ABSTRACT
Two studies were conducted to determine whether soybean meal (SBM) use in nursery pig diets can be increased by superdosing with phytase. In Exp. 1, 2,550 pigs (BW of 5.54 ± 0.09 kg) were used to evaluate the optimal level of phytase in low- or high-SBM diets. Two SBM levels (low and high) and 4 phytase doses (0, 1,250, 2,500, and 3,750 phytase units [FTU]/kg) were combined to create 8 dietary treatments in a 2 × 4 factorial arrangement. Pigs were fed a 3-phase feeding program, with each period being 10, 10, and 22 d, respectively. Inclusion of low and high SBM was 15.0 and 25.0%, respectively, for Phase 1; 19.0 and 29.0%, respectively, for Phase 2; and 32.5% for the common Phase 3 diet. Pigs fed diets with high SBM had improved G:F for Phase 1 and 2 and overall (P < 0.01) compared with low-SBM diets. Phytase quadratically improved G:F during Phase 3 and overall (P < 0.05), with the optimum phytase dose being 2,500 FTU/kg. High-SBM diets tended (P = 0.09) to decrease stool firmness (determined daily from d 1 to 10) only on d 2. In Exp. 2, 2,112 pigs (BW of 5.99 ± 0.10 kg) were used to evaluate the impact of high levels of SBM and phytase on performance, stool firmness, mortality, and morbidity in weaned pigs originating from a porcine reproductive and respiratory syndrome (PRRS) virus–positive sow farm. Pigs were fed a 3-phase feeding program as in Exp. 1. Three levels of SBM (low, medium, or high) and 2 phytase levels (600 or 2,600 FTU) were combined to create 6 dietary treatments in a 3 × 2 factorial arrangement. Inclusion of SBM was 15.0, 22.5, and 30.0% for Phase 1 and 20.0, 27.5, and 35.0% for Phase 2 for low, medium, and high SBM, respectively, and 29.0% for the common Phase 3 diet. Inclusion of SBM did not affect growth performance. The percentage of pigs removed for medical treatment linearly declined with increasing SBM levels (P = 0.04). High-SBM diets tended (P < 0.10) to decrease stool firmness during d 4 and 5 and high phytase tended (P < 0.10) to improve stool firmness on d 2 and 4. Analyzed PRRS titers in saliva samples collected on d 20 and 42 confirmed the PRRS status of the pigs; however, viral load was not impacted by dietary treatments (P ≥ 0.11). Results indicate that SBM levels in early nursery diets can be increased without decreasing growth performance and may be favorable in pigs originating from PRRS-positive sow farms by reducing costs of medical treatments. Supplementation of phytase at superdose levels can improve growth performance independently from the level of SBM in the diet.
Keywords: phytase, porcine reproductive and respiratory syndrome, soybean meal, weaned pigs
INTRODUCTION
Soybean meal (SBM) is one of the most prevalent feedstuffs used in swine diets because of its high AA content and consistent quality and availability in the market (Chiba, 2001). The use of SBM in diets of weaned pigs is typically restricted and gradually introduced due to the unfavorable effects of antinutritional factors such as trypsin inhibitor, phytate, lectins, soybean globulins, and nonstarch polysaccharides (Choct et al., 2010). Some of these effects include reductions in growth rate, impaired nutrient digestibility, and compromised intestinal villus architecture (Song et al., 2010). Isoflavones are compounds found in SBM that have antiviral properties against a wide range of viruses, including porcine reproductive and respiratory syndrome (PRRS) virus (Andres et al., 2009; Lunney et al., 2010; Sang et al., 2011). Several studies have shown improved growth performance of pigs during a PRRS infection when high levels of SBM were added to the diets (Greiner et al., 2001b; Rochell et al., 2015).
Dietary supplementation of phytase to improve the availability of phytate-bound P and superdosing with phytase to mitigate antinutritional effects of phytate and to lower phytate esters by almost complete destruction of the phytate molecule are common practices in the animal feed industry (Walk et al., 2014; Holloway et al., 2016a). Although phytase is not often used in nursery diets for young pigs immediately after weaning, it may be a means to mitigate the impact of high phytate levels associated with SBM addition. This may allow the use of higher levels of SBM, thus reducing dependence on alternative proteins that increase diet cost.
The objective of this study was to determine the impact of high inclusion levels of SBM and phytase superdosing on performance and health of newly weaned nursery pigs housed under commercial production conditions.
MATERIALS AND METHODS
Animals were treated humanely, and all practices and procedures used in these experiments were consistent with the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010). The experiments were conducted under the supervision of licensed veterinarians.
Two experiments were conducted in a commercial research facility owned and operated by The Hanor Company, Inc. (White Hall, IL). Research rooms occupy 25% of the 11,000 pig site. In both experiments, pigs were from the same genetic line (Camborough sow × PIC TR-4 sire). In each experiment, pigs were placed into 2 identical nursery rooms equipped with an automated feeding system (Feedlogic Corporation, Willmar, MN) that can blend, weigh, and record feed delivered to individual pens. All pigs were weaned at approximately 20 ± 2 d of age, and they were fed a common nursery starter diet at 0.25 kg/pig on receipt, prior to allotment and implementation of experimental diets. All diets were manufactured in a commercial feed mill (Greenfield, IL) owned and operated by The Hanor Company, Inc. The source of phytase used was of bacterial origin (Quantum Blue; AB Vista, Marlborough, UK).
Experiment 1
A total of 2,550 barrows and gilts with an average weaning age of 20.5 d and initial BW of 5.54 ± 0.09 kg were used in a 42-d trial to determine the optimal level of phytase in diets with low and high inclusion levels of SBM. Pigs were balanced and housed in pens by sex and randomly assigned to 1 of 8 dietary treatments. Pigs were placed in 2 rooms with a combined total of 102 pens (25 pigs per pen). Treatment pens were randomly distributed within each room, with equal representation.
Two SBM levels (low and high) and 4 phytase doses (0, 1,250, 2,500, and 3,750 phytase units [FTU]/kg) were combined to create 8 dietary treatments (Table 1) in a 2 × 4 factorial arrangement. The SBM level in the diet was increased by substantially reducing poultry meal. Pigs were fed a 3-phase feeding program, with each period being 10, 10, and 22 d, respectively. Phase 1 diets contained 15.5 and 25.0% SBM and Phase 2 diets contained 19.0 and 29.0% SBM for the low- and high-SBM treatments, respectively. The Phase 3 diet contained a common level of SBM of 32.5%. Phytase was not given nutrient release values in the diet formulation for Phase 1 and 2 diets, due to the limited substrate level and the inclusion of pharmacological levels of zinc oxide, to specifically determine the impact of superdosing independently of P release. However, a P release value was assigned to phytase for the simple, common SBM-containing Phase 3 diet.
Table 1.
Composition of the experimental diets for Exp. 1, as-fed basis1
| Phase 1 | Phase 2 | Phase 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low SBM2 | High SBM | Low SBM | High SBM | Common SBM | ||||||||
| Phytase, FTU3/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | ||||||||
| Ingredient | 0 | 3,750 | 0 | 3,750 | 0 | 3,750 | 0 | 3,750 | 0 | 3,750 | ||
| Corn, 8.5% CP | 18.85 | 18.75 | 13.55 | 13.38 | 53.10 | 52.97 | 45.15 | 44.91 | 51.77 | 52.72 | ||
| Soybean meal, 47.5% CP | 15.50 | 15.50 | 25.03 | 24.97 | 19.00 | 19.00 | 29.00 | 28.96 | 32.50 | 32.45 | ||
| Corn DDGS4 | 5.00 | 5.00 | 5.00 | 4.99 | 6.00 | 6.00 | 6.00 | 5.99 | 10.00 | 9.99 | ||
| Oats, steamed | 20.00 | 20.00 | 20.00 | 19.97 | – | – | – | – | – | – | ||
| Choice white grease | 3.00 | 3.05 | 5.25 | 5.29 | 3.10 | 3.15 | 4.90 | 4.94 | 2.30 | 1.85 | ||
| Poultry meal, low ash | 8.00 | 7.95 | 0.60 | 0.60 | 8.00 | 8.00 | 4.10 | 4.09 | – | – | ||
| Whey permeate5 | 21.94 | 21.96 | 21.95 | 21.92 | 6.10 | 6.10 | 6.10 | 6.09 | – | – | ||
| Plasma protein6 | 4.25 | 4.25 | 4.25 | 4.24 | 1.25 | 1.25 | 1.25 | 1.25 | – | – | ||
| L-Lys HCL | 0.48 | 0.48 | 0.42 | 0.42 | 0.60 | 0.60 | 0.41 | 0.41 | 0.33 | 0.32 | ||
| DL-Met | 0.20 | 0.20 | 0.21 | 0.20 | 0.22 | 0.22 | 0.18 | 0.17 | 0.10 | 0.09 | ||
| L-Thr | 0.11 | 0.11 | 0.10 | 0.10 | 0.17 | 0.17 | 0.10 | 0.10 | 0.08 | 0.07 | ||
| L-Trp | 0.03 | 0.03 | – | – | 0.06 | 0.06 | 0.02 | 0.01 | – | – | ||
| L-Val | – | – | – | – | 0.09 | 0.09 | – | – | – | – | ||
| Limestone, ground | 0.70 | 0.70 | 1.04 | 1.04 | 0.75 | 0.75 | 0.90 | 0.89 | 1.05 | 0.98 | ||
| Monocalcium phosphate | 0.37 | 0.38 | 1.03 | 1.03 | 0.45 | 0.45 | 0.78 | 0.77 | 1.21 | 0.58 | ||
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | ||
| Copper sulfate | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | ||
| Zinc oxide | 0.31 | 0.31 | 0.31 | 0.31 | 0.26 | 0.26 | 0.26 | 0.26 | – | – | ||
| Organic acid blend7 | 0.20 | 0.20 | 0.20 | 0.20 | – | – | – | – | – | – | ||
| Sweetener | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | – | – | ||
| Choline chloride, 60%, dry | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | ||
| Antibiotic8 | 0.30 | 0.30 | 0.30 | 0.30 | – | – | – | – | – | – | ||
| Vitamin–mineral premix9 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | ||
| Phytase10 | – | 0.08 | – | 0.08 | – | 0.08 | – | 0.08 | – | 0.08 | ||
| Iron oxide, red11 | – | – | – | 0.20 | – | – | – | 0.20 | – | 0.20 | ||
| Calculated nutrient composition | ||||||||||||
| ME, Mcal/kg | 3.53 | 3.53 | 3.57 | 3.57 | 3.49 | 3.49 | 3.54 | 3.54 | 3.37 | 3.37 | ||
| CP, % | 22.74 | 22.71 | 22.39 | 22.37 | 21.71 | 21.71 | 23.23 | 23.22 | 22.82 | 22.9 | ||
| Total Lys, % | 1.58 | 1.57 | 1.54 | 1.54 | 1.56 | 1.56 | 1.55 | 1.55 | 1.41 | 1.41 | ||
| Ca, % | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.77 | 0.64 | ||
| Available P, % | 0.45 | 0.45 | 0.45 | 0.45 | 0.37 | 0.37 | 0.37 | 0.37 | 0.38 | 0.25 | ||
| Phytate P, % | 0.16 | 0.16 | 0.19 | 0.19 | 0.20 | 0.20 | 0.23 | 0.23 | 0.24 | 0.24 | ||
| Analyzed nutrient composition | ||||||||||||
| CP, % | 23.36 | 23.4 | 22.83 | 22.95 | 22.16 | 22.06 | 23.79 | 23.80 | 22.80 | 22.90 | ||
The Phase 1 diet was fed at 1.8 kg/pig and the Phase 2 diet was fed at 5.5 kg/pig. Diets containing 1,250 and 2,500 FTU phytase/kg of were created on the farm by summit blending the appropriate portion of diets with 0 and 3,750 FTU/kg to reach the targeted phytase levels, using an automated feeding system.
SBM = soybean meal.
FTU = phytase units.
DDGS = distiller's dried grains with solubles.
Dairy Lac 80 (International Ingredient Corp., Monett, MO).
AP920 Animal Plasma (APC, Inc., Ankeny, IA).
Kem-Gest (Kemin Industries, Inc., Des Moines, IA).
Pulmotil 30 (Elanco Animal Health, Indianapolis, IN).
Supplied per kilogram of complete diet: 105 mg zinc as zinc sulfate, 100 mg iron as ferrous sulfate, 45 mg manganese as manganous oxide, 15 mg copper as copper sulfate, 0.7 mg iodine as ethylenediamine dihydroiodide, 0.3 mg selenium as sodium selenite, 9,923 IU vitamin A, 1,654 IU vitamin D3, 77.1 mg vitamin E, 3.9 mg vitamin K, 44.1 µg vitamin B12, 9.9 mg riboflavin, 33.1 mg D-pantothenic acid, 55.1 mg niacin, 3.3 mg thiamine, 5.5 mg pyridoxine, 992 µg folic acid, and 276 µg biotin.
Quantum Blue (minimum phytase activity of 5,000 FTU/g; AB Vista, Marlborough, UK).
Used to color code the dietary treatments.
Diets were manufactured by first creating the 2 dietary treatments with 0 and 3,750 FTU phytase/kg within the respective SBM levels for each phase. Diets containing 1,250 and 2,500 FTU/kg were created on the farm by summit blending the appropriate portion of diets with 0 and 3,750 FTU/kg to reach the targeted phytase levels, using the automated feeding system. The Phase 1 and 2 diets were manufactured in pellet form and the Phase 3 diet was manufactured in meal form. Representative feed samples were taken at the feed mill for chemical analysis.
Pig weights and feed disappearance were measured by pen on d 0, 10, 20, and 42 to calculate ADG, ADFI and G:F. Fecal consistency was evaluated by pen, and fecal scoring was initiated on Day 1 after placement for 10 d. The fecal consistency scoring system was the construct of licensed veterinarians who ultimately wanted a procedure to assess the extent to which dietary treatments affected the intestinal health and well-being of pigs. Observations were performed by the same trained individual every day for 10 d. The score was not intended to be a measure of the quantity of scours within a pen but rather the consistency of scour noted for each pen. The evaluator was blinded to dietary treatments being evaluated for fecal consistency within each pen. The fecal scoring system consisted of the following: 0 = normal feces, 1 = soft feces, 2 = fluid feces, and 3 = completely liquid, projectile feces (wet hindquarters).
Experiment 2
A total of 2,112 barrows and gilts with an average age of 21 d and initial BW of 5.99 ± 0.10 kg were used in a 42-d trial to evaluate the potential of high SBM levels in combination with phytase to improve growth and health of weaned pigs originating from a PRRS-positive sow farm. Assessment of PRRS status of the sow farm was based on monthly PRRS tests conducted using real-time quantitative reverse-transcription PCR. However, individual pigs used in the present study were not tested to confirm PRRS-positive status. Pigs were blocked by BW and sex and placed in a total of 96 pens (22 pigs per pen). Pens were randomly assigned within blocks to 1 of 6 dietary treatments.
Three SBM levels (low [considered normal in commercial production], medium, and high) and 2 phytase levels (600 or 2,600 FTU/kg) were combined to create 6 dietary treatments (Table 2) in a 3 × 2 factorial arrangement. Pigs were fed a 3-phase feeding program, with each period being 10, 10, and 22 d, respectively. Phase 1 diets contained 15.0, 22.5, and 30.0% SBM and Phase 2 diets contained 20.0, 27.5, and 35.0% SBM for the low-, medium-, and high-SBM treatments, respectively. The Phase 3 diet contained a common level of SBM of 29%. As in Exp. 1, phytase was not given a nutrient release value in the diet formulation for Phase 1 and 2 diets, but it was given a P release value for the simple, common SBM-containing Phase 3 diet. Representative feed samples were taken at the feed mill for chemical analysis.
Table 2.
Composition of the experimental diets for Exp. 2, as-fed basis1
| Phase 1 | Phase 2 | Phase 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low SBM2 | Medium SBM3 | High SBM | Low SBM | Medium SBM3 | High SBM | Common | |||||||||
| Phytase, FTU4/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | Phytase, FTU/kg | |||||||||
| Ingredient, % | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | |
| Corn, 8.5% CP | 18.80 | 18.80 | 11.92 | 11.92 | 5.04 | 5.04 | 45.23 | 45.23 | 40.28 | 40.28 | 35.33 | 35.33 | 50.68 | 50.64 | |
| Soybean meal, | 15.00 | 15.00 | 22.50 | 22.50 | 30.00 | 30.00 | 20.00 | 20.00 | 27.50 | 27.50 | 35.00 | 35.00 | 29.00 | 29.00 | |
| Corn DDGS5 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 9.00 | 9.00 | 7.50 | 7.50 | 6.00 | 6.00 | 10.40 | 10.40 | |
| Oat groats, steamed, | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | – | – | |
| Whey permeate6 | 21.98 | 21.98 | 21.97 | 21.97 | 21.95 | 21.95 | 6.10 | 6.10 | 6.10 | 6.10 | 6.10 | 6.10 | – | – | |
| Wheat middlings | – | – | – | – | – | – | – | – | – | – | – | – | 5.60 | 5.60 | |
| Plasma protein7 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | – | – | |
| Plant protein product8 | 9.50 | 9.50 | 8.50 | 8.50 | 7.50 | 7.50 | 7.75 | 7.75 | 6.38 | 6.38 | 5.00 | 5.00 | – | – | |
| Choice white grease | 2.15 | 2.15 | 2.93 | 2.93 | 3.70 | 3.70 | 1.50 | 1.50 | 2.15 | 2.15 | 2.80 | 2.80 | 1.30 | 1.30 | |
| L-Lys HCL | 0.39 | 0.39 | 0.20 | 0.20 | – | – | 0.41 | 0.41 | 0.24 | 0.24 | 0.07 | 0.07 | 0.42 | 0.42 | |
| DL-Met | 0.22 | 0.22 | 0.16 | 0.16 | – | – | 0.18 | 0.18 | 0.13 | 0.13 | 0.08 | 0.08 | 0.14 | 0.14 | |
| L-Thr | 0.06 | 0.06 | 0.03 | 0.03 | – | – | 0.06 | 0.06 | 0.03 | 0.03 | – | – | 0.11 | 0.11 | |
| L-Trp | 0.03 | 0.03 | 0.02 | 0.02 | – | – | 0.04 | 0.04 | 0.02 | 0.02 | – | – | 0.04 | 0.04 | |
| Limestone | 0.30 | 0.30 | 0.25 | 0.25 | 0.20 | 0.20 | 1.22 | 1.22 | 1.14 | 1.14 | 1.07 | 1.07 | 1.08 | 1.08 | |
| Monocalcium phosphate | 0.22 | 0.22 | 0.19 | 0.19 | 0.15 | 0.15 | 0.73 | 0.73 | 0.75 | 0.75 | 0.77 | 0.77 | 0.57 | 0.57 | |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.40 | 0.40 | |
| Zinc oxide | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | – | – | |
| Vitamin–mineral premix9 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | |
| Organic acid blend10 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.10 | 0.10 | |
| Choline chloride, 60% | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | – | – | |
| Antibiotic11 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | – | – | |
| Pellet binder | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | – | – | |
| Phytase premix12 | 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | |
| Calculated nutrient composition | |||||||||||||||
| ME, Mcal/kg | 3.54 | 3.54 | 3.58 | 3.58 | 3.61 | 3.61 | 3.39 | 3.39 | 3.42 | 3.42 | 3.45 | 3.45 | 3.37 | 3.37 | |
| CP, % | 23.60 | 23.60 | 25.80 | 25.80 | 28.00 | 28.00 | 23.10 | 23.10 | 24.90 | 24.90 | 26.60 | 26.60 | 22.22 | 22.22 | |
| Total Lys, % | 1.66 | 1.66 | 1.66 | 1.66 | 1.67 | 1.67 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.39 | 1.39 | |
| Standardized ileal digestible Lys, % | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.41 | 1.41 | 1.42 | 1.42 | 1.42 | 1.42 | 1.27 | 1.27 | |
| Ca, % | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.78 | 0.78 | |
| Total P, % | 0.56 | 0.56 | 0.58 | 0.58 | 0.60 | 0.60 | 0.59 | 0.59 | 0.61 | 0.61 | 0.64 | 0.64 | 0.57 | 0.57 | |
| Available P, % | 0.41 | 0.41 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | |
| Phytate P, % | 0.16 | 0.16 | 0.18 | 0.18 | 0.20 | 0.20 | 0.21 | 0.21 | 0.23 | 0.23 | 0.25 | 0.25 | 0.24 | 0.24 | |
| Analyzed nutrient composition | |||||||||||||||
| CP, % | 22.01 | 22.43 | 23.98 | 24.44 | 26.32 | 26.35 | 21.43 | 22.04 | 23.49 | 23.35 | 24.64 | 25.14 | – | – | |
The Phase 1 diet was fed at 1.8 kg/pig and the Phase 2 diet was fed at 5.5 kg/pig.
SBM = soybean meal.
Dietary treatments of medium SBM for Phase 1 and 2 were created on the farm by blending a portion of low- and high-SBM diets of the respective phase using an automated feeding system.
FTU = phytase units.
DDGS = distiller's dried grains with solubles.
Dairy Lac 80 (International Ingredient Corp., Monett, MO).
AP920 Animal Plasma (APC, Inc., Ankeny, IA).
Contained NF8 (Nutraferma, Sioux City, IA), dried fermentation biomass (Ajinomoto Heartland, Inc., Fort Lee, NJ), and NuPro (Alltech, Nicholasville, KY).
Supplied per kilogram of complete diet: 105 mg zinc as zinc sulfate, 100 mg iron as ferrous sulfate, 45 mg manganese as manganous oxide, 15 mg copper as copper sulfate, 0.7 mg iodine as ethylenediamine dihydroiodide, 0.3 mg selenium as sodium selenite, 9,923 IU vitamin A, 1,654 IU vitamin D3, 77.1 mg vitamin E, 3.9 mg vitamin K, 44.1 µg vitamin B12, 9.9 mg riboflavin, 33.1 mg D-pantothenic acid, 55.1 mg niacin, 3.3 mg thiamine, 5.5 mg pyridoxine, 992 µg folic acid, and 276 µg biotin.
AviPlus (Vetagro Inc., Chicago, IL).
Pulmotil 18G (Elanco Animal Health, Indianapolis, IN).
Quantum Blue (minimum phytase activity of 5,000 FTU/g; AB Vista, Marlborough, UK).
Diets were manufactured by first creating dietary treatments with low and high SBM levels with the respective phytase level for Phase 1 and 2. Diets containing medium SBM levels were created on the farm by blending the appropriate portion of diets with low and high SBM, using the automated feeding system. The Phase 1 and 2 diets were manufactured in pellet form and the Phase 3 diet was manufactured in meal form.
Pigs were vaccinated with modified live PRRS vaccine (2 mL of Ingelvac PRRS MLV; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) at 3 d after placement, mycoplasma vaccine (2 mL of Myco Silencer; Intervet Inc., Millsboro, DE) at 8 d after placement, and mycoplasma and circovirus vaccine (2 mL of Ingelvac FLEXCombo; Boehringer Ingelheim Vetmedica, Inc.) at 4 wk after placement.
Pig weights and feed disappearance were measured by pen on d 0, 10, 20, and 42 to calculate ADG, ADFI and G:F for each period and overall. Fecal scoring was initiated on d 1 after placement through d 10 and was assessed by pen using the same methodology as described for Exp. 1
Sick pigs that showed lethargy and anorexia were medically treated and removed from the pen for humane care. An extra pen for each dietary treatment was reserved for sick pigs. Pigs that were removed were tagged with individual identification, weighed, and placed in the appropriate pen according to their dietary treatment. Removed pigs were maintained on their original dietary treatment until the end of the study. This allowed for quantification of the number of pigs that received medicine by dietary treatment. Pigs weighing ≥11.3 kg (this BW represents the mean BW minus 3 SD) at the end of the study were considered full-value pigs. For the calculation of ADG, ADFI, and G:F, dead pigs and sick pigs were taken into account for the days they were in the test pens.
Oral fluid samples were collected to assess viral load of PRRS. Four pens for each treatment were randomly selected to collect saliva samples on d 20 and 42. The samples were taken from the same pens both times. Cotton ropes were placed in the pens to allow the pigs to chew the ropes and contribute to the oral fluid sample. Ropes were suspended within a clean area of the pen to provide access to several pigs at the same time. Ropes were suspended for a minimum of 15 min or until wet. Then, fluid in the rope was squeezed into an individual tube using clean aseptic gloves for each pen, and the tube was immediately capped. Samples were frozen after collection and they were assayed for the presence of PRRS at the Hanor Diagnostic Laboratory (Hanor, Webster City, IA). Viral load was determined using real-time quantitative reverse-transcription PCR using the MagMax-96 viral RNA isolation kit (Thermo Fisher Scientific, Inc., Waltham, MA) and VetMAX NA and EU PRRSV reagents (Thermo Fisher Scientific, Inc.). Viral load is expressed as cycle threshold (Ct) values, defined as the number of cycles required for the fluorescent signal to exceed a threshold. Cycle thresholdvalues are inversely related to the amount of viral RNA detected in the sample. Cycle threshold values of 36 and above suggest that saliva samples were PRRS negative and values of 35 and lower suggest that saliva samples were PRRS positive (Kleiboeker et al., 2005).
Statistical Analyses
Data for Exp. 1 were analyzed using SAS (SAS Inst. Inc., Cary, NC) as a complete randomized design with a 2 × 4 factorial arrangement of treatments with individual pen as the experimental unit. Growth performance and fecal score data were analyzed using the GLM procedure. The model included SBM level, phytase concentrations, room, and their interactions as fixed effects. For performance data, pen average initial BW was used as a covariate. Orthogonal contrast comparisons were conducted to determine linear and quadratic effects of phytase concentrations.
Data for Exp. 2 were analyzed using SAS as a randomized complete block design with a 3 × 2 factorial arrangement of treatments in 16 blocks and individual pen as the experimental unit. Growth performance and fecal score data were analyzed using the GLM procedure, and the model included block, SBM, phytase levels, and the interaction of SBM and phytase levels as fixed effects. Orthogonal contrast comparisons were conducted to determine linear and quadratic effect of SBM levels. The PRRS virus load data were analyzed using the MIXED procedure with repeated measures over time on each experimental unit (pen). The model included SBM, phytase levels, and time and their interactions as fixed effects and pen as a random effect. For both experiments, least squares means were reported and differences were considered statistically significant at P ≤ 0.05, with tendencies at 0.05 < P ≤ 0.10.
RESULTS
Experiment 1
No interactions between SBM levels and phytase supplementation were observed for growth performance. Pigs fed diets with high SBM had lower ADFI during Phase 2 (P < 0.001; Table 3), but ADFI was not affected during other phases or overall. High-SBM diets increased ADG during Phase 1 and 2 (P < 0.05) but not during Phase 3 when the common diet was fed or overall. An increased SBM level improved G:F for Phase 1 and 2 and the overall period (P < 0.01) compared with pigs fed low-SBM diets containing poultry meal. Phytase supplementation linearly increased ADFI and ADG during Phase 1 and improved G:F in quadratic manner during Phase 3 and the overall period (P < 0.05). The greatest G:F was observed for pigs fed the treatment with 2,500 FTU phytase/kg.
Table 3.
Effect of dietary soybean meal (SBM) levels and phytase on growth performance in nursery pigs (Exp. 1)1
| Low SBM | High SBM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phytase, FTU2/kg | Phytase, FTU/kg | ||||||||
| Item | 0 | 1,250 | 2,500 | 3,750 | 0 | 1,250 | 2,500 | 3,750 | SEM |
| BW, kg | |||||||||
| 0 d | 5.4 | 5.5 | 5.5 | 5.6 | 5.5 | 5.5 | 5.6 | 5.5 | 0.2 |
| 10 d3 | 7.1 | 7.2 | 7.2 | 7.4 | 6.9 | 7.1 | 7.1 | 7.1 | 0.1 |
| 20 d4 | 11.2 | 11.4 | 11.5 | 11.5 | 11.0 | 11.3 | 11.3 | 11.1 | 0.1 |
| 42 d | 23.0 | 22.5 | 22.8 | 22.8 | 22.2 | 23.0 | 22.9 | 22.5 | 0.3 |
| ADG, g | |||||||||
| Phase 15 (d 0–10) | 147 | 167 | 165 | 160 | 159 | 179 | 174 | 189 | 7 |
| Phase 26 (d 11–20) | 379 | 390 | 397 | 383 | 393 | 395 | 403 | 397 | 7 |
| Phase 3 (d 21–42) | 507 | 531 | 525 | 516 | 534 | 499 | 525 | 514 | 9 |
| Overall | 393 | 412 | 410 | 402 | 413 | 400 | 413 | 411 | 6 |
| ADFI, g | |||||||||
| Phase 17 (d 0–10) | 154 | 168 | 163 | 167 | 161 | 165 | 169 | 174 | 5 |
| Phase 28 (d 11–20) | 479 | 496 | 497 | 503 | 468 | 473 | 472 | 475 | 8 |
| Phase 3 (d 21–42) | 810 | 820 | 814 | 799 | 842 | 785 | 791 | 802 | 16 |
| Overall | 577 | 589 | 585 | 580 | 593 | 565 | 566 | 577 | 10 |
| G:F, g/kg | |||||||||
| Phase 19 (d 0–10) | 953 | 995 | 1,013 | 960 | 986 | 1,085 | 1,031 | 1,085 | 35 |
| Phase 210 (d 11–20) | 791 | 786 | 798 | 761 | 840 | 835 | 854 | 837 | 12 |
| Phase 311 (d 21–42) | 625 | 646 | 644 | 645 | 633 | 635 | 663 | 640 | 8 |
| Overall12 | 680 | 699 | 700 | 692 | 696 | 707 | 728 | 711 | 6 |
| Mortality, % | 2.0 | 2.4 | 3.7 | 0.9 | 2.9 | 3.6 | 3.0 | 2.2 | 0.9 |
| Full-value pigs,13 % | 98.1 | 98.3 | 97.7 | 99.1 | 97.3 | 97.0 | 97.6 | 98.1 | 0.8 |
Values represent least squares means of 12 or 13 pens per treatment with 25 pigs per pen.
FTU = phytase units.
Main effects of SBM (P = 0.006) and phytase (P = 0.04).
Main effect of SBM (P = 0.004).
Main effect of SBM (P = 0.003) and linear effect of phytase (P = 0.007).
Main effect of SBM (P = 0.039).
Linear effect of phytase (P = 0.029).
Main effect of SBM (P < 0.001).
Main effect of SBM (P = 0.006).
Main effect of SBM (P < 0.001).
Quadratic effect of phytase (P = 0.026).
Main effect of SBM (P < 0.001) and quadratic effect of phytase (P = 0.001).
Pigs with BW ≥ 11.3 kg at the end of the nursery.
No interactive effects on fecal scores of SBM levels and phytase were observed during the 10-d sampling period (Fig. 1). On d 2, pigs fed high-SBM diets tended to had looser stools compared with pigs fed low-SBM diets (P = 0.09).
Figure 1.
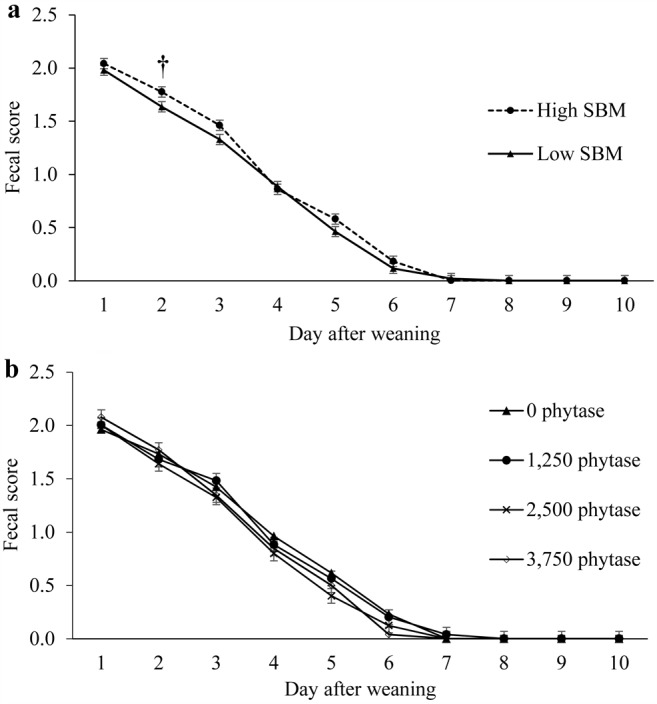
Main effect of dietary soybean meal (SBM) levels (panel a) and phytase (panel b) on fecal scores in nursery pigs for Exp. 1. Fecal scores were measured by pen during the first 10 d of the experiment. The fecal scoring system consisted of the following: 0 = normal feces, 1 = soft feces, 2 = fluid feces, and 3 = completely liquid feces. Interactions of SBM × phytase, P > 0.17. †Means tend to be different (P ≤ 0.10).
Experiment 2
No interactions were observed between SBM level and phytase supplementation (Table 4). The inclusion of SBM did not affect ADFI, ADG, or G:F. The percentage of pigs that were removed and medically treated linearly declined with increasing SBM levels (P = 0.04). The level of SBM did not affect the percentage of full-value pigs (P = 0.99).
Table 4.
Effect of dietary soybean meal (SBM) levels and phytase on growth performance in nursery pigs obtained from a porcine reproductive and respiratory syndrome (PRRS)–positive sow farm (Exp. 2)1
| Low SBM | Medium SBM | High SBM | |||||
|---|---|---|---|---|---|---|---|
| Phytase, FTU2/kg | Phytase, FTU/kg | Phytase, FTU/kg | |||||
| Item | 600 | 2,600 | 600 | 2,600 | 600 | 2,600 | SEM |
| BW, kg | |||||||
| 0 d | 5.97 | 5.98 | 5.95 | 6.00 | 5.94 | 5.93 | 0.04 |
| 10 d | 7.52 | 7.48 | 7.42 | 7.57 | 7.54 | 7.34 | 0.08 |
| 20 d | 11.84 | 11.80 | 11.70 | 11.82 | 11.74 | 11.60 | 0.16 |
| 42 d | 21.59 | 21.65 | 21.72 | 21.72 | 21.86 | 21.17 | 0.30 |
| ADG, g | |||||||
| Phase 1 (d 0–10) | 138 | 133 | 131 | 138 | 148 | 129 | 6 |
| Phase 2 (d 11–20) | 355 | 357 | 353 | 353 | 348 | 352 | 10 |
| Phase 3 (d 21–42) | 487 | 491 | 497 | 496 | 505 | 479 | 10 |
| Overall | 362 | 362 | 364 | 365 | 374 | 354 | 6 |
| ADFI, g | |||||||
| Phase 1 (d 0–10) | 138 | 133 | 128 | 133 | 142 | 129 | 5 |
| Phase 2 (d 11–20) | 443 | 445 | 431 | 445 | 438 | 434 | 12 |
| Phase 3 (d 21–42) | 785 | 779 | 779 | 791 | 796 | 769 | 15 |
| Overall | 526 | 521 | 517 | 528 | 534 | 513 | 10 |
| G:F, g/kg | |||||||
| Phase 1 (d 0–10) | 1,004 | 1,006 | 1,027 | 1,036 | 1,041 | 1,001 | 32 |
| Phase 2 (d 11–20) | 799 | 801 | 818 | 793 | 794 | 811 | 11 |
| Phase 3 (d 21–42) | 620 | 630 | 637 | 627 | 634 | 623 | 5 |
| Overall | 688 | 695 | 704 | 692 | 699 | 691 | 4 |
| Removed and treated pigs,3 % | 11.1 | 11.1 | 8.7 | 9.5 | 7.3 | 9.4 | 1.2 |
| Mortality, % | 1.1 | 0.8 | 1.9 | 1.2 | 2.3 | 1.7 | 0.5 |
| Full-value pigs,4 % | 96.5 | 98.1 | 96.8 | 98.1 | 97.6 | 96.9 | 0.8 |
Values represent least squares means of 16 pens per treatment with 22 pigs per pen.
FTU = phytase units.
Linear effect of SBM (P = 0.04).
Pigs with BW ≥ 11.3 kg at the end of the nursery.
No interactive effects on fecal scores between SBM level and phytase were observed (Fig. 2). Pigs fed high-SBM diets tended to present looser stools than pigs fed low- and medium-SBM diets during d 4 (P < 0.06) and 5 (P < 0.05). Moreover, high levels of phytase tended to reduce fecal scores on d 2 (P < 0.09) and 4 (P < 0.07).
Figure 2.
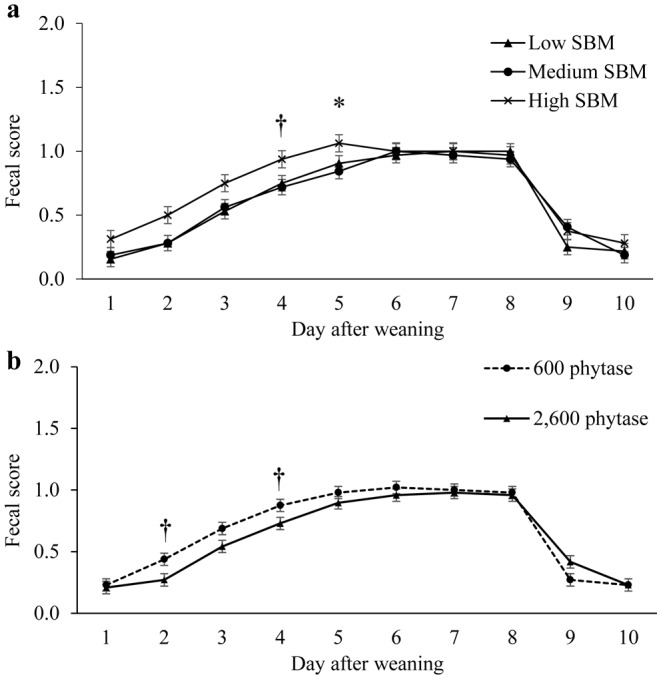
Effect of dietary soybean meal (SBM) levels (panel a) and phytase (panel b) on fecal scores in nursery pigs for Exp. 2. Fecal scores were measured by pen during the first 10 d of the experiment. The fecal scoring system consisted of the following: 0 = normal feces, 1 = soft feces, 2 = fluid feces, and 3 = completely liquid feces. Interactions of SBM × phytase, P > 0.10. †Means tend to be different (P ≤ 0.10). *Means are different (P ≤ 0.05).
No interactive effects of SBM levels, phytase, and day were observed for the viral load of PRRS in saliva samples (Fig. 3). The viral load of PRRS in saliva samples tended (P < 0.10) to be higher on d 42 than on d 20.
Figure 3.
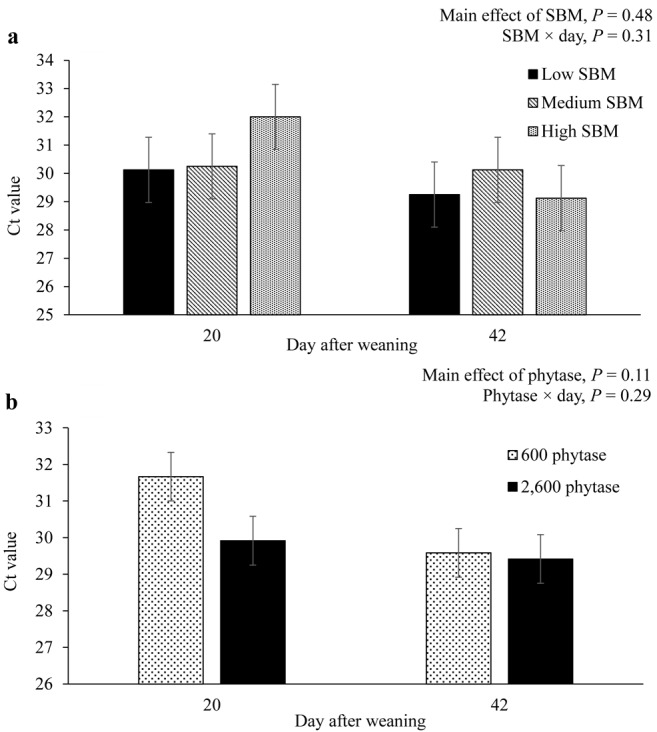
Effect of dietary soybean meal (SBM; panel a) and phytase (panel b) on oral viral load of nursery pigs obtained from a porcine reproductive and respiratory syndrome (PRRS)–positive sow farm. Viral load was determined using real-time quantitative reverse-transcription PCR. Viral load is expressed as cycle threshold (Ct) values, defined as the number of cycles required for the fluorescent signal to exceed a threshold. Cycle threshold values are inversely related to the amount of viral RNA detected in the sample. Cycle threshold values of 36 and above suggest that saliva samples were PRRS negative and values of 35 and lower suggest that saliva samples were PRRS positive (Kleiboeker et al., 2005). Interactions of SBM × phytase × day, P = 0.41, and SBM × phytase, P = 0.11. Main effect of day, P = 0.09.
DISCUSSION
High-quality protein sources are necessary for weaned pigs to avoid negative effects on postweaning performance (Li et al., 1990; Che et al., 2012). Among vegetable protein sources, SBM is the only product that can be used as an exclusive protein source in most swine diets due to its high level of AA and high-quality AA profile. Also, it is a more consistent and cost-effective protein source compared with animal protein products (Shannon and Allee, 2010). However, the use of SBM has been restricted in weaned pig diets due to the unfavorable effects of antinutritional factors.
In Exp. 1, it was postulated that supplementation of phytase at high concentrations to nursery pigs could allow for a higher inclusion level of SBM. However, no interactions between phytase and SBM on performance or stool firmness were observed. Increasing levels of SBM in early nursery diets by replacing poultry byproduct meal did not reduce growth performance. In fact, pigs fed diets with high-SBM diets had improved ADG and G:F, which supports the suggestion that SBM is a more reliable protein ingredient source for early nursery diets. Although most of the literature available shows a negative effect of SBM in young pigs (Li et al., 1990; Dréau et al., 1994; Pluske et al., 1997; Jun et al., 2009), that effect was not observed in the present study. The poultry byproduct meal used in the present study was a pet food–grade poultry byproduct meal, which is characterized by greater protein, lysine, and methionine content; lower ash and Ca concentrations; greater AA digestibility; and lower variability compared with feed-grade poultry byproduct meal (Dozier et al., 2003). Nonetheless, the reduction in growth performance in pigs fed low-SBM diets may have been due to the inclusion of an inferior poultry byproduct meal. Similar to the results of the current study, Moran et al. (2014) reported that weaned pigs fed diets with pet food–grade poultry byproduct meal had reduced growth performance compared with pigs fed high-SBM (with high levels of synthetic AA) diets. In that same study, pigs fed high levels of SBM performed equal to pigs fed diets with either fish meal or a fish meal replacement product as the main protein source.
Phytase supplementation improved pig performance regardless of the SBM level, and this response was optimal at 2,500 FTU/kg. One of the benefits of supplementing diets with phytase is related to its capacity to hydrolyze the phytate molecule and release the bound P, thereby improving P availability. The use of superdosing levels of phytase, which refers to the supplementation of high amounts of phytase to destroy at least 85% of the antinutrient phytate, is aimed at the release of P in an intermediate step in achieving the primary goal of eliminating phytate. Similar to the present results, several studies have demonstrated positive effects on growth performance of weaned, grower, and finisher pigs when superdosing phytase (Flohr et al., 2014; Santos et al., 2014; Wilcock et al., 2014; Bradley et al., 2015; Koehler et al., 2015; Holloway et al., 2016b).
In Exp. 2, it was hypothesized that high levels of SBM may modulate the response of pigs originating from a PRRS-positive sow farm by reducing systemic viral replication and improving growth performance. Soybean products contain isoflavones, which have shown antiviral properties against a wide range of viruses. Andres et al. (2009) suggested that isoflavones can interfere with virus binding and entrance into the host cell, inhibit the replication of the virus inside the host cell, and induce the production of cytokines. The main isoflavones found in SBM products are genistein and daidzein (Greiner et al., 2001b). Antiviral properties of both components have been studied in PRRS-challenged pigs. Greiner et al. (2001b) reported that supplementation of genistein improved systemic serum virus elimination and growth performance in weaned pigs inoculated with PRRS. However, in a different study (Greiner et al., 2001a), dietary soy daidzein did not impact the rate of serum virus elimination but it improved growth performance during periods of high viremia.
In Exp. 2, increasing the inclusion of SBM did not have any negative effects on ADG, ADFI or G:F, which is consistent with the results observed in Exp. 1. However, these results differ from the results reported by Rochell et al. (2015), who evaluated the effect of low and high SBM levels (17.5 and 29.0%, respectively) in weanling pigs infected with PRRS and found that high levels of SBM tended to improve ADG. However, they were unable to conclude whether the favorable effects of feeding high levels of SBM to PRRS-infected pigs was associated with isoflavones, AA, or other components found in SBM.
In practice, the use of SBM is initially restricted in early nursery diets to avoid a hypersensitivity response, which is an immune stimulation of the gastrointestinal tract in young pigs to soy glycinin and β-conglycinin and usually can induce diarrhea (Feng et al., 2007; DeRouchey et al., 2010). In both experiments of the present study, the average fecal consistency ranged from 0 to 2 of the fecal scoring system (normal feces to fluid feces) but never reached the score of 3 (completely fluid feces). In Exp. 1, fecal consistency improved from d 1 to 6, after which feces reached normal consistency (fecal score of 0). The opposite was observed in Exp. 2, in which the stool firmness decreased from d 1 to 8, which was most likely due to the health status of the pigs. High-SBM diets tended to decreased stool firmness only on d 2 in Exp. 1 and during d 4 to 5 in Exp. 2. However, in Exp. 2, the high level of phytase tended to improve the fecal consistency on d 2 and 4. Field observations suggest that loose stools are not commonly associated with reduced performance or livability, but barn managers tend to medicate the pigs when loose stools are observed. Therefore, even small differences in stool consistency may be relevant because of increased production costs associated with medications.
In Exp. 2, increasing levels of SBM linearly reduced the number of pigs that were removed from test pens and required medical treatment from 11.1 to 8.4%. The decrease in medical treatments in pigs fed high SBM compared with pigs fed low and medium SBM levels represents a positive economic impact in reducing labor cost and medication expenses. There was no improvement in the percentage of full-value pigs at the end of the experiment, indicating that increased medical treatments in pigs fed low- and medium-SBM diets may have successfully maintained pig weight gain to reach the desired minimum BW of 11.3 kg at the end of the nursery.
Based on monthly testing for PRRS activity, the sow farm that these pigs originated from was weaning a combination of negative and positive pigs for the field strain virus. Therefore, the sow farm was still positive but appeared to be stabilizing (reduction in PRRS activity). Upon arrival at the nursery facility, pigs were vaccinated with modified live virus PRRS vaccine; therefore, when oral fluids of the subset of pigs were analyzed using real-time PCR, the results were positive. This was most likely a combination of field strain and vaccine strain. Once their maternal antibodies diminished during the mid to late nursery phase, PRRS PCR cycle times decreased, which is an indicator of more virus present. This is consistent with Done et al. (1996), who reported that PRRS titers usually reach a peak at 5 to 6 wk after infection.
Levels of SBM or dietary concentration of phytase did not influence the oral PRRS viral load when measured on d 20 or 42. Greiner et al. (2001b) reported that soy genistein (pure extract) at dietary concentrations of 200 to 400 mg/kg acted as active immune modulators to improve serum virus elimination and growth performance in PRRS-challenged pigs. The concentration of genistein in dietary treatments used in Exp. 2 was estimated based on the USDA database for the isoflavone content of food (USDA, 2008), which indicates that defatted SBM contains, on average, approximately 1,147 mg/kg of genistein, resulting in a calculated dietary genistein concentration of 200, 286, and 372 mg/kg for the low-, medium-, and high-SBM treatments, respectively. Rochell et al. (2015) reported a reduction in serum PRRS viral load in pigs infected with PRRS when fed diets containing 638 mg/kg of soy genistein compared with pigs fed diets with 369 mg/kg of soy genistein. This suggests that the levels of genistein in the diets used in the present study may have been too low to elicit antiviral effects in pigs derived from a PRRS-positive sow herd or that the virus load in the pigs was too low.
Results from these studies indicate that the level of SBM in early nursery diets can be increased without decreasing growth performance or increasing the incidence of diarrhea in nursery pigs and it may be favorable in pigs that originated from a PRRS-positive sow farm by reducing costs associated with medical treatment. Supplementation of phytase at superdose levels can improve growth performance independently from the level of SBM in the diet.
FOOTNOTES
Funded in part by the United Soybean Board (Chesterfield, MO; USB number 1430-512-5207) and AB Vista Feed Ingredients, Marlborough UK. Appreciation is expressed to The Hanor Company (Franklin, KY) for providing access to the facilities and their staff for assistance in conducting the studies.
LITERATURE CITED
- Andres A., Donovan S. M., Kuhlenschmidt M. S. 2009. Soy isoflavones and virus infections. J. Nutr. Biochem. 20:563–569. doi: 10.1016/j.jnutbio.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C., Walk C. L., Walker N. D., Wilcock P. 2015. The effect of superdosing phytase with or without the addition of live yeast in diets void of spray dried plasma in pigs from weaning to 21 days post-weaning. J. Anim. Sci. 93(Suppl. 2):57 (Abstr.) [Google Scholar]
- Che L., Zhan L., Fang Z., Lin Y., Yan T., Wu D. 2012. Effects of dietary protein sources on growth performance and immune response of weanling pigs. Livest. Sci. 148:1–9. doi: 10.1016/j.livsci.2012.04.019 [DOI] [Google Scholar]
- Chiba L. 2001. Proteins supplements. In: Southern L., Lewis J. editors, Swine nutrition. CRC Press, Boca Raton, FL: p. 803–837. [Google Scholar]
- Choct M., Dersjant-Li Y., McLeish J., Peisker M. 2010. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 23:1386–1398. [Google Scholar]
- DeRouchey J. M., Goodband R. D., Tokach M. D., Nelssen J. L., Dritz S. S. 2010. Nursery swine nutrient recommendations and feeding management. In: Meisinger D. J. editor, National swine nutrition guide. U.S. Pork Center of Excellence, Ames, IA: p. 65–79. [Google Scholar]
- Done S. H., Paton D. J., White M. E. 1996. Porcine reproductive and respiratory syndrome (PRRS): A review, with emphasis on pathological, virological and diagnostic aspects. Br. Vet. J. 152:153–174. doi: 10.1016/S0007-1935(96)80071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier W. A., Dale N. M., Dove C. R. 2003. Nutrient composition of feed-grade and pet-food-grade poultry by-product meal. J. Appl. Poult. Res. 12:526–530. doi: 10.1093/japr/12.4.526 [DOI] [Google Scholar]
- Dréau D., Lallès J. P., Philouze-Romé V., Toullec R., Salmon H. 1994. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J. Anim. Sci. 72:2090–2098. doi: 10.2527/1994.7282090x [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies (FASS) 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed.FASS, Champaign, IL. [Google Scholar]
- Feng J., Liu X., Xu Z. R., Lu Y. P., Liu Y. Y. 2007. Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Dig. Dis. Sci. 52:1845–1850. doi: 10.1007/s10620-006-9705-0 [DOI] [PubMed] [Google Scholar]
- Flohr J., Goodband R. D., Tokach M. D., Langbein K. B., Dritz S. S., DeRouchey J. M., Woodworth J. 2014. Influence of a superdose of phytase on finishing pig performance and carcass characteristics. J. Anim. Sci. 92(Suppl. 2):149 (Abstr.) [Google Scholar]
- Greiner L. L., Stahly T. S., Stabel T. J. 2001a. The effect of dietary soy daidzein on pig growth and viral replication during a viral challenge. J. Anim. Sci. 79:3113–3119. doi: 10.2527/2001.79123113x [DOI] [PubMed] [Google Scholar]
- Greiner L. L., Stahly T. S., Stabel T. J. 2001b. The effect of dietary soy genistein on pig growth and viral replication during a viral challenge. J. Anim. Sci. 79:1272–1279. doi: 10.2527/2001.7951272x [DOI] [PubMed] [Google Scholar]
- Holloway C. L., Boyd R. D., Walk C. L., Patience J. F. 2016a. Impact of super-dosing phytase in diets fed to 40 kg, 60 kg and 80 kg pigs on phytate catabolism. J. Anim. Sci. 94(Suppl. 2):112–113. (Abstr.) doi:10.2527/msasas2016-238 [Google Scholar]
- Holloway C. L., Boyd R. D., Zier-Rush C. E., Walk C. L., Patience J. F. 2016b. Impact on growth performance and carcass characteristics of super-dosing phytase in growing pig diets. J. Anim. Sci. 94(Suppl. 2):113 (Abstr.) doi:10.2527/msasas2016-239 [Google Scholar]
- Jun X., Anguo Z., Zhisheng W., Dawei A. 2009. Influence of glycinin and B-conglycinin of soybean on the proliferation and immune function of suckling piglets peripheral blood mononuclear cells in in vitro culture. J. Anim. Plant Sci. 19:115–118. [Google Scholar]
- Kleiboeker S. B., Schommer S. K., Lee S. M., Watkins S., Chittick W., Polson D. 2005. Simultaneous detection of North American and European porcine reproductive and respiratory syndrome virus using real-time quantitative reverse transcriptase–PCR. J. Vet. Diagn. Invest. 17:165–170. doi: 10.1177/104063870501700211 [DOI] [PubMed] [Google Scholar]
- Koehler D. D., Corrigan B., Elsbernd A. J., Gould S. A., Holloway C. L., Patience J. F. 2015. Super-dosed phytase improves rate and efficiency of gain in nursery pigs. J. Anim. Sci. 93(Suppl. 2):56 (Abstr.) [Google Scholar]
- Li D. F., Nelssen J. L., Reddy P. G., Blecha F., Hancock J. D., Allee G. L., Goodband R. D., Klemm R. D. 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 68:1790–1799. doi: 10.2527/1990.6861790x [DOI] [PubMed] [Google Scholar]
- Lunney J. K., Benfield D. A., Rowland R. R. R. 2010. Porcine reproductive and respiratory syndrome virus: An update on an emerging and re-emerging viral disease of swine. Virus Res. 154:1–6. doi: 10.1016/j.virusres.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran K., van Heugten E., Funderburke D., Funderburke C. 2014. Influence of protein sources on nursery pig performance. J. Anim. Sci. 92(Suppl. 2):159 (Abstr.) [Google Scholar]
- Pluske J. R., Hampson D. J., Williams I. H. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Rochell S. J., Alexander L. S., Rocha G. C., Van Alstine W. G., Boyd R. D., Pettigrew J. E., Dilger R. N. 2015. Effects of dietary soybean meal concentration on growth and immune response of pigs infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 93:2987–2997. doi: 10.2527/jas.2014-8462 [DOI] [PubMed] [Google Scholar]
- Sang Y., Rowland R. R. R., Blecha F. 2011. Interaction between innate immunity and porcine reproductive and respiratory syndrome virus. Anim. Health Res. Rev. 12:149–167. doi: 10.1017/S1466252311000144 [DOI] [PubMed] [Google Scholar]
- Santos T. T., Walk C. L., Wilcock P., Cordero G., Chewning J. J. 2014. Performance and bone characteristics of growing pigs fed diets marginally deficient in available phosphorus and a novel microbial phytase. Can. J. Anim. Sci. 94:493–497. doi: 10.4141/cjas2013-190 [DOI] [Google Scholar]
- Shannon M. C., Allee G. L. 2010. Protein and amino acids sources for swine diets. In: Meisinger D. J. editor, National swine nutrition guide. U.S. Pork Center of Excellence, Ames, IA: p. 30–36. [Google Scholar]
- Song Y. S., Pérez V. G., Pettigrew J. E., Martinez-Villaluenga C., de Mejia E. G. 2010. Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim. Feed Sci. Technol. 159:41–49. doi: 10.1016/j.anifeedsci.2010.04.011 [DOI] [Google Scholar]
- USDA 2008. Database for the isoflavone content of selected foods. https://www.ars.usda.gov/ARSUserFiles/80400525/Data/isoflav/Isoflav_R2.pdf. (Accessed 25 February, 2016.)
- Walk C. L., Santos T. T., Bedford M. R. 2014. Influence of superdose of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broiler. Poult. Sci. 90:543–549. [DOI] [PubMed] [Google Scholar]
- Wilcock P., Brandley C. L., Chewning J. J., Walk C. L. 2014. The effect of superdosing phytase on inositol and phytate concentration in the gastrointestinal tract and its effect on pig performance. J. Anim. Sci. 92(Suppl. 2):383 (Abstr.) [Google Scholar]


