effects of reactive oxygen species in nerve tissue, based on animal studies. Organophosphates inhibit acetylcholinesterase leading to excessive acetylcholine, activation of glutamatergic neurons, and drive excitotoxicity and neuronal cell death.12 A proposed mechanism linking environmental toxins to MS is the activation of the immune system by toxic chemicals leading to destruction of oligodendrocytes.12 Alternately, environmental toxins may have an indirect effect by modifying native proteins, such that they are misidentified as foreign proteins, setting off a cascade of inflammation and CNS injury.10 However, it has also been suggested that the toxic effect influences the appearance of autoimmune diseases like MS, only in subjects who are genetically susceptible.13
Many environmental exposures have been explored as potential risk factors for the development of MS but exposures to household chemicals have not been studied in detail. Exposure to organic solvents, which are often used in commercial industries, have been reported to be associated with an increased risk of adult MS (OR 1.53, 95% CI 1.03–2.29).6, 10 However, in our study, childhood exposure to paint, stains, or lacquers, which generally contain organic solvents, was not associated with increased risk of pediatric onset‐MS. It is possible that the amount and duration of exposure may be important, as children generally do not get exposed to organic solvents for a large quantity or a prolonged period of time. Our study also does not preclude an association with adult‐onset MS.
Investigating occupational exposures may also elucidate environmental associations and risk for MS. We previously reported increased risk of pediatric‐onset MS in participants whose fathers had worked in agricultural occupations during the perinatal period.11 While data are limited, an Australian case‐control study of adults showed that some occupational exposures were strongly associated with increased risk of a first CNS demyelinating event, itself often followed by MS.14 For women, there was an increased risk for a first CNS demyelinating event with ≥10 years of exposure to livestock (adjusted odds ratio [AOR] 2.78, 95% CI 1.22–6.33), as well as farming for ≥6 years (AOR 2.00, 95% CI 1.23–3.25). These findings were not seen among men. Interestingly, the risk of farming was associated with exposure to livestock, rather than pesticides used on farms. Further, a large Norwegian study examining offshore workers in the petroleum industry, who are exposed to chemicals such as organic solvents, mineral oils, and other hydrocarbons, did not find an increased risk for MS.15
More work needs to be done in animals to see if exposure to any of these household chemicals can induce CNS inflammation and demyelination. In fact, toxins can induce demyelination in animal models of MS such as lysolecithin and cuprizone. Lysolecithin is an activator of phospholipase A2 and cuprizone is a copper chelator. However, toxin‐induced demyelination models do not replicate all the biological characteristics of MS, and animals remyelinate when cuprizone is removed from their diet.16
Studies like ours that investigate environmental risk factors for MS in isolation should be interpreted with caution, as the combination of environmental and genetic factors together contribute to MS risk.17, 18 In addition, we did not adjust for other potential confounding variables such as sun exposure, vitamin D intake, and infectious mononucleosis history in this study. However, we have collected and analyzed some of these variables separately as risk factors for pediatric MS in our different studies17, 19 and our analyses adjusted for BMI. Due to onset in childhood, most individuals do not have a prior history of mononucleosis and this data are not available.
Other limitations of our study include possible recall and sampling bias or possible exposure misclassification. However, recall bias is unlikely in most scenarios as people tend to remember specifics such as using professional exterminator services and obvious occupational exposures to certain products administered by self or others. Sampling bias is unlikely as we adjusted urban versus rural living using the zip codes. As our study is an exploratory study, these limitations were unavoidable but we will address these in future studies. We also plan to develop more detailed environmental questionnaires, including the dose, duration, age, and timing of the exposures to the onset of the disease to help us determine whether observed associations in this study were genuine or not.
A major strength of our study is the large sample size with cases enrolled all over the US, and high response rates. The large sample size enabled us to adjust the analyses for multiple potential confounders. Another major strength is our focus on the development of pediatric‐, rather than adult‐onset MS, leading to a much shorter time lag between exposure and MS onset and associated with better recall of childhood exposures. In addition, our cases were ascertained by pediatric MS specialists, and controls were enrolled at the same institutions than cases.
Conclusions
This study reveals an association between prior exposures to a few common household chemicals during early childhood and an increased risk for pediatric‐onset MS. Further research is necessary to confirm these findings, determine whether the association is causal, and identify the specific active ingredients associated with the increased risk of MS. Finally, the study of gene‐environment interactions may help elucidate the biological processes underlying the association we report.
Author contribution
Soe Mar‐Contributed to data acquisition, analysis and interpretation of the data, literature. search and writing of manuscript. Shannon Liang‐ Contributed to literature search and writing of manuscript. Michael Waltz‐Contributed to data analysis and interpretation of the data, performing statistical analysis. Charles Casper‐Contributed to data analysis and interpretation of the data, performing statistical Analysis. Manu Goyal‐Contributed to critical review of manuscript. Benjamin Greenberg‐Contributed to data acquisition and critical review of manuscript. Bianca Weinstock‐Guttman‐ Contributed to study design, data collection, data interpretation, manuscript review. Moses Rodriguez‐Contributed to study design, data acquisition and critical review of manuscript. Gregory Aaen‐Contributed to study design, data acquisition and critical review of manuscript. Anita Belman‐Contributed to study design, data acquisition and critical review of manuscript. Lisa Barcellos‐Contributed to study design and critical review of manuscript. John Rose‐Contributed to study design and critical review of manuscript. Mark Gorman‐Contributed to study design, data acquisition and critical review of manuscript. Leslie Benson‐Contributed to data acquisition and critical review of manuscript. Meghan Candee‐Contributed to data acquisition and critical review of manuscript. Tanjua Chitnis‐Contributed to study design, data acquisition and critical review of manuscript. Yolanda Harris‐Contributed to data acquisition and critical review of manuscript. Ilana Kahn‐Contributed to data acquisition and critical review of manuscript. Shelly Roalsted‐Contributed to study design, data acquisition and critical review of manuscript. Janace Hart‐Contributed to data acquisition and critical review of manuscript. Timothy Lotze‐Contributed to study design, data acquisition and critical review of manuscript. Manikum Moodley‐Contributed to data acquisition and critical review of manuscript. Jayne Ness‐Contributed to study design, data acquisition and critical review of manuscript. Mary Rensel‐Contributed to data acquisition and critical review of manuscript. Jennifer Rubin‐Contributed to data acquisition and critical review of manuscript. Teri Schreiner‐Contributed to data acquisition and critical review of manuscript. Jan‐Mendelt Tillema‐Contributed to study design, data acquisition and critical review of manuscript. Amy Waldman‐Contributed to data acquisition and critical review of manuscript. Lauren Krupp‐Contributed to study design, data acquisition and critical review of manuscript. Jennifer Graves – Contributed to study design; data acquisition; data preparation, analysis, and interpretation; and critical review of manuscript. Emmanuelle Waubant‐Contributed to study design, obtaining funding, data analysis, data acquisition and critical review of manuscript.
Conflict of Interest
Soe Mar, Shannon Liang, Michael Waltz, Charles Casper, Manu Goyal, Bianca Weinstock‐Guttman, Moses Rodriguez, Gregory Aaen, Anita Belman, Lisa Barcellos, Mark Gorman, Leslie Benson, Meghan Candee, Tanjua Chitnis, Yolanda Harris, Ilana Kahn, Shelly Roalsted, Janace Hart, Timothy Lotze, Manikum Moodley, Jayne Ness, Mary Rensel, Jennifer Rubin, Teri Schreiner, Jan‐Mendelt Tillema, Amy Waldman, Lauren Krupp, and Emmanuelle Waubant have no relevant disclosures. Benjamin Greenberg – Dr. Greenberg has received research funding from Medimmune, Chugai, Medday, NIH, PCORI, Guthy Jackson Charitable Foundation, NMSS and the Transverse Myelitis Association. Consulting fees from Alexion, EMD Serono and Novartis. John Rose – Dr. Rose has received research funding from: National Multiple Sclerosis Society, NIH, Guthy Jackson Charitable Foundation, Biogen, Teva Neuroscience, Friends of MS and Western Institute for Biomedical Research. Jennifer Graves – Dr. Graves has no conflict directly related to content of the manuscript. Unrelated to the manuscript she has received compensation for grants, clinical trial adjudication service and non‐promotional educational speaking activities from Genentech, Biogen, Med Day and Genzyme.
Funding information
National Multiple Sclerosis Society and the National Institute of Neurological Disorders and Stroke through: 1R01NS071463.
Funding Statement
This work was funded by National Multiple Sclerosis Society grant HC‐1509‐06233; National Institute of Neurological Disorders and Stroke grant 1R01NS071463.
References
- 1. Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune‐mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–1267. [DOI] [PubMed] [Google Scholar]
- 2. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol 2007;61:504–513. [DOI] [PubMed] [Google Scholar]
- 3. Oksenberg JR, Barcellos LF. Multiple sclerosis genetics: leaving no stone unturned. Genes Immun 2005;6:375–387. [DOI] [PubMed] [Google Scholar]
- 4. Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65. [DOI] [PubMed] [Google Scholar]
- 5. Hedstrom AK, Hillert J, Olsson T, Alfredsson L. Smoking and multiple sclerosis susceptibility. Eur J Epidemiol 2013;28:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riise T, Moen BE, Kyvik KR. Organic solvents and the risk of multiple sclerosis. Epidemiology 2002;13:718–720. [DOI] [PubMed] [Google Scholar]
- 7. Povey A, Guppy MJ, Wood M, et al. Cytochrome P2 polymorphisms and susceptibility to scleroderma following exposure to organic solvents. Arthritis Rheum 2001;44:662–665. [DOI] [PubMed] [Google Scholar]
- 8. Cai P, König R, Boor PJ, et al. Chronic exposure to trichloroethene causes early onset of SLE‐like disease in female MRL +/+ mice. Toxicol Appl Pharmacol 2008;228:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffin JM, Gilbert KM, Lamps LW, et al. CD4(+) T‐cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol Sci 2000;57:345–352. [DOI] [PubMed] [Google Scholar]
- 10. Barragan‐Martinez C, Speck‐Hernández CA, Montoya‐Ortiz G, et al. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta‐analysis. PLoS One 2012;7:e51506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graves JS, Chitnis T, Weinstock‐Guttman B, et al. Maternal and perinatal exposures are associated with risk for pediatric‐onset multiple sclerosis. Pediatrics 2017;139:e20162838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parron T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol 2011;256:379–385. [DOI] [PubMed] [Google Scholar]
- 13. Shoenfeld Y, Agmon‐Levin N. ‘ASIA’ ‐ autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun 2011;36:4–8. [DOI] [PubMed] [Google Scholar]
- 14. Valery PC, Lucas RM, Williams DB, et al. Occupational exposure and risk of central nervous system demyelination. Am J Epidemiol 2013;177:954–961. [DOI] [PubMed] [Google Scholar]
- 15. Riise T, Kirkeleit J, Aarseth JH, et al. Risk of MS is not associated with exposure to crude oil, but increases with low level of education. Mult Scler 2011;17:780–787. [DOI] [PubMed] [Google Scholar]
- 16. Denic A, Johnson AJ, Bieber AJ, et al. The relevance of animal models in multiple sclerosis research. Pathophysiology 2011;18:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gianfrancesco MA, Stridh P, Rhead B, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric‐onset MS. Neurology 2017;88:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gianfrancesco MA, Stridh P, Shao X, et al. Genetic risk factors for pediatric‐onset multiple sclerosis. Mult Scler 2017;:1352458517733551. [Epub ahead of print]. 10.1177/1352458517733551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chitnis T, Graves J, Weinstock‐Guttman B, et al. Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann Clin Transl Neurol 2016;3:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]


