Abstract
Immunotherapy based on the adoptive transfer of naturally occurring or gene-engineered T cells can mediate tumour regression in patients with metastatic cancer. Here, we discuss progress in the use of adoptively transferred T cells, focusing on how they can mediate tumour cell eradication. Recent advances include more accurate targeting of antigens expressed by tumours and the associated vasculature, and the successful use of gene engineering to re-target T cells before their transfer into the patient. We also describe how new research has helped to identify the particular T cell subsets that can most effectively promote tumour eradication.
T cells move through tissues, scanning for MHC–peptide complexes that specifically activate their T cell receptors (TCRs). T cells are also capable of sensing a variety of signals that can alert them to potentially threatening pathogens — and to cancer. Tumour-specific T cells are probably activated through encounters with tumour-associated antigens that are presented by specialized antigen-presenting cells (APCs), including dendritic cells (DCs). However, activated T cells are capable of directly recognizing antigens that are presented on the surfaces of tumour cells. Based on intravital imaging, there is a growing body of evidence indicating that the migration of tumour-specific T cells is rapidly arrested when they encounter their cognate antigens1.
It is perhaps this arrested migration of T cells following their encounter with an antigen that forms the basis of the observation that, in patients with melanoma, tumour-infiltrating lymphocyte (TIL) populations can be isolated and expanded from tumours simply by excising the tumour mass, dissociating cells into single-cell suspensions or tumour fragments, and adding the T cell growth factor interleukin-2 (IL-2) (FIG. 1). It is also possible to use genetically engineered T cells to treat a variety of tumour histologies (FIG. 2).
Figure 1 |. Isolation of tumour-infiltrating lymphocytes and expansion of tumour-specific T cell populations.
Tumours are often complex masses containing diverse cell types. These masses can be surgically resected and fragmented, and the cells can be placed in wells into which a T cell growth factor, such as interleukin-2 (IL-2), is added. T cell populations that have the desired T cell receptor (TCR) specificity can be selected and expanded, and then adoptively transferred into patients with cancer. Prior to this adoptive transfer, hosts can be immunodepleted by either chemotherapy alone or chemotherapy in combination with total-body irradiation. The combination of a lymphodepleting preparative regimen, adoptive cell transfer and a T cell growth factor (such as IL-2) can lead to prolonged tumour eradication in patients with metastatic melanoma. MDSC, myeloid-derived suppressor cell; NK, natural killer; TReg, regulatory T.
Figure 2 |. Three ways to genetically engineer T cells to confer specificity for tumour-associated antigens.
T cells can be genetically engineered to recognize tumour-associated antigens in various ways in current clinical trials. If a patient expresses a tumour-associated antigen that is recognized by an available receptor structure, autologous T cells can be genetically engineered to express the desired receptor. New receptors can be generated in a variety of ways. a | T cells can be identified and cloned from patients with particularly good antitumour responses. Their T cell receptors (TCRs) can be cloned and inserted into retroviruses or lentiviruses, which are then used to infect autologous T cells from the patient to be treated. b | Chimeric antigen receptors (CARs) can be generated in a variety of ways. Most commonly, sequences encoding the variable regions of antibodies are engineered to encode a single chain, which is then genetically engrafted onto the TCR intracellular domains that are capable of activating T cells. These CARs have antibody-like specificities, which enable them to recognize MHC-nonrestricted structures on the surfaces of target cells. c | TCRs can also be isolated from humanized mice that have been primed to recognize tumour antigens. These mice express human MHC class I or MHC class II molecules and can be immunized with the tumour antigen of interest. Mouse T cells specific for the MHC-restricted epitope of interest can then be isolated, and their TCR genes are cloned into recombinant vectors that can be used to genetically engineer autologous T cells from the patient.
This Review focuses on immunotherapy based on the adoptive transfer of naturally occurring or gene-engineered tumour-specific T cells. We summarize the state of knowledge regarding what T cells ‘see’ in tumour masses, what T cells must ‘do’ to trigger tumour cell death, and how T cells experience maturational changes that dramatically affect their phenotypes and functions. Finally, we consider how best to combine tumour-specific T cells with other therapies to treat patients with cancer.
Can tumour-infiltrating lymphocytes kill tumours?
The treatment of patients with cell populations that have been expanded ex vivo is called adoptive cell transfer (ACT). Cells that are infused back into a patient after ex vivo expansion can traffic to the tumour and mediate its destruction. ‘Preparative lymphodepletion’ — the temporary ablation of the immune system in a patient with cancer — can be accomplished using chemotherapy alone or in combination with total-body irradiation, and the addition of this step is associated with enhanced persistence of the transferred T cells. Moreover, the combination of a lymphodepleting preparative regimen with ACT and the administration of the T cell growth factor IL-2 can lead to prolonged tumour eradication in patients with metastatic melanoma or other tumour histologies (including leukaemias and synovial cell sarcomas) who have exhausted other treatment options2–7.
It seems perplexing that tumours persist despite being enriched for T cells that are specific for antigens expressed by the tumour. This is perhaps because tumour-specific T cells within the tumour are experiencing chronic activation and are bathed in immunosuppressive factors. There is a growing body of work suggesting that TILs are restrained in vivo by immunosuppressive molecules, such as T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), lymphocyte activation gene 3 protein (LAG3), programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte antigen 4 (CTLA4)8,9. However, removing T cells from this presumably immunosuppressive tumour environment enables their activation and clonal expansion. During the process of expanding TIL populations in culture, tumour cells die or are killed by activated natural killer (NK) cells or newly expanding T cell populations. The TILs are dissociated from immunosuppressive cell populations — such as myeloid-derived suppressor cells (MDSCs) — and possibly exposed to lower levels of immunosuppressive cytokines during this early period in culture. The ex vivo expansion of TIL populations to more favourable numbers followed by the transfer of these cells back into the host can trigger the death and complete eradication of the tumour, leading to the durable and complete remission — and even the ‘cure’ — of established cancers4.
How T cells target tumours
The identification of appropriate tumour antigens is perhaps the greatest challenge now facing workers in the field of cancer immunotherapy10. Antigens encoded by mutated genes are likely to be mostly unique to an individual tumour, but are there also self antigens shared by tumour cells and normal tissues that are dispensable? Is there a ‘window’ such that T cells can recognize tumour cells but not normal cells? What proportion of malignant cells in a tumour stably express the candidate antigen, and at what level? Is it strictly necessary to target all cancer cells, or will bystander killing result in tumour sterilization? Does the candidate antigenic molecule yield different antigenic epitopes, some stimulating CD8+ T cells and others CD4+ T cells?
MHC class I-restricted T cells that are specific for tumours (either naturally or as a result of gene engineering) are, theoretically, capable of directly recognizing many types of tumour cells, because virtually every nucleated cell in the body expresses MHC class I molecules. However, neoplastic cells are notoriously unstable targets, as these cells often downregulate their expression of MHC class I molecules11–13. Professional APCs process exogenous antigens and present them to T cells in the context of MHC class II molecules, or in the context of MHC class I molecules through a mechanism known as cross-priming. Such indirect recognition of tumour-associated antigens by T cells might provide an effective means of targeting tumour masses that have lost MHC expression by triggering innate immunity and vascular collapse.
Immune surveillance might eliminate many microscopic tumours before they become evident and, based on experiments in mice, some investigators have proposed that tumours experience immunoediting13,14,163. However, tumour masses that grow uncontrollably and ultimately kill their hosts demonstrably express immunologically targetable antigens. Thus, not only do tumour masses ‘escape’ recognition by eliminating antigenic targets that they express, but they also co-opt or render insufficient the adaptive immune system of the host164.
The first human tumour-associated antigen gene to be defined at the sequence level was melanoma-associated antigen 1 (MAGEA1), which encodes the antigen MZ2E15. Since then, hundreds of antigens that are naturally processed and presented on tumour cell surfaces have been identified. Although they are too numerous to mention here, some of the many hundreds of epitopes recognized by T cells are listed in the Cancer Immunity Peptide Database. Indeed, tumour immunologists now have a deeper understanding of which tumour-associated antigens T cells can recognize, with targetable molecular structures falling into five major categories that are listed below.
T cells can recognize unaltered tissue-differentiation antigens on tumours.
After transformation, tumour cells generally continue to express antigens that are characteristic of their tissue site of origin. Tissue-differentiation antigens can be excellent targets for ACT-based therapy, but only for tissues that are not essential for life. For example, patients who have successful responses to immunotherapies targeting CD19 on B cell lymphomas also experience long-term depletion of normal polyclonal CD19+ B lineage cells5,7. Likewise, T cells engineered to be specific for carcinoembryonic antigen (CEA) mediate the destruction of colon cancer in patients and mice, but at the same time cause transient destruction of the normal colonic mucosa16,17.
Melanocyte differentiation antigens (MDAs) — such as PMEL (also known as gp100), melanoma antigen recognized by T cells 1 (MART1), tyrosinase, tyrosinase-related protein 1 (TYRP1) and TYRP2 — are expressed by most melanoma tumours but are also expressed by normal melanocytes in the stratum basale of the skin, in the retinal pigmented epithelium in the eyes, and in the inner ear, where they are associated with melanin production18,19. Based on early antigen-cloning studies, non-mutated self MDAs were proposed to be strong candidates for targeting using TIL-based immunotherapy20. However, T cells with gene-engineered TCRs specific for PMEL or MART1 mediated the destruction of normal melanocytes in the skin, eyes and ears of treated patients, and these detrimental effects sometimes required intervention with corticosteroid treatment21. Ocular toxicity was also observed in a mouse model using T cells specific for PMEL22. Objective clinical responses were observed in 9 out of 36 (25%) patients treated in these trials, but only one patient experienced a complete response to treatment21.
By sharp contrast, populations of TILs can be given usually without causing evidence of eye or ear toxicity, despite having TCRs specific for the same non-mutated self antigens4,23. The induction of long-term, durable immune responses, which can kill all of the tumour cells, can usually be accomplished by the transfer of TILs without causing the toxicity attributable to T cells. This suggests that the naturally occurring TILs in melanoma might respond to antigens other than non-mutated self antigens.
Tumour-specific T cells can recognize the products of mutated genes.
Recent studies involving exomic sequencing of human melanomas have indicated the presence of a large number of mutational events24, enabling cancer immunologists to target non-synonymous mutations that result in the creation of new epitopes. The inherent genetic instability of tumours generates many potential tumour-associated antigens, which may result from somatic single-base mutations within gene-coding regions, from mutations in stop codons that extend open reading frames, from frameshift mutations or from gene rearrangements that lead to the production of fusion proteins, among other mechanisms.
Emerging data, especially from exomic sequencing, indicates that melanomas contain more mutations than other types of cancer25. Specifically, melanomas contain large numbers of mutations that are characteristic of damage from ultraviolet radiation. Pyrimidines (cyto-sine and thymine) are most vulnerable to ultraviolet radiation, which characteristically produces C to T and CC to TT transitions. Longer wavelength radiation can cause oxidative damage to DNA. Ultraviolet light is also mitogenic, as it stimulates melanocytes to divide, in part by inducing the expression of melanotropin (also known as MSH), and this potentially exacerbates its properties as a mutagen26.
Importantly, T cells potentially recognizing these neo-antigens will not have been tolerized in the thymus. Furthermore, some of these T cell-targetable mutations may be required to drive the cancer phenotype. Tumour-specific antigens that are caused by particular point mutations have been shown to be recognized by naturally-occurring T cells27.
Recognition of viral antigens.
Some cancers associated with transforming viruses can express viral products, which are attractive targets because they are not expressed by normal tissues. Although only applicable to a subset of cancer histologies, it is possible to target the oncogenic proteins encoded by cancer-associated viruses, such as those derived from Epstein–Barr virus28 and human papillomavirus (HPV)29. Experiments in mice indicate that the successful targeting of mutant tumour-associated epitopes, especially those derived from driver mutations or transforming oncogenes, can lead to complete tumour destruction30. It is likely that targeting of virus-encoded transforming proteins that are expressed by tumour cells can also promote tumour elimination.
Tumour-specific T cells can recognize antigens produced by epigenetic changes.
Evidence now indicates that epigenetic changes in cancer cells can be the primary driver of some tumour histologies31. Epigenetic changes can trigger the expression of a category of non-mutated proteins called cancer germline antigens or cancer–testis antigens32. Cancer–testis antigens are normally expressed by germline cells in the testes and fetal ovaries, but they are also expressed by many types of tumours. Cancer–testis antigens are among the most attractive immunotherapeutic targets because of their shared expression among many tumour histologies and their lack of expression in normal tissues.
Cancer–testis antigens are products of genes that are inactive in normal non-germline tissues (with the possible exception of the placenta). T cells do not recognize these antigens on male germline cells because these cells do not express MHC class I or MHC class II molecules33. Genomic DNA encoding cancer–testis antigens is often demethylated in tumours33, and the dysregulated cancer epigenome may share some characteristics with the epigenome of germline cells34. Combinatorial therapeutic approaches involving drugs that modulate the epigenome and immunotherapy that targets cancer–testis antigens have been suggested based on these data and have been pursued in mouse models35 and in human in vitro systems36. The tumours that most commonly express cancer–testis antigens include melanomas, lung carcinomas and cancers of the head and neck, oesophagus and bladder37.
There are about 110 cancer–testis antigens that can potentially be targeted by naturally occurring or gene-engineered T cells. Many of the genes encoding these antigens are part of multigene families derived from gene duplications, although little is known about their functions. Approximately 30 of these cancer–testis antigens are encoded on the X chromosome (see the CT Database) and have been designated cancer–testis X genes. These antigens in particular have stimulated great interest among cancer immunologists. Several of these antigens — including cancer–testis antigen 1 (CTAG1; also known as NY-ESO-1)6, MAGEA3 (REF. 38) and synovial sarcoma X breakpoint 2 (SSX2) — have been the targets of contemporary clinical trials. A recent report targeting CTAG1 using autologous T cells with genetically engineered TCRs showed evidence of objective clinical responses in 8 out of 17 (47%) patients with metastatic melanoma and in 8 out of 10 (80%) patients with metastatic synovial sarcoma, all of whom were heavily pretreated with standard therapies. No toxicity against normal tissues was observed6. This work validates T cell-mediated targeting of neo-antigens derived from cancer-associated alterations in the epigenome as a therapeutic approach.
Recognition of antigens on non-transformed tumour vasculature and stroma.
Many studies have focused on how T cells directly kill transformed cells in the tumour mass. However, tumours contain many other cell types, including large numbers of stromal cells and leukocytes, such as NK cells, myeloid-derived cells, B cells39 and of course different T cell populations. Recent findings indicate that targeting these cell types may also promote tumour elimination40. For example, one study showed that the ablation of tumour-infiltrating mesenchymal cells that expressed fibroblast activation protein (FAP; also known as seprase) led to tumour necrosis41. This suggests that T cells engineered to recognize these cells could also mediate antitumour immunity without directly targeting the tumour.
Finally, to obtain nutrients, growing tumours must continually acquire neovasculature, which is derived from existing host blood vessels or from bone marrow-derived endothelial cells. This neovasculature is subject to immune attack because, compared with existing vasculature, it can express higher levels of certain molecules, such as vascular endothelial growth factor receptor 2 (VEGFR2)42–44. An important caveat here is that although the vasculature in mouse tumours seems clearly distinct from that in normal tissue, mouse tumour vasculature is generally less than a month old in most studies. These new vessels might be different from the vasculature that is present in human tumours, which may have been established for several years before diagnosis.
Antitumour functions of T cells.
Given the wealth of preclinical and clinical data available, it may seem surprising that there is no consensus in the literature regarding the complex cellular mechanisms that mediate tumour rejection. T cells traffic to areas where their target antigens are expressed and can produce cytokines, chemokines and anti-angiogenic factors that affect tumour growth. T cells that mediate effective antitumour responses may also directly mediate cytotoxic responses against tumour cells, either through their expression of apoptosis-inducing molecules or through the release of cytotoxic granules.
Mature differentiated CD8+ T cells and some types of CD4+ T cells release interferon-γ (IFNγ) and tumour necrosis factor (TNF), which enhance the immune response by upregulating the expression of MHC class I and MHC class II molecules on both tumour cells and tumour-resident APCs. CD4+ T cells are capable of activating and regulating many aspects of innate and adaptive immunity, including the function of cytotoxic CD8+ T cells. They can also engage and ‘license’ APCs, which in turn recruit additional T cells and promote the activation of the innate immune system165.
ACT immunotherapies can eradicate tumours
Immunotherapy based on the adoptive transfer of tumour-specific lymphocytes has a rich history that dates back several decades45–47. It is important to distinguish these ACT-based treatments from other immunotherapies, such as therapeutic cancer vaccines, which are aimed at boosting immune reactivity in the tumour-bearing host. Therapeutic vaccines are attractive because they are easy to use and have shown low toxicities in preclinical and clinical trials at the US National Cancer Institute and elsewhere48–56. Vaccines can be augmented using prime–boost regimens, MHC anchor modifications, cytokines and co-stimulation57–62. However, broad reviews of clinical trials show that cancer vaccines only induce objective tumour regression in less than 4% of patients treated63,64. Although the therapeutic cancer vaccine sipuleucel-T — which the US Food and Drug Administration (FDA) has approved for the treatment of castration-resistant prostate cancer — increased survival by an average of 4.1 months, no significant differences in time to disease progression were measured and patients did not experience tumour regression or long-term, durable responses65. Importantly, none of the cancer vaccines that have been tested in many hundreds of clinical trials come close to the aspirational goal of a ‘cure’ of metastatic disease, and their therapeutic benefit — if it is measurable — is modest and measured in months, not years.
Current therapies based on TILs.
Immunotherapies based on the adoptive transfer of TILs are the best available treatment for patients with metastatic melanoma. However, these therapies have limitations. Not all patients can be entered into trials, as they are still limited to patients with good performance status who are capable of withstanding the rigours of the lympho depletion- and IL-2-based treatments that are currently used.
A recent study of 93 patients with stage IV melanoma described three sequential trials in which patients were treated with the adoptive transfer of autologous TILs, administered in conjunction with IL-2 following host conditioning regimens. Objective clinical response rates, assessed using response evaluation criteria in solid tumours (RECIST), ranged from 49% to 72%4. Although patients can sometimes benefit enormously from partial responses, every patient aspires to become cancer free. The difficult reality for patients with melanoma is that partial responses offer only a temporary respite in disease progression. Importantly, 20 of the 93 patients (22%) achieved complete tumour regression, and 19 of these 20 (93%) have ongoing complete regression with a minimum follow up of 57 months. Indeed, some of these patients have been alive and disease free for more than 8 years and are probably ‘cured’ of metastatic melanoma. The likelihood of achieving a complete response was similar regardless of prior therapy. Thus, ACT therapy with autologous TILs can mediate durable complete responses in patients with metastatic melanoma and has similar efficacy irrespective of prior treatment4. Recent reports document that several other groups have reproduced these findings with objective response rates of 48%66,67.
Despite these successes, the reasons why some patients respond favourably to this treatment and others do not remain unknown at present. The determinants of successful ACT-based immunotherapy have been studied in mice68, and recent insights into T cell differentiation have been obtained from human studies4, which are discussed in more detail below. It has been shown that tumours may escape from the T cell-based therapy in some instances using a variety of mechanisms11,12,69. In addition, studies in mice and humans have explored the target specificities of the transferred T cells, the nature of the micro-environment and how can it be targeted (including the use of increased-intensity lymphodepletion), and the differentiation states of the cells involved.
As described below, genetically engineered T cells can be used to destroy cancers of various histologies. However, the efficacy of naturally occurring TILs appears to be restricted to melanoma, for reasons that are not fully understood. Other tumours, such as those arising from the colon and breast, do contain T cells39,70, but the specificities and functions of these T cells remain unclear. One explanation for why melanomas yield therapeutic TILs is that melanomas contain more mutations than virtually any other type of cancer25. Some tumours — especially those arising in childhood, testicular germ cell tumours and some leukaemias — contain very few mutations, and their cancerous phenotypes appear to be largely a result of epigenetic derangements31,71. Interestingly, melanomas are not alone in having highly mutated genomes, but are joined by some primary lung tumours71, which can experience extreme environmental mutagenesis induced by tobacco smoke rather than by light. This observation leads to the speculation that the numerous mutations in lung cancer could also be targeted by T cell-based ACT.
Although naturally occurring tumour-infiltrating T cell populations are currently only isolated and expanded from melanomas, virtually all tumour cell types can be lysed by T cells engineered to recognize antigens on tumour cell surfaces5,6,16,72,73 and can trigger the release of cytokines and chemokines from these T cells. This has prompted the development of approaches to genetically engineer T cells to express specific receptors that target tumours.
Genetic modification of T cells for ACT.
T cells can be genetically engineered, using a variety of techniques, to express TCRs with a high affinity and excellent specificity for target antigens74–76. Most frequently, investigators use retroviruses or lentiviruses encoding an antigen receptor specific for the target antigen. Gene engineering can vastly increase the range of tumour histologies susceptible to treatment; this range now includes neuroblastoma77, synovial cell sarcoma6, leukaemia and lymphoma3,5,7, to name just a few examples from the recent literature. Current efforts are too numerous to summarize here: 765 of the 1119 gene-therapy protocols considered by the Recombinant DNA Advisory Committee in the US alone are focused on the treatment of cancer78. Gene engineering may well be capable of targeting virtually any cancer histology, because T cells can be engineered to target antigens expressed by transformed and non-transformed cells from within the tumour mass. More details of this recognition are described below.
The TCRs used in current clinical trials can have several origins (FIG. 2). If a high-affinity TCR can be isolated from a patient, especially from a patient who had an excellent response to ACT, genes encoding this TCR can be cloned into a retrovirus or lentivirus, which can then be used to transduce autologous T cells from other patients with matching HLA restriction elements21. The affinity of engineered receptors can be increased by changes to the complementarity-determining regions or through directed evolution, although such changes may also reduce specificity79. It is also possible to use TCRs isolated from mice. Human T cells engineered to express mouse TCRs have been used to target CEA and PMEL in human clinical trials and can yield objective clinical responses in patients who receive them, although they can also cause ‘on target’ toxicities16,21.
Chimeric antigen receptors (CARs) are another means for providing specificity to transduced T cells and can originate from antibodies (FIG. 2). The variable regions from antibody genes can be engineered to encode single-chain structures fused to TCR intracellular domains that are capable of activating T cells. Recombinant retro-viruses can then be used to transduce T cells with the CAR, which has antibody-like specificity. Thus, CARs recognize MHC-nonrestricted structures on the surfaces of target cells, whereas TCRs recognize mainly intra-cellular antigens that have been processed and presented as peptide complexes with MHC molecules80,81. Finally, it is worth noting that, rather than engineering the T cells themselves, another approach that can be used to promote T cell recognition of tumours is to engineer bi specific antibodies82,83. One subgroup of these, known as bispecific T cell engagers (BITEs), can promote tumour elimination by linking T cells to tumours, even in the absence of cognate TCR–MHC interactions.
Advantages and disadvantages of gene-engineered T cells.
Gene-engineered T cells have several advantages. These include the ability of the immunotherapist to choose the biophysical properties of the receptor used84 as well as the cell population that is genetically engineered (such as cells at early stages of differentiation)85. Genetic modification of T cells for ACT is not limited to conferring new antigen reactivity to recipient T cells, but can also be used to insert genes that improve the efficacy of the T cells that are transduced. Such genes include those encoding molecules involved in co-stimulation86, the prevention of apoptosis87, the induction of inflammation88–90 or homeostatic proliferation, as well as those encoding chemokine receptors that promote T cell homing91. Finally, it is worth mentioning that it may be possible to engineer T cells to express transcription factors that control T cell differentiation and fate. Of course, any of these manipulations can also be done on naturally occurring tumour-specific T cells, or in concert with gene engineering that confers reactivity.
There are also disadvantages to using gene-engineered T cells. Current approaches have only monoclonal specificity, and this may represent a narrow attack on the tumour, facilitating the development of antigen escape variants. In addition, mispairing of the TCR α-and β-chains can occur92, although the efficiency of this in human cells has been questioned93. Transducing haematopoietic stem cells (HSCs) suppresses mispairing by shutting off the expression of recombination-activating genes through allelic exclusion94,95. Alternatively, using transmembrane domains from mouse receptors or transducing human γδ cells might limit mispairing96.
Another problem with the use of gene-engineered T cells is the development of unexpected toxicities. ‘Off-target’ toxicity results from the recognition of an unintended structure owing to antigenic mimicry or cross-reactivity (as well as TCR mispairing92), whereas in the case of ‘on-target’ toxicity T cells recognize the intended molecular target. Both types of recognition can result in tissue destruction and can trigger secondary effects, such as a cytokine storm81,97. However, the safety of ACT therapies can be improved by new technologies that enable the rapid obliteration of T cells in the event of adverse toxicity. For example, the T cells can be modified to express an inducible form of caspase 9 that causes rapid cell death when activated by an otherwise bio inert small-molecule drug98. The biggest hurdle for gene therapy to overcome remains the identification of antigens that can be targeted to destroy the cancer without causing untoward toxicity to normal tissues.
Depleting a corrupt host microenvironment
Contrary to early claims that patients with cancer are globally immunosuppressed, meaningful immuno-suppression is not seen until late stages in most patients with cancer. However, immunosuppressive cellular elements within the tumour microenvironment have long been observed99–101. Based on the goal of removing this immunosuppression, researchers have developed pre-treatment regimens of total-body irradiation or chemo-therapy that are capable of depleting lymphoid cells. Although it seems counterintuitive that the efficacy of ACT-based tumour immunotherapy can be improved by the removal of the host immune system, the data supporting the use of lymphodepleting regimens before ACT in mice are robust and clear102,103. There is also evidence that these approaches can improve ACT-based immunotherapies in human clinical trials4,104,105.
Several mechanisms might underlie the augmented efficacy of tumour-reactive T cells in the lymphopenic environment. These factors include the elimination or reprogramming of immunosuppressive MDSCs, which are discussed elsewhere in this focus issue106. Briefly, MDSCs are a heterogeneous population of CD11b+GR1+ cells that are found at increased frequencies in tumours and in chronic infections101,107. In a classic experiment conducted nearly two decades ago, it was shown that the depletion of MDSCs using GR1-specific antibodies could result in protection from tumour challenge108. More recently, it was shown that bone marrow-derived myeloid cells could be reprogrammed to lose their immunosuppressive properties through the use of IL-12 (REFS 89,90). Thus, modulation of the suppressive activities of MDSCs might contribute to the increased antitumour T cell responses observed after ACT.
In addition, ACT is enhanced by the depletion of endogenous cells that compete for activating cytokines109, by the augmentation of adoptively transferred T cells through HSC transplantation102, and by the increased functionality of APCs, which is mediated in part by the bacterial translocation that accompanies total-body irradiation110. Finally, although effector T cells can augment ACT-based immunotherapy in mice, regulatory T (TReg) cells diminish the effectiveness of these therapies111, perhaps through direct cellular inhibition but also by decreasing the availability of IL-2 (REF. 112). This concept of a ‘yin and yang’ relationship between TReg cells and effector T cells, which is governed by IL-2, has been discussed in the literature113,114. Taken together, it is clear that strategies that aim to overcome the highly dysregulated immune microenvironment found in patients with cancer can dramatically improve T cell-based immunotherapies.
T cell differentiation state and ACT
Lymphodepletion can have a marked impact on treatment with ACT-based immunotherapies, but it is not the only factor responsible for affecting clinical responses. Emerging findings from both mouse studies and clinical trials indicate that intrinsic properties related to the differentiation state of the adoptively transferred T cell populations are crucial to the success of ACT-based approaches4,115,116.
CD8+ T cell differentiation.
CD8+ T cells trigger tumour rejection in both mice and humans and can be categorized into distinct memory subsets based on their differentiation states85,117. The available data suggest that CD8+ T cells follow a progressive pathway of differentiation from naive T cells into central memory T cell (TCM cell) and effector memory T cell (TEM cell) populations85,116,118 (FIG. 3). The extent of differentiation is determined by the strength of the TCR signal and the cytokine environment that the T cell encounters during antigen-specific activation. Experiments using TCR-transgenic mice have revealed that CD8+ T cells that are stimulated multiple times with their specific antigen and IL-2 show decreased survival and proliferation compared with cells that are stimulated only once or not at all. Although these CD8+ T cells acquired the ability to lyse target cells and to produce IFNγ, qualities thought to be important in their antitumour efficacy22, their actual antitumour efficacy declined following adoptive transfer115. This decline in the function of tumour-specific T cells has been linked to a variety of factors, including cell-intrinsic counter-regulation119. T cell differentiation is associated with increased production of granzymes and reactive oxygen species, but also with the loss of the ability to produce IL-2, to home to lymph nodes and to resist apoptotic death. These findings have led to the hypothesis that the differentiation state of CD8+ T cells is inversely related to their capacity to proliferate and persist116 (FIG. 3).
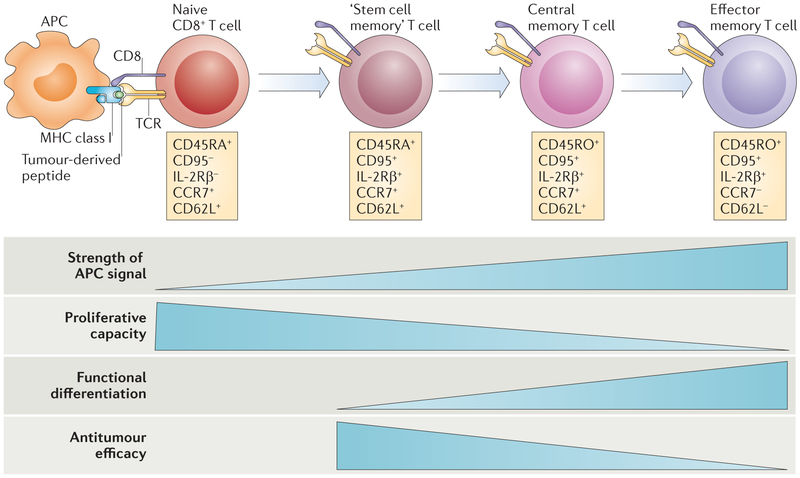
Figure 3 |. Progressive T cell differentiation diminishes proliferative and antitumour capacities.
T cells experience progressive changes in their phenotypes following antigenic stimulation. Depending on the strength and duration of the signals that they encounter during activation, they are launched on a pathway of proliferation and differentiation. T cells must be able to fully differentiate if they are to have antitumour efficacy. However, experimental evidence indicates that for adoptive transfer, T cell differentiation is inversely correlated with antitumour efficacy. The process of T cell differentiation results in the loss of proliferative and self-renewal capacity. For CD8+ T cells, T memory stem (TSCM) cells are more effective against tumours than central memory T (TCM) cells, which are more effective than effector memory T (TEM) cells. APC, antigen-presenting cell; CCR7, CC-chemokine receptor 7; IL-2Rβ, IL-2 receptor β-chain; TCR, T cell receptor. Figure is modified, with permission, from REF. 117 © (2011) Macmillan Publishers Ltd. All rights reserved.
In terms of efficacy in ACT for cancer, naive T cells have been shown to be more effective than memory T cells115,120,121, and within memory pools TCM cells show increased antitumour activity compared with TEM cells in mice122,123, non-human primates124 and humanized mouse models125. In addition, new evidence indicates that cells derived from TCM cells might persist and function in humans because of the reacquisition of CD62L126. It is clinically relevant that ‘younger’ T cells can be genetically engineered with a higher efficiency85, although it remains unclear whether the increased efficacy of these T cells depends on them being separated from fully differentiated effector cells before adoptive transfer.
More recently, there has been interest in a distinct population of CD8+ T memory stem (TSCM) cells, which were first characterized by Emerson and colleagues in transplantation models127. These cells express high levels of stem cell antigen 1 (SCA1), B cell lymphoma 2 (BCL-2) and the IL-2 receptor β-chain (IL-2Rβ) and were shown in mice to have superior antitumour properties compared with other memory T cell subsets128. In humans, TSCM cells resemble naive T cells, in that they have the phenotype CD45RA+CD45RO−. They express the IL-7 receptor α-chain, which can facilitate their survival129, as well as high levels of molecules that facilitate their homing to lymph nodes, such as CD62L and CC-chemokine receptor 7 (CCR7). In addition, human TSCM cells express co-stimulatory receptors (including CD27 and CD28), which may facilitate their proliferative capacities130, and high levels of CD95 and IL-2Rβ85,117.
TSCM cells have a genetic programme that enables them to proliferate extensively, and they can further differentiate into TCM and TEM cells (FIG. 3). Importantly, human TSCM cells show increased antitumour activity compared with TCM and TEM cells, suggesting that they will be more effective for ACT in patients with cancer85,117,128,131. Although T cells can reacquire CD62L expression124, the fundamental programme of differentiation proceeds efficiently in only one direction and so arresting or reversing T cell differentiation might be desirable in the setting of ACT for cancer under some circumstances. Future ACT-based immunotherapies may well rely more on the phenotype, telomere length, IL-2-producing capacity and TCR affinity of T cells when determining which cells should be transferred.
Modulating T cell differentiation to increase therapeutic efficacy.
As discussed above, the differentiation status of effector CD8+ T cells markedly affects their anti tumour efficacy. However, the differentiation state of the CD8+ T cells found in TIL populations that are isolated from patients is not yet clear. It is possible that, in some patients, available tumour-specific T cells are already terminally differentiated into TEM cells. In addition, the culture conditions currently used for preparing TILs for ACT — 2 weeks or more of culture with high doses of IL-2, a CD3-specific antibody and, in some cases, irradiated allogeneic ‘stimulator’ cells — may cause further differentiation of isolated TILs.
It is not clear whether limiting these powerful differentiation factors would improve the efficacy of TILs. Moreover, it is not known whether these improvements in T cell antitumour functions (if any such improvements occur) would compensate for the reduced TIL numbers that might result from restricting these factors. However, because IL-2 promotes the differentiation of effector T cells and increases their susceptibility to apoptosis, it will be important to explore whether reducing IL-2 concentrations in TIL culture conditions may be beneficial for ACT purposes. Other cytokines worth investigating are IL-15, which promotes TCM cell differentiation, and IL-21, which has been shown to arrest T cell differentiation, such that ‘younger’ cells are obtained at the end of culture132,133. In addition, pharmacological modulators of the WNT signalling pathway could have a beneficial effect85,128,134,135.
Differentiation of tumour-specific CD4+ T cells.
Much of the existing work in cancer immunotherapy has focused on CD8+ T cells. However, CD4+ T cells can also efficiently promote tumour rejection, in part through their ability to secrete IL-2 (REF. 111) and to recruit and sustain tumour-specific CD8+ T cells136. CD4+ T cells can also alter the function of APCs (especially DCs) and innate immune cells165. Furthermore, in addition to enhancing CD8+ T cell function, CD4+ T cells can have a more direct role in tumour elimination137,138. Several preclinical studies illustrate the potential uses of tumour-specific CD4+ T cells for cancer immuno-therapy95,139–144, and a recent clinical study in which nine patients with metastatic melanoma were treated with tumour-specific CD4+ T cell clones145 highlighted one patient who showed a complete response146.
The roles of CD4+ T cells in the antitumour immune response crucially depend on their polarization, which is determined by their expression of key transcription factors and has been reviewed elsewhere147. As already mentioned, TReg cells have potent immunoinhibitory functions and may contribute to the immunosuppressive tumour microenvironment. The role of TH2 cells (T helper 2 cells) in the antitumour immune response remains unclear, but TH1 cells can mediate antitumour responses in mouse models, in part through their production of IFNγ148.
New evidence suggests that adoptively transferred TH17 cells can promote long-lived antitumour immunity143,149. Although IL-17 itself has been linked to carcinogenesis150,151, it is clear that TH17-type polarization conditions can also augment the antitumour functions of CD8+ T cells152,153. TH17 cells are capable of inducing efficient tumour rejection141,142, but only if they are able to differentiate into effector T cells with TH1-like properties143. Interestingly, TH17 cells show similarities to stem cells in terms of their gene expression profile, their resistance to apoptosis, and their capacity for self-renewal and multipotency143,166.
In summary, CD4+ T cells are important for driving many distinct effector immune responses, and harnessing these cells may enable the development of better cancer therapies.
Future directions
ACT after lymphodepletion has emerged as a promising advance in cancer immunotherapy. Emerging data from preclinical and clinical studies have increased our understanding of the mechanisms that underlie successful immunotherapies and have helped us to identify the most effective T cell populations. In addition, gene engineering has expanded the potential target population that could benefit from ACT-based immunotherapies.
Importantly, ACT-based therapies are not FDA-approved and are only available in a limited number of locations worldwide. A major limitation of these therapies is their expense, and the treatments require specialized cell-production facilities and highly trained laboratory and medical staff. However, despite these limitations, there have been improvements in translating personalized cell therapies into the clinic (including advances in cell isolation and culture techniques), which have led to a proliferation of new experimental therapies. It seems plausible that blood banks could grow tumour-specific T cells for use in the clinic, or that autologous or even allogeneic cells could be mass-produced in a central facility, perhaps by a commercial enterprise154,155.
In the future, it will be important to explore methods for improving immune ablation. Although pilot trials have suggested that total-body irradiation can improve the efficacy of ACT-based therapies, randomized trials to compare high-intensity lymphodepleting regimens are currently underway (see ClinicalTrials.gov; study identifier NCT01319565). Inexpensive and routine DNA sequencing techniques may soon revolutionize cancer immunotherapy by enabling the identification of patient-specific tumour antigens (FIG. 4).
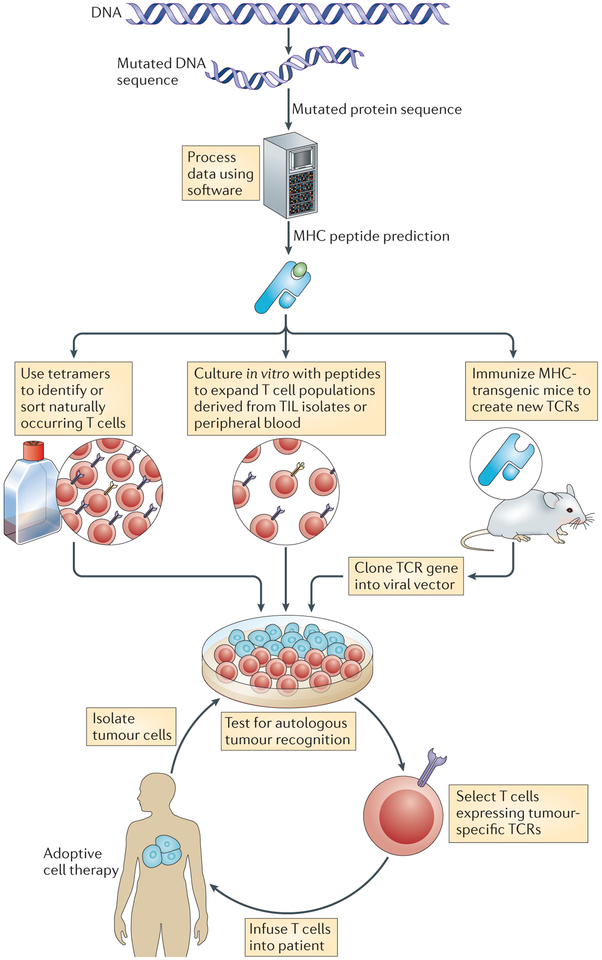
Figure 4 |. Highly personalized medicine.
Inexpensive and readily available DNA sequencing technology might revolutionize cancer immunotherapy, enabling a highly personalized approach to the identification of new tumour-associated antigens. The expressed genes from a patient’s tumour can be sequenced to identify candidate mutant T cell epitopes. Relevant epitopes that could potentially bind to the MHC molecules of the patient could be predicted using peptide prediction algorithms (for example, see the HLA Peptide Binding Predictions website). If peptides derived from mutant proteins are found to be capable of forming new MHC-restricted target structures, the candidate peptides could be used in one of at least three ways. First, scientists can identify or sort cells that express relevant antigens (such as those derived from driver oncogenes) using tetramer-like reagents. Second, candidate peptides could be used to stimulate T cells that are already present in the patient’s tumour or in their peripheral blood. Third, tumour antigens could be used to prime tumour-specific T cells in humanized mice that are transgenic for human MHC molecules. If the T cell populations generated are specific for the patient’s tumour, they could be expanded and adoptively transferred if they are of human origin. Alternatively, mouse T cells can be used to identify suitable T cell receptors (TCRs) for gene-engineering approaches. TIL, tumour-infiltrating lymphocyte.
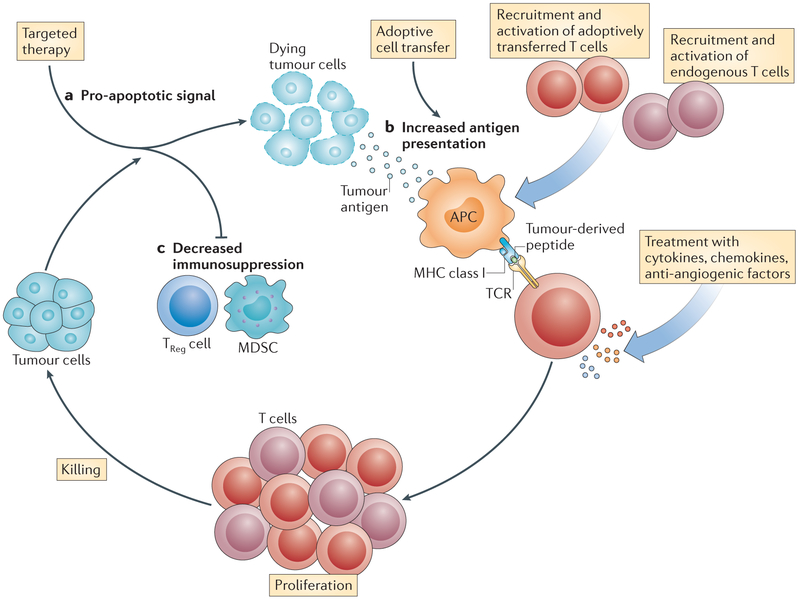
Finally, there is a strong rationale for using other cancer therapies in combination with ACT-based therapy156 (FIG. 5). Studies in mice have shown that acute activation of T cells can augment their antitumour efficacy157. This can be accomplished in vivo by administering a vaccine together with the transferred cells158. Tumour cell death after ‘oncogene withdrawal’ may provide the antigenic stimulation that can drive T cells159,160. Oncogene withdrawal may also reduce the production of immunosuppressive cytokines by tumour cells161. The use of targeted agents might change the balance of pro- and anti-apoptotic molecules in tumour cells to bias these cells towards death following encounter with tumour-specific T cells or their toxic metabolites. In addition, it has been shown that the administration of vemurafenib (a small-molecule inhibitor of the RAF–MEK–ERK signalling pathway) can lead to the upregulation of tumour-associated antigens on melanomas, thereby promoting T cell-mediated recognition of the tumour162.
Figure 5 |. The rationale for combining targeted therapies with adoptive cell transfer-based immunotherapy.
a | A targeted agent (such as vemurafenib) can be used to promote apoptosis in tumour cells. b | Antigens released by dying tumour cells can then be acquired at an increased rate by antigen-presenting cells (APCs) that are present in the tissue or in local draining lymph nodes. These APCs process the tumour antigens and present tumour-derived peptides to T cells. This can lead to the priming of adoptively transferred tumour-specific T cells, as well as the activation of other endogenous tumour-specific T cell populations. Treatment with immunostimulatory cytokines and chemokines may increase the efficiency of tumour-specific T cell activation. c | Therapies that target immunosuppressive factors or cells present in the tumour microenvironment — such as regulatory T (TReg) cells and myeloid-derived suppressor cells (MDSCs) — may also promote increased activation of tumour-specific T cells. TCR, T cell receptor.
Conclusions
To paraphrase the description of leukaemia by the physician William Castle in 1950: although the palliation of cancer is the daily task of the oncologist, its cure is our “fervent hope”. It seems clear that drug-based treatments alone generally do not kill all cancer cells — with the notable exception of germ cell tumours and some haemato-logical malignancies. Residual disease after drug therapy will ultimately grow back, with lethal consequences. However, the immune system is capable of achieving sterilizing immunity and of inducing long-term, durable responses that are probably curative. The use of adoptive T cell-based therapies to eradicate cancer is at a rare nexus of basic immunology and clinically meaningful therapy.
Acknowledgements
This work was supported by the Intramural Research Program of the Center for Cancer Research, US National Cancer Institute (NCI), National Institutes of Health. The authors would like to thank C. Klebanoff, L. Gattinoni, C. Hinrichs and P. Muranski for discussions about T cell differentiation, M. Bachinski for editorial help, E. Tran for critically reading the manuscript, and all the members of the translational immunology team at the NCI, especially J. C. Yang, P. F. Robbins, R. A. Morgan, R. M. Sherry, S. Feldman, M. Parkhurst, M. Hughes, G. Phan and U. Kammula.
Glossary
- Tumour-infiltrating lymphocyte
(TIL). A member of the heterogeneous population of T cells found in a tumour. TILs are characterized by a diversity of phenotypes, antigen specificities, avidities and functional characteristics. TIL populations can be activated and expanded ex vivo and re-infused into the tumour-bearing host.
- Interleukin-2
(IL-2). A T cell growth factor that is capable of triggering the expansion of both effector T cell and regulatory T cell populations. IL-2 is used to treat patients with melanoma and as a part of some ACT-based treatment regimes.
- Adoptive cell transfer
(ACT). The administration of tumour-specific lymphocytes (obtained from the patient (autologous) or from a donor (allogeneic)) following a lymphodepleting preparative regimen.
- Lymphodepletion
The use of total-body irradiation or cytotoxic drugs to deplete the lymphoid compartment in a patient.
- Myeloid-derived suppressor cells
(MDSCs). A group of immature CD11b+GR1+ cells (which include precursors of macrophages, granulocytes, dendritic cells and myeloid cells) that are produced in response to various tumour-derived cytokines. These cells have been shown to induce tolerance in tumour-associated CD8+ T cells.
- Cross-priming
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens onto MHC class I molecules. This property is atypical, because most cells exclusively present peptides from their endogenous proteins on MHC class I molecules.
- Immunoediting
A process by which the immune system of a host may alter the gene expression of an emerging tumour, such that the most immunogenic epitopes are removed or ‘edited’, thereby facilitating tumour escape from immune recognition.
- Carcinoembryonic antigen
A protein found in fetal gastrointestinal tissue that can be upregulated in some gastrointestinal cancers and can serve as a marker of tumour burden.
- Objective clinical responses
The response evaluation criteria in solid tumours (RECIST) define an objective response as a 30% reduction in the sum of the longest diameters of measurable tumour lesions when comparing post-treatment with pretreatment values. The World Health Organization criteria define an objective response to be a 50% reduction in the sum of the products of perpendicular diameters of measurable lesions. In both sets of criteria, no new lesions can appear. Perhaps the most important clinical end point is benefit from a treatment based on increased survival time, although this can only be assessed using controlled patient cohorts.
- Driver mutations
A nonsense mutation in a gene that causes a cancer cell to have a survival and/or growth advantage.
- Cancer–testis antigens
(Also known as cancer germline antigens). A class of >100 proteins that are expressed by many human cancers but not by normal adult tissues except in the testes and fetal ovaries. These antigens include CTAG1, MAGEA3 and SSX1.
- Complementarity-determining regions
Short amino acid sequences found in the variable domains of antigen receptor proteins that recognize an antigen and therefore provide the receptor with its specificity for that particular antigen.
- Directed evolution
A cyclic sequence of steps (including modification, selection and amplification) that is used, typically in vitro, to enrich for proteins or nucleic acids that show properties that are desired by the researcher but that are not necessarily found in nature.
- Chimeric antigen receptors
(CARs). Antigen receptors that contain sequences from more than one source, such as an antibody molecule, a T cell receptor signalling chain, and an activating motif.
- Homeostatic proliferation
A process of activation and proliferation of leukocytes in the lymphopenic environment. T cell homeostatic proliferation is driven by T cell receptor interactions with self-peptide–MHC complexes and T cell responsiveness to cytokines such as interleukin-7 (IL-7), IL-15 and possibly IL-21.
- Recombination-activating genes
These genes (Rag1 and Rag2) are expressed by developing lymphocytes. Mice that are deficient in either RAG protein fail to produce B and T cells owing to a developmental block in the gene rearrangement that is required for antigen receptor expression.
- Allelic exclusion
A mechanism that ensures that a lymphocyte expresses antigen receptors of only a single specificity at its cell surface. This is an integral step in the clonal commitment of a lymphocyte lineage.
- Cytokine storm
A sudden surge in the circulating levels of pro-inflammatory cytokines, such as interleukin-1, interleukin-6, tumour necrosis factor and interferon-γ. Clinically, this can result in hypotension, acute renal failure, poor pulmonary function and even death.
- Central memory T cell
(TCM cell). An antigen-experienced CD8+ T cell that lacks immediate effector function but is able to mediate rapid recall responses. These cells also rapidly develop the phenotype and function of effector memory T cells after re-stimulation with antigen. TCM cells retain the migratory properties of naive T cells and therefore circulate through the secondary lymphoid organs.
- Effector memory T cell
(TEM cell). A terminally differentiated T cell that lacks lymph-node-homing receptors but expresses receptors that enable it to home to inflamed tissues. TEM cells can exert immediate effector functions without the need for further differentiation.
- Telomere
The segment at the end of chromosome arms, which consists of a series of repeated DNA sequences (TTAGGG in all vertebrates) that regulate chromosomal replication at each cell division.
- Allogeneic
Inter-individual genetic variation at the MHC locus. In a partially matched transplant, for example, some MHC molecules are shared by the donor and recipient, but in addition the donor has some MHC molecules that the recipient does not.
- TH2 cells
(T helper 2 cells). A subset of CD4+ T cells that has an important role in humoral immunity and in allergic responses. TH2 cells express the transcription factors GATA3 and STAT6 and produce cytokines such as interleukin-4 (IL-4), IL-5, IL-9 and IL-13, which regulate IgE synthesis, eosinophil proliferation, mast cell proliferation and airway hyperresponsiveness, respectively. A TH2 cell pattern of cytokine expression is observed in allergic inflammation and in parasitic infections, conditions that are both associated with IgE production and eosinophilia.
- TH1 cells
(T helper 1 cells). A subset of CD4+ T cells that expresses the transcription factor T-bet and is associated with cell-mediated immunity. TH1 cells provide help for cytotoxic T cell responses by secreting high concentrations of interleukin-2, tumour necrosis factor and interferon-γ. They may also promote immunopathology in certain autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis.
- TH17 cells
(T helper 17 cells). A subset of CD4+ T helper cells that produce interleukin-17 (IL-17) and that are thought to be important in antibacterial and antifungal immunity and may also have a role in autoimmune diseases. Their generation involves IL-23 and IL-21, as well as the transcription factors RORγt and STAT3.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Deguine J, Breart B, Lemaitre F, Di Santo JP & Bousso P Intravital imaging reveals distinct dynamics for natural killer and CD8+ T cells during tumor regression. Immunity 33, 632–644 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Kochenderfer JN et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or c4hemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res 17, 4550–4557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, Bagg A & June CH Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med 365, 725–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol 29, 917–924 (2011).This study provides evidence that gene-engineered T cells can treat other solid tumour histologies (in this case, metastatic synovial cell sarcoma), in addition to melanoma.
- 7.Kochenderfer JN et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 8 December 2011. (doi:10.1182/blood-2011-10-384388).References 2, 3, 5 and 7 demonstrate the therapeutic power of genetically engineered T cells in the treatment of CD19+ lymphoma.
- 8.Baitsch L et al. Exhaustion of tumor-specific CD8 T cells in metastases from melanoma patients. J. Clin. Invest 121, 2350–2360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadzadeh M et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offringa R Antigen choice in adoptive T-cell therapy of cancer. Curr. Opin. Immunol 21, 190–199 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Restifo NP et al. Identification of human cancers deficient in antigen processing. J. Exp. Med 177, 265–272 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restifo NP et al. Loss of functional β2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl Cancer Inst 88, 100–108 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber RD, Old LJ & Smyth MJ Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Matsushita H et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Bruggen P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Parkhurst MR et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther 19, 620–626 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos R et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res 68, 8446–8455 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Overwijk WW & Restifo NP Autoimmunity and the immunotherapy of cancer: targeting the “self” to destroy the “other”. Crit. Rev. Immunol 20, 433–450 (2000). [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med 188, 277–286 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami Y et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl Acad. Sci. USA 91, 3515–3519 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LA et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer DC et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc. Natl Acad. Sci. USA 105, 8061–8066 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh S et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology 116, 981–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies MA & Samuels Y Analysis of the genome to personalize therapy for melanoma. Oncogene 29, 5545–5555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walia V, Mu EW, Lin JC & Samuels Y Delving into somatic variation in sporadic melanoma. Pigment Cell Melanoma Res 25, 155–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilchrest BA Molecular aspects of tanning. J. Invest. Dermatol 131, e14–e17 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Robbins PF et al. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med 183, 1185–1192 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner MK & Heslop HE Adoptive T cell therapy of cancer. Curr. Opin. Immunol 22, 251–257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenter GG et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med 361, 1838–1847 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Anders K et al. Oncogene-targeting T cells reject large tumors while oncogene inactivation selects escape variants in mouse models of cancer. Cancer Cell 20, 755–767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481, 329–334 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann O et al. Genome-wide analysis of cancer/testis gene expression. Proc. Natl Acad. Sci. USA 105, 20422–20427 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida LG et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res 37, D816–D819 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baylin SB & Jones PA A decade of exploring the cancer epigenome — biological and translational implications. Nature Rev. Cancer 11, 726–734 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo ZS et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res 66, 1105–1113 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wargo JA et al. Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol. Immunother 58, 383–394 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson AJ, Caballero OL, Jungbluth A, Chen YT & Old LJ Cancer/testis antigens, gametogenesis and cancer. Nature Rev. Cancer 5, 615–625 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Chinnasamy N et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol 186, 685–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruffell B et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 8 August 2011. (doi:10.1073/pnas.1104303108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engels B, Rowley DA & Schreiber H Targeting stroma to treat cancers. Semin. Cancer Biol 22, 41–49 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraman M et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 330, 827–830 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Chinnasamy D et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Invest 120, 3953–3968 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrimali RK et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 70, 6171–6180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung AS, Lee J & Ferrara N Targeting the tumour vasculature: insights from physiological angiogenesis. Nature Rev. Cancer 10, 505–514 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Fefer A Immunotherapy and chemotherapy of Moloney sarcoma virus-induced tumors in mice. Cancer Res 29, 2177–2183 (1969). [PubMed] [Google Scholar]
- 46.Greenberg PD, Cheever MA & Fefer A Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2− lymphocytes. J. Exp. Med 154, 952–963 (1981).An important and remarkable landmark paper that describes in significant detail the scientific foundations of lymphodepletion prior to adoptive immunotherapy using what are now known as CD4+ T cells.
- 47.Rosenberg SA & Terry WD Passive immunotherapy of cancer in animals and man. Adv. Cancer Res 25, 323–388 (1977). [DOI] [PubMed] [Google Scholar]
- 48.Wang M et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol 154, 4685–4692 (1995). [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer DC et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J. Immunol 173, 7209–7216 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen PW et al. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J. Immunol 156, 224–231 (1996). [PMC free article] [PubMed] [Google Scholar]
- 51.Bronte V et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J. Immunol 154, 5282–5292 (1995). [PMC free article] [PubMed] [Google Scholar]
- 52.Cormier JN et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J. Sci. Am 3, 37–44 (1997). [PMC free article] [PubMed] [Google Scholar]
- 53.Leitner WW et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nature Med 9, 33–39 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ying H et al. Cancer therapy using a self-replicating RNA vaccine. Nature Med 5, 823–827 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overwijk WW et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc. Natl Acad. Sci. USA 96, 2982–2987 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg SA et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol 175, 6169–6176 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Irvine KR et al. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J. Natl Cancer Inst 89, 1595–1601 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Rao JB et al. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7–1 expression. J. Immunol 156, 3357–3365 (1996). [PMC free article] [PubMed] [Google Scholar]
- 59.Irvine KR et al. Recombinant virus vaccination against “self” antigens using anchor-fixed immunogens. Cancer Res 59, 2536–2540 (1999). [PMC free article] [PubMed] [Google Scholar]
- 60.Irvine KR, Rao JB, Rosenberg SA & Restifo NP Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J. Immunol 156, 238–245 (1996). [PMC free article] [PubMed] [Google Scholar]
- 61.Carroll MW et al. Construction and characterization of a triple-recombinant vaccinia virus encoding B7–1, interleukin 12, and a model tumor antigen. J. Natl Cancer Inst 90, 1881–1887 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlom J Recent advances in therapeutic cancer vaccines. Cancer Biother. Radiopharm 27, 2–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg SA, Yang JC & Restifo NP Cancer immunotherapy: moving beyond current vaccines. Nature Med 10, 909–915 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klebanoff CA, Acquavella N, Yu Z & Restifo NP Therapeutic cancer vaccines: are we there yet? Immunol. Rev 239, 27–44 (2011).An update on recent vaccine trials showing that although current therapeutic cancer vaccines can extend survival in some studies (by months, not years), they are rarely if ever curative.
- 65.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 363, 411–422 (2010).Although criticized by some for the trial design, this study nevertheless served as a key basis for the FDA licensing of sipuleucel-T (Provenge; Dendreon).
- 66.Itzhaki O et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J. Immunother 34, 212–220 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Besser MJ et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res 16, 2646–2655 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Klebanoff CA et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res 17, 5343–5352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khong HT & Restifo NP Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nature Immunol 3, 999–1005 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogino S, Galon J, Fuchs CS & Dranoff G Cancer immunology-analysis of host and tumor factors for personalized medicine. Nature Rev. Clin. Oncol 8, 711–719 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stratton MR Exploring the genomes of cancer cells: progress and promise. Science 331, 1553–1558 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Louis CU et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP & Rosenberg SA Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerkar SP et al. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J. Immunother 34, 343–352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abad JD et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. J. Immunother 31, 1–6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan RA et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pule MA et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med 14, 1264–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Recombinant DNA Advisory Committee. Human gene transfer protocols. National Institutes of Health; [online], http://oba.od.nih.gov/oba/rac/protocol.pdf (2011). [Google Scholar]
- 79.Varela-Rohena A et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature Med 14, 1390–1395 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sadelain M, Brentjens R & Riviere I The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol 21, 215–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgan RA et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther 18, 843–851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baeuerle PA & Itin C Clinical experience with gene therapy and bispecific antibodies for T cell-based therapy of cancer. Curr. Pharm. Biotechnol 14 February 2012. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 83.Choi BD et al. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin. Biol. Ther 11, 843–853 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Merhavi-Shoham E, Haga-Friedman A & Cohen CJ Genetically modulating T-cell function to target cancer. Semin. Cancer Biol 22, 14–22 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Gattinoni L et al. A human memory T cell subset with stem cell-like properties. Nature Med 17, 1290–1297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephan MT et al. T cell-encoded CD80 and 4–1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature Med 13, 1440–1449 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Charo J et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res 65, 2001–2008 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pegram HJ et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 21 February 2012. (doi:10.1182/blood-2011-12-400044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerkar SP et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest 121, 4746–4757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerkar SP et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 70, 6725–6734 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng W et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res 16, 5458–5468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bendle GM et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nature Med 16, 565–570 (2010).An important paper that explores in significant detail the consequences of ‘mispairing’ of transduced TCR α- and β-chains.
- 93.Rosenberg SA Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther 18, 1744–1745 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vatakis DN et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl Acad. Sci. USA 108, e1408–e1416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ha SP et al. Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity. J. Clin. Invest 120, 4273–4288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jorritsma A, Schotte R, Coccoris M, de Witte MA & Schumacher TN Prospects and limitations of T cell receptor gene therapy. Curr. Gene Ther 11, 276–287 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Brentjens R, Yeh R, Bernal Y, Riviere I & Sadelain M Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther 18, 666–668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Stasi A et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med 365, 1673–1683 (2011).This paper describes a highly effective means of rapidly deleting gene-engineered T cells in the event of toxicity.
- 99.North RJ Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med 155, 1063–1074 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheever MA, Greenberg PD & Fefer A Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J. Immunol 125, 711–714 (1980). [PubMed] [Google Scholar]
- 101.Bronte V et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+T cells. Blood 96, 3838–3846 (2000). [PMC free article] [PubMed] [Google Scholar]
- 102.Wrzesinski C et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J. Clin. Invest 117, 492–501 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wrzesinski C et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J. Immunother 33, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dudley ME et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dudley ME et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol 26, 5233–5239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gabrilovich DI, Ostrand-Rosenberg S & Bronte V Coordinated regulation of myeloid cells by tumours. Nature Rev. Immunol 12, 253–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bronte V et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol 161, 5313–5320 (1998).One of the first descriptions of the suppressive myeloid subset later known as MDSCs.
- 108.Seung LP, Rowley DA, Dubey P & Schreiber H Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc. Natl Acad. Sci. USA 92, 6254–6258 (1995).This paper describes a classic experiment demonstrating that depletion of GR1+ cells can enhance tumour immunotherapy.
- 109.Gattinoni L et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med 202, 907–912 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paulos CM et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest 117, 2197–2204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Antony PA et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol 174, 2591–2601 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kastenmuller W et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol 187, 3186–3197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Antony PA & Restifo NP CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J. Immunother 28, 120–128 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bluestone JA The yin and yang of interleukin-2-mediated immunotherapy. N. Engl. J. Med 365, 2129–2131 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Gattinoni L et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest 115, 1616–1626 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gattinoni L, Powell DJ Jr, Rosenberg SA & Restifo NP Adoptive immunotherapy for cancer: building on success. Nature Rev. Immunol 6, 383–393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sallusto F & Lanzavecchia A Memory in disguise. Nature Med 17, 1182–1183 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Klebanoff CA, Gattinoni L & Restifo NP CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev 211, 214–224 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palmer DC & Restifo NP Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol 30, 592–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hinrichs CS et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl Acad. Sci. USA 106, 17469–17474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hinrichs CS et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 117, 808–814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klebanoff CA et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA 102, 9571–9576 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klebanoff CA et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 1969–1974 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berger C et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest 118, 294–305 (2008).An important study in non-human primates showing that cells derived from central memory T cells are more persistent than those derived from effector memory T cells.
- 125.Wang X et al. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117, 1888–1898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chapuis A et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. 5 March 2012. (doi:10.1073/pnas.1113748109).References 122 and 126 show that central memory T cells mediate superior antitumour immunity compared with effector memory T cells in murine systems.
- 127.Zhang Y, Joe G, Hexner E, Zhu J & Emerson SG Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nature Med 11, 1299–1305 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Gattinoni L et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature Med 15, 808–813 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mackall CL, Fry TJ & Gress RE Harnessing the biology of IL-7 for therapeutic application. Nature Rev. Immunol 11, 330–342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.June CH, Bluestone JA, Nadler LM & Thompson CB The B7 and CD28 receptor families. Immunol. Today 15, 321–331 (1994). [DOI] [PubMed] [Google Scholar]
- 131.Ji Y et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nature Immunol 12, 1230–1237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hinrichs CS et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh H et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res 71, 3516–3527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gattinoni L, Ji Y & Restifo NP Wnt/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res 16, 4695–4701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gattinoni L, Klebanoff CA & Restifo NP Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci. Transl. Med 1, 11ps12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pardoll DM & Topalian SL The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol 10, 588–594 (1998). [DOI] [PubMed] [Google Scholar]
- 137.Xie Y et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J. Exp. Med 207, 651–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quezada SA et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med 207, 637–650 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mumberg D et al. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl Acad. Sci. USA 96, 8633–8638 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Corthay A et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity 22, 371–383 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Martin-Orozco N et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muranski P et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112, 362–373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muranski P et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frankel TL et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J. Immunol 184, 5988–5998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yee C Adoptive therapy using antigen-specific T-cell clones. Cancer J 16, 367–373 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Hunder NN et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med 358, 2698–2703 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nakayamada S, Takahashi H, Kanno Y & O’Shea JJ Helper T cell diversity and plasticity. Curr. Opin. Immunol 15 February 2012. (doi:10.1016/j.coi.2012.01.014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nishimura T et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother. Pharmacol 46, S52–S61 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Muranski P & Restifo NP Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol 21, 200–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zou W & Restifo NP TH17 cells in tumour immunity and immunotherapy. Nature Rev. Immunol 10, 248–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muranski P & Restifo NP Does IL-17 promote tumor growth? Blood 114, 231–232 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Hinrichs CS et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 114, 596–599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yen HR et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J. Immunol 183, 7161–7168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boni A et al. Adoptive transfer of allogeneic tumor-specific T cells mediates effective regression of large tumors across major histocompatibility barriers. Blood 112, 4746–4754 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Restifo NP & Bachinski M Imagining a cure: for cancer patients, close is not good enough. The Scientist April 2011, 28–29 (2011). [Google Scholar]
- 156.Blank CU, Hooijkaas AI, Haanen JB & Schumacher TN Combination of targeted therapy and immunotherapy in melanoma. Cancer Immunol. Immunother 60, 1359–1371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Klebanoff CA et al. Programming tumor-reactive effector memory CD8+ T cells in vitro obviates the requirement for in vivo vaccination. Blood 114, 1776–1783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Overwijk WW et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med 198, 569–580 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Restifo NP Can antitumor immunity help to explain “oncogene addiction”? Cancer Cell 18, 403–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rakhra K et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).A paper that explores the immunological consequences of oncogene withdrawal.
- 161.Sumimoto H, Imabayashi F, Iwata T & Kawakami Y The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med 203, 1651–1656 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boni A et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 70, 5213–5219 (2010). [DOI] [PubMed] [Google Scholar]
- 163.DuPage M, Mazumdar C, Schmidt LM, Cheung AF & Jacks T Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482, 405–409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Staveley-O’Carroll K et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl Acad. Sci. USA 95, 1178–1183 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hung K et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med 188, 2357–2368 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kryczek I et al. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med 3, 104ra100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]