Abstract
Natural glucocorticoids, a class of cholesterol-derived hormones, modulate an array of metabolic, anti-inflammatory, immunosuppressive and cognitive signaling. The synthesis of natural glucocorticoids, largely cortisol in humans, is regulated by the hypothalamic-pituitary-adrenal (HPA) axis and exhibits pronounced circadian variation. Considering the central regulatory function of endogenous glucocorticoids, maintenance of the circadian activity of the HPA axis is essential to host survival and chronic disruption of such activity leads to systemic complications. There is a great deal of interest in synthetic glucocorticoids due to the immunosuppressive and anti-inflammatory properties and the development of novel dosing regimens that can minimize the disruption of endogenous activity, while still maintaining the pharmacological benefits of long-term synthetic glucocorticoid therapy. Synthetic glucocorticoids are associated with an increased risk of developing the pathological disorders related to chronic suppression of cortisol rhythmicity as a result of the potent negative feedback by synthetic glucocorticoids on the HPA axis precursors. In this study, a mathematical model was developed to explore the influence of chronopharmacological dosing of exogenous glucocorticoids on the endogenous cortisol rhythm considering intra-venous and oral dosing. Chronic daily dosing resulted in modification of the circadian rhythmicity of endogenous cortisol with the amplitude and acrophase of the altered rhythm dependent on the administration time. Simulations revealed that the circadian features of the endogenous cortisol rhythm can be preserved by proper timing of administration. The response following a single dose was not indicative of the response following long-term, repeated chronopharmacological dosing of synthetic glucocorticoids. Furthermore, simulations revealed the inductive influence of long-term treatment was only associated with low to moderate doses, while high doses generally led to suppression of endogenous activity regardless of the chronopharmacological dose. Finally, chronic daily dosing was found to alter the responsiveness of the HPA axis, such that a decrease in the amplitude of the cortisol rhythm resulted in a partial loss in the time-of-day dependent response to CRH stimulation, while an increase in the amplitude was associated with a more pronounced time-of-day dependence of the response.
Introduction
Natural glucocorticoids (GC) are a class of cholesterol-derived hormones secreted from the zona fasiculata of the adrenal glands (Arlt and Stewart 2005). These hormones mediate a wide array of physiological functions with potent modulatory effects on metabolic, anti-inflammatory, immunosuppressive and cognitive signaling (Arlt and Stewart 2005; Fietta et al. 2009). The synthesis of natural glucocorticoids, primarily cortisol in humans, is regulated by the hypothalamic-pituitary-adrenal (HPA) axis, which along with the sympathetic nervous system constitutes the primary physiological stress response mechanism. HPA axis activity is mediated through a signaling cascade involving the sequential release of corticotrophin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and cortisol (CORT). Cortisol transduces its physiological functions by binding to glucocorticoid receptors (Spiga et al. 2014). Upon cortisol binding, the glucocorticoid receptor complex translocates to the nucleus where it can regulate gene expression by binding to glucocorticoid response elements that subsequently activate or repress gene transcription (McMaster and Ray 2007). Importantly, the basal activity of the HPA axis hormones exhibits pronounced circadian variation, with a peak in glucocorticoid secretion during the early morning hours in humans (Spiga et al. 2014). Cortisol is critically involved in the appropriate synchronization of peripheral circadian clock genes, which further coordinate the functions of their residing tissues and promote homeostasis (Nicolaides et al. 2017). Therefore, the maintenance of homeostatic cortisol circadian rhythms is critical to overall host survival (Smith and Vale 2006).
Since the discovery of the immunosuppressive and anti-inflammatory properties of cortisone (a closely related natural analog of cortisol) by Hench and Kendall in 1948 (Hench et al. 1949), synthetic GCs have been extensively used in the treatment of chronic inflammatory conditions including asthma, skin infections, and rheumatoid arthritis as well as for their immunosuppressive effects in patients undergoing organ transplantation (Edwards 2012; Fisher et al. 1992; Kirwan et al. 2010). Synthetic glucocorticoids have complex genomic action, similar to cortisol, with anti-inflammatory effects largely mediated by transrepression of regulatory genes involved in human immunology (Alangari 2010; Buttgereit et al. 2005). Although structurally similar to natural GCs, synthetic GCs can significantly differ in their potency and metabolic clearance to their endogenous analogs (Alangari 2010; Czock et al. 2005). Despite the vast pharmacological benefits of synthetic GC administration, chronic use is associated with serious systemic adverse effects, especially during high-dose administration (Beltrametti et al. 2016; Curtis et al. 2006; Rhen and Cidlowski 2005; Xu et al. 2008). Adverse effects are attributed to the transactivation of pathways involved in diabetes and glaucoma, as well as the transrepression of the HPA axis (Alangari 2010; Buttgereit et al. 2005; Liu et al. 2013). Consistent with clinical manifestations due to cortisol exposure outside the normal physiological range, patients receiving synthetic GCs are at an increased risk of developing psychiatric disorders like depression, drug-induced hyperglycemia, long-term diabetes mellitus, osteoporosis, gastritis and cardiovascular disease (Curtis et al. 2006; Fardet et al. 2012; Hwang and Weiss 2014; Moghadam-Kia and Werth 2010; Oster et al. 2017). Considering the diverse and complex effects of synthetic GCs, the relationship between pharmacological dosing and the biochemical, physiological and behavioral processes influenced by chronic administration of synthetic glucocorticoids has yet to be fully elucidated (McMaster and Ray 2007; Ohdo et al. 2010).
Given the central regulatory function of the endogenous glucocorticoids, chronic disruption of cortisol rhythmicity is thought to result in the subsequent misalignment of peripheral circadian clocks, thus leading to the development of systemic complications (Dibner et al. 2010; Nicolaides et al. 2017). Therefore, there is a great deal of interest in the development of novel dosing regimens that can minimize the disruption of homeostatic circadian activity of cortisol and still maintain the pharmacological benefits of long-term synthetic GC therapy (Cutolo 2016). While significant progress has been made in the development of selective glucocorticoid receptor agonists that minimize transactivating properties to avoid adverse effects (Alangari 2010), a number of studies have investigated the influence of administration time of exogenous GCs on the endogenous cortisol rhythm with the aim of identifying chronopharmacological dosing regimens that minimize the disruption of the endogenous cortisol rhythm and the incidence of adrenal suppression. For example, healthy subjects administered synthetic GCs in the morning were found to exhibit the least suppression of the endogenous cortisol rhythm, while evening administration, resulted in maximal suppression of cortisol secretion and thus, found to be less physiologically compatible (Fisher et al. 1992; Haus et al. 2012a; Haus et al. 2012b; Kirwan 2011a; Xu et al. 2008). Additional studies aimed to replicate the endogenous cortisol activity for patients suffering from adrenal insufficiency (Newell-Price et al. 2008). While these studies showed that the administration time could likely be tailored to minimize disruption or to replicate the endogenous GC rhythm in the short-term, comprehensive studies on the longer-term influences of chronic dosing of synthetic GCs on the rhythmic characteristics of endogenous HPA axis activity are currently lacking.
Along with time-of-dosing, the influence of dose strength and different administration routes on endogenous HPA axis activity in the context of chronic exposure to synthetic GCs has yet to be elucidated. Adequately accounting for such factors in exploratory experimental studies can be exceedingly expensive as clinical designs grow in complexity and size. In such cases, a model-based approach can be a particularly useful tool for efficiently generating and evaluating experimentally-verifiable hypotheses related to the dose-exposure-response relationship for synthetic GCs. Through mathematical modeling, the impact of pharmacokinetics (dose, administration time, route of administration, duration of treatment, etc.) in accordance with internal circadian rhythms and external environmental influences, such as light and feeding, can be thoroughly investigated (Bae and Androulakis 2017; Hartmanshenn et al. 2016; Mavroudis et al. 2014; Pierre et al. 2016; Pierre et al. 2017). For example, physiologically-based modeling was previously implemented to understand how endogenous melatonin, a compound with strong circadian dependence, was influenced by the administration of exogenous melatonin and to elucidate the chronopharmacokinetics of exogenous melatonin for replication of the endogenous rhythm of melatonin (Peng et al. 2013).
In this study, we developed a mathematical model to explore the influence of exogenous GC dosing on the endogenous cortisol rhythm for a generic synthetic GC, considering both an intra-venous bolus and once-daily oral dosing. For these administration routes, we compared the HPA axis activity as indicated by changes in the cortisol rhythm due to a bolus of drug in systemic circulation with the pharmacological response following slower appearance rates in systemic circulation considering absorption after oral dosing. Furthermore, we attempt to determine how the response to short term treatment differs from that of chronic repeated dosing. As such, the goal of this study was to elucidate how long-term chronopharmacological dosing regimens influenced the basal cortisol activity using a model-based approach.
Methods
Description of the HPA axis model
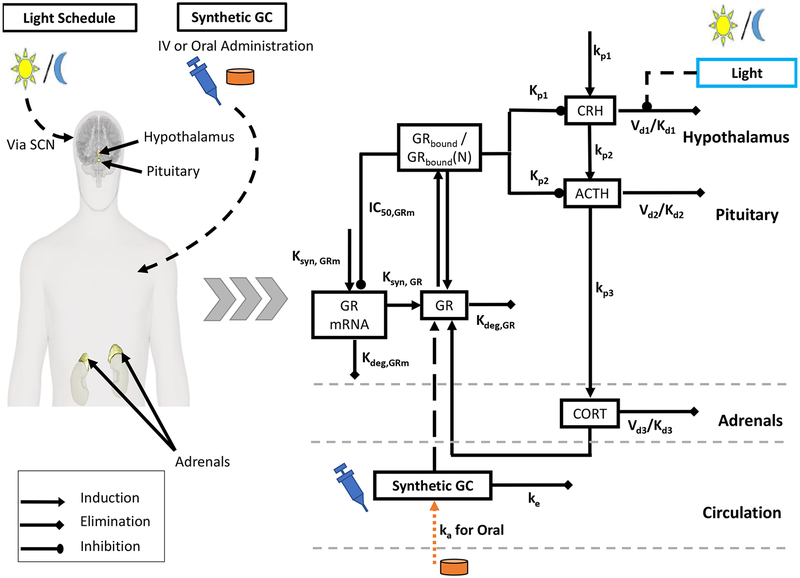
A schematic of the model is depicted in Fig. 1. The underlying form of the oscillator was originally developed by Goodwin (Goodwin 1965), and has since been modified to include the Michaelis-Menten type degradation kinetics, which obviates the need to use unrealistically large Hill coefficients (Sriram et al. 2012). Given an appropriate choice of parameters, the model equations are able to produce circadian (24-hour periodic) oscillations (Mavroudis et al. 2014; Rao et al. 2016; Rao and Androulakis 2017).
Fig. 1:
Model Schematic: A schematic of the model depicting the primary interactions in the hypothalamus-pituitary-adrenal (HPA) axis. The synthetic glucocorticoids (GC) competitively bind to the glucocorticoid receptor and contribute to the negative feedback arm of the HPA axis. Synthetic GCs are administered by either a bolus injection directly into systemic circulation or by oral administration. Appearance in systemic circulation following oral administration is indicated by the orange line.
The primary mediators of the HPA axis, CRH, ACTH and cortisol (CORT) are represented by nonlinear ordinary differential equations (ODEs). CRH induces the release of ACTH from the pituitary gland, which subsequently induces the release of CORT from the adrenal glands (Equation 7–9). The synthesis of CRH in the hypothalamus is described by zero-order kinetics, while ACTH and CORT synthesis is described by first-order kinetics. Moreover, the model accounts for the binding of CORT to the glucocorticoid receptor (GR) (Ramakrishnan et al. 2002) as well as the pharmacodynamics of the cortisol-bound receptor complex (Equation 10–13). Briefly, the model accounts for the transcription of GR mRNA (GRmRNA), (Equation 10) and the subsequent translation of GR protein (GR) (Equation 11). CORT forms a complex with cytoplasmic GR, (GRbound). A fraction of this complex translocates. to the nucleus, GRbound(N). Upon translocation, the hormone-receptor complex, (GRbound) is known to negatively regulate the expression of (GRmRNA), which is accounted for in Equation 10. Finally, Equations 7–9 account for inhibitor influence of the nucleated CORT-bound receptor complex, GRbound(N), on the release of CORT and ACTH.
Furthermore, we account for the entraining influence of light on the HPA axis via the suprachiasmatic nucleus (SCN). Light is assumed to have an inductive influence on CRH in diurnal animals by inhibiting its degradation (Kalsbeek et al. 2010). A symmetric light schedule (12hr light/12hr dark) was used to entrain our model of the HPA axis. Such a light schedule has been used previously in mathematical modeling studies to investigate the influence of light entrainment on the behavior of the endogenous circadian clock and the HPA axis hormones (Bordyugov et al. 2015; Mavroudis et al. 2014; Pierre et al. 2016; Schmal et al. 2015). While a simple 12hr light/12 dark schedule was used in the present study, more complex light schedules could be considered to investigate the influence of factors such as seasonality on the pharmacodynamic response of synthetic GCs. The model considers a 1 to 2-hour delay between the start of light exposure and the onset of the photo-induced effects in the HPA axis, denoted by the term lighteffect (Jung et al. 2010). This delay in the photo-inductive effect on the HPA axis was modeled using a series of transit compartments (Equation 3). Finally, a step function is used to model the light profile, while a Hill function is used to describe the dynamics of the phototransduction pathways (Equation 1–6). All simulations were implemented in MATLAB 2017b. Model equations were integrated using MATLAB’s built-in ode45 routine.
HPA Axis Mediators
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
Glucocorticoid Receptor Pharmacodynamics
| 11 |
| 12 |
| 13 |
Description of pharmacokinetic models for synthetic GC administration
Once-daily dosing is described using pharmacokinetic models that qualitatively captured the experimentally observed features of the drug exposure profile, such as the absorption rate and half-life, for a generic synthetic GC. While some synthetic GCs demonstrate complex pharmacokinetics due to competitive binding of the corticosteroid binding globulin (CBG) and interconversion between pharmacologically active and inactive forms by 11β-hydroxysteroid dehydrogenase type1/2 (Czock et al. 2005; Xu et al. 2007; Xu et al. 2008), linear pharmacokinetics are assumed for the model drug. Disruption of endogenous cortisol circadian rhythmicity following dosing may be evaluated under the assumption of linear pharmacokinetics to understand qualitative changes in the dose-exposure-response relationship.
To assess how the endogenous cortisol rhythm is influenced by the rate of appearance of drug into the system, pharmacokinetic models describing an intra-venous (IV) and oral dosing are used assuming absorption and elimination follow first-order rate processes. Disposition of synthetic GCs was previously described by 1 or 2 compartment models depending on the drug, administration route and dose (Czock et al. 2005). For this preliminary dosing study, a 1-compartment model is assumed to describe drug distribution within the body. The rate of disappearance of drug from systemic circulation following an injection is described by Equation 16. Disappearance from the gastrointestinal tract (GIT) after oral administration is described by Equation 17 and the amount of drug in systemic circulation is given by Equation 18. These equations are simplified from those developed by Xu et al. for IV and oral dosing of prednisolone using a 1-compartment model (Xu et al. 2007), neglecting first pass extraction and interconversion between prednisolone and prednisone for the arbitrary synthetic GC. Since the displacement of cortisol from plasma protein, metabolic enzymes, and GR binding sites due to competition with synthetic GCs is not considered, the loss of endogenous cortisol and drug from the system are independent in this model.
The 1-compartment model (Equation 18) was amended to simulate extended release of an oral dose using a series of five transit compartments (TC) as shown in Equations 19–21. The use of transit compartments has previously been implemented to delay the absorption rate in pharmacokinetic models (Cirincione et al. 2017; Mould and Upton 2013). In this study, the number of transit compartments and absorption rate constants for the slow-acting synthetic GC were selected to delay the absorption rate by approximately 3-fold while maintaining the same elimination rate constant as the fast acting GC, and ensure drug was cleared from the body within 24 hours. The pharmacokinetics of synthetic GCs as described by Equations 19–21 will herein be referred to as slow-acting synthetic GCs whereas the behavior described by Equation 18 will be referred to as the fast-acting synthetic GCs.
IV Administration
| 14 |
Oral Administration
| 15 |
| 16 |
| 17 |
| 18 |
| 19 |
Glucocorticoid Receptor Pharmacodynamics after GC Dosing
Upon dosing synthetic GCs, the equations describing the glucocorticoid receptor dynamics are modified to consider binding of the synthetic GC, as well as cortisol, to the glucocorticoid receptor, resulting in increased negative feedback to the HPA axis precursors, CRH and ACTH. GR is assumed to have the same affinity for endogenous and synthetic GCs.
| 20 |
| 21 |
Parameterization of the Model
The model is calibrated to qualitatively match the early morning peak in the endogenous cortisol circadian rhythm in healthy human subjects (Spiga et al. 2014) in order to understand how the endogenous cortisol rhythm is modified by drug administration in the absence of chronic inflammation. The model input parameters are given in the Supplementary Material.
Dosing Experiments
Several chronopharmacological dosing regimens are simulated to understand how administration time, dosing strength, administration route, and duration of treatment of synthetic GCs disrupted HPA axis activity. The once-a-day administration time of the IV bolus or oral dose of synthetic GCs is varied by 1-hour intervals throughout the simulated day. Different doses of synthetic GCs are modeled to evaluate how the strength of negative feedback via the glucocorticoid receptor dynamics influence the endogenous cortisol rhythm. Doses varied from the nominal amount of 1× are classified as low (less than 1×), intermediate (2× to 10×), and high (above 10×). For both administration routes, the pharmacological effects of short term and chronic treatments are simulated using a single dose and multiple doses with a dosing interval of 24 hours for once-a-day administration.
Changes in amplitude, acrophase, and area-under-the-curve (AUC) of the endogenous cortisol rhythm are used as metrics to quantify disruption of the HPA axis activity relative to the baseline activity. Amplitude and acrophase are determined when the cortisol rhythm reached a new stable oscillatory state after chronic once-daily dosing. The relative change in amplitude is calculated by Equation 19.
| 22 |
The AUC of the endogenous cortisol profile is determined for the 24-hour period following the first dose and after multiple doses when the cortisol rhythm reaches the new stable state. The change in 24-hour AUC for short and long term pharmacological effects is calculated by Equation 20.
| 23 |
Simulations are compared against various clinical studies that evaluated disruption of the endogenous cortisol rhythm following intra-venous and oral administration of synthetic glucocorticoids (Buttgereit et al. 2013; Haus et al. 2012a; Kirwan et al. 2010; Xu et al. 2008),
Responsiveness of the HPA Axis
We study the cortisol response to in-silico CRH stimulation in order to determine whether modulating the rhythmic characteristics of cortisol through once-daily chronopharmacological dosing of synthetic GCs (at the nominal dose) also alters the responsiveness of the HPA axis. IV administration of CRH is simulated as a single pulse perturbation in the CRH rhythm (Equation 8), a procedure used by a number of studies in order to determine differences in the HPA axis responsiveness (Kirwan 2011b; Markovic et al. 2011). We quantify the cortisol response to CRH injection by calculating the difference in AUC of the cortisol profiles between the stimulated and un-stimulated control condition for 4 hours from the application of the simulated CRH injection. Finally, we simulated the administration of CRH at multiple time points at 2-hour intervals throughout the simulated day, in order to quantify the time-of-day dependent response of the HPA axis to CRH stimulation.
Results
Pharmacokinetic Profiles for the synthetic glucocorticoid
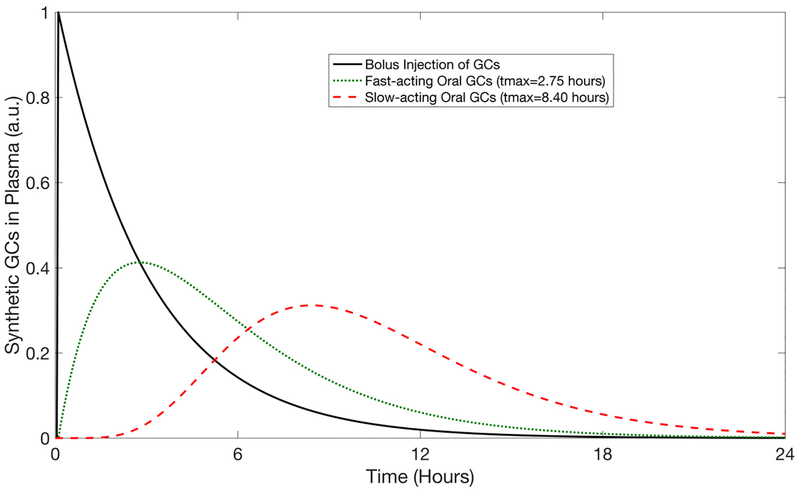
The pharmacokinetic profiles for the representative synthetic glucocorticoid administered by IV and oral administration routes are given in Fig. 2 for the nominal dose of 1×. The faster-acting oral dose resulted in a Cmax ≈ 40% of the initial dose, tmax = 2.75 hours, and 100% bioavailability (AUCIV = AUCoral). The slow-acting oral GC had a Cmax ≈ 30% of the initial dose, tmax = 8.4 hours, and 99% bioavailability.
Fig. 2:
Pharmacokinetic profiles for a bolus injection, a fast-acting oral dose, and a slow-acting oral dose of synthetic GCs. Representative profiles are shown for a nominal dose of 1×. Cortisol concentration is given in arbitrary units (a.u.).
Influence of once-daily chronopharmacological dosing of synthetic GCs on the cortisol circadian rhythm
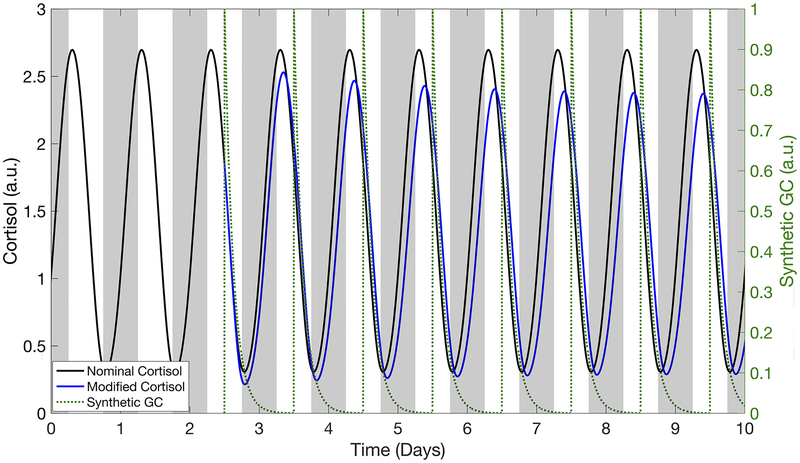
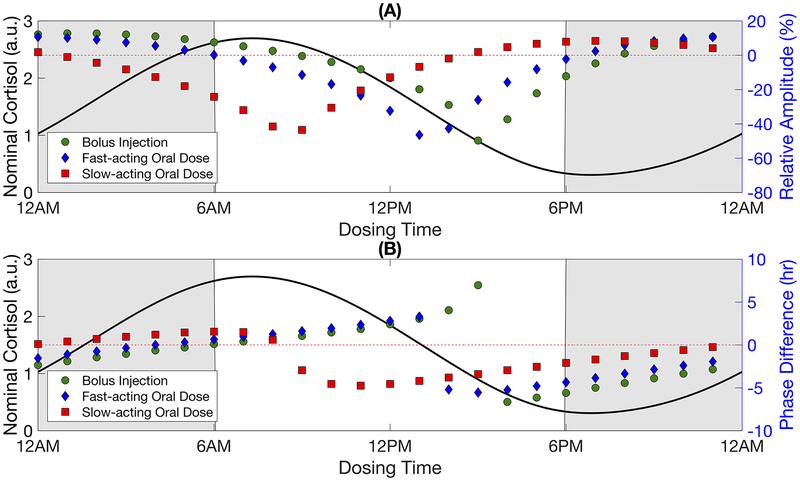
Once-a-day administration of synthetic GCs caused endogenous cortisol activity to evolve to a new stable, regular circadian rhythm (Fig. 3). Upon termination of synthetic GC intervention, the cortisol rhythm returned to the basal activity observed prior to dosing (Supplementary Fig. 1). The amplitude and acrophase of this new stable cortisol rhythm depended on the time at which the drug was administered as shown in Fig. 4. Amplitude generally decreased when the daily dosing of synthetic GCs by bolus injection was initiated during the declining phase of the nominal cortisol rhythm (Fig. 4a). The endogenous cortisol rhythm following once-a-day administration of the fast-acting and slow-acting oral doses qualitatively showed similar changes in amplitude as the bolus injection, but with an advance in dosing times by about 2 hours and 6 hours to produce the same effect on the cortisol rhythm. The shifts roughly correlated with the time needed to reach the maximum pharmacological effect following oral administration due to the absorption rates (tmax = 2.75 hours and tmax = 8.4 hours). Maximal suppression occurred when synthetic GCs were administered daily at 3:00 PM by bolus injection, 1:00 PM for the faster-acting oral dose, and 9:00 AM for the slow-acting oral dose. For all administration routes, certain once-daily chronopharmacological dosing regimens resulted in HPA axis induction, corresponding to an increase in amplitude of the endogenous cortisol rhythm. Maximal induction of the endogenous cortisol amplitude largely occurred when synthetic GCs were administered during the simulated night.
Fig 3.
Modified cortisol profiles after dosing of synthetic glucocorticoids (GC) by bolus injection at the nominal amount (1×). The modified cortisol rhythm is indicated by the blue line. The black line corresponds to the nominal cortisol profile based on endogenous HPA axis activity. The pharmacokinetic profiles for the bolus injection are indicated by the dotted green line. The grey shaded areas represent the time at which the system is not exposed to light. Cortisol concentration is given in arbitrary units (a.u.).
Fig. 4:
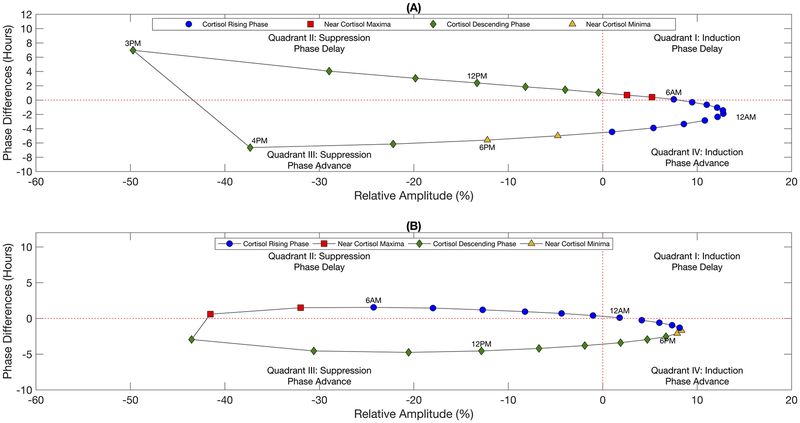
Amplitude and phase of the modified cortisol rhythm after once-daily chronopharmacological dosing of synthetic glucocorticoids. The relative amplitude and difference in the acrophase of the modified cortisol rhythm after a repeated once-a-day administration of a bolus injection, fast-acting oral dose, or slow-acting oral dose are shown in A and B, respectively. The nominal cortisol rhythm (indicated by the black line) is given for reference to show how dosing times align with the baseline circadian rhythm. The shaded areas represent the simulated night, that is the time at which the system is not exposed to light. The change in amplitude is calculated by Relative Amplitude (%) = [(Amptreatment − Ampbaseline)/Ampbaseline] × 100%. A negative value for phase difference indicates an advance in the acrophase (i.e. peaks earlier in the simulated day relative to the nominal cortisol rhythm) while a positive value indicates a delay in the acrophase (i.e. peaks later in the simulated day). Cortisol concentration is given in arbitrary units (a.u.).
A once-daily bolus injection introduced near the nadir or during the rising phase of the nominal cortisol rhythm predicted an advance in the acrophase of the cortisol rhythm, whereas initiating dosing near the peak or descending phase of the cortisol rhythm resulted in a delay of the acrophase (Fig. 4b). For both administration routes, the change in acrophase was most sensitive when synthetic GCs were administered at dosing times associated with greatest amplitude suppression for all routes of administration. Furthermore, while the change in acrophase for the bolus injection and fast-acting oral doses exhibited a discontinuity (termed Type 0 (Johnson 1992)), the acrophase response varied more smoothly (continuous, termed Type 1 (Johnson 1992)) for the slow-acting oral dose, which had a lower maximal plasma concentration. The relationship between amplitude and phase are shown Fig. 5 for the bolus injection and the slow-acting oral dose. The fast-acting oral dose revealed similar behavior to the bolus injection (data not shown). Depending on the time of synthetic GC administration, the acrophase of the new stable rhythm was found to adopt two different values for a given change in its amplitude with the difference between acrophases increasing with greater amplitude suppression as observed for the bolus injection (Fig. 5a). Similar behavior was observed for the slow-acting GC, but to a lesser extent (Fig. 5b).
Fig 5.
Relationship between the relative amplitude and phase difference of the modified cortisol rhythm after long-term once-daily chronopharmacological dosing of synthetic glucocorticoids. Amplitude and phase for the modified cortisol rhythms after chronic administration of a daily bolus injection and the slow-acting oral dose are shown in A and B, respectively. Marker labels correspond to the time of administration. Marker color indicates the administration time relative to the nominal cortisol rhythm where blue circles correspond to dosing times from 8:00 PM to 6:00 AM (ascending phase of baseline rhythm), red squares correspond to dosing times from 7:00 AM to 8:00 AM (near peak of baseline rhythm), green diamonds correspond to dosing times from 9:00 AM to 5:00 PM (descending phase of baseline rhythm), and yellow triangles correspond to dosing times from 6:00 PM to 7:00 PM (near nadir of baseline rhythm).
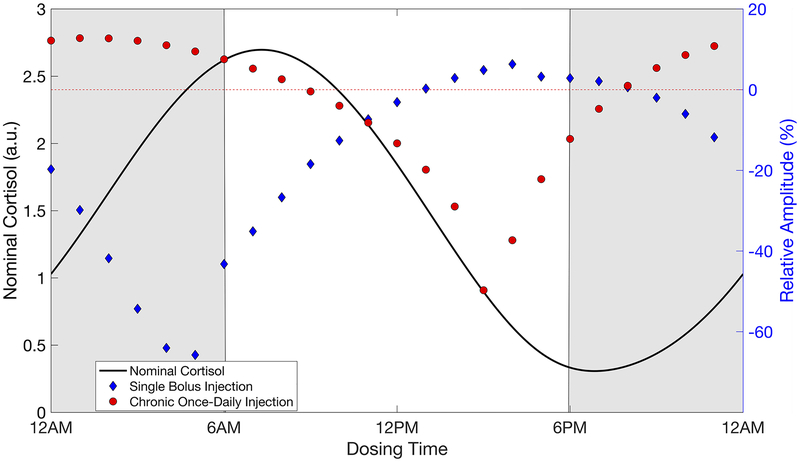
Importantly, our simulations indicated that specific chronopharmacological regimens of synthetic GC administration can minimize the disruption of the nominal GC rhythm. For example, daily administration of a nominal dose of synthetic GCs by bolus injection around 9:00 AM (Supplementary Fig 1.), resulted in a minimal change to the amplitude and acrophase of the cortisol rhythm relative to the basal activity, whereas a fast-acting oral dose at 6:00 AM or a slow-acting oral dose at midnight resulted in minimal change. Moreover, the amplitude change after a single dose was not indicative of the amplitude change after repeated administration (Fig. 6) considering that several days to weeks of once-a-day dosing was needed before the endogenous cortisol stabilized to the new rhythm. Simulations predicted similar behavior following oral administration (Supplementary Fig. 2).
Fig 6.
Amplitude of the modified cortisol rhythm after single and repeated once-daily chronopharmacological dosing of synthetic glucocorticoids by bolus injection at the nominal dose. The relative amplitude associated with the modified cortisol rhythm after a single injection and after long-term once-daily IV dosing are shown. The relative change in amplitude is calculated by Relative Amplitude (%) = [(Amptreatment − Ampbaseline)/Ampbaseline] × 100%. Cortisol concentration is given in arbitrary units (a.u.).
Influence of dosing on total cortisol exposure
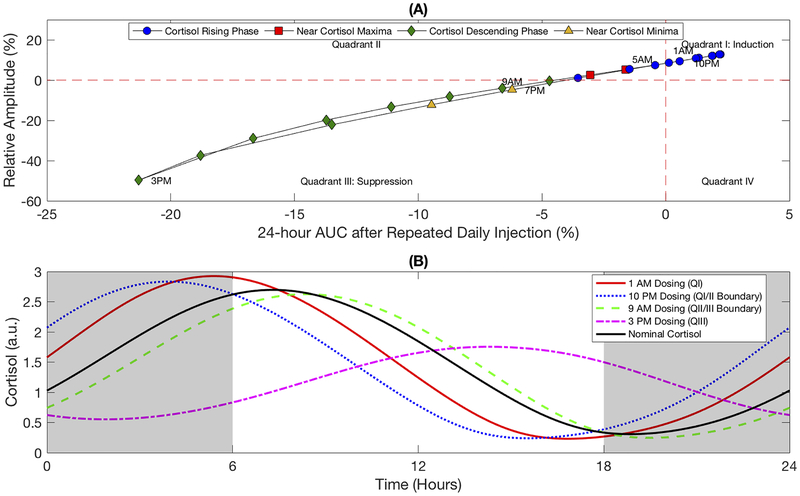
The induction of the cortisol amplitude in response to once-daily chronopharmacological dosing of synthetic GCs does not necessarily imply an increase in the total cortisol secreted by the adrenal glands in the 24-hour period following dosing, herein referred to as the total cortisol exposure. While the amplitude of the endogenous cortisol rhythm quantified the difference between the altered minima and maxima following once-a-day administration of synthetic GCs, the change in 24-hour AUC indicated how total cortisol exposure differed relative to the basal HPA axis activity. The relationship between amplitude and 24-hour AUC after repeated administration of a once-daily injection are shown in Fig 7a. Long-term dosing during the night between 10:00 PM and 5:00 AM indicated induction of the HPA axis by both the amplitude and 24-hour AUC, while chronic dosing during the day between 9:00 AM and 7:00 PM were associated with HPA axis suppression as shown by the reduced amplitude and 24-hour AUC. Interestingly, our simulations showed that dosing between 6:00 to 8:00 AM and 8:00 to 9:00 PM resulted in increased amplitude, but reduced total cortisol exposure (24-hour AUC) relative to the basal cortisol activity without synthetic GCs.
Fig. 7:
Influence of chronic once-daily chronopharmacological dosing on the total cortisol exposure. The relationship between the 24-hour AUC and amplitude change after long-term dosing of a daily bolus injection at the nominal dose (1×) is shown in A. The modified cortisol profiles after dosing and for the baseline conditions are given in B for selected dosing times. The grey shaded areas represent the simulated night, that is the time at which the system is not exposed to light. Cortisol concentration is given in arbitrary units (a.u.).
Endogenous cortisol profiles are given in Fig. 7b for selected dosing times. The cortisol rhythm associated with maximum induction (dosing at 1:00 AM) showed increased daily cortisol maxima and reduced daily cortisol minima, in which the shift in maxima was sufficient to increase the total cortisol exposure despite the reduction in minima. In contrast, the predicted cortisol profile associated with maximum HPA axis suppression (dosing at 3:00 PM) revealed a decrease in daily maxima and an increase in daily minima, leading to an overall reduction in 24-hour exposure. Cortisol profiles that fell within quadrant II of Fig. 7a showed a decreased daily cortisol nadir and an increased daily cortisol maximum, which was insufficient to overcome the decreased minima, leading to an overall decline in the total cortisol exposure (data not shown). Two additional cortisol profiles are provided in Fig. 7b characterized by a negligible change in 24-hour AUC following repeated dosing at 10:00 PM (nearest the boundary between quadrants I and II), and negligible amplitude change after repeated dosing at 9:00 AM (nearest the boundary between quadrants II and III). The former scenario showed the increase in daily cortisol was balanced by a reduction in the cortisol nadir such that the 24-hour AUC was maintained, while the latter case revealed the cortisol maxima and nadir were both reduced such that no net change was observed in the peak to trough height relative to the baseline amplitude. Similar behavior was predicted for oral administration (Supplementary Fig. 3), although no profiles were observed in Quadrant I for the slow-acting oral GC.
Influence of dosing strength on the activity of the HPA axis
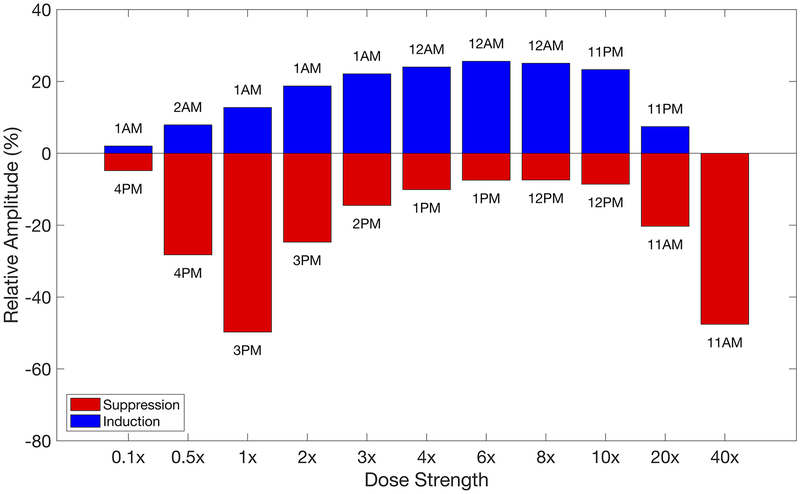
The amplitude of the cortisol rhythm associated with once-daily chronopharmacological dosing of a bolus injection that predicted the greatest suppressive and inductive effects on the HPA axis activity are given in Fig. 8 for several dosing strengths, where 1× corresponded to the nominal amount. The amplitude changes associated with all chronopharmacological injections are given in Supplementary Fig. 4a. Higher doses led to maximum suppression and induction earlier in the simulated day than low or intermediate doses. As the dose increased beyond the nominal, once-daily dosing during the simulated night led to increased inductive effects while dosing in the simulated day showed reduced suppressive effects up to 6×. Beyond 6×, the inductive effects were reduced and suppression increased until only suppression was observed at all dosing times at 40×. Furthermore, our model predicted that the acrophase of the endogenous cortisol rhythms was also sensitive to the dosing strength as shown in Supplementary Fig. 4b where low doses of the bolus injection were associated with continuous Type 1 phase response curves, and intermediate and high doses of the bolus injection were associated with discontinuous Type 0 phase response curves. Below the nominal dose, the maximal phase resetting and greatest suppression of the amplitude were predicted to occur at the same dosing time whereas the maximal phase resetting occurred at dosing times well after maximal suppression of the amplitude was observed for intermediate and high doses.
Fig. 8:
Influence of dosing strength on the relative amplitude after chronic once-daily chronopharmacological dosing of a bolus injection. The relative amplitude for the modified cortisol rhythms are given for the once-daily chronopharmacological dosing regimens that resulted in the greatest inductive and suppressive effects at each strength of the IV dose. The administration times corresponding to these changes in amplitude are indicated in the figure.
The dose-response relationship following oral administration of fast and slow-acting synthetic GCs are provided in Supplementary Fig. 5. The amplitude change associated with all chronopharmacological dosing regimens are given in Supplementary Fig. 6a and 7a, respectively. For the nominal and low doses, the bolus injection showed greatest amplitude suppression after chronic once-daily pharmacological dosing compared to the equivalent oral doses of the fast or slow acting GCs. Beyond the nominal amount, greater suppression of the amplitude was predicted for the slow-acting oral dose, while the fast-acting oral dose showed an intermediate effect at all dosing strengths. Differences between administration routes were attributed to the duration at which the synthetic GCs was maintained above a minimum pharmacologically active amount and the maximal concentration achieved at an equivalent dose. Interestingly, the bolus injection resulted in greater induction of HPA axis activity for all dose strengths, which may result due to the very high initial concentrations immediately following the bolus injection. Furthermore, administration of the fast-acting oral GCs resulted in a phase-response relationship that was qualitatively similar to that obtained during IV administration. For the slow-acting oral GCs, discontinuous Type 0 phase response curves were associated with intermediate and high doses with the discontinuity occurring at dosing times during the simulated day, except at very high doses when the phase discontinuity was observed near the start of the simulated night. The phase change associated with all chronopharmacological dosing regimens are given in Supplementary Fig. 6b and 7b. Moreover, the relationship between 24-hour AUC and amplitude following repeated administration of synthetic GCs by all administration routes are provided in Supplementary Fig. 8–10 for several dosing strengths.
Impact of synthetic GCs on the responsiveness of the HPA axis
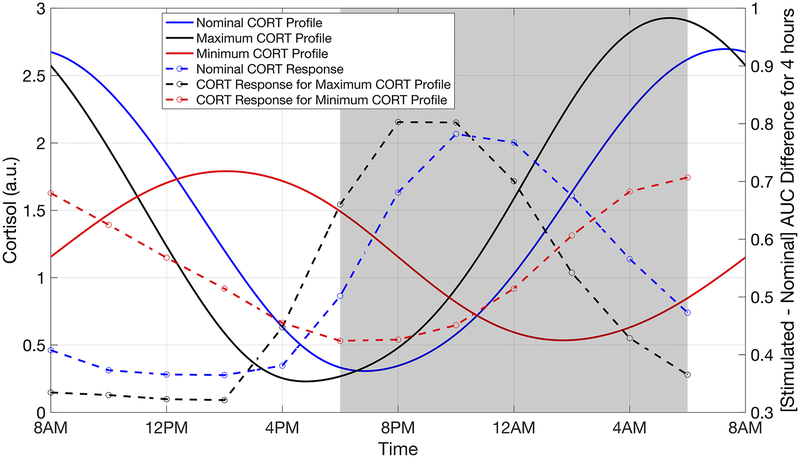
The rhythmic characteristics of the endogenous glucocorticoids have important implications for the stress-responsiveness of the HPA axis. Therefore, we determined how alterations in the cortisol circadian rhythm as a result of chronic daily administration of synthetic GCs (at the nominal dose) influenced the responsiveness of the HPA axis. In doing so, we simulated the HPA axis response to a CRH stimulation test once the cortisol rhythm attained a new stable state following repeated administration of the synthetic GCs. Moreover, possible alterations in the time-dependent response to CRH stimulation was determined. Fig. 9 compares the change in cortisol secretion following the simulated CRH stimulation test for baseline conditions without synthetic GC administration and for the dosing regimens that led to greatest disruption of the endogenous cortisol rhythm (dosing at 3:00 PM and 1:00 AM).
Fig. 9:
Influence of once-daily chronopharmacological dosing of synthetic GCs on the responsiveness of the HPA axis to CRH stimulation. The cortisol response due to simulated IV administration of the CRH is compared for the nominal cortisol profile without dosing (black), the cortisol rhythm with maximal amplitude (blue) after once-daily chronopharmacological administration of synthetic GCs, and the cortisol rhythm with minimal amplitude (red) after once-daily chronopharmacological administration of synthetic GCs. Cortisol (CORT) concentration is given in arbitrary units (a.u.). The response to CRH stimulation was determined at 2-hour intervals for each of the cortisol profiles (represented by circles). A time-of-day dependent response is observed for all profiles. A reduction in the amplitude of the cortisol rhythm is associated with a partial loss in the time-of-day dependence of the response to CRH stimulation, while an increase in the amplitude is associated with a more pronounced time-of-day dependence of the response.
A robust time-of-day dependence in the cortisol response to CRH administration was observed for all chronopharmacological dosing regimens considered (data not shown), with the maximal response occurring near the nadir of the endogenous cortisol rhythm. On the other hand, the HPA axis was minimally responsive when stimulated near the circadian maxima of the cortisol rhythm. Furthermore, the time at which the maximal response occurred was correlated to the acrophase of the cortisol rhythm. Thus, a phase advance in the cortisol rhythm as a result of the chronopharmacological dosing resulted in a phase advance in the time at which the maximal response to CRH stimulation occurred, in comparison to the nominal case in the absence of dosing (Fig. 9). Importantly, our simulations predicted that a suppression of the cortisol amplitude after synthetic GC administration was associated with a loss in the time-of-day dependence of the HPA axis response to CRH stimulation. Moreover, chronopharmacological dosing regimens that largely preserved the rhythmic characteristics of the nominal cortisol profile exhibited minimal alterations in the responsiveness of the HPA axis.
Summary of Key Results
Chronic daily dosing of synthetic GCs resulted in an alteration of the rhythmic characteristics of the endogenous cortisol, with the amplitude and the acrophase of the altered rhythm depending on the time of dosing. Our simulations showed that timing of administration can be appropriately adjusted to preserve the circadian features of the endogenous cortisol rhythm by both intra-venous and oral administration. Moreover, the suppression of the HPA axis following a single dose of synthetic GCs was not indicative of the long-term response to chronopharmacological dosing. Importantly, the inductive influence of long-term synthetic GC administration was only observed at low to moderate doses, while high doses generally led to suppressed endogenous activity regardless of dosing time. Finally, the responsiveness of the HPA axis was dependent on the timing of administration with maximal response occurring near the nadir of the endogenous cortisol rhythm and minimal response near the acrophase.
Discussion
Recognizing the functional importance of the circadian regulation underlying the signaling dynamics of complex physiological systems, such as the HPA axis, has led to great interest in the incorporation of chronobiological principles for the development of safer and more efficacious therapies (Ballesta et al. 2017; Smolensky and Peppas 2007). A major concern associated with long-term therapeutic use of GCs is the suppression of endogenous HPA axis activity (Nicolaides et al. 2017). However, chronopharmacological delivery of synthetic GCs is a promising approach to minimize the disruption of the endogenous cortisol circadian rhythmicity. In the present work, we used a semi-mechanistic mathematical model of the HPA axis to study the influence of chronic chronopharmacological intervention on endogenous HPA axis activity.
The model predicted that for all simulated dose strengths and routes of administration considered, the endogenous circadian activity of the HPA axis adapted to the repeated daily exposure to synthetic GCs by adopting a new stable circadian rhythm. Moreover, all three routes of administration of synthetic GCs resulted in qualitatively similar alterations of the cortisol circadian rhythm. However, due to differences in the duration for which synthetic GCs were maintained above a minimum pharmacologically active amount, both the oral administration routes considered resulted in a greater suppression of HPA axis activity in comparison to IV administration. For a given dosing strength, oral administration resulted in a comparable change in the rhythmic characteristics of the cortisol rhythm at earlier dosing times. This shift in response to earlier dosing times was most prominent for slow-acting oral administration, for which the maximal plasma drug concentrations were delayed the longest. Therefore, our results suggested that the exposure profile of synthetic GCs might be systematically manipulated in order to optimize the dosing time as well as the pharmacodynamic effect on the cortisol rhythm. An improved characterization of the chronopharmacological influence of synthetic GCs on HPA axis activity can lead to the development of novel dosage forms in order to improve patient compliance and limiting the incidence of adverse effects while maintaining treatment efficacy (Levi and Schibler 2007). Indeed, modified-release (MR) prednisolone tablets that delay the release of drug up to 4 hours after administration have been developed to chronopharmacologically target the late-night (2:00 to 4:00 AM) circadian rise in proinflammatory cytokines in RA patients by enabling the dose to be administered at 10:00 PM, conveniently before the patients slept (Kirwan et al. 2010). The use of MR prednisolone was shown to result in an improvement in clinical symptoms while also preventing the suppression of endogenous cortisol rhythmicity. Furthermore, once-daily dosing of extended release formulations have proven effective for improved pain relief in patients with osteoarthritis of the knee (Bodick et al. 2015) and in studies aiming to replicate endogenous cortisol rhythmicity in patients suffering from adrenal insufficiency (Forss et al. 2012; Johannsson et al. 2009), thereby replacing therapies requiring multiple doses per day. Together, these studies highlight the benefits of novel formulations with systematically manipulated exposure profiles, to aide in the development of improved chronic synthetic GC treatment options.
The maintenance of homeostatic circadian rhythms in the HPA axis is dependent on an intricate balance between the temporally-varying feedforward and feedback processes within the HPA network. Given this variation in regulatory dynamics of the HPA axis, chronopharmacological dosing can reduce the disruption of the endogenous cortisol rhythm. Indeed, our simulations suggest that once-daily administration of synthetic GCs shortly after the start of the active phase (around 6:00 AM for fast-acting oral GCs or 9:00 AM for a bolus injection, in our case) can minimize the suppression of the endogenous cortisol rhythm, by largely preserving its amplitude and acrophase. Moreover, the simulated suppression of the cortisol rhythm after the first dose is in qualitative agreement with experimental findings exploring the short-term influence of the synthetic GC administration of endogenous cortisol rhythmicity. A number of studies have found that administration of a single dose of synthetic GCs by infusion in the morning results in minimal disruption of the endogenous cortisol rhythm, while evening administration is associated with a substantial suppressive effect (Haus et al. 2012a). Long-term daily administration of synthetic GCs by bolus injection or a fast-acting oral dose in the latter half of the active phase (late afternoon in humans) is predicted to result in maximal suppression of cortisol rhythm.
In addition to the changes in amplitude, there were substantial alterations in the acrophase of the cortisol rhythm upon long-term once-daily administration of synthetic GCs. The acrophase of the circadian rhythm of critical signaling hormones, such as cortisol, is tightly regulated and is thought to enable the host to optimally separate physiologically incompatible processes to different times of the day (Buijs et al. 2003; Sukumaran et al. 2010). Disruptions in the appropriate circadian activity of cortisol are associated with a number of health problems (Potter et al. 2016). Therefore, understanding the influence of dosing on the acrophase of the endogenous cortisol rhythm is particularly important.
Interestingly, models simulations predicted that the time of dosing could be varied such that the acrophase of the cortisol rhythm adopted two different values for roughly the same change in amplitude. The acrophase of the rhythm was most sensitive for chronic dosing regimens that resulted in high plasma concentrations of synthetic GCs towards the end of active phase (late afternoon). The acrophase response exhibited a sharp discontinuity at high doses. Such an acrophase response has been well documented in the study of phase response curves (PRC) for other circadian oscillators, with transition from continuous PRCs (Type 1) to discontinuous PRCs occurring upon exposure of the circadian systems to large perturbations (Johnson 1992). Moreover, dosing times resulting in maximal amplitude suppression were also associated with the greatest resetting in the acrophase of the rhythm. These observations are in agreement with experimental studies on the phase-resetting behavior, in response to a light pulse, of the mammalian circadian clock in individual fibroblasts (Pulivarthy et al. 2007; Spoelstra et al. 2004). Therefore, daily dosing of the bolus injection or fast-acting synthetic GCs near the beginning of the subjective night and at high doses is predicted to be least favorable due to the maximal disruption of the endogenous cortisol rhythm.
Despite synthetic GCs having an apparent inhibitory influence in the short-term, certain chronopharmacological dosing regimens can result in the induction of HPA axis activity after chronic use. Put another way, the activity of the HPA axis following the first dose was not predictive of the HPA axis in response to long-term treatment. At the nominal (1×) dose strength, model simulations predicted that a once-daily bolus injection of synthetic GCs at 3:00 AM, a fast-acting daily oral dose at 12:00 AM, or a slow-acting daily oral dose at 7:00 PM, during the simulated night, resulted in an induction of HPA axis activity. Moreover, our results suggested that the observed induction in HPA axis activity is linked to a regulatory change associated with the repeated administration of the synthetic GCs at only low to moderate doses, since administration at high dose strengths leads to suppression in HPA axis activity regardless of dosing time. Interestingly, such a time-of-day dependent increase in amplitude is in agreement with observations from the study by Kirwan et al., where the daily administration of low dose (5mg) MR-prednisolone such that it was released at 2:00 AM, during the rising phase of the cortisol rhythm, resulted in an increase in the circadian maxima and a decrease in circadian minima of the cortisol rhythm in RA patients after a 2-week dosing period (Buttgereit et al. 2013; Kirwan et al. 2010). However, an increase in the cortisol amplitude does not necessarily imply an increase in the total endogenous cortisol exposure (as measured by the 24-hour AUC). Once-daily IV administration of a nominal (1×) dose of synthetic GCs near the start of the active phase resulted in a suppression of the total endogenous cortisol exposure, but either increased the amplitude (for dosing between 6:00 to 8:00 AM) or preserved the amplitude of the endogenous cortisol rhythm (for dosing around 9:00 AM). Despite the increased amplitude, the decrease in the 24-hour AUC might still be indicative of an overall suppression of HPA axis activity. On the other hand, the enhanced circadian rhythmicity might also suggest that the chronic chronopharmacological intervention enables the system to more efficiently restrict the high cortisol levels to a given time of the day, and thereby, separate conflicting downstream GC-responsive physiological processes (Sukumaran et al. 2010).
Finally, an important indicator of HPA axis activity involves its ability to mount an appropriate response to stressors. Model simulations predict that altering the rhythmic characteristics of cortisol through chronopharmacological dosing modifies the responsiveness of the HPA axis. Decreased cortisol amplitude upon chronopharmacological dosing is predicted to result in a dampening in the time-of-day dependence of the response to CRH stimulation, while an increase in the amplitude of the cortisol circadian rhythm is associated with a more robust time-of day-dependent response to CRH stimulation. Interestingly, Kirwan et al. found that RA patients who exhibited an induction in cortisol amplitude after daily administration of MR-prednisolone also had a more robust cortisol response to CRH stimulation (Kirwan 2011b). In partial agreement with these experimental results, our simulations predict that an increase in cortisol amplitude associated after chronopharmacological synthetic GC administration can lead to enhanced HPA responsiveness in a time-dependent manner.
While previous models have successfully studied the time-dependence of cortisol suppression after a single dose (Xu et al. 2008), our simulations can also explore the adaptability and responsiveness of the HPA axis following repeated administration. By accounting for a more physiologically relevant representation of the interactions between feedforward and feedback processes in the HPA network, our model predicts that synthetic GCs can have a complex non-trivial influence on HPA axis activity that might not be captured by simpler mathematical representations, which do not account for endogenous circadian rhythmicity. In doing so, we further emphasize the importance of using multiple metrics (circadian amplitude, acrophase, AUC and responsiveness) to comprehensively understand the alterations in HPA axis activity in response to chronopharmacological intervention. The current model may be augmented with a physiologically-based pharmacokinetic model that accounts for the nonlinear dynamics associated with some synthetic GCs. These complexities arise from competitive binding to the corticosteroid binding globulin (CBG) and to interconversion between pharmacologically active and inactive forms by 11β-hydroxysteroid dehydrogenase type1/2 for both endogenous and synthetic GCs (Czock et al. 2005; Xu et al. 2007; Xu et al. 2008). Furthermore, these proteins exhibit their own circadian rhythmicity (Angeli et al. 1977; Malisch et al. 2008), which can complicate the chronopharmacological relationship between dose, drug exposure, and response. The feedback mechanisms underlying dysregulation of the HPA axis are thought to be a result of the imbalance between GR and mineralocorticoid receptors (Harris et al. 2013). As such, the disruption of the HPA axis following administration of synthetic GCs can be studied more thoroughly considering the activity of both receptors.
Additionally, physiological factors, such as chronotype, sex, age, ethnicity or disease state, contribute to significant variation in pharmacokinetics (Hartmanshenn et al. 2016) as well as the underlying regulatory mechanisms controlling HPA axis activity and responsiveness (Cutolo et al. 2005; Ebner and Singewald 2017; Kouri et al. 2013; Oster et al. 2017; Rao and Androulakis 2017). To incorporate such factors, the model may be re-parameterized to reflect the physiology or clinical scenarios of interest to match the basal cortisol rhythm, and thus HPA axis activity, to the desired population. Moreover, clinical studies have shown that differences in pharmacokinetics (i.e. clearance) are often balanced by differences in pharmacodynamics (i.e. receptor affinity) such that dose adjustment of synthetic GCs may not be needed across subgroups (Czock et al. 2005; Magee et al. 2001). A model-based methodology may be particularly useful to explain the physiological mechanisms underlying these clinical outcomes, and to evaluate the need for therapeutic dose monitoring of chronic synthetic GC treatment across patient subgroups.
Supplementary Material
Acknowledgments
Funding
RTR and IPA acknowledge support from NIH Grant GM 24211. MLS is supported by a Bristol-Myers Squibb Doctoral Fellowship.
Footnotes
Declaration of Interest Statement
The authors have no conflicts of interest to declare.
References
- Alangari AA. 2010. Genomic and non-genomic actions of glucocorticoids in asthma. Ann Thorac Med. 5(3):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli A, Frajria R, Bisbocci D, Ceresa F. 1977. Temporal changes in plasma transcortin (cbg) binding capacity during the menstrual cycle. Biological Rhythm Research. 8(3–4):237–242. [Google Scholar]
- Arlt W, Stewart PM. 2005. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 34(2):293–313, viii. [DOI] [PubMed] [Google Scholar]
- Bae S-A, Androulakis IP. 2017. The synergistic role of light-feeding phase relations on entraining robust circadian rhythms in the periphery. Gene Regul Syst Bio. 11:1177625017702393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta A, Innominato PF, Dallmann R, Rand DA, Levi FA. 2017. Systems chronotherapeutics. Pharmacol Rev. 69(2):161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrametti SP, Ianniello A, Ricci C. 2016. Chronotherapy with low-dose modified-release prednisone for the management of rheumatoid arthritis: A review. Ther Clin Risk Manag. 12:1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodick N, Lufkin J, Willwerth C, Kumar A, Bolognese J, Schoonmaker C, Ballal R, Hunter D, Clayman M. 2015. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: A randomized clinical trial. J Bone Joint Surg Am. 97(11):877–888. [DOI] [PubMed] [Google Scholar]
- Bordyugov G, Abraham U, Granada A, Rose P, Imkeller K, Kramer A, Herzel H. 2015. Tuning the phase of circadian entrainment. J R Soc Interface. 12(108):20150282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. 2003. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. J Endocrinol. 177(1):17–26. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Burmester GR, Lipworth BJ. 2005. Optimised glucocorticoid therapy: The sharpening of an old spear. Lancet. 365(9461):801–803. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, Supronik J, Szombati I, Romer U, Witte S et al. 2013. Low-dose prednisone chronotherapy for rheumatoid arthritis: A randomised clinical trial (capra-2). Ann Rheum Dis. 72(2):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirincione B, Edwards J, Mager DE. 2017. Population pharmacokinetics of an extended-release formulation of exenatide following single- and multiple-dose administration. AAPS J. 19(2):487–496. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, Kovac SH, Spettell CM, Saag KG. 2006. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 55(3):420–426. [DOI] [PubMed] [Google Scholar]
- Cutolo M 2016. Glucocorticoids and chronotherapy in rheumatoid arthritis. RMD Open. 2(1):e000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Villaggio B, Otsa K, Aakre O, Sulli A, Seriolo B. 2005. Altered circadian rhythms in rheumatoid arthritis patients play a role in the disease’s symptoms. Autoimmunity Reviews. 4(8):497–502. [DOI] [PubMed] [Google Scholar]
- Czock D, Keller F, Rasche FM, Haussler U. 2005. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 44(1):61–98. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 72:517–549. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. 2017. Individual differences in stress susceptibility and stress inhibitory mechanisms. Current Opinion in Behavioral Sciences. 14:54–64. [Google Scholar]
- Edwards C 2012. Sixty years after hench--corticosteroids and chronic inflammatory disease. J Clin Endocrinol Metab. 97(5):1443–1451. [DOI] [PubMed] [Google Scholar]
- Fardet L, Petersen I, Nazareth I. 2012. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 169(5):491–497. [DOI] [PubMed] [Google Scholar]
- Fietta P, Fietta P, Delsante G. 2009. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci. 63(5):613–622. [DOI] [PubMed] [Google Scholar]
- Fisher LE, Ludwig EA, Wald JA, Sloan RR, Middleton E Jr., Jusko WJ. 1992. Pharmacokinetics and pharmacodynamics of methylprednisolone when administered at 8 am versus 4 pm. Clin Pharmacol Ther. 51(6):677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss M, Batcheller G, Skrtic S, Johannsson G. 2012. Current practice of glucocorticoid replacement therapy and patient-perceived health outcomes in adrenal insufficiency - a worldwide patient survey. BMC Endocr Disord. 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin BC. 1965. Oscillatory behavior in enzymatic control processes. Adv Enzyme Regul. 3:425–438. [DOI] [PubMed] [Google Scholar]
- Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. 2013. Mineralocorticoid and glucocorticoid receptor balance in control of hpa axis and behaviour. Psychoneuroendocrinology. 38(5):648–658. [DOI] [PubMed] [Google Scholar]
- Hartmanshenn C, Scherholz M, Androulakis IP. 2016. Physiologically-based pharmacokinetic models: Approaches for enabling personalized medicine. J Pharmacokinet Pharmacodyn. 43(5):481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E, Sackett-Lundeen L, Smolensky MH. 2012a. Rheumatoid arthritis: Circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull NYU Hosp Jt Dis. 70 Suppl 1:3–10. [PubMed] [Google Scholar]
- Haus E, Sackett-Lundeen L, Smolensky MH. 2012b. Rheumatoid arthritis: Circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull NYU Hosp Jt Dis. 70 Suppl 1:3–10. [PubMed] [Google Scholar]
- Hench PS, Kendall EC, Slocumb CH, Polley HF. 1949. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: Compound e) and of pituitary adrenocortical hormone in arthritis: Preliminary report. Ann Rheum Dis. 8(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JL, Weiss RE. 2014. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 30(2):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson G, Bergthorsdottir R, Nilsson AG, Lennernas H, Hedner T, Skrtic S. 2009. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: A pharmacokinetic study. Eur J Endocrinol. 161(1):119–130. [DOI] [PubMed] [Google Scholar]
- Johnson CH. 1992. Phase response curves: What can they tell us about circadian clocks. Circadian clocks from cell to human. 209–249. [Google Scholar]
- Jung CM, Khalsa SB, Scheer FA, Cajochen C, Lockley SW, Czeisler CA, Wright KP Jr. 2010. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 25(3):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. 2010. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 22(5):362–372. [DOI] [PubMed] [Google Scholar]
- Kirwan JR. 2011a. Targeting the time of day for glucocorticoid delivery in rheumatoid arthritis. Int J Clin Rheumatol. 6(3):273–279. [Google Scholar]
- Kirwan JR. 2011b. Targeting the time of day for glucocorticoid delivery in rheumatoid arthritis. International Journal of Clinical Rheumatology. 6(3):273–279. [Google Scholar]
- Kirwan JR, Clarke L, Hunt LP, Perry MG, Straub RH, Jessop DS. 2010. Effect of novel therapeutic glucocorticoids on circadian rhythms of hormones and cytokines in rheumatoid arthritis. Ann N Y Acad Sci. 1193:127–133. [DOI] [PubMed] [Google Scholar]
- Kouri V-P, Olkkonen J, Kaivosoja E, Ainola M, Juhila J, Hovatta I, Konttinen YT, Mandelin J. 2013. Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS ONE. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Schibler U. 2007. Circadian rhythms: Mechanisms and therapeutic implications. Annual Review of Pharmacology and Toxicology. 47(1):593–628. [DOI] [PubMed] [Google Scholar]
- Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. 2013. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 9(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee MH, Blum RA, Lates CD, Jusko WJ. 2001. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. 41(11):1180–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T Jr. 2008. Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 156(2):210–217. [DOI] [PubMed] [Google Scholar]
- Markovic VM, Cupic Z, Vukojevic V, Kolar-Anic L. 2011. Predictive modeling of the hypothalamic-pituitary-adrenal (hpa) axis response to acute and chronic stress. Endocr J. 58(10):889–904. [DOI] [PubMed] [Google Scholar]
- Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP. 2014. Mathematical modeling of light-mediated hpa axis activity and downstream implications on the entrainment of peripheral clock genes. Physiological Genomics. 46(20):766–778. [DOI] [PubMed] [Google Scholar]
- McMaster A, Ray DW. 2007. Modelling the glucocorticoid receptor and producing therapeutic agents with anti-inflammatory effects but reduced side-effects. Exp Physiol. 92(2):299–309. [DOI] [PubMed] [Google Scholar]
- Moghadam-Kia S, Werth VP. 2010. Prevention and treatment of systemic glucocorticoid side effects. International journal of dermatology. 49(3):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development-part 2: Introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ. 2008. Modified-release hydrocortisone for circadian therapy: A proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol (Oxf). 68(1):130–135. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Charmandari E, Kino T, Chrousos GP. 2017. Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact on health. Front Endocrinol (Lausanne). 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohdo S, Koyanagi S, Matsunaga N. 2010. Chronopharmacological strategies: Intra- and inter-individual variability of molecular clock. Adv Drug Deliv Rev. 62(9–10):885–897. [DOI] [PubMed] [Google Scholar]
- Oster H, Challet E, Ott V, Arvat E, Ronald de Kloet E, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. 2017. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 38(1):3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HT, Bouak F, Vartanian O, Cheung B. 2013. A physiologically based pharmacokinetics model for melatonin--effects of light and routes of administration. Int J Pharm. 458(1):156–168. [DOI] [PubMed] [Google Scholar]
- Pierre K, Schlesinger N, Androulakis IP. 2016. The role of the hypothalamic-pituitary-adrenal axis in modulating seasonal changes in immunity. Physiological Genomics. 48(10):719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Schlesinger N, Androulakis IP. 2017. The hepato-hypothalamic-pituitary-adrenal-renal axis: Mathematical modeling of cortisol’s production, metabolism and seasonal variation. Journal of Biological Rhythms. In Press. [DOI] [PubMed] [Google Scholar]
- Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ. 2016. Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocr Rev. 37(6):584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulivarthy SR, Tanaka N, Welsh DK, De Haro L, Verma IM, Panda S. 2007. Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci U S A. 104(51):20356–20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, DuBois DC, Almon RR, Pyszczynski NA, Jusko WJ. 2002. Fifth-generation model for corticosteroid pharmacodynamics: Application to steady-state receptor down-regulation and enzyme induction patterns during seven-day continuous infusion of methylprednisolone in rats. J Pharmacokinet Pharmacodyn. 29(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, DuBois D, Almon R, Jusko WJ, Androulakis IP. 2016. Mathematical modeling of the circadian dynamics of the neuroendocrine-immune network in experimentally induced arthritis. Am J Physiol Endocrinol Metab. 311(2):E310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RT, Androulakis IP. 2017. Modeling the sex differences and interindividual variability in the activity of the hypothalamic-pituitary-adrenal axis. Endocrinology. 158(11):4017–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. 2005. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 353(16):1711–1723. [DOI] [PubMed] [Google Scholar]
- Schmal C, Myung J, Herzel H, Bordyugov G. 2015. A theoretical study on seasonality. Front Neurol. 6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. 2006. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 8(4):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Peppas NA. 2007. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev. 59(9–10):828–851. [DOI] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, Lightman SL. 2014. Hpa axis-rhythms. In: Terjung R, editor. Comprehensive physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc; p. 1273–1298. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, Albrecht U, van der Horst GT, Brauer V, Daan S. 2004. Phase responses to light pulses in mice lacking functional per or cry genes. J Biol Rhythms. 19(6):518–529. [DOI] [PubMed] [Google Scholar]
- Sriram K, Rodriguez-Fernandez M, Doyle FJ. 2012. Modeling cortisol dynamics in the neuro-endocrine axis distinguishes normal, depression, and post-traumatic stress disorder (ptsd) in humans. PLoS Computational Biology. 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Almon RR, DuBois DC, Jusko WJ. 2010. Circadian rhythms in gene expression: Relationship to physiology, disease, drug disposition and drug action. Adv Drug Deliv Rev. 62(9–10):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Winkler J, Derendorf H. 2007. A pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma. J Pharmacokinet Pharmacodyn. 34(3):355–372. [DOI] [PubMed] [Google Scholar]
- Xu J, Winkler J, Sabarinath SN, Derendorf H. 2008. Assessment of the impact of dosing time on the pharmacokinetics/pharmacodynamics of prednisolone. AAPS J. 10(2):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.