Abstract
The DE and ME content (Exp. 1) as well as the apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of essential AA (EAA; Exp. 2) were compared between Chinese corn and U.S. sorghum. The effects of U.S. sorghum as a potential substitute for Chinese corn on growth performance of 114 weaned pigs (8.8 ± 1.0 kg BW; Exp. 3) and 60 growing pigs (23.4 ± 1.6 kg BW; Exp. 4) were evaluated, and the effect of protease supplementation on N utilization was determined in sorghum-based diets fed to growing pigs (Exp. 4). In Exp. 1, there was no difference in DE and ME content between corn and sorghum. In Exp. 2, the AID and SID of most EAA and the concentrations of standardized ileal digestible Lys, Met, Thr, and His were less in sorghum than in corn (P < 0.05). In Exp. 3, there was no difference in ADG and ADFI among treatments during the experimental period. The G:F and apparent total tract digestibility (ATTD) of CP was decreased for pigs fed diets with sorghum in the first 2 wk (P < 0.05) and for pigs fed diets containing 60% sorghum in the following 2 wk (P < 0.05). The fecal score for pigs fed diets with sorghum, regardless of the substitute level, was less (P < 0.05) or tended to be less (P = 0.086) than that for pigs fed diets containing 60% corn. In Exp. 4, no differences were observed in ADG and ADFI overall among pigs fed diets based on corn and soybean meal (CSBM) or sorghum and soybean meal (SSBM). Pigs fed CSBM or SSBM with protease supplementation had greater (P < 0.05) or tended to have greater (P = 0.062) G:F than pigs fed SSBM. Compared with CSBM, SSBM increased fecal N excretion by more than 25% and decreased the ATTD of CP by more than 7% during the whole experiment (P < 0.05). Protease supplementation reduced fecal N excretion by more than 12% and increased ATTD of CP by more than 6% (P < 0.05). In conclusion, based on optimal G:F and CP digestibility, diets for weaned pigs should contain less than 20% sorghum during the first 2 wk and no more than 40% during the subsequent 2 wk after weaning. Sorghum used as an alternative energy source for corn in diets fed to growing pigs decreases CP utilization by increasing manure N output, which might be partially offset by protease supplementation.
Keywords: energy, growth performance, nitrogen, pigs, protease, sorghum
INTRODUCTION
Sorghum contains a unique structure of protein bodies, which compromises N and energy utilization by physical and chemical interactions (Salinas et al., 2006; Liu et al., 2013a,c), but this could be addressed, at least partially, by exogenous protease for broilers (Xu et al., 2017) and pigs (Pan et al., 2017). Sorghum is not always comparable to corn, mainly due to its relatively high tannin content (Khoddami et al., 2015; Pan et al., 2016a). Accordingly, once favorably priced, low-tannin sorghum is usually used as an alternative energy source to reduce diet cost and an overdependence on corn (Yin et al., 2002; Paulk et al., 2015; Xie et al., 2017).
As is well known, the United States is the largest producer and exporter of sorghum grain, accounting for almost 20% of world production and 80% of world sorghum exports in 2016 and 2017 (USDA-FAS, 2017). It has been reported that more than 99% of U.S. sorghum is tannin free due to decades of breeding efforts, and the nontannin sorghum grown for livestock feed has virtually the same energy profile as corn (Awika and Rooney, 2004). Unfortunately, the majority of Chinese-produced sorghum grain is used to produce liquor and vinegar for these cultivars possessing high tannin content required for liquor and vinegar flavor, and only a very small proportion is used for human food or feed (Diao, 2017). With the price of corn soaring in recent years, China has imported large amounts of U.S. sorghum as animal feed (Diao, 2017). However, there is a dearth of studies to accurately evaluate the nutritional value of U.S. sorghum grain.
We hypothesized that sorghum imported from the United States has a similar energy content but low N utilization compared with corn produced in China and that the increased manure N output from growing pigs fed a sorghum-based diet could be offset by protease supplementation. Therefore, the objectives of 4 experiments were to compare DE and ME content (Exp. 1) as well as apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of essential AA (EAA) and standardized ileal digestible EAA composition (Exp. 2) between Chinese corn and U.S. sorghum, to evaluate effects of U.S. sorghum as a potential substitute for Chinese corn on growth performance and apparent total tract digestibility (ATTD) of nutrients in weaned (Exp. 3) and growing pigs (Exp. 4), and to determine whether protease could improve CP utilization by reducing manure N output in a sorghum-based diet fed to growing pigs (Exp. 4).
MATERIALS AND METHODS
The experimental protocols used in these experiments, including animal care and use, were approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, P.R. China).
General
These studies were conducted in the Swine Nutrition Research Center of the National Feed Engineering Technology Research Center (Chengde, P.R. China) for Exp. 1, 3, and 4 and in the Metabolism Laboratory of the Ministry of Agriculture Feed Industry Centre (China Agricultural University, Beijing, P.R. China) for Exp. 2.
Yellow dent corn (Zea mays L.) produced in China was obtained from the Swine Nutrition Research Center of the National Feed Engineering Technology Research Center. The sorghum (Sorghum bicolor) sample imported from the United States was purchased from the Tianjin Fujiadesenhe International Trading Company in Tianjin (P.R. China). Both grains were ground in a hammer mill using a 2-mm screen. The analyzed chemical composition of the corn and sorghum grain is presented in Table 1. The protease with activity of 10,000 units/g used in Exp. 4 is extracted from the porcine pancreas and small intestines and provided by Shanghai Honest Biological Technology Company (Shanghai, P.R. China).
Table 1.
Analyzed chemical composition of corn and sorghum (%, as-fed basis)1
| Item | Corn | Sorghum |
|---|---|---|
| DM | 88.25 | 87.63 |
| GE, MJ/kg | 16.28 | 16.10 |
| CP | 7.94 | 7.98 |
| Prolamin | 4.29 | 5.91 |
| Ether extract | 2.34 | 1.82 |
| Ash | 1.22 | 1.33 |
| NDF | 15.05 | 10.50 |
| ADF | 3.30 | 3.05 |
| Starch | 61.46 | 67.48 |
| Ca | 0.04 | 0.02 |
| Total P | 0.23 | 0.26 |
| Phytate | 0.37 | 0.70 |
| Tannin | 0.01 | 0.05 |
| Total phenols | 0.12 | 0.34 |
| Indispensable AA | ||
| Lys | 0.23 | 0.21 |
| Met | 0.18 | 0.16 |
| Thr | 0.29 | 0.30 |
| Trp | 0.06 | 0.07 |
| Val | 0.40 | 0.45 |
| Leu | 1.04 | 1.01 |
| Ile | 0.25 | 0.33 |
| Phe | 0.36 | 0.39 |
| His | 0.23 | 0.20 |
| Arg | 0.31 | 0.34 |
| Dispensable AA | ||
| Tyr | 0.19 | 0.17 |
| Ser | 0.35 | 0.33 |
| Glu | 1.32 | 1.60 |
| Pro | 0.65 | 0.68 |
| Gly | 0.30 | 0.27 |
| Ala | 0.55 | 0.65 |
| Cys | 0.25 | 0.22 |
| Asp | 0.49 | 0.50 |
| Total indispensable AA | 3.73 | 3.46 |
| Total dispensable AA | 4.10 | 4.42 |
| Total AA | 7.45 | 7.88 |
All values are the results of an analysis conducted in duplicate.
All diets in these experiments were fed in mash form, and the experimental pigs (Duroc × Landrace × Yorkshire) were provided by the Swine Nutrition Research Centre of the National Feed Engineering Technology Research Centre.
Experimental Design and Sample Collection
Experiment 1: Energy Measurement
The objective of this experiment was to determine DE and ME as well as ATTD of GE of corn and sorghum grain. Twelve barrows (36.7 ± 2.0 kg BW) were randomly allotted to 1 of 2 diets with 6 barrows per treatment. The diets were formulated to contain 96.9% of corn or sorghum grain and 3.1% of vitamins and minerals (Table 2).
Table 2.
Composition of experimental diets in Exp. 1 and 2 (%, as-fed basis)
| Exp. 1 | Exp. 2 | ||||
|---|---|---|---|---|---|
| Item | Corn diet | Sorghum diet | Corn diet | Sorghum diet | N-free diet |
| Ingredient | |||||
| Corn | 96.9 | – | 96.6 | – | – |
| Sorghum | – | 96.9 | – | 96.6 | – |
| Corn starch | – | – | – | – | 73.5 |
| Sucrose | – | – | – | – | 15.0 |
| Cellulose acetate | – | – | – | – | 4.0 |
| Soybean oil | – | – | – | – | 3.0 |
| Dicalcium phosphate | 1.7 | 1.7 | 1.7 | 1.7 | 2.5 |
| Limestone | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 |
| Sodium chloride | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Chromic oxide | – | – | 0.3 | 0.3 | 0.3 |
| Potassium carbonate | – | – | – | – | 0.3 |
| Magnesium oxide | – | – | – | – | 0.1 |
| Vitamin and mineral premix1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Analyzed nutrient level | |||||
| DM | 89.68 | 89.20 | 89.88 | 89.38 | 90.80 |
| CP | 7.68 | 7.76 | 7.69 | 7.75 | 0.48 |
| Ash | 3.60 | 3.62 | 3.77 | 3.81 | 5.02 |
| Ca | 0.68 | 0.65 | 0.68 | 0.65 | 0.78 |
| Total P | 0.55 | 0.57 | 0.55 | 0.57 | 0.54 |
| Indispensable AA | |||||
| Lys | 0.23 | 0.20 | 0.01 | ||
| Met | 0.17 | 0.16 | – | ||
| Thr | 0.25 | 0.26 | 0.01 | ||
| Trp | 0.06 | 0.07 | – | ||
| Val | 0.41 | 0.47 | 0.01 | ||
| Leu | 1.00 | 0.98 | 0.02 | ||
| Ile | 0.24 | 0.32 | 0.01 | ||
| Phe | 0.34 | 0.37 | 0.02 | ||
| His | 0.21 | 0.18 | 0.01 | ||
| Arg | 0.32 | 0.35 | 0.01 | ||
| Dispensable AA | |||||
| Tyr | 0.20 | 0.18 | 0.01 | ||
| Ser | 0.34 | 0.31 | 0.02 | ||
| Glu | 1.30 | 1.58 | 0.04 | ||
| Pro | 0.63 | 0.64 | 0.01 | ||
| Gly | 0.31 | 0.28 | 0.02 | ||
| Ala | 0.55 | 0.65 | 0.02 | ||
| Cys | 0.22 | 0.21 | 0.01 | ||
| Asp | 0.48 | 0.50 | 0.02 | ||
Vitamin and mineral premix provided the following per kilogram of diet: 12,000 IU vitamin A as vitamin A acetate, 2,500 IU vitamin D as vitamin D3, 30 IU vitamin E as DL-α-tocopheryl acetate, 12 μg vitamin B12, 3 mg vitamin K as menadione sodium bisulfate, 15 mg d-pantothenic acid as calcium pantothenate, 40 mg nicotinic acid, 400 mg choline as choline chloride, 30 mg Mn as manganese oxide, 80 mg Zn as zinc oxide, 90 mg Fe as iron sulfate, 10 mg Cu as copper sulfate, 0.35 mg I as ethylenediamine dihydroiodide, and 0.3 mg Se as sodium selenite.
All pigs were individually housed in stainless-steel metabolism crates (1.4 by 0.7 by 0.6 m) equipped with a feeder and a nipple drinker located in an environmentally controlled room with the temperature maintained at 22 ± 2°C. Barrows were provided ad libitum access to water and were fed an amount of feed each day equivalent to 4% of their BW determined at the beginning of the experiment. The daily feed was divided into 2 equal sized portions and provided at 0800 and 1600 h, and the amount of feed provided was recorded at each feeding time.
The experiment lasted 12 d, including 7 d for adaption to the diets and 5 d for the collection of feces and urine. During the collection period, feed refusals and spillage were collected twice daily and subsequently dried and weighed. Feces were collected from each pig as soon as they appeared in the metabolism crates and were immediately stored in plastic bags at −20°C. Urine was collected in buckets, containing 50 mL of 6 N HCl, located under the metabolism crates (Pan et al., 2016c). The volume of collected urine was measured each day, and 10% of the daily urinary collection was stored at −20°C. At the end of collection, feces and urine were separately thawed, pooled by pig, homogenized, and subsampled. Before chemical analysis, fecal subsamples were dried at 65°C in a drying oven for 72 h. Urine samples (4 mL) were dried at 65°C for 8 h with quantitative filter paper in crucibles (Li et al., 2015).
Experiment 2: Amino Acid Digestibility
This experiment was conducted to determine the AID and SID of CP and EAA and the standardized ileal digestible EAA composition in corn and sorghum. The experiment was conducted using 18 crossbred barrows (24.9 ± 1.4 kg BW) fitted with T-cannulas at the terminal ileum according to the method of Stein et al. (1998). The barrows were individually housed in stainless-steel metabolism crates (1.4 by 0.7 by 0.6 m) located in a temperature-controlled room (22 ± 2°C) and were fed 1 of 3 diets, with 6 barrows per diet, in a completely randomized design.
The N-free diet, containing 73.5% cornstarch and 15% sucrose, was used to determine basal ileal endogenous N losses (Chen et al., 2016), and the other 2 diets contained 96.6% corn or sorghum as the only source of dietary N (Table 2). Chromic oxide (0.3%) was included in all diets as an indigestible marker. Vitamins and minerals were supplemented in all diets to meet or exceed the estimated nutrient requirements for growing pigs (NRC, 2012). All pigs were fed at a daily level of 4% of BW. Two equal-sized meals were provided at 0800 and 1700 h each day.
After a full recovery period, the barrows were fed 1 of 3 diets for a 7-d period, consisting of a 5-d dietary acclimation period followed by a 2-d digesta collection, which lasted for 9 h daily beginning at 0800 h using the procedures described by Stein et al. (1998). On d 6 and 7, a plastic bag was attached to the barrel of the cannula. The bags were removed whenever they were filled with digesta or at least every 30 min and then stored at −20°C to prevent bacterial degradation of AA in the digesta (Pan et al., 2016b). At the end of the experiment, digesta samples were thawed, mixed by pig and period, subsampled, and lyophilized in a vacuum freeze-dryer (Tofflon Freezing Drying Systems, Minhang District, Shanghai, P.R. China).
Experiment 3: Growth Performance and Fecal Score of Weaned Pigs
This study evaluated the effects and optimum substitution level of the U.S. sorghum as a potential replacement for corn on growth performance, ATTD of nutrients, and fecal score in weaned pigs. A total of 144 healthy weaned pigs (8.8 ± 1.0 kg BW and 28 ± 3 d of age) were assigned to 4 treatments according to sex and weight in a randomized complete block design. Each treatment diet was fed to 6 replicate pens, with 6 pigs (3 barrows and 3 gilts) per pen.
The energy sources of 4 diets were mainly 60% corn, 40% corn and 20% sorghum, 20% corn and 40% sorghum, or 60% sorghum (Table 3). Extra EAA were incorporated into the diets based on the standardized ileal digestible EAA concentration derived from Exp. 2. All diets, with 0.25% chromic oxide added as an indigestible marker (Wu et al., 2017), were formulated to meet the nutrient requirements for pigs according to the NRC (2012).
Table 3.
Ingredient and nutrient contents of diets in Exp. 3 (%, as-fed basis)
| d 1 to 14 | d 15 to 28 | |||||||
|---|---|---|---|---|---|---|---|---|
| Corn:sorghum ratio | Corn:sorghum ratio | |||||||
| Item | 60:0 | 40:20 | 20:40 | 0:60 | 60:0 | 40:20 | 20:40 | 0:60 |
| Ingredient | ||||||||
| Corn | 60.0 | 40.0 | 20.0 | – | 60.0 | 40.0 | 20.0 | – |
| Sorghum | – | 20.0 | 40.0 | 60.0 | – | 20.0 | 40.0 | 60.0 |
| Soybean meal | 9.61 | 9.57 | 9.52 | 9.48 | 15.25 | 15.22 | 15.20 | 15.15 |
| Extruded soybean meal | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 |
| Spray-dried porcine plasma | 4.0 | 4.0 | 4.0 | 4.0 | – | – | – | – |
| Fish meal | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Whey powder | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Soybean oil | 2.9 | 2.9 | 2.9 | 2.9 | 1.6 | 1.6 | 1.6 | 1.6 |
| Dicalcium phosphate | 0.9 | 0.9 | 0.9 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 |
| Limestone | 1.0 | 1.0 | 1.0 | 1.0 | 0.8 | 0.8 | 0.8 | 0.8 |
| Salt | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| L-Lys HCl | 0.35 | 0.36 | 0.38 | 0.39 | 0.36 | 0.38 | 0.39 | 0.41 |
| DL-Met | 0.10 | 0.12 | 0.14 | 0.16 | 0.09 | 0.09 | 0.10 | 0.12 |
| L-Thr | 0.07 | 0.08 | 0.09 | 0.10 | 0.12 | 0.13 | 0.13 | 0.14 |
| L-Trp | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 |
| Chromic oxide | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin mineral premix1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Analyzed nutrient level | ||||||||
| DM | 89.8 | 89.9 | 89.7 | 90.2 | 89.4 | 89.7 | 89.7 | 89.9 |
| CP | 20.0 | 19.8 | 19.9 | 19.7 | 18.9 | 19.1 | 18.9 | 18.7 |
| Ash | 5.71 | 5.65 | 5.68 | 5.76 | 5.31 | 5.38 | 5.49 | 5.32 |
| Ca | 0.82 | 0.82 | 0.81 | 0.80 | 0.72 | 0.71 | 0.71 | 0.70 |
| Total P | 0.64 | 0.64 | 0.64 | 0.65 | 0.58 | 0.58 | 0.59 | 0.59 |
| Calculated nutrient level2 | ||||||||
| Standardized ileal digestible Lys | 1.35 | 1.35 | 1.35 | 1.35 | 1.23 | 1.23 | 1.23 | 1.23 |
| Standardized ileal digestible Met | 0.39 | 0.39 | 0.39 | 0.39 | 0.37 | 0.37 | 0.37 | 0.37 |
| Standardized ileal digestible Thr | 0.79 | 0.79 | 0.79 | 0.79 | 0.73 | 0.73 | 0.73 | 0.73 |
| Standardized ileal digestible Trp | 0.22 | 0.22 | 0.22 | 0.22 | 0.20 | 0.20 | 0.20 | 0.20 |
| ME, Mcal/kg | 3.40 | 3.40 | 3.40 | 3.40 | 3.35 | 3.35 | 3.35 | 3.35 |
Vitamin and mineral premix provided the following per kilogram of diet: 12,000 IU vitamin A as vitamin A acetate, 2,500 IU vitamin D as vitamin D3, 30 IU vitamin E as DL-α-tocopheryl acetate, 12 μg vitamin B12, 3 mg vitamin K as menadione sodium bisulfate, 15 mg d-pantothenic acid as calcium pantothenate, 40 mg nicotinic acid, 400 mg choline as choline chloride, 30 mg Mn as manganese oxide, 80 mg Zn as zinc oxide, 90 mg Fe as iron sulfate, 10 mg Cu as copper sulfate, 0.35 mg I as ethylenediamine dihydroiodide, and 0.3 mg Se as sodium selenite.
These values were calculated from data provided in Exp. 1 and 2.
Pigs were housed in pens with fully slatted floors, and all pigs had free access to feed and water throughout the 28-d experiment. The temperature of the barn was controlled between 23 and 28°C, and relative humidity was controlled at 60 to 70%. Pigs and feed were weighed at the beginning (d 1), mid stage (d 14), and end (d 28) to determine ADG, ADFI, and G:F. Piglets were observed for clinical signs of diarrhea every day (Pan et al., 2016b), and a scoring system was applied to indicate the presence and severity of diarrhea as following: 1 = hard feces; 2 = slightly soft feces; 3 = soft, partially formed feces; 4 = loose, semiliquid feces; and 5 = watery, mucous-like feces. From d 11 to 13 and from d 25 to 27, approximately 100 g of feces was collected from each pen for 3 d, and the fecal samples were stored at −20°C prior to being oven dried (Pan et al., 2017c). The 3-d collection of feces was pooled by pen and then dried at 65°C for 72 h.
Experiment 4: Effects of Protease on N Utilization
This study was conducted to determine effects of sorghum as a replacement for corn on growth performance in growing pigs and effects of protease on N utilization for growing pigs fed sorghum-based diets. Sixty growing pigs (23.4 ± 1.6 kg BW) were allotted to 3 diets with 5 replicate pens per treatment (2 barrows and 2 gilts per pen) according to sex and weight in a randomized complete block design.
The experiment lasted for 70 d, including phase 1 (d 1 to 35) and phase 2 (d 36 to 70). The 3 diets were based on corn and soybean meal (CSBM) or sorghum and soybean meal (SSBM) without or with 150 mg/kg protease supplementation (1.5 ± 0.4 units/g of diet). The energy sources of CSBM or SSBM mainly included 70.34% corn or 20% corn and 50% sorghum in phase 1 and 73.6% corn or 73.4% sorghum in phase 2 (Table 4). All diets, with 0.25% chromic oxide added as an indigestible marker, were formulated to meet or exceed the nutrient requirements for growing pigs (25 to 50 kg and 50 to 75 kg) according to the NRC (2012).
Table 4.
Ingredient and nutrient contents of diets in Exp. 4 (%, as-fed basis)1
| d 1 to 35 | d 36 to 70 | |||||
|---|---|---|---|---|---|---|
| SSBM | SSBM | |||||
| Item | CSBM | − | + | CSBM | − | + |
| Ingredient | ||||||
| Corn | 70.34 | 20.0 | 20.0 | 73.6 | – | – |
| Sorghum | – | 50.0 | 50.0 | – | 73.4 | 73.4 |
| Soybean meal | 24.0 | 24.0 | 24.0 | 21.0 | 21.0 | 21.0 |
| Wheat bran | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean oil | 0.55 | 0.55 | 0.55 | 0.43 | 0.43 | 0.43 |
| Dicalcium phosphate | 0.86 | 1.00 | 1.00 | 0.65 | 0.90 | 0.90 |
| Limestone | 0.98 | 0.85 | 0.85 | 0.96 | 0.77 | 0.77 |
| Sodium chloride | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lys HCl | 0.31 | 0.34 | 0.34 | 0.23 | 0.28 | 0.28 |
| DL-Met | 0.06 | 0.10 | 0.10 | 0.03 | 0.09 | 0.09 |
| L-Thr | 0.08 | 0.10 | 0.10 | 0.04 | 0.07 | 0.07 |
| L-Trp | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Chromic oxide | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin and mineral permix2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Analyzed nutrient level | ||||||
| DM | 88.7 | 88.9 | 89.0 | 88.7 | 89.1 | 89.0 |
| CP | 17.3 | 17.2 | 17.1 | 16.1 | 16.0 | 16.0 |
| Ash | 4.94 | 4.93 | 5.06 | 4.50 | 4.73 | 4.75 |
| Ca | 0.68 | 0.67 | 0.67 | 0.62 | 0.61 | 0.60 |
| Total P | 0.56 | 0.55 | 0.54 | 0.54 | 0.53 | 0.52 |
| Calculated nutrient level3 | ||||||
| Standardized ileal digestible Lys | 0.98 | 0.98 | 0.98 | 0.85 | 0.85 | 0.85 |
| Standardized ileal digestible Met | 0.28 | 0.28 | 0.28 | 0.24 | 0.24 | 0.24 |
| Standardized ileal digestible Thr | 0.59 | 0.59 | 0.59 | 0.52 | 0.52 | 0.52 |
| Standardized ileal digestible Trp | 0.17 | 0.17 | 0.17 | 0.15 | 0.15 | 0.15 |
| ME, Mcal/kg | 3.30 | 3.30 | 3.30 | 3.30 | 3.30 | 3.30 |
CSBM = corn and soybean meal; SSBM = sorghum and soybean meal; “−” = without protease; “+” = with 150 mg/kg protease supplementation (1.5 ± 0.4 units/g of diet).
During d 1 through 35, the vitamin and mineral premix provided the following per kilogram of diet: 6,000 IU vitamin A as vitamin A acetate, 2,400 IU vitamin D as vitamin D3, 21.6 IU vitamin E as DL-α-tocopheryl acetate, 2 mg vitamin K as menadione sodium bisulfate, 0.96 mg vitamin B1, 5.2 mg vitamin B2, 2 mg vitamin B6, 12 μg vitamin B12, 11.2 mg d-pantothenic acid as calcium pantothenate, 22 mg nicotinic acid, 400 mg choline as choline chloride, 0.4 mg folic acid, 40 μg biotin, 120 mg Fe as iron sulfate, 130 mg Cu as copper sulfate, 20 mg Mn as manganese oxide, 0.4 mg I as ethylenediamine dihydroiodide, and 0.3 mg Se as sodium selenite. During d 36 through 70, the vitamin and mineral premix provided the following per kilogram of diet: 5,600 IU vitamin A as vitamin A acetate, 2,200 IU vitamin D as vitamin D3, 21.6 IU vitamin E as DL-α-tocopheryl acetate, 1.8 mg vitamin K as menadione sodium bisulfate, 0.88 mg vitamin B1, 4 mg vitamin B2, 1.8 mg vitamin B6, 12 μg vitamin B12, 10 mg d-pantothenic acid as calcium pantothenate, 20 mg nicotinic acid, 320 mg choline as choline chloride, 0.4 mg folic acid, 40 μg biotin, 100 mg Fe as iron sulfate, 15 mg Cu as copper sulfate, 10 mg Mn as manganese oxide, 0.3 mg I as ethylenediamine dihydroiodide, and 0.3 mg Se as sodium selenite.
These values were calculated from data provided in Exp. 1 and 2.
During the experiment, pigs were housed in partially steel-slatted and concrete-floored pens (2.7 by 1.8 m). Each pen was equipped with a stainless steel self-feeder and a nipple drinker. Pigs had ad libitum access to diets and water. Pigs and feeders were weighed on d 1, 35, and 70 to calculate ADG, ADFI, and G:F. From d 32 to 34 and from d 67 to 69, approximately 200 g of feces was collected from each pen for 3 d, and the fecal samples were stored at −20°C prior to being oven dried (Pan et al., 2017). The 3-d collection of feces was pooled by pen and then dried at 65°C for 72 h.
Chemical Analysis and Calculations
All samples were ground to pass through a 1-mm screen and thoroughly mixed before analysis. All chemical analyses were conducted in duplicate. The chemical analyses of the ingredients, diets, and feces included DM (method 930.15; AOAC, 2006), CP (method 984.13; AOAC, 2006), ether extract (method 920.39; AOAC, 2006), ash (method 942.05; AOAC, 2006), Ca (method 968.08; AOAC, 2006), and P (method 946.06; AOAC, 2006). The NDF and ADF contents were determined using fiber bags (model F57; ANKOM Technology Corp., Macedon, NY) and a fiber analyzer (ANKOM200 Fiber Analyzer; ANKOM Technology Corp.) using the basic procedure of Van Soest et al. (1991) with heat-stable α-amylase and sodium sulfite and expressed inclusive of residual ash (Pan et al., 2014). The GE of ingredients, diets, feces, and urine were measured using an Automatic Isoperibol Oxygen Bomb Calorimeter (Parr 6300 Calorimeter; Parr Instrument Company, Moline, IL). Total starch was measured using method 76-13.01 of the American Association of Cereal Chemists (1976), conducted using a commercial Starch Assay Kit (STA20; Sigma-Aldrich Corp., St. Louis, MO). The sorghum and corn grains were also analyzed for prolamin (Hamaker et al., 1995), tannin (Price et al., 1978), phytate (Skoglund et al., 1997a,b), and total phenols by the Folin–Ciocalteu method (Kaluza et al., 1980). Total phenols were calculated and expressed as grams gallic acid equivalent/100-g sample. Amino acid analysis in the lab was detailed by Pan et al. (2016b). The chromium content in the diets and feces was measured using an Atomic Absorption Spectrophotometer (Z-5000; Hitachi, Ltd., Tokyo, Japan) according to the procedure of Williams et al. (1962).
Calculations for DE and ME in corn and sorghum grains (Exp. 1) were detailed by Pan et al. (2016a). Gross energy intake was calculated as the product of the GE content of the diet and the actual feed intake over the 5-d collection period. The energy lost in feces and urine was measured for each diet, and the DE and ME contents of the diets were calculated. The DE and ME contents in the diets were divided by 0.969 to calculate the DE and ME values in the corresponding ingredient samples. The ATTD of nutrients in each diet was calculated according to the procedures described by Adeola (2001): ATTD (%) = 100% × (GEi − GEf)/GEi, in which GEi is the total GE intake of each pig (kcal of DM) calculated as the product of the GE of the diet over the actual feed intake during the 5-d collection period and GEf is the GE content in feces of each pig (kcal of DM) calculated as the GE content of the feces over the dry weight of total feces obtained during the 5-d collection period.
The AID and SID of AA and CP (Exp. 2) were calculated as described by Stein et al. (2007) using the following equation: AID (%) = [1 − (AAd/AAf) × (Crf/Crd)] × 100%, in which AAd and Crd are the concentrations of AA and Cr, respectively, in the ileal digesta (g/kg of DM) and AAf and Crf are the concentrations of AA and Cr, respectively, in the test diets (g/kg of DM). The AID of CP was calculated using the equation shown above. The endogenous loss of N for each AA was measured from pigs fed the N-free diet based on the following equation: IAAend = [AAd × (Crf/Crd)], in which IAAend is the basal endogenous loss of an AA (g/kg of DM intake) and AAd and Crd represent the concentrations of AA and Cr, respectively, in the ileal digesta from the pigs fed the N-free diet. The Cr concentration in the N-free diet is represented by Crf. The endogenous loss of CP was determined using the same equation. The average IAAend for the 6 pigs fed the N-free diet was used to calculate the SID of AA in all diets. The SID value was calculated using the following equation: SID (%) = [AID + (IAAend/AAf) × 100%]. The SID value of each AA was multiplied by the concentration of AA (DM basis) to calculate the concentration of standardized ileal digestible AA (Pan et al., 2016c).
The ATTD of nutrients (Exp. 3 and 4) and estimated manure N output (Exp. 4) were calculated as described by Pan et al. (2017). Nutrient digestibility was determined by the equation as follows: ATTD of nutrient (%) = [1 − (Crdiet × nutrientfeces)/(Crfeces × nutrientdiet)] × 100%, in which, Crdiet or Crfeces represent the concentrations of Cr in the diets or feces and nutrientfeces or nutrientdiet represent the concentrations of nutrient in the diets or feces. Fecal N excretion per weight gain (g/kg) = [N intake (g/d) × (100 − ATTD of N)]/[100 × ADG (kg/d)].
Statistical Analysis
Data in Exp. 1 and 2 were analyzed using SAS (version 9.2; SAS Inst. Inc., Cary, NC) with a Student's t-test for unpaired data, with individual pig as an experimental unit. Performance and nutrient digestibility in Exp. 3 and 4 were analyzed with each pen as the experimental unit using GLM procedures of SAS followed by Tukey's multiple range tests. Differences in the fecal score were tested by the χ2 contingency test. Treatment means were calculated using the LSMEANS statement. Statistically significant differences were declared at P < 0.05, and differences at 0.05 ≤ P < 0.10 were considered a trend toward significance.
RESULTS
Energy Concentration in Exp. 1
There was no difference in DE and ME or ATTD of GE and OM between corn and sorghum grains (Table 5). The ATTD of CP in corn grain was greater than in sorghum grain (P < 0.05).
Table 5.
Concentration of DE and ME content (MJ/kg, DM basis) and apparent total tract digestibility (ATTD) of nutrients (%) in corn and sorghum grains (Exp. 1)1
| Item | Corn | Sorghum | SEM | P-value |
|---|---|---|---|---|
| DE | 16.36 | 16.35 | 0.06 | 0.95 |
| ME | 16.10 | 16.09 | 0.04 | 0.99 |
| ATTD of OM | 90.28 | 91.20 | 0.36 | 0.12 |
| ATTD of GE | 87.90 | 88.06 | 0.30 | 0.71 |
| ATTD of CP | 77.79 | 73.72 | 1.01 | 0.04 |
Values are the means of 6 observations per grain.
Digestible CP and Essential AA in Exp. 2
The AID and SID of CP and most EAA, with the exception of Val, Leu, and Phe, were greater (P < 0.05) in corn than in sorghum (Table 6). There was no difference in total standardized ileal digestible EAA between the 2 grains, whereas the concentration of standardized ileal digestible CP in sorghum was lower than in corn (P < 0.05). The concentrations of standardized ileal digestible Lys, Met, Thr, and His were lower in sorghum (P < 0.05) whereas concentrations of standardized ileal digestible Val and Ile were greater than in corn (P < 0.05).
Table 6.
Apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of CP and essential AA (EAA) and concentration of Standardized ileal digestible CP and EAA in corn and sorghum grains (DM basis, Exp. 2)1
| Item | Corn | Sorghum | SEM | P-value |
|---|---|---|---|---|
| AID value, % | ||||
| CP | 59.48 | 54.07 | 1.76 | 0.04 |
| Lys | 58.40 | 50.62 | 1.68 | 0.03 |
| Met | 83.68 | 73.02 | 1.48 | <0.01 |
| Thr | 56.98 | 51.66 | 1.32 | 0.04 |
| Trp | 58.22 | 54.98 | 1.05 | 0.04 |
| Val | 72.34 | 78.13 | 2.13 | 0.10 |
| Leu | 75.48 | 71.95 | 1.75 | 0.18 |
| Ile | 69.65 | 52.26 | 2.05 | <0.01 |
| Phe | 64.88 | 63.45 | 2.02 | 0.82 |
| His | 62.65 | 54.15 | 1.85 | 0.02 |
| Arg | 69.56 | 61.53 | 2.22 | 0.04 |
| SID value, % | ||||
| CP | 72.48 | 65.42 | 2.26 | 0.04 |
| Lys | 74.80 | 66.39 | 1.80 | 0.03 |
| Met | 89.88 | 79.17 | 1.56 | <0.01 |
| Thr | 79.82 | 74.54 | 1.32 | 0.04 |
| Trp | 85.22 | 70.53 | 2.50 | <0.01 |
| Val | 85.34 | 87.25 | 1.45 | 0.78 |
| Leu | 82.48 | 81.95 | 1.55 | 0.69 |
| Ile | 81.56 | 73.26 | 1.78 | 0.01 |
| Phe | 75.88 | 72.68 | 2.02 | 0.74 |
| His | 82.86 | 77.08 | 1.38 | 0.04 |
| Arg | 86.68 | 78.99 | 2.46 | 0.04 |
| Concentration, g/kg | ||||
| CP | 65.21 | 59.57 | 1.89 | 0.04 |
| Lys | 1.95 | 1.59 | 0.08 | 0.03 |
| Met | 1.83 | 1.45 | 0.05 | <0.01 |
| Thr | 2.63 | 2.55 | 0.03 | 0.04 |
| Trp | 0.58 | 0.56 | 0.05 | 0.25 |
| Val | 3.87 | 4.48 | 0.07 | 0.01 |
| Leu | 9.72 | 9.45 | 0.08 | 0.05 |
| Ile | 2.31 | 2.76 | 0.08 | 0.04 |
| Phe | 3.10 | 3.23 | 0.07 | 0.32 |
| His | 2.16 | 1.76 | 0.06 | <0.01 |
| Arg | 3.04 | 3.06 | 0.04 | 0.78 |
| Total | 31.18 | 30.90 | 0.56 | 0.54 |
Values for SID were calculated by correcting AID values with the basal endogenous losses. Basal endogenous losses averaged 20.90 g CP/kg DMI, 0.59 g Lys/kg DMI, 0.08 g Met/kg DMI, 0.67 g Thr/kg DMI, 0.14 g Trp/kg DMI, 0.51 g Val/kg DMI, 0.23 g Leu/kg DMI, 0.69 g Ile/kg DMI, 0.55 g Phe/kg DMI, 0.37 g His/kg DMI, and 0.95 g Arg/kg DMI.
Growth Performance, Apparent Total Tract Digestibility of Nutrients, and Fecal Score of Weaned Pigs in Exp. 3
There was no difference in ADG and ADFI among the treatments during the experimental period (Table 7). The G:F and the ATTD of CP was decreased for pigs fed diets with sorghum in the first 2 wk (P < 0.05) and for pigs fed diets containing 60% sorghum in the following 2 wk (P < 0.05). The ATTD of DM (P = 0.066), OM (P = 0.082), and GE (P = 0.088) tended to decrease for pigs fed diets with sorghum on d 14. The fecal score for pigs fed diets with sorghum, regardless of the substitute level, was less (P < 0.05) or tended to be less (P = 0.086) than that for pigs fed diets containing 60% corn.
Table 7.
Effects of corn replacement with sorghum in diets fed to weaned pigs on growth performance and apparent total tract digestibility (ATTD) of nutrients (Exp. 3)1
| Corn:sorghum ratio | ||||||
|---|---|---|---|---|---|---|
| Item | 60:0 | 40:20 | 20:40 | 0:60 | SEM | P-value |
| Performance | ||||||
| d 1 to 14 | ||||||
| ADG, g | 379 | 362 | 358 | 350 | 13.47 | 0.83 |
| ADFI, g | 568 | 551 | 549 | 536 | 18.60 | 0.70 |
| G:F | 0.668a | 0.655b | 0.652b | 0.651b | 0.003 | 0.04 |
| Fecal score | 2.94a | 2.84b | 2.83b | 2.85b | – | 0.04 |
| d 15 to 28 | ||||||
| ADG, g | 474 | 472 | 460 | 454 | 14.29 | 0.89 |
| ADFI, g | 817 | 816 | 802 | 798 | 33.76 | 0.93 |
| G:F | 0.581a | 0.579a | 0.573ab | 0.567b | 0.004 | 0.03 |
| Fecal score | 2.86 | 2.80 | 2.79 | 2.81 | – | 0.086 |
| d 1 to 28 | ||||||
| ADG, g | 428 | 415 | 408 | 401 | 11.08 | 0.84 |
| ADFI, g | 693 | 684 | 676 | 663 | 23.09 | 0.84 |
| G:F | 0.618a | 0.609ab | 0.605b | 0.603b | 0.004 | 0.04 |
| Fecal score | 2.90a | 2.82b | 2.82b | 2.83b | – | 0.02 |
| ATTD of nutrients, % | ||||||
| d 14 | ||||||
| DM | 82.47 | 80.94 | 80.92 | 80.62 | 0.57 | 0.066 |
| OM | 85.89 | 84.52 | 84.46 | 84.14 | 0.50 | 0.082 |
| GE | 83.46 | 82.09 | 82.13 | 81.78 | 0.53 | 0.088 |
| CP | 79.08a | 76.87b | 74.89c | 74.76c | 0.74 | 0.01 |
| d 28 | ||||||
| DM | 81.01 | 81.46 | 81.46 | 81.37 | 0.41 | 0.83 |
| OM | 84.48 | 84.98 | 84.88 | 84.79 | 0.35 | 0.75 |
| GE | 81.56 | 82.33 | 81.83 | 81.81 | 0.44 | 0.66 |
| CP | 76.67a | 77.39a | 76.28a | 73.80b | 0.69 | 0.01 |
a–cLeast squares means within a row with different superscripts differ (P < 0.05).
Values are least squares means for 6 pens per treatment.
Growth Performance of Growing Pigs and Effects of Protease on N Utilization in Exp. 4
No differences were observed in ADG and ADFI overall among the treatments (Table 8). Pigs fed CSBM or SSBM with protease had greater (P < 0.05) or tended to have greater (P = 0.062) G:F than pigs fed SSBM. The ATTD of DM, OM, and GE in CSBM tended to be greater on d 35 (P = 0.066, P = 0.058, and P = 0.052, respectively) and was greater on d 70 (P < 0.05) than that in SSBM.
Table 8.
Growth performance and apparent total tract digestibility (ATTD) of nutrients of growing pigs fed diets based on corn and soybean meal (CSBM) or sorghum and soybean meal (SSBM) supplemented without (−) or with (+) 150 mg/kg protease (1.5 ± 0.4 units/g of diet; Exp. 4)1
| SSBM | |||||
|---|---|---|---|---|---|
| Item | CSBM | − | + | SEM | P-value |
| Performance | |||||
| d 1 to 35 | |||||
| ADG, g | 738 | 730 | 756 | 15.7 | 0.36 |
| ADFI, g | 1,546 | 1,576 | 1,538 | 39.7 | 0.58 |
| G:F | 0.481a | 0.462b | 0.489a | 0.005 | 0.03 |
| d 36 to 70 | |||||
| ADG, g | 789 | 810 | 815 | 18.6 | 0.68 |
| ADFI, g | 2,316 | 2,454 | 2,333 | 64.2 | 0.26 |
| G:F | 0.342 | 0.327 | 0.344 | 0.006 | 0.062 |
| d 1 to 70 | |||||
| ADG, g | 758 | 769 | 784 | 15.4 | 0.52 |
| ADFI, g | 1,926 | 2,008 | 1,939 | 53.4 | 0.48 |
| G:F | 0.395 | 0.379 | 0.400 | 0.006 | 0.058 |
| ATTD of nutrients, % | |||||
| d 35 | |||||
| DM | 83.46 | 81.70 | 82.70 | 0.64 | 0.066 |
| OM | 85.30 | 83.70 | 84.70 | 0.55 | 0.058 |
| GE | 82.90 | 80.80 | 82.40 | 0.62 | 0.052 |
| CP | 75.04a | 69.60b | 74.26a | 1.32 | 0.04 |
| d 70 | |||||
| DM | 84.60a | 82.60b | 84.30a | 0.42 | 0.03 |
| OM | 86.32a | 84.70b | 86.32a | 0.50 | 0.04 |
| GE | 83.50a | 81.50b | 83.06a | 0.45 | 0.04 |
| CP | 79.72a | 68.60c | 75.60b | 1.28 | <0.01 |
a–cLeast squares means within a row with different superscripts differ (P < 0.05).
Values are least squares means for 5 pens per treatment.
Compared with CSBM, SSBM increased (P < 0.05) fecal N excretion per weight gain by more than 25% and decreased (P < 0.05) ATTD of CP by more than 7% during the whole experiment (Fig. 1). Protease supplementation reduced (P < 0.05) fecal N excretion by more than 12% and increased (P < 0.05) ATTD of CP by more than 6%.
Figure 1.
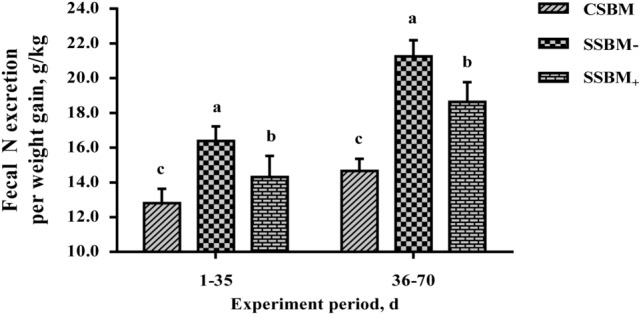
Manure N output from growing pigs fed diets based on corn and soybean meal (CSBM) or sorghum and soybean meal (SSBM) supplemented without (SSBM−) or with 150 mg/kg protease (1.5 ± 0.4 units/g of diet; SSBM+) during 70 d period experiment (Exp. 4). Values are least squares means ± SEM; n = 5/treatment. a–cMeans with different superscripts significantly differ (P < 0.05).
DISCUSSION
As expected, the sorghum grain imported from the United States had low tannin concentration and had a chemical composition that was not different from corn grown in China, which was in line with the published results (NRC, 2012; Pan et al., 2016a; Stein et al., 2016; Xu et al., 2017). However, the nutritional value of sorghum is generally considered to be only 95% of that of corn (Hancock, 2000), despite the chemical composition of sorghum being similar to or even better than corn (Liu et al., 2013a). The concentration of starch and AA in sorghum is greater (Jaworski et al., 2015) but the digestibility was lower than in maize (Pedersen et al., 2007; Cervantes-Pahm et al., 2014a,b). Therefore, it is necessary to accurately evaluate DE and ME content (Exp. 1) and standardized ileal digestible AA concentration (Exp. 2) of corn and sorghum for formulating similar diets to compare the growth performance of weaned (Exp. 3) and growing pigs (Exp. 4) fed corn- or sorghum-based diets.
The DE and ME of corn and sorghum were close to the published values (NRC, 2012; Cervantes-Pahm et al., 2014a; Li et al., 2014; Pan et al., 2016a). As hypothesized, DE and ME values of U.S. sorghum were nearly equal to those of the corn produced in China, which was in agreement with reports that the concentration of GE in sorghum is similar to that in corn, and this is also the case for the concentration of DE and ME content (Cervantes-Pahm et al., 2014a; Bolarinwa and Adeola, 2016). The lack of a difference in apparent digestibility of DM, GE, and OM between the 2 grains is also in accordance with the published results (Cervantes-Pahm et al., 2014a; Lowell et al., 2015). It indicates that U.S. sorghum could replace Chinese corn as an energy source in term of the effective energy in this study. As a result, to compare effects of 2 different energy sources (corn or sorghum) on growth performance of weaned or growing pigs (Exp. 3 and 4), sorghum directly replaced corn by different proportions without any additional energy supplementation in the diet formulation based on the similar DE content (Exp. 1).
The concentrations of AA in corn and sorghum used in the present experiment are within the range of published values (Pedersen et al., 2007; NRC, 2012; Stein et al., 2016). The AID and SID values obtained for corn and sorghum were less than values reported by the NRC (2012) but close to those for most EAA reported by Cervantes-Pahm et al. (2014b) and Stein et al. (2016). The variation may be the results of differences in cultivar, growing conditions, and antinutritional factors in grains (Fuller et al., 1989; Mariscal-Landín et al., 2004). The AID and SID values for corn were greater than those for sorghum, which were in line with published values (Pedersen et al., 2007; Stein et al., 2016). Amino acid digestibility in sorghum is negatively influenced by many factors including tannin (Jansman, 1993; Mariscal-Landín et al., 2004; Liu et al., 2013b), polyphenols (Liu et al., 2013c, 2016), kafirin (Selle et al., 2010; Liu et al., 2013a), phytate (Selle et al., 2003; Stein et al., 2016), and fiber (Bach Knudsen and Munck, 1985; Jaworski et al., 2015).
By taking the ileal digestibility of AA into consideration, a lack of available AA in compound feeds may be prevented while avoiding an excessive protein intake (Stein et al., 2007). Actually, the concentration of AA differs among cereal grains; therefore, the concentration of standardized ileal digestible AA may be more practical. The standardized ileal digestible AA concentration represents the amount of AA in the cereal grain that is assumed to be available for protein synthesis after absorption from the intestinal tract. The low contribution of standardized ileal digestible Lys, Met, and Thr from sorghum indicates that greater protein or AA supplementation is needed when sorghum is used in diets than when corn is being used. The results also support the observation that more protein supplementation is required where sorghum is the major source of energy and protein in diets (Bwibo and Neumann, 2003). Therefore, more crystalline Lys, Met, and Thr were incorporated into diets based on sorghum than diets based on corn to meet the standardized ileal digestible EAA requirements for pigs in Exp. 3 and 4.
Sorghum may be used as the sole cereal grain to replace corn in diets fed to pigs without reducing the growth performance of weaned (Hongtrakul et al., 1998; Sotak et al., 2014; Woyengo et al., 2014), growing (Shelton et al., 2004), or finishing pigs (Benz et al., 2011; Paulk et al., 2015). In these studies, complete or partial substitution of corn with U.S. sorghum did not affect the feed intake, suggesting that the inclusion of sorghum had no effect on the palatability of the diets fed to weaned or growing pigs. There was no difference in ADG of weaned or growing pigs fed corn- and sorghum-based diets, which was in agreement with published data (Sotak et al., 2014; Paulk et al., 2015). Taking only the ADG and ADFI into consideration, sorghum may be equivalent to corn for weaned or growing pigs without negative effects on growth performance.
However, substituting sorghum for corn in diets reduced or tended to reduce G:F, for both weaned and growing pigs, especially for weaned pigs in the first 2 wk after weaning. In spite of no difference in ADG, a significant decrease in G:F was found in growing pigs fed SSBM relative to those fed CSBM (Shelton et al., 2004). The decrease in G:F was consistent with the decrease in nutrient digestibility, especially ATTD of CP, and the decrease in G:F and nutrient digestibility could be offset partially by protease supplementation in sorghum-based diets fed to growing pigs. Given the G:F and ATTD of CP in weaned pigs in the present study, the optimum inclusion of U.S. sorghum in diets should be less than 20% during the first 2 wk and no more than 40% during subsequent 2 wk after weaning.
Sorghum grain is not the preferred feed ingredient for pigs because of the poor digestibility of its protein in nonruminant animals (Selle et al., 2010). As shown in these 4 studies, the CP digestibility was lower for pigs fed diets based on sorghum than for pigs fed diets based on corn. As reviewed by Duodu et al. (2003), the poor protein digestibility in sorghum is due to an array of exogenous (grain structure, polyphenols, phytate, and cell wall components) and endogenous (disulfide crosslinking, kafirin hydrophobicity, and protein secondary structure) factors. Therefore, more work needs to be done to address the decreased CP digestibility in sorghum-based diets according to the corresponding factors.
In pigs, the decreased CP digestibility in sorghum-based diets would result in surplus N emission from animal manure (Yin et al., 1993; Yin, 1994; Pan et al., 2017), which may damage the natural environment (Pan et al., 2016c; Loss et al., 2017; Monteiro et al., 2017). Protease has been confirmed to improve N digestibility in monogastric animals (Piao et al., 1999; Liu et al., 2013a; Yu et al., 2016). Similarly, in this study, protease significantly increased the ATTD of CP by more than 6% and reduced fecal N excretion by more than 12%, which were similar to the values reported in recent studies that reported that proteases increased the ATTD of CP by more than 8% and decreased the fecal N excretion by more than 10% (Pan et al., 2017). Therefore, protease could improve N utilization by offsetting the increase in fecal N output for growing pigs fed sorghum-based diets, and supplementing with protease may be a potential way to address the low N utilization in sorghum-based diets.
It was interesting that the energy digestibility responded to the protease, which was in accordance with similar reports that exogenous proteases could improve the protein and energy utilization in sorghum (Liu et al., 2013c; Selle et al., 2013; Pan et al., 2017; Xu et al., 2017). It may be related to the unique structure of protein bodies that the kafirin protein fraction could impede protein and energy utilization because of its inherent hydrophobicity and disulfide cross-linking (Duodu et al., 2003; Salinas et al., 2006; Selle et al., 2010; Liu et al., 2013c). It follows that any improvements in protein digestibility may indirectly enhance the digestion of energy in sorghum (Selle et al., 2010; Liu et al., 2013c). Results indicated that protease supplementation with the capacity to degrade kafirin may be beneficial to energy utilization. The improved ATTD of nutrients perhaps resulted in the trend toward the increased G:F for pigs fed sorghum-based diets supplemented with protease.
The fecal score reflected the clinical signs of diarrhea, and the higher the fecal score is, the worse the diarrhea (Pan et al., 2016b). The fecal score was decreased in diets with inclusion of sorghum, irrespective of the substitute proportion. Unfortunately, few comparable studies have been completed to compare effects of diets based on corn and sorghum on fecal scores in weaned pigs. The exact mechanism is unclear, and it may be associated with the polyphenols in sorghum grain. Polyphenol extracts differ in properties, and some may reduce Escherichia coli–induced diarrhea for postweaning pigs, most likely by inactivating labile toxins (Verhelst et al., 2010, 2014). Polyphenol-containing plants are widely used as additives in various feed or food products because of their antioxidative (Lu et al., 2011) and antibacterial properties (Gordon and Wareham, 2010). Furthermore, plant polyphenols have been reported to inactivate enterotoxin in vitro (Morinaga et al., 2005). In clinical medicine, a sorghum-based oral rehydration solution may be used as an alternative in the treatment of diarrhea (Pelleboer et al., 1990; Mustafa et al., 1995). Even so, further studies are needed to verify the positive effect and to determine if sorghum may provide a new approach to alleviating diarrhea in weaned pigs.
In conclusion, to meet dietary requirements for EAA in pigs, diets based on sorghum require more EAA supplementation, especially Lys, Met, and Thr, than diets based on corn in these studies, although sorghum has a DE and ME content and total standardized ileal digestible EAA composition similar to that of corn. Corn may be substituted with sorghum in diets fed to weaned or growing pigs without negative effects on growth performance. The inclusion of sorghum in diets may alleviate diarrhea in weaned pigs, but given the optimal G:F and N digestibility, less than 20% sorghum during the first 2 wk and no more than 40% during the subsequent 2 wk after weaning is recommended. Sorghum used as an alternative energy source for corn in growing pig diets could increase manure N output and thus decrease N utilization, and this could be addressed, at least partially, by protease supplementation. In terms of only one source of sorghum and corn used in these studies and the variability among different sources of the same cereal grains, further investigation is warranted to confirm the above results.
Footnotes
Financial support was received from the National Natural Science Foundation (numbers 31372316 and 31772612) and the 111 Project (B16044).
LITERATURE CITED
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis J., Southern L. L. editors, Swine nutrition. CRC Press, Washington, DC: p. 903–916. [Google Scholar]
- American Association of Cereal Chemists 1976. Approved methods of analysis. American Association of Cereal Chemists, St. Paul, MN. [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed.AOAC Int., Arlington, VA. [Google Scholar]
- Awika J. M., Rooney L. W. 2004. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 65:1199–1221. doi: 10.1016/j.phytochem.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K. E., Munck L. 1985. Dietary fibre contents and compositions of sorghum and sorghum-based foods. J. Cereal Sci. 3:153–164. doi: 10.1016/S0733-5210(85)80025-4 [DOI] [Google Scholar]
- Benz J. M., Tokach M. D., Dritz S. S., Nelssen J. L., DeRouchey J. M., Sulabo R. C., Goodband R. D. 2011. Effects of increasing choice white grease in corn- and sorghum-based diets on growth performance, carcass characteristics, and fat quality characteristics of finishing pigs. J. Anim. Sci. 89:773–782. doi: 10.2527/jas.2010-3033 [DOI] [PubMed] [Google Scholar]
- Bolarinwa O. A., Adeola O. 2016. Regression and direct methods do not give different estimates of digestible and metabolizable energy values of barley, sorghum and wheat for pigs. J. Anim. Sci. 94:610–618. doi: 10.2527/jas.2015-9766 [DOI] [PubMed] [Google Scholar]
- Bwibo N. O., Neumann C. G. 2003. The need for animal source foods by Kenyan children. J. Nutr. 133:3936S–3940S. [DOI] [PubMed] [Google Scholar]
- Cervantes-Pahm S. K., Liu Y., Stein H. H. 2014a. Comparative digestibility of energy and nutrients and fermentability of dietary fibre in eight cereal grains fed to pigs. J. Sci. Food Agric. 94:841–849. doi: 10.1002/jsfa.6316 [DOI] [PubMed] [Google Scholar]
- Cervantes-Pahm S. K., Liu Y., Stein H. H. 2014b. Digestible indispensable amino acid score and digestible amino acids in eight cereal grains. Br. J. Nutr. 111:1663–1672. doi: 10.1017/S0007114513004273 [DOI] [PubMed] [Google Scholar]
- Chen Y. F., Wu F., Li P. L., Lyu Z. Q., Liu L., Lyu M. B., Wang F. L., Lai C. H. 2016. Energy content and amino acid digestibility of flaxseed expellers fed to growing pigs. J. Anim. Sci. 94:5295–5307. doi: 10.2527/jas.2016-0578 [DOI] [PubMed] [Google Scholar]
- Diao X. M. 2017. Production and genetic improvement of minor cereals in China. Crop J. 5:103–114. doi: 10.1016/j.cj.2016.06.004 [DOI] [Google Scholar]
- Duodu K. G., Taylor J. R. N., Belton P. S., Hamaker B. R. 2003. Factors affecting sorghum protein digestibility. J. Cereal Sci. 38:117–131. doi: 10.1016/S0733-5210(03)00016-X [DOI] [Google Scholar]
- Fuller M. F., Cadenhead A., Brown D. S., Brewer A. C., Carver M., Robinson R. 1989. Varietal differences in the nutritive value of cereal grains for pigs. J. Agric. Sci. 113:149–163. doi: 10.1017/S0021859600086706 [DOI] [Google Scholar]
- Gordon N. C., Wareham D. W. 2010. Antimicrobial activity of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against clinical isolates of Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents 36:129–131. doi: 10.1016/j.ijantimicag.2010.03.025 [DOI] [PubMed] [Google Scholar]
- Hamaker B. R., Mohamed A. A., Habben J. E., Huang C. P., Larkins B. A. 1995. Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method. Cereal Chem. 72:583–588. [Google Scholar]
- Hancock J. D. 2000. Value of sorghum and sorghum co-products in diets for livestock. In: Smith C. W., Frederiksen R. A. editors, Sorghum: Origin, history, technology, and production. John Wiley & Sons., New York, NY: p. 731–749. [Google Scholar]
- Hongtrakul K., Goodband R. D., Behnke K. C., Nelssen J. L., Tokach M. D., Bergström J. R., Nessmith W. B., Jr, Kim I. H. 1998. The effects of extrusion processing of carbohydrate sources on weanling pig performance. J. Anim. Sci. 76:3034–3042. doi: 10.2527/1998.76123034x [DOI] [PubMed] [Google Scholar]
- Jansman A. J. M. 1993. Tannins in feedstuffs for simple-stomached animals. Nutr. Res. Rev. 6:209–236. [DOI] [PubMed] [Google Scholar]
- Jaworski N. W., Lærke H. N., Bach Knudsen K. E., Stein H. H. 2015. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J. Anim. Sci. 93:1103–1113. doi: 10.2527/jas.2014-8147 [DOI] [PubMed] [Google Scholar]
- Kaluza W. Z., McGrath R. M., Roberts T. C., Schröder H. H. 1980. Separation of phenolics of Sorghum bicolor (L.) Moench grain. J. Agric. Food Chem. 28:1191–1196. doi: 10.1021/jf60232a039 [DOI] [Google Scholar]
- Khoddami A., Truong H. H., Liu S. Y., Roberts T. H., Selle P. H. 2015. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Anim. Feed Sci. Technol. 210:190–199. doi: 10.1016/j.anifeedsci.2015.09.029 [DOI] [Google Scholar]
- Li P., Li D. F., Zhang H. Y., Li Z. C., Zhao P. F., Zeng Z. K., Xu X., Piao X. S. 2015. Determination and prediction of energy values in corn distillers dried grains with solubles sources with varying oil content for growing pigs. J. Anim. Sci. 93:3458–3470. doi: 10.2527/jas.2014-8782 [DOI] [PubMed] [Google Scholar]
- Li Q. F., Zang J. J., Liu D. W., Piao X. S., Lai C. H., Li D. F. 2014. Predicting corn digestible and metabolizable energy content from its chemical composition in growing pigs. J. Anim. Sci. Biotechnol. 5:11–18. doi: 10.1186/2049-1891-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Selle P. H., Court S. G., Cowieson A. J. 2013a. Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion. Anim. Feed Sci. Technol. 183:175–183. doi: 10.1016/j.anifeedsci.2013.05.006 [DOI] [Google Scholar]
- Liu S. Y., Selle P. H., Cowieson A. J. 2013b. Influence of white- and red-sorghum varieties and hydrothermal component of steam-pelleting on digestibility coefficients of amino acids and kinetics of amino acids, nitrogen and starch digestion in diets for broiler chickens. Anim. Feed Sci. Technol. 186:53–63. doi: 10.1016/j.anifeedsci.2013.08.006 [DOI] [Google Scholar]
- Liu S. Y., Selle P. H., Cowieson A. J. 2013c. Strategies to enhance the performance of pigs and poultry on sorghum-based diets. Anim. Feed Sci. Technol. 181:1–14. doi: 10.1016/j.anifeedsci.2013.01.008 [DOI] [Google Scholar]
- Liu S. Y., Truong H. H., Khoddami A., Moss A. F., Thomson P. C., Roberts T. H., Selle P. H. 2016. Comparative performance of broiler chickens offered ten equivalent diets based on three grain sorghum varieties as determined by response surface mixture design. Anim. Feed Sci. Technol. 218:70–83. doi: 10.1016/j.anifeedsci.2016.05.008 [DOI] [Google Scholar]
- Loss A., Lourenzi C. R., Junior E. S., Junior C. A. M., Benedet L., Pereira M. G., Piccolo M. C., Brunetto G., Lovato P. E., Comin J. J. 2017. Carbon, nitrogen and natural abundance of 13C and 15N in biogenic and physicogenic aggregates in a soil with 10 years of pig manure application. Soil Tillage Res. 166:52–58. doi: 10.1016/j.still.2016.10.007 [DOI] [Google Scholar]
- Lowell J. E., Liu Y. H., Stein H. H. 2015. Comparative digestibility of energy and nutrients in diets fed to sows and growing pigs. Arch. Anim. Nutr. 69:79–97. doi: 10.1080/1745039X.2015.1013664 [DOI] [PubMed] [Google Scholar]
- Lu N., Chen P., Yang Q., Peng Y. Y. 2011. Anti- and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxicol. In Vitro 25:833–838. doi: 10.1016/j.tiv.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Mariscal-Landín G., Avellaneda J. H., Reis de Souza T. C., Aguilera A., Borbolla G. A., Mar B. 2004. Effect of tannins in sorghum on amino acid ileal digestibility and on trypsin (E.C.2.4.21.4) and chymotrypsin (E.C.2.4.21.1) activity of growing pigs. Anim. Feed Sci. Technol. 117:245–264. doi: 10.1016/j.anifeedsci.2004.09.001 [DOI] [Google Scholar]
- Monteiro A. N. T. R., Bertol T. M., de Oliveira P. A. V., Dourmad J. Y., Coldebella A., Kessler A. M. 2017. The impact of feeding growing-finishing pigs with reduced dietary protein levels on performance, carcass traits, meat quality and environmental impacts. Livest. Sci. 198:162–169. doi: 10.1016/j.livsci.2017.02.014 [DOI] [Google Scholar]
- Morinaga N., Iwamaru Y., Yahiro K., Tagashira M., Moss J., Noda M. 2005. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 280:23303–23309. doi: 10.1074/jbc.M502093200 [DOI] [PubMed] [Google Scholar]
- Mustafa S. A., Karrar Z. E., Suliman J. I. 1995. Cereal-based oral rehydration solutions in Sudanese children with diarrhoea: A comparative clinical trial of rice-based and sorghum-based oral rehydration solutions. Ann. Trop. Paediatr. 15:313–319. doi: 10.1080/02724936.1995.11747791 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 10th rev. ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pan L., Bu D. P., Wang J. Q., Cheng J. B., Sun X. Z., Zhou L. Y., Qin J. J., Zhang X. K., Yuan Y. M. 2014. Effects of Radix Bupleuri extract supplementation on lactation performance and rumen fermentation in heat-stressed lactating Holstein cows. Anim. Feed Sci. Technol. 187:1–8. doi: 10.1016/j.anifeedsci.2013.09.008 [DOI] [Google Scholar]
- Pan L., Li P., Ma X. K., Xu Y. T., Tian Q. Y., Liu L., Li D. F., Piao X. S. 2016a. Tannin is a key factor in the determination and prediction of energy content in sorghum grains fed to growing pigs. J. Anim. Sci. 94:2879–2889. doi: 10.2527/jas.2016-0457 [DOI] [PubMed] [Google Scholar]
- Pan L., Ma X. K., Wang H. L., Xu X., Zeng Z. K., Tian Q. Y., Zhao P. F., Zhang S., Yang Z. Y., Piao X. S. 2016b. Enzymatic feather meal as an alternative animal protein source in diets for nursery pigs. Anim. Feed Sci. Technol. 212:112–121. doi: 10.1016/j.anifeedsci.2015.12.014 [DOI] [Google Scholar]
- Pan L., Shang Q. H., Ma X. K., Wu Y., Long S. F., Wang Q. Q., Piao X. S. 2017. Coated compound proteases improve nitrogen utilization by decreasing manure nitrogen output for growing pigs fed sorghum soybean meal based diets. Anim. Feed Sci. Technol. 230:136–142. doi: 10.1016/j.anifeedsci.2017.05.014 [DOI] [Google Scholar]
- Pan L., Zhao P. F., Yang Z. Y., Long S. F., Wang H. L., Tian Q. Y., Xu Y. T., Xu X., Zhang Z. H., Piao X. S. 2016c. Effects of coated compound proteases on apparent total tract digestibility of nutrients and apparent ileal digestibility of amino acids for pigs. Asian-Australas. J. Anim. Sci. 29:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulk C. B., Hancock J. D., Fahrenholz A. C., Wilson J. M., McKinny L. J., Behnke K. C. 2015. Effects of sorghum particle size on milling characteristics and growth performance in finishing pigs. Anim. Feed Sci. Technol. 202:75–80. doi: 10.1016/j.anifeedsci.2015.01.017 [DOI] [Google Scholar]
- Pedersen C., Boersma M. G., Stein H. H. 2007. Energy and nutrient digestibility in NutriDense corn and other cereal grains fed to growing pigs. J. Anim. Sci. 85:2473–2483. doi: 10.2527/jas.2006-620 [DOI] [PubMed] [Google Scholar]
- Pelleboer R. A., Felius A., Goje B. S., Van Gelderen H. H. 1990. Sorghum-based oral rehydration solution in the treatment of acute diarrhea. Trop. Geogr. Med. 42:63–68. [PubMed] [Google Scholar]
- Piao X. S., Han K., Kim J. H., Cho W. T., Kim Y. H., Liang C. 1999. Effects of Kemzyme, phytase and yeast supplementation on the growth performance and pollution reduction of broiler chicks. Asian-Australas. J. Anim. Sci. 12:36–41. [Google Scholar]
- Price M. L., Steve V. S., Butler L. C. 1978. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 26:1214–1218. doi: 10.1021/jf60219a031 [DOI] [Google Scholar]
- Salinas I., Pro A., Salinas Y., Sosa E., Becerril C. M., Cuca M., Cervantes M., Gallegos J. 2006. Compositional variation amongst sorghum hybrids: Effect of kafirin concentration on metabolizable energy. J. Cereal Sci. 44:342–346. doi: 10.1016/j.jcs.2006.08.008 [DOI] [Google Scholar]
- Selle P. H., Cadogan D. J., Li X., Bryden W. L. 2010. Implication of sorghum in broiler chicken nutrition. Anim. Feed Sci. Technol. 156:57–74. doi: 10.1016/j.anifeedsci.2010.01.004 [DOI] [Google Scholar]
- Selle P. H., Liu S. Y., Cai J., Cowieson A. J. 2013. Steam-pelleting temperatures, grain variety, feed form and protease supplementation of mediumly-ground, sorghum-based broiler diets: Influences on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Anim. Prod. Sci. 53:378–387. doi: 10.1071/AN12363 [DOI] [Google Scholar]
- Selle P. H., Walker A. R., Bryden W. L. 2003. Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Aust. J. Exp. Agric. 43:475–479. doi: 10.1071/EA02155 [DOI] [Google Scholar]
- Shelton J. L., Matthews J. O., Southern L. L., Higbie A. D., Bidner T. D., Fernandez J. M., Pontif J. E. 2004. Effect of nonwaxy and waxy sorghum on growth, carcass traits, and glucose and insulin kinetics of growing-finishing barrows and gilts. J. Anim. Sci. 82:1699–1706. doi: 10.2527/2004.8261699x [DOI] [PubMed] [Google Scholar]
- Skoglund E., Carlsson N. G., Sandberg A. S. 1997a. Analysis of inositol mono- and diphosphate isomers using high-performance ion chromatography and pulsed amperometric detection. J. Agric. Food Chem. 45:4668–4673. doi: 10.1021/jf970184+ [DOI] [Google Scholar]
- Skoglund E., Carlsson N. G., Sandberg A. S. 1997b. Determination of isomers of inositol mono- to hexaphosphates in selected foods and intestinal contents using high-performance ion chromatography. J. Agric. Food Chem. 45:431–436. doi: 10.1021/jf9603238 [DOI] [Google Scholar]
- Sotak K. M., Goodband R. D., Tokach M. D., Dritz S. S., Derouchey J. M., Nelssen J. L. 2014. Nutrient database for sorghum distillers dried grains with solubles from ethanol plants in the western plains region and their effects on nursery pig performance. J. Anim. Sci. 92:292–302. doi: 10.2527/jas.2013-6599 [DOI] [PubMed] [Google Scholar]
- Stein H. H., Lagos L. V., Casas G. A. 2016. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 218:33–69. doi: 10.1016/j.anifeedsci.2016.05.003 [DOI] [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., de Lange C. F. M. 2007. Invited review: Amino acid bioavailability and digestibility in pig feed ingredients: Terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas.2005-742 [DOI] [PubMed] [Google Scholar]
- Stein H. H., Shipley C. F., Easter R. A. 1998. Technical note: A technique for inserting a T-cannula into the distal ileum of pregnant sows. J. Anim. Sci. 76:1433–1436. doi: 10.2527/1998.7651433x [DOI] [PubMed] [Google Scholar]
- USDA – Foreign Agriculture Service (USDA-FAS) 2017. data. https://www.fas.usda.gov/data/grain-world-markets-and-trade (Accessed October 12, 2017.)
- Van Soest P. J., Robertson J. B., Lewis B. A. 1991. Methods for dietary fiber neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Verhelst R., Schroyen M., Buys N., Niewold T. 2010. The effects of plant polyphenols on enterotoxigenic Escherichia coli adhesion and toxin binding. Livest. Sci. 133:101–103. doi: 10.1016/j.livsci.2010.06.035 [DOI] [Google Scholar]
- Verhelst R., Schroyen M., Buys N., Niewold T. 2014. Dietary polyphenols reduce diarrhea in enterotoxigenic Escherichia coli (ETEC) infected post-weaning piglets. Livest. Sci. 160:138–140. doi: 10.1016/j.livsci.2013.11.026 [DOI] [Google Scholar]
- Williams C. H., David D. J., Iismaa O. 1962. The determination of chromic oxide in feces samples by atomic absorption spectrophotometry. J. Agric. Sci. 59:381–385. doi: 10.1017/S002185960001546X [DOI] [Google Scholar]
- Woyengo T. A., Beltranena E., Zijlstra R. T. 2014. Nonruminant nutrition symposium: Controlling feed cost by including alternative ingredients into pig diets: A review. J. Anim. Sci. 92:1293–1305. doi: 10.2527/jas.2013-7169 [DOI] [PubMed] [Google Scholar]
- Wu Y., Pan L., Shang Q. H., Ma X. K., Long S. F., Xu Y. T., Piao X. S. 2017. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed Sci. Technol. 230:126–135. doi: 10.1016/j.anifeedsci.2017.05.013 [DOI] [Google Scholar]
- Xie F., Pan L., Li Z. C., Shi M., Liu L., Li Y. K., Huang C. F., Li D. F., Piao X. S., Cao Y. H. 2017. Comparative digestibility of energy in four cereal grains fed to barrows at four body weights. Anim. Feed Sci. Technol. 232:215–221. doi: 10.1016/j.anifeedsci.2016.09.007 [DOI] [Google Scholar]
- Xu X., Wang H. L., Pan L., Ma X. K., Tian Q. Y., Xu Y. T., Long S. F., Zhang Z. H., Piao X. S. 2017. Effects of coated proteases on the performance, nutrient retention, gut morphology and carcass traits of broilers fed corn or sorghum based diets supplemented with soybean meal. Anim. Feed Sci. Technol. 223:119–127. doi: 10.1016/j.anifeedsci.2016.10.015 [DOI] [Google Scholar]
- Yin Y. L. 1994. Nutritive value of feedstuffs and diets for pigs, II. Apparent post-ileal digestibility and interrelationship between dietary constituents and fecal and ileal digestibility. Anim. Feed Sci. Technol. 45:243–255. doi: 10.1016/0377-8401(94)90030-2 [DOI] [Google Scholar]
- Yin Y.-L., Gurung N. K., Jeaurond E. A., Sharpe P. H., de Lange C. F. M. 2002. Nutrient digestibility of Canadian-developed sorghum and pearl millet grains fed to growing pigs compared to traditional cereal grains. Can. J. Anim. Sci. 82:385–391. doi: 10.4141/A01-086 [DOI] [Google Scholar]
- Yin Y. L., Huang R. L., Zhang H. Y., Chen C. M., Li T. J., Pan Y. F. 1993. Nutritive value of feedstuffs and diets for pigs. I. Chemical composition, apparent ileal and fecal digestibility. Anim. Feed Sci. Technol. 44:1–27. doi: 10.1016/0377-8401(93)90034-H [DOI] [Google Scholar]
- Yu G. X., Chen D. W., Yu B., He J., Zheng P., Mao X. B., Huang Z. Q., Luo J. Q., Zhang Z. H., Yu J. 2016. Coated protease increases ileal digestibility of protein and amino acids in weaned piglets. Anim. Feed Sci. Technol. 214:142–147. doi: 10.1016/j.anifeedsci.2016.02.006 [DOI] [Google Scholar]


