ABSTRACT
Interactive effects of supplemental Zn and zilpaterol hydrochloride (ZH) were evaluated in feedlot steers (n = 40; 652 kg ± 14 initial BW) to determine their impact on feedlot performance, blood constituents, and carcass traits. The study was conducted as a randomized complete block design with a 2 × 2 factorial treatment arrangement. Steers were blocked by BW and randomly assigned to treatments. Factors consisted of supplemental Zn (60 or 300 mg/kg diet DM) and ZH (0 or 8.33 mg/kg) in the diets. For diets supplemented with 300 mg Zn/kg DM, 60 mg Zn/kg was supplemented as zinc sulfate and 240 mg Zn/kg was supplemented as zinc oxide, and the diet was fed for 24 d. Zilpaterol hydrochloride was fed for 21 d followed by a 3-d withdrawal. Cattle were housed in partially covered individual feeding pens equipped with automatic waterers and fence-line feed bunks and were fed once daily for ad libitum intake. Plasma samples were collected on d 0 and 21 to assess changes in Zn, plasma urea nitrogen (PUN), glucose, and lactate concentrations, and serum samples were collected on d 21 to assess IGF-1 concentration. On d 25, cattle were weighed and transported 450 km to a commercial abattoir for harvest; HCW and incidence of liver abscesses were recorded. Carcass data were collected after 36 h of refrigeration. Data were analyzed as a mixed model with Zn, ZH, and Zn × ZH as fixed effects; block as a random effect; and steer as the experimental unit. No interaction or effects of Zn or ZH were observed for IGF-1 concentration, plasma glucose, or lactate concentrations (P ≥ 0.25). No interaction between Zn and ZH was observed for PUN concentration, but PUN decreased with ZH (P < 0.01). There were no effects of ZH or Zn on ADG, DMI, final BW, feed efficiency, HCW, back fat, KPH, quality grade, or incidence of liver abscesses (P > 0.05). Zinc supplementation tended (P = 0.08) to improve the proportion of carcasses grading USDA Choice. Feeding ZH decreased yield grade (P = 0.05) and tended to increase LM area (P = 0.07). In conclusion, increasing dietary concentrations of Zn does not impact response to ZH, but feeding ZH altered circulating concentrations of PUN.
Keywords: insulin-like growth factor 1, plasma urea nitrogen, steer, zilpaterol hydrochloride, zinc oxide
INTRODUCTION
Zilpaterol hydrochloride (ZH) is a synthetic β2–adrenergic agonist fed to cattle during the final 20 to 40 d of finishing to enhance skeletal muscle mass while simultaneously reducing adipose tissue deposition. Zilpaterol HCl supplementation commonly results in improved ADG and feed efficiency (Montgomery et al., 2009; Scramlin et al., 2010). In the meta-analysis conducted by Lean et al. (2014), ZH supplementation increased HCW, LM area, and dressing percentage, whereas it decreased 12th-rib fat thickness. Plasma components related to nitrogen metabolism (plasma urea nitrogen [PUN]) have either decreased (Parr et al., 2014) or remained unchanged (Cônsolo et al., 2015) in response to ZH, whereas plasma constituents related to carbohydrate metabolism (i.e., glucose and lactate) were unaffected (Van Bibber-Krueger et al., 2015).
Zinc is an essential component of over 300 enzymes and over 2,000 transcription factors in microorganisms, plants, and animals (Vallee and Falchuk, 1993; Jeong and Eide, 2013) and is essential for protein synthesis through the activation of RNA polymerase (Wu and Wu, 1987). Zinc has also been shown to inhibit protein degradation (Whipple and Koohmaraie, 1991) and is a positive allosteric modulator of agonists binding β2–adrenergic receptors (Swaminath et al., 2002). In addition, feeding less than 100 mg Zn/kg in combination with ractopamine hydrochloride resulted in variable responses in growth (Edenburn et al., 2016; Genther-Schroeder et al., 2016).
We hypothesized that feeding increased concentrations of supplemental Zn in combination with ZH could further enhance skeletal muscle accretion and further alter blood metabolites associated with nitrogen, carbohydrate, and lipid metabolism in cattle. The objectives of this study were to assess changes in plasma zinc and other blood constituents and to evaluate effects on growth and carcass characteristics of finishing steers supplemented with or without ZH and 60 or 300 mg/kg diet DM supplemental Zn.
MATERIALS AND METHODS
Protocols and procedures followed in this study were approved by the Kansas State University Institutional Animal Care and Use Committee. The study was conducted at the Kansas State University Beef Cattle Research Center in Manhattan, KS.
Animal Processing and Handling
Upon arrival at the Kansas State University Beef Cattle Research Center, crossbred steers were allowed ad libitum access to ground brome hay. Approximately 24 h after arrival, steers were assigned a numbered ear tag unique to each animal and individual BW were recorded. Steers received a topical parasiticide (Dectomax pour-on; Zoetis Inc., Florham Park, NJ), a 5-way viral vaccine (Bovi-Shield Gold 5; Zoetis Inc.), and a 7-way clostridial vaccine (Ultrabac 7 Somnubac; Zoetis Inc.). Sixteen days after arrival, steers were implanted with Component E-S with Tylan (200 mg progesterone, 20 mg estradiol benzoate, and 29 mg tylosin tartrate; Elanco Animal Health, Greenfield, IN). Steers were reimplanted with Component TE-IS with Tylan (16 mg estradiol, 80 mg trenbolone acetate, and 29 mg tylosin tartrate; Elanco Animal Health) 3 mo after the initial implant. Steers were housed as a group in dirt-surfaced pens prior to the experiment and fed a finishing ration consisting of 93% concentrate and 7% roughage. Approximately 4 mo after arrival, steers were weighed and placed back into their pens, and 3 d later, they were sorted into experimental pens and fed their respective diets. Body weights were measured prior to feeding on d 0 and 21 and prior to shipment at 0700 h on d 25. To minimize effects of gut fill and potential differences in initial BW, initial BW multiplied by 0.96 were used for performance calculations.
Experimental Design
The study was conducted as a randomized complete block design with a 2 × 2 factorial arrangement of treatments. Factors consisted of 1) diets containing 0 or 8.33 mg ZH/kg diet DM (Merck Animal Health, Millsboro, DE) and 2) diets containing 60 or 300 mg supplemental Zn/kg dietary DM (60Zn or 300Zn, respectively). Sixty milligrams Zn per kilogram diet DM was commonly supplemented to cattle at the Kansas State University Beef Cattle Research Center and is within the range recommended by feedlot consulting nutritionists (Vasconcelos and Galyean, 2007). The high supplemental Zn concentration (300Zn) was above values recommended by consulting feedlot nutritionists (Vasconcelos and Galyean, 2007), but below the maximum tolerable level of 500 mg Zn/kg recommended by the NRC (2000). Both diets contained 60 mg/kg Zn from ZnSO4 provided by the premade trace mineral premix that was included in the final experimental vitamin/mineral premix. For the 300Zn diets, an additional 240 mg/kg diet DM supplemental Zn was included as zinc oxide (Table 1). Zilpaterol hydrochloride was administered for 21 d followed by a 3-d withdraw prior to harvest. Forty crossbred steers (652 kg ± 14 initial BW) were stratified by BW and randomly assigned, within strata (10 blocks), to individual feeding pens. Pens were randomly assigned to treatment.
Table 1.
Diet composition of steers fed 0 or 8.33 mg/kg diet DM zilpaterol hydrochloride (ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively) for 24 d
| No ZH | ZH1 | |||
|---|---|---|---|---|
| Item | 60Zn | 300Zn | 60Zn | 300Zn |
| Ingredient, % DM basis | ||||
| Steam-flaked corn | 53.55 | 53.52 | 53.55 | 53.52 |
| Wet corn gluten feed | 35.00 | 35.00 | 35.00 | 35.00 |
| Wheat straw | 7.00 | 7.00 | 7.00 | 7.00 |
| Feed additive premix2 | 2.16 | 2.16 | 2.16 | 2.16 |
| Vitamin/mineral premix3 | 0.17 | 0.17 | 0.17 | 0.17 |
| Limestone | 1.82 | 1.82 | 1.82 | 1.82 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Zinc oxide | – | 0.03 | – | 0.03 |
| Calculated nutrient composition4 | ||||
| CP, % | 14.0 | 14.0 | 14.0 | 14.0 |
| Ca, % | 0.75 | 0.75 | 0.75 | 0.75 |
| P, % | 0.51 | 0.51 | 0.51 | 0.51 |
| K, % | 0.70 | 0.70 | 0.70 | 0.70 |
| Zn, mg/kg | 93 | 333 | 93 | 333 |
| NDF, % | 22.7 | 22.7 | 22.7 | 22.7 |
Zilpaterol HCl (Merck Animal Health, Millsboro, DE) was added to the feed additive premix and fed for 21 d at 8.33 mg/kg of diet DM followed by a 3-d withdrawal prior to harvest.
Formulated to provide 300 mg/d monensin and 90 mg/d tylosin (Elanco Animal Health, Greenfield, IN) in a ground corn carrier.
Formulated to provide (on a DM basis) the following added nutrient levels: 2,200 IU/kg vitamin A, 22 IU/kg vitamin E (alpha tocopherol acetate), 0.10 mg/kg Co (cobalt carbonate), 10 mg/kg Cu (copper sulfate), 0.6 mg/kg I (ethylenediamine dihydriodide), 60 mg/kg Mn (manganous sulfate), 0.25 mg/kg Se (sodium selenite), and 60 mg Zn/kg (zinc sulfate).
Calculated from NRC (2000) values for individual ingredients.
Blood Collection
Blood was collected from each steer via jugular venipuncture approximately 2 h prior to feeding on d 0 and 21 using two 6-mL trace mineral–free blood collection tubes (Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ) containing K2 EDTA as anticoagulant for analysis of plasma Zn, glucose, lactate, and PUN concentrations. Additionally, blood was collected using a 10-mL serum sampling tube (Vacutainer; Becton, Dickinson and Company) on d 21 for analysis of serum IGF-1 concentration. Blood samples for plasma analyses were immediately placed on ice and centrifuged within 10 min of sampling. Samples used for analysis of Zn, glucose, lactate, and PUN were centrifuged at 2,494 × g for 20 min at room temperature to recover plasma. Plasma was transferred by pipette to 5-mL plastic tubes, capped, and frozen at −20°C until analysis. Serum tubes were allowed to coagulate for 1 h at 4°C and centrifuged at 2,494 × g for 20 min at room temperature, and serum was transferred by pipette to storage vials and frozen at −20°C.
Housing and Diet Preparation
Steers were randomly assigned to individual, partially covered feeding pens equipped with concrete floors, automatic waterers, and fence-line feed bunks (10 pens/treatment). Steers were housed in 2 barns, each containing 20 individual concrete-surfaced pens. Each pen measured 1.5 by 6 m. Pens were partially covered and were equipped with individual feed bunks; water fountains were shared between adjacent pens. Feed bunks were visually inspected, and allocations of feed were adjusted daily, leaving 0.23 kg/animal of residual feed before feeding the following day. Zilpaterol hydrochloride (8.33 mg/kg diet DM) was incorporated into the feed additive premix, which was subsequently mixed into complete diets. Diets were mixed once daily and hand delivered to individual animals at 0900 h, and animals were allowed ad libitum access to feed. Weights of fresh feed were recorded daily, and unconsumed feed was removed from the bunk and weighed on d 21 and 24 or as needed to determine actual feed intake. A subsample of unconsumed feed was dried in a forced-air oven at 55°C for 48 h to determine DM content.
Harvest
Final BW (gross BW × 0.96) were determined immediately before transporting cattle 450 km to a commercial abattoir in Holcomb, KS. Hot carcass weights and incidence and severity of liver abscesses were recorded the day of harvest. Liver abscesses were scored according to the procedure described by Brown et al., 1975; Elanco Animal Health): 0 = no abscesses; A− = 1 or 2 small abscesses or abscess scars; A0 = 2 to 4 small, well-organized abscesses; and A+ = 1 or more large or active abscesses with or without adhesions). Following 36 h of refrigeration, marbling score, 12th-rib s.c. fat thickness, LM area, and yield grade were obtained using camera (VBG 2000; E+V Technology GmbH & Co. KG, Oranienburg, Germany) images provided by the abattoir, USDA quality grade and incidence and severity of dark cutting beef were determined by a certified USDA grader, and percentage KPH was determined by trained research personnel.
Chemical Analyses
Glucose and lactate concentrations in plasma were analyzed using a YSI 2300 STAT Plus Glucose and L-Lactate Analyzer (YSI Inc., Yellow Springs, OH). Concentrations of PUN were determined according to procedure described by Marsh et al. (1965) using an Auto Analyzer II (Seal Analytical, Inc., Mequon, WI). Insulin-like growth factor-1 was measured using an IGF-1 ELISA kit (Immunodiagnostic Systems Inc., Fountain Hills, AZ) and analyzed using a Wallac Victor 2 1420 Multilabel Counter (PerkinElmer, Inc., Waltham, MA). Plasma samples for Zn analysis were prepared by diluting 1 mL plasma with 3 mL 0.2% lanthanum oxide diluent. Following dilution, samples were analyzed using an atomic absorption spectrometer (PerkinElmer Atomic Absorption Spectrometer 3110; PerkinElmer, Inc.) set at a wavelength of 214 nm.
Statistical Analyses
The study was conducted as a randomized complete block design with a 2 × 2 factorial arrangement of treatments. Feedlot performance and blood constituents were analyzed using the MIXED procedure of SAS version 9.4 (SAS Inst. Inc., Cary, NC). The model included fixed effects of Zn, ZH, day, and 2- and 3-way interactions. The random effect was block, and the experimental unit was steer. Daily feed allocations were analyzed as repeated measures using Zn, ZH, day, and 2- and 3-way interactions as fixed effects; block as the random effect; and steers as the experimental unit. Day was included in the repeated measures statement with animal nested within treatment as the subject and compound symmetry as the covariance structure. Hot carcass weight, dressed yield, KPH, LM area, 12th-rib s.c. fat, yield grade, and marbling score were analyzed using the MIXED procedure of SAS with a model statement that included fixed effects of Zn, ZH, and the interaction between Zn and ZH. The random effect was block and experimental unit was steer. Categorical data, such as liver abscess incidence and quality grade, were analyzed using the GLIMMIX procedure of SAS with fixed effects of Zn, ZH, and interaction between Zn and ZH. The random effect was block, and the experimental unit was steer. Least squares means were separated using the PDIFF option for separation of means. Means were determined to be different at α level ≤ 0.05, and tendencies were declared at 0.05 ≤ P ≤ 0.10.
RESULTS AND DISCUSSION
Growth Performance
Multiple studies have documented effects of ZH and Zn supplementation on finishing cattle, but few have documented the potential for interactive effects between ZH and Zn. We observed no Zn × ZH interactions or effects of Zn on ADG, DMI, or G:F (P ≥ 0.11; Table 2). In agreement, Edenburn et al. (2016) did not observe any interactions for ADG, DMI, or feed efficiency when zinc propionate was supplemented (97 mg/kg total dietary Zn) in combination with ractopamine HCl supplemented at a rate of 400 mg/d. Similarly, Genther-Schroeder et al. (2016) observed no Zn × ractopamine HCl interaction on DMI; however, they observed a linear improvement in ADG and feed efficiency when Zn was supplemented at rates of 30, 60, and 90 mg/kg as a Zn–AA complex in combination with ractopmaine HCl. Differences among results could be attributed to the source or concentration of Zn or the type of β-adrenergic agonist.
Table 2.
Feedlot performance over 24-d feeding period of steers fed 0 or 8.33 mg/kg zilpaterol hydrochloride (ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively)1
| No ZH | ZH | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | 60Zn2 | 300Zn2 | 60Zn2 | 300Zn2 | SEM | Zn | ZH | Zn × ZH |
| Initial BW,3 kg | 648 | 645 | 644 | 638 | 13.62 | 0.21 | 0.10 | 0.66 |
| Final BW,3 kg | 671 | 658 | 676 | 666 | 15.15 | 0.11 | 0.36 | 0.83 |
| DMI, kg/d | 10.57 | 10.22 | 10.90 | 10.28 | 0.53 | 0.35 | 0.71 | 0.80 |
| ADG, kg/d | 0.97 | 0.55 | 1.36 | 1.19 | 0.30 | 0.32 | 0.10 | 0.68 |
| G:F | 0.0860 | 0.0220 | 0.1240 | 0.1140 | 0.0337 | 0.27 | 0.06 | 0.42 |
Zilparterol hydrochloride (Merck Animal Health, Millsboro, DE) was fed for 21 d followed by a 3-d withdrawal prior to harvest.
Sixty milligrams Zn per kilogram was provided as zinc sulfate, and the remaining 240 mg Zn/kg in the 300Zn diet was fed as zinc oxide.
Calculated as BW × 0.96 for all treatments.
Arelovich et al. (2008) and Greene et al. (1988) concluded that supplementing 430 mg Zn/kg diet or 360 mg Zn/d, respectively, had no impact on DMI, ADG, or feed efficiency. In addition, lower Zn supplementation levels ranging from 10 (Kessler et al., 2003) to 75 mg/kg DM (Nunnery et al., 2007) had no effects on DMI, ADG, or feed efficiency when compared with control diets without supplemental Zn. Malcolm-Callis et al. (2000) fed Zn at 20, 100, or 200 mg/kg of diet DM as zinc sulfate to finishing steers and observed no change in ADG, feed efficiency, or final BW during a 112-d finishing trial, but the authors did observe a tendency for decreased DMI with increasing Zn concentration. Additionally, Spears and Kegley (2002) observed that final BW was unaffected by supplementing Zn at 25 mg/kg DM as zinc oxide or zinc proteinate. When assessing overall feedlot performance during ZH supplementation, results from the current study suggest that high concentrations of Zn are not warranted to improve feedlot performance.
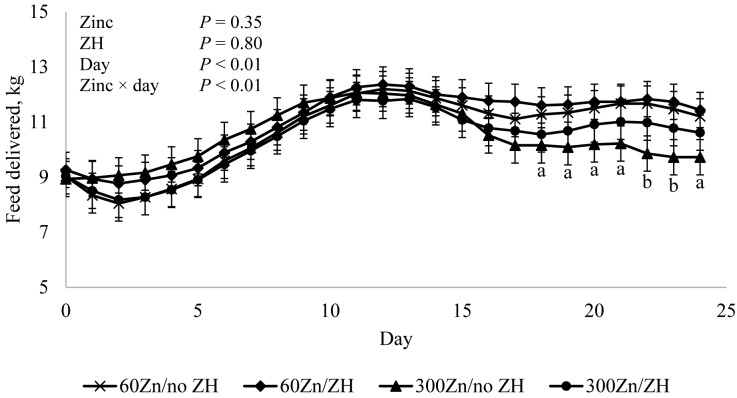
No Zn × ZH × day, Zn × ZH, or ZH × day interactions (P ≥ 0.63; Fig. 1) or effects of Zn or ZH (P ≥ 0.35) were detected for amount of feed delivered daily, but there was an effect of day (P < 0.01). In addition, amounts of feed consumed by steers each day declined near the end of the experimental period for steers fed 300Zn, resulting in a Zn × day interaction (P < 0.01). Changes in the amount of feed delivered were in response to amounts of unconsumed feed remaining in feed bunks 24 h after delivery, reflecting differences in feed consumption. Differences in feed allocated to cattle supplemented with 60Zn or 300Zn did not manifest until d 19 (P > 0.10). Day 18 thru 21 and d 24, cattle supplemented with 300Zn tended to consume less feed than their counterparts supplemented with 60Zn (P ≤ 0.08), and on d 22 and 23, cattle supplemented with 300Zn consumed less feed than cattle supplemented with 60Zn (P = 0.03). Edenburn et al. (2016) and Genther-Schroeder et al. (2016) observed no Zn × ractopamine HCl interactions on DMI when cattle were supplemented with 97 mg/kg Zn as zinc propionate or up to 90 mg Zn/kg as a Zn–AA complex, respectively. Supplementing up to 97 mg Zn/kg in combination with ractopamine HCl may not have been sufficient enough to observe the adverse effect on DMI that was observed toward the end of the present study.
Figure 1.
Feed delivered daily to steers fed 0 or 8.33 mg/kg zilpaterol hydrochloride (ZH; no ZH or ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively). Zilpaterol hydrochloride (Merck Animal Health, Millsboro, DE) was fed for 21 d followed by a 3-d withdrawal prior to harvest. Bunks were monitored and adjusted daily so trace amounts of residual feed remained the following day. Feed delivered was recorded daily on as-fed basis and then converted to DM based on ingredient DM analyses. No zinc × ZH × day, zinc × ZH, or ZH × day interactions (P = 0.63, P = 0.67, and P = 0.92, respectively) were observed. aWithin day, the average feed delivered to cattle supplemented with 300 mg Zn/kg tended to decrease (P ≤ 0.08) compared with the average feed delivered to cattle supplemented with 60 mg Zn/kg, regardless of ZH supplementation. bWithin day, the average feed delivered to cattle supplemented with 300 mg Zn/kg decreased (P ≤ 0.03) compared with the average feed delivered to cattle supplemented with 60 mg Zn/kg, regardless of ZH supplementation.
Malcolm-Callis et al. (2000) supplemented cattle with 20, 100, or 200 mg Zn/kg diet DM and observed that Zn supplementation tended to linearly decrease DMI d 0 to 112. The authors suggested that increased Zn concentration as zinc sulfate may negatively influence palatability; however, it is conceivable that excess Zn adversely affects gut microbiota or may interfere with absorption of other nutrients. Ivan and Grieve (1975) observed a decrease in liver Cu concentration when supplemental Zn was increased to 100 mg/kg, suggesting that Cu absorption may be suppressed by supplementing 100 mg Zn/kg DM. In addition, increased supplemental Zn has been shown to inhibit cellulose digestion (Martinez and Church, 1970).
Zilpaterol hydrochloride supplementation had no effect on DMI (P = 0.71; Table 2) but tended to increase ADG (P = 0.10) and tended to improve feed efficiency (P = 0.06). Responses to ZH supplementation in ADG, DMI, and feed efficiency have varied in previous research. Cônsolo et al. (2015) observed a 17 and 23% increase in ADG and 19 and 21% improvement in feed efficiency when ZH was fed for 20 or 30 d, respectively. Similar improvements in ADG and G:F were observed in earlier literature (Montgomery et al., 2009; Baxa et al., 2010; Parr et al., 2011). Conversely, Boyd et al. (2015) did not observe any differences in ADG, DMI, or G:F when ZH was supplemented for 21 d. In addition, Hilscher et al. (2015) observed no difference in DMI or ADG, but G:F improved when steers were supplemented with ZH for 20 d compared with unsupplemented steers. Hales et al. (2014) and Scramlin et al. (2010) reported a 7 to 9% decrease in DMI with ZH supplementation for 21 or 30 d, respectively, whereas Avendaño-Reyes et al. (2006), Montgomery et al. (2009), and Parr et al. (2011) observed no change in DMI due to ZH. Furthermore, the results of the present study are in contrast with results from work we have previously published (Van Bibber-Krueger et al., 2015) in which ZH supplementation decreased DMI but ADG and G:F were not different between ZH-supplemented steers and control steers. In the meta-analysis conducted by Lean et al. (2014), ZH supplementation increased ADG by 0.15 kg/d, final BW by 8 kg, and G:F by 0.024 but decreased DMI by 0.12 kg/d. Factors such as study design, diet formulation, genetic variation among breeds, age, sex, and environmental factors can contribute to the lack of congruency among research (Mersmann, 1998, 2002). In addition, the low number of experimental units may have contributed to the lack of congruency between the current trial and previous research.
Carcass Characteristics
The effects of Zn and ZH on carcass characteristics are summarized in Table 3. There were no Zn × ZH interactions detected for any carcass traits (P ≥ 0.37). In agreement with the present study, Edenburn et al. (2016) and Genther-Schroeder et al. (2016) did not observe any added benefit to supplementing 1 g/steer Zn from zinc propionate or 30, 60, or 90 mg/kg diet DM Zn from a Zn–AA complex, respectively, in combination with ractopamine HCl for carcass characteristics. In the present trial, it is possible too much Zn was supplemented to the cattle to observe any interactions. Hergenreder (2014) concluded that cAMP production and β-AR protein and mRNA abundance were not affected when bovine cells were treated with ZnCl2 combined with ZH. These results suggest that increasing Zn supplementation may result in increased extracellular free Zn resulting in β-adrenergic receptors becoming overstimulated and an ultimate decrease in synthesis of cAMP (Hergenreder, 2014). There may be a threshold at which Zn supplementation in combination with β-adrenergic agonists begin to have negative effects on β-adrenergic receptors, which may explain the lack of interactions in the present study.
Table 3.
Carcass traits of steers receiving 0 or 8.33 mg/kg zilpaterol hydrochloride (ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively) for a 24-d experiment1
| No ZH | ZH | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | 60Zn2 | 300Zn2 | 60Zn2 | 300Zn2 | SEM | Zn | ZH | Zn × ZH |
| HCW, kg | 438 | 433 | 445 | 440 | 10.15 | 0.83 | 0.21 | 0.96 |
| Dressed yield, % | 65.2 | 65.8 | 65.7 | 65.9 | 0.47 | 0.37 | 0.52 | 0.72 |
| Liver abscess, % | 30.0 | 20.0 | 30.0 | 30.0 | 14.81 | 0.72 | 0.72 | 0.72 |
| LM area, cm2 | 97.2 | 93.5 | 99.4 | 100.0 | 2.52 | 0.46 | 0.07 | 0.37 |
| 12th-rib s.c. fat, cm | 1.32 | 1.37 | 1.22 | 1.35 | 0.10 | 0.37 | 0.48 | 0.76 |
| USDA yield grade | 2.7 | 3.0 | 2.4 | 2.4 | 0.22 | 0.50 | 0.05 | 0.50 |
| Marbling score3 | 481 | 492 | 503 | 523 | 23.8 | 0.52 | 0.27 | 0.85 |
| KPH, % | 1.60 | 1.70 | 1.45 | 1.75 | 0.17 | 0.09 | 0.66 | 0.39 |
| Select, % | 20 | 0 | 10 | 0 | 8.33 | 0.08 | 0.55 | 0.55 |
| Choice, % | 80 | 100 | 90 | 100 | 8.33 | 0.08 | 0.55 | 0.55 |
Zilpaterol hydrochloride (Merck Animal Health, Millsboro, DE) was fed for 21 d followed by a 3-d withdrawal prior to harvest.
Sixty milligrams Zn per kilogram was provided as zinc sulfate, and the remaining 240 mg Zn/kg in the 300Zn diet was fed as zinc oxide.
Marbling scores were determined by a USDA grader: Small = 400 to 499; Modest = 500 to 599.
Regardless of ZH supplementation, carcasses from cattle supplemented with 300Zn tended to have a greater KPH percentage (P = 0.09), fewer carcass that graded Select (P = 0.08), and 15% more carcasses that graded Choice (P = 0.08) compared with carcasses from cattle supplemented with 60Zn. There were no effects of Zn supplementation for HCW, dressing percentage, percent liver abscesses, LM area, 12th-rib fat thickness, or USDA yield grade or marbling score (P ≥ 0.37). In agreement, Greene et al. (1988), Huerta et al. (2002), and Nunnery et al. (2007) observed no differences in carcass traits from steers supplemented with Zn at 200 mg/kg DM (from zinc sulfate or zinc methionine), 360 mg/d (from zinc oxide), or 75 mg/kg diet DM (from zinc sulfate, zinc methionine, or zinc propionate), respectively. Conversely, Malcolm-Callis et al. (2000) observed a quadratic decrease for 12th-rib fat thickness and quadratic increase for yield grade in carcasses from cattle supplemented with 20, 100, or 200 mg/kg diet DM Zn as zinc sulfate. Spears and Kegley (2002) observed an increase in marbling score and quality grade and a tendency for increased yield grade and 12th-rib fat thickness in carcasses from steers supplemented with Zn at 25 mg/kg DM, regardless of source, compared with carcasses from nonsupplemented steers. The authors suggested that the Zn content of the basal ration was slightly below NRC (2000) recommendations, which may have resulted in a greater response in carcass traits compared with studies in which the basal ration contained adequate Zn. In addition, differences may be attributed to the initial Zn status of animals prior to starting experiments, which can potentially impact responses to Zn supplementation. Zinc is a diverse trace mineral that directly or indirectly participates in numerous enzymes within mammals, making it difficult to determine the precise cause for lack of congruence among studies. In an in vitro trial, Oh and Choi (2004) reported an increase in lipogenic activity in bovine intramuscular adipocytes with increasing Zn concentration. These results may partially explain the tendency for increased quality grade and percent KPH in the current study and the increased yield grade and 12th-rib fat thickness observed by Malcolm-Callis et al. (2000) and Spears and Kegley (2002).
Zilpaterol hydrochloride supplementation reduced USDA yield grade (P = 0.05) and tended to increase LM muscle area (P = 0.07) compared with carcasses from cattle that were not fed ZH; however, there were no differences in HCW, dressed yield percentage, liver abscess percentage, 12th-rib fat thickness, marbling score, percent KPH, and carcasses grading Select or Choice (P ≥ 0.27) between carcasses of cattle fed diets with ZH and carcasses of cattle fed diets without ZH. The lack of an effect on HCW and dressing percentage in the current trial was not expected and is in disagreement with previously published literature. Previous results indicate increased HCW ranged from 11 kg when ZH was fed for 20 or 40 d (Holland et al. [2010] and Plascencia et al. [2008], respectively) to 22 kg when ZH was fed for 30 d (Avendaño-Reyes et al., 2006). In the meta-analysis by Lean et al. (2014), ZH was noted to increase HCW by 15 kg and dressing percentage by 1.7%. Conversely, Cônsolo et al. (2015) observed no difference in HCW when cattle were supplemented with ZH for 20 d, but after 30 d of supplementation, HCW increased with ZH supplementation whereas dressing percentage increased regardless of duration of supplementation. Elam et al. (2009) fed ZH for 0, 20, 30, or 40 d and observed linear increases in HCW. Cattle in the current trial were supplemented with ZH for 21 d, which may have been insufficiently long to detect a difference in HCW, given the small number of cattle. Decreased yield grade in the present study is consistent with results previously reported by Scramlin et al. (2010), Hales et al. (2014), and Hilscher et al. (2015) with ZH supplementation, and the 4.36 cm2 increase in LM area in the current study is less than the average increase (8 cm2) reported by Lean et al. (2014), although it is within the range of observations used in their meta-analysis.
Other than yield grade, carcass trait responses associated with adipose tissue (i.e., 12th-rib fat thickness, marbling score, KPH, and quality grade) have not been consistent in carcasses from ZH-supplemented cattle compared with carcasses from unsupplemented cattle. In agreement with the current results, Van Bibber-Krueger et al. (2015) observed no difference in percent KPH, 12th-rib fat thickness, marbling score, or percent of carcasses that graded Choice or Select when ZH was supplemented for 23 d, whereas Holland et al. (2010) observed fewer carcasses grading Choice and more carcasses grading Select with ZH administration following 20 d of ZH administration, regardless of withdrawal time. Elam et al. (2009) observed poorer marbling scores, decreased KPH, and decreased 12th-rib fat thickness with ZH supplementation, which is in agreement with Vasconcelos et al. (2008). Miller et al. (2012) indicated ZH had minimal effects on adipose tissue metabolism, which, in combination with genetic variation among breeds, age of cattle, and the low number of experimental units in the current trial, could contribute to the differences observed among trials for marbling score, 12th-rib fat thickness, and quality grade. Additionally, the lack of differences in the present study could be attributed to the size of the steers at study initiation. Steers in the present study were heavier when ZH was supplemented compared with most cattle in previous research, suggesting heavier cattle supplemented with ZH may not respond as profoundly as lighter cattle supplemented with ZH.
Blood Metabolites
The second objective of this trial was to assess some of the underlying metabolic changes occurring with Zn and ZH supplementation. There were no Zn × ZH × day interactions for plasma glucose, plasma lactate, PUN, or plasma Zn (P ≥ 0.16; Table 4).
Table 4.
Concentrations of plasma metabolites on d 0 and 21 from steers fed 0 or 8.33 mg/kg zilpaterol hydrochloride (ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively)1
| Day 0 | Day 21 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No ZH | ZH | No ZH | ZH | ||||||
| Item | 60Zn2 | 300Zn2 | 60Zn2 | 300Zn2 | 60Zn2 | 300Zn2 | 60Zn2 | 300Zn2 | SEM |
| Glucose, mM | 5.80 | 4.88 | 4.61 | 5.50 | 5.74 | 6.46 | 5.43 | 5.54 | 0.61 |
| Lactate, mM | 6.26 | 4.28 | 4.79 | 5.32 | 3.96 | 5.69 | 3.34 | 3.83 | 1.19 |
| PUN,3,4,5,6 mM | 4.72 | 4.82 | 4.51 | 4.40 | 4.21 | 4.61 | 3.47 | 3.33 | 0.22 |
| Zinc,6,7,8 mg/L | 1.32 | 1.40 | 1.45 | 1.39 | 1.46 | 1.81 | 1.53 | 1.67 | 0.07 |
Zilpaterol hydrochloride (Merck Animal Health, Millsboro, DE) was fed for 21 d followed by a 3-d withdrawal prior to harvest.
Sixty milligrams Zn per kilogram was provided as zinc sulfate, and the remaining 240 mg Zn/kg in the 300Zn diet was fed as zinc oxide.
PUN = plasma urea nitrogen.
Zilpaterol hydrochloride × day interaction (P = 0.03).
Effect of ZH (P < 0.01).
Effect of day (P < 0.01).
Zinc × day interaction (P = 0.01).
Effect of Zn (P = 0.01).
Zinc plays an important role in the production, storage, and action of insulin (Bellia et al., 2013). Therefore, plasma glucose concentration may be affected by increased concentrations of supplemental Zn. There were no Zn × ZH or Zn × day interactions or effects of Zn or day for plasma glucose or lactate (P ≥ 0.16). Arelovich et al. (2008) fed Zn at 430 mg/kg DM (zinc chloride) to beef cattle and observed no change in serum glucose concentration between steers supplemented with Zn and control steers. In agreement, no changes in serum or plasma glucose concentrations were observed when 80 and 140 mg Zn/kg DM (as zinc sulfate) were added to the diets of buffalo calves (Ramulu et al., 2015) or when 50, 100, or 150 mg Zn/kg DM (as zinc sulfate) was included in diets for lambs (Wang et al., 2006). In contrast, Daghash and Mousa (1999) observed increased serum glucose concentration due to Zn supplementation in buffalo calves under heat stress. This difference may be due to the environment in which the calves were maintained.
Glucose and lactate concentrations are important components of cellular metabolism and could potentially provide energy necessary for greater protein deposition during periods of β-agonist administration. Limited research is available comparing the effects of ZH supplementation on plasma glucose and lactate. No ZH × day interaction or effect of ZH was observed for plasma glucose or lactate (P ≥ 0.29). In agreement with the current results, Van Bibber-Krueger et al. (2015) observed no change in plasma glucose or lactate concentration between control and ZH-supplemented steers after 23 d of ZH administration. These results are further supported by Cônsolo et al. (2015), who reported no change in serum glucose concentration following ZH supplementation for 30 d. Eisemann et al. (1988) observed an initial change in glucose concentration after 8 mg/d of clenbuterol was administered to steers, but after 9 d of feeding clenbuterol, the glucose concentration was not different between control animals and clenbuterol-supplemented animals. Plasma lactate concentrations previously reported in steers revealed an increased plasma lactate concentration with addition of cimaterol (Byrem et al., 1998) and clenbuterol (Eisemann et al., 1988), suggesting an increase in peripheral glycolysis (Eisemann et al., 1988). Lack of congruence among results may be due to differences in β-agonist administered; however, plasma glucose concentrations in the present study are in agreement with results previously reported by Van Bibber-Krueger et al. (2015).
Plasma urea nitrogen can be used as an indicator of protein intake and protein degradation. Froetschel et al. (1990) indicated that feeding 1,142 mg Zn/kg DM decreased ruminal AA digestion, decreased synthesis of bacterial AA, and decreased abomasal passage of total AA as a percentage of AA intake. These results suggest that increased concentrations of supplemental Zn may affect ruminal protein metabolism. In addition, Zn is an essential component of glutamic dehydrogenase and is required for urea synthesis (Vallee, 1959) and RNA polymerase (Vallee and Falchuk, 1993), making it essential for protein synthesis. There were no Zn × ZH or Zn × day interactions or effects of Zn on PUN concentration (P ≥ 0.25) in the current trial. In agreement, there were no differences observed in serum urea nitrogen concentrations due to supplementation of bulls with 10 mg/kg DM Zn as zinc oxide, zinc proteinate, or zinc polysaccharide (Kessler et al., 2003) or in heifers supplemented with 200 mg/kg DM Zn as zinc sulfate or zinc methionine (Huerta et al., 2002). Daghash and Mousa (1999) observed decreased serum urea nitrogen in buffalo calves receiving 50 or 100 mg Zn/kg diet compared with control calves. The reduced serum urea nitrogen concentration observed by Daghash and Mousa (1999) may be a reflection of increased Zn requirements for younger ruminants compared with the older animals in the current trial.
Zilpaterol hydrochloride has consistently increased carcass protein deposition (Leheska et al., 2009; Shook et al., 2009), and therefore, urea nitrogen concentration might be expected to decrease in response to ZH supplementation (Parr et al., 2014). A ZH × day interaction was detected for PUN concentration, with PUN concentration decreasing following ZH supplementation. These results suggest decreased protein degradation or increased protein synthesis in response to ZH supplementation. Consistent with current results, supplementing cattle with clenbuterol (Ricks et al., 1984), cimaterol (Quirke et al., 1988), or ZH (Parr et al., 2014; Van Bibber-Krueger et al., 2015) resulted in decreased urea nitrogen concentrations. Conversely, Cônsolo et al. (2015) observed no change in serum urea nitrogen concentrations in heifers supplemented with ZH for 30 d. Differences in results may be reflective of the duration of ZH administration. It is conceivable that cattle may be more responsive to ZH supplementation early in the feeding period, but over time, cells may become desensitized to ZH supplementation; however, current research suggested PUN concentration was reduced when ZH was administered for 21 d.
No Zn × ZH or ZH × day interactions were observed (P ≥ 0.17) for plasma Zn concentration. A Zn × day interaction was detected (P = 0.01), in which plasma Zn concentrations were greater for cattle supplemented with 300Zn on d 21 compared with plasma Zn concentrations on d 0. Results for plasma or serum Zn concentration have been variable in previous literature. In agreement, Huerta et al. (2002) observed increased serum Zn concentration after supplementing heifers 200 mg/kg diet DM Zn as zinc sulfate or zinc methionine. In contrast, supplementing 430 mg/kg diet DM Zn as zinc chloride (Arelovich et al., 2008); 20, 100, or 200 mg/kg diet DM Zn as zinc sulfate (Malcolm-Callis et al., 2000); or 360 mg/d Zn as zinc oxide or zinc methionine (Greene et al., 1988) resulted in no increase in serum Zn. Stress or disease causes rapid redistribution of Zn from extracellular tissue, thereby reducing concentrations of Zn in blood (Hambidge et al., 1986), which may contribute to differences observed for plasma or serum Zn concentrations among studies.
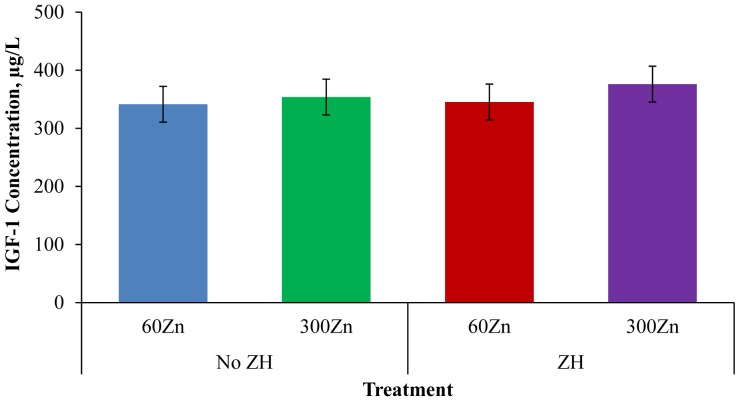
Insulin-like growth factor-1 production is stimulated, in part, by GH and potentially could be impacted by the addition of Zn or ZH to finishing cattle diets. In particular, Zn deficiency consistently resulted in decreased circulating IGF-1 concentrations, which may reflect the role of Zn in IGF-1 induction of cell proliferation (MacDonald, 2000). Insulin-like growth factor-1 concentrations were analyzed on d 21 following ZH supplementation (Fig. 2). No Zn × ZH interaction was observed (P = 0.75), and IGF-1 concentration was not affected by Zn supplementation (P = 0.45). Jafarpour et al. (2015) supplemented 58, 118, or 163 mg/kg diet DM Zn as zinc methionine to sheep and observed a linear increase in plasma IGF-1 concentration as supplemental Zn increased. The difference in results may be due to different sources of Zn used, species, or initial trace mineral status of the animals.
Figure 2.
Day-21 serum IGF-1concentrations of steers fed 0 or 8.33 mg/kg zilpaterol hydrochloride (ZH) and 60 or 300 mg supplemental Zn/kg diet DM (60Zn or 300Zn, respectively). The first 60 mg Zn/kg was provided as zinc sulfate and the remaining 240 mg Zn/kg in the 300Zn diet was supplemented as zinc oxide. Zilpaterol hydrochloride (Merck Animal Health, Millsboro, DE) was fed for 21 d followed by a 3-d withdrawal prior to harvest. No Zn × ZH interaction, effect of Zn, or effect of ZH (P = 0.75, P = 0.45, and P = 0.65, respectively) was detected.
Serum IGF-1 concentration was not impacted by ZH supplementation in the current trial (P = 0.65). Parr et al. (2014) observed no difference in IGF-1 mRNA concentrations in the LM of cattle supplemented with or without ZH for 20 d after correcting for initial IGF-1 mRNA differences between treatments prior to ZH supplementation. In agreement with the current results, Dawson et al. (1993) observed no difference in plasma IGF-1 concentration from steers that received 1.5 mg/kg diet cimaterol for 23 wk. In contrast, Beermann et al. (1987) observed decreased plasma IGF-1 levels in lambs supplemented with 10 mg cimaterol/kg diet. Differences in response to cimaterol supplementation may be due to species differences, but the current results with ZH supplementation are in agreement with Dawson et al. (1993), who suggested that growth promoting effects in cattle may not be directly mediated through increased IGF-1 concentration.
In conclusion, supplementing increased concentrations of Zn in diets of finishing steers fed ZH during the final 21 d of finishing does not further enhance feedlot performance, carcass traits, or blood parameters associated with increased growth; however, increased dietary concentrations of supplemental Zn may have adverse effects on feed consumption with prolonged feeding.
FOOTNOTES
Contribution number 17-363-J from the Kansas Agricultural Experiment Station.
LITERATURE CITED
- Arelovich H. M., Laborde H. E., Amela M. I., Torrea M. B., Martínez M. F. 2008. Effects of dietary addition of zinc and (or) monensin on performance, rumen fermentation and digesta kinetics in beef cattle. Span. J. Agric. Res. 6:362–372. doi: 10.5424/sjar/2008063-329 [DOI] [Google Scholar]
- Avendaño-Reyes L., Torres-Rodríguez V., Meraz-Murillo F. J., Pérez-Linares C., Figueroa-Saavedra F., Robinson P. H. 2006. Effects of two β-adrenergic agonists on finishing performance, carcass characteristics, and meat quality of feedlot steers. J. Anim. Sci. 84:3259–3265. doi: 10.2527/jas.2006-173 [DOI] [PubMed] [Google Scholar]
- Baxa T. J., Hutcheson J. P., Miller M. F., Brooks J. C., Nichols W. T., Streeter M. N., Yates D. A., Johnson B. J. 2010. Additive effects of a steroidal implant and zilpaterol hydrochloride on feedlot performance, carcass characteristics, and skeletal muscle messenger ribonucleic acid abundance in finishing steers. J. Anim. Sci. 88:330–337. doi: 10.2527/jas.2009-1797 [DOI] [PubMed] [Google Scholar]
- Bellia F., Pietropaolo A., Grasso G. 2013. Formation of insulin fragments by insulin-degrading enzyme: The role of zinc (II) and cystine bridges. J. Mass Spectrom. 48:135–140. doi: 10.1002/jms.3060 [DOI] [PubMed] [Google Scholar]
- Beermann D. H., Butler W. R., Hogue D. E., Fishell V. K., Dalrymple R. H., Ricks C. A., Scanes C. G. 1987. Cimaterol-induced muscle hypertrophy and altered endocrine status in lambs. J. Anim. Sci. 65:1514–1524. doi: 10.2527/jas1987.6561514x [DOI] [PubMed] [Google Scholar]
- Boyd B. M., Shackelford S. D., Hales K. E., Brown-Brandl T. M., Bremer M. L., Spangler M. L., Wheeler T. L., King D. A., Erickson G. E. 2015. Effects of shade and feeding zilpaterol hydrochloride to finishing steers on performance, carcass quality, heat stress, mobility, and body temperature. J. Anim. Sci. 93:5801–5811. doi: 10.2527/jas.2015-9613 [DOI] [PubMed] [Google Scholar]
- Brown H., Bing R. F., Grueter H. P., McAskill J. W., Cooley C. O., Rathmacher R. P. 1975. Tylosin and chlortetracycline for the prevention of liver abscesses, improved weight gains and feed efficiency in feedlot cattle. J. Anim. Sci. 40:207–213. doi: 10.2527/jas1975.402207x [DOI] [PubMed] [Google Scholar]
- Byrem T. M., Beermann D. H., Robinson T. F. 1998. The beta-agonist cimaterol directly enhances chronic protein accretion in skeletal muscle. J. Anim. Sci. 76:988–998. [DOI] [PubMed] [Google Scholar]
- Cônsolo N. R. B., Rodriguez F. D., Goulart R. S., Frasseto M. O., Ferrari V. B., Silva L. F. 2015. Zilpaterol hydrochloride improves feed efficiency and changes body composition in nonimplanted Nellore heifers. J. Anim. Sci. 93:4948–4955. doi: 10.2527/jas.2015-9291 [DOI] [PubMed] [Google Scholar]
- Daghash H. A., Mousa S. M. 1999. Zinc sulfate supplementation to ruminant rations and its effects on digestibility in lamb; growth, rectal temperature and some blood constituents in buffalo calves under heat stress. Assiut Vet. Med. J. 40:128–146. [Google Scholar]
- Dawson J. M., Craigon J., Buttery P. J., Beever D. E. 1993. Influence of diet and β-agonist administration on plasma concentrations of growth hormone and insulin-like growth factor-1 in young steers. Br. J. Nutr. 70:93–102. doi: 10.1079/BJN19930107 [DOI] [PubMed] [Google Scholar]
- Edenburn B. M., Kneeskern S. G., Bohrer B. M., Rounds W., Boler D. D., Dilger A. C., Felix T. L. 2016. Effects of supplementing zinc or chromium to finishing steers fed ractopamine hydrochloride on growth performance, carcass characteristics, and meat quality. J. Anim. Sci. 94:771–779. doi: 10.2527/jas.2015-9979 [DOI] [PubMed] [Google Scholar]
- Eisemann J. H., Huntington G. B., Ferrell C. L. 1988. Effects of dietary clenbuterol on metabolism of the hindquarters in steers. J. Anim. Sci. 66:342–353. doi: 10.2527/jas1988.662342x [DOI] [PubMed] [Google Scholar]
- Elam N. A., Vasconcelos J. T., Hilton G., VanOverbeke D. L., Lawrence T. E., Montgomery T. H., Nichols W. T., Streeter M. N., Hutchinson J. P., Yates D. A., Galyean M. L. 2009. Effect of zilpaterol hydrochloride duration of feeding on performance and carcass characteristics of feedlot cattle. J. Anim. Sci. 87:2133–2141. doi: 10.2527/jas.2008-1563 [DOI] [PubMed] [Google Scholar]
- Froetschel M. A., Martin A. C., Amos H. E., Evans J. J. 1990. Effects of zinc sulfate concentration and feeding frequency on ruminal protozoal numbers, fermentation patterns and amino acid passage in steers. J. Anim. Sci. 68:2874–2884. [DOI] [PubMed] [Google Scholar]
- Genther-Schroeder O. N., Branine M. E., Hansen S. L. 2016. The effects of increasing supplementation of zinc-amino acid complex on growth performance, carcass characteristics, and inflammatory response of beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 94:3389–3398. doi: 10.2527/jas.2015-0209 [DOI] [PubMed] [Google Scholar]
- Greene L. W., Lunt D. K., Byers F. M., Chirase N. K., Richmond C. E., Knutson R. E., Schelling G. T. 1988. Performance and carcass quality of steers supplemented with zinc oxide or zinc methionine. J. Anim. Sci. 66:1818–1823. doi: 10.2527/jas1988.6671818x [DOI] [PubMed] [Google Scholar]
- Hales K. E., Shackelford S. D., Wells J. E., King D. A., Hayes M. D., Brown-Brandl T. M., Kuehn L. A., Freetly H. C., Wheeler T. L. 2014. Effects of feeding dry-rolled corn-based diets with and without wet distillers grains with solubles and zilpaterol hydrochloride on performance, carcass characteristics, and heat stress in finishing beef steers. J. Anim. Sci. 92:4023–4033. doi: 10.2527/jas.2014-7638 [DOI] [PubMed] [Google Scholar]
- Hambidge K. M., Casey C. E., Krebs N. F. 1986. Zinc. In: Mertz W. editor, Trace minerals in human and animal nutrition. Vol. 2. Academic Press, Orlando, FL: p. 1–137. doi: 10.1016/B978-0-08-092469-4.50005-4 [DOI] [Google Scholar]
- Hergenreder J. E. 2014. Growth promotant supplementation alters live performance and carcass characteristics while impacting cellular metabolism, gene expression, and protein synthesis. PhD Diss., Texas Tech Univ., Lubbock. TX. [Google Scholar]
- Hilscher F. H., Hussey E. M., Nuttelman B. L., Burken D. B., Griffin W. A., Vander Pol K. J., Hutcheson J. P., Erickson G. E. 2015. Impact of sorting before feeding zilpaterol hydrochloride on feedlot performance and carcass characteristics of yearling steers. J. Anim. Sci. 93:2285–2296. doi: 10.2527/jas.2014-8579 [DOI] [PubMed] [Google Scholar]
- Holland B. P., Krehbiel C. R., Hilton G. G., Streeter M. N., VanOverbeke D. L., Shook J. N., Step D. L., Burciaga-Robules L. O., Stein D. R., Yates D. A., Hutcheson J. P., Nichols W. T., Montgomery J. L. 2010. Effect of extended withdrawal of zilpaterol hydrochloride on performance and carcass traits in finishing beef steers. J. Anim. Sci. 88:338–348. doi: 10.2527/jas.2009-1798 [DOI] [PubMed] [Google Scholar]
- Huerta M., Kincaid R. L., Cronrath J. D., Busboom J., Johnson A. B., Swenson C. K. 2002. Interaction of dietary zinc and growth implants on weight gain, carcass traits and zinc in tissues of growing beef steers and heifers. Anim. Feed Sci. Technol. 95:15–32. doi: 10.1016/S0377-8401(01)00334-0 [DOI] [Google Scholar]
- Ivan M., Grieve C. M. 1975. Effects of zinc, copper, and manganese supplementation of high-concentrate ration on digestibility, growth, and tissue content of Holstein calves. J. Dairy Sci. 58:410–415. doi: 10.3168/jds.S0022-0302(75)84579-6 [DOI] [PubMed] [Google Scholar]
- Jafarpour N., Khorvash M., Rahmani H. R., Pezeshki A., Hosseini Ghaffari M. 2015. Dose–responses of zinc-methionine supplements on growth, blood metabolites and gastrointestinal development in sheep. J. Anim. Physiol. Anim. Nutr. 99:668–675. doi: 10.1111/jpn.12286 [DOI] [PubMed] [Google Scholar]
- Jeong J., Eide D. J. 2013. The SLC39 family of zinc transporters. Mol. Aspects Med. 34:612–619. doi: 10.1016/j.mam.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J., Morel I., Dufey P. A., Gutzwiller A., Stern A., Geyer H. 2003. Effect of organic zinc sources on performance, zinc status and carcass, meat and claw quality in fattening bulls. Livest. Prod. Sci. 81:161–171. doi: 10.1016/S0301-6226(02)00262-2 [DOI] [Google Scholar]
- Lean I. J., Thompson J. M., Dunshea F. R. 2014. A meta-analysis of zilpaterol and ractopamine effects on feedlot performance, carcass traits and shear strength of meat in Cattle. PLoS One 9:e115904. doi: 10.1371/journal.pone.0115904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leheska J. M., Montgomery J. L., Krehbiel C. R., Yates D. A., Hutcheson J. P., Nichols W. T., Streeter M., Blanton J. R., Jr, Miller M. F. 2009. Dietary zilpaterol hydrochloride. II. Carcass composition and meat palatability of beef cattle. J. Anim. Sci. 87:1384–1393. doi: 10.2527/jas.2008-1168 [DOI] [PubMed] [Google Scholar]
- MacDonald R. S. 2000. The role of zinc in growth and cell proliferation. J. Nutr. 130:1500S–1508S. [DOI] [PubMed] [Google Scholar]
- Malcolm-Callis K. J., Duff G. C., Gunter S. A., Kegley E. B., Vermeire D. A. 2000. Effects of supplemental zinc concentration and source on performance, carcass characteristics, and serum values in finishing beef steers. J. Anim. Sci. 78:2801–2808. [DOI] [PubMed] [Google Scholar]
- Marsh W. H., Fingerhut B., Miller H. 1965. Automated and manual direct methods for the determination of blood urea. Clin. Chem. 11:624–627 [PubMed] [Google Scholar]
- Martinez A., Church D. C. 1970. Effect of various mineral elements on in vitro rumen cellulose digestion. J. Anim. Sci. 31:982–990. doi: 10.2527/jas1970.315982x [DOI] [PubMed] [Google Scholar]
- Mersmann H. J. 1998. Overview of the effects of β-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 76:160–172. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J. 2002. Beta-adrenergic receptor modulation of adipocyte metabolism and growth. J. Anim. Sci. 80 (E. Suppl. 1):E24–E29. doi: 10.2527/animalsci2002.0021881200800ES10005x [DOI] [Google Scholar]
- Miller E. K., Chung K. Y., Hutcheson J. P., Yates D. A., Smith S. B., Johnson B. J. 2012. Zilpaterol hydrochloride alters abundance of β-adrenergic receptors in bovine muscle cells but has little effect on de novo fatty acid biosynthesis in bovine subcutaneous adipose tissue explants. J. Anim. Sci. 90:1317–1327. doi: 10.2527/jas.2011-4589 [DOI] [PubMed] [Google Scholar]
- Montgomery J. L., Krehbiel C. R., Cranston J. J., Yates D. A., Hutcheson J. P., Nichols W. T., Streeter M. N., Bechtol D. T., Johnson E., TerHune T., Montgomery T. H. 2009. Dietary zilpaterol hydrochloride. I. Feedlot performance and carcass traits of steers and heifers. J. Anim. Sci. 87:1374–1383. doi: 10.2527/jas.2008-1162 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th rev. ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Nunnery G. A., Vasconcelos J. T., Parsons C. H., Salyer G. B., Defoor P. J., Valdez F. R., Galyean M. L. 2007. Effects of source of supplemental zinc on performance and humoral immunity in beef heifers. J. Anim. Sci. 85:2304–2313. doi: 10.2527/jas.2007-0167 [DOI] [PubMed] [Google Scholar]
- Oh Y. S., Choi C. B. 2004. Effects of zinc on lipogenesis of bovine intramuscular adipocytes. Asian-Australas. J. Anim. Sci. 17:1378–1382. doi: 10.5713/ajas.2004.1378 [DOI] [Google Scholar]
- Parr S. L., Brown T. R., Ribeiro F. R. B., Chung K. Y., Hutcheson J. P., Blackwell B. R., Smith P. N., Johnson B. J. 2014. Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride. J. Anim. Sci. 92:3348–3363. doi: 10.2527/jas.2013-7221 [DOI] [PubMed] [Google Scholar]
- Parr S. L., Chung K. Y., Galyean M. L., Hutcheson J. P., DiLorenzo N., Hales K. E., May M. L., Quinn M. J., Smith D. R., Johnson B. J. 2011. Performance of finishing beef steers in response to anabolic implant and zilpaterol hydrochloride supplementation. J. Anim. Sci. 89:560–570. doi: 10.2527/jas.2010-3101 [DOI] [PubMed] [Google Scholar]
- Plascencia A., Torrentera N. G., Zinn R. A. 2008. Influence of the beta-agonist, zilpaterol, on growth performance and carcass characteristics of feedlot steers. J. Anim. Vet. Adv. 7(10):1257–1260. [Google Scholar]
- Quirke J. F., Allen P., Moloney A. P., Sommer M., Hanrahan J. P., Sheehan W., Roche J. F. 1988. Effects of the beta-agonist cimaterol on blood metabolite and hormone concentrations, growth and carcass composition in finishing Friesian steers. J. Anim. Physiol. Anim. Nutr. 60:128–136. doi: 10.1111/j.1439-0396.1988.tb00186.x [DOI] [Google Scholar]
- Ramulu S. P., Nagalakshmi D., Kumar M. K. 2015. Effect of zinc supplementation on haematology and serum biochemical constituents in Murrah buffalo calves. Indian J. Anim. Res. 49:482–486. doi: 10.5958/0976-0555.2015.00095.3 [DOI] [Google Scholar]
- Ricks C. A., Dalrymple R. H., Baker P. K., Ingle D. L. 1984. Use of a β-agonist to alter fat and muscle deposition in steers. J. Anim. Sci. 59:1247–1255. doi: 10.2527/jas1984.5951247x [DOI] [Google Scholar]
- Scramlin S. M., Platter W. J., Gomez R. A., Choat W. T., McKeith F. K., Killefer J. 2010. Comparative effects of ractopamine hydrochloride and zilpaterol hydrochloride on growth performance, carcass traits, and longissimus tenderness of finishing steers. J. Anim. Sci. 88:1823–1829. doi: 10.2527/jas.2009-2405 [DOI] [PubMed] [Google Scholar]
- Shook J. N., VanOverbeke D. L., Kinman L. A., Krehbiel C. R., Holland B. P., Streeter M. N., Yates D. A., Hilton G. G. 2009. Effects of zilpaterol hydrochloride and zilpaterol hydrochloride withdrawal time on beef carcass cutability, composition, and tenderness. J. Anim. Sci. 87:3677–3685. doi: 10.2527/jas.2009-1816 [DOI] [PubMed] [Google Scholar]
- Spears J. W., Kegley E. B. 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80:2747–2752. [DOI] [PubMed] [Google Scholar]
- Swaminath G., Steenhuis J., Kobilka B., Lee T. W. 2002. Allosteric modulation of β2–adrenergic receptor by Zn2+. Mol. Pharmacol. 61:65–72. doi: 10.1124/mol.61.1.65 [DOI] [PubMed] [Google Scholar]
- Vallee B. L. 1959. Biochemistry, physiology, and pathology of zinc. Physiol. Rev. 39:443–490. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Falchuk K. H. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118. [DOI] [PubMed] [Google Scholar]
- Van Bibber-Krueger C. L., Miller K. A., Parsons G. L., Thompson L. K., Drouillard J. S. 2015. Effects of zilpaterol hydrochloride on growth performance, blood metabolites, and fatty acid profiles of plasma and adipose tissue in finishing steers. J. Anim. Sci. 93:2419–2427. doi: 10.2527/jas.2014-8771 [DOI] [PubMed] [Google Scholar]
- Vasconcelos J. T., Galyean M. L. 2007. Nutritional recommendations of feedlot consulting nutritionists: The 2007 Texas Tech University survey. J. Anim. Sci. 85:2772–2781. doi: 10.2527/jas.2007-0261 [DOI] [PubMed] [Google Scholar]
- Vasconcelos J. T., Rathmann R. J., Reuter R. R., Leibovich J., McMeniman J. P., Hales K. E., Covey T. L., Miller M. F., Nichols W. T., Galyean M. L. 2008. Effects of duration of zilpaterol hydrochloride feeding and days on the finishing diet on finishing cattle performance and carcass traits. J. Anim. Sci. 86:2005–2015. doi: 10.2527/jas.2008-1032 [DOI] [PubMed] [Google Scholar]
- Wang R., Zhu X., Guo F., Zhang W., Jia Z. 2006. Influence of different dietary levels of zinc on performance, vitamin B12, and blood parameters in lambs. Int. J. Vitam. Nutr. Res. 76:353–358. doi: 10.1024/0300-9831.76.6.353 [DOI] [PubMed] [Google Scholar]
- Whipple G., Koohmaraie M. 1991. Degradation of myofibrillar proteins by extractable lysosomal enzymes and m-calpain, and the effects of zinc chloride. J. Anim. Sci. 69:4449–4460. doi: 10.2527/1991.69114449x [DOI] [PubMed] [Google Scholar]
- Wu F., Wu C. 1987. Zinc in DNA replication and transcription. Annu. Rev. Nutr. 7:251–272. doi: 10.1146/annurev.nu.07.070187.001343 [DOI] [PubMed] [Google Scholar]