Abstract
Activated immune cells become obligate glucose utilizers, and a large i.v. lipopolysaccharide (LPS) dose causes insulin resistance and severe hypoglycemia. Therefore, study objectives were to quantify the amount of glucose needed to maintain euglycemia following an endotoxin challenge as a proxy of leukocyte glucose requirements. Fifteen fasted crossbred gilts (30.3 ± 1.7 kg) were bilaterally jugular catheterized and assigned 1 of 2 i.v. bolus treatments: control (CON; 10 mL sterile saline; n = 7) or LPS challenge + euglycemic clamp (LPS-Eu; Escherichia coli 055:B5; 5 μg/kg BW; 50% dextrose infusion to maintain euglycemia; n = 8). Following administration, blood glucose was determined every 10 min and dextrose infusion rates were adjusted in LPS-Eu pigs to maintain euglycemia for 8 h. Pigs were fasted for 8 h prior to the bolus and remained fasted throughout the challenge. Rectal temperature was increased in LPS-Eu pigs relative to CON pigs (39.8 vs. 38.8°C; P < 0.01). Relative to the baseline, CON pigs had 20% decreased blood glucose from 300 to 480 min postbolus (P = 0.01) whereas circulating glucose content in LPS-Eu pigs did not differ (P = 0.96) from prebolus levels. A total of 116 ± 8 g of infused glucose was required to maintain euglycemia in LPS-Eu pigs. Relative to CON pigs, overall plasma insulin, blood urea nitrogen, β-hydroxybutrate, L-lactate, and LPS-binding protein were increased in LPS-Eu pigs (295, 108, 29, 133, and 13%, respectively; P ≤ 0.04) whereas NEFA was decreased (66%; P < 0.01). Neutrophils in LPS-Eu pigs were decreased 84% at 120 min postbolus and returned to CON levels by 480 min (P < 0.01). Overall, lymphocytes, monocytes, eosinophils, and basophils were decreased in LPS-Eu pigs relative to CON pigs (75, 87, 70, and 50%, respectively; P ≤ 0.05). These alterations in metabolism and the large amount of glucose needed to maintain euglycemia indicate nutrient repartitioning away from growth toward the immune system. Glucose is an important fuel for the immune system, and data from this study established that the glucose requirements of an intensely and acutely activated immune system in growing pigs are approximately 1.1 g/kg BW0.75/h.
Keywords: glucose, immune system, insulin, requirement
INTRODUCTION
Infection and inflammation are likely experienced by all production animals to varying degrees at some point in their life cycle. Inflammation has negative economic consequences to animal agriculture because it decreases production, feed efficiency, reproduction and increases health care costs. An activated immune system demands a large amount of energy and nutrients (Lochmiller and Deerenberg, 2000; Johnson, 2012), which reprioritizes the hierarchy of nutrient partitioning away from productive purposes. For example, skeletal muscle proteolysis is an important source of AA for different components of the immune response. The AA requirement during immunoactivation has been extensively studied (Klasing and Austic, 1984a,b; Reeds and Jahoor, 2001; Johnson, 2012); however, accurately quantifying the energetic requirements of the immune system is more difficult because there are immune cells within virtually every tissue and leukocyte distribution and flux change dynamically during immunoactivation. Whole-body energy expenditure and glucose utilization are estimated to increase approximately 50% during an infection (Lang and Dobrescu, 1991; Lang et al., 1993; Plank et al., 1998). Recently, Huntley et al. (2017) estimated that immunoactivation increases maintenance requirements of growing pigs by ∼25%. We believe increased glucose utilization by an activated immune system makes up a majority of this increased energy cost because stimulated leukocytes switch their metabolism from oxidative phosphorylation to aerobic glycolysis to support energetic and substrate demand (Palsson-McDermott and O'Neill, 2013). Despite homeorhetic efforts to synchronously ensure glucose availability, including increased liver glucose export and decreased glucose disposal in insulin-sensitive tissues, hypoglycemia and hyperlactemia often develop following a lipopolysaccharide (LPS) challenge (McGuinness, 2005). Previously, we have used a LPS–euglycemic clamp technique to estimate glucose requirements of an activated immune system in growing calves and lactating cows (Kvidera et al., 2016a, 2017b), but whether this model yields similar results across species is not clear. Therefore, experimental objectives were to use the LPS–euglycemic clamp technique to estimate the amount of glucose required to fuel an acute immune response in growing pigs.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Iowa State University Institutional Animal Care and Use Committee. Fifteen crossbred gilts (30.3 ± 1.7 kg BW) were randomly assigned to individual pens (57 by 221 cm) at the Iowa State University Swine Nutrition Farm (Ames, IA). Pigs were weighed on pen assignment, and this BW was used to calculate the LPS dose. Pigs were allowed to acclimate for 2 d before they were implanted with bilateral jugular catheters using the percutaneous technique as we have previously described (Sanz Fernandez et al., 2015). Following catheterization, pigs were allowed 3 d to recover before they were assigned to 1 of 2 treatments: control (CON; 10 mL sterile saline; n = 7) or LPS challenge + euglycemic clamp (LPS-Eu; 5 μg/kg BW; Escherichia coli 055:B5; Sigma-Aldrich Corp., St. Louis, MO; n = 8). During acclimation and the postsurgery period, pigs were fed a diet formulated to meet or exceed the predicted requirements of energy, protein, minerals, and vitamins (Supplemental Table S1 [see the online version of the article at http://journalofanimalscience.org]; NRC, 2012) and were allowed to consume feed ad libitum. Feed was removed 8 h prior to treatment administration, and animals remained fasted during the 480-min data collection period.
Rectal temperatures were obtained −30 and 0 min relative to bolus administration and hourly postbolus using a digital thermometer (M700; GLA Agricultural Electronics, San Luis Obispo, CA). Baseline blood samples were obtained at −30, −20, and 0 min relative to bolus administration to establish baseline glucose levels. Each respective treatment bolus was administered immediately following the 0-min blood sample collection. Samples for blood glucose measurements were obtained hourly in CON pigs and every 10 min in LPS-Eu pigs and analyzed with a glucometer (TRUEbalance glucometer; McKesson Corp., San Francisco, CA). Dextrose infusion in LPS-Eu pigs began when blood glucose content declined below baseline levels, and its infusion rate was adjusted as necessary to maintain blood glucose concentration at baseline levels (±10%). The rate of 50% dextrose infusion (mL/h) was transformed to the rate of glucose infusion (ROGI; g/h). The total glucose infused for each pig was calculated using the ROGI for each 10-min interval (48 intervals in total) according to the following equation: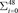 ROGI (g/h)i × (1 h/60 min) × 10 min.
ROGI (g/h)i × (1 h/60 min) × 10 min.
Additional blood samples for further analysis were obtained from pigs in both treatments at −30, −20, and 0 min relative to the bolus and hourly postbolus. Blood samples (10 mL) were collected in glass tubes containing 50 µL sterile heparin (Sagent Pharmaceuticals, Inc., Schaumburg, IL). Plasma was harvested following centrifugation at 1,500 × g for 15 min at 4°C and subsequently frozen at −20°C until analysis. Plasma insulin, NEFA, glucose, β-hydroxybutyrate (BHB), LPS-binding protein (LBP), L-lactate, and blood urea nitrogen (BUN) concentrations were determined using commercially available kits validated for use in our laboratory (insulin [Mercodia AB, Uppsala, Sweden], NEFA [Wako Chemicals USA, Inc., Richmond, VA], BHB [Pointe Scientific, Inc., Canton, MI], LBP [Hycult Biotech, Uden, the Netherlands], L-lactate [Biomedical Research Service Center, Buffalo, NY], and BUN [Teco Diagnostics, Anaheim, CA]). The inter- and intra-assay coefficients for the insulin, NEFA, glucose, BHB, L-lactate, LBP, and BUN assays were 8.5 and 6.2%, 7.1 and 6.8%, 20.1 and 3.9%, 14.4 and 13.5%, 2.3 and 3.7%, 26.1 and 5.6%, 14.2 and 5.7%, respectively. For white blood cell counts, a 3-mL blood sample was also collected (K2 EDTA; Becton, Dickinson and Company, Franklin Lakes, NJ) and stored at 4°C before submission to the Iowa State Department of Veterinary Pathology (Ames, IA) within 24 h of collection for complete blood count analysis with automated differential.
Each animal's respective parameter was analyzed using repeated measures with an autoregressive covariance structure for blood, ROGI, and rectal temperature data and spatial power law for complete blood count parameters. The repeated effect was minute after bolus administration. The effect of time was analyzed for ROGI and 10-min interval blood glucose data from LPS-Eu pigs, and no covariates were used for these analyses. For all remaining parameters, each specific variable's prebolus value served as a covariate. For blood samples, values obtained from −30, −20, and 0 min samples relative to the bolus were averaged and this value was used as a covariate. Effects of treatment, time, and treatment × time interaction were assessed as a completely randomized design using PROC MIXED (SAS Inst. Inc., Cary, NC). Data are reported as least squares means and are considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10.
RESULTS
Overall rectal temperature was increased in LPS-Eu pigs relative to CON pigs (39.8 vs. 38.8°C, respectively; P < 0.01; Fig. 1). Pigs administered LPS became lethargic and vomited within 30 min postbolus. Post hoc analysis revealed that, relative to the baseline, CON pigs became mildly hypoglycemic near the end of the experiment (20% decrease in samples from 300 to 480 min postbolus; P = 0.01; Fig. 2A). In LPS-Eu pigs, hyperglycemia occurred for 20 to 30 min postbolus (approximately 30%; P ≤ 0.05) relative to the baseline. Glucose infusion began 61 ± 11 min after LPS administration (range 20–110 min), and infusion rates increased with time (P < 0.01; Fig. 2B). From 110 min onward, circulating glucose in the LPS-Eu pigs did not differ from the baseline (P > 0.10; indicating successful maintenance of euglycemia). An average of 116 ± 8 g of glucose was infused to maintain euglycemia during the entire 480-min clamp.
Figure 1.
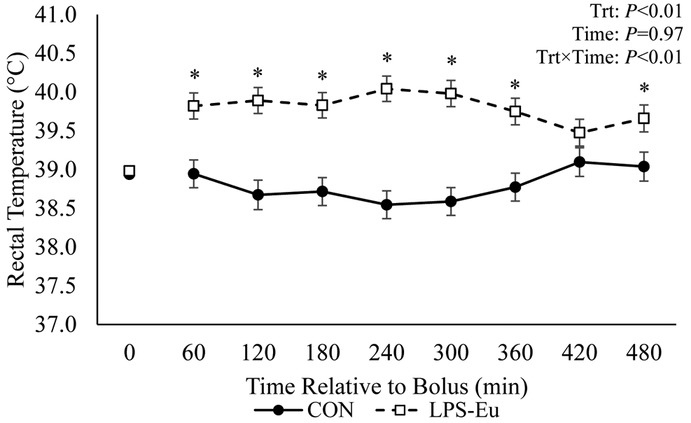
Rectal temperature in pigs administered a bolus of saline (control [CON]) or lipopolysaccharide challenge + euglycemic clamp [LPS-Eu]. Results are expressed as least squares means ± SEM. Time 0 represents an average of measurements taken −30, −20, and 0 min relative to bolus administration. *Denotes treatment differences (P < 0.05) at the indicated time point. Trt = treatment.
Figure 2.
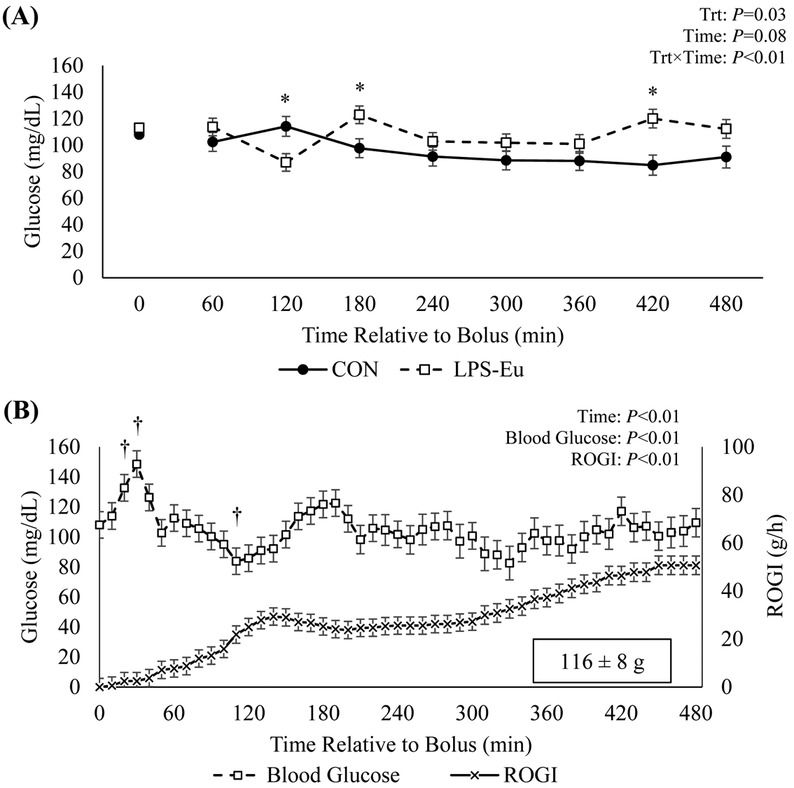
(A) Blood glucose levels in pigs administered a bolus of saline (control [CON]) or lipopolysaccharide challenge + euglycemic clamp (LPS-Eu) and (B) the 10-min interval blood glucose levels and average rate of glucose infusion (ROGI) in LPS-Eu pigs. Results are expressed as least squares means ± SEM. Time 0 represents an average of samples taken −30, −20, and 0 min relative to bolus administration. *Denotes treatment differences (P < 0.05) at the indicated time point. †Indicates difference relative to the baseline value (P ≤ 0.05). Trt = treatment.
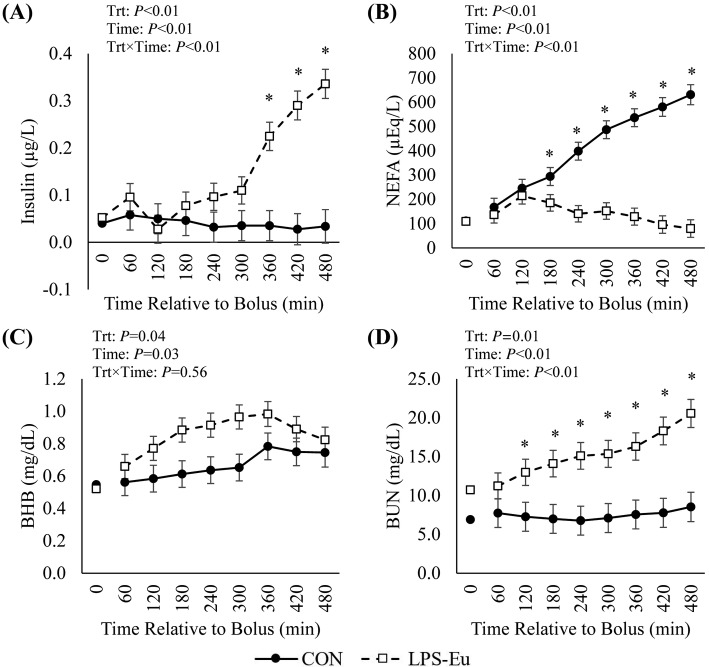
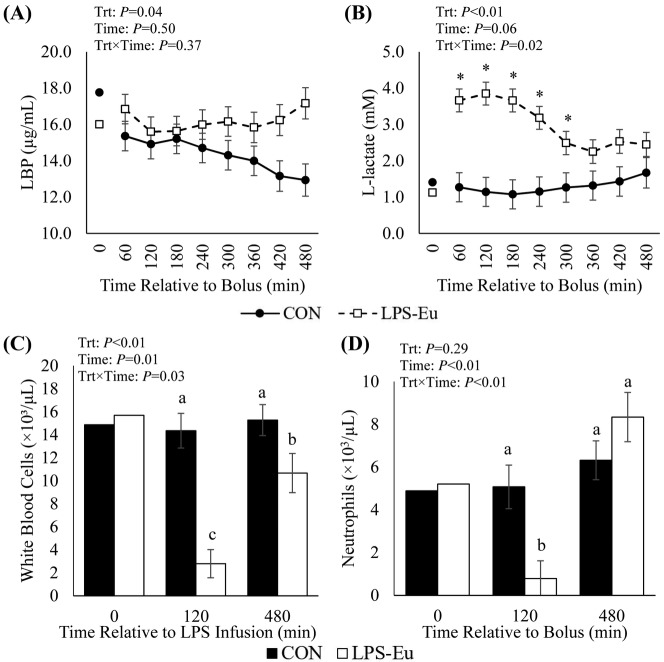
There was a treatment × time interaction (P < 0.01) in circulating insulin, as it did not differ between treatments for the first 300 min, but it markedly and progressively increased from 360 min onward (10-fold increased at 480 min) in the LPS-Eu pigs versus the CON pigs (Fig. 3A). Circulating NEFA progressively increased from 60 to 480 min in CON pigs (267%; P < 0.01), whereas no changes were observed in LPS-Eu pigs (P = 0.25), resulting in a treatment × time interaction where NEFA was increased almost 8-fold in CON pigs vs. LPS-Eu pigs at the end of the challenge (P < 0.01; Fig. 3B). Relative to CON pigs, overall circulating BHB was increased in LPS-Eu pigs (29%; P = 0.04; Fig. 3C). There was a treatment × time interaction (P < 0.01) in BUN, as it progressively increased (>140% by 480 min) in LPS-Eu pigs but did not change in CON pigs (Fig. 3D). Lipopolysaccharide-binding protein was increased 13% overall in LPS-Eu pigs compared with CON pigs (P = 0.04; Fig. 4A). There was a treatment × time interaction (P = 0.02; Fig. 4B) as relative to CON pigs, L-lactate was increased approximately 185% in LPS-Eu pigs during the first 300 min postbolus, but this difference became less pronounced near the end of the experiment. At 120 min postbolus, circulating white blood cells and neutrophils were decreased in LPS-Eu pigs versus CON pigs, and white blood cells remained decreased at 480 min postbolus whereas neutrophils returned to CON levels (P ≤ 0.03; Fig. 4C and 4D). Overall circulating lymphocytes, monocytes, eosinophils, basophils, and platelets were decreased in LPS-Eu pigs compared with CON pigs (75, 87, 70, 50, and 57%, respectively; P ≤ 0.05; Table 1). Red blood cells and the neutrophil-to-lymphocyte ratio were increased in LPS-Eu pigs versus CON pigs (12 and 251%, respectively; P < 0.01; Table 1).
Figure 3.
Circulating (A) insulin, (B) NEFA, (C) β-hydroxybutyrate (BHB), and (D) blood urea nitrogen (BUN) in pigs administered a bolus of saline (control [CON]) or lipopolysaccharide challenge + euglycemic clamp (LPS-Eu). Results are expressed as least squares means ± SEM. Time 0 represents an average of samples taken −30, −20, and 0 min relative to bolus administration. *Denotes treatment differences at the indicated time point. Trt = treatment.
Figure 4.
Circulating (A) lipopolysaccharide-binding protein (LBP), (B) L-lactate, (C) white blood cells, and (D) neutrophils in pigs administered a bolus of saline (control [CON]) or lipopolysaccharide challenge + euglycemic clamp (LPS-Eu). Results are expressed as least squares means ± SEM. Time 0 represents an average of samples taken −30, −20, and 0 min relative to bolus administration. *Denotes treatment differences (P < 0.05) at the indicated time point. a–cMeans with different letters differ (P ≤ 0.05). Trt = treatment.
Table 1.
Complete blood count parameters in pigs given a bolus of saline (control [CON]) or lipopolysaccharide challenge + euglycemic clamp (LPS-Eu)1
| Treatment | P-value | |||||
|---|---|---|---|---|---|---|
| Parameter | CON | LPS-Eu | SEM | Treatment | Time | Treatment × time |
| Lymphocytes, ×103/μL | 7.72 | 1.94 | 0.47 | <0.01 | 0.94 | 0.71 |
| Monocytes, ×103/μL | 0.92 | 0.12 | 0.07 | <0.01 | 0.83 | 0.58 |
| Eosinophils, ×103/μL | 0.23 | 0.07 | 0.03 | <0.01 | 0.63 | 0.06 |
| Basophils, ×103/μL | 0.04 | 0.02 | 0.01 | 0.05 | 0.08 | 0.64 |
| Red blood cells, ×106/μL | 5.53 | 6.18 | 0.07 | <0.01 | <0.01 | <0.01 |
| Platelets, ×103/μL | 491 | 211 | 21 | <0.01 | 0.39 | 0.38 |
| Neutrophil:lymphocyte ratio | 0.74 | 2.60 | 0.27 | <0.01 | <0.01 | <0.01 |
Samples were obtained 180 and 480 min postbolus.
DISCUSSION
Infection compromises animal efficiency by redistributing nutrients away from productive products (e.g., skeletal muscle, milk, fetuses) toward the immune system. Whole-body energy expenditure is estimated to increase approximately 50% during infection (Lang and Dobrescu, 1991; Lang et al., 1993; Plank et al., 1998). Increased glucose oxidation could be used to facilitate a febrile response following LPS administration; however, this occurs independently of rectal temperature changes during sepsis in experimental animal models (Lang et al., 1987). Furthermore, prolonged hypoglycemia develops despite mild and transient fever in our previous ruminant models (Kvidera et al., 2016a, 2017b). Therefore, the carbohydrate metabolic adjustments occurring herein are likely not an attempt to increase body temperature.
Upon antigen exposure, activated immune cells increase glucose consumption (Calder et al., 2007; Maratou et al., 2007; MacIver et al., 2008). In an attempt to “spare glucose” for the immune system, insulin-sensitive tissues (muscle and adipose tissue) become insulin refractory and hepatic glucose output increases through both glycogenolysis and gluconeogenesis (Wolfe et al., 1977; Filkins, 1978; Spitzer et al., 1985; McGuinness, 1994, 2005). Despite the coordinated homeorhetic efforts to supply the proper type and amount of fuel for the immune system, rapid leukocyte glucose utilization results in hypoglycemia and hyperlactemia (McGuinness, 2005). Because of the aforementioned metabolic adjustments, we are able to use a LPS challenge coupled with a euglycemic clamp to estimate glucose requirements of an activated immune system as we have described in growing and lactating ruminants (Kvidera et al., 2016a, 2017b). Hence, study objectives were to quantify activated immune system glucose requirements in a growing monogastric model.
The immune system was successfully activated by LPS, as indicated by pyrexia (1.0°C increase; Fig. 1) as well as hyperlactemia and altered circulating immune cell dynamics (Fig. 4B, 4C, and 4D). Glucose infusion began approximately 60 min after LPS administration. Glucose infusion rates steadily increased over time (Fig. 2B), and from the accumulated ROGI, we estimate the acutely activated immune system in the current study used approximately 116 g of glucose in a 480-min period. On a metabolic BW basis, the glucose requirement was approximately 1.1 g/kg BW0.75/h, which is comparable to data we generated in other LPS–euglycemic clamp experiments in growing steers (1.0 g/kg BW0.75/h; Kvidera et al., 2016a) and lactating cows (0.66 and 1.0 g/kg BW0.75/h; Kvidera et al., 2017b; E. A. Horst and L. H. Baumgard, unpublished data). Therefore, the immune system uses a large quantity of glucose following immunoactivation, and the amount is seemingly conserved across species and physiological states.
The current experimental design has some limitations. In contrast with our previous LPS–euglycemic clamp experiments in ruminants, the current study is limited by the absence of an LPS control treatment, which would have confirmed immunoactivation-induced hypoglycemia. However, hypoglycemia following an acute LPS challenge in pigs is well documented (Myers et al., 1997; Leininger et al., 2000; Bruins et al., 2003; S. K. Kvidera and L. H. Baumgard, unpublished data). The lack of hepatic glucose output measurements is a second limitation to our experimental design, because it prevents us from estimating the liver's contribution to the circulating glucose pool. However, endotoxemia-induced increased hepatic glycogenolysis and gluconeogenesis is well characterized in multiple species (Wolfe et al., 1977; Filkins, 1978; Lang et al., 1985; Spitzer et al., 1985; McGuinness, 1994, 2005). The hyperglycemic phase (i.e., within minutes of LPS administration) results from orchestrated peripheral insulin insensitivity coupled with enhanced hepatic glucose output, which provides glucose in surplus of immune cell utilization capacity. Unfasted animals have a more robust hepatic glycogenolytic response, a scenario that causes us to underestimate the quantity of glucose entering the circulating pool and, subsequently, the total amount of glucose used by the activated immune system. Therefore, by incorporating fasting in the current protocol, we are more accurately determining immune system glucose utilization. The current experimental design is also constrained by the unmeasured extent of peripheral tissue glucose consumption. However, many studies have demonstrated reduced insulin sensitivity and glucose utilization in both muscle and adipose tissue during endotoxemia both in vivo (Raymond et al., 1981; Ling et al., 1994; Poggi et al., 2007; Mulligan et al., 2012) and in vitro (Song et al., 2006; Liang et al., 2013). The mechanism behind this phenomenon is thought to be inflammation-induced activation of c-Jun amino-terminal kinases, which interfere with proper insulin receptor substrate phosphorylation and, thus, insulin action (Aguirre et al., 2000). Furthermore, tissues with a large immune compartment, such as the liver, lung, and ileum, increase glucose utilization following immune activation, and this increase is more pronounced in animals with exogenous glucose infusions to maintain euglycemia (Lang et al., 1993).
Euglycemia was successfully maintained in LPS-Eu pigs, whereas glucose was mildly decreased (20%) in CON pigs after the 300th minute (13th hour of fasting; Fig. 2A). Hypoglycemia in CON animals at this stage of the challenge differs from our previous LPS–euglycemic clamp studies, likely because pigs were fasted for 8 h prior to bolus administration and, therefore, hepatic glycogen was ostensibly becoming depleted. In agreement, the hyperglycemic period immediately after LPS administration lasted only approximately 20 min (Fig. 2B) and glucose infusion began 61 min postbolus; both parameters are considerably less than the approximately 3-h hyperglycemic phase observed in well-fed growing and lactating ruminants (Kvidera et al., 2016a, 2017b). Interestingly, despite the transient hyperglycemic period, circulating insulin began to progressively increase about 360 min postbolus and it was increased 10-fold by the end of the experiment. This indicates hyperglycemia is not causal to LPS-induced hyperinsulinemia and substantiates data from others demonstrating LPS directly acts on the pancreas (Vives-Pi et al., 2003; Bhat et al., 2014). Rationale for why marked hyperinsulinemia occurs is not clear, but insulin is important for immune cell glucose uptake and development during activation (Shimizu et al., 1983; Helderman, 1984; Calder et al., 2007; Maratou et al., 2007), and recent reports indicate that it has potent anti-inflammatory actions (Chalmeh et al., 2013). Moreover, circulating NEFA were substantially blunted in LPS-Eu pigs, and this is likely due to insulin's potent antilipolytic role (Vernon, 1992). Despite decreased NEFA, circulating BHB was increased 29% in LPS-Eu pigs. This conflicts with literature on rodent studies, which demonstrates reduced ketone synthesis following immunoactivation, possibly due to hyperinsulinemia (Neufeld et al., 1980) or direct antiketogenic effects of inflammatory cytokines (Memon et al., 1992). Reasons for increased circulating BHB in the current study are unclear but may be due to species differences, LPS dose, and severity of challenge. We have observed increased circulating BHB in chronic low-dose LPS–challenged pigs (Kvidera et al., 2016b) and cows (Kvidera et al., 2017a), whereas in ruminant models of acute immune activation, we have reported decreased circulating BHB (Kvidera et al., 2016a, 2017b). Regardless of why, a better understanding of how endotoxemia influences lipid and ketone metabolism is important to understanding the overall energetics of the immune response.
Hyperlactemia is another endotoxemia characteristic (Wolfe et al., 1977; Michaeli et al., 2012) that is likely due to enhanced glucose utilization through aerobic glycolysis and decreased pyruvate entry into the tricarboxylic acid cycle in activated immune cells (Palsson-McDermott and O'Neill, 2013). In the current study, LPS-administered pigs had a >200% increase in circulating L-lactate during the first 300 min of the clamp. Other potential sources include skeletal muscle, which may export L-lactate (a process akin to the Warburg effect) as an oxidative fuel for nonimmune cells in an attempt to spare glucose for the immune system (Tannahill and O'Neill, 2011). Skeletal muscle catabolism occurs during infection to provide AA as substrates for gluconeogenesis (Wannemacher et al., 1980) and for synthesis of leukocytes and acute phase proteins (Iseri and Klasing, 2013). Circulating BUN, a biomarker of muscle catabolism, was markedly increased in LPS-Eu pigs relative to CON pigs (108% overall; Fig. 3D), and this agrees with other pig LPS models (Myers et al., 1997; Webel et al., 1997; Bruins et al., 2003). Interestingly, LBP did not change in LPS-Eu pigs whereas it steadily decreased in CON pigs, which is in contrast to previous patterns observed in ruminants (Kvidera et al., 2016a, 2017b). Reasons why LBP did not increase relative to baseline values over time in LPS-infused animals are not clear but may be due to dose, species, or fasting, because energy status can have an impact on the acute phase response (Doherty et al., 1993; Reid et al., 2002), although it still remains responsive (Ling et al., 2004).
White blood cell distribution radically changes following LPS administration as demonstrated by leukopenia observed in this and other studies where concentrations of circulating immune cells are highly dependent on time relative to immune activation (Fig. 4C and 4D; Lee et al., 2000; Williams et al., 2009). Leukopenia is likely due to leukocyte extravasation into tissues, especially those with important immune function such as the liver, kidney, spleen, and lung (Lang and Dobrescu, 1991; Mészáros et al., 1991). Intravenous LPS administration also presumably causes white blood cell infiltration into tissues such as adipose and muscle (Caesar et al., 2012; Pillon et al., 2013), highlighting the difficulty of pinpointing glucose consumption by simply measuring whole-tissue glucose uptake as immune and resident cells within the tissue are utilizing glucose at different rates. Accordingly, Mészáros et al. (1991) examined different hepatic cell fractions after an i.v. LPS challenge and demonstrated that glucose uptake did not change in parenchymal cells but markedly increased in Kupffer cells and neutrophils. This demonstrates increased glucose import into immune cells while extra-immune cells decrease glucose utilization, which supports our assumption that infused glucose was primarily utilized by immune cells rather than by cells from peripheral tissue.
Herein we describe the immune system as a substantial glucose user, and this has implications to several practical on-farm scenarios. For instance, heat-stressed animals have compromised intestinal barrier function and, therefore, increased intestinal-derived LPS infiltration (Baumgard and Rhoads, 2013). Nutrient-restricted animals also have reduced intestinal barrier function (Pearce et al., 2013; Zhang et al., 2013; Kvidera et al., 2017c), and both voluntary and involuntary nutrient restriction is common in a variety of situations including weaning, shipping, gestation, drought, and overcrowding. In addition to the gastrointestinal tract, other obvious sources of immunoactivation include metritis, mastitis, laminitis, and respiratory disease, and this immunological stress reduces production efficiency (Johnson, 1997, 2012). Immunoactivation during situations such as the aforementioned examples results in glucose becoming a critical metabolite to mount an immune response. In the current study, 116 g of glucose (equivalent to 476 kcal) was utilized by the immune system in 8 h. If extrapolated to 24 h and incorporating the energetic costs of protein accretion (1 g protein synthesis requires 10 kcal ME; Patience, 2012), then approximately 143 g of protein or approximately 476 g of lean tissue synthesis is being compromised per day in a 30-kg pig due to acute immune activation. This, coupled with increased muscle catabolism to provide gluconeogenic precursors and AA, results in severely compromised productivity in growing animals raised for lean tissue production.
Conclusion
We estimate the activated immune system uses approximately 116 g of glucose within 480 min. This is likely underestimated because we are unable to account for immune system glucose utilization during the transient hyperglycemic phase as well as the liver's gluconeogenic contribution to the circulating pool. On a metabolic BW basis, the extent of glucose utilization is interestingly consistent between the current experiment and other LPS–euglycemic clamp experiments we have performed, despite different animal ages, physiological status, and species. This suggests that the reprioritization and extent of fuel utilization by immune cells following intense activation is highly conserved. This inflammatory response directs nutrients away from productive purposes, and therefore, infection and inflammation have a large impact on energy availability for the synthesis of economically relevant products. Having a better understanding of energetic and nutrient requirements of the immune response is a prerequisite to developing strategies aimed at minimizing productivity losses during immunoactivation.
LITERATURE CITED
- Aguirre V., Uchida T., Yenush L., Davis R., White M. F. 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275:9047–9054. doi: 10.1074/jbc.275.12.9047 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., Rhoads R. P. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Bhat U. G., Ilievski V., Unterman T. G., Watanabe K. 2014. Porphyromonas gingivalis lipopolysaccharide upregulates insulin secretion from pancreatic β cell line MIN6. J. Periodontol. 85:1629–1636. doi: 10.1902/jop.2014.140070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins M. J., Deutz N. E., Soeters P. B. 2003. Aspects of organ protein, amino acid and glucose metabolism in a porcine model of hypermetabolic sepsis. Clin. Sci. (Lond.) 104:127–141. doi: 10.1042/cs1040127 [DOI] [PubMed] [Google Scholar]
- Caesar R., Reigstad C. S., Bäckhed H. K., Reinhardt C, Ketonen M., Lundén G., Cani P. D., Bäckhed F. 2012. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 61:1701–1707. doi: 10.1136/gutjnl-2011-301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Dimitriadis G., Newsholme P. 2007. Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr. Opin. Clin. Nutr. Metab. Care 10:531–540. doi: 10.1097/MCO.0b013e3281e72ad4 [DOI] [PubMed] [Google Scholar]
- Chalmeh A., Badiei K., Pourjafar M., Nazifi S. 2013. Anti-inflammatory effects of insulin regular and flunixin meglumine on endotoxemia experimentally induced by Escherichia coli serotype O55:B5 in an ovine model. Inflamm. Res. 62:61–67. doi: 10.1007/s00011-012-0551-6 [DOI] [PubMed] [Google Scholar]
- Doherty J. F., Golden M. H., Raynes J. G., Griffin G. E., McAdam K. P. 1993. Acute-phase protein response is impaired in severely malnourished children. Clin. Sci. (Lond.) 84:169–175. doi: 10.1042/cs0840169 [DOI] [PubMed] [Google Scholar]
- Filkins J. P. 1978. Phases of glucose dyshomeostasis in endotoxicosis. Circ. Shock 5:347–355. [PubMed] [Google Scholar]
- Helderman J. H. 1984. Acute regulation of human lymphocyte insulin receptors. Analysis by the glucose clamp. J. Clin. Invest. 74:1428–1435. doi: 10.1172/JCI111554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., Patience J. F. 2017. Immune system stimulation increases nursery pig maintenance energy requirements. J. Anim. Sci. 95(E-Suppl. 5):145 (Abstr.) [Google Scholar]
- Iseri V. J., Klasing K. C. 2013. Dynamics of the systemic components of the chicken (Gallus domesticus) immune system following activation by Escherichia coli; Implications for the costs of immunity. Dev. Comp. Immunol. 40:248–257. doi: 10.1016/j.dci.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 2012. Fueling the immune response: What's the cost? In: Patience J. editor, Feed efficiency in swine. Wageningen Academic Publ., Wageningen, the Netherlands: p. 211–223. [Google Scholar]
- Klasing K. C., Austic R. E. 1984a. Changes in protein degradation in chickens due to an inflammatory challenge. Proc. Soc. Exp. Biol. Med. 176:292–296. doi: 10.3181/00379727-176-41873 [DOI] [PubMed] [Google Scholar]
- Klasing K. C., Austic R. E. 1984b. Changes in protein synthesis due to an inflammatory challenge. Proc. Soc. Exp. Biol. Med. 176:285–291. doi: 10.3181/00379727-176-41872 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Dickson M. J., Horst E. A., Ydstie J. A., Shouse C. S., Bidne K. L., Mayorga E. J., Al-Qaisi M., Ramirez Ramirez H. A., Keating A. F., Baumgard L. H. 2017a. Effects of continuous and increasing lipopolysaccharide infusion on basal metabolism in lactating cows. J. Dairy Sci. 100(E-Suppl. 2):88 (Abstr.) [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., Baumgard L. H. 2016a. Technical note: A procedure to estimate glucose requirements of an activated immune system in steers. J. Anim. Sci. 94:4591–4599. doi: 10.2527/jas.2016-0765 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., Baumgard L. H. 2017b. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi: 10.3168/jds.2016-12001 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Laughlin E. J., Mayorga E. J., Seibert J. T., Abuajamieh M., Al-Qaisi M., Lei S., Keating A. F., Ross J. W., Baumgard L. H. 2016b. Effects of repeated LPS infusion on hematologic, metabolic, and intestinal histology parameters. FASEB J. 30:925.14. (Abstr.) [Google Scholar]
- Kvidera S. K., Horst E. A., Sanz Fernandez M. V., Abuajamieh M., Ganesan S., Gorden P. J., Green H. B., Schoenberg K. M., Trout W. E., Keating A. F., Baumgard L. H. 2017c. Characterizing effects of feed restriction and glucagon-like peptide 2 administration on biomarkers of inflammation and intestinal morphology. J. Dairy Sci.doi: 10.3168/jds.2017-13229 [DOI] [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Blakesley H. L., Spitzer J. J. 1987. Fever is not responsible for the elevated glucose kinetics in sepsis. Proc. Soc. Exp. Biol. Med. 185:455–461. doi: 10.3181/00379727-185-42569 [DOI] [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Spitzer J. J. 1985. Glucose kinetics and body temperature after lethal and nonlethal doses of endotoxin. Am. J. Physiol. 248:R471–R478. [DOI] [PubMed] [Google Scholar]
- Lang C. H., Dobrescu C. 1991. Sepsis-induced increases in glucose uptake by macrophage-rich tissues persist during hypoglycemia. Metabolism 40:585–593. doi: 10.1016/0026-0495(91)90048-2 [DOI] [PubMed] [Google Scholar]
- Lang C. H., Spolarics Z., Ottlakan A., Spitzer J. J. 1993. Effect of high-dose endotoxin on glucose production and utilization. Metabolism 42:1351–1358. doi: 10.1016/0026-0495(93)90137-D [DOI] [PubMed] [Google Scholar]
- Lee D. N., Shen T. F., Yen H. T., Weng C. F., Chen B. J. 2000. Effects of chromium supplementation and lipopolysaccharide injection on the immune response of weanling pigs. Asian-Australas. J. Anim. Sci. 13:1414–1421. doi: 10.5713/ajas.2000.1414 [DOI] [Google Scholar]
- Leininger M. T., Portocarrero C. P., Schinckel A. P., Spurlock M. E., Bidwell C. A., Nielsen J. N., Houseknecht K. L. 2000. Physiological response to acute endotoxemia in swine: Effect of genotype on energy metabolites and leptin. Domest. Anim. Endocrinol. 18:71–82. doi: 10.1016/S0739-7240(99)00064-8 [DOI] [PubMed] [Google Scholar]
- Liang H., Hussey S. E., Sanchez-Avila A., Tantiwong P., Musi N. 2013. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One 8:e63983. doi: 10.1371/journal.pone.0063983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P. R., Bistrian B. R., Mendez B., Istfan N. W. 1994. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: Relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism 43:279–284. doi: 10.1016/0026-0495(94)90093-0 [DOI] [PubMed] [Google Scholar]
- Ling P. R., Smith R. J., Kie S., Boyce P., Bistrian B. R. 2004. Effects of protein malnutrition on IL-6-mediated signaling in the liver and the systemic acute-phase response in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287:R801–R808. doi: 10.1152/ajpregu.00715.2003 [DOI] [PubMed] [Google Scholar]
- Lochmiller R., Deerenberg C. 2000. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 88:87–98. doi: 10.1034/j.1600-0706.2000.880110.x [DOI] [Google Scholar]
- MacIver N. J., Jacobs S. R., Wieman H. L., Wofford J. A., Coloff J. L., Rathmell J. C. 2008. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 84:949–957. doi: 10.1189/jlb.0108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratou E., Dimitriadis G., Kollias A., Boutati E., Lambadiari V., Mitrou P., Raptis S. A. 2007. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur. J. Clin. Invest. 37:282–290. doi: 10.1111/j.1365-2362.2007.01786.x [DOI] [PubMed] [Google Scholar]
- McGuinness O. P. 1994. The impact of infection on gluconeogenesis in the conscious dog. Shock 2:336–343. doi: 10.1097/00024382-199411000-00007 [DOI] [PubMed] [Google Scholar]
- McGuinness O. P. 2005. Defective glucose homeostasis during infection. Annu. Rev. Nutr. 25:9–35. doi: 10.1146/annurev.nutr.24.012003.132159 [DOI] [PubMed] [Google Scholar]
- Memon R. A., Feingold K. R., Moser A. H., Doerrler W., Adi S., Dinarello C. A., Grunfeld C. 1992. Differential effects of interleukin-1 and tumor necrosis factor on ketogenesis. Am. J. Physiol. 263:E301–E309. [DOI] [PubMed] [Google Scholar]
- Mészáros K., Bojta J., Bautista A. P., Lang C. H., Spitzer J. J. 1991. Glucose utilization by Kupffer cells, endothelial cells, and granulocytes in endotoxemic rat liver. Am. J. Physiol. 260:G7–G12. [DOI] [PubMed] [Google Scholar]
- Michaeli B., Martinez A., Revelly J. P., Cayeux M. C., Chioléro R. L., Tappy L, Berger M. M. 2012. Effects of endotoxin on lactate metabolism in humans. Crit. Care 16:R139. doi: 10.1186/cc11444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan K. X., Morris R. T., Otero Y. F., Wasserman D. H., McGuinness O. P. 2012. Disassociation of muscle insulin signaling and insulin-stimulated glucose uptake during endotoxemia. PLoS One 7:e30160. doi: 10.1371/journal.pone.0030160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. J., Farrell D. E., Evock-Clover C. M., McDonald M. W., Steele N. C. 1997. Effect of growth hormone or chromium picolinate on swine metabolism and inflammatory cytokine production after endotoxin challenge exposure. Am. J. Vet. Res. 58:594–600. [PubMed] [Google Scholar]
- Neufeld H. A., Pace J. G., Kaminski M. V., George D. T., Jahrling P. B., Wannemacher R. W., Beisel W. R. 1980. A probable endocrine basis for the depression of ketone bodies during infectious or inflammatory state in rats. Endocrinology 107:596–601. doi: 10.1210/endo-107-2-596 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev ed.Natl. Acad. Press; Washington, DC. [Google Scholar]
- Palsson-McDermott E. M., O'Neill L. A. 2013. The Warburg effect then and now: From cancer to inflammatory diseases. BioEssays 35:965–973. doi: 10.1002/bies.201300084 [DOI] [PubMed] [Google Scholar]
- Patience J. F. 2012. The influences of dietary energy of feed efficiency in grow-finish swine. In: Patience J. editor, Feed efficiency in swine. Wageningen Academic Publ., Wageningen, the Netherlands: p. 101–129. [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., Gabler N. K. 2013. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi: 10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Pillon N. J., Bilan P. J., Fink L. N., Klip A. 2013. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 304:E453–E465. doi: 10.1152/ajpendo.00553.2012 [DOI] [PubMed] [Google Scholar]
- Plank L. D., Connolly A. B., Hill G. L. 1998. Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann. Surg. 228:146–158. doi: 10.1097/00000658-199808000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi M., Bastelica D., Gual P., Iglesias M. A., Gremeaux T., Knauf C., Peiretti F., Verdier M., Juhan-Vague I., Tanti J. F., Burcelin R., Alessi M. C. 2007. C3H/HeJ mice carrying a Toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50:1267–1276. doi: 10.1007/s00125-007-0654-8 [DOI] [PubMed] [Google Scholar]
- Raymond R. M., Harkema J. M., Emerson T. E. 1981. In vivo skeletal muscle insulin resistance during E coli endotoxin shock in the dog. Circ. Shock 8:425–433. [PubMed] [Google Scholar]
- Reeds P. J., Jahoor F. 2001. The amino acid requirements of disease. Clin. Nutr. 20(Suppl. 1):15–22. doi: 10.1054/clnu.2001.0402 [DOI] [Google Scholar]
- Reid M., Badaloo A., Forrester T., Morlese J. F., Heird W. C., Jahoor F. 2002. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am. J. Clin. Nutr. 76:1409–1415. [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C., Rhoads R. P., Baumgard L. H. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu F., Kahn C. R., Garzelli C., Hooks J. J., Notkins A. L. 1983. The binding of insulin to mouse leucocytes during viral infections. Diabetologia 25:521–524. doi: 10.1007/BF00284463 [DOI] [PubMed] [Google Scholar]
- Song M. J., Kim K. H., Yoon J. M., Kim J. B. 2006. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 346:739–745. doi: 10.1016/j.bbrc.2006.05.170 [DOI] [PubMed] [Google Scholar]
- Spitzer J. A., Nelson K. M., Fish R. E. 1985. Time course of changes in gluconeogenesis from various precursors in chronically endotoxemic rats. Metabolism 34:842–849. doi: 10.1016/0026-0495(85)90109-X [DOI] [PubMed] [Google Scholar]
- Tannahill G. M., O'Neill L. A. 2011. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. 585:1568–1572. doi: 10.1016/j.febslet.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Vernon R. G. 1992. Effects of diet on lipolysis and its regulation. Proc. Nutr. Soc. 51:397–408. doi: 10.1079/PNS19920053 [DOI] [PubMed] [Google Scholar]
- Vives-Pi M., Somoza N., Fernández-Alvarez J., Vargas F., Caro P., Alba A., Gomis R., Labeta M. O., Pujol-Borrell R. 2003. Evidence of expression of endotoxin receptors CD14, Toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin. Exp. Immunol. 133:208–218. doi: 10.1046/j.1365-2249.2003.02211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R. W., Beall F. A., Canonico P. G., Dinterman R. E., Hadick C. L., Neufeld H. A. 1980. Glucose and alanine metabolism during bacterial infections in rats and rhesus monkeys. Metabolism 29:201–212. doi: 10.1016/0026-0495(80)90061-X [DOI] [PubMed] [Google Scholar]
- Webel D. M., Finck B. N., Baker D. H., Johnson R. W. 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514–1520. doi: 10.2527/1997.7561514x [DOI] [PubMed] [Google Scholar]
- Williams P. N., Collier C. T., Carroll J. A., Welsh T. H., Laurenz J. C. 2009. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest. Anim. Endocrinol. 37:139–147. doi: 10.1016/j.domaniend.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Elahi D., Spitzer J. J. 1977. Glucose and lactate kinetics after endotoxin administration in dogs. Am. J. Physiol. 232:E180–E185. [DOI] [PubMed] [Google Scholar]
- Zhang S., Albornoz R. I., Aschenbach J. R., Barreda D. R., Penner G. B. 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. J. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]