Abstract
One hundred eight Angus × Hereford steers, originating from 7 cow–calf were obtained from an auction yard on d −2 and transported by road (800 km; 12 h) to an experimental feedlot facility. Upon arrival on d −1, shrunk BW was recorded and steers were grouped with free-choice access to grass hay, mineral supplement, and water. On d 0, steers were ranked by source and shrunk BW and assigned to 1 of 18 pens (6 steers/pen). Pens were allocated to 1) no immunomodulatory ingredient supplementation during feedlot receiving (CON), 2) supplementation with OmniGen-AF (OMN; 22 g/steer daily, as-fed basis; Phibro Animal Health Corp., Teaneck, NJ) from d 0 to 30, or 3) 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA) from d 7 to 30 (IPF). From d 0 to 80, steers had free-choice access to grass hay and water and received a corn-based concentrate. Feed DMI was recorded from each pen, and steers were assessed for bovine respiratory disease (BRD) signs daily. Steers were vaccinated against BRD pathogens on d 0 and 21. Final shrunk BW was recorded on d 81, and blood samples were collected on d 0, 3, 7, 10, 14, 21, 31, 42, 56, and 73. Steer ADG and final BW were greater (P ≤ 0.05) in CON steers than in OMN and IPF steers (1.23, 0.76, and 1.06 kg/d [SEM 0.06], respectively, and 320, 282, and 307 kg [SEM 4], respectively) and (P < 0.01) in IPF steers than in OMN steers. No treatment effects were detected (P ≥ 0.76) for BRD incidence (66 ± 4%) and DMI, whereas G:F was greater (P < 0.01) in OMN steers than in CON steers. Mean plasma cortisol concentration was greater (P = 0.01) in CON steers than in OMN and IPF steers. Plasma haptoglobin concentrations tended (P = 0.10) to be greater in CON steers than in IPF steers on d 3, were greater (P = 0.04) in IPF steers than in CON steers on d 7, and tended (P = 0.10) to be less in OMN steers than in IPF and CON steers on d 21. Blood mRNA expression of interleukin 8 was greater (P ≤ 0.05) in OMN and IPF steers than in CON steers on d 3 and in OMN steers than in CON and IPF steers on d 14. Blood mRNA expression of tumor necrosis-α was greater (P ≤ 0.05) in OMN and IPF steers than in CON steers on d 10. Plasma IGF-I concentrations, serum antibody titers to BRD pathogens, and blood mRNA expression of chemokine ligand 5, cyclooxygenase 2, interleukin 8 receptor, and L-selectin did not differ (P ≥ 0.21) among treatments. Collectively, the immunomodulatory feed ingredients evaluated herein impacted adrenocortical and innate immune responses but failed to mitigate BRD incidence and improve performance of receiving cattle.
Keywords: immunomodulatory ingredients, innate immunity, performance, receiving cattle, respiratory disease
INTRODUCTION
Feedlot receiving is one of the most critical phases of the beef production cycle, when cattle are exposed to a multitude of stress and health challenges that directly impact their welfare and productivity (Duff and Galyean, 2007). As examples, receiving cattle often experience long road transport and are immediately subjected to commingling with different animals and exposure to novel diets and environments, which are known to impair their immune system and growth metabolism (Cooke, 2017). Accordingly, the incidence of bovine respiratory disease (BRD) is elevated during feedlot receiving, despite vaccination against BRD pathogens and efforts to minimize the aforementioned stressors (Kirkpatrick et al., 2008).
Prophylactic medication with feed-grade antimicrobials is often effective in mitigating BRD incidence during feedlot receiving (Wilson et al., 2017). However, with increased regulations regarding the use of feed-grade antimicrobials in livestock systems (U.S. Food and Drug Administration, 2015), alternative dietary strategies that enhance the immune function of receiving cattle are warranted. These include the use of nonantibiotic feed ingredients with immunomodulatory properties, such as OmniGen-AF (OMN; Phibro Animal Health Corp., Teaneck, NJ) and Immune Primer formulas (2 oral capsules of Stocker Immune Primer [Ramaekers Nutrition, Santa Cruz, CA] on d 0 + 15 g/steer daily [as-fed basis] of Stocker Preconditioned Premix [Ramaekers Nutrition] from d 7 to 30 [IPF]). The OMN was recently shown to improve milk production and innate immunity parameters in transition dairy cows (Brandão et al., 2016). The products contained in the IPF treatment are based on transfer factor proteins and lactate-producing probiotics, which are associated with improved immunity in humans and cattle (Fudenberg and Fudenberg, 1989; Krehbiel et al., 2003). Based on this information, we hypothesized that OMN or IPF are dietary alternatives to improve cattle immunocompetence and productivity during feedlot receiving. Therefore, this experiment evaluated the effects of supplementing OMN or IPF products on performance, health, and physiological responses of receiving cattle.
MATERIALS AND METHODS
This experiment was conducted at the Oregon State University – Eastern Oregon Agricultural Research Center (Union station; Union, OR). All animals were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the Oregon State University Institutional Animal Care and Use Committee (number 4851).
Animals and Treatments
One hundred eight Angus × Hereford steers were purchased from a commercial auction yard (Producers Livestock Marketing Association, Vale, OR) and used in this experiment (d 0 to 80). Steers originated from 7 cow–calf operations located in eastern and central Oregon, and no health or management history of the steers was available at the time of purchase (d −2). Steers were loaded into a double-deck commercial livestock trailer (Legend 50-foot [15.24 m] cattle liner; Barrett Trailers, LLC, Purcell, OK) at the auction yard (d −2; 1800 h) and transported for 800 km to elicit the stress of a long haul (Cooke et al., 2013). During transport, the driver stopped once after 6 h of driving to rest for 60 min, whereas total transport time was 12 h. Steers remained in the truck throughout the 12-h transportation period. Minimum, maximum, and average environmental temperatures during transport were −1, 23, and 11°C, respectively, whereas average humidity was 37% and no precipitation was observed.
On d −1, steers were unloaded (0600 h) at the Eastern Oregon Agricultural Research Center, immediately weighed (220 ± 2 kg initial shrunk BW), and maintained as a single group in a drylot pen (80 by 40 m) with ad libitum access to orchardgrass (Dactylis glomerata L.) hay, water, and a commercial mineral mix (described in Table 1) for 24 h. On d 0, steers were ranked according to source and shrunk BW and allocated to 1 of 18 drylot pens (35 by 15 m; 6 steers/pen) in a manner such that pens had equivalent initial shrunk BW and steers from 3 different sources to stimulate the stress of commingling (Step et al., 2008). Pens were assigned to receive 1 of 3 treatments: 1) no immunomodulatory ingredient supplementation during feedlot receiving (CON; n = 6), 2) supplementation with OMN (22 g/steer daily, as-fed basis; Phibro Animal Health Corp.) from d 0 to 30 (n = 6), or 3) administration of IPF products (Ramaekers Nutrition), which was 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix from d 7 to 30 (n = 6). Pens were assigned to treatments in a manner such that the OMN, IPF, and CON were balanced for initial shrunk BW and calf source and they contained steers from each of the 7 cow–calf sources.
Table 1.
Ingredient composition and nutrient profile of concentrate offered during the experiment (d 0 to 80)1
| Item | Day 0 to 7 | Day 8 to 19 | Day 20 to 30 | Day 31 to 80 |
|---|---|---|---|---|
| Ingredient (as-fed basis) | ||||
| Whole corn, kg/d | 0.91 | 2.27 | 4.09 | 5.23 |
| Soybean meal, kg/d | 0.36 | 0.36 | 0.55 | 0.68 |
| Mineral mix,2 kg/d | 0.05 | 0.05 | 0.05 | 0.05 |
| Nutrient profile3 (DM basis) | ||||
| TDN, % | 83 | 86 | 87 | 87 |
| NEm, Mcal/kg | 2.11 | 2.14 | 2.16 | 2.16 |
| NEg, Mcal/kg | 1.46 | 1.47 | 1.49 | 1.49 |
| NDF, % | 8.4 | 8.1 | 8.1 | 8.1 |
| ADF, % | 4.6 | 4.1 | 4.0 | 4.0 |
| CP, % | 20.3 | 14.3 | 13.7 | 13.5 |
Steers had free-choice access to orchardgrass (Dactylis glomerata L.) hay and water throughout the experimental period. Hay and concentrate were offered separately in different sections of the feed bunk.
Cattleman's Choice (Performix Nutrition Systems, Nampa, ID) containing 14% Ca, 10% P, 16% NaCl, 1.5% Mg, 3,200 mg/kg Cu, 65 mg/kg I, 900 mg/kg Mn, 140 mg/kg Se, 6,000 mg/kg Zn, 136,000 IU/kg vitamin A, 13,000 IU/kg vitamin D3, and 50 IU/kg vitamin E.
Based on nutritional profile of each ingredient, which were analyzed using wet chemistry procedures by a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY). Calculation for TDN used the equation proposed by Weiss et al. (1992), whereas NEm and NEg were calculated using the equations proposed by the NRC (1996).
According to the manufacturer, OMN contains a mixture of active dried Saccharomyces cerevisiae, dried Trichoderma longibrachiatum fermentation product, niacin, vitamin B12, riboflavin-5-phosphate, D-calcium pantothenate, choline chloride, biotin, thiamine monohydrate, pyridoxine hydrochloride, menodione dimethylpyrimidinol bisulfate, folic acid, calcium aluminosilicate, sodium aluminosilicate, diatomaceous earth, calcium carbonate, rice hulls, and mineral oil (the full formulation is proprietary). Both IPF products, Stocker Immune Primer capsules and the Stocker Preconditioned Premix, have similar composition and are based on transfer factor proteins extracted from bovine colostrum and egg yolks, plant-derived heteropolysaccharides, lactate-producing probiotics, vitamins, and minerals. The inclusion and administration rate of OMN and IPF products were according to manufacturer's recommendations for growing cattle.
From d 0 to 80, steers had free-choice access to orchardgrass (D. glomerata L.) hay and water and received a corn-based concentrate (Table 1). Hay and concentrate were offered (0800 h) separately in different sections of the feed bunk (d 0 to 80). The OMN (d 0 to 30) and IPF Stocker Preconditioned Premix (d 7 to 30) were mixed daily with the concentrate, whereas IPF oral capsules of Stocker Immune Primer were administered (d 0) using a bolus applicator provided by the manufacturer (Ramaekers Nutrition). From d 31 to 80, all steers received diets without the addition OMN or IPF (Table 1). On d 0, steers were vaccinated against Clostridium and Mannheimia haemolytica (One Shot Ultra 7; Zoetis Inc., Florham Park, NJ) and infectious bovine rhinotracheitis, bovine viral diarrhea complex, parainfluenza-3 virus (PI3), and bovine respiratory syncytial virus (BRSV; Bovi-Shield Gold 5; Zoetis Inc.) and were administered an anthelmintic (Dectomax; Zoetis Inc.). On d 21, steers were revaccinated against Clostridium (Ultrabac 8; Zoetis Inc.) and infectious bovine rhinotracheitis virus, bovine viral diarrhea complex, PI3, and BRSV (Bovi-Shield Gold 5; Zoetis Inc.).
Sampling
Samples of hay and concentrate ingredients were collected weekly, pooled across all weeks, and analyzed for nutrient content by a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY). All samples were analyzed by wet chemistry procedures for concentrations of CP (method 984.13; AOAC, 2006), ADF (method 973.18, modified for use in an Ankom 200 fiber analyzer [ANKOM Technology Corp., Fairport, NY]; AOAC, 2006), and NDF (Van Soest et al., 1991; modified for use in an Ankom 200 fiber analyzer [ANKOM Technology Corp.]). Calculations for TDN used the equation proposed by Weiss et al. (1992), whereas NEm and NEg were calculated with the equations proposed by the NRC (1996). The hay nutritional profile was (DM basis) 57% TDN, 59.7% NDF, 38.1% ADF, 1.12 Mcal/kg of NEm, 0.57 Mcal/kg of NEg, and 11.0% CP. The nutrient profile of the concentrate is described in Table 1.
Steer full BW was recorded on d 0, 3, 7, 10, 14, 21, 31, 42, 73, and 80 of the experiment at 0700 h, prior to the hay and concentrate feeding of the day. Shrunk BW was recorded on d 81, after 16 h of water and feed withdrawal. Shrunk BW values from d −1 and 81 were used to calculate steer ADG during the experiment. Concentrate, hay, and total DMI were evaluated daily from d 0 to 80 from each pen by collecting and weighing offered and nonconsumed feed. All samples were dried for 96 h at 50°C in forced-air ovens for DM calculation. Hay, concentrate, and total daily DMI of each pen were divided by the number of steers within each pen and expressed as kilograms per steer per day. Total BW gain and DMI of each pen were used for G:F calculation.
Steers were observed daily for BRD signs according to the DART system (Zoetis Inc.) and received antimicrobial treatment as described by Wilson et al. (2015). Moreover, IPF steers diagnosed with BRD also received 2 oral capsules of Stocker Immune Primer (Ramaekers Nutrition) concurrently with each antimicrobial administration, as recommended by the manufacturer.
Blood samples were collected from all steers, concurrently with full BW evaluation from d 0 to 73, into commercial blood collection tubes (Vacutainer, 10 mL; Becton, Dickinson and Company, Franklin Lakes, NJ) containing no additive or containing freeze-dried sodium heparin for serum and plasma collection, respectively. Blood samples were also collected from 3 steers/pen, which were randomly selected on d −1, into PAXgene tubes (BD Diagnostic Systems, Sparks, MD) for whole-blood RNA extraction. These samples were collected on d 0, 3, 7, 10, and 14 for mRNA expression analysis of innate immunity genes (Table 2) to assess such response during the initial 2 wk of feedlot receiving, when cattle are coping with the stressors associated with feedlot entry (Duff and Galyean, 2007; Cooke, 2017; Wilson et al., 2017).
Table 2.
Primer sequences, accession number, and reference for all gene transcripts analyzed by real-time reverse-transcription PCR
| Target gene | Primer sequence | Accession no. | Source |
|---|---|---|---|
| Cyclooxygenase-2 | |||
| Forward | AATCATTCACCAGGCAAAGG | AF031699 | Silva et al. (2008) |
| Reverse | TAGGGCTTCAGCAGAAAACG | ||
| Tumor necrosis factor α | |||
| Forward | AACAGCCCTCTGGTTCAAAC | NM_173966 | Riollet et al. (2000) |
| Reverse | TCTTGATGGCAGACAGGATG | ||
| L-selectin | |||
| Forward | GACACTTCCCTTCAGCCGTAC | NM_174182.1 | Playford et al. (2014) |
| Reverse | AGTTCTTTGCTTCTTCAGTGAGAG | ||
| Interleukin-8 | |||
| Forward | ACACATTCCACACCTTTCCAC | NM_173925.2 | Kliem et al. (2013) |
| Reverse | ACCTTCTGCACCCACTTTTC | ||
| Interleukin-8 receptor | |||
| Forward | CGGGTCATCTTTGCTGTCG | NM_174360.3 | Playford et al. (2014) |
| Reverse | ATGAGGGTGTCCGCGATC | ||
| CCL5 | |||
| Forward | GCCCTGCTGCTTTGCCTATAT | NM_175827.2 | Buza et al. (2003) |
| Reverse | TCCACCCTAGCTCAACTCCAA | ||
| β-actin | |||
| Forward | CTGGACTTCGAGCAGGAGAT | AY141970 | Gifford et al. (2007) |
| Reverse | GGATGTCGACGTCACACTTC | ||
| β2-microglobulin | |||
| Forward | GGGCTGCTGTCGCTGTCT | NM_173893 | Silva et al. (2008) |
| Reverse | TCTTCTGGTGGGTGTCTTGAGT | ||
Blood Laboratorial Analyses
Plasma and Serum Samples
After collection, all blood samples were immediately placed on ice, centrifuged (2,500 × g for 30 min at 4°C) for plasma or serum harvest, and stored at −80°C on the same day of collection. Plasma samples collected from d 0 to 31 were analyzed for cortisol (Immulite 1000; Siemens Medical Solutions Diagnostics, Inc., Los Angeles, CA) and haptoglobin (Cooke and Arthington, 2013) concentrations, given that adrenocortical and acute-phase protein responses in receiving cattle return to baseline levels within 4 wk after feedlot entry (Cooke, 2017). Plasma samples collected on d 0, 21, 42, and 73 were analyzed for IGF-I concentrations (Immulite 1000; Siemens Medical Solutions Diagnostics, Inc.) to metabolically assess steer nutritional status throughout the experimental period (Hess et al., 2005). The intra- and interassay CV for haptoglobin were 2.4 and 8.0%, respectively. Plasma IGF-I and cortisol were analyzed within single assays, and the intra-assay CV were 3.1 and 4.1%, respectively. Serum samples collected on d 0, 10, 21, 31, and 42 were analyzed for antibody titers against BRSV, bovine herpesvirus-1 (BHV-1), bovine viral diarrhea virus-1 (BVD-1), and PI3 using virus neutralization tests and for antibodies against M. haemolytica using a quantitative agglutination test (Texas A&M Veterinary Medical Diagnostic Laboratory, Amarillo, TX).
PAXgene Samples
Total RNA was extracted using the PAXgene Blood RNA Kit (QIAGEN Inc., Valencia, CA). Quantity and quality of isolated RNA were assessed using UV absorbance (NanoDrop Lite; Thermo Fisher Scientific Inc., Wilmington, DE) at 260 nm and a 260:280 nm ratio, respectively (Fleige and Pfaffl, 2006). Extracted RNA (120 ng) was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit with random hexamers (Applied Biosystems, Inc., Foster City, CA). Real-time reverse-transcription PCR was completed using the Fast SYBR Green Master Mix (Applied Biosystems, Inc.) and gene-specific primers (20 pM each; Table 2) with the StepOne Real-Time PCR System (Applied Biosystems, Inc.), according to procedures described by Cooke et al. (2008). At the end of each reverse-transcription PCR, amplified products were subjected to a dissociation gradient (95°C for 15 s, 60°C for 30 s, and 95°C for 15 s) to verify the amplification of a single product by denaturation at the anticipated temperature. Responses were quantified based on the threshold cycle (CT), the number of PCR cycles required for target amplification to reach a predetermined threshold. Responses from genes of interest were quantified based on the CT and normalized to the geometrical mean of the CT values from β2-microglobulin and β-actin (Vandesompele et al., 2002). The CV for the geometrical mean of the β2-microglobulin and β-actin CT values across all samples was 2.0%. Results are expressed as relative fold change (2−ΔΔCT) as described by Ocón-Grove et al. (2008).
Statistical Analysis
Pen was considered the experimental unit for all analyses. Quantitative data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), whereas binary data were analyzed using the GLIMMIX procedure of SAS with a binomial distribution and logit link function. All data were analyzed using Satterthwaite approximation to determine the denominator degrees of freedom for tests of fixed effects, with pen(treatment) and steer(pen) as random variables, except for DMI and G:F, which used pen(treatment) as a random variable. The model statement for initial and final BW, ADG, G:F, and morbidity-related and mortality results contained the effects of treatment. The model statement for DMI, cumulative BRD incidence, full BW change, and blood variables contained the effects of treatment, day, and the resultant interaction, in addition to results from d 0 as an independent covariate only for blood variables. Steer source was also included as an independent covariate for mRNA expression analysis of innate immunity genes, given that steers were randomly selected within each pen for blood mRNA sampling. The specified term for all repeated statements was day, with pen(treatment) as the subject for DMI and steer(pen) as the subject for all other analyses. The covariance structure used was first-order autoregressive, which provided the smallest Akaike information criterion and, hence, the best fit for all variables analyzed. All results are reported as least squares means except for blood variables, which are reported as covariately adjusted least squares means. Significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤ 0.10. Results are reported according to main effects if no interactions were significant or according to the highest-order interaction detected.
RESULTS
Performance and Health Variables
A treatment effect was detected (P < 0.01) for ADG, which was greater (P ≤ 0.05) in CON steers than in IPF and OMN steers and greater (P < 0.01) in IPF steers than in OMN steers (Table 3). Accordingly, a treatment effect was also detected (P < 0.01) for final shrunk BW (d 81), which was greater (P ≤ 0.05) in CON steers than in IPF and OMN steers and greater (P < 0.01) in IPF steers than in OMN steers (Table 3). No treatment effects were detected (P ≥ 0.77) for hay, concentrate, or total DMI (Table 3). Based on the concentrate intake of each pen, IPF steers consumed a mean of 14.5 g/d (SE 0.1) of Stocker Preconditioned Premix from d 7 to 30, whereas OMN was consumed at a mean of 20.2 g/d (SE 0.3) from d 0 to 30 by OMN steers. A treatment effect was detected (P = 0.01) for G:F, which was less (P ≤ 0.04) in OMN steers than in CON and IPF steers (Table 3) but did not differ (P = 0.33) between IPF steers and CON steers, despite differences in ADG and equivalent DMI between these latter treatments (Table 3).
Table 3.
Performance parameters during an 80-d feedlot receiving from beef steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with immunostimulant ingredients (IPF1 [n = 6] and OMN2 [n = 6]) from d 0 to 30 of the receiving period3
| Item | CON | IPF | OMN | SEM | P-value |
|---|---|---|---|---|---|
| Initial BW (d −1), kg | 219 | 220 | 220 | 7 | 0.99 |
| Final BW (d 81), kg | 320a | 307b | 282c | 4 | <0.01 |
| ADG, kg/d | 1.23a | 1.06b | 0.76c | 0.06 | <0.01 |
| DMI, kg/d | |||||
| Hay | 3.19 | 3.06 | 3.10 | 0.25 | 0.93 |
| Concentrate | 4.64 | 4.63 | 4.69 | 0.06 | 0.77 |
| Total | 7.83 | 7.69 | 7.79 | 0.30 | 0.94 |
| G:F,4 g/kg | 173a | 152a | 107b | 14 | 0.01 |
Values within rows with different superscripts differ (P ≤ 0.05).
IPF = 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA) from d 7 to 30.
OMN = supplementation with OmniGen-AF (22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ).
Steer shrunk BW was obtained after road transport (800 km for 12 h) on d −1 and after 16 h of water and feed withdrawal on d 81. Steer ADG was calculated using initial and final BW. Feed intake was recorded daily from d 0 to 80 by measuring feed offered and refusals from each pen, divided by the number of steers within each pen, and expressed as kilograms per steer per day.
Calculated using total DMI from d 0 to 80 and BW gain of each pen from d −1 to d 81.
It should be noted, however, that full BW did not differ (P ≥ 0.69) among treatments until d 56 and differed (P ≤ 0.09) among treatments only on d 73 and 80 (treatment × day interaction, P < 0.01; Fig. 1a). Moreover, DMI parameters did not differ among treatments throughout the experiment (treatment × day interaction, P ≥ 0.94; Fig. 1b). Hence, G:F from d 56 to 80 was reduced (P < 0.01) in OMN steers compared with CON and IPF steers (50, 230, and 176 g/kg [SEM 27], respectively) and also tended to be less (P = 0.09) in IPF steers than in CON steers.
Figure 1.
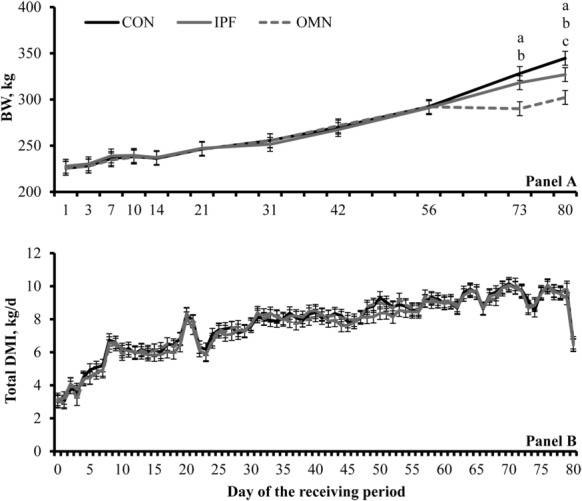
Body weight (panel A) and DMI (hay + concentrate; panel B) during a 80-d feedlot receiving from beef steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with 1) 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA; n = 6) from d 7 to 30 (IPF) or 2) supplementation with OmniGen-AF (OMN; 22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ; n = 6). A treatment × day interaction was detected (P < 0.01) for BW but not for DMI (P = 0.97). Within day, letters indicate the following treatment differences: aCON vs. OMN (P < 0.01), bIPF vs. OMN (P ≤ 0.04), and cIPF vs. CON (P = 0.09).
No treatment differences were detected (P ≥ 0.55) for BRD incidence (Table 4), whereas BRD signs were observed only during the initial 30 d of feedlot receiving (Fig. 2; day effect, P < 0.01). No treatment differences were detected (P = 0.36) for other morbidity reasons (i.e., bloat), number of antimicrobial treatments required on BRD diagnosis, and percentage of cattle that required ≥1 antimicrobial treatment on BRD diagnosis as well as mortality rate during the experiment (Table 4).
Table 4.
Morbidity and mortality parameters during a 80-d feedlot receiving from beef steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with immunostimulant ingredients (IPF1 [n = 6] and OMN2 [n = 6]) from d 0 to 30 of the receiving period3
| Item | CON | IPF | OMN | SEM | P-value |
|---|---|---|---|---|---|
| Incidence of bovine respiratory disease symptoms, % | 69.4 | 61.1 | 69.4 | 9.1 | 0.76 |
| Number of antimicrobial treatments required | 1.13 | 1.32 | 1.31 | 0.13 | 0.55 |
| Calves that required ≥ 1 antimicrobial treatment, % | 13.1 | 23.3 | 27.3 | 9.9 | 0.59 |
| Other morbidity reasons,4 % | 8.3 | 8.3 | 2.8 | 4.1 | 0.55 |
| Mortality, % | 2.8 | 5.5 | 0.0 | 2.7 | 0.36 |
IPF = 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA) from d 7 to 30.
OMN = supplementation with OmniGen-AF (22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ).
Steers were observed daily for bovine respiratory disease symptoms according to the DART system (Zoetis Inc., Florham Park, NJ) and received antimicrobial treatment as described by Wilson et al. (2015).
All non–bovine respiratory disease–related morbidity was due to bloat, with steers receiving 60 mL (oral drench, mixed with 500 mL of water) of Therabloat (Zoetis Inc.) when bloat was detected (Meyer and Bartley, 1972).
Figure 2.
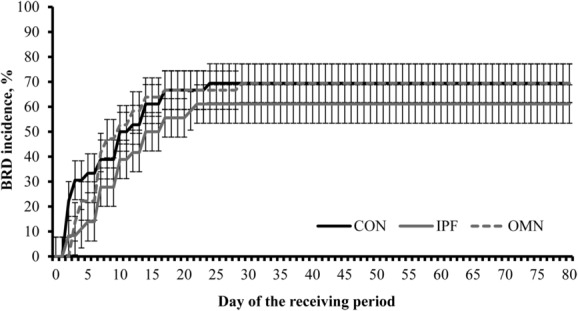
Cumulative incidence of bovine respiratory disease (BRD) symptoms during a 80-d feedlot receiving from in steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with 1) 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA; n = 6) from d 7 to 30 (IPF) or 2) supplementation with OmniGen-AF (OMN; 22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ; n = 6). Steers were observed daily for BRD symptoms according to the DART system (Zoetis Inc., Florham Park, NJ) and received medication as described by Wilson et al. (2015). No treatment or treatment × day interaction were detected (P ≥ 0.59), whereas a day effect was significant (P < 0.01).
Physiological Variables
A treatment effect was detected for plasma cortisol (P = 0.02), given that mean plasma cortisol concentration was greater (P = 0.01) in CON steers than in IPF and OMN steers and did not differ (P = 0.93) between IPF steers and OMN steers (Table 5). A treatment × day interaction was detected (P = 0.05; Fig. 3) for plasma haptoglobin concentration, which tended (P = 0.10) to be greater in CON steers than in IPF steers on d 3, was greater (P = 0.04) in IPF steers than in CON steers on d 7, and tended (P ≤ 0.10) to be less in OMN steers than in IPF and CON steers on d 21 of the experiment (Fig. 3). No treatment effects were detected (P = 0.41) for plasma IGF-I concentrations (Table 5). Moreover, day effects were detected (P ≤ 0.01) for all plasma and serum variables (Fig. 3; Table 6),
Table 5.
Metabolic, humoral, and gene expression responses in beef steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with immunostimulant ingredients (IPF1 [n = 6] and OMN2 [n = 6]) from d 0 to 30 of the receiving period3,4
| Item | CON | IPF | OMN | SEM | P-value |
|---|---|---|---|---|---|
| Metabolic variables | |||||
| Plasma cortisol, ng/mL | 21.89a | 18.74b | 18.63b | 0.92 | 0.02 |
| Plasma IGF-I, ng/mL | 178 | 166 | 175 | 7 | 0.41 |
| Serum antibody variables, titer log 2 | |||||
| Mannheimia haemolytica | 10.44 | 10.41 | 10.23 | 0.23 | 0.79 |
| Parainfluenza-3 virus | 6.65 | 5.99 | 6.18 | 0.51 | 0.66 |
| Bovine respiratory syncytial virus | 5.59 | 5.47 | 4.81 | 0.43 | 0.39 |
| Bovine viral diarrhea virus-1 | 5.77 | 6.19 | 6.16 | 0.51 | 0.81 |
| Bovine herpesvirus-1 | 3.53 | 3.02 | 4.15 | 0.43 | 0.21 |
| Blood mRNA expression | |||||
| Chemokine ligand 5 | 4.47 | 3.22 | 2.89 | 0.61 | 0.24 |
| Cyclooxygenase 2 | 3.93 | 3.34 | 3.88 | 0.53 | 0.69 |
| Interleukin 8 receptor | 4.86 | 4.73 | 5.44 | 0.97 | 0.85 |
| L-selectin | 1.83 | 1.78 | 1.75 | 0.12 | 0.91 |
Values within rows with different superscripts differ (P ≤ 0.05).
IPF = 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA) from d 7 to 30.
OMN = supplementation with OmniGen-AF (22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ).
On d 0, steers were vaccinated against Clostridium and Mannheimia haemolytica (One Shot Ultra 7; Zoetis Inc., Florham Park, NJ) and infectious bovine rhinotracheitis, bovine viral diarrhea complex, parainfluenza-3 virus, and bovine respiratory syncytial virus (Bovi-Shield Gold 5; Zoetis Inc.) and were administered an anthelmintic (Dectomax; Zoetis Inc.). On d 21, steers were revaccinated against Clostridium (Ultrabac 8; Zoetis Inc.) and infectious bovine rhinotracheitis virus, bovine viral diarrhea complex, parainfluenza-3 virus, and bovine respiratory syncytial virus (Bovi-Shield Gold 5; Zoetis Inc.).
Blood samples were collected on d 0, 3, 7, 10, 14, 21, 31, 42, and 73 and analyzed for cortisol (d 0 to 31), IGF-I (d 0, 21, 42, and 73), serum antibody variables (d 0, 10, 21, 31, and 42), and whole-blood mRNA expression (d 0 to 14).
Figure 3.
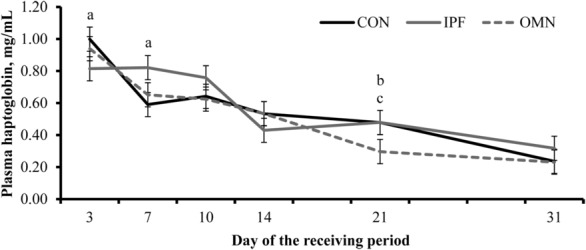
Plasma haptoglobin concentrations in feedlot receiving steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with 1) 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA; n = 6) from d 7 to 30 (IPF) or 2) supplementation with OmniGen-AF (OMN; 22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ; n = 6). A treatment × day interaction was detected (P ≤ 0.05). Blood samples collected on d 0 were a significant covariate (P = 0.02) but did not differ among treatments (0.21, 0.25, and 0.28 mg/mL [SEM 0.04] in CON, IPF, and OMN steers, respectively). Within day, letters indicate the following treatment differences: aIPF vs. CON (P ≤ 0.10), bOMN vs. CON (P = 0.10), and cOMN vs. IPF (P = 0.09).
Table 6.
Concentrations of plasma cortisol (ng/mL) and IGF-I (ng/mL); serum titers against Mannheimia haemolytica (MH), parainfluenza-3 virus (PI3), bovine respiratory syncytial virus (BRSV), bovine viral diarrhea virus-1 (BVD-1), and bovine herpesvirus-1 (BHV-1); and whole-blood mRNA expression of chemokine ligand 5 (CCL5), cyclooxygenase 2 (COX2), interleukin 8 receptor (IL8R), and L-selectin (SELL) in beef steers during an 80-d feedlot receiving1,2
| Plasma variables | Serum antibody titers | Blood mRNA expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Cortisol | IGF-I | MH | PI3 | BRSV | BVD-1 | BHV-1 | CCL5 | COX2 | IL8R | SELL |
| 0 | 22.9a | 113c | 7.44d | 5.11c | 0.55d | 3.78dc | 0.77d | 5.21a | 3.59b | 5.13b | 1.68b |
| 3 | 21.3ab | – | – | – | – | – | – | 3.41bc | 5.18a | 7.05a | 1.96a |
| 7 | 19.4bc | – | – | – | – | – | – | 2.90c | 2.51c | 3.89b | 1.54b |
| 10 | 18.8c | – | 8.89c | 6.38a | 2.78c | 5.17c | 3.72b | 3.82b | 3.64b | 4.93b | 1.82ab |
| 14 | 19.9bc | – | – | – | – | – | – | 3.93b | 3.48b | 4.15b | 1.81ab |
| 21 | 20.1bc | 132b | 11.05a | 5.83b | 5.39b | 6.17b | 2.77c | – | – | – | – |
| 31 | 18.9c | – | 11.28a | 6.55a | 6.67a | 6.00b | 4.44a | – | – | – | – |
| 42 | – | 192a | 10.22b | 6.33ab | 6.33a | 6.83a | 3.33b | – | – | – | – |
| 73 | – | 196a | – | – | – | – | – | – | – | – | – |
| SEM | 0.97 | 5 | 0.21 | 0.38 | 0.30 | 0.38 | 0.28 | 0.52 | 0.43 | 0.87 | 0.12 |
| P-value | 0.01 | <0.01 | <0.01 | <0.03 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.05 | 0.05 |
a–dValues within columns with different superscripts differ (P ≤ 0.05).
On d 0, steers were vaccinated against Clostridium and Mannheimia haemolytica (One Shot Ultra 7; Zoetis Inc., Florham Park, NJ) and infectious bovine rhinotracheitis, bovine viral diarrhea complex, PI3, and BRSV (Bovi-Shield Gold 5; Zoetis Inc.) and were administered an anthelmintic (Dectomax; Zoetis Inc.). On d 21, steers were revaccinated against Clostridium (Ultrabac 8; Zoetis Inc.) and infectious bovine rhinotracheitis virus, bovine viral diarrhea complex, PI3, and BRSV (Bovi-Shield Gold 5; Zoetis Inc.).
Blood samples were collected on d 0, 3, 7, 10, 14, 21, 31, 42, and 73 and analyzed for cortisol (d 0 to 31), IGF-I (d 0, 21, 42, and 73), serum antibody variables (d 0, 10, 21, 31, and 42), and whole-blood mRNA expression (d 0 to 14).
No treatment effects were detected (P = 0.21) for serum titers against M. haemolytica, PI3, BRSV, BVD-1, and BHV-1 (Table 5), whereas day effects were detected (P ≤ 0.03) for all these variables (Table 6). Tendencies for treatment × day interactions were detected (P ≤ 0.08; Fig. 4) for blood mRNA expression of interleukin 8 and tumor necrosis-α. Blood mRNA expression of interleukin 8 was greater (P ≤ 0.05) in OMN and IPF steers than in CON steers on d 3 and greater in OMN steers than in CON and IPF steers on d 14 (Fig. 4a). Blood mRNA expression of tumor necrosis-α was greater (P ≤ 0.05) in OMN and IPF steers than in CON steers on d 10 (Fig. 4b). No treatment effects were detected (P ≥ 0.24) for blood mRNA expression of chemokine ligand 5, cyclooxygenase 2, interleukin 8 receptor, and L-selectin (Table 5). Day effects were also detected (P ≤ 0.05) for blood mRNA expression variables (Fig. 4; Table 6).
Figure 4.
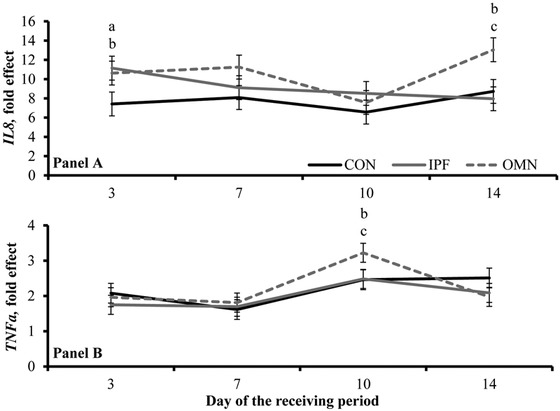
Whole-blood mRNA expression of interleukin 8 (IL8; panel A) and tumor necrosis-α (TNFa; panel B) in feedlot receiving steers supplemented or not (no immunomodulatory ingredient supplementation during feedlot receiving [CON]; n = 6) with 1) 2 oral capsules of Stocker Immune Primer on d 0 + 15 g/steer daily (as-fed basis) of Stocker Preconditioned Premix (Ramaekers Nutrition, Santa Cruz, CA; n = 6) from d 7 to 30 (IPF) or 2) supplementation with OmniGen-AF (OMN; 22 g/steer daily, as-fed basis, from d 0 to 30; Phibro Animal Health Corp., Teaneck, NJ; n = 6). Blood samples collected on d 0 were significant covariates (P ≤ 0.04) but did not differ among CON, IPF, and OMN steers (8.1-, 7.3-, and 7.4-fold effect [SEM 1.7], respectively, for interleukin 8 mRNA expression and 3.6-, 3.5-, and 3.3-fold effect [SEM 0.3], respectively, for tumor necrosis-α mRNA expression). Treatment × day interactions were detected (P ≤ 0.08). Within day, letters indicate the following treatment differences: aIPF vs. CON (P = 0.05), bOMN vs. CON (P ≤ 0.05), and cOMN vs. IPF (P ≤ 0.01).
DISCUSSION
As is common in commercial feedlot operations, the management history of steers prior to the initiation of this experiment was not fully known; therefore, they were considered high risk (Wilson et al., 2017). Furthermore, steers experienced the stress of weaning, auction, transportation, vaccination, and feedlot entry within a 72-h period, whereas the combination of these stressors are known to stimulate neuroendocrine and inflammatory responses that impact cattle immunocompetence and performance (Cooke, 2017). Accordingly, day effects observed for plasma cortisol and haptoglobin corroborate that steers experienced an adrenocortical and subsequent acute-phase protein response elicited by transport, vaccination, and feedlot entry (Cooke et al., 2011). Day effects detected for mRNA expression of whole-blood genes suggest immune activation on feedlot entry, given that interleukin 8, tumor necrosis-α, chemokine ligand 5, cyclooxygenase 2, interleukin 8 receptor, and L-selectin are key inflammatory components of the innate immune system (Abbas and Lichtman, 2007). Collectively, these stress-induced inflammatory processes are linked with the BRD complex in receiving cattle (Berry et al., 2004; Cooke, 2017), supporting the substantial incidence of BRD observed in the present experiment, which is comparable to research efforts conducted at commercial receiving yards (Snowder et al., 2006; Marques et al., 2016).
Contrary to our hypothesis, however, the OMN- and IPF-based products failed to mitigate BRD incidence and improve steer receiving performance. These ingredients were tested due to 1) their immunomodulatory potential (Fudenberg and Fudenberg, 1989; Krehbiel et al., 2003; Brandão et al., 2016), 2) the need for alternative dietary strategies to enhance immunocompetence of receiving cattle, and 3) the negative association among BRD incidence and cattle productivity (Snowder et al., 2006; Schneider et al., 2009). Moreover, OMN- and IPF-based products were administered to cattle during the initial 30 d of feedlot receiving, when the majority of BRD is observed in feedlot cattle (Snowder et al., 2006). The IPF products are based on transfer factor proteins extracted from bovine colostrum and egg yolks as well as lactate-producing probiotics. Transfer factor is a component of dialyzable leukocyte extracts produced in small quantities by T-lymphocytes (Rozzo and Kirkpatrick, 1992) that, once extracted and administered into the recipient, is able to transfer cell-mediated immunity for specific pathogens (Fudenberg and Fudenberg, 1989). Treatment with transfer factor has been shown to provide delayed hypersensitivity to Eimeria bovis in calves (Klesius and Kristensen, 1977). Published research examining its use in cattle have been scarce; however, Montgomery et al. (2008) investigated using transfer factor delivered as an oral drench in receiving heifers compared with treatment with tilmicosin phosphate. Similar to the results seen in the current study, treatment with transfer factor did not improve DMI, ADG, or feed efficiency during a 36-d receiving period; however, the incidence of BRD was increased in heifers receiving transfer factor (Montgomery et al., 2008). Hence, these authors concluded that transfer factor was not as effective as tilmicosin phosphate in preventing incidence of BRD and attributed this outcome to extensive rumen degradation of transfer factor. Alternatively, a direct-fed microbial such as lactic acid–producing bacteria have been shown to improve performance and decrease morbidity in receiving cattle (Krehbiel et al., 2003; McDonald et al., 2005); therefore, the lactic acid–producing bacteria in the IPF-based products were also expected to mitigate BRD incidence and enhance steer performance in the present experiment.
The OMN is based on components that have been shown to modulate the innate immune system, particularly yeast-based ingredients. More specifically, yeast products contain pathogen-associated molecular patterns such as β-glucans that are recognized by the immune system trigger innate immune responses by binding to Toll-like receptors and preparing the immune system against pathogens (Heine and Ulmer, 2005; Broadway et al., 2015). Feeding yeast cell wall components to newly received feedlot heifers alleviated inflammatory and serum cortisol responses to lipopolysaccharide administration (Burdick Sanchez et al., 2013). Accordingly, OMN supplementation has been shown to impact leukocytes gene expression related to the inflammatory response in dairy cows (Wang et al., 2009; Nace et al., 2014). These include improved leukocyte function, surface L-selectin concentration, and phagocytosis of extracellular pathogens in addition to fewer incidents of udder edema and mastitis following OMN supplementation (Wang et al., 2009; Ryman et al., 2013; Nace et al., 2014). In beef cattle, Armstrong et al. (2016) observed altered mRNA expression in a variety of genes relevant to innate immune function, suggesting that OMN regulates antigen presentation and signal transduction in cattle. However, no research evaluating the impacts of OMN supplementation on performance and immunocompetence of high-risk receiving cattle had been conducted to date.
Corroborating their immunomodulatory properties, supplementing OMN or IPF products to receiving steers herein impacted blood parameters that indicate enhanced innate immunity. More specifically, treatment differences in whole-blood mRNA expression of interleukin 8 and tumor necrosis-a suggest heightened inflammation, particularly in OMN steers, as previously observed by others (Burdick et al., 2012; Burdick Sanchez et al., 2014; Brandão et al., 2016). Treatment effects on plasma haptoglobin concentrations, an acute-phase protein whose synthesis is stimulated by proinflammatory cytokines (Carroll and Forsberg, 2007), also suggest an altered acute-phase response typical of feedlot receiving (Cooke, 2017) in OMN and IPF steers. These outcomes also can be associated with treatment differences in plasma cortisol concentrations, which serves as an effector molecule on proinflammatory and acute-phase reactions (Steiger et al., 1999; Carroll et al., 2009; Cooke et al., 2012). In turn, a potential enhancement in innate immunity from OMN and IPF supplementation may have contributed to a lessened adrenocortical response to the stress and immune challenges associated with feedlot receiving (Carroll and Forsberg, 2007; Cooke, 2017). Others have also reported reduced circulating cortisol concentrations when OMN was supplemented to dairy cows exposed to heat-stress conditions or receiving lipopolysaccharide administration (Hall et al., 2014; McBride et al., 2016). Moreover, pathological conditions such as BRD elicit inflammatory and innate immune reactions (Ackermann et al., 2010). Given that BRD incidence did not differ among treatments throughout the experiment, treatment differences observed in plasma haptoglobin, cortisol, and blood mRNA expression of interleukin 8 and tumor necrosis-a were not caused by morbidity differences among OMN, IPF, and CON steers.
Heightened innate immunity often results in enhanced acquired immunity on vaccination (Durum and Muegge, 1996). However, supplementing OMN and IPF did not improve acquired humoral immunity against BRD pathogens, which likely contributed to the similar incidence of BRD among treatments (Callan, 2001). Perhaps treatment differences in innate immune parameters were not sufficient to improve acquired humoral responses to vaccinated antigens. It should be noted that serum antibody titers against BRD pathogens increased across all treatments during the experiment, indicating that steers effectively acquired humoral immunity against these pathogens on vaccination and revaccination (Howard et al., 1989; Bolin and Ridpath, 1990; Richeson et al., 2008). Moreover, vaccination against BRD pathogens is also known to further contribute to the adrenocortical and inflammatory responses reported herein (Rodrigues et al., 2015; Lippolis et al., 2016), and it is typically performed on feedlot arrival and a few weeks later due to lack of health history in high-risk receiving cattle (Richeson et al., 2008; Edwards, 2010). Therefore, and as previously mentioned, vaccination against BRD also contributed to the increase in cortisol, haptoglobin, and whole-blood mRNA expression parameters on feedlot arrival observed across treatments.
Supplementing OMN and IPF also failed to improve feedlot receiving performance. In fact, both treatments impaired steer growth and feed efficiency during the 80-d receiving period compared with CON steers, with a greater disadvantage to steers receiving OMN. Nevertheless, treatment differences in BW were noted only after d 56 of the receiving period, despite similar DMI throughout the experiment. Hence, treatment differences on ADG and final BW should be mainly associated with the impaired G:F of IPF and OMN steers after d 56. Yet the OMN and IPF treatments were administered until d 30 of feedlot receiving, when cattle are constantly exposed to a multitude of stressors that directly impair their growth metabolism (Duff and Galyean, 2007; Cooke, 2017). Moreover, BRD symptoms were observed only during the initial 30 d of feedlot receiving (Snowder et al., 2006) and did not differ among OMN, CON, and IPF steers and, therefore, should not be associated with treatment differences on BW and ADG (Schneider et al., 2009). Differences in ADG and BW between treatments were also not reflected by plasma IGF-I concentrations, which is positively associated with cattle growth rates (Bishop et al., 1989; Ellenberger et al., 1989; Elsasser et al., 1989). Hence, the exact reasons why OMN and IPF steers experienced reduced growth rates compared with CON steers after d 56 of the experiment cannot be properly elucidated, particularly because all steers were receiving the same overall and nutritional management during this period.
In summary, this experimental model fully represented the stress and health challenges that commercial feeder cattle experience during feedlot receiving, resulting in elevated BRD incidence and morbidity. However, neither OMN nor IPF supplementation were capable of mitigating BRD incidence and improving receiving performance, despite their impacts on adrenocortical and inflammatory responses on feedlot entry. As previously mentioned, published literature investigating the inclusion of OMN or IPF products into feedlot receiving diets is extremely limited. Based on the results from Montgomery et al. (2008), perhaps the transfer factor contained in the IPF products was extensively degraded in the rumen, whereas the lactic acid–producing bacteria failed to improve immunocompetence and productive traits of receiving cattle (Krehbiel et al., 2003). Alternatively, OMN supplementation elicited immunomodulatory effects in cattle administered lipopolysaccharide when offered for 28 d prior to the challenge (Burdick Sanchez et al., 2014). In fact, research suggests cattle should be adapted to OMN supplementation for several weeks prior to the stress or immune insult (Nace et al., 2014; Ryman et al., 2013). Hence, one can speculate that the OMN supplementation failed to improve health and performance of receiving cattle due to the lack of a previous adaption period, such as during a preconditioning program (Pritchard and Mendez, 1990). Consequently, additional research is warranted to further evaluate the use of OMN and IPF products as well as identify other nonantibiotic feed ingredients that enhance cattle immunocompetence and productivity during feedlot receiving.
Footnotes
Financial support for this research was provided by the Oregon Beef Council.
LITERATURE CITED
- Abbas A. K., Lichtman A. H. 2007. Cellular and molecular immunology. 6th ed.Saunders Co., Philadelphia, PA. [Google Scholar]
- Ackermann M. R., Derscheid R., Roth J. A. 2010. Innate immunology of bovine respiratory diseases. Vet. Clin. North Am.: Food Anim. Pract. 26:215–228. doi: 10.1016/j.cvfa.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed.AOAC Int., Arlington, VA. [Google Scholar]
- Armstrong S. A., McLean D. J., Schell T. H., Bobe G., Bionaz M. 2016. Evaluation of immune function markers in OmniGen-AF® supplemented steers. J. Anim. Sci. 94(E. Suppl. 5):46 (Abstr.) [Google Scholar]
- Berry B. A., Confer A. W., Krehbiel C. R., Gill D. R., Smith R. A., Montelongo M. 2004. Effects of dietary energy and starch concentrations for newly received feedlot calves: II. Acute-phase protein response. J. Anim. Sci. 82:845–850. doi: 10.2527/2004.823845x [DOI] [PubMed] [Google Scholar]
- Bishop M. D., Simmen R. C. M., Simmen F. A., Davis M. E. 1989. The relationship of insulin-like growth factor-1 with post-weaning performance in Angus beef cattle. J. Anim. Sci. 67:2872–2880. doi: 10.2527/jas1989.67112872x [DOI] [PubMed] [Google Scholar]
- Bolin S. R., Ridpath J. F. 1990. Range of viral neutralizing activity and molecular specificity of antibodies induced in cattle by inactivated bovine viral diarrhea virus-vaccines. Am. J. Vet. Res. 51:703–707. [PubMed] [Google Scholar]
- Brandão A. P., Cooke R. F., Corrá F. N., Piccolo M. B., Gennari R., Leiva T., Vasconcelos J. L. M. 2016. Physiologic, health, and production responses of dairy cows supplemented with an immunomodulatory feed ingredient during the transition period. J. Dairy Sci. 99:5562–5572. doi: 10.3168/jds.2015-10621 [DOI] [PubMed] [Google Scholar]
- Broadway P. R., Carroll J. A., Burdick Sanchez N. C. 2015. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: A review. Microorganisms 3:417–427. doi: 10.3390/microorganisms3030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick N. C., Carroll J. A., Chapman J. D., Welsh T. H., Jr, Vann R. C., Randel R. D. 2012. OmniGen-AF supplementation modulates the physiological and acute phase response of Brahman heifers to an endocrine challenge. J. Anim. Sci. 90(E-Suppl. 3):221. [Google Scholar]
- Burdick Sanchez N. C., Buntyn J. O., Carroll J. A., Wistuba T., DeHann K., Sieren S. E., Jones S. J., Schmidt T. B. 2014. Enhancement of the acute phase response to lipopolysaccharide in feedlot steers supplemented with OmniGen-AF. J. Anim. Sci. 92(E-Suppl. 2):37–38. (Abstr.) [Google Scholar]
- Burdick Sanchez N. C., Young T. R., Carroll J. A., Corley J. R., Rathmann R. J., Johnson B. J. 2013. Yeast cell wall supplementation alters aspects of the physiological and acute phase responses of crossbred heifers to an endotoxin challenge. Innate Immun. 19:411–419. doi: 10.1177/1753425912469673 [DOI] [PubMed] [Google Scholar]
- Buza J. J., Mori Y., Bari A. M., Hikono H., Aodon G., Hirayama S., Shu Y., Momotani E. 2003. Mycobacterium avium subsp. paratuberculosis infection causes suppression of RANTES, monocyte chemoattractant protein 1, and tumor necrosis factor alpha expression in peripheral blood of experimentally infected cattle. Infect. Immun. 71:7223–7227. doi: 10.1128/IAI.71.12.7223-7227.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan R. J. 2001. Fundamental considerations in developing vaccination protocols. Bovine Pract. 34:14–22. [Google Scholar]
- Carroll J. A., Forsberg N. E. 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am.: Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Reuter R. R., Chase C. C., Jr, Coleman S. W., Riley D. G., Spiers D. E., Arthington J. D., Galyean M. L. 2009. Profile of the bovine acute-phase response following an intravenous lipopolysaccharide challenge. Innate Immun. 15:81–89. doi: 10.1177/1753425908099170 [DOI] [PubMed] [Google Scholar]
- Cooke R. F. 2017. Invited paper: Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33(1):1–11. [Google Scholar]
- Cooke R. F., Arthington J. D. 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., Lamb G. C., Ealy A. D. 2008. Effects of supplementation frequency on performance, reproductive, and metabolic responses of Brahman-crossbred females. J. Anim. Sci. 86:2296–2309. doi: 10.2527/jas.2008-0978 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Bohnert D. W., Moriel P., Hess B. W., Mills R. R. 2011. Effects of polyunsaturated fatty acid supplementation on forage digestibility, performance, and physiological responses of feeder cattle. J. Anim. Sci. 89:3677–3689. doi: 10.2527/jas.2010-3515 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Carroll J. A., Dailey J., Cappellozza B. I., Bohnert D. W. 2012. Bovine acute-phase response following different doses of corticotrophin-release hormone challenge. J. Anim. Sci. 90:2337–2344. doi: 10.2527/jas.2011-4608 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Guarnieri Filho T. A., Cappellozza B. I., Bohnert D. W. 2013. Rest stops during road transport: Impacts on performance and acute-phase protein responses of feeder cattle. J. Anim. Sci. 91:5448–5454. doi: 10.2527/jas.2013-6357 [DOI] [PubMed] [Google Scholar]
- Duff G. C., Galyean M. L. 2007. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durum S. K., Muegge K. 1996. Cytokines linking the immune and inflammatory systems: IL-1, TNF, IL-6, IFN-ab, and TGF-b. In: Rich R. R., Fleisher T. A., Schwartz B. D., Shearer W. T., Strober W. editors, Clinical immunology, principles and practice. Mosby, St. Louis, MO: p. 350–362. [Google Scholar]
- Edwards T. A. 2010. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. North Am.: Food Anim. Pract. 26:273–284. doi: 10.1016/j.cvfa.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Ellenberger M. A., Johnson D. E., Carstens G. E., Hossner K. L., Holland M. D., Nett T. M., Nockels C. F. 1989. Endocrine and metabolic changes during altered growth rates in beef cattle. J. Anim. Sci. 67:1446–1454. doi: 10.2527/jas1989.6761446x [DOI] [PubMed] [Google Scholar]
- Elsasser T. H., Rumsey T. S., Hammond A. C. 1989. Influence of diet on basal and growth hormone-stimulated plasma concentrations of IGF-1 in beef cattle. J. Anim. Sci. 67:128–141. doi: 10.2527/jas1989.671128x [DOI] [PubMed] [Google Scholar]
- Fleige S., Pfaffl M. W. 2006. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27:126–139. doi: 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Fudenberg H. H., Fudenberg H. H. 1989. Transfer factor: Past, present and future. Annu. Rev. Pharmacol. Toxicol. 29:475–516. doi: 10.1146/annurev.pa.29.040189.002355 [DOI] [PubMed] [Google Scholar]
- Gifford C. A., Racicot K., Clark D. S., Austin K. J., Hansen T. R., Lucy M. C., Davies C. J., Ott T. L. 2007. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 90:274–280. doi: 10.3168/jds.S0022-0302(07)72628-0 [DOI] [PubMed] [Google Scholar]
- Hall L. W., Rivera F. A., Villar F., Chapman J. D., Long N. M., Collier R. J. 2014. Evaluation of OmniGen-AF® in lactating heat-stressed Holstein cows. In: 25th Annu. Fla. Ruminant Nutr. Symp., University of Florida, Gainesville, FL: p. 16–26. [Google Scholar]
- Heine H., Ulmer A. J. 2005. Recognition of bacteria products by Toll-like receptors. Chem. Immunol. Allergy 86:99–119. doi: 10.1159/000086654 [DOI] [PubMed] [Google Scholar]
- Hess B. W., Lake S. L., Scholljegerdes E. J., Weston T. R., Nayigihugu V., Molle J. D. C., Moss G. E. 2005. Nutritional controls of beef cow reproduction. J. Anim. Sci. 83:E90–E106. [Google Scholar]
- Howard C. J., Clarke M. C., Brownlie J. 1989. Protection against respiratory infection with bovine virus diarrhea virus by passively acquired antibody. Vet. Microbiol. 19:195–203. doi: 10.1016/0378-1135(89)90066-7 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J. G., Step D. L., Payton M. E., Richards J. B., McTague L. F., Saliki J. T., Confer A. W., Cook B. J., Ingram S. H., Wright J. C. 2008. Effect of age at the time of vaccination on antibody titers and feedlot performance in beef calves. J. Am. Vet. Med. Assoc. 233:136–142. doi: 10.2460/javma.233.1.136 [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Kristensen F. 1977. Bovine transfer factor: Effect on bovine and rabbit coccidiosis. Clin. Immunol. Immunopathol. 7:240–252. doi: 10.1016/0090-1229(77)90051-4 [DOI] [PubMed] [Google Scholar]
- Kliem H., Rodler D., Ulbrich S. E., Sinowatz F., Berisha B., Meyer H. H. D., Schams D. 2013. Dexamethasone-induced eosinopenia is associated with lower progesterone production in cattle. Reprod. Domest. Anim. 48:137–148. doi: 10.1111/j.1439-0531.2012.02116.x [DOI] [PubMed] [Google Scholar]
- Krehbiel C. R., Rust S. R., Zang G., Gilliland S. E. 2003. Bacterial direct-fed microbials in ruminant diets: Performance response and mode of action. J. Anim. Sci. 81:E120–E132. [Google Scholar]
- Lippolis K. D., Cooke R. F., Schubach K. M., Brandão A. P., da Silva L. G. T., Marques R. S., Bohnert D. W. 2016. Altering the time of vaccination against respiratory pathogens to enhance vaccine efficacy and performance of feeder cattle. J. Anim. Sci. 94:3987–3995. doi: 10.2527/jas.2016-0673 [DOI] [PubMed] [Google Scholar]
- Marques R. S., Cooke R. F., Rodrigues M. C., Cappellozza B. I., Larson C. K., Moriel P., Bohnert D. W. 2016. Effects of organic or inorganic Co, Cu, Mn, and Zn supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J. Anim. Sci. 94:1215–1226. doi: 10.2527/jas.2015-0036 [DOI] [PubMed] [Google Scholar]
- McBride M. L., Burdick Sanchez N. C., Carroll J. A., Broadway P. R., Ortiz X. O., Collier J. L., McLean D., Chapman J. D., Kattesh H. G., Collier R. J. 2016. Omnigen-AF reduces basal plasma cortisol as well as cortisol release to adrenocorticotropic hormone or corticophin releasing hormone and vasopressin in lactating dairy cows under thermoneutral or acute heat stress conditions. J. Anim. Sci. 94(E-Suppl. 5):541–542. [DOI] [PubMed] [Google Scholar]
- McDonald A., Anderson P., DeFoor P., Botts R. 2005. DFMs improve health, performance of cattle. Feedstuffs 77:12–13. [Google Scholar]
- Meyer R. M., Bartley E. E. 1972. Bloat in cattle. XVI. Development and application of techniques for selecting drugs to prevent feedlot bloat. J. Anim. Sci. 34:234–240. [DOI] [PubMed] [Google Scholar]
- Montgomery S. P., Sindt J. J., Greenquist M. A., Loe E. R., Drouillard J. S. 2008. Comparison of bovine transfer factor and tilmicosin phosphate: Effects on health and growth performance of newly arrived feedlot heifers. Int. J. Appl. Res. Vet. Med. 6(3):175–180. [Google Scholar]
- Nace E. L., Nickerson S. C., Kautz F. M., Breidling S., Wochele D., Ely L. O., Hurley D. J. 2014. Modulation of innate immune function and phenotype in bred dairy heifers during the periparturient period induced by feeding an immunostimulant for 60 days prior to delivery. Vet. Immunol. Immunopathol. 161:240–250. doi: 10.1016/j.vetimm.2014.08.013 [DOI] [PubMed] [Google Scholar]
- NRC 1996. Nutrient requirements of beef cattle. 7th rev. ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Ocón-Grove O. M., Cooke F. N. T., Alvarez I. M., Johnson S. E., Ott T. L., Ealy A. D. 2008. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest. Anim. Endocrinol. 34:135–145. doi: 10.1016/j.domaniend.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Playford M., Dawson K., Playford S., Smith A. N., Page S. W., Collins K., Forsberg N. 2014. Effect of an immunomodulatory feed additive on markers of immunity in pasture-fed dairy cows. Aust. Vet. J. 92:479–481. doi: 10.1111/avj.12269 [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Mendez J. K. 1990. Effects of preconditioning on pre- and post-shipment performance of feeder calves. J. Anim. Sci. 68:28–34. doi: 10.2527/1990.68128x [DOI] [PubMed] [Google Scholar]
- Richeson J. T., Beck P. A., Gadberry M. S., Gunter S. A., Hess T. W., Hubbell D. S., Jones C. 2008. Effects of on-arrival versus delayed modified-live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly-received beef calves. J. Anim. Sci. 86:999–1005. doi: 10.2527/jas.2007-0593 [DOI] [PubMed] [Google Scholar]
- Riollet C., Rainard P., Poutrel B. 2000. Kinetics of cells and cytokines during immune-mediated inflammation in the mammary gland of cows systemically immunized with Staphylococcus aureus α-toxin. Inflamm. Res. 49:486–496. doi: 10.1007/s000110050621 [DOI] [PubMed] [Google Scholar]
- Rodrigues M. C., Cooke R. F., Marques R. S., Cappellozza B. I., Arispe S. A., Keisler D. H., Bohnert D. W. 2015. Effects of vaccination against respiratory pathogens on feed intake, metabolic and inflammatory responses in beef heifers. J. Anim. Sci. 93:4443–4452. doi: 10.2527/jas.2015-9277 [DOI] [PubMed] [Google Scholar]
- Rozzo S. J., Kirkpatrick C. H. 1992. Purification of transfer factors. Mol. Immunol. 29:167–182. doi: 10.1016/0161-5890(92)90098-I [DOI] [PubMed] [Google Scholar]
- Ryman V. E., Nickerson S. C., Kautz F. M., Hurley D. J., Ely L. O., Wang Y. Q., Forsberg N. E. 2013. Effect of dietary supplementation on the antimicrobial activity of blood leukocytes isolated from Holstein heifers. Res. Vet. Sci. 95:969–974. doi: 10.1016/j.rvsc.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Schneider M. J., Tait R. G., Jr, Busby W. D., Reecy J. M. 2009. An evaluation of bovine respiratory disease complex in feedlot cattle: Impact on performance and carcass traits using treatment records and lung scores. J. Anim. Sci. 87:1821–1827. doi: 10.2527/jas.2008-1283 [DOI] [PubMed] [Google Scholar]
- Silva E., Gaivão M., Leitão S., Amaro A., Lopes da Costa L., Mateus L. 2008. Blood COX-2 and PGES gene transcription during the peripartum period of dairy cows with normal puerperium or with uterine infection. Domest. Anim. Endocrinol. 35:314–323. doi: 10.1016/j.domaniend.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Snowder G. D., Van Vleck L. D., Cundiff L. V., Bennett G. L. 2006. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 84:1999–2008. doi: 10.2527/jas.2006-046 [DOI] [PubMed] [Google Scholar]
- Steiger M., Senn M., Altreuther G., Werling D., Sutter F., Kreuzer M., Langhans W. 1999. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers. J. Anim. Sci. 77:2523–2532. doi: 10.2527/1999.7792523x [DOI] [PubMed] [Google Scholar]
- Step D. L., Krehbiel C. R., DePra H. A., Cranston J. K., Fulton R. W., Kirkpatrick J. G., Gill D. R., Payton M. E., Montelongo M. A., Confer A. W. 2008. Effects of commingling beef calves from different sources and weaning protocols during a forty-two-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 86:3146–3158. doi: 10.2527/jas.2008-0883 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration 2015. Fact sheet: Veterinary feed directive final rule and next steps. Accessed June 10, 2017. http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/ucm449019.htm.
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research0034.1. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., Lewis B. A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Wang Y. Q., Puntenney S. B., Burton J. L., Forsberg N. E. 2009. Use of gene profiling to evaluate the effects of a feed additive on immune function in periparturient dairy cattle. J. Anim. Physiol. Anim. Nutr. 93:66–75. doi: 10.1111/j.1439-0396.2007.00780.x [DOI] [PubMed] [Google Scholar]
- Weiss W. P., Conrad H. R., St. Pierre N. R. 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 39:95–110. doi: 10.1016/0377-8401(92)90034-4 [DOI] [Google Scholar]
- Wilson B. K., Richards C. J., Step D. L., Krehbiel C. R. 2017. Best management practices for newly weaned calves for improved health and well-being. J. Anim. Sci. 95:2170–2182. doi: 10.2527/jas.2016.1006 [DOI] [PubMed] [Google Scholar]
- Wilson B. K., Step D. L., Maxwell C. L., Wagner J. J., Richards C. J., Krehbiel C. R. 2015. Evaluation of multiple ancillary therapies used in combination with an antimicrobial in newly received high-risk calves treated for bovine respiratory disease. J. Anim. Sci. 93:3661–3674. doi: 10.2527/jas.2015-9023 [DOI] [PMC free article] [PubMed] [Google Scholar]


