Abstract
Objective
To investigate the association between the perfusion magnetic resonance imaging (MRI) and vertebrobasilar dolichoectasia (VBD) in vertigo patients and at least one vascular risk factor.
Methods
We studied 289 patients with vertigo (spinning, swaying, nausea, vomiting, and unsteady gait) who performed multimode MRI. Maximum diameter and tortuous parameters of the basilar artery and vertebral arteries were calculated using magnetic resonance angiography. Relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), mean transit time (MTT), and time to peak (TTP) maps were evaluated by dynamic susceptibility contrast‐enhanced perfusion imaging. Association of perfusion MRI and VBD was evaluated by nonparametric tests and receiver‐operating characteristic curve was constructed to predict posterior ischemic stroke in VBD patients.
Results
The prevalence of VBD was 26.6% (n = 77/289) in our study. Male gender was the risk factor of VBD by multivariate analysis. BA diameter was significant statistics between ischemic stroke and nonischemic stroke patients. TTP in bilateral lower cerebellum, superior cerebellum, bilateral pons, and occipital and temporal lobes region of interests was significantly delayed in VBD versus non‐VBD patients, while rCBF, rCBV, and MTT parameters were not significant differences. TTP in the right temporal lobe delayed by 21.96 ms was the best predictive value and the mean TTP predictive threshold value in all ROIs was 22.67 ± 1.48 ms.
Interpretation
VBD leads to the hypoperfusion of posterior circulation territory characterized by delayed TTP. Delayed TTP in cerebellum, pons, and occipital and temporal lobes fed by vertebrobasilar arteries predicted the occurrence of posterior ischemic stroke in VBD patients.
Introduction
Vertebrobasilar dolichoectasia (VBD) was a clinical entity characterized by elongated, dilated and/or tortuous vertebral artery, and basilar artery (BA),1, 2, 3 ranging from 0.05% to 18%.4, 5, 6 VBD presentation includes symptoms by cerebral ischemia in the vertebrobasilar territory.2, 3, 4, 5, 6
Morphology changes of vertebrobasilar arteries subsequently changing the hemodynamics in vertebrobasilar system.7, 8 Transcranial Doppler ultrasound has been in utilization of detecting a significantly decreased arterial blood flow velocity in VBD patients.9 A higher fluid attenuated inversion recovery vascular hyperintensity (FVH) grade representing slow arterial blood flow has been observed common in patients with transient ischemic attack (TIA)/stroke related to VBD and the moderate/severe dilated BA had the higher grade of FVH.10 Similarly, CT perfusion was utilized in undetermined isolated vertigo patients to find the hypoperfusion in the territories of the basilar artery curve cohort.11 Recent studies have found that perfusion weighted imaging (PWI) added diagnostic value by depicting regions with delayed perfusion with negative DWI in acute transient vestibular syndrome of unknown cause or TIA patients.12, 13 However, the utilization of PWI to detect the hypoperfusion in posterior circulation which contribute to identify ischemia caused by VBD has less been reported yet.
The purpose of this study was to evaluate the association between VBD and hypoperfusion in posterior circulation detected by dynamic susceptibility contrast‐enhanced (DSC) perfusion imaging, and was to evaluate the parameters of predicting the occurrence of posterior ischemic stroke in VBD patients with vertigo and who have at least one vascular risk factor.
Methods
Patients
We collected information about 524 consecutive patients hospitalized at the Department of Neurology of Zhengzhou People's Hospital from December 2014 to May 2017. For patients consenting to screening, the study trained neurologist (D.P.Z.) conducted a neurological and vestibular examination according to a standard protocol. Most also underwent confirmatory caloric testing of vestibular function. If they fulfilled the following criteria: chiefly complaint for vertigo (main symptoms including spinning, swaying, nausea, vomiting, and unsteady gait); age over 18 years; with at least one vascular risk factor; and have negative results of Dixhall‐pike test and Roll test. Those with definitive diagnosis of benign paroxysmal positional vertigo (BPPV), Meniere's Disease, aural vertigo, recurrent ischemic strokes, atrial fibrillation, or congenital heart disease history were ineligible for inclusion. All patients were evaluated by CT scan (n = 524), MRI (n = 395) or MRA (n = 382), and PWI (n = 321). Two hundred and thirty‐five patients were excluded because of encephalorrhagia (n = 4), refusing to underwent MRI/MRA (n = 142) and PWI (n = 10), obtaining poor images (n = 27) or incomplete data (n = 52). Ultimately, 289 patients were selected into the study group. Through the utilization of MRA, patients were divided into VBD group and non‐VBD group.
For the purpose of this study, the following concomitants were examined: history of hypertension (previous diagnosis of arterial hypertension: systolic blood pressure > 140 mmHg or diastolic > 90 mm Hg or both or past or present use of antihypertensive agents), diabetes mellitus (previous diagnosis of diabetes or past or present use of antidiabetic agents), obesity (body mass index ≥ 28.0), hyperlipidemia (cholesterol > 5.17 mmol/L, triglycerides > 1.71 mmol/L, or both), hyperhomocysteinemia (>15.0 μmol/L), drinking at least once a week (1 standard alcohol consumption is equivalent to 120 mL of wine, 360 mL of beer, or 45 mL of distilled spirits), smoking (continuously or cumulatively smoke for more than 6 months and at least one cigarette per day), history of coronary artery disease (CAD), and vertebrobasilar arteries stenosis (the stenosis of vertebral artery, BA or posterior cerebral artery > 50% evaluated by MRA). All patients with vascular risk factors had been previously diagnosed as such and/ or were already taking medications for these conditions.
The study was approved by the local ethics committee and all participants signed an informed consent form before their enrollment.
MR protocols
MRI and MRA were performed with a 3.0‐T scanner (GE Medical, Piscataway, NJ) within the first week of patients’ hospitalization. Conventional sequences included: axial T1‐weighted images, axial, sagittal, and coronal T2‐weighted images, fluid‐attenuated inversion recovery sequence (FLAIR) images as well as diffusion‐weighted imaging (DWI). Images were obtained in the axial plane with section of 5 mm in thickness and 1 mm in length. Three‐dimensional time‐of‐flight (TOF) MRA was performed with a repetition time of 24 msec, echo time of 6 msec, FOV of 24 × 24 cm, and section thickness of 0.8–1.6 mm. The scanning results were obtained by reconstructing the image with maximum intensity projection. The diameter of BA was measured at the mid‐pons level and the diameter of vertebral artery was measured from the bilateral vertebral artery junction, 3 mm apart, at three consecutive points.
MRI/MRA data were reviewed by two experienced radiologists blinded to clinical and demographic data. Basilar artery length (BAL) refers to a standard line length which was drawn from the top of the BA to union of both VAs. Bending length (BL) refers to the vertical length between the middle point of the width of the BA at the point of greatest bending and the standard line14 (Fig. 1). For both vertebral arteries, the actual length was measured by tracing the course of the vessel from its origin to the vertebral level of C2, and the straight distance was calculated by measuring the linear distance from origin to the end of the vessel.15 Tortuosity index (TI) of the BA and vertebral arteries were calculated in the current study. TI was defined as: [(actual length of the vessel/ straight‐line length of same vessel ‐1) ×100].
Figure 1.
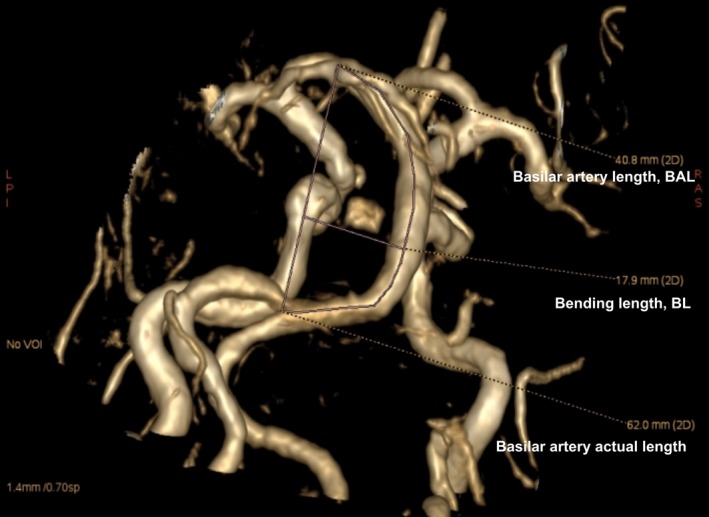
A vertebrobasiar dolichoectasia (VBD) patient who was measured basilar artery tortuous parameters. The picture showed that basilar artery length (BAL) is 40.80 mm, bending length (BL) is 17.90 mm and actual length of basilar artery is 62.0 mm. Tortuosity index (TI) of the BA = (62.0/40.8‐1)×100 = 51.9.
VBD was defined as the following one of criteria: (1) the maximum diameter of BA was greater than 4.5 mm; (2) the BAL was greater than 29.5 mm; (3) the BL was greater than 10.0 mm; (4) the maximum diameter of VA was greater than 4.0 mm.8, 16 Patients included into the study with VBD were clarified into the study group (VBD group), and those included into study but without VBD were clarified into the control group (non‐VBD group).
Perfusion weighted imaging
Perfusion weighted imaging was performed with a dynamic, susceptibility‐weighted, contrast‐enhanced perfusion (DSC) method, using a gradient recalled T2*‐weighted echo‐planar imaging sequence (Signa HDX 3.0‐T MR, GE Medical). The parameters used were as follows: TR/TE = 1500/30 msec, flip angle = 90°, FOV = 23 × 23 cm, matrix size = 128 × 128, and slice thickness = 4 mm (with a 1.2‐mm gap). Image acquisition started 5 seconds before contrast agent being injected to establish a precontrast baseline. Five seconds after the start of image acquisiton, 0.2 mL/kg of gadopentetate dimeglumine (Gd‐DTPA) at a rate of 4 mL/sec was injected manually through a 18‐gauge venous cannula into the antecubital vein, followed by 0.2 mL/kg of saline. A total of 60 single‐shot echo‐planar images were obtained from each of 19 sections over an interval of 98 sec. According to the different territories fed by different branches of vertebrobasilar arteries,17 ten regions of interest (ROI) were chosen: the surface of bilateral lower cerebellum (supplied by posterior inferior cerebellar artery), bilateral superior cerebellum (supplied by superior cerebellar artery), bilateral pons at the level of mid‐pons (supplied by BA), and bilateral occipital and temporal lobes (supplied by posterior cerebral artery). Perfusion parameter maps (for rCBF, rCBV, MTT, and TTP) were obtained using the Brainstat AIF software (GE 4.6 work station).
Statistical analysis
Patients’ demographic and clinical characteristic between posterior circulation ischemic stroke individuals and those without posterior circulation ischemic stroke were compared by the Student's t test for continuous factors and the χ2 test for dichotomous factors (Fisher's exact test was used when the expected cell frequency was <5). Significant risk factors (P < 0.05) were included in the multivariate analysis with adjustment for age and gender. Patients’ demographic and clinical characteristic between the VBD group and non‐VBD group were also compared subsequently. The Mann‐Whitney U test was performed to compare the rCBF, rCBV, MTT, TTP between two groups. Posterior circulation ischemic stroke patients were divided into two subgoups: VBD subgroup and non‐VBD subgroup, and the rCBF, rCBV, MTT, TTP between two subgroups were compared by the Mann‐Whitney U test. Nonposterior circulation ischemic stroke patients were dealt with the same method.
Receiver‐operating characteristic (ROC) analysis was constructed to determine the rCBF, rCBV, MTT, and TTP values of predicting the occurrence of posterior circulation ischemic stroke in VBD patients. Youden index was calculated to determine the best threshold values and their sensitivity and specificity. Significance was assumed at P < 0.05. Data were analyzed with IBM SPSS version 20.0.
Results
Subject characteristics
The study enrolled 524 vertigo patients between 2014 and 2017, and 289 patients with available MRA and PWI were taken into analysis. The mean age of patients was 60.9 (standard deviation, 12.5) and 53% were male. Of the 289 patients, 38.8% (n = 112) were diagnosed with posterior circulation ischemic stroke, of which 39 patients involving pons, 19 cerebellums, nine occipital and temporal lobes, six medulla oblongata, two occipital lobes, two pons and cerebellum, one temporal lobes, one mesencephalon, one pons and mesencephalon, and eight concurrently involving basal ganglia and pons, five frontal, parietal, occipital and temporal lobes, four cerebellum and frontal lobe, four pons and the centrum semiovale, three cerebellum and basal ganglia, three cerebellum and periventricular, three basal ganglia and occipital and temporal lobes, one corpus callosums, one pons, and periventricular. As the respect of risk factors, arterial hypertension (OR, 2.407, 95% CI, 1.327–4.367), diabetes (OR = 2.839, 95% CI: 1.640–4.916), current smoking (OR = 2.657, 95% CI: 1.269–5.565), the history of stroke (OR = 2.117, 95% CI: 1.105–4.057), vertebrobasilar arteries stenosis (OR = 0.422, 95% CI: 0.252–0.705), and dilated BA were the risk factors of the occurrence of posterior circulation ischemic stroke (Table 1). The prevalence of VBD was 26.6% (n = 77/289) in the current study. In VBD group, 35.1% (n = 27/77) were identified with posterior circulation ischemic stroke. The rate of posterior circulation ischemic stroke in the VBD patients was not different from patients without VBD (χ2 = 1.282, P = 0.527), however, BA diameter was significant statistics between ischemic stroke and nonischemic stroke (U = 2.914, P < 0.01). Male gender was the risk factor of VBD, however, there were no relevant differences in terms of arterial hypertension, diabetes, dyslipidemia, CAD, hyperhomocysteinemia, hyperuricemia, current smoking, drinking, history of vertigo/stroke, and carotid arteries plaques between VBD and non‐VBD patients (Table 2).
Table 1.
Baseline characteristic of the study patients between posterior circulation ischemic stroke and nonposterior circulation ischemic stroke
| IS (n = 112) | Non‐IS (n = 177) | OR (univariate) | OR (multivariate) | |
|---|---|---|---|---|
| Age, y | 62 ± 12 | 60 ± 13 | NS | ‐ |
| Male, n (%) | 68 (61) | 86 (49) |
0.800 [0.647–0.990] P < 0.05 |
0.677 [0.344–1.332] |
| Hypertension, n (%) | 90 (80) | 101 (57) |
0.710 [0.607–0.831] P < 0.01 |
2.407 [1.327–4.367] P < 0.01 |
| Diabetes, n (%) | 63 (56) | 51 (29) |
0.512 [0.386–0.680] P < 0.01 |
2.839 [1.640–4.916] P < 0.01 |
| Dyslipidemia, n (%) | 67 (60) | 108 (61) | 1.020 [0.842–1.236] | NS |
| CAD, n (%) | 22 (20) | 43 (24) | 1.237 [0.784–1.951] | NS |
| Hyperhomocysteinemia, n (%) | 36 (32) | 40 (23) | 0.703 [0.479–1.031] | NS |
| Hyperuricemia, n (%) | 0 | 7 (4) | NS | ‐ |
| Smoking, n (%) | 50 (45) | 40 (23) |
0.506 [0.360–0.713] P < 0.01 |
2.657 [1.269–5.565] P < 0.05 |
| Alcoholism, n (%) | 31 (28) | 24 (14) |
0.490 [0.304–0.790] P < 0.01 |
1.559 [0.718–3.384] |
| History of vertigo, n (%) | 1 (0.9) | 6 (3.) | 3.797 [0.463–31.120] | NS |
| History of strokes, n (%) | 36 (32) | 23 (13) |
0.404 [0.254–0.645] P < 0.01 |
2.117 [1.105–4.057] P < 0.05 |
| Vertebrobasilar arteries stenosis, (>50%) n (%) | 30 (27) | 20 (11) |
0.422 [0.252–0.705] P < 0.01 |
|
| BA parameters, median (interquartile range) | ||||
| BAL | 24.90 (5.00) | 23.60 (6.80) | NS | |
| BL | 2.80 (2.90) | 2.65 (2.60) | NS | |
| TI | 5.47 (8.13) | 5.08 (7.05) | NS | |
| Diameter | 3.60 (0.90) | 3.30 (0.90) | P < 0.01 | |
| VA parameters, median (interquartile range) | ||||
| Left VA | ||||
| Diameter | 2.80 (1.10) | 2.90 (1.30) | 0.275 | |
| TI | 0.10 (0.11) | 0.10 (0.11) | 0.996 | |
| Right VA | ||||
| Diameter | 2.60 (1.28) | 2.60 (1.30) | 0.930 | |
| TI | 0.07 (0.05) | 0.06 (0.06) | 0.598 | |
IS indicates ischemic stroke; CAD, coronary artery disease; VA, vertebral artery; BA, basilar artery; BAL, basilar artery length; BL, bending length; TI, tortuosity index.
Table 2.
Baseline characteristic of VBD and non‐VBD patients
| VBD (n = 77) | Non‐VBD (n = 212) | OR | |
|---|---|---|---|
| Age, y, mean ± SD | 63 ± 13 | 60 ± 12 | NS |
| Male, n (%) | 51 (66) | 102 (48) |
0.734 [0.594–0.906] P < 0.01 |
| Hypertension, n (%) | 48 (62) | 142 (67) | 1.082 [0.888–1.318] |
| Diabetes, n (%) | 29 (38) | 84 (40) | 1.065 [0.764–1.482] |
| Dyslipidemia, n (%) | 44 (57) | 130 (61) | 1.081 [0.867–1.348] |
| CAD, n (%) | 19 (25) | 46 (22) | 0.879 [0.551–1.402] |
| Hyperhomocysteinemia, n (%) | 25 (32) | 50 (24) | 0.741 [0.496–1.107] |
| Hyperuricemia, n (%) | 2 (3) | 5 (2) | 0.908 [0.180–4.584] |
| Medical history | |||
| Vertigo, n (%) | 2 (3) | 5 (2) | 0.908 [0.180–4.584] |
| Strokes, n (%) | 19 (25) | 40 (19) | 0.765 [0.473–1.235] |
| Smoking, n (%) | 28 (36) | 62 (29) | 0.804 [0.560–1.155] |
| Alcoholism, n (%) | 15 (19) | 40 (19) | 0.969 [0.568–1.651] |
| Vertebrobasilar arteries stenosis (>50%), n (%) | 14 (18) | 36 (17) | 0.934 [0.534–1.634] |
VBD indicates vertebrobasilar dolichoectasia; CAD, coronary artery disease; VA, vertebral artery; BA, basilar artery.
PWI analysis and VBD
The perfusion in the more proximal portions of the posterior circulation fed by vertebrobasilar arteries‐ the lower cerebellum and pons were studied in all patients shown in Table 3, rCBF and rCBV were not significantly decreased and MTT was not signal delayed in any ROIs of bilateral lower cerebellum and pons in VBD patients. TTP in bilateral lower cerebellum and bilateral pons ROIs were significantly delayed in VBD patients than that in those without VBD (lower cerebellum: right P = 0.001; left P = 0.003; pons: right P = 0.016; left: P = 0.009, respectively).
Table 3.
Perfusion values in the more proximal portions of the posterior circulation between VBD patients and in non‐VBD patients
| VBD (n = 77) | Non‐VBD (n = 212) | P valuea | |
|---|---|---|---|
| Right cerebellum | |||
| rCBF | 24.42 (14.97, 34.30) | 26.28 (17.22, 35.72) | NS |
| rCBV | 1.95 (1.45, 2.58) | 2.07 (1.37, 2.87) | NS |
| MTT | 6.29 (5.26, 7.82) | 6.00 (5.19, 7.11) | NS |
| TTP | 21.87 (20.11, 24.72) | 20.58 (18.94, 22.99) | 0.001 |
| Left cerebellum | |||
| rCBF | 25.40 (15.52, 36.02) | 27.23 (17.72, 36.84) | NS |
| rCBV | 2.09 (1.40, 2.79) | 2.22 (1.44, 3.11) | NS |
| MTT | 6.40 (5.60, 7.81) | 6.19 (5.43, 7.63) | NS |
| TTP | 22.03 (19.64, 24.54) | 20.50 (18.91, 22.87) | 0.003 |
| Right pon | |||
| rCBF | 25.27 (16.14, 36.43) | 24.25 (15.98, 34.62) | NS |
| rCBV | 2.10 (1.38, 3.24) | 2.17 (1.47, 3.02) | NS |
| MTT | 6.45 (5.79, 8.05) | 6.20 (5.46, 7.57) | NS |
| TTP | 21.55 (19.73, 25.23) | 20.08 (19.11, 23.40) | 0.016 |
| Left pon | |||
| rCBF | 24.58 (14.53, 33.07) | 25.13 (17.20, 37.09) | NS |
| rCBV | 2.21 (1.38, 3.20) | 2.12 (1.61, 3.10) | NS |
| MTT | 6.56 (5.45, 8.24) | 6.31 (5.39, 7.49) | NS |
| TTP | 21.98 (19.72, 25.02) | 20.60 (19.03, 23.16) | 0.009 |
rCBV indicates relative cerebral blood volume; rCBF, relative cerebral blood flow; MTT, mean transit time; and TTP, time to peak. Values were expressed as median (percentiles of 25th and 75th).
P values were calculated by the Mann‐Whitney U test.
Imperfectly, we tried to evaluate the perfusion in occipital and temporal lobes fed by vertebrobasilar arteries, however, 20 patients’ original data were lost during the storage and 10 patients’ perfusion images of occipital and temporal lobes were not available because of poor image processing. And thus, the perfusion in the distal portion of the posterior circulation‐ the superior cerebellum and occipital and temporal lobes were studied in 259 patients shown in Table 4. Similarly, rCBF and rCBV were not significantly decreased and MTT was not signal delayed in any ROIs of superior cerebellum and occipital and temporal lobes in VBD patients. TTP in superior cerebellum and occipital and temporal lobes ROIs were significantly delayed in VBD patients compared with VBD negative patients (superior cerebellum: right P = 0.010; left P = 0.020; occipital lobes: right P = 0.042; left: P = 0.034, temporal lobes: right P = 0.053; left: P = 0.006, respectively).
Table 4.
Perfusion values in the distal portion of the posterior circulation between VBD patients and in non‐VBD patients
| VBD (n = 73) | Non‐VBD (n = 186) | P Valuea | |
|---|---|---|---|
| Right superior cerebellum | |||
| rCBF | 17.89 (13.23) | 20.32 (12.86) | NS |
| rCBV | 1.85 (1.29) | 2.03 (1.22) | NS |
| MTT | 7.01 (2.16) | 7.19 (2.11) | NS |
| TTP | 22.43 (4.70) | 21.31 (4.72) | 0.010 |
| Left superior cerebellum | |||
| rCBF | 16.53 (13.16) | 18.68 (12.96) | NS |
| rCBV | 1.78 (1.32) | 1.79 (1.07) | NS |
| MTT | 7.34 (2.40) | 6.88 (1.95) | NS |
| TTP | 22.41 (4.54) | 21.25 (4.67) | 0.020 |
| Right occipital lobe | |||
| rCBF | 14.39 (10.11) | 13.67 (9.83) | NS |
| rCBV | 1.34 (0.86) | 1.30 (0.88) | NS |
| MTT | 6.93 (1.91) | 6.73 (1.97) | NS |
| TTP | 22.53 (4.74) | 21.64 (4.06) | 0.042 |
| Left occipital lobe | |||
| rCBF | 14.24 (11.93) | 13.91 (10.82) | NS |
| rCBV | 1.37 (0.83) | 1.40 (1.03) | NS |
| MTT | 6.87 (2.33) | 6.87 (2.19) | NS |
| TTP | 22.65 (4.34) | 21.75 (3.97) | 0.034 |
| Right temporal lobe | |||
| rCBF | 15.49 (11.18) | 14.60 (9.43) | NS |
| rCBV | 1.36 (0.84) | 1.34 (0.92) | NS |
| MTT | 6.32 (1.79) | 6.48 (2.13) | NS |
| TTP | 21.93 (4.56) | 20.82 (4.26) | 0.053b |
| Left temporal lobe | |||
| rCBF | 12.98 (12.29) | 14.57 (9.05) | NS |
| rCBV | 1.33 (1.18) | 1.41 (0.88) | NS |
| MTT | 6.70 (2.22) | 6.55 (1.94) | NS |
| TTP | 21.85 (5.75) | 20.53 (4.40) | 0.006 |
rCBV indicates relative cerebral blood volume; rCBF, relative cerebral blood flow; MTT, mean transit time; and TTP, time to peak. Values were expressed as median (interquartile range).
P values were calculated by the Mann‐Whitney U test.
P < 0.05 and the differences were considered to be significant.
We further divided ischemic stroke patients into two subgroups: VBD subgroup and non‐VBD subgroup to compare the rCBF, rCBV, MTT, TTP between subgroups. As shown in Table 5, TTP in all ROIs were novel delayed in posterior circulation ischemic stroke patients with VBD than those without VBD (lower cerebellum: right P = 0.002; left P = 0.011; pon: right P = 0.018; left P = 0.027, superior cerebellum: right P = 0.010; left P = 0.020; occipital lobes: right P = 0.042; left P = 0.034; temporal lobes: right P = 0.053; left P = 0.006), while, rCBF and rCBV were not significant decreased and MTT were not signal delayed in any ROIs in posterior circulation ischemic stroke patients with VBD (Fig. 2). Non‐posterior circulation ischemic stroke patients were also divided into two subgroups. TTP in bilateral lower cerebellum ROIs was signal delayed in nonposterior circulation ischemic stroke patients with VBD (lower cerebellum: right P = 0.026; left P = 0.003; respectively). Though TTP in the right occipital lobe ROIs were not signal delayed in VBD subgroup (P = 0.297), TTP in bilateral superior cerebellum, pons, left occipital, and temporal lobes ROIs still had its tendency to be delayed (All P < 0.20). While, no significant differences in rCBF, rCBV, MTT were found in any ROIs of posterior circulation territories (Table 5).
Table 5.
Perfusion values between VBD and non‐VBD cohort in posterior circulation ischemic stroke patients or nonposterior circulation ischemic stroke patients
| IS | Non‐IS | |||||
|---|---|---|---|---|---|---|
| VBD (n = 27) | Non‐VBD (n = 85) | P Value | VBD (n = 50) | Non‐VBD (n = 127) | P Value | |
| Right lower cerebellum | ||||||
| rCBF | 20.76 (21.17) | 23.66 (17.15) | 0.981 | 25.41 (18.86) | 27.10 (16.47) | 0.210 |
| rCBV | 2.10 (1.18) | 2.02 (1.71) | 0.681 | 1.94 (1.27) | 2.11 (1.39) | 0.302 |
| MTT | 6.93 (3.58) | 6.13 (2.19) | 0.366 | 6.00 (2.24) | 5.97 (1.89) | 0.328 |
| TTP | 23.78 (4.56) | 21.19 (3.95) | 0.002a | 21.42 (4.47) | 20.22 (3.74) | 0.026a |
| Left lower cerebellum | ||||||
| rCBF | 25.40 (20.52) | 24.51 (21.62) | 0.894 | 25.51 (21.03) | 27.64 (17.55) | 0.238 |
| rCBV | 2.37 (1.35) | 2.07 (2.31) | 0.822 | 2.03 (1.54) | 2.26 (1.52) | 0.263 |
| MTT | 6.55 (2.58) | 6.35 (2.37) | 0.772 | 6.34 (2.04) | 6.08 (2.21) | 0.349 |
| TTP | 23.89 (5.16) | 21.29 (4.44) | 0.011a | 21.32 (4.47) | 20.32 (3.87) | 0.033a |
| Right pon | ||||||
| rCBF | 24.62 (16.95) | 23.04 (18.24) | 0.527 | 25.88 (23.51) | 25.85 (17.98) | 0.637 |
| rCBV | 2.07 (1.58) | 2.09 (1.64) | 0.531 | 2.11 (2.09) | 2.23 (1.59) | 0.752 |
| MTT | 6.95 (2.62) | 6.39 (2.31) | 0.380 | 6.41 (1.53) | 6.07 (1.89) | 0.183 |
| TTP | 23.59 (5.16) | 21.48 (5.05) | 0.018a | 21.20 (5.33) | 20.51 (3.76) | 0.159b |
| Left pon | ||||||
| rCBF | 24.43 (20.03) | 22.62 (17.83) | 0.749 | 24.87 (18.73) | 25.71 (20.89) | 0.238 |
| rCBV | 2.24 (1.86) | 2.01 (1.44) | 0.905 | 2.21 (1.90) | 2.18 (1.53) | 0.541 |
| MTT | 6.80 (4.17) | 6.56 (2.60) | 0.825 | 6.54 (2.19) | 6.03 (1.93) | 0.181 |
| TTP | 23.55 (5.90) | 21.21 (5.06) | 0.027 | 21.56 (4.64) | 20.36 (3.51) | 0.085b |
| Right superior cerebellum▵ | ||||||
| rCBF | 18.03 (16.31) | 20.25 (12.72) | 0.982 | 17.84 (13.85) | 20.38 (13.22) | 0.265 |
| rCBV | 2.01 (1.38) | 2.02 (1.43) | 0.784 | 1.85 (1.23) | 2.03 (1.12) | 0.528 |
| MTT | 8.21 (2.42) | 7.24 (2.50) | 0.508 | 6.87 (1.89) | 6.88 (2.05) | 0.584 |
| TTP | 24.38 (6.31) | 22.05 (4.60) | 0.006a | 22.01 (4.73) | 21.43 (3.99) | 0.135b |
| Left superior cerebellum▵ | ||||||
| rCBF | 18.52 (14.43) | 18.78 (14.60) | 0.610 | 15.98 (11.43) | 18.54 (12.42) | 0.199 |
| rCBV | 2.03 (1.38) | 1.80 (1.33) | 0.436 | 1.65 (0.90) | 1.74 (0.94) | 0.389 |
| MTT | 8.17 (2.87) | 7.02 (2.07) | 0.128 | 6.94 (1.90) | 6.77 (2.01) | 0.841 |
| TTP | 23.91 (6.17) | 21.66 (5.05) | 0.030a | 21.88 (4.16) | 21.08 (3.61) | 0.123b |
| Right occipital lobe▵ | ||||||
| rCBF | 14.48 (10.79) | 14.07 (10.93) | 0.855 | 14.19 (10.52) | 13.04 (8.48) | 0.436 |
| rCBV | 1.45 (0.94) | 1.32 (0.99) | 0.948 | 1.33 (0.83) | 1.29 (0.88) | 0.350 |
| MTT | 7.11 (2.50) | 6.84 (2.51) | 0.918 | 6.88 (1.87) | 6.71 (1.68) | 0.901 |
| TTP | 24.71 (6.03) | 22.28 (3.95) | 0.012a | 21.87 (3.89) | 21.41 (3.56) | 0.297 |
| Left occipital lobe▵ | ||||||
| rCBF | 15.87 (13.66) | 13.44 (12.47) | 0.442 | 14.20 (12.86) | 13.95 (10.33) | 0.937 |
| rCBV | 1.34 (1.01) | 1.45 (1.11) | 0.840 | 1.42 (0.84) | 1.32 (0.88) | 0.641 |
| MTT | 6.96 (2.42) | 7.00 (2.72) | 0.654 | 6.77 (2.25) | 6.81 (2.12) | 0.921 |
| TTP | 24.76 (6.92) | 22.51 (4.16) | 0.024a | 22.29 (4.10) | 21.69 (3.48) | 0.169b |
| Right temporal lobe▵ | ||||||
| rCBF | 17.44 (7.93) | 14.53 (10.00) | 0.173 | 12.81 (11.61) | 14.60 (9.09) | 0.257 |
| rCBV | 1.41 (0.78) | 1.34 (1.03) | 0.676 | 1.28 (0.95) | 1.33 (0.81) | 0.470 |
| MTT | 6.44 (2.07) | 6.58 (3.26) | 0.535 | 6.11 (1.67) | 6.38 (1.71) | 0.550 |
| TTP | 22.94 (4.39) | 21.66 (4.85) | 0.062b | 21.10 (3.40) | 20.39 (3.80) | 0.166b |
| Left temporal lobe▵ | ||||||
| rCBF | 15.88 (11.68) | 14.51 (9.74) | 0.349 | 11.83 (12.16) | 14.62 (8.92) | 0.131 |
| rCBV | 1.45 (1.07) | 1.45 (0.86) | 0.365 | 1.12 (1.22) | 1.35 (0.92) | 0.437 |
| MTT | 6.95 (1.84) | 6.79 (2.31) | 0.553 | 6.36 (2.17) | 6.27 (1.58) | 0.378 |
| TTP | 23.19 (5.27) | 21.59 (4.51) | 0.014a | 21.74 (5.18) | 20.17 (3.86) | 0.067b |
IS indicates ischemic stroke; VBD, vertebrobasilar dolichoectasia; rCBV, relative cerebral blood volume; rCBF, relative cerebral blood flow; MTT, mean transit time; and TTP, time to peak. Values were expressed in median (interquartile range). P values were calculated by the Mann‐Whitney U test.
▵ Perfusion values in bilateral superior cerebellum and occipital and temporal lobes were studied in 259 patients because 30 patients were lack of completed data.
P < 0.05 and the differences were considered to be significant.
P < 0.20 were considered to likely have significant difference.
Figure 2.
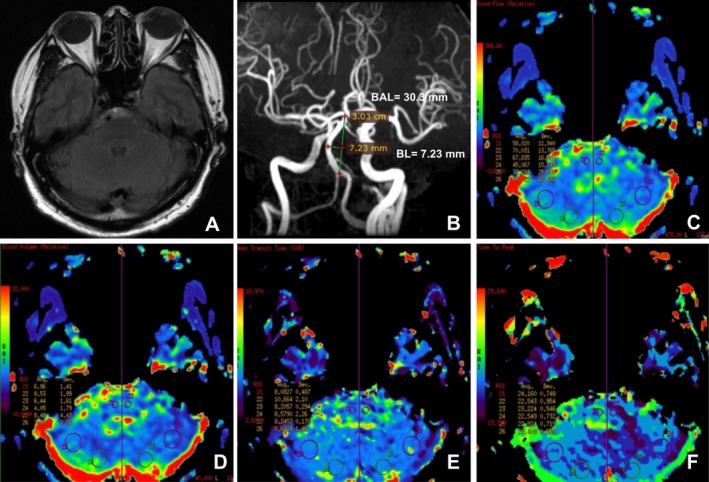
A vertebrobasiar dolichoectasia (VBD) patient without infarction but a relative hypoperfusion in the left cerebellum and the right pon. (A) T2‐FLAIR showed basilar artery deviation midline of pons. (B) MRA showed basilar artery length (BAL) is 30.30 mm and bending length (BL) is 7.23 mm. (C) The relative cerebral blood flow (rCBF) is lower in the left cerebellum ROIs (45.067 mL/100g/min vs. 67.835 mL/100g/min) and the right pon ROIs (58.020 mL/100g/min vs. 70.031 mL/100g/min). (D) Relative cerebral blood volume (rCBV) is lower in the left cerebellum ROIs (4.05 mL/100g vs. 6.44 mL/100 g) and the right pon ROIs (6.96 mL/100g vs. 8.53 mL/100g). (E) Mean transit time (MTT) is 8.0827s in the right pon ROIs and 10.664s in the left pon ROIs. (F) Time to peak (TTP) is 24.160s in the right pon ROIs and 22.543s in the left pon ROIs.
ROC curve analysis
Although rCBF, rCBV, and MTT in any ROIs of posterior circulation territories had no values of predicting the occurrence of posterior circulation ischemic stroke in VBD patients (data have not shown), TTP in the more proximal and the distal portion of posterior circulation ROIs all had their predictive values (Fig. 3).
Figure 3.
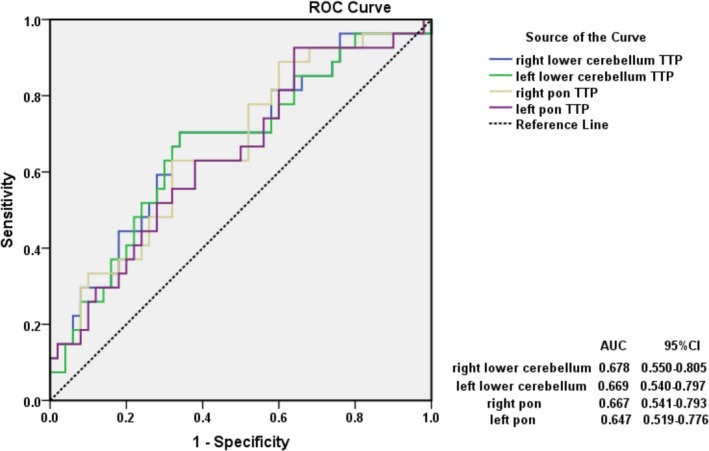
Receiver‐operating characteristic (ROC) curve was constructed to determine the time to peak (TTP) values of predicting the occurrence of posterior circulation ischemic stroke in vertebrobasilar dolichoectasia (VBD) patients. The prediction values of delayed TTP in the proximal portions of the posterior circulation ROIs: right lower cerebellum, AUC = 0.678 (95%CI, 0.550–0.805), Youden index (YI) =0.364; left lower cerebellum, AUC = 0.669 (95% CI, 0.540–0.797), YI = 0.364; right pon, AUC = 0.667 (95%CI, 0.541–0.79), YI = 0.310; left pon, AUC = 0.647 (95%CI, 0.517–0.776), YI = 0.286.
The TTP predictive threshold value in the right lower cerebellum ROIs was 22.69 msec with a sensitivity of 70.4% and a specificity of 66.0%, while in the left lower cerebellum ROIs was slightly shorter (22.37 msec) with the same sensitivity and specificity. In addition, the TTP predictive threshold value in the right pon ROIs was 22.64 msec with sensitivity of 63.0% and specificity of 68.0%, respectively. The TTP predictive threshold value in the left pon ROIs (19.75 msec) was shorter than the right with a higher sensitivity (92.6%), but the specificity was too low (36.0%, Fig. 3).
On the other hand, the TTP predictive threshold value in the right superior cerebellum ROIs was 24.06 msec with a sensitivity of 60% and a specificity of 72.9%, and was 23.66 msec with a sensitivity of 56% and a specificity of 72.9% in the left superior cerebellum ROIs. The TTP predictive threshold value in the occipital lobes were 24.36 msec in the right side (a sensitivity of 56% and a specificity of 77.1%) and 24.71 msec in the left side (a sensitivity of 52% and a specificity of 83.3%), respectively. Furthermore, the TTP predictive threshold value in the right temporal lobes ROIs was 21.96 msec with a sensitivity of 76% and a specificity of 66.7%, and was 21.02 msec with a sensitivity of 80% and a specificity of 54.2% in the left temporal lobes ROIs (Fig. 4).
Figure 4.
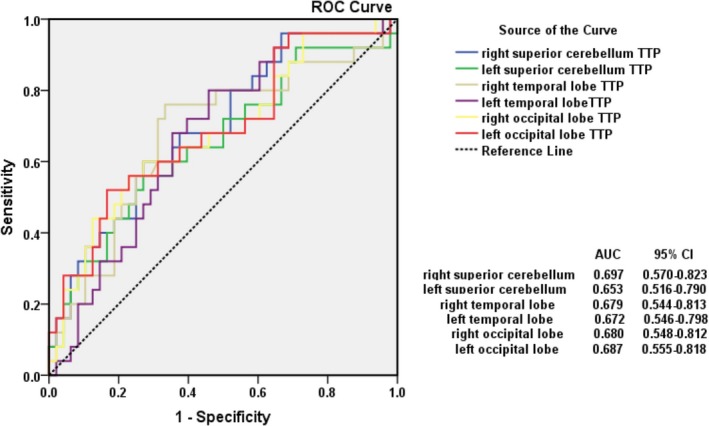
The prediction values of delayed TTP in the distal portions of the posterior circulation right superior cerebellum (ROIs), AUC = 0.697 (95%CI, 0.570–0.823), Youden index (YI) = 0.329; left superior cerebellum, AUC = 0.653 (95%CI, 0.516–0.790), YI = 0.289; right temporal lobe, AUC = 0.679 (95%CI, 0.544–0.813), YI = 0.427; left temporal lobe, AUC = 0.672 (95%CI, 0.546–0.798), YI = 0.342; right occipital lobe, AUC = 0.680 (95%CI, 0.548–0.812), YI = 0.331; left occipital lobe, AUC = 0.687 (95%CI, 0.555–0.818), YI = 0.353.
Comparing the Youden index (YI) in different ROIs (Fig. 3), we found that TTP in the right temporal lobe had a better value of predicting the occurrence of posterior ischemic stroke in VBD patients. Furthermore, we summed up the best TTP values in all ROIs and calculated the mean TTP value and its standard deviation, obtaining that the mean TTP predictive threshold value was 22.67 ± 1.48 msec.
Discussion
VBD is an arteriopathy characterized by dilated, elongated and/or tortuous vertebral artery or basilar artery, which leads to hypoperfusion of posterior circulation territory characterized by delayed TTP. The most interesting finding in this study was that delayed TTP in lower cerebellum, superior cerebellum, pons, and occipital and temporal lobes has value of predicting the occurrence of posterior circulation ischemic stroke in VBD patients. TTP in the right temporal lobe delayed by 21.96 msec is the best predictive value in the current study and the mean TTP predictive threshold value in posterior circulation is 22.67 ± 1.48 msec. If confirmed, DSC‐PWI, in particular TTP maps, could be a candidate early image marker to determine the diagnosis of posterior circulation ischemic stroke in VBD patients with vertigo who should be followed up diffusion‐weighted image of MRI.
Cerebellar strokes were classified into five categories based on etiology, using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification: (1) large‐artery atherosclerosis, (2) cardioembolic stroke, (3) small‐artery occlusion (lacunes), (4) stroke of other determined cause, and (5) stroke of undetermined cause. In the current study, 80 patients were solely involving pons, cerebellums, medulla oblongata, occipital lobes, corpus callosums, temporal lobes, or mesencephalon, of which the etiology were mainly thought to be small‐artery occlusion or large‐artery atherosclerosis. On the other hand, 32 patients were concurrently involving the anterior and posterior circulation which might be caused by cardioembolism or other undetermined etiology.
In the current study, arterial hypertension, diabetes, current smoking, the history of stroke, vertebrobasilar arteries stenosis, and dilated BA were the risk factors of the occurrence of posterior circulation ischemic stroke. Increased BA diameter is the risk factor of future stroke.1, 18 In the GENIC study, Pico et al19 even found that BA diameter was associated with an increased 5‐year stroke mortality rate in patients with stroke and a diameter >4.3 mm might be a marker for a high risk of fatal stroke. However, in the SPS3 trial, Nakajima et al20 found that vertebrobasilar ectasia was not the risk of recurrent stroke, which is contradictory to our findings. Patients’ characteristic, and subtype of stroke may explain the different findings. In addition, we did not found that BAL, BL or TI of the BA were the risk factors of posterior circulation ischemic stroke, suggesting that dilation of BA was associated with perfusion of posterior circulation but tortuosity and elongation were not associated with the perfusion in territories of posterior circulation. A recent review on dolichoectasia demonstrated that dolichoectatic vessels that had large diameters seem to be associated with the greatest risk of stroke, while novel elongation and tortuosity were associated with compressive cranial neuropathies,1 this may support our findings. Though some VBD patients were involved with vertebral artery and some patients were determined to be ischemic stroke located in the areas fed by vertebral artery, we did not find that dilation or tortuosity of vertebral artery was the risk of posterior circulation ischemic stroke. The small proportion of patients determined to be ischemic stroke located in the areas fed by vertebral artery in our study might be the explanation.
Previous studies have shown that morphology changes of vertebrobasilar arteries would change the hemodynamics in vertebrobasilar system.7, 9, 10 A significantly decreased arterial blood flow velocity detected with TCD in VBD patients has been reported in several studies.9 In addition, FLAIR vascular hyperintensities (FVH) has been evaluated to represent the slow arterial blood flow. Föster et al10 did a retrospective study focusing on VBD patients and found the presence of FVH in dilated BA and the diameter of the BA moderately correlated with the extent of FVH. They also found that a higher FVH grade was common in patients with TIA/stroke related to VBD comparing to patients with other etiology and asymptomatic patients.
On the other hand, occurring the VBD, hypoperfusion in the vertebrobasilar system territory may subsequently exhibit posterior circulation ischemic. Undetermined isolated vertigo patients with BA tortuosity has the hypoperfusion in the territories of the BA has been reported in a retrospective study with the utilization of the whole‐brain computed tomography perfusion (CTP).11 Grams et al12 found that PWI included Tmax and CBF parametric maps added diagnostic value by depicting regions with delayed perfusion or post‐ischemic hyperperfusion in transient ischemic attack patients with negative DWI. Choi et al13 demonstrated that patients with acute transient vestibular syndrome (12%) showed unilateral cerebellar hypoperfusion on PWI without an infarction on DWI, and 80% of them had a focal stenosis or hypoplasia of the corresponding vertebral artery. In the current study, we focused on vertigo patients with at least one vascular risk factor and found that VBD was associated with hypoperfusion in posterior circulation which was characterized by delayed TTP. And we deeply observed that the hypoperfusion occurred not only in VBD patients with posterior circulation ischemic stroke but also in those without posterior circulation ischemic stroke. Taken together, we speculated that the morphological changes of vertebrobasilar arteries, especially the BA, leads to the hemodynamics change, consequently the delayed TTP in posterior circulation territories and posterior circulation ischemic occur.
Another interesting finding in the current study was that delayed TTP had value of predicting the occurrence of posterior circulation ischemic stroke in VBD patients. TTP in the right temporal lobe delayed by 21.96 ms (a sensitivity of 76% and a specificity of 66.7%) was the best predictive value in the current study. We hypothesized that VBD patients with TTP delayed by the threshold values may be at a higher risk of posterior circulation ischemic. A meta‐analysis demonstrated that 6.8% patients with acute ischemic stroke had a negative DWI scan and patients with posterior circulation ischemia had five times the odds of having a negative DWI.21 It has previously been shown that the glial‐specific S100 calcium‐binding protein β (S100β) provides a useful serum marker of stroke.22, 23 That seems to be a good candidate to detect stroke for patients with negative DWI imaging. However, Purrucker et al22 focused on patients presenting with acute vertigo and found that S100β had a high sensitivity of 94.4% for detecting stroke in patients with vertigo but the specificity was low (31.8%). The Youden index was 0.262 which was not only lower than that of TTP in the right temporal lobe but in other ROIs in our study, demonstrating that delayed TTP might be a better predictive factor for detecting posterior circulation ischemic stroke, especially in VBD patients. Therefore, we suggested that delayed TTP might be a better candidate early image marker to detect stroke for patients with negative DWI imaging. However, delayed TTP in the right temporal ROIs in the current study is not capable of revealing the hypoperfusion in other areas of posterior circulation‐ cerebellum, pons, and occipital lobes. We calculated the mean TTP predictive threshold value in all ROIs of posterior circulation, to some extent, might be credible to reveal the hypoperfusion in posterior circulation.
This study has several limitations. First, it is a prospective, cross‐sectional study in a single center and asymptomatic VBD patients were excluded in the current study, thus our data were limited and selection bias might be inevitable. Second, because it is difficult to evaluate the perfusion in other territories of posterior circulation, thus we chose to evaluate the perfusion in lower and superior cerebellum, pons, and occipital and temporal lobes, which might be entitled to represent the perfusion of posterior circulation. Another limitation of this study was that, PWI utilized for evaluating the perfusion of the brain, in particularly the posterior circulation has its limitation, and therefore, the TTP predictive threshold value we obtained in the current study is a relative value. Future studies will go after expanding sample size as well as conducting regular patient follow‐up.
In conclusion, VBD might lead to the hypoperfusion in posterior circulation territory characterized by delayed TTP. Delayed TTP has significant value of predicting the occurrence of posterior circulation ischemic stroke in VBD patients with vertigo. TTP in the right temporal lobe delayed by 21.96 msec is the best predictive value and the mean TTP predictive threshold value is 22.67 ± 1.48 msec. Clinically, to evaluate the high risk of posterior circulation ischemic stroke, it is necessary for VBD patients, especially those without infarction or those with a negative DWI imaging, to conduct PWI examination.
Conflicts of Interest
None declared.
Funding Information
This study was supported by the National Natural Science Foundation of China (81471203).
Funding Statement
This work was funded by the National Natural Science Foundation of China grant 81471203.
References
- 1. Gutierrez J, Sacco RL, Wright CB. Dolichoectasia‐an evolving arterial disease. Nat Rev Neurol 2011;7:41–50. [DOI] [PubMed] [Google Scholar]
- 2. Lou M, Caplan LR. Vertebrobasiar dilatative arteriopathy (dolichoectasia). Ann N Y Acad Sci 2010;1184:121–133. [DOI] [PubMed] [Google Scholar]
- 3. Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology 2008;70:66–72. [DOI] [PubMed] [Google Scholar]
- 4. Pico F, Labreuche J, Amarenco P. Pathophysiology, presentation, prognosis, and management of intracranial arterial dolichoectasia. Lancet Neurol 2015;14:833–845. [DOI] [PubMed] [Google Scholar]
- 5. Del Brutto VJ, Ortiz JG, Biller J. Intracranial arterial dolichoectasia.Front Neurol 2017;17(8):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samim M, Goldstein A, Schindler J, Johnson MH. Multimodality imaging of vertebrobasilar dolichoectasia: clinical presentations and imaging spectrum. Radiographics 2016;36:1129–1146. [DOI] [PubMed] [Google Scholar]
- 7. Hong JM, Chung CS, Bang OY, et al. Vertebral artery dominance contributes to basilar artery curvature and peri‐vertebrobasilar junctional infarcts. J Neurol Neurosurg Psychiatry 2009;80:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by maganetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry 2004;75:22–26. [PMC free article] [PubMed] [Google Scholar]
- 9. Kumral E, Kisabay A, Atac C, et al. The mechanism of ischemic stroke in patients with dolichoectatic basilar artery. Eur J Neurol 2005;12:437–444. [DOI] [PubMed] [Google Scholar]
- 10. Föster A, Kerl HU, Wenz H, et al. Fluid attenuated inversion recovery vascular hyperintensities possibly indicate slow arterial blood flow in vertebrobasilar dolichoectasia. J Neuroimaging 2015;25:608–613. [DOI] [PubMed] [Google Scholar]
- 11. Li W, Feng Y, Lu W, et al. Evaluating the morphological changes of intracranial arteries and whole‐brain perfusion in undetermined isolated vertigo. J Neurol Sci 2016;370:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grams RW, Kidwell CS, Doshi AH, et al. Tissue‐negative transient ischemic attack: is there a role for perfusion MRI? AJR Am J Roentgenol 2016;207:157–162. [DOI] [PubMed] [Google Scholar]
- 13. Choi JH, Park MG, Choi SY, et al. Acute transient vestibular syndrome: prevalence of stroke and efficacy of bedside evaluation. Stroke 2017;48:556–562. [DOI] [PubMed] [Google Scholar]
- 14. Nishikata M, Hirashima Y, Tomita T, et al. Measurement of basilar artery bending and elongation by magnetic resonance cerebral angiography: relationship to age, sex and vertebral artery dominance. Arch Gerontol Geriatr 2004;38:251–259. [DOI] [PubMed] [Google Scholar]
- 15. Morris SA, Orbach DB, Geva T, et al. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 2011;124:388–396. [DOI] [PubMed] [Google Scholar]
- 16. Smoker WR, Price MJ, Keyes WD, et al. High‐resolution computed tomography of the basilar artery: 1. Normal size and position. AJNR Am J Neuroradiol 1986;7:55–60. [PMC free article] [PubMed] [Google Scholar]
- 17. Tatu L, Moulin T, Vuillier F, Bogousslavsky J. Arterial territories of the human brain. Front Neurol Neurosci 2012;30:99–110. [DOI] [PubMed] [Google Scholar]
- 18. Gutierrez J, Cheung K, Bagci A, et al. Brain arterial diameters as a risk factor for vascular events. J Am Heart Assoc 2015;4:e002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pico F, Labreuche J, Gourfinkel‐An I, Amarenco P. GENIC Investigators. Basilar artery diameter and 5‐year mortality in patients with stroke. Stroke 2006;37:2342–2347. [DOI] [PubMed] [Google Scholar]
- 20. Nakajima M, Pearce LA, Ohara N, et al. Vertebrobasilar ectasia in patients with lacunar stroke: the SPS3 trial. J Stroke Cerebrovasc Dis 2015;24:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI‐negative acute ischemic stroke. Neurology 2017;89:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Purruckera JC, Herrmanna O, Lutsch JK, et al. Serum protein S100β is a diagnostic biomarker for distinguishing posterior circulation stroke from vertigo of nonvascular causes. Eur Neurol 2014;72:278–284. [DOI] [PubMed] [Google Scholar]
- 23. Kartal AG, Yılmaz S, Yaka E, et al. Diagnostic value of S100β protein in the differential diagnosis of acute vertigo in the emergency department. Acad Emerg Med 2014;21:736–741. [DOI] [PubMed] [Google Scholar]


