Abstract
Cancer immunotherapy using the adoptive transfer of autologous tumor-infiltrating lymphocytes results in objective cancer regression in 49–72% of patients with metastatic melanoma. In a pilot trial combining cell transfer with a maximum lymphodepleting regimen, complete durable responses were seen in 40% of patients, with complete responses ongoing beyond 3 to 7 years. Current approaches to cell transfer therapy using autologous cells genetically engineered to express conventional or chimeric T-cell receptors have mediated cancer regression in patients with metastatic melanoma, synovial sarcoma, neuroblastoma and refractory lymphoma. Adoptive cell transfer immunotherapy is a rapidly developing new approach to the therapy of metastatic cancer in humans. This Review will emphasize the current available applications of cell transfer immunotherapy for patients with cancer.
Introduction
Attempts to develop effective immunotherapies for the treatment of patients with metastatic cancer fall into three major categories: nonspecific stimulation of the immune system, active immunization using cancer vaccines, and adoptive cell transfer immunotherapy. Nonspecific approaches include the upregulation of immune reactivity either by general immune stimulation (such as the administration of interleukin [IL]-2) or the blockade of inhibitory influences (such as the use of an anti-CTLA4 antibody).1 These approaches can mediate tumor regression in about 10–15% of patients with metastatic melanoma and renal cell carcinoma,2 these tumors seem to be responsive because of their ability to naturally give rise to high levels of antitumor T cells that can be stimulated by these immune modulators. Active immunization with cancer vaccines is an attractive therapy approach because of its ease of administration and lack of toxi city; however, no approach has yet been developed that can reproducibly mediate the regression of metastatic cancers at clinically meaningful levels. Adoptive cell transfer immunotherapy is the most effective form of immunotherapy and involves the transfer of immune cells with antitumor activity into cancer patients. It has been shown to mediate the objective regression of metastatic melanoma in up to 72% of patients, including the induction of up to 40% of complete durable responses.3 Recent developments involving the introduction of genes encoding antitumor T-cell receptors (TCRs) into lymphocytes have provided immune cells capable of mediating cancer regressions in patients with several different cancer types and this approach is under vigorous development at a variety of academic centers. In this Review, I present a brief history of the development of immunotherapy for patients with metastatic cancer and describe the current state of development of adoptive cell transfer immunotherapy.
A very brief history of cancer immunotherapy
Following the first quantitative descriptions of antibodies in the 1880s, studies of the humoral arm of the immune system dominated immunology until the 1960s. The importance of the cellular arm of the immune system was not known and the word ‘lymphocyte’ was not listed in the index of the 1958 issue of the Journal of Immunology. Two advances in the 1940s were harbingers of the future development of cellular and cancer immunology. In 1942, Landsteiner and Chase demonstrated that delayed hyper sensitivity could be transferred between mice using immune cells obtained from sensitized donors,4 and a year later Gross showed that syngeneic mice immunized against tumors in the same inbred strain could reject a subsequent tumor challenge.5 This work, however, received little attention until the 1960s when many studies demonstrated the importance of cellular immunology as a mediator of allograft rejection as well as protection against the transfer of mouse tumors. Studies were hindered by the inability to manipulate lymphocytes and sustain their survival outside the body. The identification of a T-cell growth factor (IL-2) in 19766 provided, for the first time, a means to grow T lymphocytes in vitro, although the tiny amounts of IL-2 available from in vitro cell lines severely limited its application to cancer immunotherapy. The identification of the DNA sequence of the gene encoding IL-2 in 1983,7 and the expression of this gene in Escherichia coli and biological characterization of recombinant IL-2 a year later8 provided new opportunities for therapy in humans.
The demonstration in 1985 that the administration of IL-2 to patients could mediate the regression of large, established, invasive human cancers9 represented the first demonstration that manipulation of the human immune system could reproducibly lead to tumor regression. These data ultimately led to the FDA approval of IL-2 for the treatment of patients with metastatic renal cell carcinoma in 1992 and for metastatic melanoma in 1998. Durable complete responses of metastatic disease in 5% to 10% of patients have been observed more than 20 years after IL-2 administration.10 Other nonspecific immunotherapy approaches, such as the administration of the monoclonal antibody ipilimumab that can block inhibitory influences (for example CTLA-4 engagement on lymphocytes), have also been shown to lead to tumor regression in patients with melanoma.11
The ability of IL-2 to support the growth of human lymphocytes in the laboratory with maintenance of their immunologic activity led to the molecular characterization of the first human cancer antigen in 1991.12 In the next two decades, hundreds of antigens and antigenic epitopes expressed on cancer cells recognized by the immune system were described,13,14 which led to a myriad of clinical trials assessing immunization with peptides, proteins, dendritic cells, recombinant viruses, whole cells, and plasmid DNA. With very few exceptions these trials have failed to demonstrate a clinical benefit. Recently, a dendritic cell vaccine was reported to prolong the survival of patients with prostate cancer by about 4 months, though there were no tumor regressions or prolongation of progression-free survival in treated patients.15 There are many difficulties in vaccine approaches, including the inability to generate large numbers of antitumor cells with high affinity for tumor antigen, as well as an immunosuppressive micro environment at the tumor site that can suppress potent effector mechanisms.16 Many of these difficulties have been overcome by the use of adoptive cell transfer of antitumor immune cells—this treatment approach, referred to as adoptive cell therapy (ACT), provides the best direct evidence that the immune system is capable of curing patients with metastatic cancer.17–19 Although most studies of adoptive immunotherapy have dealt with the treatment of patients with metastatic melanoma, there are now examples of the successful application of this treatment to patients with other types of malignancies.20–25
ACT with tumor-infiltrating lymphocytes
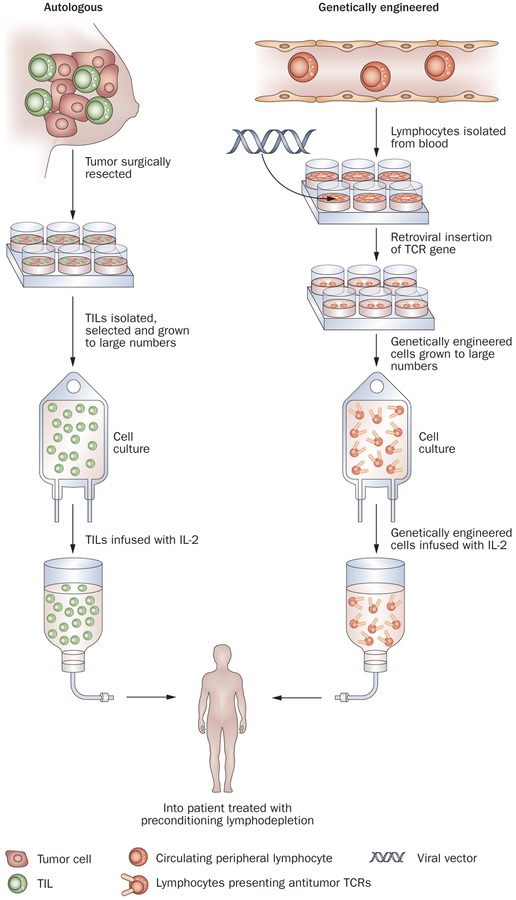
ACT is a treatment approach that involves the identification, in vitro, of autologous lymphocytes with anti-tumor activity, the in vitro expansion of these cells to large numbers and their infusion into the cancer-bearing host.1 Lymphocytes used for adoptive transfer can either be derived from the stroma of resected tumors or genetically engineered to express antitumor TCRs (Figure 1). ACT has several theoretical advantages compared with other forms of immunotherapy. Large numbers of cells (often up to 1011 cells) with antitumor activity can be grown in vitro and cells that exhibit high recognition of tumor antigens can be selected for infusion using in vitro assays. The cells can be activated ex vivo to exhibit anti-tumor effector functions and thus overcome the suppressive influences that exist in vivo that can limit the antitumor activity of T cells. Most importantly, the host can be manipulated before the cell transfer to provide an altered microenvironment for the transferred cells; multiple suppressive mechanisms, such as T-regulatory lymphocytes or myeloid-derived suppressor cells can significantly interfere with the antitumor activity of lymphocytes and the ability to eliminate these suppressor cells before the administration of antitumor effector cells represents a unique advantage of ACT.
Figure 1 |.
Adoptive cell transfer immunotherapy using either autologous TILs obtained from resected tumors or using peripheral lymphocytes genetically transduced with retroviruses to express antitumor T-cell receptors. Cells are expanded in vitro to large numbers (up to 1011) and infused after patients have received a preparative lymphodepleting regimen. Abbreviations: TCR, T-cell receptor; TIL, tumor-infiltrating lymphocyte.
Studies in animal models and patients with melanoma demonstrated that lymphocytes infiltrating into growing tumors can often exhibit in vitro evidence of antitumor activity when removed from the tumor and grown in vitro.26–28 In patients with melanoma, these tumor-infiltrating lymphocytes (TILs) can directly lyse tumor cells and secrete cytokines such as γ-interferon, IL-2 and tumor necrosis factor when encountering tumor anti-gens.27,28 In the initial studies of the administration of these TILs to patients with metastatic melanoma, transient tumor reductions were seen; however, the inability of these cells to persist in vivo following adoptive transfer severely limited their antitumor activity.28,29 An important modification of this approach demonstrated that the administration of a non-myeloablative lymphodepleting preparative regimen consisting of cyclophosphamide and fludarabine before the adoptive transfer of anti-tumor effector cells could lead to dramatic increases in the persistence of the transferred cells in vivo and a dramatic increase in their antitumor activity.17,18 This regimen depleted circulating lymphocytes for about 8 days before host hematopoietic cells recovered. TILs with antitumor activity, administered at a time of maximum lymphodepletion, could be found circulating in patients many months after adoptive transfer, and in some patients comprised 75% of all circulating CD8 cells.17 The cells expanded almost a thousandfold in vivo as they circulated, infiltrated into organs and became activated by tumor deposits throughout the body. Early studies showed that almost 50% of 43 heavily pretreated patients with metastatic melanoma experienced objective tumor regressions following cell transfer18 and, in updated results,3 13% experienced durable, complete regressions ongoing beyond 5 years and are likely cured (Table 1; S. A. Rosenberg, unpublished data).
Table 1 |.
Efficacy of adoptive cell transfer therapy
| Treatment | n | PR, n (duration, months) | CR, n (duration, months)* | OR‡ |
|---|---|---|---|---|
| No TBI | 43 | 16 (84, 36, 29, 28, 14, 12, 11, 7, 7, 7, 7, 4, 4, 2, 2, 2) | 5 (>86, >84, >83, >82, >69) | 49% |
| 2 Gy TBI | 25 | 8 (14, 9, 6, 6, 5, 4, 3, 3) | 5 (>73, >70, >65, >62, >59) | 52% |
| 12 Gy TBI | 25 | 8 (21, 13,7,6,6,5,3,2) | 10 (>53, >50, >49, >49, >44, >43, >43, >43,>42, 19) | 72% |
20 CRs: 19 ongoing at 42–86 months.
52 responding patients: 42 had prior IL-2 therapy, 22 had prior IL-2 and chemotherapy. Abbreviations: CR, complete response; IL, interleukin; OR, overall response; PR, partial response; TBI, total body irradiation.
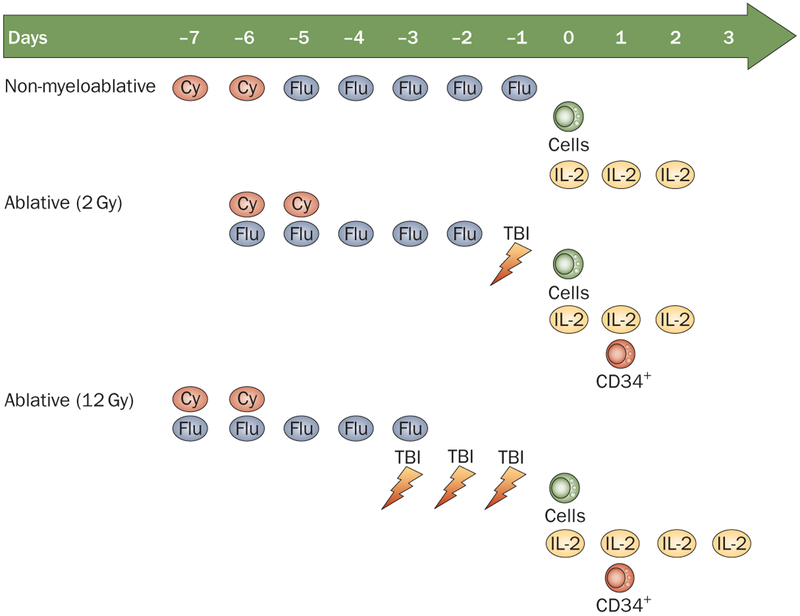
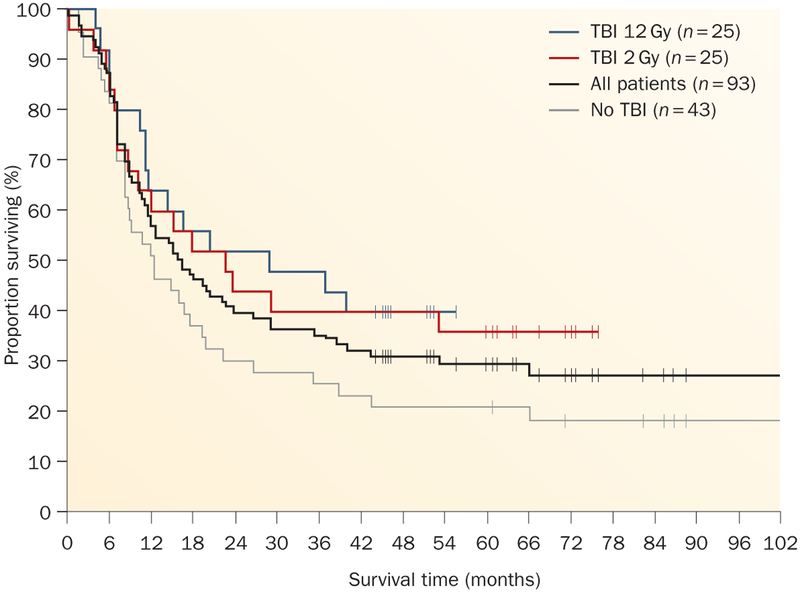
At the same time the early clinical studies were being conducted, animal experiments demonstrated that there was a direct correlation between the intensity of the lymphodepletion and the antitumor effects of the transferred cells.30 Thus, two pilot trials were conducted in 25 patients each in which either 2 Gy or 12 Gy total body irradiation was added to the cyclophosphamide–fludarabine lymphodepleting preparative regimen (Figure 2).30 These treatments resulted in the highest levels of objective and complete responses ever seen in the treatment of patients with metastatic melanoma3 (Table 1 and Figure 3; Rosenberg, S.A. unpublished data). Objective response rates using RECIST criteria were seen in 49% to 72% of all patients. The complete response rate in the last trial (12 Gy total body irradiation) was 40%. Of the 93 patients that took part in these trials, 20 patients experienced a complete regression of all metastatic cancer and 19 patients have ongoing complete regressions from 3–7 years and might be cured. The 5-year overall survival rate was 29% (Figure 3; S. A. Rosenberg, unpublished data).
Figure 2 |.
Preparative regimens for cell transfer. A comparison of the lymphodepleting methods used: non-myeloablative and TBI (using 2 Gy and 12 Gy).3 Abbreviations: Cy, cyclophosphamide; Flu, fludarabine; IL, interleukin; TBI, total body irradiation.
Figure 3 |.
Survival of patients with metastatic melanoma treated with autologous tumor-infiltrating lymphocytes. Patients were treated in three sequential trials using a cyclophosphamide–fludarabine lymphodepleting regimen either alone (no TBI) or plus 2 Gy or 12 Gy TBI (Figure 2). Median follow up of 69 months. Abbreviation: TBI, total body irradiation.
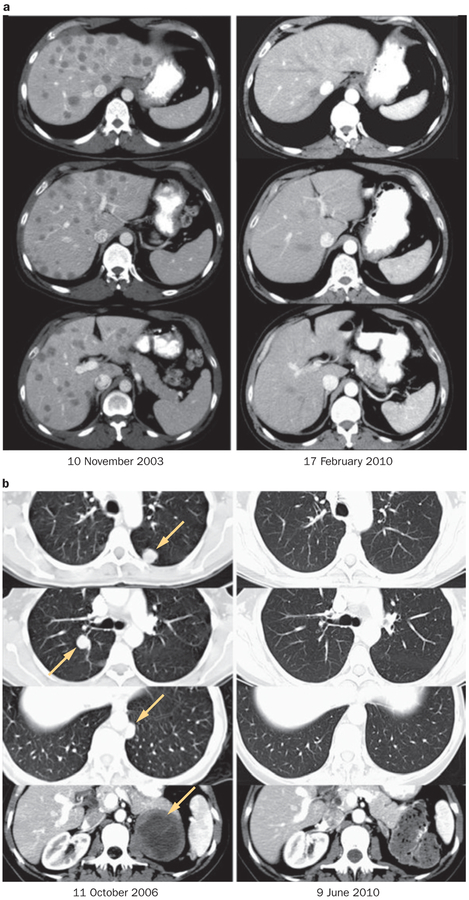
Of particular importance was the ability of ACT to mediate durable complete regressions in heavily pretreated patients with extensive tumor burdens (Figure 4). A large proportion (86%) of the 93 patients in these trials had visceral disease (stage M1b or M1c) as did 17 of the 20 complete responders.3 All but five of the 93 patients and all but two of the complete responders had progressive disease following systemic treatment before receiving ACT. In addition, 83% of the patients were refractory to prior treatment with IL-2 and 40% had received both IL-2 and chemotherapy. The same complete regression rate was seen regardless of whether patients had received IL-2, chemotherapy, γ-interferon or anti-CTLA-4 monoclonal antibody either alone or in combination.3
Figure 4 |.
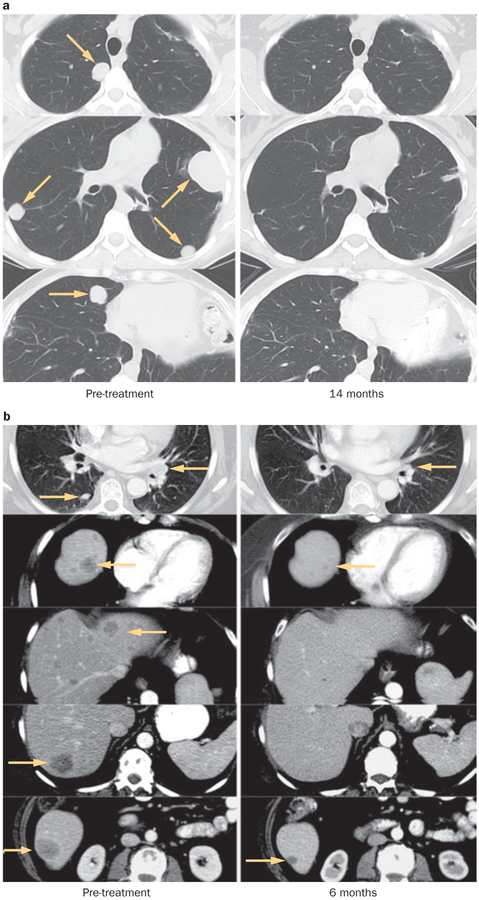
Examples of complete durable responses in patients receiving adoptive cell therapy.3 a | Regression of multiple liver metastases (patient received non-myeloablative lymphodepletion; Figure 2). b | Regression of multiple lung metastases and an adrenal metastasis (patient received total body irradiation 12 Gy lymphodepletion; Figure 2). Permission obtained from the American Association for Cancer Research © Rosenberg, S. A. et al. Clin. Cancer Res. 17, 4550–4557 (2011).
A comparison of ACT with available treatments for patients with metastatic melanoma is shown in Table 2. Only two treatments are approved by the FDA for patients with metastatic melanoma: dacarbazine chemotherapy and IL-2. Several experimental treatments have been reported, including the use of ipilimumab (an anti-CTLA-4 antibody),11 and the administration of a BRAF inhibitor, the latter suitable for the approximately half of patients that have mutated BRAF.31 Complete response rates for these treatments vary between approximately 1% and 6%, compared with the 21.5% complete response rate seen with ACT. The ability to cure patients with metastatic cancer is dependent on the mediation of durable complete responses, and the capacity of ACT to do so has resulted in impressive long-term survival of patients receiving adoptive immunotherapy.
Table 2 |.
Objective responses in patients with metastatic melanoma
| Treatment | n | CR | PR | OR |
|---|---|---|---|---|
| Dacarbazine60 | 149 | 4 (2.7%) | 14 (9.4%) | 18 (12.1%) |
| lnterleukin-261 | 270 | 17 (6.3%) | 26 (9.6%) | 43 (17.9%) |
| Ipilimumab11 | 540 | 3 (0.6%) | 35 (6.4%) | 38 (7.0%) |
| Vemurafenib31 | 219 | 2 (0.9%) | 104 (47.5%) | 106 (48.4%) |
| Adoptive cell transfer3 | 93 | 20 (21.5%) | 32 (34.4%) | 52 (55.9%) |
Abbreviations: CR, complete response; OR, overall response; PR, partial response.
Studies in murine models and humans have defined the mechanisms of action of the lymphodepleting chemo therapy before cell transfer.32,33 Lymphodepletion has a variety of positive impacts including the elimination of T-regulatory cells or myeloid-derived suppressor cells and the elimination of endogenous lymphocytes that provide a sink for growth promoting cytokines such as IL-7 and IL-15. Although IL-15 is not normally detected in the sera of patients, high levels of IL-15 appear in the blood following lymphodepletion and provide a homeostatic growth stimulus to the adoptively transferred lymphocytes.18 The lymphodepleting regimen can also lead to activation of antigen-presenting cells, in part by increased susceptibility to toll-like receptor stimulation.34
Multiple studies have identified the characteristics of cells that are likely to mediate tumor regression. In concert with studies in animal models,33 the administration of cells with a high proliferative potential—such as cells with longer telomeres,35 or cells that express markers indicative of less differentiation (such as CD27 and CD28)—is associated with higher levels of clinical response following ACT.36 The persistence of the cells in the circulation 1 month after transfer37 and a decrease in the reappearance of CD4+Foxp3+ T-regulatory cells in the circulation as hematopoietic reconstitution occurs also correlate with response (X. Yao et al. unpublished data).
ACT using autologous TILs is not suitable for all patients with metastatic melanoma. Patients must be able to tolerate the lymphodepleting chemotherapy and must remain suitable for treatment during the 4–6 weeks required to grow the cells in vitro. A simplified method for generating TILs for therapy using shorter culture times has recently been described,38 and early studies of the infusion of these ‘younger’ TILs have reported tumor regressions.39,40 In addition to other requirements, patients must have tumor deposits that can be resected for growth of TILs and although most patients with metastatic melanoma have resectable lesions, for those patients that do not have resectable lesions this requirement can be overcome by genetically engineering circulating lymphocytes to exhibit antitumor activity.
ACT with genetically modified lymphocytes
The success of ACT using autologous TILs in patients with metastatic melanoma suggested that this approach could be effective for the treatment of patients with other cancer types. Melanoma, however, is unique among cancers in that it naturally gives rise to high levels of antitumor T cells infiltrating into tumors as evidenced in part by the objective responses seen to nonspecific immune modulators such as IL-2 and ipilimumab.9–11 Only rare cells with antitumor activity can be identified from patients with other types of cancer. The ability to transduce genes into lymphocytes with 80–90% efficiency using gamma retroviruses or lentiviruses provides an opportunity to genetically engineer lymphocytes with genes encoding TCRs that recognize tumor antigens or with genes encoding molecules that increase their antitumor activity. A variety of genetic alterations of lymphocytes for possible use in cell transfer are shown in Table 3. Genes encoding cytokines such as IL-2 or IL-15 can enable antitumor T cells to generate their own growth-promoting cytokines,41,42 lessen the need for the systemic administration of these substances and sustain cell survival following cytokine withdrawal. The survival and function of antitumor T cells used for ACT can be improved by the introduction of co-stimulatory molecules such as CD80, co-receptor molecules such as CD8 or molecules such as BCL2 to inhibit apoptosis, molecules such as CD62L or CCR7 that facilitate the traffic of cells to appropriate in vivo locations,43 and the introduction of telomerase that prevents telomere shortening and thus enhances the proliferative potential of the transferred cells.35
Table 3 |.
Potential gene alterations to improve the efficacy of cell transfer therapy
| Genetic alterations | Target |
|---|---|
| Expand tumor recognition | T-cell receptors or chimeric T-cell receptors that recognize cancer antigens |
| Cytokines | IL-2, IL-12, IL-15, IL-17, IL-21, IL-23 |
| Co-stimulatory molecules | CD8, CD27, CD80, 41BBL, OX40L |
| Antiapoptotic molecules | BCL2, BCL2L1, FLIP, TIPE-2 |
| Reverse inhibitory influences | Knock out: SHP-1, PD-1, CTLA4, SOCS, CIS; dominant negative: TGF-β, CBLB |
| Trafficking molecules | CD62L, CCR7, CXCR2, CXCR4 |
| Improve cell survival | Telomerase; knock out: TP53 |
The introduction of genes encoding receptors that recognize cancer antigens can be used to convert normal circulating peripheral lymphocytes into cells with anti-tumor activity. This function provides the reproducible ability to generate cells with antitumor activity against cancers other than melanoma and widens the application of ACT to patients with frequently occurring cancers. Using this gene therapy approach it is only necessary to identify very few cells with antitumor activity that can then serve as the basis for the isolation of the genes encoding these antitumor receptors. Once these genes are incorporated into transducing vectors they can be used to generate cells for the treatment of large numbers of patients.
Conventional TCRs are composed of alpha and beta chains that form a heterodimer that recognizes peptides derived from intracellular cancer-associated antigens and are presented on the surface of MHC molecules on tumor cells. Genes encoding TCRs that recognize a wide variety of cancer antigens have now been identified including the recognition of melanoma–melanocyte antigens and differentiation antigens and a variety of cancer–testes antigens expressed on common epithelial cancers.44,45 The affinity of these transduced TCRs can be further amplified by modifying individual amino acids in their antigen combining regions.46 Other genetic modifications of the TCR, such as the introduction of cysteines to form interchain disulfide bonds or the introduction of murine constant regions, can prevent mispairing of introduced alpha and beta chains of the modified TCR with chains from the endogenous TCR.47,48
The choice of target antigen is critical to the success of ACT. The first example of the successful treatment of patients with genetically modified lymphocytes involved the introduction of genes encoding TCRs that recognized the MART1 and gp100 melanoma–melanocyte differentiation antigens.49 Up to 30% of patients receiving cells transduced with these TCRs exhibited objective cancer regressions. When targeting melanoma–melanocyte antigens the transferred T cells also target normal melanocytes in the eye and in the ear that can result in visual or auditory dysfunction.50 The local application of steroids abrogated these side effects without interfering with the systemic tumor regression mediated by these transferred T cells.
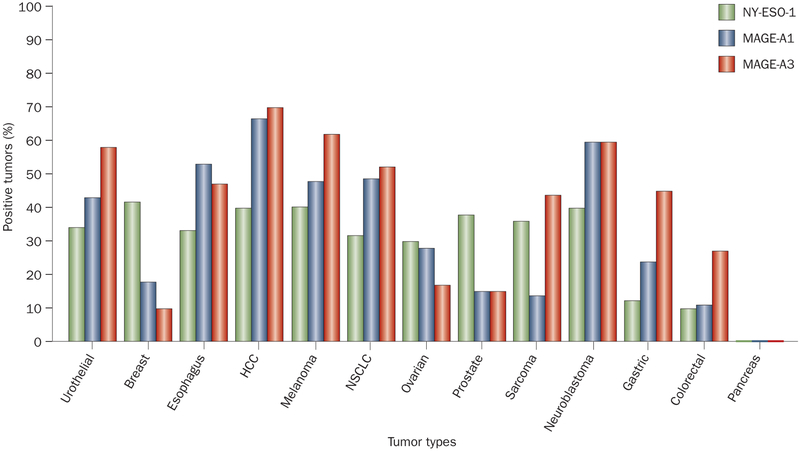
Cancer–testes antigens are expressed during fetal development and represent ideal targets for ACT because they are re-expressed in cells from common epithelial cancers but are not expressed in any adult tissue except the testes (which does not express MHC antigens and is thus protected from immune attack). Dozens of cancer–testes antigens have been described encompassing most tumor types (Figure 5).51,52 ACT using gene-modified cells to target the NY-ESO-1 cancer–testes antigen resulted in objective cancer regressions in five of seven patients with heavily pretreated synovial cell sarcomas, a tumor type that expresses high levels of the NY-ESO-1 cancer–testes antigen.20 Objective cancer regressions were observed in five of 12 patients with heavily pretreated metastatic melanomas after treatment with autologous NY-ESO-1 TCR gene-engineered T cells.20 Examples of responses of patients with synovial cell sarcomas are shown in Figure 6. TCRs against the MAGE-A3 cancer–testes antigen are entering clinical trials.51 ACT using gene-modified cells that recognize cancer–testes anti-gens could potentially be suitable for the treatment of 20–30% of patients with common epithelial cancers and these studies are actively being pursued.
Figure 5 |.
Expression of three cancer–testes antigens (NY-ESO-1, MAGE-A1, MAGE-A3) in a variety of cancer types. Abbreviations: HCC, hepatocellular carcinoma; NSCLC, non-small-cell lung cancer.
Figure 6 |.
Examples of cancer regression in patients with synovial cell sarcoma treated with autologous T cells transduced with the gene encoding an anti-NYESO-1 T-cell receptor. a | Regression of multiple lung metastases. B | Regression of multiple lung and liver metastases. Permission for part a obtained from the American Society of Clinical Oncology © Robbins, P. F. et al. J. Clin. Oncol. 29, 917–924 (2011).
The use of ACT for cancer treatment is limited mainly by the ability to generate TCRs that specifically recognize cancer-associated antigens. To broaden the application of this approach, techniques have been developed to generate TCRs reactive with cancer antigens by immunizing HLA transgenic mice with tumor-antigen sequences that are different from sequences found in the mouse. Thus, the problems of tolerance of humans to these antigens can be overcome and high affinity TCRs can be identified. This approach has been successfully used to generate high-affinity TCRs against the gp100 melanoma–melanocyte antigen used to treat patients with metastatic melanoma,50 and TCRs that recognize carcinoembyronic antigen overexpressed on tumors from patients with colorectal and other cancers.22
Chimeric antigen receptors
Conventional TCRs are restricted to the recognition of antigens presented on specific MHC molecules. A technique for the development of antitumor T cells based on the recognition of antibodies (called chimeric antigen receptors [CAR]) was developed by Eshhar and co-workers.53 In this approach, the antigen-combining regions of the heavy and light chains of antibodies are genetically linked and attached to T-cell intracellular signaling molecules. When these genetic constructs are transduced into lymphocytes, the lymphocyte gains the ability to recognize antigens based on the recognition of the antibody rather than that of a conventional TCR. Cells expressing these CAR have significantly widened the potential for the application of adoptive immunotherapy. T cells expressing CAR targeting the GD2 antigen have been used to mediate tumor regression in patients with neuroblastoma.23 Carbonic anhydrase-8 is overexpressed on renal cancers and the use of CAR targeting this antigen was reported, although toxicity to the hepatobiliary tree was observed that limited its use.54 The most successful application of this approach to date has been the use of CARs that target the CD19 B-cell antigen highly expressed in over 80% of patients with non-Hodgkin lymphomas and chronic lymphocytic leukemia. Successful regression of bulky lymphoma deposits in heavily pretreated patients refractory to standard treatment has been achieved in four of six patients receiving ACT using CAR targeting the CD19 gene (J. Kochenderfer, unpublished observations).21 Recently, CAR targeting VEGFR2, which is overexpressed in the tumor vasculature, entered clinical testing.55
There are potential dangers associated with the use of ACT that are based on the appropriate selection of the target antigen; the potency of genetically modified T cells to kill target cells as well as secrete large amounts of cytokine.56,57 Although concern has been expressed about the induction of graft-versus-host disease in mouse models when the introduced TCR chains recombine with endogenous TCR chains,58 no evidence of graft-versus-host disease has been seen in over 100 patients treated with this gene therapy approach.59
Conclusions
ACT using autologous TILs represents the most-effective approach for the curative treatment of patients with metastatic melanoma. The need to grow a patient’s own cells for therapy represents the ultimate in ‘personalized’ medicine since a new ‘drug’ is created for each patient, a treatment paradigm that does not fit into the ‘off-the-shelf’ requirement of pharmaceutical and bio-technology companies. Currently, only a few academic cancer centers offer ACT for the treatment of patients with metastatic melanoma though recent simplifications of the methods for cell growth could facilitate the production of treatment-quality cells by academic blood banks or individual academic laboratories. The success of ACT using TILs for the treatment of melanoma and gene-modified cells for the treatment of many refractory cancer types is providing a stimulus for the more widespread application of this treatment approach.
Medscape education Continuing Medical Education online.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Nature Publishing Group. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/nrclinonc; (4) view/print certificate.
Released: 2 August 2011; Expires: 2 August 2012
Learning objectives
Upon completion of this activity, participants should be able to:
Describe the practice of cell transfer immunotherapy
Compare cell transfer immunotherapy with other treatments for metastatic melanoma
Evaluate the role of immune suppression in cell transfer immunotherapy
Assess uses of cell transfer immunotherapy for cancers other than melanoma
Key points.
Adoptive cell transfer immunotherapy can mediate the objective regression of metastatic melanoma in 49–72% of patients
Complete durable regressions using cell transfer immunotherapy have been seen in up to 40% of patients and it is likely curative in many patients
The high incidence of durable complete regressions in patients with melanoma receiving cell transfer immunotherapy is similar, independent of the patient’s prior treatment
Cell transfer immunotherapy can be extended to additional cancer types by using autologous lymphocytes that are genetically transduced to express antitumor T-cell receptors (TCRs) or chimeric antigen receptors (CARs)
Using these TCR or CAR gene transduced cells, objective regressions have been seen in patients with synovial cell sarcoma, lymphoma, and melanoma
The opportunity to genetically modify autologous lymphocytes with a variety of genes that can improve their antitumor function is opening new possibilities for developing effective cancer treatments
Review criteria.
A formal literature search for this Review was not performed. This Review includes a summary of the author’s work and knowledge based on reading the oncology and immunology literature. Knowledge gained from regular attendance at conferences, workshops, and other national and international meetings was also included.
Acknowledgments
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
The author, the journal Chief Editor L. Hutchinson and the CME questions author C. P. Vega declare no competing interests.
References
- 1.Rosenberg SA, Robbins PF & Restifo NP Cancer immunotherapy in Cancer: Principles & Practice of Oncology (Eds DeVita VT, Lawrence TS & Rosenberg SA) 332–344 (Lippincott Williams & Wilkins, Philadelphia, 2011). [Google Scholar]
- 2.Slingluff CL, Flaherty K, Rosenberg SA & Read PW Cutaneous Melanoma in Cancer: Principles & Practice of Oncology (Eds DeVita VT, Lawrence TS & Rosenberg SA) 1643–1691 (Lippincott Williams & Wilkins, Philadelphia, 2011). [Google Scholar]
- 3.Rosenberg SA et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landsteiner K & Chase MW Experiments on transfer of cutaneous sensitivity to simple compounds. Proc. Soc. Exp. Biol. Med 49, 688–690 (1942). [Google Scholar]
- 5.Gross L Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res. 3, 326–333 (1943). [Google Scholar]
- 6.Morgan DA, Ruscetti FW & Gallo RG Selective in vitro growth of T-lymphocytes from normal bone marrow. Science 193, 1007–1008 (1976). [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi T, Matsui H & Fujita T Structure and expression of a cloned cDNA for human interleukin-2. Nature 302, 305–307 (1983). [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA et al. Biological activity of recombinant human interleukin-2 produced in E. coli. Science 223, 1412–1414 (1984). [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med 313, 1485–1492 (1985). [DOI] [PubMed] [Google Scholar]
- 10.Smith FO et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin. Cancer Res. 14, 5610–5618 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Bruggen P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 10, 281–287 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Robbins PF, Wang R-F & Rosenberg SA Tumor antigens recognized by cytotoxic lymphocytes in Cytotoxic Cells: Basic Mechanisms and Medical Applications (Eds Sitkovsky MV & Henkart PA) 363–383 (J. B. Lippincott, Philadelphia, 2000). [Google Scholar]
- 15.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostrate cancer. N. Engl. J. Med 363, 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol 175, 6169–6176 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Dudley ME et al. Cancer regression and autoimmunity in patients after clonal repopulation with anti-tumor lymphocytes. Science 298, 850–854 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol 26, 5233–5239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA & Dudley ME Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol 21, 233–240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins PF et al. Tumor regression in patients with metastatic synovial sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol 29, 917–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkhurst MR et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther 19, 620–626 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pule MA et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med 14, 1264–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straathof K et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood 105, 1898–1904 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Rooney CM et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92, 1549–1555 (1998). [PubMed] [Google Scholar]
- 26.Rosenberg SA, Spiess P & Lafreniere R A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233, 1318–1321 (1986). [DOI] [PubMed] [Google Scholar]
- 27.Muul LM, Spiess PJ, Director EP & Rosenberg SA Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol 138, 989–995 (1987). [PubMed] [Google Scholar]
- 28.Rosenberg SA et al. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. Preliminary report. N. Engl. J. Med 319, 1676–1680 (1988). [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA et al. Treatment of patients with metastatic melanoma using autologous tumor-infiltrating lymphocytes and interleukin-2. J. Natl Cancer Inst. 86, 1159–1166 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Wrzesiniski C et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J. Immunother 33, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman PB et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med 364, 2507–2516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 9, 2973–2980 (2003). [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L, Powell DJ, Rosenberg SA & Restifo NP Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol 6, 383–393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulos CM et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest 117, 2197–2204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Shen X, Hodes RJ, Rosenberg SA & Robbins P Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol 175, 7046–7052 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J. Immunol 176, 7726–7735 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins PF et al. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol 173, 7125–7130 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran KQ et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother 31, 742–751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besser MJ et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16, 2646–2655 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Dudley ME et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 16, 6122–6131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K & Rosenberg SA Transduction of an interleukin-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J. Immunol 167, 6356–6365 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu C et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood 109, 5168–5177 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinrichs CS et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 117, 808–814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan RA, Dudley ME & Rosenberg SA Adoptive cell therapy: Genetic modification to redirect effector cell specificity. Cancer J. 16, 336–341 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinnasamy N et al. A TCR targeting the HLA-A*0201–restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol 186, 685–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins PF et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol 180, 6116–6131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA & Morgan RA Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CJ et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan RA et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LA et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ & Chen YT Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol. Rev 188, 22–32 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Simpson AJ, Caballero OL, Jungbluth A, Chen YT & Old LJ Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 5, 625 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Gross G, Waks T & Eshhar Z Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamers CHJ et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol 24, e20–e22 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Chinnasamy D et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Invest 120, 3953–3968 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brentjens R, Yeh R, Bernal Y, Riviere I & Sadelain M Treatment of chronic lymphotic leukemia with genetically targeted autologous T cells: Case report of an unforeseen adverse event in a phase 1 clinical trial. Mol. Ther 18, 666–668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan RA et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther 18, 843–851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendle GM et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med 16, 565–570 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg SA Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther 18, 1744–1755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Middleton MR et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol 18, 158–166 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Atkins MB et al. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol 17, 2105–2116 (1999). [DOI] [PubMed] [Google Scholar]