Abstract
Microcin J25 (MccJ25) is an antimicrobial peptide produced by a fecal strain of Escherichia coli containing 21 AA. This study was performed primarily to evaluate the effects of MccJ25 as a potential substitute for antibiotics (AB) on growth performance, nutrient digestibility, fecal microbiota, and intestinal barrier function in weaned pigs. In the present study, 180 weaned pigs (7.98 ± 0.29 kg initial BW) were randomly assigned to 1 of 5 treatments, including a basal diet (CON) and CON supplemented with AB (20 mg/kg colistin sulfate; ABD) or 0.5, 1.0, and 2.0 mg/kg MccJ25. On d 0 to 14, dietary supplementation with MccJ25 and ABD had positive effects on ADG, ADFI, diarrhea incidence, and G:F (P < 0.05). Pigs fed the 2.0 mg/kg MccJ25 diet had greater ADG (P < 0.05) and marginally greater G:F (P < 0.10) compared with pigs fed the ABD diet. Compared with the CON diet, the 2.0 mg/kg MccJ25 diet sharply improved (P < 0.05) ADG and G:F and decreased (P < 0.05) diarrhea incidence (d 15 to 28 and d 0 to 28). Apparent digestibility of nutrients in pigs fed 1.0 and 2.0 mg/kg MccJ25 was improved (P < 0.05) compared with that of pigs fed CON and ABD. The serum cytokines IL-6 and IL-1β and tumor necrosis factor-α levels in pigs fed MccJ25 were greater than in pigs fed CON (P < 0.05). Additionally, the IL-10 concentration in pigs fed MccJ25 was sharply increased (P < 0.05) compared with that of pigs fed CON. Pigs fed 1.0 and 2.0 mg/kg MccJ25 diets had remarkably decreased D-lactate, diamine oxidase, and endotoxin concentrations and fecal E. coli numbers (P < 0.05) and improved fecal Lactobacillus and Bifidobacterium numbers (P < 0.05). Compared with the ABD diet, the diet containing 2.0 mg/kg MccJ25 did not increase D-lactate, diamine oxidase, and endotoxin (d 14) concentrations (P < 0.05) or decrease the Lactobacillus and Bifidobacterium (d 28) numbers (P < 0.05). The diets containing 1.0 and 2.0 mg/kg MccJ25 and ABD (d 28) improved lactate concentration and short-chain fatty acid concentrations, including acetate, propionate, and butyrate, in feces (P < 0.05). Moreover, the pigs fed 2.0 mg/kg MccJ25 had greater lactate, butyrate (d 14), and propionate concentrations than the pigs fed the ABD diet (P < 0.05). In conclusion, dietary supplemented MccJ25 effectively improved performance, attenuated diarrhea and systematic inflammation, enhanced intestinal barrier function, and improved fecal microbiota composition of weaned pigs. Therefore, MccJ25 could be a potential effective alternative to AB for weaned pigs.
Keywords: antibiotics, antimicrobial peptides, diarrhea incidence, growth performance, microcin J25, weaned pigs
INTRODUCTION
Pigs are always challenged by postweaning stress, which ultimately can result in poor performance and disruptions to the immune system, microecology homeostasis, and intestinal barrier function (Moeser et al., 2006; Smith et al., 2010; Rong et al., 2015), as well as high susceptibility to pathogen infection, including Escherichia coli, Clostridium spp., and Salmonella spp., and an increased incidence of diarrhea (Heo et al., 2013; Britton and Young, 2014; Stensland et al., 2015). Therefore, it is necessary to develop possible strategies to control the negative effects of postweaning stress.
For decades, antibiotics (AB) have been widely used in the animal feed industry as growth promoters and a medicated feed additive to reduce susceptibility to infectious disease and improve the composition of intestinal microflora to decrease the risk of diarrhea (Barton, 2000; Vondruskova et al., 2010; Lin, 2011). Unfortunately, misuse or widespread use of AB has resulted in increasing bacterial resistance (Yin et al., 2013; Gill et al., 2015), AB residue in edible animal products, and an imbalance of the composition of intestinal microflora and reduction of the immune defenses of young animals (Millet and Maertens, 2011; McNamee et al., 2013). Hence, safe, effective, and natural products are required as alternatives to AB, and this requirement has attracted considerable research interest (Wang et al., 2007; Wang et al., 2011; Thacker, 2013).
Antimicrobial peptides (AMP) represent a potential alternative to available AB (Hancock and Sahl, 2006; Fjell et al., 2011). These peptides are present in all living organisms and are evolutionarily conserved short cationic molecules that support the host's defense against microbial infections (Zasloff, 2002). Unlike conventional AB, it is difficult for bacteria to develop resistance to these AMP because they act either through membrane lysis or by attacking intracellular targets (Giuliani et al., 2007; Chou et al., 2010). Therefore, AMP are potentially promising candidates to replace conventional AB. Some researchers have reported that AMP could be used in animal feed to replace AB as growth promoters (Peng et al., 2016; Wan et al., 2016; Yi et al., 2016).
Microcin J25 (MccJ25) is a 2106-Da, plasmid-encoded, small, ribosomally synthesized AMP that is isolated from a fecal strain of E. coli containing 21 AA residues (Salomón and Farias, 1992). This natural product has been shown to be extraordinarily stable and withstand high temperature, chemical denaturation, low pH, and proteolysis (Rebuffat et al., 2004; Knappe et al., 2009). It exhibits strong bacteriocidal activity in the 5 to 500 nM range and is active against several strains of Gram-negative bacteria, including pathogenic E. coli, Salmonella, and Shigella (Blond et al., 1999; Sable et al., 2000), with no hemolytic activity against human red blood cells (Lopez et al., 2007). These features might make it useful for various industrial applications, such as in the swine industry. At present, to the authors' knowledge, little is known about the use of MccJ25 as a feed additive in the pig industry. Therefore, the objective of this study was to determine the efficacy of MccJ25 as a replacement for conventional AB to enhance the growth performance and intestinal heath of pigs after weaning.
MATERIALS AND METHODS
All procedures used in these experiments were conducted in accordance with the Chinese Guidelines for Animal Welfare and were approved by the China Agricultural University Institutional Animal Care and Use Committee (Beijing, China).
Microcin J25 Sample Preparation
A novel expression vector was generated using standard recombinant DNA procedures. Specifically, the codon-optimized genes coding for MccJ25 were synthesized by Genscript manufacturing and then cloned into pBR322 to generate expression vector pMJ25. Then, pMJ25 was transformed into E. coli DH5α. The recombinant bacteria were cultured in a sucrose-complex medium at 37°C with 100 µg/L ampicillin in a 10-L fermenter for 22 h. After incubation, the cell supernatant was harvested. The MccJ25 was purified using an AKTA Purifier System (Amersham Biosciences, Piscataway, NJ). The purity of the MccJ25 was above 99.95%. The AA sequence of the peptides determined by automated Edman degradation (model 494 Procise Protein/Peptide Sequencer; Applied Biosystems, Foster City, CA) and a mass spectrometer (Q-TOF Mass Analyzer; Micromass Ltd., Manchester, UK) was GGAGHVPEYFVGIGTPISFYG. The relative molecular mass was 2,106.01 Da, determined using matrix-assisted laser desorption/ionization time-of-flight mass spectrophotometry with a Voyager instrument (Applied Biosystems).
Pigs and Dietary Treatments
A total of 180 pigs weaned at 25 d (Landrace × Yorkshire × Duroc; 7.98 ± 0.29 kg average initial BW; 90 males and 90 females) was used in a 28-d growth study at the Swine Nutrition Research Center of the National Feed Engineering Technology Research Center (Chengde, Hebei Province, China). All pigs were housed in temperature-controlled nursery rooms (25°C ± 3°C). The rooms were equipped with identical woven mesh floors (1.2 × 1.5 m) with automatic ventilation systems. Each pen had 1 feeder and a low-pressure drinking nipple. Feed and water were available to the pigs ad libitum.
The pigs were randomly assigned to 1 of 5 treatments with 6 pigs per pen and 6 replicate pens per treatment by gender and BW in a randomized complete block design. The proportion of barrows to gilts was equal in each pen. Dietary treatments included a corn–soybean meal control diet (CON) as well as a similar CON diet supplemented with AB (20 mg/kg colistin sulfate; ABD) and CON supplemented with 0.5, 1.0, and 2.0 mg/kg MccJ25, respectively. The 3 levels of MccJ25 selected in the present study were based on results of minimum inhibitory concentration against E. coli k88 (0.2 μg/mL; H. T. Yu, unpublished data). Pigs were fed diets formulated to meet or exceed NRC-recommended nutrient requirements (NRC, 2012) and did not contain any in-diet AB, expect that the positive control diet contained colistin sulfate. The ingredients and chemical composition of the CON are shown in Table 1.
Table 1.
Composition and nutrient levels of the basal diet (as-fed basis, antibiotic free)
| Item | Content |
|---|---|
| Ingredient, % | |
| Corn, yellow | 33 |
| Extruded corn | 20 |
| Extruded soybean | 10 |
| Soybean meal, dehulled (48% CP) | 9 |
| Fish meal (64% CP) | 5 |
| Whey powder | 10 |
| Soybean protein concentrate | 6.8 |
| Soybean oil | 2.2 |
| Dicalcium phosphate | 1.7 |
| Limestone | 0.57 |
| Salt | 0.26 |
| L-Lys HCl, 78.8% | 0.36 |
| DL-Met, 98.5 | 0.10 |
| L-Thr, 98.5% | 0.12 |
| L-Trp, 98.5% | 0.02 |
| Choline chloride | 0.12 |
| Mineral and vitamin premix1 | 0.50 |
| Cr2O3 | 0.25 |
| Total | 100.00 |
| Chemical composition, calculated | |
| DE, kcal/kg | 3,566 |
| ME, kcal/kg | 3,419 |
| NE, kcal/kg | 2,612 |
| CP | 18.92 |
| Standardized ileal digestible Lys | 1.35 |
| Standardized ileal digestible Met | 0.41 |
| Standardized ileal digestible Thr | 0.81 |
| Standardized ileal digestible Trp | 0.23 |
| Ca | 0.91 |
| Total P | 0.76 |
| Analyzed composition | |
| DE, kcal/Kg | 4,118 |
| CP | 19.89 |
| Ca | 0.91 |
| Total P | 0.79 |
| Lys | 1.33 |
Vitamin and mineral premix provided the following per kilogram of diet: 12,000 IU vitamin A as vitamin A acetate, 2,500 IU vitamin D as vitamin D3, 30 IU vitamin E as DL-α-tocopherol acetate, 12 μg of vitamin B12, 3 mg vitamin K as menadione sodium bisulfate, 15 mg D-pantothenic acid as calcium pantothenate, 40 mg of nicotinic acid, 400 mg choline as choline chloride, 30 mg Mn as manganese oxide, 90 mg Fe as iron sulfate, 10 mg Cu as copper oxide, 0.35 mg I as ethylenediamine dihydroiodide, and 0.3 mg Se as sodium selenite.
Sample Collection
Pigs were individually weighed on an empty stomach at the start of the trial and on d 14 and 28. Feed consumption was recorded on d 14 and 28. All feed remaining in the feeder was weighed and subtracted from the daily allowance to determine the actual daily feed intake. Feed wastage was considered minimal, so feed disappearance was determined to be a reliable estimate of feed consumption. Feed consumption and the ADFI and G:F were calculated. To evaluate the effect of dietary treatments on the apparent total tract digestibility (ATTD) of GE, DM, and CP, Cr2O3 (0.25%) was used as an inert indigestible indicator in the diets from d 22 to 28. Fresh fecal samples were collected from each pen on the last 3 d to determine the apparent nutrient digestibility. The fecal samples were pooled by pen, then oven-dried at 60°C for 72 h, and subsequently ground with a 1-mm screen. Additionally, fresh fecal samples were collected from 2 pigs per pen on d 14 and 28. The collected samples were immediately placed on ice (1 to 2 h) and transported to the refrigerator at −80°C for microbial composition analysis. Blood samples were collected by anterior vena cava puncture from 1 randomly selected pig in each pen using anticoagulant-free Vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ). Serum was obtained after centrifugation (Biofuge22R; Heraeus, Hanau, Germany) at 3,000 × g for 15 min at 4°C and then stored at −80°C until analysis.
Fecal Consistency and Incidence of Diarrhea
Health status, diarrhea incidence, and mortality of pigs were recorded every day. The occurrence of diarrhea, health status, and mortality for each pig was visually recorded at 0900 and 1700 h. The severity of diarrhea was measured using a fecal consistency scoring system. Fecal consistency was visually assessed each day by observers who were blind to the treatments. Fresh excreta were graded by using the following scores: 0 = normal, solid; 1 = possible, semisolid, slight diarrhea; 2 = definitely semiliquid; and 3 = very watery and frothy diarrhea. A cumulative diarrhea score per diet and day was then calculated (Wu et al., 2012). The occurrence of diarrhea was defined as the maintenance of fecal scores of 2 or 3 for 2 consecutive days and was determined with the following formula: diarrhea incidence (%) = [(number of pigs with diarrhea within a treatment)/(number of pigs × total experimental days)] × 100, in which “number of pigs with diarrhea” was the total number of pigs with diarrhea observed each day.
Chemical Analyses
Before chemical analysis, samples of all experimental diets and dried feces were ground through a 1-mm screen and thoroughly mixed. The chemical analyses of the experimental diets and excreta samples included DM (method 930.15; AOAC, 2006), CP (method 984.13; AOAC, 2006), Ca (method 968.08; AOAC, 2006), and P (method 946.06; AOAC, 2006) as described by AOAC International (AOAC, 2006). Gross energy was measured with an automatic adiabatic oxygen bomb calorimeter (model 6300; Parr Instrument Co., Moline, IL), and the Cr concentration in all experimental diets and excreta samples was determined using a polarized Zeeman Atomic Absorption Spectrometer (Hitachi Z2000; Tokyo, Japan) after nitric acid–perchloric acid wet ash sample preparation. All analyses were conducted in duplicate. The ATTD of nutrients was calculated using the formula ATTD (g/kg) = {1 − [Crfeed × Nutrientfeces/(Crfeces × Nutrientfeed)]} × 1,000, in which Crfeed is the Cr concentration in the feed (g/kg DM), Nutrientfeces is the nutrient concentration in feces (g/kg DM), Crfeces is the Cr concentration in feces (g/kg DM), and Nutrientfeed is the nutrient concentration in the feed (g/kg DM)
Determination of D-Lactate, Diamine Oxidase, and Endotoxin Concentrations in Serum
Serum samples were thawed and thoroughly mixed immediately before testing. Serum levels of D-lactate (catalog number DG50344p) and diamine oxidase (DAO; catalog number DG50059p) were determined using commercially available porcine ELISA kits (Beijing Winter Song Boye Biotechnology Co. Ltd., Beijing, China) according to the manufacturer's protocol. Data were acquired by a microplate reader using MPM 6.1 software (Bio-Rad Laboratories, Hercules, CA).
Serum endotoxin level was measured by a quantitative chromogenic end point Tachypleus amebocyte lysate endotoxin detection kit following the manufacturer's instructions (catalog number KTE20; Xiamen TAL Experimental Plant Co., Ltd., Anhui, China).
Enzyme-Linked Immunosorbent Assays of Cytokine Concentration in Serum
Sandwich ELISA assays were used to quantify immune indices. Serum samples were thawed and thoroughly mixed immediately before testing. The levels of IL-6 (catalog number H007), IL-1β (catalog number H002), IL-10 (catalog number H009), and tumor necrosis factor-α (TNF-α; catalog number H052) in serum were determined using commercially available porcine ELISA kits according to standard procedures described by the manufacture. All of the assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). Data were acquired by a microplate reader using MPM 6.1 software (Bio-Rad Laboratories).
Fecal Microbial Composition Analysis
The total genomic DNA from the different reference strains that were used to generate standard curves was extracted using a QIAamp UCP Pathogen Mini Kit (Qiagen, Munich, Germany; catalog number 50214). The bacteria genomic DNA was extracted from feces using a QIAamp DNA Stool Mini Kit (Qiagen; catalog number 51504) according to the manufacturer's instructions. Deoxyribonucleic acid concentration was determined by spectrophotometry (NanoDrop 2000). The gene-specific PCR primers for quantitative detection of total bacteria (200 bp), E. coli (96 bp), Bifidobacterium (121 bp), and Lactobacillus (126 bp) were as follows: 5ʹ-ACTCCTACGGGAGGCAGCAG-3ʹ and 5ʹ-ATTACCGCGGCTGCTGG-3ʹ for total bacteria, 5ʹ-CATGCCGCGTGTATGAAGAA-3ʹ and 5ʹ-CGGGTAACGTCAATGAGCAAA-3ʹ for E. coli, 5ʹ-CGCGTCCGTTGTGAAG-3ʹ and 5ʹ-CTTCCCGATATCTACACATTCCA-3ʹ for Bifidobacterium, and 5ʹ-GAGGCAGCAGTAGGGAATCTT-3ʹ and 5ʹ-CAACAGTTACTCTGACACCCGTTC-3ʹ for Lactobacillus. All primers and fluorescent oligonucleotide probes were commercially synthesized by Invitrogen (Shanghai, China). The quantitative detections of total bacteria, E. coli, Lactobacillus, and Bifidobacterium were performed using real-time PCR using a StepOne Plus System as previously described (Gao et al., 2013). All samples were run in duplicate.
Ion Chromatographic Assays of Short-Chain Fatty Acid and Lactate Concentrations in Feces
Fecal samples were thawed and thoroughly mixed immediately before testing. Concentrations of short-chain fatty acids (SCFA) in feces were determined using ion chromatography (ICS 3000; Thermo, New York, NY). Briefly, 1 g of feces was weighted into a 10-mL polypropylene tube, and 8 mL double-distilled H2O was added; the polypropylene tube was placed in an ultrasonic bath for 20 min, and then the mixture was centrifuged for 15 min at 10,000 × g at 4°C. Then, 0.16 mL of supernatant was mixed with 8 mL double-distilled H2O and filtered through a 0.22-μm filter. The organic acids in a 25-μL extracted sample solution were analyzed by high-performance ion chromatography (ICS-3000; Dionex, Sunnyvale, CA) with a conductivity detector. The organic acids were separated on an AS11 analytical column (250 × 4 mm) and an AG11 guard column under the following gradient conditions: 0.8 to 1.5 mM for 0 to 5 min, 1.5 to 2.5 mM for 5 to 10 min, and 2.5 mM for10 to 15 min. The gradient was performed with potassium hydroxide, and the flow rate was 1.0 mL/min. Short-chain fatty acid and lactate concentrations were normalized to feces weight as micrograms per milliliter.
Statistical Analysis
Differences in diarrhea incidence among treatments were tested with the χ2 contingency test. Other data were subjected to ANOVA using the GLM procedure of SAS (version 9.0; SAS Inst. Inc., Cary, NC). The pen was the experimental unit for analysis of performance data and apparent nutrient digestibility. Individual pig was the experimental unit for other parameters. Pens were considered blocks, and dietary treatment was the only fixed effect. Polynomial contrasts were conducted to determine linear and quadratic effects of supplemental MccJ25. The significance of differences between treatments was tested using the Student-Newman-Keuls multiple-range test. Data are presented as mean values with their SE; P < 0.05 was considered statistically significant, and P < 0.10 indicated a trend.
RESULTS
Growth Performance and Diarrhea Incidence
On d 28, pigs fed diets supplemented with MccJ25 had significantly increased ADG (linear, P = 0.003) with increasing MccJ25 supplementation (Table 2). Pigs given 2.0 mg/kg MccJ25 had greater ADG (P = 0.006) and G:F (P = 0.024) and lower diarrhea incidence (P = 0.003) compared with the CON pigs. There was no significant difference in ADG and G:F among all MccJ25 and ABD treatments. Additionally, remarkable differences in ADFI among all treatments were not evident (Table 2).
Table 2.
Effects of microcin J25 (MccJ25) on the growth performance and diarrhea incidence of weaned pigs
| Dietary MccJ25,1 mg/kg | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON1 | ABD1 | 0.5 | 1.0 | 2.0 | SEM2 | ANOVA | Linear | Quadratic |
| d 0 to 14 | |||||||||
| ADG, g | 309c | 355b | 352b | 376a | 383a | 6.17 | <0.001 | <0.001 | 0.001 |
| ADFI, g | 487b | 514a | 515a | 528a | 524a | 7.82 | 0.009 | 0.008 | 0.069 |
| G:F | 1.56a | 1.45b | 1.46b | 1.41b,c | 1.36c | 0.02 | 0.019 | 0.012 | 0.171 |
| DI,3 % | 17.26a | 11.70b | 11.30b | 10.11b | 9.12b | 1.64 | 0.021 | — | — |
| d 15 to 28 | |||||||||
| ADG, g | 536c | 569a,b | 553b,c | 571a,b | 584a | 5.86 | <0.001 | 0.014 | 0.717 |
| ADFI, g | 997 | 1004 | 1006 | 982 | 994 | 21.92 | 0.946 | 0.688 | 0.494 |
| G:F | 1.66a | 1.57a,b | 1.60a,b | 1.52b | 1.51b | 0.04 | 0.048 | 0.149 | 0.619 |
| DI, % | 7.54a | 5.55a,b | 6.15a,b | 5.35a,b | 4.36b | 0.46 | 0.018 | — | — |
| d 0 to 28 | |||||||||
| ADG, g | 436b | 453a,b | 441a,b | 452a,b | 467a | 6.94 | 0.045 | 0.003 | 0.977 |
| ADFI, g | 747 | 754 | 744 | 751 | 745 | 15.3 | 0.988 | 0.538 | 0.442 |
| G:F | 1.60a | 1.54a,b | 1.55a,b | 1.51b | 1.47b | 0.03 | 0.046 | 0.149 | 0.619 |
| DI, % | 12.40a | 8.62b | 8.70a,b | 7.73b | 6.74b | 1.20 | 0.033 | — | — |
Means in the same row with different superscripts differ (P < 0.05).
CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
n = 6.
DI = diarrhea incidence: (number of pigs with diarrhea × diarrhea days)/(total number of pigs × total days) ×100.
During d 0 to 14, the ADG (linear, P < 0.001, and quadratic, P = 0.001), ADFI (linear, P = 0.008), and G:F (linear, P = 0.012) of pigs were significantly improved with increasing MccJ25 supplementation. Compared with the CON pigs, the pigs fed MccJ25 and ABD had sharply improved ADG (P < 0.001), ADFI (P = 0.009), and G:F (P = 0.019) and decreased (P = 0.021) diarrhea incidence. Pigs given 2.0 mg/kg MccJ25 diet had greater (P = 0.005) and a trend toward improved G:F (P = 0.094) compared with the ABD-fed pigs. There was no remarkable difference in ADFI and diarrhea incidence between ABD-fed pigs and MccJ25-fed pigs (Table 2).
During d 15 to 28, pigs had significantly increased (linear, P = 0.014) ADG as the MccJ25 supplementation increased. Compared with the CON pigs, pigs supplemented with 1.0 and 2.0 mg/kg MccJ25 had sharply increased ADG (P = 0.007 and P = 0.039, respectively) and marginally improved G:F (P = 0.060 and P = 0.054, respectively) and decreased diarrhea incidence (P = 0.04 and P = 0.026, respectively). There was no significant difference in growth performance and diarrhea incidence between pigs fed MccJ25 and pigs fed the ABD (Table 2).
Apparent Total Tract Digestibility of Nutrients
The ATTD of OM (linear, P < 0.001), CP (linear, P < 0.001), and GE (linear, P < 0.001, and quadratic, P = 0.006) was significantly increased in response to increasing MccJ25 supplementation (Table 3). On d 28, the ATTD of OM, CP, and GE in pigs fed 1.0 and 2.0 mg/kg MccJ25 and ABD were greater (P < 0.001) than those in pigs fed CON. The pigs fed 2.0 mg/kg MccJ25 had greater ATTD of OM (P = 0.014) and GE (P < 0.001) than pigs fed ABD, whereas there was no significant difference in ATTD of CP between pigs fed 2.0 mg/kg MccJ25 and pigs fed ABD (Table 3).
Table 3.
Effects of microcin J25(MccJ25) on apparent total tract digestibility of nutrients in weaned pigs for 28 d (% of DM)
| Dietary MccJ25,1 mg/kg | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | ABD | 0.5 | 1.0 | 2.0 | SEM2 | ANOVA | Linear | Quadratic |
| OM | 87.59c | 88.69b | 87.83c | 88.83b | 89.50a | 0.21 | <0.001 | <0.001 | 0.692 |
| CP | 81.94c | 84.83a,b | 83.42,bc | 85.33a | 85.99a | 0.59 | <0.001 | <0.001 | 0.085 |
| GE | 85.78c | 87.13b | 86.08c | 89.48a | 89.72a | 0.24 | <0.001 | <0.001 | 0.006 |
Means in the same row with different superscripts differ (P < 0.05).
CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
n = 6.
The Concentrations of D-Lactate, Diamine Oxidase, and Endotoxins
On d 14, pigs had sharply decreased (linear, P < 0.001, and quadratic, P = 0.001) levels of DAO and D-lactate and decreased concentrations of endotoxins (linear, P < 0.001, and quadratic, P = 0.010) in serum with increasing MccJ25 supplementation (Table 4). Compared with CON, the MccJ25 and ABD treatments significantly decreased (P < 0.001) the serum D-lactate, DAO, and endotoxin concentrations. The pigs fed diets containing 1.0 and 2.0 mg/kg MccJ25 did not have increased concentrations of D-lactate (P = 0.013 and P < 0.001, respectively) and DAO (P = 0.004 and P = 0.001, respectively) compared with the pigs fed the ABD diet. Additionally, a significant difference (P = 0.017) in the serum endotoxin concentrations was observed between pigs fed 2.0 mg/kg MccJ25 and pigs fed ABD. (Table 4)
Table 4.
Effects of MccJ25 (MccJ25) on intestinal permeability of weaned pigs
| Dietary MccJ25,1 mg/kg | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | ABD | 0.5 | 1.0 | 2.0 | SEM2 | ANOVA | Linear | Quadratic |
| d 14 | |||||||||
| D-Lactate, µmol/mL | 13.95a | 9.75b | 9.71b | 8.12c | 7.14c | 0.45 | <0.001 | <0.001 | 0.001 |
| DAO,3 pg/mL | 5.12a | 4.30b | 4.07b | 3.54c | 3.41c | 0.16 | <0.001 | <0.001 | 0.001 |
| Endotoxins,4 EU/L | 20.09a | 16.95b | 15.93b | 13.58b,c | 13.06c | 0.91 | <0.001 | 0.001 | 0.010 |
| d 28 | |||||||||
| D-Lactate, µmol/mL | 8.11a | 7.41b | 7.32b | 6.62c | 6.40c | 0.29 | 0.002 | <0.001 | 0.003 |
| DAO, pg/mL | 3.64a | 3.21b | 3.15b | 2.95c | 2.93c | 0.06 | <0.001 | 0.001 | 0.045 |
| Endotoxins, EU/L | 18.96a | 15.89b | 15.84b | 14.86b | 13.99b | 0.53 | <0.001 | <0.001 | 0.004 |
Means in the same row with different superscripts differ (P < 0.05).
CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
n = 6.
DAO = diamine oxidase.
EU = endotoxin unit.
On d 28 d, the serum concentrations of D-lactate (linear, P < 0.001, and quadratic, P = 0.003), DAO (linear, P = 0.001, and quadratic, P = 0.045), and endotoxins (linear, P < 0.001, and quadratic, P = 0.004) were sharply decreased with increasing MccJ25 supplementation in pigs (Table 4). The pigs fed the ABD and MccJ25 diets had lower (P < 0.001) serum concentrations of D-lactate, DAO, and endotoxins compared with pigs fed the CON. Compared with pigs fed the ABD, pigs fed diets containing 1.0 and 2.0 mg/kg MccJ25 had remarkably decreased serum D-lactate (P = 0.029 and P = 0.007, respectively) and DAO (P = 0.011 and P = 0.006, respectively) concentrations. However, the concentration of serum endotoxins was similar between pigs fed MccJ25 and pigs fed the ABD (Table 4).
Cytokine Levels in Serum
The cytokine concentrations in serum are presented in Fig. 1. At d 14 and 28, results indicated that pigs fed the ABD and MccJ25 diets had significantly decreased concentrations of the cytokines IL-6, IL-1β, and TNF-α and increased IL-10 levels (P < 0.001) compared with the pigs fed the CON diet (Fig. 1A and 1B). However, these cytokines in all of the pigs fed the MccJ25 and ABD diets were similar.
Figure 1.
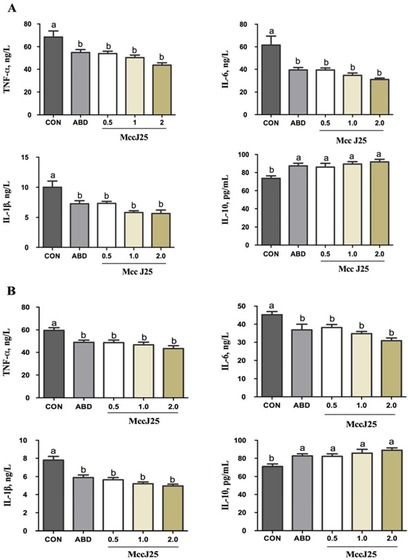
Effects of microcin J25 (MccJ25) on the immune response of weaned pigs. (A) Serum levels of the proinflammatory tumor necrosis factor-α (TNF-α), IL-6, IL-1β, and anti-inflammatory IL-10 for 14 d. (B) Serum levels of the proinflammatory TNF-α, IL-6, IL-1β, and anti-inflammatory IL-10 for 28 d. a,bTreatments that are significantly different from each other at P < 0.05 are indicated by different letters. Values are least squares means ± SEM; n = 6. CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
Microbial Composition Levels in Feces
Results for microbial populations in feces are presented in Fig. 2. There were no significant differences in total bacterial numbers in feces among all treatments during the experiment period (Fig. 2A and 2B). On d 14, the numbers of fecal Lactobacillus in pigs fed the ABD and MccJ25 were greater (P = 0.021) than in the CON pigs. A significant reduction (P < 0.001) in fecal E. coli numbers was found in pigs fed 1.0 mg/kg MccJ25, 2.0 mg/kg MccJ25, and the ABD compared with pigs fed the CON. Additionally, compared with the CON, the diet supplemented with MccJ25 significantly increased (P < 0.001) the numbers of Bifidobacterium in feces (Fig. 2A). Pigs fed 1.0 and 2.0 mg/kg MccJ25 had greater fecal Lactobacillus counts (P < 0.001) and lower fecal E. coli counts (P < 0.001) than pigs fed ABD, but Bifidobacterium counts remained the same in pigs fed ABD and MccJ25.
Figure 2.
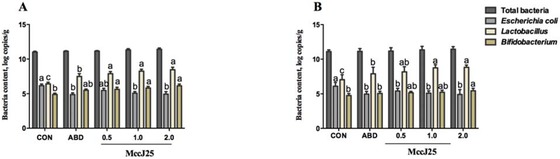
Effects of microcin J25 (MccJ25) on microbial composition in feces of weaned pigs. (A) Total bacteria, Escherichia coli, Lactobacillus, and Bifidobacterium content in feces of weaned pigs on d 14 of the trial. (B) Total bacteria, Escherichia coli, Lactobacillus, and Bifidobacterium content in feces of weaned pigs on d 28 of the trial. a–cTreatments that are significantly different from each other at P < 0.05 are indicated by different letters. Values are least squares means ± SEM; n = 6. CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
On d 28, pigs fed MccJ25 and ABD had lower E. coli numbers (P = 0.002) and greater Lactobacillus numbers (P = 0.001) in feces than the CON pigs (Fig. 2B). The pigs fed the 2.0 mg/kg MccJ25 diet had significantly increased (P = 0.001) Bifidobacterium numbers compared with pigs fed CON (Fig. 2B). Moreover, compared with the ABD diet, the diet containing 2.0 mg/kg MccJ25 sharply increased the numbers of Lactobacillus (P = 0.027) and Bifidobacterium (P = 0.025), but no remarkable difference existed between the ABD and MccJ25 diets concerning the numbers of E. coli in feces (Fig. 2B). Additionally, pigs fed 1.0 mg/kg MccJ25 also had increased (P = 0.045) fecal Lactobacillus counts compared with pigs fed ABD.
Lactate Concentrations in Feces
The concentration of lactate (d 14) in feces was significantly increased (linear, P = 0.002) with increasing MccJ25 supplementation in pigs (Fig. 3A). On d 14, pigs fed MccJ25 and ABD had greater lactate concentrations than pigs fed CON (P = 0.008). Pigs fed 2.0 mg/kg MccJ25 and ABD had improved lactate concentrations compared with pigs fed 0.5 and 1.0 mg/kg MccJ25 (P < 0.001), whereas pigs fed ABD and 2.0 mg/kg MccJ25 diets had no significant difference in the concentration of lactate (Fig. 3A).
Figure 3.
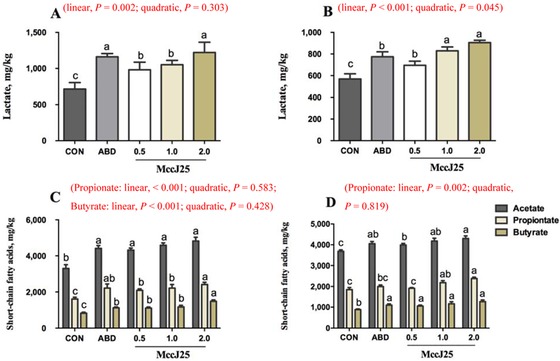
Effects of microcin J25 (MccJ25) on lactate and short-chain fatty acid (SCFA) levels in feces of weaned pigs. (A) Lactate concentration in feces of weaned pigs on d 14 of the trial. (B) Lactate concentration in feces of weaned pigs on d 28 of the trial. (C) The concentrations of acetate, propionate, and butyrate in feces on d 14 of the trail. (D) The concentrations of acetate, propionate, and butyrate in feces on d 28 of the trail. Lactate and SCFA levels were determined by ion chromatography, and the data were normalized to feces weight (per gram). a–cTreatments that are significantly different from each other at P < 0.05 are indicated by different letters. Values are least squares means ± SEM; n = 6. CON = basal diet; ABD = CON supplemented with antibiotics (20 mg/kg colistin sulfate); 0.5 mg/kg MccJ25 = CON supplemented with 0.5 mg/kg MccJ25; 1.0 mg/kg MccJ25 = CON supplemented with 1.0 mg/kg MccJ25; 2.0 mg/kg MccJ25 = CON supplemented with 2.0 mg/kg MccJ25.
On d 28, the concentration of lactate in feces was significantly increased (linear, P < 0.001, and quadratic, P = 0.045) with increasing MccJ25 supplementation in pigs (Fig. 3B). Compared with pigs fed CON, pigs fed MccJ25 and ABD had greater lactate concentration (P < 0.001), and 1.0 and 2.0 mg/kg MccJ25 dramatically increased the (P < 0.001) lactate concentration compared with ABD (Fig. 3B).
Short-Chain Fatty Acid Concentrations in Feces
Results for SCFA concentrations in all dietary treatments are shown in Fig. 3C and 3D. On d 14, the concentrations of propionate and butyrate in pigs were increased (linear, P < 0.001) with increasing MccJ25 supplementation. Pigs fed MccJ25 and ABD had greater (P < 0.001) acetate, propionate, and butyrate concentrations compared with pigs fed CON. Compared with pigs fed ABD, pigs fed 2.0 mg/kg MccJ25 had a dramatically increased (P < 0.001) butyrate concentration, a marginally improved propionate concentration (P = 0.063), and a trend toward an increased (P = 0.082) acetate concentration (Fig. 3C).
On d 28, the concentration of propionate was increased (linear, P = 0.002) with increasing MccJ25 supplementation in pigs (Fig. 3D). Compared with the pigs fed CON, the pigs fed MccJ25 and ABD had greater (P < 0.001) acetate and butyrate concentrations, but pigs fed MccJ25 and ABD maintained the same level of acetate and butyrate concentrations. The concentration of propionate in feces was significantly increased in pigs fed 1.0 (P = 0.007) and 2.0 mg/kg MccJ25 (P < 0.001) compared with pigs fed CON. Pigs fed the 2.0 mg/kg MccJ25 diet had a greater (P = 0.002) concentration of propionate in feces compared with pigs fed the ABD diet (Fig. 3D).
DISCUSSION
Microcin J25 is one of the most well studied lasso peptides and has received attention because of its unique structure and potent antibacterial activity (Pan, 2012). In this study, we have demonstrated that dietary supplementation with 1.0 and 2.0 mg/kg MccJ25 can improve the growth performance of weaned pigs and effectively attenuate the incidence of postweaning diarrhea. The positive effects for growth performance and diarrhea with supplementation of AMP to weaned pig diets have been previously reported (Wu et al., 2012; Yoon et al., 2013; Xiong et al., 2014). Wan et al. (2016) reported that improved ADG and G:F as well as a reduced incidence of diarrhea were achieved in weaned pigs fed diets containing the AMP plectasin (Ple). Similarly, Yi et al. (2016) showed that pigs fed diets supplemented with the AMP Cathelicidin-WA (CWA), derived from the endemic Bungarus fasciatus, had improved ADG and it effectively attenuated postweaning diarrhea. Peng et al. (2016) also observed that a diet containing recombinant porcine β-defensin 2 improved the growth performance of weaned pigs and reduced the incidence of postweaning diarrhea more effectively than AB supplementation. In the present study, microcin J25 achieved improvement of performance and diarrhea incidence similar to or greater than that of colistin sulfate in weaned pigs. Therefore, these results indicate for the first time that 1.0 and 2.0 mg/kg MccJ25 can produce positive effects similar to those of colistin sulfate on the growth performance and the incidence of diarrhea of weaned pigs
In the current study, greater ATTD of OM and GE were observed in weaned pigs treated with MccJ25 diets compared with pigs fed CON and ABD, and the ATTD of CP was also improved compared with that of pigs fed CON. The positive results in the ATTD of nutrients in the present study are consistent with previous studies that reported that pigs fed diets supplemented with AMP Ple or colistin sulfate can tend to have improved ATTD of DM and GE (Wan et al., 2016). Similar to the effects of the current study, improved ATTD of nutrients in weaned pigs fed diets supplemented with AB or AMP have been previously reported (Jin et al., 2008; Choi et al., 2011; Yoon et al., 2013, 2014).
Cytokines play an important role in the immune response and inflammation and are also important mediators for protection against or susceptibility to infection and some gastrointestinal dysfunctions (Ye et al., 2006; Praveena et al., 2010). Some studies, both in vitro and in vivo, have reported that uncontrolled synthesis of proinflammatory cytokines has a negative impact on gut integrity and epithelial function, including an increase in permeability to macromolecules and transport of nutrients and ions (McKay and Baird, 1999). Proinflammatory cytokine concentrations, including those of IL-1β, IL-6, and TNF-α, are typically increased in the small intestines of pigs after weaning (Pié et al., 2004). These 3 major cytokines play important roles in cellular immunity; for example, a high IL-6 concentration can induce tissue damage (Johnson, 1997; Pié et al., 2004). Interleukin-1β has been shown to mediate the host inflammatory response to prevent infection (Al-Sadi and Ma, 2007). Tumor necrosis factor-α can also disrupt epithelial barrier function in several ways, including altering lipid composition in the membrane microdomains of the tight junction (Li et al., 2008; Capaldo and Nusrat, 2009).
In the present study, the MccJ25 and ABD diets showed potential in significantly decreasing the levels of IL-1β, IL-6, and TNF-α in serum accompanied by an increase in the level of the anti-inflammatory cytokine IL-10. Yi et al. (2016) reported that diets supplemented with CWA can reduce levels of the proinflammatory cytokines IL-6 in serum. These results may be because MccJ25 and AB killed pathogenic bacteria, limited the expansion of competing Enterobacteriaceae (including pathogens and pathobionts), and improved the amounts of beneficial intestinal microbials. On the other hand, some AMP can confer protection by immune modulation, which may be a reason for the results observed in the present study. In this study, the findings were consistent with previous studies that reported AMP or AB can improve the immune response in weaned pigs (Tang et al., 2012; Wu et al., 2012).
The function of the intestinal barrier is related to many factors, including D-lactate, DAO, and endotoxins in serum (Song, 2009; Guo et al., 2010; Zhao et al., 2011; Rong et al., 2015). D-Lactate is a metabolite end product of intestinal bacteria. Mammals neither produce D-lactate nor have D-lactate dehydrogenase; therefore, the body retains a lower level of D-lactate under healthy conditions (Murray et al., 1993). An increasing D-lactate level in serum is usually accompanied by damage to the intestinal barrier function (Zhao et al., 2014). Diamine oxidase is one of the DAO catalyzed by deaminases. It is mainly a secretion of intestinal epithelial cells and is expressed in the small intestine but rarely in serum under normal circumstances (Smith et al., 1986; Wolvekamp and Debruin, 1994; Chen et al., 1998). With damage to intestinal barrier integrity, tissue DAO levels decrease, and serum DAO concentrations increase (Chen et al., 1998; Zhao et al., 2014). Endotoxins are one of the secretions of E. coli, and their increasing activity indicates injury to intestinal barrier function or increased intestinal permeability (Smith et al., 2010).
In the present study, lower serum concentrations of D-lactate, DAO, and endotoxins were observed in weaned pigs fed 1.0 and 2.0 mg/kg MccJ25 compared with pigs fed ABD and CON. These findings are consistent with previously published studies, such as the study of Tang et al. (2012), who reported there were lower D-lactate concentrations in the serum of weaned pigs fed diets supplemented with an AMP (lactoferrampin-lactoferricin), and a report that showed that pigs treated with casein glycomacropeptide (CGMP) had lower serum DAO and D-lactate levels than pigs challenged with enterotoxigenic E. coli (ETEC) K88, indicating the ability of CGMP to protect the barrier function of the intestinal mucosa (Rong et al., 2015). However, Tang et al. (2012) observed that there was no effect of the AMP (lactoferrampin and lactoferricin) on serum levels of DAO. The reason for this discrepancy may be a possible variation in the level of dietary supplementation or the origin of the peptides (natural or synthetic). In the current study, significantly decreased serum concentrations of D-lactate, DAO, and endotoxins in pigs fed MccJ25-supplemented diets may be due to modulation of the gut environment, enhancing gut barrier function by increasing occludin, zonula occludens-1, and tight junction protein expression and neutralization of endotoxins (Lee et al., 2015; Wan et al., 2016; Yi et al., 2016). Additionally, positive responses in concentrations of D-lactate, DAO, and endotoxins may be associated with improvement in microbiota composition by limiting the increase of competing Enterobacteriaceae, including pathogens and pathobionts, improving beneficial intestinal microbial balance.
After weaning, major changes occur in the composition of intestinal microbiota, providing an opportunity for pathogenic coliforms and other bacteria to invade, causing gastric disorders and hence reduced performance (Jin et al., 2008a,b). Recently, many studies reported that dietary supplementation with AMP, such as a fusion peptide of lactoferricin and lactoferrampin, potato protein, AMP A5 (A3), and cecropin AD, beneficially affects the host animal by reducing the total numbers of aerobes while simultaneously enhancing the total numbers of anaerobes and beneficial Lactobacillus in the intestines of weaned pigs (Jin et al., 2008a; Tang et al., 2012; Wu et al., 2012; Yoon et al., 2013, 2014). In the current study, real-time PCR was performed for quantitative detection of total bacteria, E. coli, Lactobacillus, and Bifidobacterium. Results showed that the pigs fed diets supplemented with MccJ25 had decreased numbers of E. coli and increased amounts of Lactobacillus and Bifidobacterium in feces; the pigs fed a diet supplemented with colistin sulfate had increased numbers of Lactobacillus, but the numbers of Bifidobacterium were not affected. These findings for microflora populations are consistent with a study that found a diet supplemented with the AMP Ple can increase Bifidobacterium compared with CON but that colistin sulfate did not significantly affect the numbers of Bifidobacterium compared with CON (Wan et al., 2016). Additionally, several previous studies have observed positive effects of AMP and AB on microbiota composition (Li et al., 2000; Blake et al., 2003; Yi et al., 2016). Recently, a study reported that microcin-producing E. coli Nissle 1917 limited growth of competitors in an inflamed intestine, including commensal E. coli, adherent-invasive E. coli, and Salmonella enterica (Sassone-Corsi et al., 2016). In this study, pigs fed 1.0 and 2.0 mg/kg MccJ25 and ABD had significantly increased concentrations of lactate and SCFA in feces. Furthermore, the 2.0 mg/kg MccJ25 diet improved butyrate and propionate concentrations more effectively than the ABD diet on d 14 and 28, respectively. These findings are consistent with the study of Yi et al. (2016), who reported that CWA increased the ratio of Lactobacillus to total bacteria and increased SCFA levels in feces. These results provide evidence that MccJ25 not only modulates microbial composition but also improves the metabolic capabilities of the microbiome in the intestines of a host.
Before the establishment and development of a developed immune system, which generally occurs 2 wk after weaning, neonatal weaned pigs are very susceptible to pathogens and stressors caused by intestinal flora change (Peng et al., 2016). At this stage, given its effects on inflammation and the intestinal barrier and microbiota composition, dietary supplementation with innate immunity factors is important for the growth performance and gut health of weaned pigs (Yoon et al., 2013; Wan et al., 2016; Yi et al., 2016). The optimal dosage of MccJ25 treatment for pig growth, incidence of diarrhea, and gut health was 2.0 mg/kg.
In conclusion, the present study demonstrated that dietary supplementation with MccJ25 can improve growth performance and effectively attenuate diarrhea in weaned pigs. It does so by enhancing intestinal barrier function, improving microbiota composition, or activating the immune response. Therefore, this study indicates that MccJ25 could potentially be used as an alternative to traditional AB feed additives for weaned pigs.
Footnotes
Financial support for this research was provided by the National Key Research and Development Program of China (number 2016YFD0501308) and Agro-scientific Research in the Public Interest (number 201403047).
LITERATURE CITED
- Al-Sadi R. M., Ma T. Y. 2007. IL-1β causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 178:4641–4649. doi: 10.4049/jimmunol.178.7.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed.AOAC Int., Arlington, VA. [Google Scholar]
- Barton M. D. 2000. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 13:279–299. doi: 10.1079/095442200108729106 [DOI] [PubMed] [Google Scholar]
- Blake D. P., Hillman K., Fenlon D. R. 2003. The use of a model ileum to investigate the effects of novel and existing antimicrobials on indigenous porcine gastrointestinal microflora: Using vancomycin as an example. Anim. Feed Sci. Technol. 103:123–139. doi: 10.1016/S0377-8401(02)00286-9 [DOI] [Google Scholar]
- Blond A., Péduzzi J., Goulard C., Chiuchiolo M. J., Barthelemy M., Prigent Y., Salomon R. A., Farias R. N., Moreno F., Rebuffat S. 1999. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur. J. Biochem. 259:747–756. doi: 10.1046/j.1432-1327.1999.00085.x [DOI] [PubMed] [Google Scholar]
- Britton R. A., Young V. B. 2014. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 146:1547–1553. doi: 10.1053/j.gastro.2014.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C. T., Nusrat A. 2009. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788:864–871. doi: 10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. L., Cheng H. C., Wu W. T., Liu Y. J., Liu S. Y. 1998. Supplementation of konjac glucomannan into a low-fiber Chinese diet promoted bowel movement and improved colonic ecology in constipated adults: A placebo-controlled, diet-controlled trial. J. Am. Coll. Nutr. 27:102–108. [DOI] [PubMed] [Google Scholar]
- Choi J. Y., Shinde P. L., Ingale S. L., Kim J. S., Kim Y. W., Kim K. H., Kwon I. K., Chae B. J. 2011. Evaluation of multi-microbe probiotics prepared by submerged liquid or solid substrate fermentation and antibiotics in weaning pigs. Livest. Sci. 138:144–151. doi: 10.1016/j.livsci.2010.12.015 [DOI] [Google Scholar]
- Chou H. T., Wen H. W., Kuo T. Y., Lin C. C., Chen W. J. 2010. Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides 31:1811–1820. doi: 10.1016/j.peptides.2010.06.021 [DOI] [PubMed] [Google Scholar]
- Fjell C. D., Hiss J. A., Hancock R. E., Schneider G. 2011. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 11:37–51. [DOI] [PubMed] [Google Scholar]
- Gao Y., Han F., Huang X., Rong Y., Yi H., Wang Y. 2013. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J. Anim. Sci. 91:5614–5625. doi: 10.2527/jas.2013-6528 [DOI] [PubMed] [Google Scholar]
- Gill E. E., Franco O. L., Hancock R. E. 2015. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 85:56–78. doi: 10.1111/cbdd.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A., Pirri G., Nicoletto S. 2007. Antimicrobial peptides: An overview of a promising class of therapeutics. Open Life Sci. 2:1–33. doi: 10.2478/s11535-007-0010-5 [DOI] [Google Scholar]
- Guo Y. Y., Liu M. L., He X. D., Jiang C. Q., Liu R. L. 2010. Functional changes of intestinal mucosal barrier in surgically critical patients. World J. Emerg. Med. 1:205–208. [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Sahl H. G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557. doi: 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- Heo J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., Nyachoti C. M. 2013. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Jin Z., Yang Y. X., Choi J. Y., Shinde P. L., Yoon S. Y., Hahn T. W., Lim H. T., Park Y. K., Hahm K. S., Joo J. W., Chae B. J. 2008a. Effects of potato (Solanum tuberosum L. cv. Golden valley) protein having antimicrobial activity on the growth performance, and intestinal microflora and morphology in weaning pigs. Anim. Feed Sci. Technol. 140:139–154. doi: 10.1016/j.anifeedsci.2007.12.006 [DOI] [Google Scholar]
- Jin Z., Yang Y. X., Choi J. Y., Shinde P. L., Yoon S. Y., Hahn T. W., Lim H. T., Park Y. K., Hahm K. S., Joo J. W., Chae B. J. 2008b. Potato (Solanum tuberosum L. cv. Golden valley) protein as a novel antimicrobial agent in weanling pigs. J. Anim. Sci. 86:1562–1572. doi: 10.2527/jas.2007-0414 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Knappe T. A., Linne U., Robbel L., Marahiel M. A. 2009. Insights into the biosynthesis and stability of the lasso peptide capistruin. Chem. Biol. 16:1290–1298. doi: 10.1016/j.chembiol.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Lee J. K., Seo C. H., Luchian T., Park Y. 2015. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob. Agents Chemother. 60:495–506. doi: 10.1128/AAC.01998-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. F., Zhang S. M., Li T. Z., Qiao Q. Y., Thacker P. A., Kim J. H. 2000. Effect of feed antibiotics on the performance and intestinal microflora of weaning pigs in China. Asian-Australas. J. Anim. Sci. 13:1554–1560. doi: 10.5713/ajas.2000.1554 [DOI] [Google Scholar]
- Li Q., Zhang Q., Wang M., Zhao S., Ma J., Luo N., Li N., Li Y., Xu G., Li J. 2008. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin. Immunol. 126:67–80. doi: 10.1016/j.clim.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Lin J. 2011. Effect of antibiotic growth promoters on intestinal microbiota in food animals: A novel model for studying the relationship between gut microbiota and human obesity? Front. Microbiol. 2:53. doi: 10.3389/fmicb.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez F. E., Vincent P. A., Zenoff A. M., Salomon R. A., Farias R. N. 2007. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J. Antimicrob. Chemother. 59:676–680. doi: 10.1093/jac/dkm009 [DOI] [PubMed] [Google Scholar]
- McKay D. M., Baird A. W. 1999. Cytokine regulation of epithelial permeability and ion transport. Gut 44:283–289. doi: 10.1136/gut.44.2.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee S. E., Cunningham R., Elliott C. T. 2013. Simultaneous immunochemical detection of four banned antibiotic growth promoters in raw and cooked poultry tissue. Food Addit. Contam., Part A, Chem. Anal. Control Expo. Risk Assess. 30:1270–1278. doi: 10.1080/19440049.2013.801087 [DOI] [PubMed] [Google Scholar]
- Millet S., Maertens L. 2011. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 187:143–144. doi: 10.1016/j.tvjl.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Moeser A. J., Klok C. V., Ryan K. A., Wooten J. G., Little D., Cook V. L., Blikslager A. T. 2006. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G173–G181. doi: 10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- Murray M. J., Barbose J. J., Cobb C. F. 1993. Serum d-lactate levels as a predictor of acute intestinal ischemia in a rat model. J. Surg. Res. 54:507–509. doi: 10.1006/jsre.1993.1078 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl.Acad. Press, Washington, DC. [Google Scholar]
- Pan S. J. 2012. Biosynthesis and engineering of lasso peptides. PhD Diss. Princeton Univ., Princeton, NJ. [Google Scholar]
- Peng Z. X., Wang A. R., Xie L. Q., Song W. P., Wang J., Yin Z., Zhou D. S., Li F. Q. 2016. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned pigs. Sci. Rep. 6:26790. doi: 10.1038/srep26790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J. P., Blazy F., Laffitte J., Sève B., Oswald I. P. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of pigs. J. Nutr. 134:641–647. [DOI] [PubMed] [Google Scholar]
- Praveena P. E., Periasamy S., Kumar A. A., Singh N. 2010. Cytokine profiles, apoptosis and pathology of experimental Pasteurella multocida serotype A1 infection in mice. Res. Vet. Sci. 89:332–339. doi: 10.1016/j.rvsc.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Rebuffat S., Blond A., Destoumieux-Garzon D., Goulard C., Peduzzi J. 2004. Microcin J25, from the macrocyclic to the lasso structure: Implications for biosynthetic, evolutionary and biotechnological perspectives. Curr. Protein Pept. Sci. 5:383–391. doi: 10.2174/1389203043379611 [DOI] [PubMed] [Google Scholar]
- Rong Y. L., Lu Z. Q., Zhang H. W., Zhang L., Song D. G., Wang Y. Z. 2015. Effects of casein glycomacropeptide supplementation on growth performance, intestinal morphology, intestinal barrier permeability and inflammatory responses in Escherichia coli K88 challenged pigs. Anim. Nutr. 1:54–59. doi: 10.1016/j.aninu.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable S., Pons A. M., Gendron-Gaillard S., Cottenceau G. 2000. Antibacterial activity evaluation of microcin J25 against diarrheagenic Escherichia coli. Appl. Environ. Microbiol. 66:4595–4597. doi: 10.1128/AEM.66.10.4595-4597.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomón R. A., Farias R. N. 1992. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J. Bacteriol. 174:7428–7435. doi: 10.1128/jb.174.22.7428-7435.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi M., Nuccio S. P., Liu H., Hernandez D., Vu C. T., Takahashi A. A., Edwards R. A., Raffatellu M. 2016. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 540:280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F., Clark J. E., Overman B. L., Tozel C. C., Huang J. H., Rivier J. E., Blikslager A. T., Moeser A. J. 2010. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G352–G363. doi: 10.1152/ajpgi.00081.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. K., Buccini F. 1986. Use of d-lactic acid measurements in the diagnosis of bacterial infections. J. Infect. Dis. 154:658–664. doi: 10.1093/infdis/154.4.658 [DOI] [PubMed] [Google Scholar]
- Song W. B. 2009. Soluble intercellular adhesion molecule-1, d-lactate and diamine oxidase in patients with inflammatory bowel disease. World J. Gastroenterol. 15:3916–3919. doi: 10.3748/wjg.15.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensland I., Kim J., Bowring B., Collins A., Mansfield J., Pluske J. 2015. A comparison of diets supplemented with a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex, or zinc oxide, on post-weaning diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with enterotoxigenic Escherichia coli. Animal 5:1147–1168. doi: 10.3390/ani5040403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. S., Fatufe A. A., Yin Y. L., Tang Z. R., Wang S. P., Liu Z. Q., Wu X., Li T. J. 2012. Dietary supplementation with recombinant lactoferrampin-lactoferricin improves growth performance and affects serum parameters in pigs. J. Anim. Vet. Adv. 11:2548–2555. doi: 10.3923/javaa.2012.2548.2555 [DOI] [Google Scholar]
- Thacker P. A. 2013. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 4:35. doi: 10.1186/2049-1891-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondruskova H., Slamova R., Trckova M., Zraly Z., Pavlik I. 2010. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned pigs: A review. Vet. Med. (Praha) 55:199–224. [Google Scholar]
- Wan J., Li Y., Chen D. W., Yu B., Chen G., Zheng P., Mao X. B., Yu J., He J. 2016. Recombinant plectasin elicits similar improvements in the performance and intestinal mucosa growth and activity in weaned pigs as an antibiotic. Anim. Feed Sci. Technol. 211:216–226. doi: 10.1016/j.anifeedsci.2015.12.003 [DOI] [Google Scholar]
- Wang X. Q., Yang F., Liu C., Zhou H. J., Wu G. Y., Qiao S. Y., Li D. F., Wang J. J. 2011. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weaning pigs. J. Nutr. 142:7–13. doi: 10.3945/jn.111.147074 [DOI] [PubMed] [Google Scholar]
- Wang Y. Z., Shan T. Z., Xu Z. R., Feng J., Wang Z. Q. 2007. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weaning pigs. Anim. Feed Sci. Technol. 135:263–272. doi: 10.1016/j.anifeedsci.2006.07.013 [DOI] [Google Scholar]
- Wolvekamp M. C. J., Debruin R. W. F. 1994. Diamine oxidase-an overview of historical, biochemical and functional aspects. Dig. Dis. 12:2–14. doi: 10.1159/000171432 [DOI] [PubMed] [Google Scholar]
- Wu S. D., Zhang F. R., Huang Z. M., Liu H., Xie C. Y., Zhang J., Thacker P. A., Qiao S. Y. 2012. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned pigs challenged with Escherichia coli. Peptides 35:225–230. doi: 10.1016/j.peptides.2012.03.030 [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H. S., Li L., Wang Y. F., Huang R. L., Li F. N., Wang S. P., Qiu W. 2014. Effects of antimicrobial peptides in nursery diets on growth performance of pigs reared on five different farms. Livest. Sci. 167:206–210. doi: 10.1016/j.livsci.2014.04.024 [DOI] [Google Scholar]
- Ye D., Ma I., Ma T. 2006. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G496–G504. doi: 10.1152/ajpgi.00318.2005 [DOI] [PubMed] [Google Scholar]
- Yi H. B., Zhang L., Gan Z. S., Xiong H. T., Yu C. H., Du H. H., Wang Y. Z. 2016. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6:25679. doi: 10.1038/srep25679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X. X., Song F. J., Gong Y. H., Tu X. C., Wang Y. X., Cao S. Y., Liu J. N., Lu Z. X. 2013. A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 68:2445–2452. doi: 10.1093/jac/dkt223 [DOI] [PubMed] [Google Scholar]
- Yoon J. H., Ingale S. L., Kim J. S., Kim K. H., Lee S. H., Park Y. K., Lee S. C., Kwon I. K., Chae B. J. 2014. Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weaning pigs. Livest. Sci. 159:53–60. doi: 10.1016/j.livsci.2013.10.025 [DOI] [Google Scholar]
- Yoon J. H., Ingale S. L., Kim J. S., Kim K. H., Lohakare J., Park Y. K., Park J. C., Kwon I. K., Chae B. J. 2013. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weaning pigs. J. Sci. Food Agric. 93:587–592. doi: 10.1002/jsfa.5840 [DOI] [PubMed] [Google Scholar]
- Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- Zhao L., Luo L., Jia W. K., Xiao J., Huang G., Tian G., Li J. W., Xiao Y. B. 2014. Serum diamine oxidase as a hemorrhagic shock biomarker in a rabbit model. PLoS One 9:e102285. doi: 10.1371/journal.pone.0102285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Qin G. X., Sun Z. W., Che D. S., Bao N., Zhang X. D. 2011. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 12:8502–8512. doi: 10.3390/ijms12128502 [DOI] [PMC free article] [PubMed] [Google Scholar]


