Abstract
To generate chimeric Ag receptors (CARs) for the adoptive immunotherapy of cancer patients with ErbB2-expressing tumors, a single-chain Ab derived from the humanized mAb 4D5 Herceptin (trastuzumab) was initially linked to T cell signaling domains derived from CD28 and the CD3ζ to generate a CAR against ErbB2. Human PBLs expressing the 4D5 CAR demonstrated Ag-specific activities against ErbB2+ tumors. However, a gradual loss of transgene expression was noted for PBLs transduced with this 4D5 CAR. When the CD3ζ signaling domain of the CAR was truncated or mutated, loss of CAR expression was not observed, suggesting that the CD3ζ signaling caused the transgene decrease, which was supported by the finding that T cells expressing 4D5 CARs with CD3ζ ITAM mutations were less prone to apoptosis. By adding 4–1BB cytoplasmic domains to the CD28-CD3ζ signaling moieties, we found increased transgene persistence in 4D5 CAR-transduced PBLs. Furthermore, constructs with 4–1BB sequences demonstrated increased cytokine secretion and lytic activity in 4D5 CAR-transduced T cells. More importantly, PBLs expressing this new version of the 4D5 CAR could not only efficiently lyse the autologous fresh tumor digests, but they could strongly suppress tumor growth in a xenogenic mouse model.
Adoptive cell therapy (ACT)3 has emerged as the most effective treatment for patients with metastatic melanoma. An immunodepleting preparative regimen followed by ACT of tumor-reactive autologous tumor-infiltrating lymphocytes (TILs) resulted in the clonal repopulation of patients with antitumor T cells (1). Of patients with metastatic melanoma refractory to all other treatments, 50% experienced objective clinical regression, some with complete responses (2). Intensifying the lymphodepletion by adding total-body irradiation to the chemo-therapy conditioning regimen improved the therapeutic results of ACT, resulting in a 72% objective response rate (3). However, TILs with high avidity for tumor Ags can only be generated from patients with melanoma and, therefore, a need exists for the generation of T cells with broad reactivity against shared cancer-associated Ags present on common epithelial tumors.
The ability to introduce genes into circulating human lymphocytes provides the flexibility to introduce Ag receptors as well as molecules that can provide the cell with enhanced properties required for effective ACT (4–8). The first clinical trial to successfully mediate the regression of human cancer by ACT using genetically engineered autologous lymphocytes was recently published (9). Seventeen patients were treated with a TCR that was reactive with the MART-1 melanoma Ag isolated from highly reactive TILs. Two patients with metastatic melanoma who received ACT of their autologous normal lymphocytes transduced with genes encoding this MART-1 TCR experienced long-term objective regression of metastatic tumor, and 2 of an additional 14 patients also experienced objective tumor regressions for a response rate of 13% (10). The decreased response rate using TCR-modified lymphocytes for the treatment of cancer patients, compared with the use of TILs, implies that further optimization of TCR gene therapy is required. Additionally, current TCR-based gene therapies target HLA-A2-restricted epitopes, which greatly lessens the number of patients that can be treated by adoptive immunotherapy.
A chimeric Ag receptor (CAR) is an artificially constructed hybrid protein containing the Ag binding domains of a single-chain Ab (scFv) linked to T cell signal domains. The main characteristics of CARs are their ability to redirect T cell specificity and killing/effector activity toward a selected target in a non-MHC-restricted manner, exploiting the Ag-binding properties of mAbs (11, 12). This non-MHC-restricted Ag recognition gives T cells expressing CARs the potential ability to recognize tumor cells in patients independent of HLA status, and thus these cells may be able to effectively treat tumors that have lost or down-regulated HLA (a major mechanisms of tumor immune escape). While CAR-engineered T cells would not be impacted by HLA down-regulation, Ag loss has been observed in murine models of ACT, and such events would abrogate the function of any CAR-based gene therapy (13, 14).
ErbB2 (HER-2/Neu) represents one of the most studied targets for cancer-specific therapy. Herceptin (trastuzumab), a mAb directed against the extracellular domain of ErbB2, is therapeutically active in ErbB2 overexpressing breast carcinomas. However, a consistent number of ErbB2-positive tumors are not responsive to Herceptin-driven therapy, indicating the need for a better understanding of the mechanism of action of this Ab in vivo and suggesting the need to develop additional therapies targeting ErbB2. CARs against ErbB2 have been well characterized and tested in vitro and in animal models (15–19). Nevertheless, several limitations need to be overcome before ErbB2 CAR-modified T cells can be applied clinically. ErbB2-based CARs reported thus far are composed of scFv from murine mAbs, which have been shown to induce anti-CAR immune responses in human clinical trials (20,21). scFv-based CARs engineered to contain a signaling domain from CD3ζ or FcRγ have been shown to deliver a potent signal for T cell activation and function, but they were not sufficient to elicit substantial IL-2 secretion in the absence of a concomitant costimulatory signal (12, 22). It has been demonstrated that CARs containing the signal transduction domain of CD28 and/or 4–1BB, or other costimulatory molecules, enhanced the function of the gene-modified T cells (12, 23, 24). Therefore, a new generation of CARs containing both humanized scFv and optimized costimulatory signaling domains may be optimal for clinical trials using CAR-based genetically engineered T cells for the treatment of cancer patients.
We have assessed the function of human T cells expressing a CAR that is composed of scFv derived from a humanized mAb 4D5 (Herceptin) (25) followed by signaling domains of CD28 and CD3ζ. We found a consistent transgene decrease over time that was associated with activation-induced cell death (AICD) of transduced T cells expressing low levels of ErbB2. Here we describe that optimization of this CAR by the addition of costimulatory signaling domains from both CD28 and 4–1BB lead to maintained transgene expression by increasing the expression of an antiapoptosis gene Bcl-xL, as well as increased cytokine secretion, lytic activity, and in vivo antitumor activity of the transduced T cells.
Materials and Methods
PBLs and cell lines
All of the PBMCs used in this study were from metastatic melanoma patients treated under approved protocols at the Surgery Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD. Melanoma cell line 624.38mel (HLA-A2+, NY-ESO-1+, ErbB2+), and fresh tumor digests were generated at the Surgery Branch. ErbB2-positive tumor lines SK-OV3, SK-BR3, BT-474, MDA361, MDA231, MCF-7, CLL-222, and CRL-1740 and ErbB2-negative tumor lines MDA468 and CCRF-CEM (CEM) were provided by American Type Culture Collection. All cell lines were cultured in medium consisting of RPMI 1640 (or DMEM) supplemented with 10% heat-inactivated FBS (Biofluids), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Lymphocytes were cultured in AIM-V medium (Invitrogen) supplemented with 5% human AB serum (Valley Biomedical) and 300 IU/ml IL-2 (Chiron) at 37°C and 5% CO2.
Generation of retroviral constructs
The ErbB2-specific scFv 4D5 (25) sequence derives from the humanized mAb that was used to produce Herceptin. The sequence for the anti-VEGFR2-specific scFv 2C6 (26) was derived from a human Ab. Both CARs were designed and synthesized by PCR using a web-based DNA codon optimization algorithm (27). Supplemental Fig. 1 shows the sequences of the 4D5 and 2C6 scFvs (including the linker sequence) and the primers used to synthesis these genes.4 The synthesized DNA fragments were sequence confirmed and subcloned in frame into MSGV-1-based vector (28) containing CD28 and CD3ζ signaling moieties (12) to generate MSGV-4D5–28Z or MSGV-2C6–28Z. Variant signaling domains were constructed by mega-primer overlap PCR of specific signaling domains and assembled in the order described in figure legends (details available upon request). An MSGV-1-based vector encoding TCRα and TCRβ specific for NY-ESO-1 (MSGV-1G4-AIB) was described previously (29). FMC63–28Z is a CAR vector in which scFv against human CD19 derived from mAb FMC63 as described (30) was constructed and subcloned into MSGV-1-based retroviral vector containing CD28-CD3ζ cassette. A trinitrophenyl-specific CAR, SP6–28Z CAR (22), and a CAR containing the extracellular domain of LNGFR were used as controls.
Retrovirus vector production and transduction of T cells
Retroviral vector supernatant was produced from stable packaging cell lines as described (29) or by transient transfection. To generate transient viral supernatant, 293GP cells (Clontech Laboratories) were cotransfected with retroviral vector plasmid and envelope encoding plasmid RD114 using Lipofectamine 2000 reagent (Invitrogen). Supernatants containing the retrovirus were collected 48 and 72 h later. OKT3-activated T cells were transduced with retroviral vectors as described (29). Briefly, PBMCs were activated with OKT3 at a final concentration of 50 ng/ml with recombinant human IL-2 at a final concentration of 300 IU/ml. Cells were harvested for retroviral transduction on day 2 and applied to RetroNectin (CH-296; Takara Shuzo)-coated non-tissue culture-treated 24-well plates. After transduction, the cells were cultured in the presence of 300 IU/ml IL-2 until use.
Real-time PCR
Genomic DNA was isolated using a QIAamp DNA blood kit (Qiagen) according to the manufacturer’s instruction. One hundred nanograms of each DNA was used for the real-time quantitative PCR assay (TaqMan; Applied Biosystems). Total RNA was isolated using RNeasy Mini Kit (Qiagen). One microgram of total RNA was used in the first strand of cDNA synthesis reaction using ThermoScript RT-PCR system (Invitrogen) and diluted 10-fold with RNA-free water after the reaction. One tenth of the diluted reaction mix was used later for the real-time quantitative PCR. All PCR reactions were performed using an ABI 7500 Fast real-time PCR system instrument (Applied Biosystems). The TaqMan gene specific assay was designed by ABI Assays-by-Designs software (Applied Biosytems). Primers and probe used for detection of ErbB2 are: ErbB2 forward, 5’-GCCTCCACTTCAACCACAGT-3’; ErbB2 reverse, 5’-TCAAACGTG TCTGTGTTGTAGGT-3’; ErbB2 probe, FAM-CAGTGCAGCTCACA GATG. Primers and probe used for detection of MSGV 3’LTR that is transcribed together with the transgenes are: LTR forward, 5’-TGCAAG GCATGGAAAATACATAACTGA-3’; LTR reverse, 5’-CACAGAT ATCCTGTTTGGCCCATAT-3’; and LTR probe, FAM-TCTCTCTGTTC CTAACCTTG. The reference standard curve was established using the plasmid DNA encoding target sequence. A TaqMan β-actin control re-agents kit (Applied Biosytems) was used to normalize reactions to input RNA/DNA amounts.
FACS analysis
Abs were obtained from the following suppliers: anti-human CD3, CD4, CD8, and NKG2D (BD Biosciences), Ab to Bcl-xL (Santa Cruz Biotechnology), and anti-human Vβ13.1 reactive with the NY-ESO-1 TCR 1G4 (Immunotech). Matched isotype control Abs were used in all analyses. Cell surface expression of ErbB2 was detected by anti-ErbB2 affibody (Abcam). ErbB2 and VEGFR2 specific CAR expression was detected by ErbB2-Fc or VEGFR2-Fc fusion protein (R&D Systems), respectively, followed by PE-conjugated anti-human IgG Fc Ab (eBioscience). For analysis, the relative log fluorescence of live cells was determined using a FACScan flow cytometer with CellQuest software (BD Biosciences).
Cytokine release assays
PBL cultures were tested for reactivity in cytokine release assays using commercially available ELISA kits (IFN-γ, IL-2, and TNF-α; Endogen). Stimulator cells and responder cells were cocultured for 24 h as described(8). Cytokine secretion was measured in culture supernatants diluted to be in the linear range of the assay.
51Cr release assay
The ability of the T cells to lyse tumor target cells was measured using a 51Cr release assay as described previously (31). Briefly, 1 × 106 target cells were labeled for 1 h at 37°C with 200 μCi of 51Cr sodium chromate (GE Healthcare). Labeled target cells (5 × 103) were incubated with effector cells at the ratios indicated in the text for 4 h at 37°C in 0.2 ml of culture medium. Harvested supernatants were counted using a Wallac 1470 Wizard gamma counter (PerkinElmer). Total and spontaneous 51Cr release was determined by incubating 5 × 103 labeled targets in either 2% SDS or culture medium for 4 h at 37°C. Each datum point was determined as an average of quadruplicate wells. The percentage specific lysis was calculated as: % specific lysis = [(specific 51Cr release – spontaneous 51Cr release)/(total 51Cr release – spontaneous 51Cr release)] × 100.
Ab blocking assay
Tumor cell lines (5 × 104 cells/100 μl) were incubated with each mAb at a concentration of 10 μg/ml for 30 min at 37°C in a flat-bottom 96-well plate.
T cells (5 × 104 cells/well) were then added and incubated with target cells overnight at 37°C. The supernatants were harvested and assayed for IFN-γ production by ELISA. The anti-ErbB2 Abs used were mAb N29(15) and an anti-ErbB2 IgG fraction (Austral Biologicals). An anti-MAGE1 mAb (Austral Biologicals) was used as control.
Mice and tumor model
SCID mice were transplanted with pellets of 1β-estradiol at day–2 and then injected orthotopically (into the mammary fat pad) with 3 × 106 BT-474 human breast cancer cells (with Matrigel) on day 0. At day 7 mice received 200 mg/kg cyclophosphamide (i.p.). At day 10, 12 mice were i.v. injected with 1.5–2 × 106 human PBLs transduced with the indicated CARs when the tumor was palpable. All mice received 2000 IU of IL-2(i.p.) twice a day for 7 days following cell administration. HBSS (no PBL) and SP6 CAR (anti-trinitrophenyl) served as controls. All protocols were approved by the Weizmann Institute of Science animal use committee.
Results
PBLs redirected with CARs derived from Herceptin were specifically reactive to ErbB2+ tumor lines
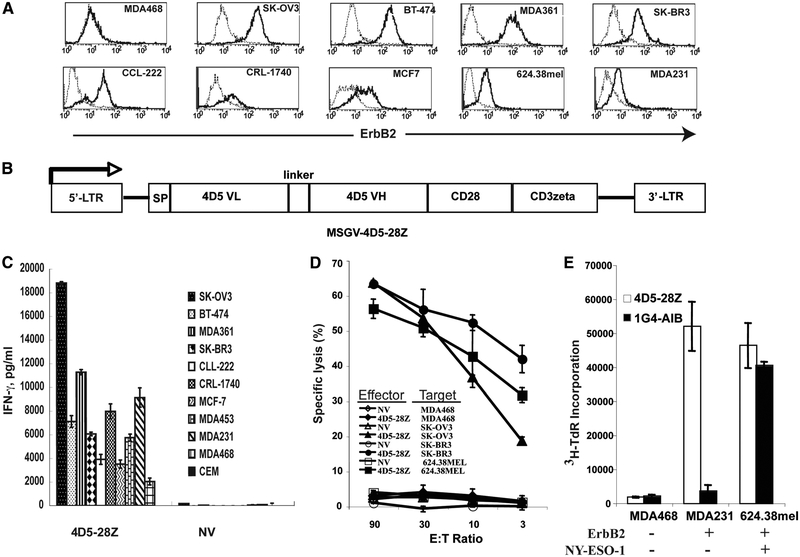
ErbB2 expression of a panel of tumor cell lines was examined via flow cytometry by staining the cells with an anti-ErbB2 Affibody molecule. The results show that ErbB2 expression was easily detected not only for breast tumor lines BT-474, MDA361, SK-BR3, MCF7, and MDA231, but also for the tumors from other origins, such as ovarian (SK-OV3), prostate (CRL-1740), colon (CCL-222), and melanoma (624.38mel) (Fig. 1A). A Herceptin-based CAR, consisting of scFv of mAb 4D5 linked to CD28 and CD3ζ signaling moieties (supplemental Fig. 1A), was constructed and cloned into a retroviral vector MSGV-1 and named MSGV-4D5–28Z (4D5–28Z) (Fig. 1B). To test if T cells expressing 4D5–28Z were capable of specifically recognizing tumor lines expressing ErbB2, the transduced PBLs were coincubated with a panel of tumor cell lines and the amount of secreted effector cytokine IFN-γ was determined. 4D5–28Z-transduced PBLs recognized all of the ErbB2+ tumor lines and secreted IFN-γ at high levels (Fig. 1C). A low level of IFN-γ was observed in cocultures with the MDA468 cell line, which was negative for ErbB2 expression by FACS analysis, while a second negative control line CEM did not induce cytokine release by engineered PBLs.
FIGURE 1.
Recognition of ErbB2-expressing tumor cell lines by Herceptin 4D5 CAR-transduced PBLs. A, ErbB2 expression of tumor cell lines. An Affibody reagent specific for ErbB2 was used to determine expression on tumor cell lines (solid line), and an isotype Ab was used as negative control (dotted line). B, A schematic representation of the retroviral vector MSGV-4D5–28Z (4D5–28Z) encoding the Herceptin (4D5)-based CAR against ErbB2. SP, signal peptide; VL, variable L chain, VH, variable H chain. Supplemental Fig. 1 shows the complete sequence of the CAR and primers used for synthesizing the scFv. C, Cytokine production of 4D5 CAR-transduced T cells. IFN-γ secretion of 4D5–28Z-transduced T cells following coculture with the indicated tumor lines. Nontransduced T cells (NV) were used as a negative control. D, Specific tumor cell lysis by 4D5 CAR-transduced T cells. 4D5 CAR-transduced PBLs (4D5–28Z) were tested in a 51Cr release assay, where nontransduced PBLs (NV) served as a control. PBLs were cocultured with 51Cr-labeled control tumor line MDA468 (ErbB2–), and ErbB2+ tumor lines SK-OV3, SK-BR3, and 624.38mel at the indicated E:T ratio for 4 h, after which the percentage lysis of target cells was calculated. E, Proliferation of 4D5 CAR-transduced PBLs. 4D5–28Z or NY-ESO-1 TCR (1G4-AIB)-transduced PBLs were cocultured with tumor lines MDA468 (ErbB2–/NY-ESO-1–), MDA231 (ErbB2+/NY-ESO-1–), and 624.38mel (ErbB2+/NY-ESO-1+) for 3 days. [3H]thymidine ([3H]TdR) was added for the final 17 h of culture and incorporation was measured. Data are representative of three experiments.
In addition to cytokine secretion, naturally occurring tumor-reactive T cells can both lyse tumor targets and are capable of Ag-driven proliferation. We next assayed for these abilities in 4D5-CAR-engineered T cells. As demonstrated by 51Cr release assay, 4D5–28Z-transduced PBLs were capable of specifically killing ErbB2+ tumors of different histology, including breast (SK-Br3), ovarian (SK-OV3), and melanoma (624.38mel) (Fig. 1D). Moreover, 4D5–28Z-engineered PBLs proliferated upon stimulation with ErbB2+ tumors (MDA231 and 624.38mel), similar to TCR-engineered cells when they were cocultured with Ag-positive tumor cells (Fig. 1E, control anti-NY-ESO-1 TCR 1G4-AIB and NY-ESO-1+ tumor line 624.38mel).
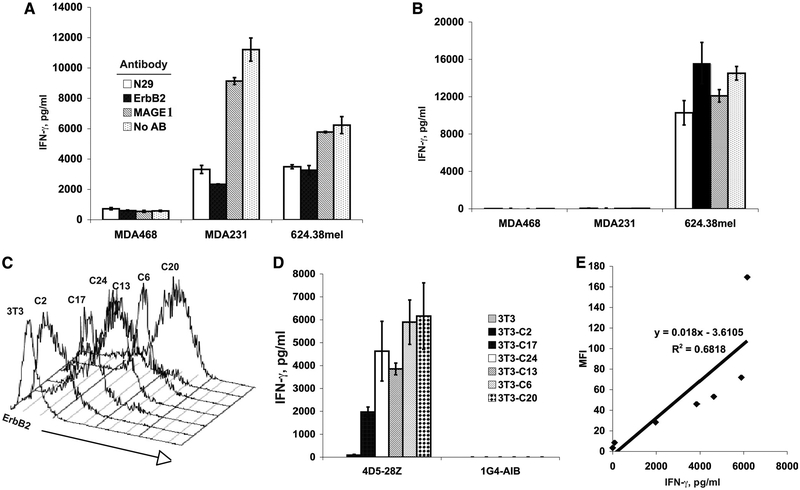
The specificity of the tumor recognition was confirmed by Ab blocking experiments. As shown in Fig. 2A, anti-ErbB2 Abs blocked the recognition of 4D5–28Z-transduced PBLs against ErbB2+ tumors MDA231 and 624.38mel, while no blocking was observed with an anti-MAGE1 control Ab. The lack of a blocking effect on NY-ESO-1 TCR-transduced PBLs (Fig. 2B) indicated that ErbB2 Ab blocking was Ag specific. To determine the correlation between ErbB2 expression and cytokine production, NIH3T3 cells were transfected with a plasmid DNA encoding human ErbB2 cDNA, and clones expressing ErbB2 at different levels were selected (Fig. 2C). IFN-γ secretion of 4D5–28Z-transduced PBLs upon coculture with these ErbB2-expressing NIH3T3 cell clones showed that cytokine production was highly correlated with ErbB2 expression on the surface of NIH3T3 cell lines (Fig. 2, D and E) and further confirmed that the 4D5 CAR recognition was Ag dependent.
FIGURE 2.
Recognition of anti-ErbB2 CAR-transduced T cells is Ag specific and correlates with Ag expression levels. Ab-blocking experiments of 4D5 CAR (A) and control NY-ESO-1 TCR (B) are shown. The transduced PBLs were cocultured with tumor cell lines in the presence of two anti-ErbB2 Abs: mouse mAb N29(15), or a commercially available anti-ErbB2 IgG fraction (ErbB2) as described in Materials and Methods. Controls included an anti-MAGE1 mAb (MAGE1) and a culture without Ab (No AB). Twenty hours after coculture, IFN-γ secretion was measured by ELISA. C, ErbB2 expression of the clones selected from ErbB2 cDNA plasmid-transfected NIH3T3 cells. D, IFN-γ production of 4D5 CAR (4D5–28Z)-transduced PBLs or control NY-ESO-1 TCR (1G4-AIB) transduced PBLs were cocultured with clones from ErbB2 cDNA-transfected NIH3T3 cells as shown in C for 20 h, and IFN-γ production was measured by ELISA. E, Correlation of ErbB2 expression (mean fluorescence intensity (MFI)) on ErbB2-transfected NIH3T3 clones was plotted vs the production of IFN-γ by 4D5 CAR-transduced PBLs cocultured with these NIH3T3 clones. Data are representative of two experiments.
Loss of transgene expression in 4D5–28Z CAR-transduced PBLs is mediated by CD3ζ signaling
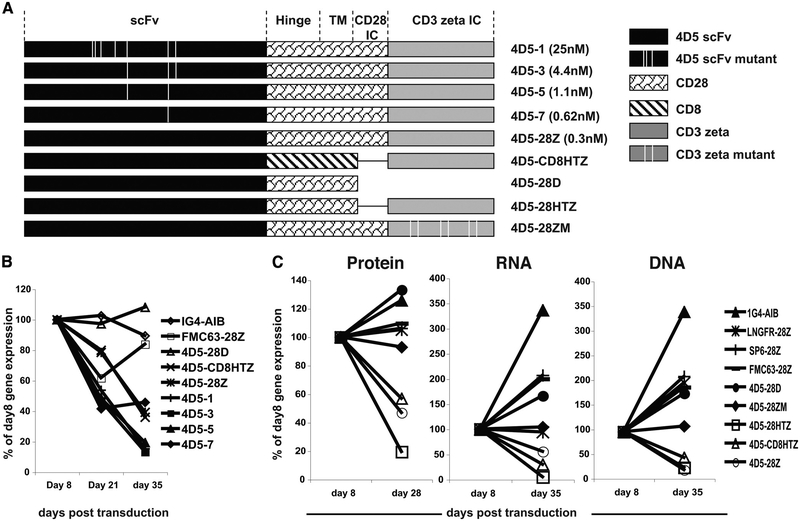
In our current T cell gene therapy clinical trials, transduced PBLs undergo strong T cell activation followed by transduction with γ-retroviral vectors and expansion ex vivo for 2–3 wk before being infused into patients. When 4D5–28Z-transduced PBLs were monitored for transgene expression over time, we consistently found that PBLs expressing 4D5–28Z showed decreased transgene expression over time (data not shown). To explore the mechanism for this observation, multiple 4D5-CAR constructs were assembled with alternative signaling domains and using variants of 4D5 with different affinities (Fig. 3A). Deleting all intracellular domains in construct 4D5–28D resulted in sustained transgene expression, similar to TCR- or control CAR-engineered cells (Fig. 3B). In construct 4D5-CD8HTZ, in which the signaling domain contained only the CD3ζ moiety and hinge and transmembrane regions from human CD8, transgene expression was also reduced, suggesting that the involvement of signals within CD3ζ were mediating the loss of expression. Similar to parent 4D5–28Z construct (with a native Ab affinity of 0.3 nM), all CARs using lower affinity scFvs showed transgene decrease as well, which indicated that the transgene decrease could not be prevented by decreasing the affinity of the ErbB2 scFv to 25 nM. These results strongly suggested that signaling from CD3ζ was responsible for the transgene decrease observed in transduced PBLs. To determine whether the 4D5-CAR target Ag ErbB2 was expressed in PBLs, ErbB2 expression in PBLs was assayed for by quantitative RT-PCR in five different donors (Table I). ErbB2 expression was observed in both the starting PBMC population and continued following T cell stimulation required to expand PBLs (a decrease in the total ErbB2 level was observed in all five donors during culture, and some variation in expression levels was noted).
FIGURE 3.
Transgene decrease was correlated with loss of transduced cells from the culture and was associated with CD3ζ signaling in the CAR. A, A schematic representation of the 4D5-based CAR constructs, including scFv, hinge region (Hinge), transmembrane region (TM), CD28 intracellular domain (CD28 IC), CD3ζ intracellular domain (CD3 ζ IC), and the hinge and transmembrane region of CD8α (CD8). All of the 4D5 mutant scFvs and CD3ζ mutations were generated by PCR-based site-directed mutagenesis using the parent 4D5–28Z as template according to the published sequences (25). The published affinity of the different 4D5 Ab variants is: parent 4D5–28Z (3 × 10–10 M), 4D5–1 (2.5 × 10–8 M), 4D5–3 (4.4 × 10–9 M), 4D5–5 (1.1 × 10–9 M), and 4D5–7 (6.2 × 10–10 M). The vertical line indicates the approximate location of the mutation. B, Transgene expression over time after transduction. PBLs were transduced with 4D5–28Z-based CARs with scFv at different affinities (4D5–28Z, 4D5–1, 4D5–3, 4D5–5, 4D5–7), a 4D5 CAR with hinge and transmembrane regions from human CD8α without CD28 signaling domain (4D5-CD8HTZ), and a 4D5 CAR with both CD28 and CD3ζ signaling domains being truncated (4D5–28D). Construct organization was as depicted in A. Controls included a CAR against CD19 (FMC63–28Z) and an anti-NY-ESO-1 TCR (1G4-AIB). Transgene expression was monitored at days 8, 21, and 35 after transduction by flow cytometry staining of the transduced PBLs using gene-specific reagents. Data shown are percentages of day 8 transgene expression of each transduced PBL population. C, PBLs were transduced with 4D5-based CARs with various signaling domain alterations: 4D5 CAR with CD28 and CD3ζ signaling domains being truncated (4D5–28D), 4D5 CAR with all three ITAMs within CD3ζ being mutated (4D5–28ZM), CD28 signaling domain being truncated from 4D5–28Z (4D5–28HTZ), 4D5 CAR without CD28 signaling domain and hinge/transmembrane region from CD8α (4D5-CD8HTZ), and the parent 4D5–28Z (4D5–28Z). As controls, PBLs were transduced with anti-NY-ESO-1 TCR (1G4-AIB), LNGFR CAR (LNGFR-28Z), SP6 CAR (SP6–28Z), and a CD19 CAR (FMC63–28Z). All mutant constructs were as depicted in A. Transgene expression was detected at indicated time by flow cytometry for protein using gene-specific reagents (left panel), for RNA using real-time quantitative RT-PCR (middle panel), and for DNA copy number determined by real-time quantitative PCR (right panel). Data shown are percentages of day 8 transgene expression of each transduced PBL population. Data are representative of three experiments.
Table I.
ErbB2 expression of OKT3-stimulated PBMCs (ErbB2 copies/106 β-actin)a
| Day 0 | Day 2 | Day 7 | Day 15 | Day 22 | |
|---|---|---|---|---|---|
| PBMC1 | 8,970 | 608 | 1,133 | 3,874 | 1,265 |
| PBMC2 | 6,451 | 1,528 | 1,918 | 1,870 | 780 |
| PBMC3 | 22,210 | 5,619 | 1,617 | 1,208 | 930 |
| PBMC4 | 8,344 | 522 | 2,095 | 1,355 | 2,422 |
| PBMC5 | 12,756 | 518 | 1,404 | 1,016 | 5,606 |
ErbB2 expression was detected by quantitative RT-PCR for PBMCs from five donors at time points as indicated. Cells were stimulated on day 0 with 50 ng/ml OKT3 and 300 IU/ml IL-2 followed by maintenance in IL-2-containing medium.
To further analyze the loss of transgene expression, gene activity and transduced cell persistence of the parent and mutant ErbB2-CARs were retested along with several control constructs. The control constructs included the NY-ESO-1 TCR (1G4-AIB), a construct made by linking the extracellular portion of LNGFR to CD28-CD3ζ (LNGFR-28Z), and two unrelated scFv CARs specific for a hapten molecule (SP6–28Z) or the B cell Ag CD19 (FMC63–28Z). All constructs were used to transduce PBLs and were examined for transgene expression by flow cytometry (Fig. 3C, left panel), vector-mediated expression at the RNA level by RT-quantitative PCR (Fig. 3C, middle panel), and vector copy number by quantitative PCR (Fig. 3C, right panel). Results demonstrated that the surface protein expression levels were highly correlated with RNA and DNA copy numbers. Decrease in trans-gene expression was found for ErbB2-directed CARs with an intact CD3ζ signaling moiety, such as 4D5–28HTZ (intact CD3ζ with hinge and transmembrane from CD28), 4D5-CD8HTZ (intact CD3ζ with hinge and transmembrane from CD8), and the parent 4D5–28Z. No transgene decrease was observed for the TCR (1G4-AIB) or any of the alternate CARs (LNGFR-28Z, SP6–28Z, and FMC63–28Z). The 4D5 CAR without CD3ζ (4D5–28D) or the 4D5 CAR in which all immunoreceptor tyrosine-based activation motifs (ITAM) were inactivated by mutations (4D5–28ZM) did not display transgene decrease. These results suggested that the trans-gene decrease was caused by the gradual loss of transduced PBLs from the culture and that the loss of transduced cells may be the result of AICD due to signaling transduced through CD3ζ.
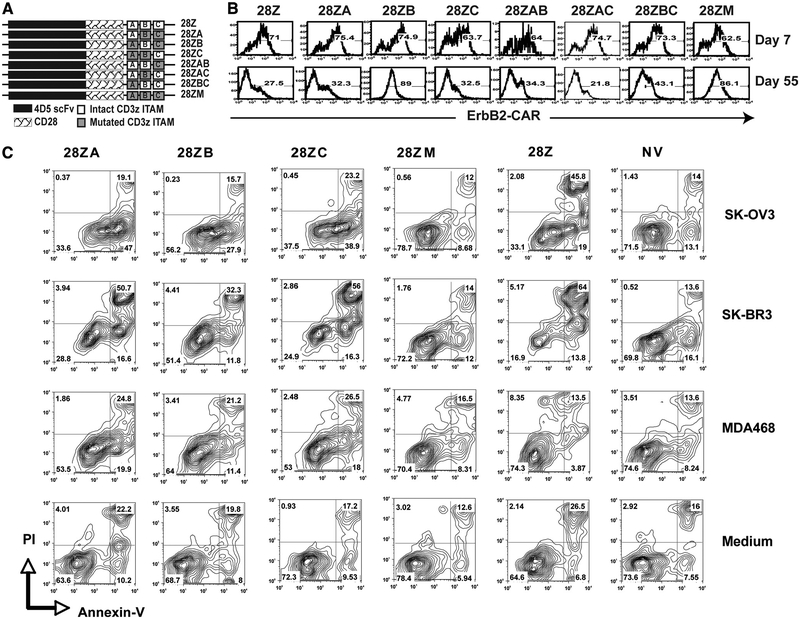
To determine which ITAMs in CD3ζ were responsible for the transgene decrease, a panel of 4D5–28Z-based CARs was generated where the three ITAMs were mutated individually or in combinations and then used to transduce PBLs (Fig. 4A). We observed that when ITAMs at both position A and C were mutated (28ZB), transgene expression was maintained (Fig. 4B), suggesting that PBLs expressing the 4D5 CAR with one ITAM at position B was less prone to apoptosis. This result is consistent with previous reports that the CD3ζ-chain could promote apoptosis by both a quantitative effect of the presence of multiple ITAMs as well as qualitative contributions made by individual ITAMs, and that the ITAMs in CD3ζ were functionally different in terms of their ability to induced apoptosis and T cell activation (32).
FIGURE 4.
Effect of specific ITAM mutations on expression, function, and AICD. A, Schematic representation of 4D5–28Z-based CARs with different ITAMs in CD3ζ signaling moiety being mutated (tyrosine to phenylalanine). B, Transgene expression of PBLs transduced with CD3ζ ITAM-mutated 4D5 CAR constructs at days 7 and 55 after transduction. C, Annexin V and PI staining of PBLs transduced with 4D5 CARs with CD3ζ ITAM mutants following coculture with ErbB2+ tumor lines SK-OV3 or SK-BR3, with an ErbB2– tumor line MDA468, and without a tumor line (Medium). Percentages of positive cells were as shown in each quadrate.
To determine the role of apoptosis in the loss of transgene expression, transduced PBLs were stimulated with the ErbB2-positive tumor lines SK-OV3 and SK-BR3 and stained for annexin V and propidium iodide (PI) (Fig. 4C). We observed that the level of apoptotic (annexin V+) cells in 4D5–28Z CAR-transduced PBLs was more than twice that of nontransduced control (NV) cells (78% vs 30% in coculture with SK-BR3) and that when all of the ITAMs were mutated (4D5–28ZM) that the level was similar (26%) to that of control cells. PBLs transduced with the 4D5 CAR with only ITAM B intact in CD3ζ (4D5–28ZB) showed a notably lower level of annexinV+ cells than did PBLs transduced with the CD3ζ ITAM mutants A or C (Fig. 4C). These results suggested that ITAM A and C in the CD3ζ signaling moiety could transmit a stronger apoptosis signal than could ITAM B. Together with the finding that ErbB2 could be detected by real-time RT-PCR at low levels in PBMCs (Table I), these data support the hypothesis that the observed transgene decrease was caused by the recognition of some ErbB2-expressing cells in the PBMCs and that CD3ζ signaling promoted these transduced PBLs to undergo AICD.
Adding 4–1BB signaling domains maintains transgene expression and enhances effector function of 4D5 CAR-transduced PBLs
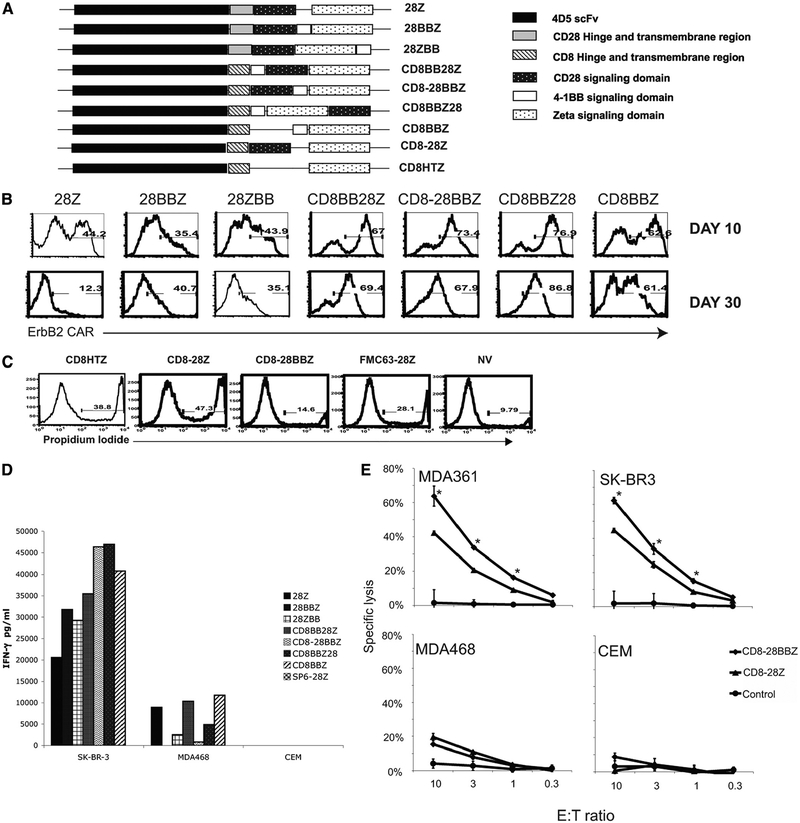
4–1BB is a TNF receptor family member and a costimulatory molecule in the activation of T cells, with signaling being independent of CD28. Signaling via 4–1BB has been shown to cause T cell expansion, cytokine induction, up-regulation of anti-apoptotic genes, and prevention of AICD (33–36), suggesting that the addition of the 4–1BB intracellular signaling domain may enhance CAR function. Placing the cytoplasmic domain of human 4–1BB between the CD28 and CD3ζ (construct 28BBZ) or after CD3ζ (construct 28ZBB) resulted in reduced transgene expression at day 10 posttransduction compared with the PBLs transduced with the original CAR (28Z) (based on percentage of CAR expression, Fig. 5B, or mean fluorescence intensity, data not shown). Reduced transgene expression was also observed in similar constructs when scFv was replaced with an scFv from another Ab (mouse mAb N29 against ErbB2) or when the CD3ζ signaling domain was replaced with FcRγ chain (our unpublished data). Codon optimization of the signaling moieties showed no improvement in transgene expression (our unpublished data).
FIGURE 5.
Adding 4–1BB signaling moiety enhanced function and maintained transgene expression of 4D5 CAR-transduced PBLs. A, A schematic representation of variant 4D5 CAR constructs with hinge and transmembrane region from either CD8α or CD28 preceding the intracellular signaling domains of CD28, 4–1BB, or CD3ζ. The signaling domains were combined in the different orders as shown. B, Transgene expression. PBLs were transduced with 4D5 CAR-based constructs with different protein domains as diagrammed in A. Transgene expression was monitored at days 10 and 30 posttransduction by flow cytometry, and the percentage of transduction was as shown. C, PBLs transduced with a 4–1BB containing CARs were less prone to AICD. PBLs were transduced with 4D5 CARs with transmembrane region and hinge region from CD8α followed by CD3ζ (CD8HTZ), both CD28 and CD3ζ (CD8–28Z), or by a construct with 4–1BB inserted between CD28 and CD3ζ (CD8–28BBZ). Controls were an unrelated CAR, CD19 CAR (FMC63–28Z), and untransduced cells (NV). Three days posttransduction, PBLs were subjected to staining with PI to determine the percentage of dead cells. The flow cytometry results shown were the cells without any gating; the number shows the percentage of PI+ cells. D, Effector cytokine production. PBLs transduced with retroviral vectors expressing the constructs shown in A were tested by coculture with ErbB2+ (SK-BR3) and ErbB2– (MDA468 and CEM) cell lines. Effector cytokine production (IFN-γ) was determined by ELISA following overnight coculture. E, Specific lysis by 4D5 CAR-transduced T cells. 4D5-CD8–28BBZ (CD8–28BBZ)- and 4D5-CD8–28Z (CD8–28Z)-transduced PBLs, with control nontransduced PBLs (NV), were cocultured with 51Cr-labeled tumor lines MDA361 (ErbB2+), SK-BR3 (ErbB2+), MDA468 (ErbB2–), and CEM (ErbB2–) at the indicated E:T ratio. Data were the percentage lysis of specific target cells as calculated (see Materials and Methods). Significant differences (where p < 0.01 by two-way ANOVA test) between the mean values for 4D5-CD8–28BBZ and 4D5-CD8–28Z are indicated by asterisks. Data are representative of three experiments.
To investigate if CD28/4–1BB protein-protein interaction was responsible for the low transgene expression, the hinge and trans-membrane regions of CD28 were replaced with the sequence from human CD8α-chain, and the signaling domains of CD28, 4–1BB, and CD3ζ were placed in different orders (Fig. 5A). PBLs were transduced with these constructs and their functions were compared with PBLs transduced with parent 4D5–28Z CAR. When the transduced PBLs were examined for the expression of transduced CAR at 10 and 30 days posttransduction (Fig. 5B), transgene expression of PBLs transduced with the original 4D5–28Z (28Z) decreased from 44.2% to 12.3%, while the transgene expression of PBLs engineered with CARs containing 4–1BB signaling domains maintained higher levels of transgene expression at day 30 (35.1–86.8%), suggesting that the anti-apoptosis function of 4–1BB signaling protected the transduced cells from AICD. This observation was further supported by determining the percentage of dead cells in PBL cultures following transduction with 4D5-CAR constructs containing the CD8 hinge and transmembrane regions either with CD28 ((4D5-CD8–28Z) or without CD28 (4D5-CD8HTZ) compared with the vector with 4–1BB (4D5-CD8–28BBZ). Flow cytometry demonstrated a greater percentage of dead cells (cells that take up PI) when PBLs were transduced with 4D5-CD8HTZ(38.8% PI+) and 4D5-CD8–28Z (47.3% PI+) compared with PBLs transduced with the 4–1BB containing construct 4D5-CD8–28BBZ(14.6% PI+) (Fig. 5C).
To compare the biological activity of the 4–1BB containing CARs, transduced PBLs were cocultured with the SK-BR3 (ErbB2+) tumor cell line (Fig. 5D). PBLs transduced with 4D5-based CAR containing the 4–1BB and CD28 signaling domains secreted up to twice the amount of IFN-γ (Fig. 5D) and equivalent amounts of TNF-α (supplemental Fig. 2). We further observed that PBLs expressing the 4D5 CAR with signaling domains on the order of CD8 hinge and transmembrane/CD28 cytoplasmic domain/4–1BB cytoplasmic domain/CD3ζ cytoplasmic domain (4D5-CD8–28BBZ) demonstrated sustained transgene expression (Fig. 5B), lower background recognition of the MDA468 cell line (Fig. 5D), and increased IL-2 production compared with other 4D5-based CARs (supplemental Fig. 2). To determine the lytic ability of 4–1BB-containing constructs, transduced PBLs were sorted based on the expression of the 4D5 CAR binding to an ErbB2-Fc-soluble fusion protein and used in a standard 51Cr release assay. As shown in Fig. 5E, 4–1BB bearing CAR-transduced PBLs showed significantly enhanced lytic activity compared with 4D5 CAR with only CD28 and CD3ζ signaling domains. These results indicated that CD28 and 4–1BB signaling could synergistically enhance T cell function and that the order of the signaling moieties can affect their function as previously reported (12, 24).
4–1BB signaling in CAR-transduced T cells promoted the up-regulation of Bcl-xL and NKG2D
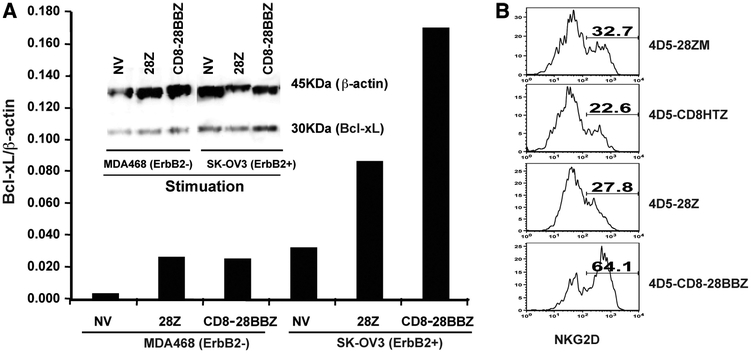
The enhanced expression of two genes that are involved in T cell function and survival, Bcl-xL and NKG2D, were reported to be correlated with 4–1BB signaling (37–40). To investigate Bcl-xL expression in CAR-transduced cells, transduced PBLs were stimulated with plate-coated ErbB2-Fc fusion protein, or VEGFR2-Fc fusion protein as a control, and expression of Bcl-xL was detected by flow cytometry intracellular staining. As shown in supplemental Fig. 3, PBLs stimulated with ErbB2-Fc showed increased Bcl-xL expression in 4D5-CD8–28BBZ (59.8%)-transduced PBLs compare with 4D5–28Z-engineered PBLs (38.7%). Significant Bcl-xL up-regulation was also evidenced by Western blot analysis. PBLs were transduced with the parent 4D5–28Z (28Z) or 4D5-CD8–28BBZ (CD8–28BBZ) and then cocultured with ErbB2-positive (SK-OV3) or -negative (MDA468) tumor lines (Fig. 6A). While both the parent 28Z and 4–1BB CAR-engineered PBLs demonstrated Bcl-xL up-regulation, there was greater Bcl-xL protein detected in cells engineered with the 4–1BB-containing vector. Taking together, Bcl-xL up-regulation of CAR-transduced PBLs was observed in an Ag-specific manner, and CD28 plus 4–1BB signaling mediated greater Bcl-xL expression over CD28 alone.
FIGURE 6.
Adding 4–1BB signaling moiety in the CAR increased both Bcl-xL and NKG2D expression upon specific Ag stimulation. A, Increased Bcl-xL expression in cells transduced with 4–1BB containing CAR. PBLs were transduced with 4D5-CD8–28BBZ (CD8–28BBZ), 4D5–28Z (28Z), or nontransduced (NV) and then cocultured with ErbB2+ tumor SK-OV3 or ErbB2– tumor MDA468 for 20 h. PBLs were separated from tumor cells by enriching for T cells with CD3 magnetic beads and subjected to Western blot for the detection of Bcl-xL. Data were quantitated and expression relative to β-actin was plotted. B, Increased NKG2D expression in cells transduced with 4–1BB containing CAR. Transduced PBLs as indicated were stimulated with ErbB2-Fc-coated plates for 20 h. The cells were harvested and stained with NKG2D and ErbB2-Fc (for 4D5 CAR) and subjected to flow cytometry. Dead cells were excluded by PI gating and CAR-transduced cells were analyzed for NKG2D expression by gating on ErbB2-Fc+ population. Percentages of positive cells were as indicated. Data are representative of two experiments.
NKG2D is an activation receptor on NK cells and CTLs that binds MHC class I-like ligands expressed primarily on virally infected and neoplastic cells. To determine whether signaling via 4–1BB in the form of a CAR could up-regulate NKG2D expression, 4D5 CAR-transduced T cells were stimulated with plate-coated ErbB2, and NKG2D expression of the stimulated T cells were subjected to flow cytometry detection. As shown in Fig. 6B, the T cells transduced with 4D5-CD8–28BBZ showed increased expression of NKG2D (64.1%), compared with T cells transduced with CARs lacking 4–1BB (22.6–32.7%).
Reactivity against fresh tumor and in vivo tumor treatment efficiency of Herceptin-based CAR-transduced T cells
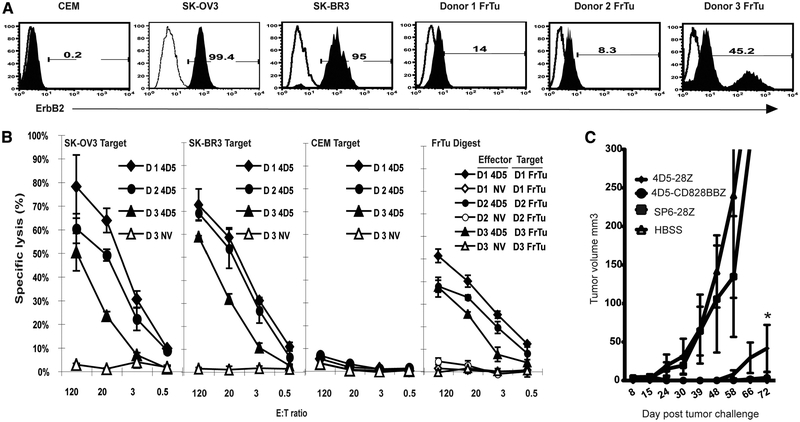
The Herceptin-based CAR constructed in this study holds promise for application in a clinical trial for the treatment of cancer patients with tumors overexpressing ErbB2. To test the lytic activity of T cells transduced with 4D5-CD8–28BBZ against autologous fresh tumor digests, PBLs from three melanoma patients were transduced with the 4D5-CD8–28BBZ and tested in a 51Cr lysis assay. As shown in Fig. 7A, all three fresh tumor digests expressed detectable levels of ErbB2. PBLs from these patients were transduced with 4D5-CD8–28BBZ and demonstrated significant lytic activity against the autologous fresh tumor digests (Fig. 7B). To evaluate the in vivo potential of 4D5 CAR-redirected T cells, we established a mammary gland cancer model with a human breast carcinoma line BT-474 implanted into the mammary fat pad of SCID mice. Mice were injected i.v. with transduced PBLs 10 days after tumor challenge and the tumor growth was monitored. As shown in Fig. 7C, there was no treatment effect of the PBLs transduced with control CAR, SP6–28Z, compared with mice treated with HBSS, while dramatic tumor growth inhibition was observed for mice treated with both 4D5–28Z- and 4D5-CD8–28BBZ-transduced PBLs. Moreover, PBLs transduced with 4D5-CD8–28BBZ showed stronger treatment efficacy compared with PBLs transduced with 4D5–28Z, which was evidenced by significantly smaller tumor volume at day 72 posttreatment (p < 0.05).
FIGURE 7.
Reactivity against fresh tumor and in vivo tumor treatment efficiency of Herceptin-based 4D5-CAR-transduced T cells. A, ErbB2 expression on fresh tumor samples. ErbB2 expression was determined using flow cytometry using an anti-ErbB2 Affibody moleculer (filled histogram) and an isotype control (open histogram) on fresh tumor digests from three melanoma patients (donors 1–3 FrTu), two ErbB2+ tumor lines (SK-OV3 and SK-BR3), and one ErbB2– line (CEM) as control. B, Tumor cell lysis. Lytic activity of 4D5 CAR-transduced PBLs against tumor cell lines and autologous fresh tumor digests (FrTu Digest). 4D5-CD8–28BBZ-transduced PBLs from different donors (D1, D2, and D3) were cocultured with 51Cr-labeled tumor lines at the indicated E:T ratios using SK-OV3 (ErbB2+), SK-BR3 (ErbB2+), CEM (ErbB2–), and autologous fresh tumor digests (FrTu) as targets. Data were the percentages of lysis of specific target cells as calculated (see Materials and Methods). Nontransduced PBLs from the same donors served as negative controls (NV). C, Treatment of a breast cancer xenograft by ErbB2-CAR-transduced PBLs. SCID mice bearing human breast tumor line BT-474, implanted in the mammary gland 10 days before treatment, were established as described in Materials and Methods. Adoptively transferred (i.v.) 4D5–28Z or 4D5-CD8–28BBZ- or SP6–28Z-transduced PBLs were administered to animals with palpable tumors. No treatment control mice received HBSS (n = 7–10/group). Tumor volume was monitored at time points as indicated. Significant difference (where p < 0.05 by two-way ANOVA test) between the mean values at day 72 for 4D5–28Z and 4D5-CD8–28BBZ is indicated by asterisks.
Discussion
Most tumor-associated Ags currently used in cancer immunotherapy are differentiation Ags that are expressed in a restricted tissue pattern or are expressed at low levels in normal tissues, but are preferentially up-regulated in malignancy (41, 42). ErbB2 is a tumor-associated Ag that is expressed on some normal tissues at low levels (43, 44) and is overexpressed on a substantial percentage of multiple malignancies, including breast cancer. Clinical use of the humanized anti-ErbB2 4D5 Ab Herceptin was demonstrated to lead to improved outcomes (45), but it is associated with an increased incidence of cardiac dysfunction (46). In trials using ErbB2-targeted immunotherapy for cancer patients, either with tumor vaccines (47, 48) or the adoptive cell transfer of ErbB2-reactive CTLs (49), no toxicities resulting from these immunotherapies was reported. Caution should be taken in extrapolating the safety of these initial reports, as the vaccine studies did not effectively treat tumors, and naturally occurring CTLs may be of low avidity due to immunological tolerance. While PBLs transduced with the ErbB2 CARs do not produce cytokine without tumor stimulation, they do manifest AICD (Figs. 3–5), suggesting recognition of low levels of ErbB2 in hematopoietic cells (Table I). Additionally, when highly active ErbB2 CAR-transduced PBLs were cocultured with the ErbB2-negative (based on FACS analysis, Fig. 1)-expressing cell line MDA468, considerable cytokine production was noted in some experiments, suggesting that background recogni tion of normal cells may be a significant risk in the clinical application of ErbB2-directed CARs.
The property of CARs to recognize normal tissues might be a common concern for all of the CARs that are composed of high-affinity scFvs targeting self-tumor-associated Ags. It has been reported that patients with metastatic renal carcinoma treated with adoptive transfer of autologous T cells that had been gene-transduced to express a CAR (scFv(G250)) against carboxyanhydrase IX (CAIX) manifested liver toxicity resulting from the specific immune reaction of the retargeted T cells directed against CAIX expressed by epithelial cells lining the bile ducts (20, 21). In clinical trials using TCR-engineered autologous PBLs for the treatment of melanoma, we recently found that infusion of autologous PBLs redirected with high-affinity TCRs (MART-1 and gp100(154)) resulted in on-target toxicity against normal cells expressing the target Ags (50). While melanocyte differentiation Ags, such as MART-1 and gp100, are expressed at similar levels in normal tissues and malignancies, ErbB2 has been reported to be overex-pressed in a substantial percentage of tumor cells from cancer patients. Thus, careful selection of patients with ErBb2 overexpression will likely be important to minimize any potential toxicity related to anti-ErbB2 CAR administration.
Data in this study demonstrated transgene decrease for PBLs transduced with the 4D5 CARs that target ErbB2, but there was no transgene decrease observed for an anti-NY-ESO-1 TCR or control CAR-transduced PBLs grown under similar conditions. Inactivation of CD3ζ prevented the transgene decrease, suggesting that the transgene decrease was associated with AICD resulting from CD3ζ signaling caused by the recognition of low levels of endogenous ErbB2 (Table I). Consistent with the finding from other investigators working with ErbB2-specific CAR (51), we found that decreased transgene expression of PBLs transduced with Herceptin-based CARs in the configuration of scFv-CD3ζ (with or without CD28 signaling moiety) was accompanied by significantly increased apoptotic/dead cells. This phenomenon was not found for PBLs transduced with CAR against CD19, the control CARs LNGFR-28Z and SP6–28Z, or CARs without CD3ζ. Further investigation suggested that CAR-associated transgene decrease and cell death was due to apoptotic signaling transmitted from ITAMs within CD3ζ stimulated by a low level of ErbB2 expression in PBMCs (Table I and Fig. 4). Our analysis confirmed previous findings that the signaling by individual ITAMs in CD3ζ was not equivalent (32). Our data also support the assumption that CARs designed to signal from the single ITAM-containing Fc receptor FcRγ might be less prone to apoptosis (33). Interestingly, we found that PBLs expressing CARs where at least one ITAM was intact functioned equally as well as PBLs transduced with the wild-type CAR in terms of Ag-specific IFN-γ secretion and lytic activity (our unpublished data).
It has been reported that the ErbB2 recognition sensitivity of CAR-expressing T cells could be adjusted by changing the binding affinity of the ErbB2-specific scFv (KD from 1.5 × 10–11 to 3.2 × 10–7 M) (18). We monitored the transgene expression of T cells transduced with CARs derived from 4D5 scFv mutants with different affinity (KD from 3 × 10−10 to 2.5 × 10–8 M, Fig. 4) and found that the CAR transgene decreased for all CARs, independent of affinity. At the same time, the low-affinity CAR (4D5–1) had dramatically reduced ability to recognize tumor cell lines (our unpublished data). As T cell avidity correlated with the efficient in vivo elimination of tumors and virally infected cells (52, 53), the enhanced specificity of Ab/Ag recognition using high-affinity scFv for CAR design may be necessary to maximize antitumor efficacy in vivo.
Based on these observations we sought strategies to protect transduced cells from undergoing AICD by utilizing the known anti-apoptosis properties of the 4–1BB molecule. 4–1BB transmits a potent costimulatory signal to T cells, promoting differentiation and enhancing long-term survival of CD8 T lymphocytes (37, 54–56), as well as up-regulating effector molecules, such as perforin, granzyme A, and cytokines (57, 58). Apart from its costimulatory function, a major effect of 4–1BB appears to be on T cell survival (37, 54, 59). Furthermore, it has been reported that signaling from both CD28 and 4–1BB has synergic effects on T lymphocytes in the induction of IL-2 and its functional activity (55, 60). Incorporating both CD28 and 4–1BB signaling in a single CAR construct may yield engineered T cells that respond to antigenic stimulus with increased abilities of cytokine production, proliferation, and T cell survival after Ag stimulation. Wang et al. reported that polyclonal T cells expressing a CD20-specific CAR construct containing CD28 and 4–1BB costimulatory domains in cis- with the CD3ζ intracellular signaling domain showed improved CTL activation, proliferation, and cytotoxicity (24). In our present study, we found that the addition of 4–1BB signaling to 4D5 CARs greatly reduced the AICD of the transduced T cells, and thus the transduced cells could be efficiently expanded in culture, an essential feature for clinical applications. Moreover, the 4–1BB signaling not only increased expression of Bcl-xL and NKG2D but also was associated with higher effector cytokine production and Ag-specific tumor cell lysis.
The induction of NKG2D expression by 4–1BB signaling may have CAR-independent antitumor properties. Human NKG2D interacts with MHC class I chain-related proteins A and B (MICA and MICB). MIC molecules are not detected on most healthy tissues, but are induced by stress such as heat shock, viral infection, or malignant transformation (61, 62). Persistent expression of MIC results in impaired NK and CD8 T cell immune responses, and this negative effect of MIC is mainly due to pronounced down-modulation of NKG2D (63). A recent study found that 4–1BB costimulation could restore down-regulated NKG2D and induce cytotoxic activity of CD8 T cells and that the 4–1BB-induced NKG2D was refractory to TGF-β down-regulation (40). Thus, enhancing 4–1BB costimulation through appropriate CAR design could promote the generation of protective antitumor immune responses by a variety of mechanisms (64–68).
The finding that forced expression of 4–1BB permitted survival and expansion of CD8 T cells both in vitro and in vivo suggests that 4–1BB stimulation will be useful in adoptive immunotherapy of cancer (55, 69). However, 4–1BB signaling has also been shown to promote expansion of regulatory T cells (70–72), which might explain in part the findings that treatment of autoimmune-prone strains of mice with 4–1BB mAb did not accelerate the syndromes but in many cases actually appeared to ameliorate them (73–76). While the induction of regulatory T cells may offset the therapeutic effect of using 4–1BB costimulation, providing 4–1BB signaling through CAR could specifically benefit only the T cells that express the CAR, thus preventing the enhancement of Tregs.
Persistence of adoptively transferred T cells was shown to be highly correlated with objective response for patients treated with either tumor-reactive TILs (77) or highly active TCR-engineered PBLs (9, 50). While the use of viral-specific PBLs as CAR targets has recently resulted in positive clinical findings (78), all of the prior clinical trials using CAR-engineered bulk PBLs for adoptive cell therapy of cancer patients showed no objective response and very short-term persistence of adoptively transferred lymphocytes (20, 78–80). T lymphocytes redirected with CARs composed of scFv of mouse origin or the use of T cell signaling domains that only contain CD3ζ, with or without CD28 intracellular regions, may not function or persist in vivo in cancer patients due to mechanisms previously discussed. Adoptive transfer of T lymphocytes engineered with CARs that are composed of human or humanized Abs with optimized T cell signaling moieties, such as 4–1BB, together with proper conditional regimens, or the transduction of virus-specific CTLs (78) holds great promise to enhance current adoptive immunotherapy for both cancer and infectious diseases.
Supplementary Material
Acknowledgments
The authors thank Arnold Mixon and Shawn Farid for assistance with flow cytometry and cell sorting.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Z.E. was supported in part by and the Sixth Framework Programme of the European Commission consortium ATTACK.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
Abbreviations used in this paper: ACT, adoptive cell therapy; AICD, activation-induced cell death; CAR, chimeric Ag receptor; PI, propidium iodide; TIL, tumor-infiltrating lymphocyte.
The online version of this article contains supplemental material.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P,Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL,Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. 2005. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol 23: 2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U,Robbins PF, Huang J, Citrin DE, Leitman SF, et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol 26: 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadelain M, Riviere I, and Brentjens R. 2003. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 3: 35–45. [DOI] [PubMed] [Google Scholar]
- 5.Murphy A, Westwood JA, Teng MW, Moeller M, Darcy PK, and Kershaw MH. 2005. Gene modification strategies to induce tumor immunity. Immunity 22: 403–414. [DOI] [PubMed] [Google Scholar]
- 6.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, and Schumacher TN. 2001. Immunotherapy through TCR gene transfer. Nat. Immunol 2: 957–961. [DOI] [PubMed] [Google Scholar]
- 7.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, and Nishimura MI. 1999. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol 163: 507–513. [PubMed] [Google Scholar]
- 8.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, and Morgan RA. 2005. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines.J. Immunol 174: 4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC,Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. 2006. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, and Dudley ME. 2008. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshhar Z, Waks T, Gross G, and Schindler DG. 1993. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 90: 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher J, Brentjens RJ, Gunset G, Riviere I, and Sadelain M. 2002. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol 20: 70–75. [DOI] [PubMed] [Google Scholar]
- 13.Kmieciak M, Knutson KL, Dumur CI, and Manjili MH. 2007. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur. J. Immunol 37: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worschech A, Kmieciak M, Knutson KL, Bear HD, Szalay AA, Wang E,Marincola FM, and Manjili MH. 2008. Signatures associated with rejection or recurrence in HER-2/neu-positive mammary tumors. Cancer Res 68: 2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, and Eshhar Z. 1993. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J. Immunol 151: 6577–6582. [PubMed] [Google Scholar]
- 16.Pinthus JH, Waks T, Kaufman-Francis K, Schindler DG, Harmelin A,Kanety H, Ramon J, and Eshhar Z. 2003. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. Cancer Res 63: 2470–2476. [PubMed] [Google Scholar]
- 17.Moritz D, Wels W, Mattern J, and Groner B. 1994. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc. Natl. Acad. Sci. USA 91: 4318–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmielewski M, Hombach A, Heuser C, Adams GP, and Abken H. 2004. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol 173: 7647–7653. [DOI] [PubMed] [Google Scholar]
- 19.Kershaw MH, Jackson JT, Haynes NM, Teng MW, Moeller M,Hayakawa Y, Street SE, Cameron R, Tanner JE, Trapani JA, et al. 2004. Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer.J. Immunol 173: 2143–2150. [DOI] [PubMed] [Google Scholar]
- 20.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R,Gratama JW, Stoter G, and Oosterwijk E. 2006. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol 24: e20–e22. [DOI] [PubMed] [Google Scholar]
- 21.Lamers CH, Langeveld SC, Groot-van Ruijven CM, Debets R, Sleijfer S, and Gratama JW. 2007. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol. Immunother 56: 1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D, and Eshhar Z. 2005. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood 105: 3087–3093. [DOI] [PubMed] [Google Scholar]
- 23.Finney HM, Akbar AN, and Lawson AD. 2004. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRζ chain. J. Immunol 172: 104–113. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, Till B,Raubitschek A, Forman SJ, Qian X, et al. 2007. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther 18: 712–725. [DOI] [PubMed] [Google Scholar]
- 25.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL,Rowland AM, Kotts C, Carver ME, and Shepard HM. 1992. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 89: 4285–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, Witte L, and Zhu Z. 2003. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J. Biol. Chem 278: 43496–43507. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Rzewski A, Sun H, Robbins PD, and Gambotto A. 2004. UpGene: application of a web-based DNA codon optimization algorithm. Biotechnol. Prog 20: 443–448. [DOI] [PubMed] [Google Scholar]
- 28.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y,Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, and Morgan RA. 2005. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum. Gene Ther 16: 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y,Molloy PE, Dunn SM, Jakobsen BK, et al. 2007. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol 179: 5845–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM,Zola H, and Hohmann AW. 1997. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol. Immunol 34: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 31.Topalian SL, Solomon D, and Rosenberg SA. 1989. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J. Immunol 142: 3714–3725. [PubMed] [Google Scholar]
- 32.Combadiere B, Freedman M, Chen L, Shores EW, Love P, and Lenardo MJ. 1996. Qualitative and quantitative contributions of the T cell receptor ζ chain to mature T cell apoptosis. J. Exp. Med 183: 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eshhar Z 2008. The T-body approach: redirecting T cells with antibody spec-ificity. Handb. Exp. Pharmacol 2008: 329–342. [DOI] [PubMed] [Google Scholar]
- 34.Croft M 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol 3: 609–620. [DOI] [PubMed] [Google Scholar]
- 35.Sica G, and Chen L. 2000. Modulation of the immune response through 4–1BB. Adv. Exp. Med. Biol 465: 355–362. [DOI] [PubMed] [Google Scholar]
- 36.Watts TH 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol 23: 23–68. [DOI] [PubMed] [Google Scholar]
- 37.Hurtado JC, Kim YJ, and Kwon BS. 1997. Signals through 4–1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol 158: 2600–2609. [PubMed] [Google Scholar]
- 38.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, and Kwon BS. 2002. 4–1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl−1. J. Immunol 169: 4882–4888. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, and Mackall CL. 2007. 4–1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J. Immunol 179: 4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YJ, Han MK, and Broxmeyer HE. 2008. 4–1BB regulates NKG2D costimulation in human cord blood CD8+ T cells. Blood 111: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilboa E 1999. The makings of a tumor rejection antigen. Immunity 11: 263–270. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg SA 1999. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 10: 281–287. [DOI] [PubMed] [Google Scholar]
- 43.Yazici H, Dolapcioglu K, Buyru F, and Dalay N. 2000. Utility of c-erbB-2 expression in tissue and sera of ovarian cancer patients. Cancer Invest 18: 110–114. [DOI] [PubMed] [Google Scholar]
- 44.Press MF, Cordon-Cardo C, and Slamon DJ. 1990. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5: 953–962. [PubMed] [Google Scholar]
- 45.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A,Fleming T, Eiermann W, Wolter J, Pegram M, et al. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 46.Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr., Ewer M, Keefe D,Shannon RP, Swain SM, Brown A, Fehrenbacher L, et al. 2005. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without Herceptin as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol 23: 7811–7819. [DOI] [PubMed] [Google Scholar]
- 47.Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB,Storrer CE, Fisher C, Shriver CD, Ioannides CG, and Ponniah S. 2005. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol 23: 7536–7545. [DOI] [PubMed] [Google Scholar]
- 48.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA,Knutson KL, and Schiffman K. 2002. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J. Clin. Oncol 20: 2624–2632. [DOI] [PubMed] [Google Scholar]
- 49.Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nahrig J,Fend F, Weber W, Busch DH, and Peschel C. 2008. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol. Immunother 57: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC,Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. 2009. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turatti F, Figini M, Alberti P, Willemsen RA, Canevari S, and Mezzanzanica D. 2005. Highly efficient redirected anti-tumor activity of human lymphocytes transduced with a completely human chimeric immune receptor.J. Gene Med 7: 158–170. [DOI] [PubMed] [Google Scholar]
- 52.Zeh HJ 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, and Yang JC. 1999. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol 162: 989–994. [PubMed] [Google Scholar]
- 53.Alexander-Miller MA, Leggatt GR, and Berzofsky JA. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93: 4102–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi C, Mittler RS, and Vella AT. 1999. Cutting edge: 4–1BB is a bona fide CD8 T cell survival signal. J. Immunol 162: 5037–5040. [PubMed] [Google Scholar]
- 55.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K,Riley JL, and June CH. 2002. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nat. Biotechnol 20: 143–148. [DOI] [PubMed] [Google Scholar]
- 56.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, and Watts TH. 2008. ERK-dependent Bim modulation downstream of the 4–1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J. Immunol 180: 8093–8101. [DOI] [PubMed] [Google Scholar]
- 57.Bukczynski J, Wen T, and Watts TH. 2003. Costimulation of human CD28– T cells by 4–1BB ligand. Eur. J. Immunol 33: 446–454. [DOI] [PubMed] [Google Scholar]
- 58.Bukczynski J, Wen T, Ellefsen K, Gauldie J, and Watts TH. 2004. Costimulatory ligand 4–1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 101: 1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H,Okumura K, and Watts TH. 2001. 4–1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol 167: 1313–1324. [DOI] [PubMed] [Google Scholar]
- 60.Wen T, Bukczynski J, and Watts TH. 2002. 4–1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J. Immunol 168: 4897–4906. [DOI] [PubMed] [Google Scholar]
- 61.Vivier E, Tomasello E, and Paul P. 2002. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr. Opin. Immunol 14: 306–311. [DOI] [PubMed] [Google Scholar]
- 62.Raulet DH 2003. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol 3: 781–790. [DOI] [PubMed] [Google Scholar]
- 63.Groh V, Wu J, Yee C, and Spies T. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419: 734–738. [DOI] [PubMed] [Google Scholar]
- 64.Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, and Hellstrom KE. 2002. Gene therapy for cancer using single-chain Fv fragments specific for 4–1BB. Nat. Med 8: 343–348. [DOI] [PubMed] [Google Scholar]
- 65.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA,Hellstrom KE, Mittler RS, and Chen L. 1997. Monoclonal antibodies against the 4–1BB T-cell activation molecule eradicate established tumors. Nat. Med 3: 682–685. [DOI] [PubMed] [Google Scholar]
- 66.May KF Jr., Chen L, Zheng P, and Liu Y. 2002. Anti-4–1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res 62: 3459–3465. [PubMed] [Google Scholar]
- 67.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI,Strome SE, Pease LR, and Chen L. 2002. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J. Clin. Invest 109: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L,Giangrande PH, Sullenger B, and Gilboa E. 2008. Multivalent 4–1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest 118: 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV,Heller G, and Sadelain M. 2007. T cell-encoded CD80 and 4–1BBL induce auto-and transcostimulation, resulting in potent tumor rejection. Nat. Med 13: 1440–1449. [DOI] [PubMed] [Google Scholar]
- 70.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, and Shirwan H. 2007. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4–1BB signaling. J. Immunol 179: 7295–7304. [DOI] [PubMed] [Google Scholar]
- 71.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A,Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, et al. 2008. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4–1BB expressed on artificial antigen-presenting cells. Blood 112: 2847–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng G, Wang B, and Chen A. 2004. The 4–1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J. Immunol 173: 2428–2434. [DOI] [PubMed] [Google Scholar]
- 73.Foell J, Strahotin S, O’Neil SP, McCausland MM, Suwyn C, Haber M,Chander PN, Bapat AS, Yan XJ, Chiorazzi N, et al. 2003. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. J. Clin. Invest 111: 1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J, Lee EN, Kim EY, Park HJ, Chang CY, Jung DY, Choi SY,Lee SK, Lee KW, Kwon GY, et al. 2005. Administration of agonistic anti-4–1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol. Lett 101: 210–216. [DOI] [PubMed] [Google Scholar]
- 75.Foell JL, Diez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS,Hoffmann MK, Mittler RS, and Dannecker GE. 2004. Engagement of the CD137 (4–1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology 113: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK,Vinay DS, and Kwon BS. 2004. 4–1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med 10: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Dudley ME, Rosenberg SA, and Robbins PF. 2004. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J. Immunol 173: 7622–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G,Huls MH, Liu E, Gee AP, Mei Z, et al. 2008. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med 14: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z,Mavroukakis SA, White DE, Wunderlich JR, Canevari S,Rogers-Freezer L, et al. 2006. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res 12: 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J,Meechoovet HB, Bautista C, Chang WC, Ostberg JR, and Jensen MC. 2007. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther 15: 825–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.