Abstract
Background and aim:
Chronic hepatitis C virus (HCV) infection is associated with several extrahepatic manifestations (EHMs). Data on the effect of sustained virological response (SVR) on the risk of EHMs are limited.
Methods:
We conducted a retrospective cohort study using data of patients from the U.S. Veterans Affairs HCV Clinical Case Registry who had a positive HCV RNA test between 10/1999 and 8/2009. Patients receiving interferon-based antiviral therapy (AVT) were identified. SVR was defined as negative HCV RNA at least 12 weeks after end of AVT. Risk of 8 incident EHMs were evaluated in Cox proportional hazards regression models.
Results:
Of the 160,875 HCV-infected veterans, 31,143 (19.4%) received AVT, of whom 10,575 (33.9%) experienced SVR. EHM risk was reduced in the SVR group compared to untreated patients for mixed cryoglobulinemia (adjusted hazard ratio [aHR]=0.61; 95%CI=0.39–0.94), glomerulonephritis (aHR=0.62; 95%CI=0.48–0.79), porphyria cutanea tarda (PCT) (aHR=0.41; 95%CI=0.20–0.83), non-Hodgkin lymphoma (NHL) (aHR=0.64; 95%CI=0.43–0.95), diabetes (aHR=0.82; 95%CI=0.76–0.88), and stroke (aHR=0.84; 95%CI=0.74–0.94), but not for lichen planus (aHR=1.11; 95%CI=0.78–1.56) or coronary heart disease (aHR=1.12; 95%CI=0.81–1.56). Risk reductions were also observed when patients with SVR were compared to treated patients without SVR for mixed cryoglobulinemia, glomerulonephritis, PCT, and diabetes. Significant reductions in the magnitude of aHRs towards the null with increasing time to initiation of AVT after HCV diagnosis were observed for glomerulonephritis, NHL, and stroke.
Conclusions:
Risks of several EHMs of HCV infection are reduced after AVT with SVR. However, early initiation of AVT may be required to reduce the risk of glomerulonephritis, NHL, and stroke.
Keywords: Hepatitis C virus, extrahepatic manifestations, antiviral therapy, sustained virological response
INTRODUCTION
Hepatitis C virus (HCV) infection is a global public health issue and approximately 185 million individuals around the world are estimated to be infected with the virus.[1] HCV infection leads to chronic hepatitis, cirrhosis, and hepatocellular carcinoma, and is the leading reason for liver transplantation in the US.[2] Chronic HCV infection is also associated with several extrahepatic manifestations (EHMs), including essential mixed cryoglobulinemia, some subtypes of B-cell non-Hodgkin lymphoma (NHL), membranoproliferative glomerulonephritis, porphyria cutanea tarda (PCT) and lichen planus.[3–5] Furthermore, there is consistent epidemiological evidence supporting the association of chronic HCV infection with type-2 diabetes mellitus, atherosclerosis, and stroke.[6–14]
Antiviral therapy (AVT) for HCV infection along with attainment of sustained virological response (SVR) -- defined as undetectable viral load at least 12 weeks after treatment completion [15] -- normalizes liver enzymes, halts the progression of liver disease, and reduces the risk of liver failure and hepatocellular carcinoma.[16–19] SVR with interferon-based AVT leads to significant reduction in the all-cause and liver-related mortality in infected individuals.[18,20] However, data on the effect of SVR on the risk of EHMs are limited. Studies conducted in Japan and Taiwan demonstrated that SVR reduces the risk of type-2 diabetes, stroke, renal disease, and acute coronary syndrome in HCV-infected individuals.[21–24] People with active HCV infection may also have a higher risk of malignant lymphoma compared to those who achieved SVR.[25] Symptoms of mixed cryoglobulinemia improve and some indolent NHLs regress after AVT.[26–29] There have been no population-based studies that evaluated the risk of EHMs after SVR in HCV-infected people in the US. As some EHMs such as mixed cryoglobulinemia, PCT, and lichen planus are rare, the role of SVR in reducing the risk of these EHMs has not been evaluated.
For nearly two decades, interferon-containing AVT has formed the basis for anti-HCV treatment. The recent introduction of all oral direct-acting antivirals (DAAs) has substantially increased the possibility of achieving SVR and preventing hepatic outcomes.[15] As some extrahepatic outcomes of chronic HCV infection are rare and data on extrahepatic benefits of SVR for DAAs are still sparse, we studied the effect of SVR after interferon-based AVT on the risk of EHMs in a large cohort of HCV-infected US military veterans.
PATIENTS AND METHODS
Data Source
The Department of Veterans Affairs (VA) HCV Clinical Case Registry (HCV-CCR) contains health information on all known HCV-infected veterans who obtained care at any of the 128 VA medical facilities in the US.[30] The HCV-CCR automatically identifies individuals with positive HCV antibody tests, as well as any HCV-related International Classification of Diseases, version 9 (ICD-9) diagnosis codes from the VA electronic medical records. HCV-CCR includes data on demographics, laboratory test results, and VA pharmacy data from October 1, 1995 through January 1, 2010. This study was approved by Baylor College of Medicine’s Institutional Review Board.
Study design and study population
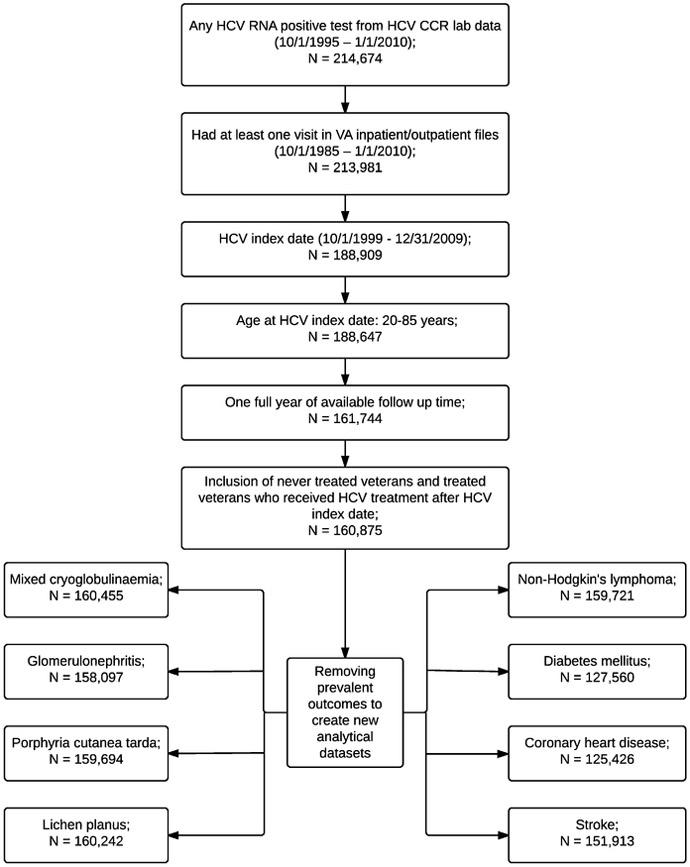
We conducted a retrospective cohort study using data from the HCV-CCR. We included individuals who: (1) had at least one positive test for HCV RNA in plasma by qualitative or quantitative assays between October 1, 1999 and August 31, 2009; (2) were aged 20 to 85 years at the earliest positive HCV RNA date (henceforth, known as the “HCV index date”); and (3) had at least one year of follow-up after the index date to allow a reasonable amount of time to ascertain baseline characteristics. We excluded treated subjects who had an HCV treatment start date (defined below) before the HCV index date.
Ascertainment of exposure, outcomes, and other characteristics
Our main exposure variable was receipt of AVT and its result (SVR or non-SVR). As defined previously,[31] veterans were considered to be treated for HCV infection if they had at least one filled prescription of any interferon with or without ribavirin any time after the HCV index date. The date of the first such interferon prescription at any VA pharmacy was considered as the start date of HCV treatment. Any further treatment occurring after a gap of > 65 days was ignored, and only the first course with interferon after the HCV index date was considered. The AVT end date was defined as the last date covered by the final interferon prescription after the AVT start date. Subjects were classified as untreated for HCV infection if they did not have a VA prescription for interferon.
Subjects were considered to have attained SVR when all subsequent HCV RNA measurements after the AVT end date were undetectable, with at least 1 negative HCV RNA test at least 12 weeks after that date.[31] We used 12 weeks instead of the clinically standard 24-week period post-AVT in the interferon era, since 98% of relapses occur within 12 weeks after treatment completion, and there is a high concordance between being free of the virus at 12 and 24 weeks.[32,33] Subjects with detectable HCV RNA after AVT end date were classified as non-SVR whereas subjects who did not have any HCV RNA measurements after the AVT start date were censored as untreated at the AVT start date.
We evaluated the following 8 EHMs of HCV infection: mixed cryoglobulinemia, glomerulonephritis, PCT, lichen planus, NHL, diabetes mellitus, coronary heart disease (CHD), and stroke, starting 12 months after the HCV index date. Incident cases were identified by the presence of at least one inpatient or outpatient ICD-9 diagnosis and/or procedure codes for the corresponding outcomes (see Supplementary Table 1 for definitions). For each outcome, we excluded the prevalent cases from the study; i.e. if the outcome was present any time before, or within 12 months after the HCV index date.
We assessed potential confounders, including age at the HCV index date, gender, race/ethnicity, period of military service (pre-Vietnam, Vietnam, post-Vietnam eras), HCV genotype, body mass index (kg/m2), smoking, alcohol intake, hypertension, and infections with hepatitis B virus (HBV) and human immunodeficiency virus (HIV). Liver fibrosis was ascertained using the AST to platelet ratio index (APRI) with a score ≥ 2 considered to represent advanced liver fibrosis. HBV infection was defined as a positive HBV surface antigen test, and HIV infection was defined as positive HIV antibody or HIV RNA test in serum. ICD-9 codes were used to define smoking, alcohol use, and hypertension (Supplementary Table 1). All the characteristics were ascertained at baseline, i.e. any time before, or within 12 months after HCV index date.
Statistical analyses
We compared the characteristics of veterans who ever received AVT to those who were never treated using chi-squared tests. We calculated the incidence rate of each EHM beginning 12 months after the HCV index date, for three groups: untreated, treated with SVR, and treated without SVR. The untreated group included individuals who were never treated, and the follow-up time before the AVT start date in those who ever received treatment (they transitioned to the treated group at the start of AVT). EHMs that occurred during AVT were ignored, since changing HCV RNA levels during the AVT may make it difficult to classify the exposure. Thus, we evaluated treated individuals beginning at the date of SVR documentation, or for those without SVR, beginning 12 weeks after the end of AVT. For each outcome, follow-up ended at the time of an event, death, or last follow-up visit at any VA facility, whichever occurred earlier.
To determine the association between AVT and risk of EHMs, separate multivariable Cox proportional hazards regression models were constructed for each outcome. Using time-dependent treatment variables, risks of EHMs were compared between those who had AVT with SVR vs. no AVT, and those who did not have SVR vs. no AVT. The covariates included in the main models were selected based on a priori knowledge of important factors that affect the risk of EHMs and comprised age at HCV index date, gender, sex, race/ethnicity, period of service, BMI, smoking, and alcohol abuse for all outcomes, and additional adjustment for diabetes and hypertension (at baseline) for glomerulonephritis, CHD and stroke. Missing information was treated as a separate category for each variable in the analyses. We further compared risks for treated patients with SVR vs. without SVR using multivariable Cox proportional hazards regression models as described above. Adjustment of p values for multiple comparisons was conducted by using the Benjamini and Hochberg method with a false discovery rate of 10%.[34]
The proportional hazards (PH) assumption was tested by including interaction terms for the effect of treatment response (AVT with/without SVR) with follow-up time and evaluating the significance of that interaction term. When the PH assumption was not satisfied, we introduced terms for the interaction of treatment response with ‘time from the HCV index date to initiation of AVT’, since the effect of AVT may depend on when it is started after HCV diagnosis. For the models with the interaction, the PH assumption was then tested again as done previously and found to be met. Results are presented for main effects of treatment response (when the PH assumption was satisfied) or with interaction effects centered at the median time to initiation of AVT (when the PH assumption was not satisfied). Plots for adjusted hazard ratios (aHRs) with their corresponding 95% confidence intervals (CIs) were constructed for the models with interaction terms when AVT was initiated at 1, 2, 3, 4, and 5 years after the HCV index date.
We also conducted some sensitivity and exploratory subgroup analyses. First, we excluded all people with HBV or HIV infection since these infections are associated with some outcomes such as NHL. Second, we used a stricter definition of the EHMs by requiring at least one inpatient or two outpatient diagnoses codes at least 30 days apart to improve the specificity of the outcome definitions. Finally, we analyzed the risk of EHMs in various subgroups formed by the presence or absence of advanced liver disease at baseline (APRI score ≥2 and <2, respectively), and the three main HCV genotypes (genotypes 1, 2, and 3) to determine whether the results differ by the liver disease status or HCV genotypes.
RESULTS
Study population
We included 160,875 veterans (Figure 1) who had a median follow-up of 5.1 years (interquartile range [IQR], 2.9–7.2 years). Most veterans were 50–59 years of age (52.1%), male (97.1%), and had served in the Vietnam War era (69.2%) (Table 1). A large proportion of subjects were non-Hispanic white (44.9%), overweight (BMI 25 to <30 kg/m2, 38.5%) or obese (BMI ≥30 kg/m2, 27.3%), smokers (59.2%) or alcohol abusers (58.3%), and had APRI score ≥2 (24.8%). The most common HCV genotype was genotype 1 (54.7%), although many individuals were not tested for genotype (31.4%). HBV and HIV co-infections were present in 1.3% and 3.2% of HCV-infected veterans, respectively.
Figure 1:
Flow chart to determine the selection of patients in the study cohort
Table 1:
Characteristics of HCV-infected veterans and comparison between those who received antiviral therapy vs. never received antiviral therapy.
| Characteristics | All veterans (N = 160,875) |
Never-treated (N = 129,732) |
Ever-treated (N = 31,143) |
p value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age at HCV index date, years | |||||||
| 20 – 39 | 4,417 | 2.8 | 3,310 | 2.6 | 1,107 | 3.6 | < 0.0001 |
| 40 – 49 | 54,748 | 34.0 | 42,055 | 32.4 | 12,693 | 40.8 | |
| 50 – 59 | 83,834 | 52.1 | 68,116 | 52.5 | 15,718 | 50.5 | |
| 60 – 69 | 12,611 | 7.8 | 11,118 | 8.6 | 1,493 | 4.8 | |
| 70 + | 5,265 | 3.3 | 5,133 | 4.0 | 132 | 0.4 | |
| Gender | < 0.0001 | ||||||
| Males | 156,253 | 97.1 | 126,273 | 97.3 | 29,980 | 96.3 | |
| Females | 4,622 | 2.9 | 3,459 | 2.7 | 1,163 | 3.7 | |
| Race/ethnicity | < 0.0001 | ||||||
| Non-Hispanic whites | 72,197 | 44.9 | 55,122 | 42.5 | 17,075 | 54.8 | |
| Non-Hispanic blacks | 48,950 | 30.4 | 42,011 | 32.4 | 6,939 | 22.3 | |
| Hispanics | 7,005 | 4.4 | 5,388 | 4.2 | 1,617 | 5.2 | |
| Others | 2,160 | 1.3 | 1,688 | 1.3 | 472 | 1.5 | |
| Unknown | 30,563 | 19.0 | 25,523 | 19.7 | 5,040 | 16.2 | |
| Period of service | < 0.0001 | ||||||
| Pre-Vietnam war | 10,353 | 6.4 | 9,631 | 7.4 | 722 | 2.3 | |
| Vietnam war | 111,360 | 69.2 | 89,797 | 69.2 | 21,563 | 69.2 | |
| Post-Vietnam war/Others | 39,162 | 24.3 | 30,304 | 23.4 | 8,858 | 28.4 | |
| Average annual no. of outpatient visits | |||||||
| 0 – 7 | 42,715 | 26.5 | 36,650 | 28.2 | 6,065 | 19.5 | < 0.0001 |
| > 7 to 13 | 39,543 | 24.6 | 30,455 | 23.5 | 9,088 | 29.2 | |
| > 13 to 25 | 38,947 | 24.2 | 29,815 | 23.0 | 9,132 | 29.3 | |
| > 25 | 39,670 | 24.7 | 32,812 | 25.3 | 6,858 | 22.0 | |
| Body mass index (kg/m2) | |||||||
| < 25.0 | 54,029 | 33.6 | 46,007 | 35.5 | 8,022 | 25.8 | < 0.0001 |
| 25.0 to < 30.0 | 61,920 | 38.5 | 49,150 | 37.9 | 12,770 | 41.0 | |
| ≥ 30.0 | 43,931 | 27.3 | 33,646 | 25.9 | 10,285 | 33.0 | |
| Unknown | 995 | 0.6 | 929 | 0.7 | 66 | 0.2 | |
| HCV genotype | < 0.0001 | ||||||
| Genotype 1 | 87,979 | 54.7 | 66,481 | 51.2 | 21,498 | 69.0 | |
| Genotype 2 | 13,016 | 8.1 | 8,396 | 6.5 | 4,620 | 14.8 | |
| Genotype 3 | 8,292 | 5.1 | 5,400 | 4.2 | 2,892 | 9.3 | |
| Other genotypes | 1,107 | 0.7 | 853 | 0.7 | 254 | 0.8 | |
| Unknown | 50,481 | 31.4 | 49,457 | 38.1 | 1,879 | 6.0 | |
| HBV infection* † | 2,037 | 1.3 | 1,716 | 1.3 | 321 | 1.0 | < 0.0001 |
| HIV infection* † | 5,086 | 3.2 | 4,350 | 3.4 | 736 | 2.4 | < 0.0001 |
| APRI score ≥ 2* ‡ | 37,842 | 24.8 | 29,992 | 24.5 | 7,850 | 26.4 | < 0.0001 |
| Smoking* | 95,154 | 59.2 | 78,195 | 60.3 | 16,959 | 54.5 | < 0.0001 |
| Alcohol abuse* | 93,713 | 58.3 | 78,605 | 60.6 | 15,108 | 48.5 | < 0.0001 |
| Diabetes* | 33,315 | 20.7 | 27,879 | 21.5 | 5,436 | 17.5 | < 0.0001 |
| Hypertension* | 82,801 | 51.5 | 68,404 | 52.7 | 14,397 | 46.2 | < 0.0001 |
Abbreviations: APRI, Aspartate aminotransferase to platelet ratio index; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus
Conditions were diagnosed any time before, or within 1 year after the HCV index date.
HBV infection was defined as positive hepatitis B surface antigen in serum; HIV infection was defined as positive HIV antibody or HIV RNA in serum.
APRI score was tested at baseline in 94.69% of veterans (data not available for 8,537 veterans).
Approximately 19% of veterans (n=31,143) received AVT during the follow-up, of whom 34% (n=10,575) achieved SVR. The median duration of AVT was 24.2 weeks (IQR, 10.9–46 weeks). As shown in Table 1, compared with never-treated veterans, treated veterans were more likely to be younger (median age, 50.8 vs. 52.3 years), non-Hispanic white (54.8% vs. 42.5%), have higher BMI (median, 27.9 vs. 26.7 kg/m2) and APRI ≥2 (26.4% vs. 24.5%); less likely to have coinfections with HBV (1.0% vs. 1.3%) or HIV (2.4% vs. 3.4%), or hypertension (46.2% vs. 52.7%); and less likely to be smokers (54.5% vs. 60.3%) or alcohol abusers (48.5% vs. 60.6%).
Risk of EHMs by treatment status
Most EHMs were rare (incidence rates <1 per 1,000 person-years), except for glomerulonephritis, diabetes, and stroke (Table 2). In general, EHM incidence was lower in the treated groups when compared to untreated veterans (except for lichen planus). The lowest incidence rates were observed in individuals who received AVT with attainment of SVR, for all outcomes except for CHD and stroke.
Table 2:
Crude incidence rates (events per 1,000 person-years) of the outcomes according to the antiviral treatment status
| Outcomes | No treatment* | Treatment without SVR | Treatment with SVR | |||
|---|---|---|---|---|---|---|
| Events | IR/1000 PYs (95% CI) | Events | IR/1000 PYs (95% CI) | Events | IR/1000 PYs (95% CI) | |
| Mixed cryoglobulinemia | 506 | 0.72 (0.66-0.78) | 62 | 0.52 (0.41-0.67) | 21 | 0.33 (0.21-0.50) |
| Glomerulonephritis | 1,957 | 2.83 (2.71-2.96) | 190 | 1.62 (1.41-1.87) | 70 | 1.09 (0.87-1.38) |
| Porphyria cutanea tarda | 367 | 0.52 (0.47-0.58) | 44 | 0.37 (0.28-0.50) | 10 | 0.16 (0.08-0.29) |
| Lichen planus | 478 | 0.68 (0.62-0.74) | 84 | 0.71 (0.57-0.88) | 36 | 0.56 (0.40-0.77) |
| Non-Hodgkin lymphoma | 638 | 0.91 (0.84-0.98) | 65 | 0.55 (0.43-0.70) | 28 | 0.43 (0.30-0.63) |
| Diabetes mellitus | 11,423 | 21.6 (21.2-22.0) | 1,459 | 17.0 (16.1-17.9) | 717 | 13.9 (12.9-15.0) |
| Coronary heart disease | 544 | 1.01 (0.93-1.10) | 54 | 0.58 (0.44-0.76) | 39 | 0.75 (0.55-1.03) |
| Stroke | 5,973 | 9.14 (8.91-9.37) | 524 | 4.64 (4.26-5.06) | 314 | 5.10 (4.57-5.70) |
Abbreviations: CI, confidence intervals; IR, incidence rate; PYs, person-years; SVR, sustained virological response
‘No treatment’ group includes the period before initiation of antiviral treatment in those who were later treated.
After adjusting for potential confounders and multiple comparisons, individuals who received AVT with attainment of SVR had significantly lower risk of mixed cryoglobulinemia (aHR=0.61; 95%CI=0.39–0.94), glomerulonephritis (aHR=0.62; 95%CI=0.48–0.79), PCT (aHR=0.41; 95%CI=0.20–0.83), NHL (aHR=0.64; 95%CI=0.43–0.95), diabetes (aHR=0.82; 95%CI=0.76–0.88), and stroke (aHR=0.84; 95%CI=0.74–0.94) compared with untreated veterans (Table 3). EHM risk was not lowered in those who did not achieve SVR, except for glomerulonephritis (aHR=0.82, 95%CI=0.69–0.96) and stroke (aHR=0.82, 95%CI=0.75–0.90), and was increased for lichen planus (aHR=1.56, 95%CI=1.22–1.99) and diabetes (aHR=1.14, 95%CI=1.08–1.20).
Table 3:
Associations of SVR and lack of SVR following antiviral therapy with the risk of extrahepatic outcomes of hepatitis C virus infection
| Outcome | Total no. of patients |
No. of events |
Treatment without SVR vs. No treatment |
Treatment with SVR vs. No treatment |
Treatment with SVR vs. Treatment without SVR |
|||
|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) * | p value | aHR (95% CI) * | p value | aHR (95% CI) * | p value | |||
| Mixed cryoglobulinemia | 160,455 | 589 | 1.11 (0.85-1.45) | 0.4518 | 0.61 (0.39-0.94) | 0.0264 | 0.55 (0.33-0.90) | 0.0170 |
| Glomerulonephritis† | 158,097 | 2,217 | 0.82 (0.69-0.96) | 0.0132 | 0.62 (0.48-0.79) | 0.0001 | 0.75 (0.57-0.99) | 0.0490 |
| Porphyria cutanea tarda† | 159,694 | 421 | 1.33 (0.97-1.84) | 0.0780 | 0.41 (0.20-0.83) | 0.0133 | 0.31 (0.14-0.65) | 0.0022 |
| Lichen planus† | 160,242 | 598 | 1.56 (1.22-1.99) | 0.0004 | 1.11 (0.78-1.56) | 0.5627 | 0.71 (0.48-1.05) | 0.0895 |
| Non-Hodgkin lymphoma† | 159,721 | 731 | 0.91 (0.70-1.19) | 0.4902 | 0.64 (0.43-0.95) | 0.0267 | 0.71 (0.45-1.11) | 0.1346 |
| Diabetes mellitus | 127,560 | 13,599 | 1.14 (1.08-1.20) | < 0.0001 | 0.82 (0.76-0.88) | < 0.0001 | 0.72 (0.65-0.78) | < 0.0001 |
| Coronary heart disease | 125,426 | 637 | 0.93 (0.70-1.24) | 0.6122 | 1.12 (0.81-1.56) | 0.4958 | 1.21 (0.80-1.82) | 0.3704 |
| Stroke† | 151,913 | 6,811 | 0.82 (0.75-0.90) | < 0.0001 | 0.84 (0.74-0.94) | 0.0030 | 1.02 (0.89-1.18) | 0.7573 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence intervals; SVR, sustained virological response
Hazard ratios were adjusted for age categories (20-39, 40-49, 50-59, 60-69, and 70+ years), sex, race, period of service, average annual number of outpatient visits, body mass index (<25.0, 25 to <30, 30+ kg/m2), smoking, and alcohol abuse. Additional adjustments for baseline diabetes mellitus and hypertension were conducted in models for glomerulonephritis, coronary heart disease, and stroke. Underlined aHRs and 95%CI along with their corresponding p values were significant by Benjamini and Hochberg method for multiple comparisons.
Because of non-proportionality, an interaction with time to initiation of antiviral therapy was included in some models. Thus, the aHRs presented for glomerulonephritis, porphyria cutanea tarda, lichen planus, non-Hodgkin lymphoma, and stroke are estimated at median time to initiation of antiviral therapy.
When the analysis was restricted to treated patients, veterans with SVR had lower risk for mixed cryoglobulinemia (aHR=0.55, 95%CI=0.33–0.90), glomerulonephritis (aHR=0.75, 95%CI=0.57–0.99), PCT (aHR=0.31, 95%CI=0.14–0.65), and diabetes (aHR=0.72, 95%CI=0.65–0.78) compared to those without SVR (Table 3).
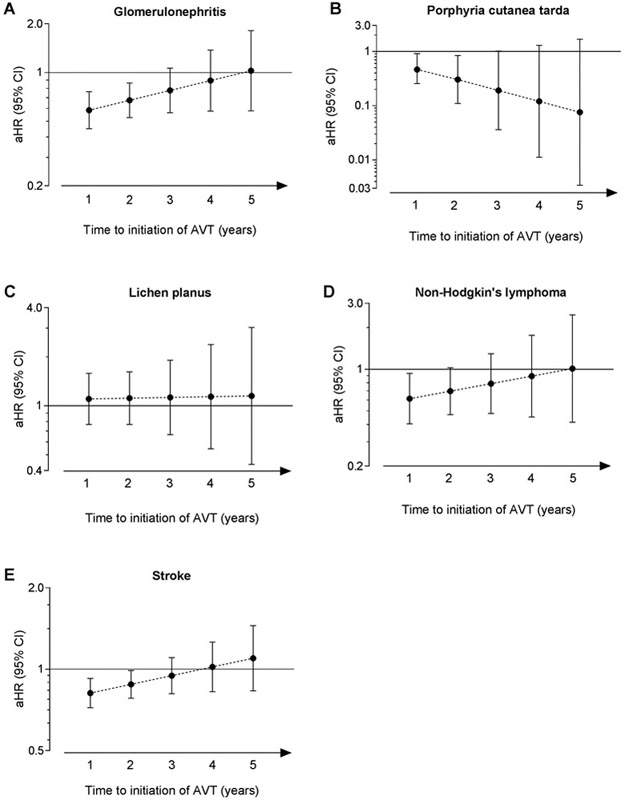
In comparing individuals with SVR to those who did not receive AVT, the PH assumption was satisfied for the main models for mixed cryoglobulinemia, diabetes, and CHD, and for models containing the interaction terms with ‘time from the HCV index date to initiation of AVT’ for glomerulonephritis, PCT, lichen planus, NHL, and stroke. We observed gradual reductions in the magnitude of protective aHRs towards the null with increasing time to initiation of AVT for glomerulonephritis, NHL, and stroke (Figure 2). The aHRs were significantly protective only when AVT was initiated at 1, or 2 years after the HCV index date for glomerulonephritis and stroke, and 1 year for NHL. For PCT, the aHRs became more protective with longer time between the HCV index date and AVT, although the aHRs were significant only when AVT was initiated at 1 or 2 years. The aHRs for lichen planus were not significant and changed minimally over time.
Figure 2:
The risk of extrahepatic manifestations in HCV-infected veterans according to time to initiation of antiviral therapy
The figure depicts the adjusted hazard ratios and the corresponding 95% confidence intervals (Y-axes) that represent the risk of extrahepatic manifestations in HCV-infected veterans. Comparisons were conducted between individuals who received antiviral therapy with SVR and those who were untreated. The time to initiation of antiviral therapy from HCV-index date is represented on the X-axes.
Abbreviations: aHR, adjusted hazard ratios; AVT, antiviral therapy; CI, confidence intervals; HCV, hepatitis C virus; SVR, sustained virological response
Sensitivity and subgroup analyses
Associations were similar to those in the primary analyses when individuals with HBV or HIV coinfections were excluded (Supplementary Table 2). When we used a stricter definition for the outcomes, significance of the association was no longer maintained for PCT and NHL when patients with SVR were compared to untreated patients (Supplementary Table 3). When HCV-infected veterans who achieved SVR were compared to those who were never treated, the risk of mixed cryoglobulinemia was reduced in those with advanced liver disease, the risk of glomerulonephritis and PCT was reduced in those without advanced liver disease, and the risk of diabetes and stroke was reduced irrespective of the baseline APRI score (Supplementary Table 4). However, the confidence intervals for the risk estimates were wide and overlapped each other due to small number of outcomes in the 2 subgroups. Similarly, overlapping wide confidence intervals for the risk estimates were obtained when we evaluated the risk of EHMs according to different genotypes (Supplementary Table 5).
DISCUSSION
In this large cohort study of HCV-infected US veterans, we found that SVR reduces the risk of some HCV-associated EHMs, such as mixed cryoglobulinemia, glomerulonephritis, PCT, NHL, diabetes, and stroke. These findings further strengthen the epidemiological evidence for their association with HCV infection. Furthermore, HCV-related EHMs carry a significant economic burden due to direct medical costs and indirect costs due to loss of productivity.[35] The results of our study emphasize the extrahepatic benefits of SVR.
Our findings also have important clinical implications. With the advent of DAAs, substantial improvements in SVR rates have been achieved with a shorter duration of AVT and fewer side effects than interferon-based AVT.[15] As more patients are treated with DAAs, we expect greater benefits of SVR including reduced risk of EHMs of chronic HCV infection, assuming that the clinical benefits of SVR remain similar with interferon-based compared to interferon-free treatment regimens. Recent reports suggest that the risk of hepatocellular carcinoma may not decrease in-spite of increased SVR rates with the DAAs,[36–38] which may be due to differences in characteristics of patients who received interferon-free versus interferon-based AVT.[36,38] Hence, our results need to be replicated in a cohort of HCV-infected patients who received DAAs.
Chronic HCV infection is associated with lymphoproliferative conditions such as mixed cryoglobulinemia and B-cell NHLs.[39,40] AVT for HCV infection is beneficial for patients with HCV-associated mixed cryoglobulinemia or indolent NHLs, as they can have complete resolution of symptoms of cryoglobulinemia and lymphoma regression.[27–29,41] We found that AVT with SVR also significantly reduces the risk of mixed cryoglobulinemia. Similarly, a Japanese study found that, individuals with SVR had a lower risk of development of malignant lymphoma when compared to those with active HCV infection.[25] We observed that AVT with SVR led to a moderate reduction in risk of B-cell NHLs when compared to untreated patients. Moreover, the benefit persisted after excluding individuals who had HIV infection, thus reducing the possibility that the protective effect could be attributed to antiretroviral therapy for HIV. However, this risk reduction was not observed when AVT was started 2 or more years after the HCV index date.
Glomerulonephritis, a common renal EHM of chronic HCV infection, is characterized by sub-endothelial deposits of HCV-related antigen-antibody complexes in the renal glomeruli.[42,43] HCV eradication can reduce the risk of proteinuria and nephrotic syndrome,[42] and end-stage renal disease.[24] In our study, we found reduced risk of glomerulonephritis after SVR, affirming the benefits of AVT in preventing HCV-associated renal disease.
HCV is also associated with dermatological conditions such as PCT and lichen planus.[44,45] The effect of AVT on PCT or lichen planus has been inconsistent, with reports of exacerbation of symptoms of lichen planus in those who received interferon-containing regimens.[46,47] A similar process may explain the increased risk for lichen planus that we observed for patients who were treated but did not attain SVR. We found that the risk of PCT was reduced after SVR when compared to untreated individuals. The magnitude of risk reduction seemed to increase with longer time to AVT initiation, but small number of observations along with wide confidence intervals prevents us from making precise conclusions.
We observed a significantly reduced risk of diabetes in treated patients with SVR compared to untreated people. Several studies have reported an association between chronic HCV infection and type 2 diabetes mellitus.[11,12,14,48,49] AVT with SVR may lead to improvements in insulin resistance and pancreatic β-cell function.[21] We found that the risk of diabetes was increased in treated veterans who did not achieve SVR, which was also seen in another cohort study.[22] This increase may be due to the effect of interferon and persistent viral replication. Interferon therapy has been reported to induce type 1 diabetes;[50] however, whether it can lead to type 2 diabetes is not known.
Although several studies have shown that HCV infection is associated with cardiovascular diseases [6–8,13,51] and stroke,[9,10] it is unclear whether HCV itself leads to these conditions. Studies from Taiwan have shown that interferon-based AVT reduces the risk of stroke and acute coronary syndrome.[23,24] In our study, we did not observe a reduced risk of CHD after AVT with SVR. Risk factor distributions for CHD vary between US and Asian countries, and the prevalence of other risk factors may affect the HCV-CHD association. It was possible that confounding due to unmeasured risk factors such as diet may have affected our results. However, we did observe a significant reduction in the risk of stroke in both SVR and non-SVR groups. Chronic infection may trigger immune and inflammatory responses, either locally within the blood vessels or via systemic inflammatory mediators and favor atherothrombosis [52]. HCV infection is associated with high levels of circulating cryoglobulins in blood which may lead to vasculitis and development of vascular thrombi.[53] Hence, treating the infection could remove the inflammatory stimuli and prevent development of stroke. But since the risk reductions were observed in both the SVR and non-SVR groups, our results may reflect the effect of interferon therapy rather than SVR and need to be verified in HCV-infected patients who were treated with interferon-free regimens.
Our study has some limitations. First, due to the nature of the veteran population being primarily male and of low socioeconomic status, the results are not generalizable to other populations. Second, the study is limited by the use of ICD-9 codes to ascertain outcomes, since there is a possibility of underreporting or misreporting of codes by the physicians. Also, conditions such as mixed cryoglobulinemia are often not detected clinically and may be underdiagnosed. Furthermore, only patient encounters that required a hospitalization and/or multiple outpatient visits to a physician are likely to be captured by these definitions. However, we expect the resulting bias to be non-differential between the treated and untreated individuals and acting towards the null. We also used stricter definitions of EHMs to improve the specificity of our definitions and confirmed most results in a sensitivity analysis. Third, we did not have detailed data on potential confounding variables such as duration of smoking, number of alcoholic drinks consumed, and dietary history. We used diagnostic codes to identify smoking and alcohol use, which would have likely captured those who were heavy smokers or alcohol abusers. Other possible confounders such as duration of infection, physical activity, and genetic risk factors could not be measured and may affect the risk estimates. Moreover, our study was population-based and evaluated the risk of 8 extrahepatic outcomes which have different risk factors and it was not possible to ascertain the potential confounders for each individual outcome. Fourth, it is possible that some patients who had spontaneous resolution of HCV infection may have been included in the untreated group. But that would likely reduce the risk of outcomes in the untreated patients and the resulting bias would be towards the null. Fifth, treated patients may have different unmeasured characteristics compared to those who were not treated. Hence, we compared the risk of extrahepatic manifestations among the treated patients who did and did not achieve SVR and confirmed most of our findings. Sixth, differences in the receipt of AVT according to the baseline liver disease status or HCV genotype was possible. Hence, we conducted subgroup analyses to evaluate the risk of EHMs according to liver disease status and HCV genotype. However, the small number of outcomes in the strata meant that our risk estimates in the subgroup analyses were not precise. Finally, some subjects may seek care and get treated outside the VA system, leading to misclassification of exposure or outcome status.
A strength of our study is the use of a large cohort of more than 150,000 HCV-infected veterans in the US. The large size allowed us to systematically evaluate the effect of AVT with SVR on the risk of EHMs, particularly for some rare conditions such as mixed cryoglobulinemia, PCT, lichen planus, and NHL. The HCV-CCR provides access to data on medical diagnoses and allows evaluation of laboratory results and pharmacy prescriptions. Evaluation of EHMs was also facilitated by the long follow-up available. We also conducted several sensitivity analyses, which generally confirmed our findings, although a few associations lost statistical significance when we used a strict definition to define the outcomes.
To summarize, we observed a significant reduction in the risk of several EHMs of chronic HCV infection with AVT and attainment of SVR. However, early initiation of AVT may be required to reduce the risk of glomerulonephritis, NHL, and stroke. Although future studies will need to evaluate the effects of DAAs, the benefits associated with SVR following interferon-based AVT suggest great promise in reducing the risk of EHMs.
Supplementary Material
SIGNIFICANCE OF THIS STUDY.
What is already known on this subject?
Chronic hepatitis C virus (HCV) infection is associated with several extrahepatic manifestations that affect various systems of the human body.
Antiviral therapy with attainment of sustained virological response (SVR), which is considered to be virological cure, has several hepatic benefits such as normalization of liver enzymes, halting the progression of liver disease, reduction in the risk of hepatocellular carcinoma.
However, data on the effect of attaining SVR on the risk of extrahepatic manifestations of HCV infection are limited.
What are the new findings?
Compared to HCV-infected individuals who did not receive treatment, SVR attainment was associated with a reduced risk of mixed cryoglobulinemia, glomerulonephritis, porphyria cutanea tarda, non-Hodgkin lymphoma, diabetes mellitus, and stroke, but not lichen planus or coronary heart disease.
Risk reductions were also observed when patients with SVR were compared to treated patients without SVR for mixed cryoglobulinemia, glomerulonephritis, porphyria cutanea tarda, and diabetes.
How might it impact on clinical practice in the foreseeable future?
With the introduction and increased use of direct-acting antivirals that have much better efficacy than interferon-containing regimens, we expect a reduction in the risk of some extrahepatic manifestations of chronic HCV infection.
Extrahepatic benefits of SVR attainment should be included in the cost-benefit analyses of new antiviral drugs for HCV infection.
ACKNOWLEDGEMENT
We thank Dr. Peter A. Richardson at the Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, for his help in generating the analytical dataset from the Veterans’ Affairs HCV Clinical Case Registry.
List of abbreviations (in the order of their appearance):
- HCV
hepatitis C virus
- EHM
extrahepatic manifestation
- NHL
non-Hodgkin lymphoma
- PCT
porphyria cutanea tarda
- AVT
antiviral therapy
- SVR
sustained virological response
- DAAs
direct-acting antivirals
- VA
Veterans’ Affairs
- HCV-CCR
HCV Clinical Case Registry
- ICD-9
International Classification of Diseases, version 9
- RNA
ribonucleic acid
- CHD
coronary heart disease
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- APRI
AST to platelet ratio index
- aHR
adjusted hazard ratio
- CI
confidence interval
- IQR
interquartile range
Footnotes
Conflicts of interest: HAT is a consultant for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., Dynavax Technologies, Vertex Pharmaceuticals, and Genentech, and has received research grants from Gilead Sciences, Merck and Co., and Vertex Pharmaceuticals. Other authors have no conflicts of interest to declare.
Financial support: Eric Engels was supported by the Intramural Research Program of the National Cancer Institute.
REFERENCES
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–42. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 2005;41:790–800. [DOI] [PubMed] [Google Scholar]
- 4.Negro F, Forton D, Craxi A, et al. Extrahepatic Morbidity and Mortality of Chronic Hepatitis C. Gastroenterology 2015;149:1345–60. [DOI] [PubMed] [Google Scholar]
- 5.Ferri C, Ramos-Casals M, Zignego AL, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev 2016;15:1145–60. [DOI] [PubMed] [Google Scholar]
- 6.Alyan O, Kacmaz F, Ozdemir O, et al. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 2008;72:1960–5. [DOI] [PubMed] [Google Scholar]
- 7.Butt AA, Xiaoqiang W, Budoff M, et al. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009;49:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu YH, Muo CH, Liu CY, et al. Hepatitis C virus infection increases the risk of developing peripheral arterial disease: a 9-year population-based cohort study. J Hepatol 2015;62:519–25. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Yang HI, Wang CH, et al. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 2010;41:2894–900. [DOI] [PubMed] [Google Scholar]
- 10.Liao CC, Su TC, Sung FC, et al. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One 2012;7:e31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003;38:50–6. [DOI] [PubMed] [Google Scholar]
- 12.Montenegro L, De Michina A, Misciagna G, et al. Virus C hepatitis and type 2 diabetes: a cohort study in southern Italy. Am J Gastroenterol 2013;108:1108–11. [DOI] [PubMed] [Google Scholar]
- 13.Vassalle C, Masini S, Bianchi F, et al. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 2004;90:565–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CS, Wang ST, Yao WJ, et al. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol 2007;166:196–203. [DOI] [PubMed] [Google Scholar]
- 15.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932–54. [DOI] [PubMed] [Google Scholar]
- 16.Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329–37. [DOI] [PubMed] [Google Scholar]
- 17.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002;122:1303–13. [DOI] [PubMed] [Google Scholar]
- 18.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama 2012;308:2584–93. [DOI] [PubMed] [Google Scholar]
- 19.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007;147:677–84. [DOI] [PubMed] [Google Scholar]
- 20.Nahon P, Bourcier V, Layese R, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2017;152:142–56.e2. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol 2007;102:570–6. [DOI] [PubMed] [Google Scholar]
- 22.Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009;49:739–44. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CS, Kao JH, Chao YC, et al. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther 2013;38:415–23. [DOI] [PubMed] [Google Scholar]
- 24.Hsu YC, Ho HJ, Huang YT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 2015;64:495–503. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y, Ikeda K, Arase Y, et al. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am J Med 2007;120:1034–41. [DOI] [PubMed] [Google Scholar]
- 26.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992;327:1490–5. [DOI] [PubMed] [Google Scholar]
- 27.Arcaini L, Bruno R. Hepatitis C virus infection and antiviral treatment in marginal zone lymphomas. Curr Clin Pharmacol 2010;5:74–81. [DOI] [PubMed] [Google Scholar]
- 28.Arcaini L, Vallisa D, Rattotti S, et al. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol 2014;25:1404–10. [DOI] [PubMed] [Google Scholar]
- 29.Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 2002;347:89–94. [DOI] [PubMed] [Google Scholar]
- 30.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc 2009;16:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backus LI, Boothroyd DB, Phillips BR, et al. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology 2007;46:37–47. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013;144:1450–5.e2. [DOI] [PubMed] [Google Scholar]
- 33.Zeuzem S, Heathcote EJ, Shiffman ML, et al. Twelve weeks of follow-up is sufficient for the determination of sustained virologic response in patients treated with interferon alpha for chronic hepatitis C. J Hepatol 2003;39:106–11. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 35.Younossi Z, Park H, Henry L, et al. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology 2016;150:1599–608. [DOI] [PubMed] [Google Scholar]
- 36.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727–33. [DOI] [PubMed] [Google Scholar]
- 37.Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol 2016;65:856–8. [DOI] [PubMed] [Google Scholar]
- 38.Toyoda H, Tada T, Takaguchi K, et al. Differences in background characteristics of patients with chronic hepatitis C who achieved sustained virologic response with interferon-free versus interferon-based therapy and the risk of developing hepatocellular carcinoma after eradication of hepatitis C virus in Japan. J Viral Hepat 2016. [DOI] [PubMed] [Google Scholar]
- 39.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008;6:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. Jama 2007;297:2010–7. [DOI] [PubMed] [Google Scholar]
- 41.Dammacco F, Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med 2013;369:1035–45. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RJ, Gretch DR, Couser WG, et al. Hepatitis C virus-associated glomerulonephritis. Effect of alpha-interferon therapy. Kidney Int 1994;46:1700–4. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993;328:465–70. [DOI] [PubMed] [Google Scholar]
- 44.Shengyuan L, Songpo Y, Wen W, et al. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009;145:1040–7. [DOI] [PubMed] [Google Scholar]
- 45.Gisbert JP, Garcia-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol 2003;39:620–7. [DOI] [PubMed] [Google Scholar]
- 46.Berk DR, Mallory SB, Keeffe EB, et al. Dermatologic disorders associated with chronic hepatitis C: effect of interferon therapy. Clin Gastroenterol Hepatol 2007;5:142–51. [DOI] [PubMed] [Google Scholar]
- 47.Protzer U, Ochsendorf FR, Leopolder-Ochsendorf A, et al. Exacerbation of lichen planus during interferon alfa-2a therapy for chronic active hepatitis C. Gastroenterology 1993;104:903–5. [DOI] [PubMed] [Google Scholar]
- 48.Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 2000;133:592–9. [DOI] [PubMed] [Google Scholar]
- 49.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol 2008;49:831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura K, Kawasaki E, Imagawa A, et al. Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 2011;34:2084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adinolfi LE, Restivo L, Zampino R, et al. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 2012;221:496–502. [DOI] [PubMed] [Google Scholar]
- 52.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 53.Misiani R, Bellavita P, Fenili D, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med 1992;117:573–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.