ABSTRACT
Bivariate animal models were used to estimate phenotypic and genetic correlations between 9 carcass merit and meat tenderness traits with 25 individual and grouped fatty acids in the subcutaneous adipose tissue of a population of 1,366 Canadian beef cattle finishing heifers and steers. In general, phenotypic correlations were low (<0.25 in magnitude) except for moderate phenotypic correlations of 9c-17:1 (−0.29 ± 0.16), 18:0 (0.26 ± 0.14), 11c-18:1 (−0.33 ± 0.15), 11t-18:1 (0.35 ± 0.14) with Warner–Bratzler shear force measured 3 d postmortem and between 14:0 (−0.36 ± 0.1), 9c-14:1 (−0.34 ± 0.08), 9c-16:1 (−0.36 ± 0.08), 9c-18:1 (0.26 ± 0.07), and sum of branched-chain fatty acids (BCFA; −0.27 ± 0.06) and back fat thickness (BFAT). Genetic correlations were also low for most of the traits. However, moderate to moderately high genetic correlations (0.25 to 0.50 in magnitude) were detected for some traits, including 17:0 (0.4 ± 0.11), 18:0 (0.44 ± 0.12), 9c-14:1 (−0.47 ± 0.11), 9c-16:1 (−0.43 ± 0.11), and the n-6:n-3 PUFA ratio (−0.5 ± 0.15) with HCW; 9c-14:1 (−0.41 ± 0.13) and 9c-16:1 (−0.42 ± 0.13) with BFAT; ai17:0 (0.43 ± 0.19) and BCFA (0.45 ± 0.19) with lean meat yield; 13c-18:1 (0.40 ± 0.15) with carcass marbling score; sum of CLA (0.45 ± 0.22), 18:2n-6 (0.47 ± 0.17), and sum of PUFA (0.48 ± 0.17) with overall tenderness measured 3 d postmortem; the n-6:n-3 PUFA ratio (0.41 ± 0.22) and sum of CLA (0.42 ± 0.25) with overall tenderness measured 29 d postmortem; and BCFA (0.41 ± 0.27) with Warner–Bratzler shear force measured 29 d postmortem. The genetic correlations observed in this study suggest that contents of some fatty acids in beef tissue and carcass merit and meat tenderness traits are likely influenced by a subset of the same genes in beef cattle. Due to some antagonistic genetic correlations, multiple-trait economic indexes are recommended when fatty acid composition, carcass merit, and meat tenderness traits are included in the breeding objective.
Keywords: beef cattle, carcass and tenderness, correlation, fatty acid composition
INTRODUCTION
Beef is an excellent source of protein, essential vitamins, and minerals in the human diet (Scollan et al., 2006). However, excessive dietary fat intake associated with consumption of red meat, including beef, is believed to be linked with atherosclerosis and other cardiovascular diseases, cancers, and diabetes in humans (Pan et al., 2012; Michas et al., 2014). Therefore, restriction of dietary fat intake is commonly recommended by health practitioners. However, clinical studies have revealed that the type of dietary fat (or the fatty acid composition) has a more profound impact on human health than the amount of fat in the diet (Hu et al., 2001; Woodside and Kromhout, 2005), and the risk of cardiovascular diseases can be moderately reduced by decreasing intake of SFA or by replacing SFA with a combination of PUFA and MUFA (Scollan et al., 2006; Michas et al., 2014). Moreover, preliminary studies in human clinical trials have shown that some trans fatty acids naturally produced by ruminant animals, including trans-11-18:1 (vaccenic acid) and cis-9, trans-11, 18:2, an isomer of CLA, have a number of potential health benefits (Scollan et al., 2006; Lim et al., 2014). Therefore, increasing the content of beneficial fatty acids and/or reducing the concentration of harmful fatty acids in beef will benefit human health and thus will add value to beef products (McCluskey et al., 2005; Lusk and Parker, 2009).
Genetic variation in fatty acid concentrations of individual and grouped fatty acids in beef tissues have been observed in many studies (Malau-Aduli et al., 2000; Pitchford et al., 2002; Ekine-Dzivenu et al., 2014), indicating that beef fatty acid profiles can be improved through genetic selection. The recent development of genomic prediction tools for fatty acids based on DNA markers in beef cattle has facilitated genomic selection on the trait (Saatchi et al., 2013; Chen et al., 2015). In addition, genomic predictions for carcass merit and other meat quality traits have also been reported (Weber et al., 2012; Akanno et al., 2014), providing opportunities for genomic selection on multiple traits simultaneously based on the same DNA marker genotypes of selection candidates. However, multiple-trait genetic selection requires an understanding of correlations between the traits to maximize the economic gain due to selection. Therefore, the objective of this study was to estimate phenotypic and genetic correlations of major individual and grouped fatty acids in subcutaneous adipose (SQ) tissue with carcass merit and meat quality traits in Canadian beef cattle.
MATERIALS AND METHODS
Animal Population and Management
A total of 1,366 finishing steers and heifers that originated from Alberta, Canada, were used in this study. Descriptions of animal populations were previously provided (Basarab et al., 2011; Chen et al., 2015; Zhang et al., 2017). Briefly, the animals were from the beef cattle research herd of the Lacombe Research and Development Centre, Lacombe, AB, Canada, composed of purebred Aberdeen Angus (n = 6), Hereford–Angus crossbreeds (HEAN; n = 120), and Charolais–Red Angus crossbreeds (n = 93). Animals from commercial herds consisted of terminal crossbred (TXX; n = 938) and Hereford-Angus and Gelbvieh-Angus (HEANGV) crossbreed steers and heifers (n = 209). The TXX calves were produced from 2- or 3-way crossbreeding systems involving terminal composite bulls (TXX) and crossbred cows of multiple beef breeds. The HEANGV steers and heifers were produced from HEAN cows mated to Gelbvieh–Aberdeen Angus bulls and Gelbvieh–Aberdeen Angus cows mated to HEAN bulls.
The animals were born between 2008 and 2011, and the management of the animals was previously described (Basarab et al., 2011; Lopez-Campos et al., 2013). Briefly, after weaning, the calves were managed under 1 of 4 production systems: 1) calf-fed with growth implants, 2) calf-fed with no growth implants, 3) yearling-fed with growth implants, and 4) yearling-fed with no growth implants. The calf-fed animals were fed a high-forage diet for a 27- to 42-d period of dietary adjustment and then finished on a high-grain diet for an average of 162 d in the feedlot. Animals under the production system of calf-fed with growth implants were implanted with 200 mg progesterone (Synovex-S, Zoetis - Kirkland, Quebec, Canada) and 20 mg estradiol benzoate at weaning and subsequently reimplanted with 120 mg trenbolone acetate and 24 mg estradiol (Revalor-S , Merck - Kirkland, Quebec, Canada) 90 to 100 d before slaughter. Yearling-fed animals rotationally grazed alfalfa (Medicago sativa L.)/meadow bromegrass (Bromus riparius Rehm.) on fall pasture for 31 to 52 d. When snow prevented grazing, they were then placed on a high-forage grower diet for an average of 191 d and then returned to summer pasture for an average of 66 d before entering the feedlot. In the feedlot, they were allowed a 21- to 23-d adjustment period to adapt to a high-grain diet before finishing in an average of 124 d. Animals under the production system of yearling-fed with growth implants were implanted with 200 mg progesterone (Synovex-S) at weaning, and this was subsequently reimplanted at 83, 154, and 240 d after weaning. They were then implanted with 24 mg estradiol Revalor-S 90 d before slaughter. All animals were cared for according to the guidelines established by the Canadian Council on Animal Care (Olfert et al., 1993).
Carcass Trait and Meat Tenderness Measurement
All animals were targeted for slaughter at a back fat thickness (BFAT) of 9 to 10 mm between the 12th and 13th ribs as determined by individual ultrasound measurement using an Aloka 500V diagnostic real time ultrasound machine with a 17-cm 3.5-Mhz linear array transducer (Overseas Monitor Corporation Ltd., Richmond, BC, Canada). This corresponded to 11 to 14 mo of age for the calf-fed cattle and 19 to 23 mo of age for the yearling-fed cattle. Collection of carcass data was previously described (Akanno et al., 2014). Briefly, each carcass was split during dressing about 45 min postmortem, and each side was weighed and summed to obtain HCW in kilograms and then chilled at 2°C for 48 h. The left side of the carcasses was ribbed at the grading site between the 12th and 13th ribs and assessed by a trained personnel for BFAT in millimeters, longissimus thoracis, rib eye area (REA) in square centimeters, and carcass marbling score (CMAR). Carcass marbling score was measured as the flecks of fat deposit interspersed between the muscle fibers (i.e., intramuscular fat) of the longissimus thoracis, and it was measured and analyzed as a continuous variable (100–399 = trace marbling or less, 400–499 = slight marbling, 500–799 = small to moderate marbling, and 800–1199 = slightly abundant or more marbling). Lean meat yield (LMY), an estimate of saleable meat in the carcass, was calculated as LMY, % = 57.96 + (0.202 × REA, cm2) − (0.027 × HCW, kg) − (0.703 × BFAT, mm) as described by Basarab et al. (2003).
Collection of meat tenderness data was previously reported by Akanno et al. (2014). Briefly, at 48 h postmortem, the left longissimus muscle (longissimus lumborum [LL]) of each animal was removed from the carcass, vacuum-packed and boxed, chilled at 2°C, and transported on the same day from a commercial meat processor plant located near High River, AB, Canada, to the Lacombe Research and Development Centre in a refrigerated truck. Steaks with a 2.5-cm thickness were collected from the anterior portion of each LL muscle for immediate measurement of meat quality traits. The remaining portion of the LL muscle was labeled, vacuum-packed (Multivar AGW; MULTIVAC, Inc., Kansas City, MO), and aged in a cooler at 2°C (wind speed of 0.5 m/s).
Meat tenderness was measured on 1.9-cm cores from the first steak of each carcass as shear force in kilograms. For the shear force measurement, a spear point temperature probe (10 cm) was inserted into midpoint of the steak and then the steak was grilled (Garland Grill ED30B; Condon Barr Food Equipment Ltd., Edmonton, AB, Canada) to an internal temperature of 35°C and then turned and cooked to a final internal temperature of 71°C (Hewlett-Packard HP34970 Data Logger; Hewlett-Packard Co., Boise, ID). To prevent further cooking, the steaks were placed in polyethylene bags, sealed, and immediately immersed into an ice-water bath. They were then transferred to a 4°C cooler and held for 24 h. Six cores 1.9 cm in diameter from each steak were removed parallel to the fiber grain. Peak shear force was determined on each core perpendicular to the fiber grain using a TA-XTplus Texture Analyzer equipped with a Warner–Bratzler shear head at a crosshead speed of 200 mm/min and a 30-kg load cell using texture Exponent 32 software (Texture Technologies Corp., Hamilton, MA). The Warner–Bratzler shear force measured 3 d postmortem (WBSF_3d) was estimated as the average of the 6 cores.
Overall tenderness was assessed on the second steak of each carcass. The steaks were cooked to a final internal temperature of 71°C and then cut into 1.3-cm cubes, avoiding connective tissues and large areas of fat. Eight cubes from each sample were randomly assigned to an 8- member trained taste panel. Samples were placed in glass jars in a circulating water bather (Lindberg/Blue model WB1120A-1; Kendro Laboratory Products, Asheville, NC) and allowed to equilibrate to 71°C prior to evaluation. Sensory descriptors were defined on a 9-point scale, from 1 to 9 (1 = extremely tough to 9 = extremely tender), and overall tenderness was assessed just prior to expelling the sample. Overall tenderness measured 3 d postmortem (OT_3d) was then measured as the average of overall tenderness scores of the 8 members. After 26 d of aging, steaks were collected from the remaining portion of the LL muscle to assess Warner–Bratzler shear force measured 29 d postmortem (WBSF_29d) and overall tenderness measured 29 d postmortem (OT_29d) using the same methods.
Subcutaneous Adipose Tissue and Fatty Acid Analyses
Fatty acid analyses was conducted on the SQ tissue of each carcass as previously described (Chen et al., 2015; Zhang et al., 2017). Briefly, a sample of approximately 5 g of SQ overlying each LL muscle was taken, vacuum-packed, and frozen at −80°C for subsequent measurement of fatty acids. The procedures of Cruz-Hernandez et al. (2004) and Dugan et al. (2007) were used to extract lipid from SQ, and fatty acid methyl esters (FAME) were derivatized from the lipid extract using sodium methoxide. Separation and quantification of FAME was conducted using a 2-step gas chromatography procedure (i.e., 2 separate gas chromatography analyses using 150°C and 175°C plateau temperature programs) as outlined by Kramer et al. (2008). Quantification of individual CLA isomers was further achieved using silver-ion HPLC as described by Cruz-Hernandez et al. (2004), and the isomers were identified using standard UC-59M (Nu-Chek Prep, Inc., Elysian, MN). Individual fatty acids were quantified as a percentage of total FAME for each sample. Concentrations of grouped fatty acids were obtained by summing up the percentages of individual fatty acids within the fatty acid group as follows: sum of trans-18:1 = 6t/8t-18:1 + 9t-18:1 + 10t-18:1 + 11t-18:1 + 12t-18:1 + 13t/14t-18:1 + 15t-18:1 + 16t-18:1; sum of CLA = 8t,10c-18:2 + 9c,11t-18:2 + 7t,9c-18:2 + 9t,11c-18:2 + 10t,12c-18:2 + 11c,13t-18:2 + 11t,13c-18:2 + 12t,14c-18:2 + 12c,14t-18:2 + 9c,11c-18:2 + 10c,12c-18:2 + 6t,8t-18:2 + 9t,11t-18:2 + 11t,13t-18:2 + 12t,14t-18:2 + 10t,12t-18:2 + 8t,10t-18:2 + 7t,9t-18:2; sum of SFA = 10:0 + 12:0 + 13:0 + 14:0 + 15:0 + 16:0 + 17:0 + 18:0 + 20:0 + 23:0; sum of MUFA = 9c-14:1 + 9c-15:1 + 7c-16:1 + 9c-16:1 + 9c-17:1 + 6t/7t/8t-18:1 + 9t-18:1 + 10t-18:1 + 11t-18:1 + 12t-18:1 + 13t/14t-18:1 + 15t-18:1 + 16t-18:1 + 9c-18:1 + 11c-18:1 + 12c-18:1 + 13c-18:1 + 14c-18:1 + 16c-18:1 + 9c-20:1 + 11c-20:1; sum of PUFA = 18:2n-6 + 18:3n-6 + 18:3n-3 + 20:2n-6 + 20:3n-9 + 20:3n-6 + 20:4n-6 + 22:4n-6 + 22:5n-3; sum of branched-chain fatty acids (BCFA) = iso14:0 + iso15:0 + ai15:0 + iso16:0 + iso17:0 + ai17:0 + iso18:0; sum of SFA + sum of BCFA; sum of n-6 = 18:2n-6 + 18:3n-6 + 20:2n-6 + 20:3n-6 + 20:4n-6 + 22:4n-6; sum of n-3 = 18:3n-3 + 22:5n-3; and the n-6:n-3 PUFA ratio. The Health Index is a modification of the atherogenicity index proposed by Ulbricht and Southgate (1991) and it was computed as HI = (MUFA + PUFA)/(4 × 14:0 + 16:0; Zhang et al., 2008). Fatty acids were grouped based on their structure and convention that was used in dietary recommendations. Individual and groups of fatty acids with an average content of 0.48% or more were analyzed in this study, and they included 15 individual and 10 grouped fatty acids.
Statistical Analysis
A bivariate animal model as implemented in ASReml 4.0 (VSN International Ltd., Hemel Hempstead, UK; Gilmour et al., 2015) was used for statistical analyses. The bivariate animal model was similar to that previously described (Zhang et al., 2017) and can be written as follows:
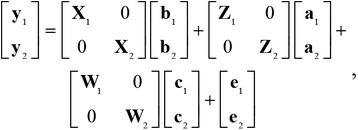 |
in which y1 and y2 are vectors of phenotypic values for 2 traits under a pairwise combination of the 25 fatty acid traits with 9 carcass merit and meat tenderness traits, b1 and b2 are vectors of fixed effects, a1 and a2 are vectors of random additive genetic effects, c1 and c2 are vectors of random contemporary group effects, and e1 and e2 are vectors of random residual effects for trait 1 and trait 2, respectively. The matrices X1, Z1, and W1 and X2, Z2, and W2 are known design matrices relating the phenotypic values to the fixed, random additive, and random contemporary group effects for trait 1 and trait 2, respectively. For each trait, phenotypic values that were greater or less than 3 SD from the mean were replaced with a missing value, resulting in 1,339 to 1,363 animals with phenotypic values of fatty acid traits, 1,127 to 1,132 animals with phenotypic values for meat tenderness traits, and 1,309 to 1,362 animals with phenotypes for carcass quality traits (Table 1). Fixed effects for fatty acid traits included breed type (TXX, HEANGV, Aberdeen Angus, HEAN, or Charolais–Red Angus crossbreeds), gender (steer or heifer), production system (calf-fed with growth implants, calf-fed with no growth implants, yearling-fed with growth implants, and yearling-fed with no growth implants), and linear covariates of individual animal's age at slaughter, days between slaughter and fatty acid extraction, and ME content of diet. The fixed effects for the carcass merit and meat tenderness traits included breed type, gender, production system as described above, and a linear covariate of the individual animal's age at slaughter. The random contemporary group effects were defined as the combination of feedlot test location and year (20 levels). Multivariate normal distributions with means equal to 0 were assumed for the random effects of a, c, and e, leading to E(y) = Xb. The variance–covariance matrix for the random effects was similar to that previously described (Zhang et al., 2017), which can be defined by
Table 1.
Descriptive statistics of mean and SD, coefficient of variation in % (CV), additive genetic variance (σa2 ± SE), and heritability (h2 ± SE) of carcass merit and meat quality traits in Canadian beef cattle
| Trait1 | N | Mean (SD) | CV | σa2 ± SE | h2 ± SE |
|---|---|---|---|---|---|
| HCW, kg | 1340 | 348.17 (39.06) | 11.22 | 498.21 ± 84.66 | 0.59 ± 0.10 |
| BFAT, mm | 1331 | 10.58 (4.12) | 38.90 | 6.30 ± 1.40 | 0.44 ± 0.10 |
| REA, cm2 | 1331 | 86.74 (10.36) | 11.95 | 37.19 ± 8.37 | 0.35 ± 0.08 |
| LMY, % | 1362 | 58.51 (4.16) | 7.11 | 5.23 ± 1.32 | 0.36 ± 0.09 |
| CMAR | 1309 | 384.50 (68.8) | 17.89 | 1232.53 ± 271.68 | 0.45 ± 0.10 |
| WBSF_ 3d, kg | 1127 | 7.77 (1.71) | 21.99 | 0.49 ± 0.20 | 0.15 ± 0.06 |
| WBSF _29d, kg | 1127 | 4.82 (0.84) | 17.43 | 0.09 ± 0.05 | 0.13 ± 0.07 |
| OT_3d | 1131 | 5.75 (0.95) | 16.53 | 0.30 ± 0.08 | 0.33 ± 0.09 |
| OT_29d | 1132 | 6.69 (0.6) | 9.00 | 0.09 ± 0.03 | 0.24 ± 0.08 |
HCW, kg: Hot carcass weight in kg; BFAT, mm: Backfat thickness in mm; REA,cm2: Rib eye area in cm2; LMY, %:Lean meat yield in %; CMAR: Carcass marbling score; WBSF_ 3d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 3 d post mortem; WBSF_ 29d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 29 d post mortem; OT_3d: Overall tenderness measured 3 d post mortem with a scale of 9 = extremely tender, 8 = very tender, 7 = moderately tender, 6 = slightly tender, 5 = neither tender nor tough, 4 = slightly tough, 3 = moderately tough, 2 = very tough, 1 = extremely tough; OT_29d: Overall tenderness measured 29 d post mortem using the same scale as OT_3d.
 |
in which 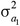 and
and 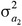 are the additive genetic variances for trait 1 and trait 2, respectively, and
are the additive genetic variances for trait 1 and trait 2, respectively, and 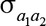 is their additive genetic covariance; A is the additive genetic relationship matrix constructed from the pedigree that was traced back 1 generation including 1,366 animals and their 130 sires;
is their additive genetic covariance; A is the additive genetic relationship matrix constructed from the pedigree that was traced back 1 generation including 1,366 animals and their 130 sires; 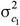 and
and 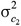 are the variance of contemporary group effects for trait 1 and trait 2, respectively, and
are the variance of contemporary group effects for trait 1 and trait 2, respectively, and 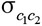 is the covariance between the 2 traits due to the same contemporary groups. The matrix
is the covariance between the 2 traits due to the same contemporary groups. The matrix 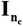 is the identity matrix with dimension nc × nc, in which nc is the number of random contemporary groups;
is the identity matrix with dimension nc × nc, in which nc is the number of random contemporary groups; 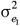 and
and 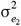 are the residual variances for trait 1 and trait 2, respectively and
are the residual variances for trait 1 and trait 2, respectively and 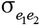 is their residual covariance; and
is their residual covariance; and 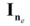 is the identity matrix with dimension ne × ne, in which ne is the number of animals. Initial values of variances were obtained using a preliminary univariate animal model analysis for subsequent pairwise bivariate analyses of the fatty acid traits with carcass merit and tenderness traits, and their variance and covariance components,
is the identity matrix with dimension ne × ne, in which ne is the number of animals. Initial values of variances were obtained using a preliminary univariate animal model analysis for subsequent pairwise bivariate analyses of the fatty acid traits with carcass merit and tenderness traits, and their variance and covariance components, 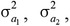 ,
,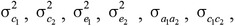 , and
, and 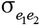 were estimated using REML. Subsequently, phenotypic variance and covariance were calculated as σp2 = σa2 + σc2 + σe2 and
were estimated using REML. Subsequently, phenotypic variance and covariance were calculated as σp2 = σa2 + σc2 + σe2 and 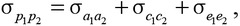 , respectively. The phenotypic and genetic correlations were then estimated as
, respectively. The phenotypic and genetic correlations were then estimated as 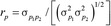 and
and 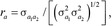 , respectively, and their SE were approximated as described by Falconer and Mackay (1996). The heritability estimate was defined as h2 = σa2/σp2, and the heritability estimates reported for the carcass merit and tenderness traits in Table 1 were the average over all bivariate analyses of the trait.
, respectively, and their SE were approximated as described by Falconer and Mackay (1996). The heritability estimate was defined as h2 = σa2/σp2, and the heritability estimates reported for the carcass merit and tenderness traits in Table 1 were the average over all bivariate analyses of the trait.
RESULTS AND DISCUSSION
Heritability Estimates of Carcass and Tenderness Traits
Descriptive statistics of mean and SD, CV, additive genetic variance, and heritability estimate for each of the 5 carcass and 4 tenderness traits are presented in Table 1. Means, SD, additive genetic variances, and heritability estimates of the 15 individual and 10 grouped fatty acid traits were previously reported (Chen et al., 2015; Zhang et al., 2017). The heritability estimates of the individual and grouped fatty acids obtained in this study were not significantly different from that reported by Zhang et al. (2017) using the A matrix when SE were considered.
For the 5 carcass merit traits analyzed, there were considerable variations observed, with a CV from 7.11% for LMY to 38.9% for BFAT. The estimates of heritability for the 5 carcass merit traits ranged from 0.35 ± 0.08 for REA to 0.59 ± 0.10 for HCW. The heritability estimates of carcass traits HCW, BFAT, REA, LMY, and CMAR are in line with those for Canadian beef cattle populations reported in other studies (Nkrumah et al., 2007; Mao et al., 2013; Miar et al., 2014). However, the heritability estimate of 0.59 ± 0.10 for HCW was greater whereas the heritability estimate of 0.35 ± 0.08 for REA was smaller than those previously reported (0.42 ± 0.14 for HCW and 0.77 ± 0.18 for REA) by Akanno et al. (2014) based on data of a smaller sample size from the same population. The variation of heritability estimates from different studies is likely due to differences in sample sizes, sampling errors, and statistical models used.
Variation in the tenderness measures was also observed, with a CV ranging from 9% for OT_29d to 21.99% for WBSF_3d. The CV for OT_29d and WBSF_29d (9% and 17.43%, respectively) were lower than those of OT_3d and WBSF_3d (16.53% and 21.99%, respectively). These decreases in CV suggest that variability in tenderness was reduced with aging, a process in which proteolytic enzymes reduce cross-linking in the meat by degrading the protein (Aaslyng, 2009). The average Warner Bratzler shear force (WBSF) dropped from 7.77 kg measured 3 d postmortem (WBSF_3d) to 4.82 kg measured 29 d postmortem (WBSF_29d), suggesting the meat became more tender. This is also reflected in the increase in the taste panel subjective measurement of overall tenderness, which increased from 5.75 (OT_3d) to 6.69 (OT_29d) with the aging process.
The estimates of heritability for the 4 tenderness traits were from 0.13 ± 0.07 for WBSF_29d to 0.33 ± 0.09 for OT_3d. Even though WBSF_3d and WBSF_29d had greater CV than OT_3d and OT_29d (21.99%, 17.43%, 16.53%, and 9%, respectively), heritability estimates for OT_3d and OT_29d were greater than those for WBSF_3d and WBSF_29d (0.33 ± 0.09 and 0.24 ± 0.08 vs. 0.15 ± 0.06 and 0.13 ± 0.07, respectively. However, the heritability estimates of WBSF_3d (0.15 ± 0.06) and WBSF_29d (0.13 ± 0.07) from this study were smaller than those previously reported by Akanno et al. (2014; i.e., 0.36 ± 0.17 for shear force on d 3 and 0.45 ± 0.20 for shear force on d 29). This discrepancy might also be due to differences in sample sizes, associated sampling errors, and statistical models used. In the study by Akanno et al. (2014), contemporary groups were defined based on sex, herd of origin, birth year, and management and were included in the mixed model as a fixed effect. In this study, the contemporary groups were defined as a combination of feedlot test location and year and were fitted in the model as a random effect. Also, Akanno et al. (2014) estimated heritability from a model in which the observed data and molecular breeding value predictions for the same trait were fitted as a 2-trait animal model, whereas in this study, heritability was estimated as the average of bivariate analyses of pairwise combinations of the fatty acid, carcass, and tenderness traits. Estimates for heritability of meat tenderness substantially vary in the literature for Bos taurus cattle from 0.00 to 0.92, according to the review by Burrow et al. (2001), even though estimates in the current study are close to the weighted average of 0.21 for WBSF in B. taurus presented by Burrow et al. (2001). Johnston et al. (2003) presented a heritability estimate of 0.11 for WBSF in temperate-adapted cattle breeds, which is in agreement with our results. However, our estimates of 0.15 ± 0.06 for WBSF_3d and 0.13 ± 0.07 for WBSF_29d were lower than those presented by Minick et al. (2004) for Angus and Charolais (0.34 ± 0.25, and 0.43 ± 0.22) but close to their estimates for Hereford and Simmental cattle (0.12 ± 0.11 and 0.16 ± 0.14). Heritability estimates for OT_3d and OT_29d presented in this study are similar to the 0.37 reported by Dikeman et al. (2005) and 0.26 ± 0.08 reported by Nephawe et al. (2004). In the present study, estimates of heritability for OT_3d and WBSF_3d (0.33 ± 0.09 and 0.15 ± 0.06, respectively) were higher than their counterpart measures OT_29d and WBSF_29d (0.24 ± 0.08 and 0.13 ± 0.07, respectively), indicating a decrease in genetic variance with aging (Table 1), which is in line with decreased variation of tenderness among samples due to the aging process as observed in this study (Table 1). However, other studies observed inconsistent or opposite changes in heritability estimates of shear force with increase in aging time (Wulf et al.,1996; Akanno et al., 2014), which may be reflective of differences in pre- and postslaughter practices, muscle sampled, sample preparation, cooking techniques, sample sizes, sampling errors, and statistical models used.
Phenotypic Correlations of Fatty Acids with Carcass and Tenderness Traits
Phenotypic correlation coefficients of the 25 fatty acids with the 5 carcass merit traits were generally low (<0.25 in magnitude; Table 2). However, relatively greater phenotypic correlation coefficients were observed between a few fatty acid traits with BFAT, including 14:0 (−0.36 ± 0.10), 15:0 (−0.25 ± 0.10), 9c-14:1 (−0.34 ± 0.08), 9c-16:1 (−0.36 ± 0.08), 9c-18:1 (0.26 ± 0.07), BCFA (−0.27 ± 0.06), and Health Index (0.25 ± 0.08). Phenotypic correlation coefficients of the 25 fatty acids with the 4 meat tenderness traits were also generally low (<0.25 in magnitude; Table 2) except for positive correlations of 0.26 ± 0.14 and 0.35 ± 0.14 for 18:0 and 11t-18:1, respectively, with WBSF_3d and negative correlations of −0.29 ± 0.16 and −0.33 ± 0.15 observed for fatty acids 9c-17:1 and 11c-18:1, respectively, with WBSF_3d. In a study of the same population of animals, Zhang et al. (2017) reported a similar trend of low negative phenotypic correlations between ultrasound BFAT and 14:0 (−0.26 ± 0.06), 15:0 (−0.27 ± 0.07), 16:0 (−0.21 ± 0.06), 9c-14:1 (−0.18 ± 0.06), 9c-16:1 (−0.17 ± 0.07), and sum of BCFA (−0.28 ± 0.05) and low positive phenotypic correlations with 9c-18:1 (0.33 ± 0.06), sum of MUFA (0.21 ± 0.06), and Health Index (0.24 ± 0.05) in the SQ tissue. Kelly et al. (2013) also reported low phenotypic correlations of fatty acids 14:0, 16:0, 18:0, 9c-16:1, and 9c-18:1 in subcutaneous fat of a multibreed beef cattle population with fat depth at the P8 site (−0.13 to 0.08). In a study by Pitchford et al. (2002), weak phenotypic correlations of MUFA in subcutaneous fat were reported for HCW, fat depth at the P8 site, and muscle intramuscular fat content (0.04 to 0.12) in a population of crossbred beef cattle. Westerling and Hedrick (1979) also reported that 16:0, 9c-16:1, 18:0, 18:2n-6, and sum of SFA in the subcutaneous fat of steers and heifers fed fescue grass and grain had a negligible to low phenotypic relationship with tenderness score (−0.24 to 0.17).
Table 2.
Phenotypic correlations ( ± SE) of 25 major fatty acids in the subcutaneous adipose tissue with 9 carcass merit and meat quality traits in Canadian beef cattle
| Trait1 | HCW, kg | BFAT, mm | REA, cm2 | LMY, % | CMAR | WBSF_ 3d, Kg | WBSF _29d, kg | OT_3d | OT_29d |
|---|---|---|---|---|---|---|---|---|---|
| 14:0 | -0.24 ± 0.10 | -0.36 ± 0.1 | -0.19 ± 0.14 | 0.16 ± 0.09 | 0.01 ± 0.10 | -0.13 ± 0.15 | 0.04 ± 0.12 | -0.01 ± 0.12 | -0.17 ± 0.11 |
| 15:0 | -0.18 ± 0.12 | -0.25 ± 0.1 | -0.15 ± 0.16 | 0.11 ± 0.10 | 0.09 ± 0.10 | -0.20 ± 0.17 | 0.04 ± 0.14 | 0.01 ± 0.14 | -0.17 ± 0.13 |
| 16:0 | -0.14 ± 0.10 | -0.18 ± 0.08 | -0.21 ± 0.12 | 0.02 ± 0.08 | -0.03 ± 0.08 | 0.01 ± 0.14 | 0.12 ± 0.08 | -0.1 ± 0.10 | -0.16 ± 0.09 |
| 17:0 | -0.08 ± 0.12 | 0 ± 0.12 | -0.10 ± 0.13 | -0.07 ± 0.09 | 0.13 ± 0.07 | -0.19 ± 0.14 | -0.02 ± 0.12 | 0.06 ± 0.11 | -0.03 ± 0.13 |
| 18:0 | -0.01 ± 0.09 | 0.17 ± 0.08 | -0.03 ± 0.1 | -0.13 ± 0.06 | -0.16 ± 0.08 | 0.26 ± 0.14 | 0.03 ± 0.11 | -0.21 ± 0.13 | 0.10 ± 0.11 |
| ai17:0 | -0.05 ± 0.09 | -0.21 ± 0.08 | -0.06 ± 0.12 | 0.13 ± 0.07 | -0.05 ± 0.09 | -0.04 ± 0.14 | 0.15 ± 0.09 | -0.08 ± 0.10 | -0.19 ± 0.09 |
| 9c-14:1 | -0.12 ± 0.09 | -0.34 ± 0.08 | -0.05 ± 0.11 | 0.23 ± 0.06 | 0.08 ± 0.09 | -0.16 ± 0.14 | 0.06 ± 0.10 | 0.01 ± 0.11 | -0.17 ± 0.10 |
| 9c-16:1 | -0.10 ± 0.09 | -0.36 ± 0.08 | -0.09 ± 0.11 | 0.21 ± 0.07 | 0.07 ± 0.09 | -0.10 ± 0.13 | 0.07 ± 0.09 | -0.02 ± 0.10 | -0.17 ± 0.09 |
| 9c-17:1 | -0.07 ± 0.13 | -0.16 ± 0.12 | -0.08 ± 0.16 | 0.08 ± 0.10 | 0.22 ± 0.10 | -0.29 ± 0.16 | -0.05 ± 0.15 | 0.11 ± 0.14 | -0.09 ± 0.16 |
| 9c-18:1 | 0.22 ± 0.10 | 0.26 ± 0.07 | 0.17 ± 0.14 | -0.14 ± 0.09 | 0 ± 0.10 | 0.11 ± 0.16 | -0.09 ± 0.11 | 0.03 ± 0.12 | 0.18 ± 0.11 |
| 11c-18:1 | 0.07 ± 0.11 | -0.16 ± 0.10 | 0.05 ± 0.13 | 0.13 ± 0.08 | 0.22 ± 0.10 | -0.33 ± 0.15 | -0.15 ± 0.13 | 0.21 ± 0.13 | -0.06 ± 0.14 |
| 13c-18:1 | 0.06 ± 0.06 | -0.01 ± 0.05 | 0.07 ± 0.07 | 0 ± 0.05 | 0.21 ± 0.05 | -0.05 ± 0.07 | -0.06 ± 0.10 | 0.06 ± 0.06 | 0.05 ± 0.06 |
| 10t-18:1 | -0.16 ± 0.10 | -0.12 ± 0.1 | -0.09 ± 0.13 | 0.09 ± 0.08 | 0.03 ± 0.09 | -0.16 ± 0.13 | 0.03 ± 0.10 | 0.04 ± 0.10 | -0.09 ± 0.11 |
| 11t-18:1 | -0.05 ± 0.12 | 0.11 ± 0.11 | -0.03 ± 0.14 | -0.08 ± 0.1 | -0.21 ± 0.10 | 0.35 ± 0.14 | 0.15 ± 0.12 | -0.23 ± 0.11 | -0.01 ± 0.14 |
| 18:2n-6 | -0.16 ± 0.07 | -0.09 ± 0.06 | 0.09 ± 0.08 | 0.13 ± 0.06 | -0.03 ± 0.06 | -0.14 ± 0.09 | -0.05 ± 0.07 | 0.12 ± 0.07 | 0.05 ± 0.07 |
| SFA | -0.14 ± 0.10 | -0.13 ± 0.08 | -0.19 ± 0.11 | -0.01 ± 0.07 | -0.09 ± 0.07 | -0.01 ± 0.13 | 0.08 ± 0.08 | -0.08 ± 0.09 | -0.12 ± 0.09 |
| BCFA | -0.15 ± 0.07 | -0.27 ± 0.06 | -0.05 ± 0.12 | 0.21 ± 0.06 | -0.09 ± 0.08 | -0.05 ± 0.11 | 0.13 ± 0.08 | -0.08 ± 0.08 | -0.19 ± 0.08 |
| SFA+BCFA | -0.15 ± 0.09 | -0.15 ± 0.08 | -0.19 ± 0.11 | 0.01 ± 0.07 | -0.10 ± 0.07 | -0.01 ± 0.14 | 0.08 ± 0.09 | -0.08 ± 0.09 | -0.13 ± 0.09 |
| MUFA | 0.16 ± 0.09 | 0.15 ± 0.07 | 0.17 ± 0.11 | -0.03 ± 0.07 | 0.09 ± 0.06 | 0.03 ± 0.13 | -0.08 ± 0.08 | 0.06 ± 0.09 | 0.13 ± 0.09 |
| Sum of trans18:1 | -0.13 ± 0.08 | -0.09 ± 0.08 | -0.03 ± 0.11 | 0.1 ± 0.06 | -0.04 ± 0.07 | -0.13 ± 0.11 | 0.03 ± 0.08 | 0.04 ± 0.09 | -0.07 ± 0.09 |
| SumCLA | 0.03 ± 0.08 | 0.05 ± 0.06 | 0.03 ± 0.07 | 0 ± 0.05 | 0 ± 0.05 | 0.17 ± 0.09 | 0.15 ± 0.08 | -0.11 ± 0.09 | -0.16 ± 0.08 |
| PUFA | -0.15 ± 0.07 | -0.08 ± 0.06 | 0.12 ± 0.08 | 0.13 ± 0.06 | -0.04 ± 0.06 | -0.13 ± 0.09 | -0.05 ± 0.07 | 0.11 ± 0.07 | 0.06 ± 0.06 |
| n-6 | -0.15 ± 0.07 | -0.07 ± 0.06 | 0.11 ± 0.08 | 0.13 ± 0.06 | -0.04 ± 0.06 | -0.15 ± 0.10 | -0.06 ± 0.07 | 0.11 ± 0.07 | 0.06 ± 0.06 |
| n-6: n-3 PUFA ratio | -0.13 ± 0.10 | -0.02 ± 0.09 | -0.01 ± 0.12 | 0.04 ± 0.08 | -0.10 ± 0.08 | -0.11 ± 0.18 | -0.16 ± 0.10 | 0.15 ± 0.12 | 0.05 ± 0.12 |
| Health Index | 0.23 ± 0.10 | 0.25 ± 0.08 | 0.23 ± 0.13 | -0.06 ± 0.09 | 0.04 ± 0.09 | 0.06 ± 0.16 | -0.10 ± 0.11 | 0.07 ± 0.11 | 0.18 ± 0.11 |
The concentrations of fatty acids were expressed as a percentage of total fatty acid methyl esters (FAME) quantified; c = cis, t = trans, ai = anteiso; Only fatty acids with a concentration greater than 0.48% of total FAME are presented. SFA+BCFA: sum of saturated (SFA) and branched chain fatty acids (BCFA); Sum trans18:1: sum of trans-18:1; MUFA: sum of all cis and all trans mono-unsaturated fatty acids (MUFA) analyzed; SumCLA: sum of conjugated linoleic acids (CLA) analyzed; PUFA: sum of polyunsaturated fatty acids (PUFA); n-6: n-3 PUFA ratio: ratio between n-6 and n-3 PUFA; Health Index: (total MUFA + total PUFA)/(4 x 14:0 + 16:0); HCW, kg: Hot carcass weight in kg; BFAT, mm: Backfat thickness in mm; REA,cm2: Rib eye area in cm2; LMY, %:Lean meat yield in %; CMAR: Carcass marbling score; WBSF_ 3d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 3 d post mortem; WBSF_ 29d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 29 d post mortem; OT_3d: Overall tenderness measured 3 d post mortem with a scale of 9 = extremely tender, 8 = very tender, 7 = moderately tender, 6 = slightly tender, 5 = neither tender nor tough, 4 = slightly tough, 3 = moderately tough, 2 = very tough, 1 = extremely tough; OT_29d: Overall tenderness measured 29 d post mortem using the same scale as OT_3d.
The generally low phenotypic correlations of fatty acid contents in subcutaneous fat of beef cattle with carcass merit and tenderness traits suggest that the environment conditions influencing fatty acid composition in adipose tissue largely differ from those that influence carcass and meat tenderness traits, because rumen microorganisms likely play a more important role in determining fatty acid composition in beef tissues than in affecting carcass merit and meat tenderness in beef cattle.
Genetics Correlations of Fatty Acids with Carcass and Tenderness Traits
Genetic correlation coefficients of the 25 fatty acid traits with most of the carcass merit and meat tenderness traits were low (<0.25 in magnitude; Table 3). However, relatively stronger genetic correlations (>0.25 in magnitude) with one or more carcass merit and meat tenderness trait were observed for all fatty acids except for 15:0, 16:0, and 11t-18:1, for which their genetic correlations with the 9 carcass merit and meat tenderness traits analyzed were all weak (<0.25 in magnitude; Table 3).
Table 3.
Genetic correlations ( ± SE) of 25 major fatty acids in the subcutaneous adipose tissue with 9 carcass merit and meat quality traits in Canadian beef cattle
| Trait1 | HCW, kg | BFAT, mm | REA, cm2 | LMY, % | CMAR | WBSF_ 3d, Kg | WBSF _29d, kg | OT_3d | OT_29d |
|---|---|---|---|---|---|---|---|---|---|
| 14:0 | -0.25 ± 0.1 | -0.30 ± 0.08 | -0.02 ± 0.13 | 0.23 ± 0.08 | 0.06 ± 0.12 | 0.13 ± 0.19 | 0.08 ± 0.20 | -0.06 ± 0.15 | -0.22 ± 0.17 |
| 15:0 | -0.07 ± 0.12 | -0.04 ± 0.14 | 0.16 ± 0.14 | 0.12 ± 0.15 | -0.13 ± 0.14 | 0.04 ± 0.20 | -0.01 ± 0.21 | -0.01 ± 0.16 | -0.16 ± 0.17 |
| 16:0 | 0.04 ± 0.14 | -0.06 ± 0.15 | 0.04 ± 0.15 | 0.06 ± 0.16 | 0.18 ± 0.15 | -0.13 ± 0.22 | -0.16 ± 0.25 | 0.10 ± 0.18 | -0.19 ± 0.20 |
| 17:0 | 0.40 ± 0.11 | 0.35 ± 0.12 | 0.16 ± 0.13 | -0.22 ± 0.13 | -0.07 ± 0.14 | -0.02 ± 0.19 | -0.24 ± 0.20 | 0.03 ± 0.15 | 0.17 ± 0.16 |
| 18:0 | 0.44 ± 0.12 | 0.24 ± 0.14 | 0.02 ± 0.15 | -0.18 ± 0.15 | -0.04 ± 0.15 | 0.09 ± 0.22 | 0.23 ± 0.24 | -0.27 ± 0.18 | -0.11 ± 0.19 |
| ai17:0 | 0.02 ± 0.18 | -0.30 ± 0.19 | 0.36 ± 0.19 | 0.43 ± 0.19 | -0.38 ± 0.19 | -0.09 ± 0.28 | 0.22 ± 0.29 | 0.10 ± 0.23 | -0.08 ± 0.25 |
| 9c-14:1 | -0.47 ± 0.11 | -0.41 ± 0.13 | -0.08 ± 0.15 | 0.30 ± 0.14 | 0.22 ± 0.16 | -0.05 ± 0.21 | -0.05 ± 0.24 | 0.07 ± 0.17 | -0.14 ± 0.19 |
| 9c-16:1 | -0.43 ± 0.11 | -0.42 ± 0.13 | -0.11 ± 0.15 | 0.22 ± 0.15 | 0.11 ± 0.15 | 0.03 ± 0.21 | 0.07 ± 0.23 | -0.02 ± 0.17 | -0.05 ± 0.19 |
| 9c-17:1 | -0.06 ± 0.13 | 0.09 ± 0.15 | 0.09 ± 0.15 | -0.09 ± 0.16 | -0.08 ± 0.15 | -0.14 ± 0.21 | -0.30 ± 0.22 | 0.17 ± 0.17 | 0.36 ± 0.18 |
| 9c-18:1 | 0.08 ± 0.14 | 0.11 ± 0.16 | -0.04 ± 0.16 | -0.13 ± 0.17 | -0.05 ± 0.16 | 0 ± 0.23 | 0.02 ± 0.25 | -0.04 ± 0.18 | 0.27 ± 0.20 |
| 11c-18:1 | -0.34 ± 0.14 | -0.16 ± 0.16 | -0.10 ± 0.16 | 0.04 ± 0.17 | 0.11 ± 0.17 | -0.13 ± 0.24 | 0.11 ± 0.25 | 0.15 ± 0.19 | 0.16 ± 0.22 |
| 13c-18:1 | -0.22 ± 0.14 | -0.05 ± 0.16 | -0.14 ± 0.16 | -0.04 ± 0.17 | 0.40 ± 0.15 | -0.04 ± 0.23 | -0.19 ± 0.24 | 0.14 ± 0.18 | 0.07 ± 0.20 |
| 10t-18:1 | 0.29 ± 0.15 | 0.14 ± 0.16 | 0.38 ± 0.16 | 0.12 ± 0.17 | -0.28 ± 0.17 | -0.15 ± 0.22 | -0.14 ± 0.25 | 0.15 ± 0.18 | 0.14 ± 0.20 |
| 11t-18:1 | -0.18 ± 0.15 | -0.07 ± 0.17 | -0.19 ± 0.17 | -0.06 ± 0.19 | 0.24 ± 0.18 | 0.07 ± 0.24 | 0.12 ± 0.26 | -0.08 ± 0.19 | -0.05 ± 0.21 |
| 18:2n-6 | -0.18 ± 0.14 | -0.01 ± 0.16 | -0.04 ± 0.16 | 0.05 ± 0.17 | -0.07 ± 0.17 | -0.22 ± 0.23 | -0.14 ± 0.25 | 0.47 ± 0.17 | 0.28 ± 0.19 |
| SFA | 0.22 ± 0.13 | 0.07 ± 0.14 | 0.05 ± 0.14 | -0.02 ± 0.15 | 0.11 ± 0.14 | -0.02 ± 0.21 | -0.02 ± 0.23 | -0.08 ± 0.17 | -0.26 ± 0.19 |
| BCFA | 0.03 ± 0.19 | -0.32 ± 0.19 | 0.35 ± 0.19 | 0.45 ± 0.19 | -0.26 ± 0.2 | 0.10 ± 0.28 | 0.41 ± 0.27 | -0.12 ± 0.23 | -0.34 ± 0.23 |
| SFA+BCFA | 0.20 ± 0.13 | 0.04 ± 0.14 | 0.06 ± 0.14 | 0.01 ± 0.15 | 0.09 ± 0.14 | -0.01 ± 0.21 | 0.01 ± 0.23 | -0.08 ± 0.17 | -0.28 ± 0.19 |
| MUFA | -0.18 ± 0.13 | -0.04 ± 0.15 | -0.07 ± 0.15 | -0.02 ± 0.16 | -0.09 ± 0.15 | 0.04 ± 0.22 | -0.01 ± 0.24 | 0.02 ± 0.18 | 0.28 ± 0.20 |
| Sum of trans18:1 | 0.26 ± 0.15 | 0.15 ± 0.16 | 0.32 ± 0.17 | 0.10 ± 0.18 | -0.29 ± 0.17 | -0.02 ± 0.24 | -0.08 ± 0.26 | 0.08 ± 0.19 | 0.07 ± 0.21 |
| sumCLA | -0.34 ± 0.17 | -0.16 ± 0.18 | -0.05 ± 0.19 | 0.06 ± 0.2 | -0.10 ± 0.19 | -0.22 ± 0.28 | -0.26 ± 0.31 | 0.45 ± 0.22 | 0.42 ± 0.25 |
| PUFA | -0.14 ± 0.14 | -0.02 ± 0.16 | 0 ± 0.16 | 0.07 ± 0.17 | -0.08 ± 0.17 | -0.25 ± 0.23 | -0.14 ± 0.25 | 0.48 ± 0.17 | 0.27 ± 0.20 |
| n-6 | -0.17 ± 0.14 | 0 ± 0.16 | -0.01 ± 0.16 | 0.06 ± 0.17 | -0.04 ± 0.17 | -0.18 ± 0.23 | -0.1 ± 0.25 | 0.04 ± 0.19 | 0.25 ± 0.19 |
| n-6: n-3 PUFA ratio | -0.50 ± 0.15 | 0.07 ± 0.20 | -0.09 ± 0.2 | 0.06 ± 0.21 | -0.17 ± 0.20 | 0.18 ± 0.28 | -0.17 ± 0.31 | 0.09 ± 0.22 | 0.41 ± 0.22 |
| Health Index | 0.06 ± 0.12 | 0.14 ± 0.14 | -0.06 ± 0.14 | -0.12 ± 0.15 | -0.05 ± 0.14 | -0.02 ± 0.21 | 0.03 ± 0.22 | -0.01 ± 0.16 | 0.28 ± 0.18 |
The concentrations of fatty acids were expressed as a percentage of total fatty acid methyl esters (FAME) quantified; c = cis, t = trans, ai = anteiso; SFA+BCFA: sum of saturated (SFA) and branched chain fatty acids (BCFA); Sum trans18:1: sum of trans-18:1; MUFA: sum of all cis and all trans mono-unsaturated fatty acids (MUFA) analyzed; SumCLA: sum of conjugated linoleic acids (CLA) analyzed; PUFA: sum of polyunsaturated fatty acids (PUFA); n-6: n-3 PUFA ratio: ratio between n-6 and n-3 PUFA; Health Index: (total MUFA + total PUFA)/(4 x 14:0 + 16:0); HCW, kg: Hot carcass weight in kg; BFAT, mm: Backfat thickness in mm; REA,cm2: Rib eye area in cm2; LMY, %: Lean meat yield in %; CMAR: Carcass marbling score; WBSF_ 3d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 3 d post mortem; WBSF_ 29d, kg: Warner Bratzler shear force on 1.9 cm cores in kg measured 29 d post mortem; OT_3d: Overall tenderness measured 3 d post mortem with a scale of 9 = extremely tender, 8 = very tender, 7 = moderately tender, 6 = slightly tender, 5 = neither tender nor tough, 4 = slightly tough, 3 = moderately tough, 2 = very tough, 1 = extremely tough; Overall tenderness measured 29 d post mortem using the same scale as OT_3d.
Saturated fatty acids are generally considered unhealthy. However, this is mainly due to the serum cholesterol effect of lauric (12:0), myristic (14:0), and palmitic acid (16:0), because stearic acid is said to have a neutral effect on serum cholesterol levels (Mensink, 2005). Therefore, selection for lower concentrations of harmful SFA in beef tissues will improve the fatty acid composition and thus the healthfulness of beef products. Myristic acid (14:0) and palmitic acid (16:0) are considered the most harmful SFA to human health as indicated by the greater weighting (4x for 14:0) in the Heath Index. The negative genetic correlations of 14:0 with HCW (−0.25 ± 0.10) and BFAT (−0.30 ± 0.08) suggest that selection for reduced 14:0 in the SQ tissue tends to result in beef cattle with larger HCW and more BFAT whereas selection for 16:0 will have minimal impacts on the carcass merit and tenderness traits due to their relatively weak genetic correlations (−0.19 ± 0.20 to 0.18 ± 0.15). The weak genetic correlations of 15:0 with all the carcass and tenderness traits (−0.16 ± 0.17 to 0.16 ± 0.14) also indicate that selection for 15:0 will not likely lead to genetic changes in the carcass merit and tenderness traits. However, the positive and moderate to moderately high genetic correlations of 17:0 and 18:0 with HCW (0.40 ± 0.11 and 0.44 ± 0.12, respectively) and with BFAT (0.35 ± 0.12 and 0.24 ± 0.14, respectively) suggests that selection to alter 17:0 and 18:0 in the SQ tissue will likely result in a commensurate change in HCW and BFAT of beef cattle.
The BCFA ai17:0 had low genetic correlations with HCW, WBSF_3d, WBSF_29d, OT_3d, and OT_29d (−0.09 ± 0.28 to 0.22 ± 0.29). However, it showed relatively greater positive genetic correlations with REA and LMY (0.36 ± 0.19 and 0.43 ± 0.19, respectively) but negative genetic correlations with BFAT and CMAR (−0.30 ± 0.19 and −0.38 ± 0.19, respectively). The BCFA ai17:0 is believed to be primarily synthesized by rumen microorganisms (Drackley, 2000). However, its heritability estimate in the SQ tissue was from 0.15 ± 0.05 to 0.22 ± 0.07 (Chen et al., 2015; Zhang et al., 2017), indicating to some extent the influence of host genetics on its concentration in the SQ tissue. The positive genetic correlations of ai17:0 with REA and LMY and negative genetic correlations with BFAT and CMAR implies possible correlated genetic changes on the carcass traits as a result of selection on ai17:0.
Monounsaturated fatty acids including 9c-14:1, 9c-16:1, 9c-17:1, and 9c-18:1 are considered healthy fatty acids, and 9c-18:1 is the most abundant individual fatty acid in the SQ tissue (Chen et al., 2015; Zhang et al., 2017). Genetic correlations of the MUFA were found to be relatively low for most of the carcass merit and meat tenderness traits analyzed (<0.25 in magnitude; Table 3). However, 9c-14:1 and 9c-16:1 showed relatively greater and negative genetic correlations with HCW and BFAT. Their genetic correlation coefficients ranged from −0.41 ± 0.13 to −0.47 ± 0.11, indicating that selection for a higher concentration of 9c-14:1 and 9c-16:1 in the SQ tissue will lead to cattle with smaller HCW and less BFAT or a leaner but lighter carcass. On the other hand, due to low genetic correlations (<0.25 in magnitude), genetic selection for 9c-17:1 and 9c-18:1 concentration in the SQ tissue will cause negligible genetic changes on the carcass merit and tenderness traits in this study except for OT_29d and WBSF_29d. The negative genetic correlation of 9c-17:1 with WBSF_29d (−0.30 ± 0.22) and positive correlation of 9c-17:1 and 9c-18:1 with OT_29d (0.36 ± 0.18 and 0.27 ± 0.20, respectively) suggest a possible genetic improvement of meat tenderness if genetic selection for higher concentrations of 9c-17:1 and 9c-18:1 in the SQ tissue is conducted. It has been reported that increased amount of oleic acid 9c-18:1 in beef is associated with increased palatability of beef products (Waldman et al., 1968; Westerling and Hedrick, 1979; Smith et al., 2006).
Intermediates 10t-18:1, 11c-18:1, 11t-18:1, and 13c-18:1 are largely generated by the microbial lipolysis of dietary fatty acids and by the incomplete biohydrogenation of PUFA in the rumen (Harfoot and Hazlewood, 1997), of which vaccenic acid 11t-18:1 was reported to have beneficial effects on human health (Bauman et al., 2000; Lock et al., 2005) whereas 10t-18:1 has been associated with increased cardiovascular health risks in humans and animal models (Hodgson et al., 1996; Bauchart et al., 2007; Roy et al., 2007). Therefore, increasing amount of 11t-18:1 and reducing the concentration of 10t-18:1 in beef products would be favorable to both the beef industry and consumers. In this study, 11t-18:1 showed low genetic correlations with carcass merit and meat tenderness traits (−0.19 ± 0.17 to 0.24 ± 0.18), suggesting that selection for 11t-18:1 in the SQ tissue will not lead to obvious genetic changes on the carcass and tenderness traits. However, genetic selection for a lower level of 10t-18:1 in the SQ tissue might result in cattle that tend to have smaller HCW and less REA but with increased marbling, due to its positive genetic correlations with HCW (0.29 ± 0.15) and REA (0.38 ± 0.16) and negative genetic correlation with CMAR (−0.28 ± 0.17; Table 3). However, it is noted that genetic selection for increased level of 13c-18:1 will improve CMAR, as supported by the positive genetic correlation of 0.40 ± 0.15.
Linoleic acid (18:2n-6) is not synthesized by the animal but arises primarily from dietary sources (Bezard et al., 1994). However, linoleic acid content in the SQ tissue showed a relatively strong heritability, with estimates ranging from 0.39 ± 0.08 to 0.53 ± 0.07 (Chen et al., 2015; Zhang et al., 2017), indicating influence of host genetics on the concentration of 18:2n-6 in the SQ tissue, likely due to genetic effects of the host on absorption, transportation, and deposition in the tissue. Relatively greater genetic correlations of 18:2n-6 with OT_3d (0.47 ± 0.17) were detected in this study, indicating that selection for a reduced amount of 18:2n-6 might genetically lead to a reduction in overall meat tenderness measured 3 d postmortem.
Genetic correlations of grouped fatty acids with the carcass merit and meat tenderness traits largely reflect the genetic correlations of their individual fatty acids (Table 3). Total SFA had lower genetic correlations with all the 9 carcass merit and meat tenderness traits (−0.26 ± 0.19 to 0.22 ± 0.13), likely due to opposite directions of genetic correlation coefficients of certain individual SFA with carcass merit and meat tenderness traits and generally low genetic correlations observed for some individual SFA. Total BCFA had a similar trend of genetic correlations with the carcass merit and meat tenderness traits as that observed for ai17:0, except for WBSF_29d and OT_29d, for which their genetic correlations with BCFA became stronger, from 0.22 ± 0.29 (ai17:0 with WBSF_29d) and −0.08 ± 0.25 (ai17:0 with OT_29d) to 0.41 ± 0.27 (BCFA with WBSF_29d) and −0.34 ± 0.23 (BCFA with OT_29d). The relatively greater genetic correlations of BCFA with WBSF_29d and OT_29d indicate that genetic selection for a reduced amount of total BCFA in the SQ tissue will genetically improve carcass tenderness and overall meat tenderness measured 29 d postmortem. This might be related to the fact that BCFA are involved in maintaining fluidity in the cell membrane (Christie, 2012).
Total SFA and BCFA (SFA + BCFA) had low genetic correlations with all carcass and tenderness traits (−0.28 ± 0.19 to 0.20 ± 0.13; Table 3). Similarly, total MUFA also showed low genetic correlations with the carcass merit and tenderness traits analyzed (−0.18 ± 0.13 to 0.28 ± 0.20; Table 3). Genetic correlations of the sum of trans-18:1 with the carcass merit and tenderness traits were found to be very similar to that of the individual fatty acid 10t-18:1, in both magnitude and direction.
Individual CLA were not analyzed in this study due to their low concentrations in the SQ tissue. However, the sum of CLA content in the SQ tissue was found to be genetically associated with HCW (−0.34 ± 0.17), WBSF_29d (−0.26 ± 0.31), OT_3d (0.45 ± 0.22), and OT_29d (0.42 ± 0.25), indicating that genetic selection for an increased amount of total CLA will result in genetic improvement of overall meat tenderness, although HCW is compromised.
Total PUFA, n-6, and the n-6:n-3 PUFA ratio were all associated, because they are all calculated from individual PUFA. The genetic correlations of PUFA with the carcass merit and meat tenderness traits have a high similarity to 18:2n-6, both in magnitude and direction (Table 3). Therefore, genetic selection for an improved level of total PUFA in the SQ tissue will also lead to genetic improvement of overall meat tenderness. However, genetic selection for n-6 will not have substantial impacts on the carcass merit and meat tenderness traits, because the genetic correlations were relatively low (−0.18 ± 0.23 to 0.25 ± 0.19). For the n-6:n-3 PUFA ratio, a relatively stronger negative genetic correlation for HCW (−0.50 ± 0.15) and positive genetic correlation for OT_29d (0.41 ± 0.22) were detected. Polyunsaturated fatty acids are essential to human health (Borsonelo and Galduroz, 2008; Zuliani et al., 2009; Perica and Delaš, 2011). However, an n-6:n-3 ratio of less than 5:1 has been recommended for human diets (World Health Organization, 2003), due to evidence that excessive levels of n-6 PUFA relative to n-3 PUFA may be associated with increased risks of chronic inflammatory diseases and depressive disorder (Hibbeln et al., 2006; Patterson et al., 2012; Husted and Bouzinova, 2016). In Canada, studies have found a greater than 5:1 n-6:n-3 ratio, on average, in commercial retail beef (Aldai et al., 2009; Ekine-Dzivenu et al., 2014; Chen et al., 2015). Therefore, selection for a reduced ratio of n-6 over n-3 is preferred by both the industry and consumers. The negative genetic correlation of the n-6:n-3 PUFA ratio with HCW (−0.50 ± 0.15) and positive genetic correlation with OT_29d (0.41 ± 0.22) indicates that genetic selection of a lower ratio of n-6 over n-3 will lead to cattle with greater HCW and reduced overall meat tenderness after 29 d of ageing.
The Health Index exhibited similar low genetic correlations with carcass merit and meat tenderness traits as its major fatty acids components, MUFA and 16:0, with genetic correlation coefficients ranging from −0.12 ± 0.15 to 0.28 ± 0.18. The relatively greater and positive genetic correlations of Health Index with OT_29d (0.28 ± 0.18) suggests that selection for a healthier fatty acid profile as measured by the Health Index in the SQ tissue might lead to a slight genetic improvement of overall meat tenderness at 29 d postmortem.
Reports on genetic correlations of fatty acid composition in subcutaneous fat with carcass merit and meat quality traits are limited to a few studies (Pitchford et al., 2002; Kelly et al., 2013). In a study of a crossbred population of multiple beef breeds, Pitchford et al. (2002) detected low genetic correlations of −0.10 and 0.07 between MUFA in subcutaneous fat with HCW and fat depth at the P8 site, which are in line with the low genetic correlations observed in this study. However, Pitchford et al. (2002) found a relatively greater genetic correlation of MUFA with intramuscular fat content (−0.27) in comparison with what we observed for marbling in this study (−0.09 ± 0.15) without considering the SE. Compared with the results of this study, Kelly et al. (2013) found relatively low genetic correlations for back fat depth at the P8 position with 14:0, 16:0, and 9c-16:1 (−0.21 to 0.15) in subcutaneous fat in a multibreed beef cattle population. However, the genetic correlations of 18:0 and 9c-18:1 with fat depth at the P8 position were greater in magnitude (−0.46 and 0.56, respectively) than what we observed in this study (0.24 ± 0.14 and 0.11 ± 0.16, respectively). Different estimates of genetic correlations from different studies may be due to differences in animal population or breed composition, statistical models used, sample sizes, and associated sampling errors. Studies with information of deeper pedigree will likely lead to more accurate estimates of the genetic parameters. Moreover, the P8 position is located at the intersection of a line parallel to the spine from the tuber ischium and a line perpendicular to it from the spinous process of the third sacral vertebra (Kelly et al., 2013). Therefore, fat depth at the P8 position and BFAT measured between the 12th and 13th ribs in this study are 2 different fat depth measures in beef cattle.
Sustainable beef production depends on continuous improvement not only for production efficiency but also for end-product quality to meet consumers' demands. Fatty acid composition is emerging as an equally important trait as carcass merit and meat tenderness due to consumers' increasing demand for healthier beef products. Although generally low genetic correlations between fatty acids and carcass and meat tenderness traits were detected in this study, moderate to moderately strong genetic correlations (0.25 to 0.50 in magnitude) were also observed. Based on documented implications of dietary fatty acids on human health, the genetic correlations of the fatty acids with carcass and meat tenderness traits can be considered favorable, neutral, and unfavorable. For instance, positive genetic correlations of the sum of CLA with OT_3d (0.45 ± 0.22) and OT_29d (0.42 ± 0.25) are favorable whereas its negative correlation with HCW (−0.34 ± 0.17) can be viewed as unfavorable. On the other hand, the negative correlation of the n-6:n-3 PUFA ratio with HCW (−0.50 ± 0.15) is favorable, because genetic selection for a lower n-6:n-3 PUFA ratio will lead to an increase in HCW, whereas its positive genetic correlation with OT_29d (0.41 ± 0.22) is antagonistic, because selection for a lower n-6:n-3 PUFA ratio will decrease overall meat tenderness after 29 d aging. Therefore, multiple-trait economic selection indexes are recommended for genetic selection to maximize beef production profitability when fatty acid composition, carcass merit, and meat tenderness traits are included in the breeding objective.
The estimates of correlations between fatty acid composition, carcass merit, and meat tenderness traits in beef cattle will not only provide valuable genetic parameters for multiple-trait economic selection index construction but will also shed some light on host genetic influence on lipid metabolism and therefore end-product traits in beef cattle such as fatty acid synthesis, fat deposition, carcass quality, and meat tenderness. Previous studies have identified that genes related to lipid metabolism including fatty acid syntheses (FASN), stearoyl-CoA desaturase (SCD), and fatty acid binding protein 4 (FABP4) are associated with fatty acid composition in beef tissues (Taniguchi et al., 2004; Zhang et al., 2008; Abe et al., 2009; Narukami et al., 2011; Li et al., 2012). These genes also showed effects on carcass and meat quality traits (Matsuhashi et al., 2011; Rempel et al., 2012; Zhao et al., 2012). Matsuhashi et al. (2011) reported that SCD showed a significant effect on luster, firmness, and texture of beef meat. They also observed that animals with decreased 18:0, increased 14:1, and increased MUFA abundance tended to have beef with greater scores of luster, firmness, and texture. Dunner et al. (2013) reported that the procollagen-lysine, 2-oxoglutarate 5-dioxygenase 3 precursor (PLOD3) gene was associated with carcass performance and fatty acids in 15 breeds of European cattle. PLOD3 encodes lysyl hydroxylase 3 (LH3) that also is correlated with collagen formation and skeletal muscle development (Heikkinen et al., 2000). The moderate to moderately high genetic correlations of some fatty acids with carcass merit and meat tenderness traits observed in this study provide additional evidence that fatty acid composition in beef tissue and carcass and meat quality traits might be regulated by a subset of the same genes in beef cattle.
Implications
Phenotypic correlations of fatty acid composition in SQ of beef cattle with carcass merit and tenderness traits were generally weak. However, moderate to moderately high genetic correlations (0.25–0.50 in magnitude) were observed between some fatty acids in the SQ of beef cattle and certain carcass merit and meat tenderness traits. Due to the antagonistic genetic correlations, multiple-trait economic indexes are recommended when genetic improvement on fatty acid composition, carcass merit, and meat tenderness traits are simultaneously considered in the breeding objective. In addition, the moderate to moderately high genetic correlations observed in this study indicates that some fatty acid, carcass merit, and meat tenderness traits are likely regulated by a subset of same genes in beef cattle.
FOOTNOTES
The authors thank Ivy Larsen for assistance on beef tissue collection, Shurong Xiong for fatty acid analyses, Ereddad Kharraz and Jonathan Curtis for assistance on fatty acid analyses, Zhiquan Wang and Erasmus Okine for valuable comments on the study, and Liuhong Chen for assistance on data analyses. This work was supported by the Alberta Livestock and Meat Agency (ALMA; 2010R038R to C. Li). Support to the project (in kind) from Beefbooster Inc., Alberta, Canada was also highly appreciated.
LITERATURE CITED
- Aaslyng M. D. 2009. Trends in meat consumption and the need for fresh meat and meat products of improved quality. In: Kerry J. P., Ledward D. editors, Improving the sensory and nutritional quality of fresh meat. Woodhead Publishing Ltd, Cambridge, UK: p. 3–18. doi: 10.1533/9781845695439.1.3 [DOI] [Google Scholar]
- Abe T., Saburi J., Hasebe H., Nakagawa T., Misumi S., Nade T., Nakajima H., Shoji N., Kobayashi M., Kobayashi E. 2009. Novel mutations of the FASN gene and their effect on fatty acid composition in Japanese black beef. Biochem. Genet. 47:397–411. doi: 10.1007/s10528-009-9235-5 [DOI] [PubMed] [Google Scholar]
- Akanno E. C., Plastow G., Woodward B. W., Bauck S., Okut H., Wu X.-L., Sun C., Aalhus J. L., Moore S. S., Miller S. P., Wang Z., Basarab J. A. 2014. Reliability of molecular breeding values for Warner-Bratzler shear force and carcass traits of beef cattle – An independent validation study. J. Anim. Sci. 92:2896–2904. doi: 10.2527/jas.2013-7374 [DOI] [PubMed] [Google Scholar]
- Aldai N., Dugan M. E. R., Rolland D. C., Kramer J. K. G. 2009. Survey of the fatty acid composition of Canadian beef: Backfat and longissimus lumborum muscle. Can. J. Anim. Sci. 89:315–329. doi: 10.4141/CJAS08126 [DOI] [Google Scholar]
- Basarab J. A., Colazo M. G., Ambrose D. J., Novak S., McCartney D., Baron V. S. 2011. Residual feed intake adjusted for backfat thickness and feeding frequency is independent of fertility in beef heifers. Can. J. Anim. Sci. 91:573–584. doi: 10.4141/cjas2011-010 [DOI] [Google Scholar]
- Basarab J. A., Price M. A., Aalhus J. L., Okine E. K., Snelling W. M., Lyle K. L. 2003. Residual feed intake and body composition in young growing cattle. Can. J. Anim. Sci. 83:189–204. doi: 10.4141/A02-065 [DOI] [Google Scholar]
- Bauchart D., Roy A., Lorenz S., Chardigny J. M., Ferlay A., Gruffat D., Sebedio J. L., Chilliard Y., Durand D. 2007. Butters varying in trans 18:1 and cis-9,trans-11 conjugated linoleic acid modify plasma lipoproteins in the hypercholesterolemic rabbit. Lipids 42:123–133. doi: 10.1007/s11745-006-3018-0 [DOI] [PubMed] [Google Scholar]
- Bauman D. E., Baumgard L. H., Corl B. A., Griinari J. M. 2000. Biosynthesis of conjugated linoleic acid in ruminants. J. Anim. Sci. 77:1–15. doi: 10.2527/jas2000.77E-Suppl1f [DOI] [Google Scholar]
- Bézard J., Blond J. P., Bernard A., Clouet P. 1994. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod. Nutr. Dev. 34(6):539–568. doi: 10.1051/rnd:19940603 [DOI] [PubMed] [Google Scholar]
- Borsonelo E. C., Galduróz J. C. F. 2008. The role of polyunsaturated fatty acids (PUFAs) in development, aging and substance abuse disorders: Review and propositions. Prostaglandins Leukot. Essent. Fatty Acids 78:237–245. doi: 10.1016/j.plefa.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Burrow H. M., Moore S. S., Johnston D. J., Barendse W., Bindon B. M. 2001. Quantitative and molecular genetic influences on properties of beef: A review. Aust. J. Exp. Agric. 41:893–919. doi: 10.1071/EA00015 [DOI] [Google Scholar]
- Chen L., Ekine-Dzivenu C., Vinsky M., Basarab J., Aalhus J., Dugan M. E. R., Fitzsimmons C., Stothard P., Li C. 2015. Genome-wide association and genomic prediction of breeding values for fatty acid composition in subcutaneous adipose and longissimus lumborum muscle of beef cattle. BMC Genet. 16(1):135. doi: 10.1186/s12863-015-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie W. 2012. Fatty acids: Branched-chain structures, occurrence and biosynthesis. The Lipid Library; http://aocs.files.cms-plus.com/LipidsLibrary/images/Importedfiles/lipidlibrary/Lipids/fa_branc/file.pdf. [Google Scholar]
- Cruz-Hernandez C., Deng Z., Zhou J., Hill A. R., Yurawecz M. P., Delmonte P., Mossoba M. M., Dugan M. E., Kramer J. K. 2004. Methods for analysis of conjugated linoleic acids and trans-18:1 isomers in dairy fats by using a combination of gas chromatography, silver-ion thin-layer chromatography/gas chromatography, and silver-ion liquid chromatography. J. AOAC Int. 87:545–562. [PubMed] [Google Scholar]
- Dikeman M. E., Pollak E. J., Zhang Z., Moser D. W., Gill C. A., Dressler E. A. 2005. Phenotypic ranges and relationships among carcass and meat palatability traits for fourteen cattle breeds, and heritabilities and expected progeny differences for Warner-Bratzler shear force in three beef cattle breeds. J. Anim. Sci. 83(10):2461–2467. doi: 10.2527/2005.83102461x [DOI] [PubMed] [Google Scholar]
- Drackley J. K. 2000. Lipid metabolism. In: D'Mello J. P. F. editor, Farm animal metabolism and nutrition. CABI Publishing, Wallingford, UK: p. 97–119. doi: 10.1079/9780851993782.0097 [DOI] [Google Scholar]
- Dugan M. E., Kramer J. K., Robertson W. M., Meadus W. J., Aldai N., Rolland D. C. 2007. Comparing subcutaneous adipose tissue in beef and muskox with emphasis on trans 18:1 and conjugated linoleic acids. Lipids 42(6):509–518. doi: 10.1007/s11745-007-3051-7 [DOI] [PubMed] [Google Scholar]
- Dunner S., Sevane N., García D., Cortés O., Valentini A., Williams J. L., Mangin B., Cañón J., Levéziel H., GeMQual Consortium 2013. Association of genes involved in carcass and meat quality traits in 15 European bovine breeds. Livest. Sci. 154(1–3):34–44. doi: 10.1016/j.livsci.2013.02.020 [DOI] [Google Scholar]
- Ekine-Dzivenu C., Chen L., Vinsky M., Aldai N., Dugan M. E. R., McAllister T. A., Wang Z., Okine E., Li C. 2014. Estimates of genetic parameters for fatty acids in brisket adipose tissue of Canadian commercial crossbred beef steers. Meat Sci. 96:1517–1526. doi: 10.1016/j.meatsci.2013.10.011 [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C. 1996. Introduction to quantitative genetics. 4th ed.Pearson/Prentice Hall, New York, NY. [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., Welham S. J., Thompson R. 2015. ASReml user guide release 4.1 structural specification. VSN International Ltd., Hemel Hempstead, UK. [Google Scholar]
- Harfoot C. G., Hazlewood G. P. 1997. Lipid metabolism in the rumen. In: Hobson P. N., Stewart C. S. editors, The rumen microbial ecosystem. Chapman & Hall, London, UK. doi: 10.1007/978-94-009-1453-7_9 [DOI] [Google Scholar]
- Heikkinen J., Risteli M., Wang C., Latvala J., Rossi M., Valtavaara M., Myllylä R. 2000. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J. Biol. Chem. 275:36158–36163. doi: 10.1074/jbc.M006203200 [DOI] [PubMed] [Google Scholar]
- Hibbeln J. R., Nieminen L. R., Blasbalg T. L., Riggs J. A., Lands W. E. 2006. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 83(6 Suppl):1483S–1493S. [DOI] [PubMed] [Google Scholar]
- Hodgson J. M., Wahlqvist M. L., Boxall J. A., Balazs N. D. 1996. Platelet trans fatty acids in relation to angiographically assessed coronary artery disease. Atherosclerosis 120:147–154. doi: 10.1016/0021-9150(95)05696-3 [DOI] [PubMed] [Google Scholar]
- Hu F. B., Manson J. E., Willett W. C. 2001. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 20:5–19. doi: 10.1080/07315724.2001.10719008 [DOI] [PubMed] [Google Scholar]
- Husted K. S., Bouzinova E. V. 2016. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina (Kaunas) 52:139–147. [DOI] [PubMed] [Google Scholar]
- Johnston D. J., Reverter A., Ferguson D. M., Thompson J. M., Burrow H. M. 2003. Genetic and phenotypic characterization of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 3. Meat quality traits. Aust. J. Agric. Res. 54(2):135–147. doi: 10.1071/AR02087 [DOI] [Google Scholar]
- Kelly M. J., Tume R. K., Newman S., Thompson J. M. 2013. Genetic variation in fatty acid composition of subcutaneous fat in cattle. Anim. Prod. Sci. 53:129–133. doi: 10.1071/AN12154 [DOI] [Google Scholar]
- Kramer J. K. G., Hernandez M., Cruz-Hernandez C., Kraft J., Dugan M. E. R. 2008. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids 43:259–273. doi: 10.1007/s11745-007-3143-4 [DOI] [PubMed] [Google Scholar]
- Li C., Aldai N., Vinsky M., Dugan M. E. R., McAllister T. A. 2012. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim. Genet. 43:93–97. doi: 10.1111/j.1365-2052.2011.02217.x [DOI] [PubMed] [Google Scholar]
- Lim J. N., Oh J. J., Wang T., Lee J. S., Kim S. H., Kim Y. J., Lee H. G. 2014. Trans-11 18:1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF-7 human mammary adenocarcinoma cells. Nutrients 6:627–636. doi: 10.3390/nu6020627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock A. L., Parodi P. W., Bauman D. E. 2005. The biology of trans fatty acids: Implications for human health and the dairy industry. Aust. J. Dairy Technol. 60:134–142. [Google Scholar]
- López-Campos Ó., Aalhus J. L., Okine E. K., Baron V. S., Basarab J. A. 2013. Effects of calf- and yearling-fed beef production systems and growth promotants on production and profitability. Can. J. Anim. Sci. 93:171–184. doi: 10.4141/cjas2012-035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk J. L., Parker N. 2009. Consumer preferences for amount and type of fat in ground beef. J. Agric. Appl. Econ. 41:75–90. doi: 10.1017/S107407080000256X [DOI] [Google Scholar]
- Malau-Aduli A. E. O., Edriss M. A., Siebert B. D., Bottema C. D. K., Deland M. P. B., Pitchford W. S. 2000. Estimates of genetic parameters for triacylglycerol fatty acids in beef cattle at weaning and slaughter. J. Anim. Physiol. Anim. Nutr. 83:169–180. doi: 10.1046/j.1439-0396.2000.00256.x [DOI] [Google Scholar]
- Mao F., Chen L., Vinsky M., Okine E., Wang Z., Basarab J., Crews D. H., Li C. 2013. Phenotypic and genetic relationships of feed efficiency with growth performance, ultrasound, and carcass merit traits in Angus and Charolais steers. J. Anim. Sci. 91:2067–2076. doi: 10.2527/jas.2012-5470 [DOI] [PubMed] [Google Scholar]
- Matsuhashi T., Maruyama S., Uemoto Y., Kobayashi N., Mannen H., Abe T., Sakaguchi S., Kobayashi E. 2011. Effects of bovine fatty acid synthase, stearoyl-coenzyme A desaturase, sterol regulatory element-binding protein 1, and growth hormone gene polymorphisms on fatty acid composition and carcass traits in Japanese Black cattle. J. Anim. Sci. 89:12–22. doi: 10.2527/jas.2010-3121 [DOI] [PubMed] [Google Scholar]
- McCluskey J. J., Wahl T. I., Li Q., Wandschneider P. R. 2005. US grass-fed beef: Marketing health benefits. J. Food Distrib. Res. 36(3):1–8. [Google Scholar]
- Mensink R. P. 2005. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids 40(12):1201–1205. doi: 10.1007/s11745-005-1486-x [DOI] [PubMed] [Google Scholar]
- Miar Y., Plastow G. S., Bruce H. L., Moore S. S., Durunna O. N., Nkrumah J. D., Wang Z. 2014. Estimation of genetic and phenotypic parameters for ultrasound and carcass merit traits in crossbred beef cattle. Can. J. Anim. Sci. 94:273–280. doi: 10.4141/cjas2013-115 [DOI] [Google Scholar]
- Michas G., Micha R., Zampelas A. 2014. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 234:320–328. doi: 10.1016/j.atherosclerosis.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Minick J. A., Dikeman M. E., Pollak E. J., Wilson D. E. 2004. Heritability and correlation estimates of Warner-Bratzler shear force and carcass traits from Angus-, Charolais-, Hereford-, and Simmental-sired cattle. Can. J. Anim. Sci. 84(4):599–609. doi: 10.4141/A03-060 [DOI] [Google Scholar]
- Narukami T., Sasazaki S., Oyama K., Nogi T., Taniguchi M., Mannen H. 2011. Effect of DNA polymorphisms related to fatty acid composition in adipose tissue of Holstein cattle. Anim. Sci. J. 82:406–411. doi: 10.1111/j.1740-0929.2010.00855.x [DOI] [PubMed] [Google Scholar]
- Nephawe K. A., Cundiff L. V., Dikeman M. E., Crouse J. D., Dale Van Vleck L. 2004. Genetic relationships between sex-specific traits in beef cattle: Mature weight, weight adjusted for body condition score, height and body condition score of cows, and carcass traits of their steer relatives. J. Anim. Sci. 82(3):647–653. doi: 10.2527/2004.823647x [DOI] [PubMed] [Google Scholar]
- Nkrumah J. D., Keisler D. H., Crews D. H., Basarab J. A., Wang Z., Li C., Price M. A., Okine E. K., Moore S. S. 2007. Genetic and phenotypic relationships of serum leptin concentration with performance, efficiency of gain, and carcass merit of feedlot cattle. J. Anim. Sci. 85:2147–2155. doi: 10.2527/jas.2006-764 [DOI] [PubMed] [Google Scholar]
- Olfert E. D., Cross B. M., McWilliams A. A. editors. 1993. Guide to the care and use of experimental animals. Vol. 1. Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Pan A., Sun Q., Bernstein A. M., Schulze M. B., Manson J. E., Stampfer M. J., Willett W. C., Hu F. B. 2012. Red meat consumption and mortality results from 2 prospective cohort studies. Arch. Intern. Med. 172:555–563. doi: 10.1001/archinternmed.2011.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E., Wall R., Fitzgerald G. F., Ross R. P., Stanton C. 2012. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012. doi: 10.1155/2012/539426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perica M. M., Delaš I. 2011. Essential fatty acids and psychiatric disorders. Nutr. Clin. Pract. 26:409–425. doi: 10.1177/0884533611411306 [DOI] [PubMed] [Google Scholar]
- Pitchford W. S., Deland M. P. B., Siebert B. D., Malau-Aduliand A. E. O., Bottema C. D. K. 2002. Genetic variation in fatness and fatty acid composition of crossbred cattle. J. Anim. Sci. 80:2825–2832. doi: 10.2527/2002.80112825x [DOI] [PubMed] [Google Scholar]
- Rempel L. A., Casas E., Shackelford S. D., Wheeler T. L. 2012. Relationship of polymorphisms within metabolic genes and carcass traits in crossbred beef cattle. J. Anim. Sci. 90:1311–1316. doi: 10.2527/jas.2011-4302 [DOI] [PubMed] [Google Scholar]
- Roy A., Chardigny J.-M., Bauchart D., Ferlay A., Lorenz S., Durand D., Gruffat D., Faulconnier Y., Sébédio J., Chilliard Y. 2007. Butters rich either in trans-10-C18:1 or in trans-11-C18:1 plus cis-9, trans-11 CLA differentially affect plasma lipids and aortic fatty streak in experimental atherosclerosis in rabbits. Animal 1:467–476. doi: 10.1017/S175173110770530X [DOI] [PubMed] [Google Scholar]
- Saatchi M., Garrick D. J., Tait R. G., Jr, Mayes M. S., Drewnoski M., Schoonmaker J., Diaz C., Beitz D. C., Reecy J. M. 2013. Genome-wide association and prediction of direct genomic breeding values for composition of fatty acids in Angus beef cattle. BMC Genomics 14:730. doi: 10.1186/1471-2164-14-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollan N., Hocquette J.-F., Nuernberg K., Dannenberger D., Richardson I., Moloney A. 2006. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 74:17–33. doi: 10.1016/j.meatsci.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Smith S. B., Lunt D. K., Chung K. Y., Choi C. B., Tume R. K., Zembayashi M. 2006. Adiposity, fatty acid composition, and delta-9 desaturase activity during growth in beef cattle. Anim. Sci. J. 77:478–486. [Google Scholar]
- Taniguchi M., Utsugi T., Oyama K., Mannen H., Kobayashi M., Tanabe Y., Ogino A., Tsuji S. 2004. Genotype of stearoyl-CoA desaturase is associated with fatty acid composition in Japanese Black cattle. Mamm. Genome 15:142–148. doi: 10.1007/s00335-003-2286-8 [DOI] [PubMed] [Google Scholar]
- Ulbricht T. L. V., Southgate D. A. T. 1991. Coronary heart disease: Seven dietary factors. Lancet 338:985–992. doi: 10.1016/0140-6736(91)91846-M [DOI] [PubMed] [Google Scholar]
- Waldman R. C., Suess G. G., Brungardt V. H. 1968. Fatty acids of certain bovine tissue and their association with growth, carcass and palatability traits. J. Anim. Sci. 27:632–635. doi: 10.2527/jas1968.273632x [DOI] [Google Scholar]
- Weber K. L., Thallman R. M., Keele J. W., Snelling W. M., Bennett G. L., Smith T. P. L., McDaneld T. G., Allan M. F., Van Eenennaam A. L., Kuehn L. A. 2012. Accuracy of genomic breeding values in multibreed beef cattle populations derived from deregressed breeding values and phenotypes. J. Anim. Sci. 90:4177–4190. doi: 10.2527/jas.2011-4586 [DOI] [PubMed] [Google Scholar]
- Westerling D. B., Hedrick H. B. 1979. Fatty acid composition of bovine lipids as influenced by diet, sex and anatomical location and relationship to sensory characteristics. J. Anim. Sci. 48:1343–1348. doi: 10.2527/jas1979.4861343x [DOI] [Google Scholar]
- Woodside J. V., Kromhout D. 2005. Fatty acids and CHD. Proc. Nutr. Soc. 64:554–564. doi: 10.1079/PNS2005465 [DOI] [PubMed] [Google Scholar]
- World Health Organization 2003. Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation. WHO Tech. Rep. Ser. 916. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- Wulf D. M., Tatum J. D., Green R. D., Morgan J. B., Golden B. L., Smith G. C. 1996. Genetic influences on beef longissimus palatability in Charolais- and Limousin-sired steers and heifers. J. Anim. Sci. 74:2394–2405. doi: 10.2527/1996.74102394x [DOI] [PubMed] [Google Scholar]
- Zhang F., Ekine-Dzivenu C., Vinsky M., Basarab J. A., Aalhus J. L., Dugan M. E. R., Li C. 2017. Phenotypic and genetic relationships of residual feed intake measures and their component traits with fatty acid composition in subcutaneous adipose of beef cattle. J. Anim. Sci. 95(7):2813–2824. doi: 10.2527/jas.2017.1451 [DOI] [PubMed] [Google Scholar]
- Zhang S., Knight T. J., Reecy J. M., Beitz D. C. 2008. DNA polymorphisms in bovine fatty acid synthase are associated with beef fatty acid composition. Anim. Genet. 39:62–70. doi: 10.1111/j.1365-2052.2007.01681.x [DOI] [PubMed] [Google Scholar]
- Zhao C. P., Tian F., Yu Y., Luo J., Hu Q., Bequette B. J., Baldwin R. L., Liu G., Zan L. S., Updike M. S., Song J. Z. 2012. Muscle transcriptomic analyses in Angus cattle with divergent tenderness. Mol. Biol. Rep. 39:4185–4193. doi: 10.1007/s11033-011-1203-6 [DOI] [PubMed] [Google Scholar]
- Zuliani G., Galvani M., Leitersdorf E., Volpato S., Cavalieri M., Fellin R. 2009. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr. Pharm. Des. 15:4087–4093. doi: 10.2174/138161209789909773 [DOI] [PubMed] [Google Scholar]


