Abstract
Mares grazing endophyte-infected (Epichloë coenophiala) tall fescue (Lolium arundinaceum) typically exhibit reproductive dysfunction rather than problems associated with peripheral vasoconstriction as a primary sign of the fescue toxicosis syndrome. Research using Doppler ultrasonography demonstrated that consumption of endophyte-infected tall fescue seed causes measurable vasoconstriction in the medial palmar artery. The objective of this study was to evaluate contractile responses of medial palmar artery and vein to increasing concentrations of various tall fescue alkaloids. Medial palmar arteries and veins were collected immediately following euthanasia from 23 horses of mixed breed, age, and gender from both forelimbs, and uterine arteries were collected from females (n = 12). Vessels were separated, cleaned of excess connective and adipose tissue, divided into 2- to 3-mm cross-sections, and suspended in a multimyograph chamber with continuously oxygenated Krebs–Henseleit buffer (95% O2/5% CO2; pH 7.4; 37°C). Following a 90-min equilibration and recovery from reference compound exposure, increasing concentrations of norepinephrine, 5-hydroxytryptamine, ergotamine, and ergonovine for the palmar artery and vein and uterine artery and ergovaline, ergocryptine, ergocristine, ergocornine, and lysergic acid for the palmar artery and vein were added to assess vasoactivity. Data were normalized as a percentage of contractile response induced by the reference compound addition and analyzed as a completely randomized design. Both norepinephrine and serotonin were vasoactive in all 3 types of blood vessels. Neither ergotamine nor ergonovine were vasoactive in the uterine artery. All alkaloids tested with the palmar artery and vein produced a contractile response, except that neither the palmar artery nor the palmar vein responded to lysergic acid (P > 0.05). Ergovaline was the most vasoactive ergot alkaloid in both the palmar artery and the palmar vein (P < 0.05) followed by ergonovine, whereas out of the 4 remaining ergopeptine alkaloids tested, ergocristine induced the lowest contractile response. Although horses do not outwardly appear to be affected by peripheral vasoconstriction as observed in cattle, these data indicate that tall fescue alkaloids are vasoactive and suggest that potential exists for peripheral vascular effects of tall fescue alkaloids in horses. This does not appear to be the case for the uterine artery, and future research should be directed at understanding how ergot alkaloids cause equine reproductive dysfunction.
Keywords: equine, ergot alkaloids, palmar artery, palmar vein, uterine artery, vasoconstriction
INTRODUCTION
Hallmarks of equine fescue toxicosis are the reproductive abnormalities in the gravid mare that can often result in the death of the foal (Cross et al., 1995). This is primarily a consequence of consumption of toxic endophyte-infected (Epichloë coenophiala) tall fescue [Lolium arundinaceum (Shreb.) Darbysh.] by the mare during gestation. Removal of pregnant mares from sources of tall fescue during gestation is a typical solution. Given that tall fescue covers over 15 million ha in the eastern United States (Hannaway et al., 2009), this is not a sustainable solution for all horses year-round. Data on other aspects of fescue toxicosis in horses is limited. Although not outwardly subjected to the vasoconstrictive effects of ergot alkaloids that are reported in cattle (Klotz, 2015), ergot alkaloids may still have an effect on blood flow in the horse and could contribute to the occurrence of pasture laminitis (Rohrbach et al., 1995). Using horses fed ground endophyte-infected tall fescue seed, McDowell et al. (2013) demonstrated with Doppler ultrasonography that the equine distal palmar artery was vasoconstricted compared with that of endophyte-free control horses. Abney et al. (1993) demonstrated that ergotamine and ergonovine were moderately vasoactive in isolated equine lateral saphenous veins and the metatarsal artery of the hind limb. However, these are different vessels than evaluated by McDowell et al. (2013). Also, there are numerous vasoactive ergot alkaloids (Fig. 1) produced by the endophyte E. coenophiala found in tall fescue (Lyons et al., 1986; Yates and Powell, 1988). Therefore, the objective of this study was to evaluate the vasoactivity of ergot alkaloids found in toxic endophyte-infected tall fescue using preparations of the equine medial palmar artery and vein and uterine artery.
Figure 1.
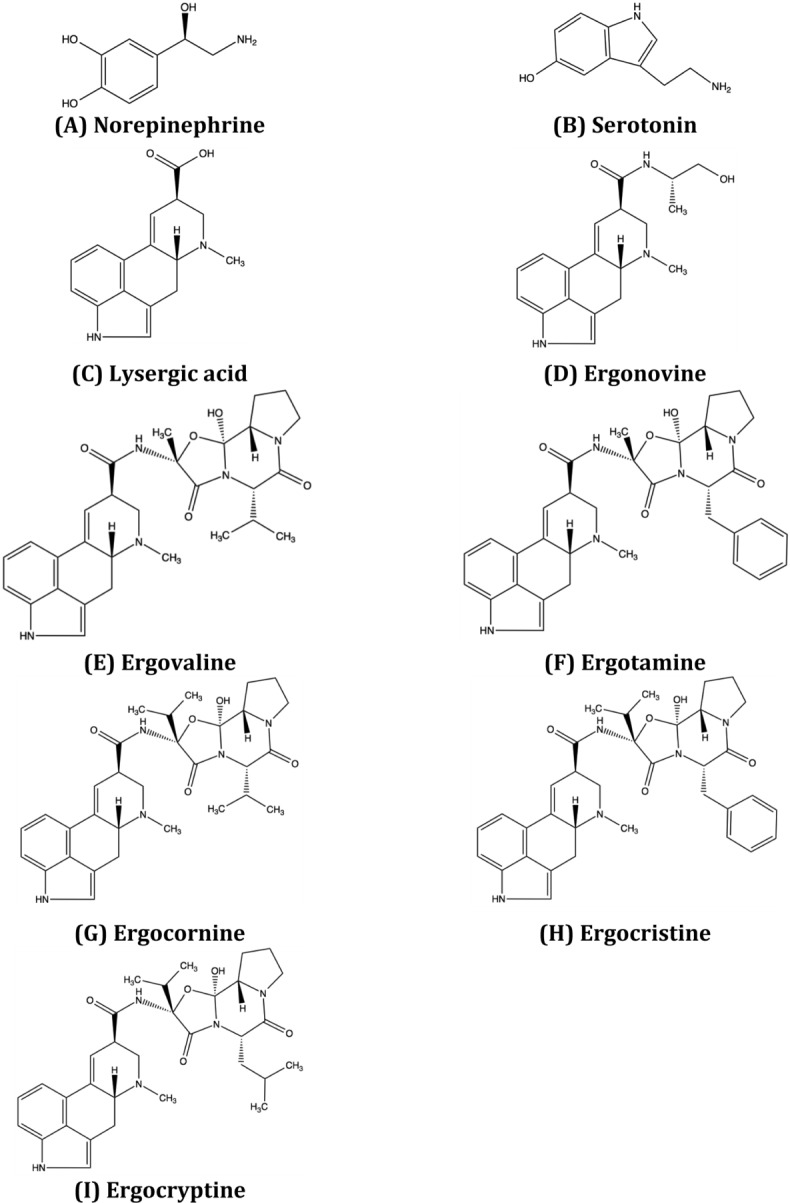
Chemical structures of biogenic amines (A) norepinephrine and (B) serotonin; ergoline alkaloids (C) lysergic acid and (D) ergonovine; and ergopeptine alkaloids (E) ergovaline, (F) ergotamine, (G) ergocornine, (H) ergocristine, and (I) ergocryptine.
MATERIALS AND METHODS
The University of Kentucky Institutional Animal Care and Use Committee approved procedures associated with euthanasia of horses described in this study.
Animals and Blood Vessels
Blood vessels were collected from adult nonpregnant mixed breed and mixed gender horses (n = 23) immediately following euthanasia. Prior to euthanasia, 12 of the horses received xylazine HCl (100 mg/mL) and the other 11 received detomidine hydrochloride (10 mg/mL) and butorphanol tartrate (10 mg/mL) as sedatives. All horses were euthanized with sodium pentobarbital (1 mL/4.5 kg BW; 390 mg/mL) that contained sodium phenytoin (50 mg/mL).
Following confirmation of death, an incision was made through the skin between the carpal and fetlock joints in the medial aspect of the forelimbs, and segments (4 to 5 cm in length) of the palmar artery and vein bundle were removed from the metacarpal region along the third metacarpal proximal to the fetlock joint of each forelimb. These segments were placed in a modified Krebs–Henseleit oxygenated buffer solution (95% O2/5% CO2; pH 7.4; composition: 11.1 mMD-glucose, 1.2 mM MgSO4, 1.2 mM KH2PO4, 4.7 mM KCl, 118.1 mM NaCl, 3.4 mM CaCl2, and 24.9 mM NaHCO3; K3753; Sigma Chemical Co., St. Louis, MO) for transport to the laboratory. Following collection of the forelimb vessels, an incision was made through the central caudal abdomen into the pelvic cavity, and reproductive tracts of euthanized females were exteriorized (n = 12). Segments (4 to 5 cm) of the right and left uterine arteries were identified, collected, and placed into the modified Krebs–Henseleit buffer. All uterine and forelimb blood vessel samples were kept on ice until processed.
The medial palmar artery and vein were separated, and all perivascular fat and connective tissue were carefully removed from the palmar and uterine vessels. The arteries and vein were sliced into 2- to 3-mm cross-sections. Cross-sections were examined under a dissecting microscope (Stemi 2000-C; Carl Zeiss AG, Oberkochen, Germany) at 12.5x magnification to verify physical integrity of tissue, and to assure a consistent segment size, dimensions were measured (Axiovision, version 20; Carl Zeiss AG). Duplicate cross-sections of each blood vessel from each animal were horizontally suspended in a tissue bath (DMT610M Multi-Chamber myograph; Danish Myo Technologies USA Inc., Atlanta, GA) containing 5 mL of continuously gassed (95% O2/5% CO2) modified Krebs–Henseleit incubation buffer (37°C; pH 7.4). The buffer used for incubations was the same as the transport buffer, except that desipramine (3 × 10−5M; D3900; Sigma Chemical Co.) was added to inactivate catecholamine neuronal uptake. Blood vessel sections were allowed to equilibrate under a resting tension of 1 g for 90 min. The buffer solution was replaced at 15-min intervals during this equilibration period. Preliminary experiments revealed that uterine artery cross-sections were minimally responsive to norepinephrine exposure, and this resulted in the use of KCl as a reference compound. Therefore, in order to assure tissue responsiveness and for use as a reference for normalization of subsequent contractile responses, medial palmar artery and vein cross-sections were exposed to the α-adrenergic agonist norepinephrine bitartrate (1 × 10−4M; A0937; Sigma Chemical Co.) and the uterine artery was exposed to 0.12 M KCl.
Concentration–Response Experiments
Cross-sections of each blood vessel used for a concentration–response experiment were run in duplicate for each animal used to create a data set. Following recovery from the norepinephrine addition (medial palmar artery and vein) or KCl addition (uterine artery) and the reestablishment of a 1-g baseline tension, the compounds being evaluated were added from least to greatest concentrations in 15-min intervals for the medial palmar vein and 20-min intervals for the medial palmar and uterine arteries (arterial preparations used in preliminary concentration–response experiments did not achieve a maximal response in the 9-min incubation period that had been validated in previous bioassays [Klotz et al., 2006; Klotz and Barnes, 2014]). The incubation periods were 9 and 14 min for venous and arterial preparations, respectively. These incubation periods were followed by two 2.5-min washout periods and a 1-min recovery interval prior to the next addition and subsequent incubation period. Increasing concentrations of biogenic amines and ergot alkaloids were added at stock concentrations such that the final added volume did not exceed 0.5% of the 5-mL incubation buffer in the myograph chamber. Test compounds were norepinephrine (Sigma Chemical Co.), serotonin HCl (100% purity; H9523; Sigma Chemical Co.), lysergic acid dihydrate (95% purity; Acros Organics, Geel, Belgium), ergonovine maleate (100% purity; E6500; Sigma Chemical Co.), ergovaline (93% purity; supplied by Forest Smith, Auburn University, Auburn, AL), ergocornine (>95% purity; E131; Sigma Chemical Co.), ergocristine (Research Plus Inc., Barnegat, NJ), α-ergocryptine (99% purity; E5625; Sigma Chemical Co.), and ergotamine D-Tartrate (97% purity; 45510; Aldrich Chemical Co., Milwaukee, WI). Concentrations ranged from 1 × 10−9, 1 × 10−8, 1 × 10−7, 1 × 10−6, 1 × 10−5, and 1 × 10−4M for all compounds except ergotamine, where the maximal concentration was 1 × 10−5 due to limitations in solubility. Due to limitations in the availability of the uterine artery samples, only norepinephrine, serotonin, ergonovine, and ergotamine concentration–response experiments were conducted.
Data Collection and Statistical Analysis
Isometric contraction of a blood vessel was recorded as grams of tension in response to exposure to norepinephrine or KCl and the subsequent alkaloid additions. Data were digitized and recorded using a Powerlab/8sp data acquisition system (ADInstruments, Colorado Springs, CO) and Chart software (version 7; ADInstruments). Contractile response was recorded as the greatest gram contractile response within the 9-min incubation period for the medial palmar vein or within the 14-min incubation period for the medial palmar and uterine arteries. All maximal values measured were corrected by the baseline measured during the interval preceding addition of the 1 × 10−4M norepinephrine or 0.12 M KCl reference treatment, thus generating a cumulative concentration response. To compensate for variation of tissue responsiveness due to differences in tissue size or individual animal variation, values were normalized as a percentage of the maximal contraction produced by the respective reference compound. Data are presented as percent means ± SE of the maximal contraction induced by norepinephrine or KCl. Data were plotted using nonlinear regression (Prism 5.0f; GraphPad Software, Inc., San Diego, CA) to illustrate the contractile response of the equine blood vessel to a treatment with the following 4-parameter equation:
in which the top and bottom are the plateaus in contractile response (y-axis) and the hill slope is the numerical steepness of the curve. This equation permitted the determination of a compound's potency as the effective concentration of the compound required to produce 50% of the observed contractile response (EC50).
Data were analyzed using the mixed model procedure of SAS (version 9.3; SAS Inst. Inc., Cary, NC) as a completely randomized design. The model included alkaloid, concentration, and an alkaloid × concentration interaction. Horse was the experimental unit. Analysis of variance was conducted, and pairwise comparisons of least squares means (±SEM) were performed if the probability of a greater F-statistic was significant for a tested effect. Mean separation was done using least significant difference features in SAS. Probabilities of P < 0.05 are discussed as significant, unless otherwise noted.
RESULTS
Palmar Artery and Vein
The cross-sections of the palmar arteries and veins used in these experiments had average lengths of 2.71 ± 0.02 and 2.25 ± 0.01, respectively; i.d. of 1.05 ± 0.01 and 1.47 ± 0.02, respectively; and o.d. of 4.63 ± 0.03 and 3.93 ± 0.02 mm, respectively. The biogenic amines norepinephrine and serotonin were vasoactive in both the palmar artery (Fig. 2A) and the palmar vein (Fig. 2B), indicating the presence of adrenergic and serotonergic receptor populations. Norepinephrine induced greater contractile responses in both the palmar artery and the palmar vein (P < 0.05) than serotonin. Onset of the contractile response to both norepinephrine and serotonin occurred at 1 × 10−6M, but the response generated by norepinephrine was greater than that generated by serotonin in the palmar artery (P < 0.05). Mean maximal responses were observed at the greatest concentrations of norepinephrine and serotonin (1 × 10−4M) for the palmar artery at 68.1 ± 1.7 and 46.2 ± 1.7%, respectively, and for the palmar vein at 61.6 ± 1.4 and 19.8 ± 1.4%, respectively.
Figure 2.
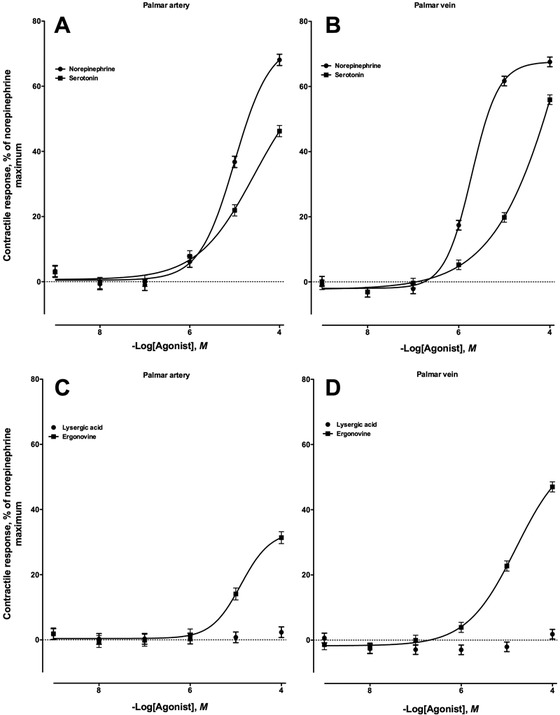
Contractile response of the nonpregnant equine (A) medial palmar artery and (B) vein to increasing concentrations of norepinephrine (artery, n = 10; −logEC50 = 4.99 ± 0.09, in which EC50 is the effective concentration of the compound required to produce 50% of the observed contractile response; vein, n = 10; −logEC50 = 5.71 ± 0.06) and serotonin (artery, n = 10; −logEC50 = 4.56 ± 0.59; vein, n = 10; −logEC50 = 3.42 ± 1.44). Contractile response of the nonpregnant equine (C) medial palmar artery and (D) vein to increasing concentrations of lysergic acid (artery, n = 11; −logEC50 was not determined; vein, n = 10; −logEC50 was not determined) and ergonovine (artery, n = 9; −logEC50 = 4.88 ± 0.12; vein, n = 9; −logEC50 = 4.80 ± 0.10).
Of the 2 ergoline alkaloids evaluated in the current study, only ergonovine induced a significant contractile response in both the palmar artery and the palmar vein (Fig. 2C and 2D, respectively). Lysergic acid was not vasoactive in either the palmar artery (Fig. 2C) or the palmar vein (Fig. 2D) at any concentration evaluated (P > 0.05). The onset of contractile response caused by exposure to ergonovine occurred at 1 × 10−5M in both the palmar artery and the palmar vein preparations. The greatest contractile intensity produced by ergonovine was 31.3 ± 1.8% for the palmar artery and 47.0 ± 1.5% for the palmar vein at the 1 × 10−4M addition.
Five different ergopeptine alkaloids were evaluated for vasoactivity in the equine palmar artery and vein (Fig. 3A and 3B, respectively), and all 5 tested produced contractile responses in both vessel types. Ergovaline had the earliest onset of contraction (P < 0.05) for the artery and vein (1 ×10−6 and 1 ×10−7M, respectively) as well as the greatest contractile intensity (P < 0.05) at the 1 × 10−4M addition (60.2 ± 2.4% for the artery and 56.8 ± 2.1% for the vein) compared with the other 4 ergopeptine alkaloids. Second to ergovaline was the response generated by exposing the vessels to increasing concentrations of ergotamine (Fig. 3). Contractile responses to ergotamine peaked at 20.6 ± 0.7% for the palmar artery and 15.8 ± 0.9% for the palmar vein at the highest concentration evaluated. These responses were greater than those for ergocornine, ergocryptine, and ergocristine at the 1 × 10−5M addition (P < 0.05). The onset of contractile response for ergocornine, ergocryptine, and ergocristine was 1 × 10−5M for the palmar artery and vein (Fig. 3). Exposure of palmar artery and vein to increasing concentrations of ergocristine produced the smallest contractile response of the ergopeptine alkaloids evaluated (P < 0.05).
Figure 3.
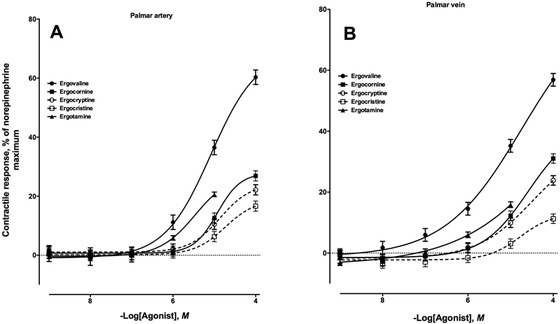
Contractile response of the nonpregnant equine (A) medial palmar artery and (B) vein to increasing concentrations of ergovaline (artery, n = 5; −logEC50 = 5.08 ± 0.08, in which EC50 is the effective concentration of the compound required to produce 50% of the observed contractile response; vein, n = 5; −logEC50 = 4.73 ± 0.29), ergocornine (artery, n = 10; −logEC50 = 4.93 ± 0.07; vein, n = 9; −logEC50 = 4.59 ± 0.41), ergocryptine (artery, n = 10; −logEC50 = 4.86 ± 0.06; vein, n = 9; −logEC50 = 4.48 ± 0.59), ergocristine (artery, n = 10; −logEC50 = 4.73 ± 0.15; vein, n = 9; −logEC50 = 4.77 ± 0.66), and ergotamine (artery, n = 10; −logEC50 = 5.45 ± 0.03; vein, n = 9; −logEC50 was not determined).
Uterine Artery
The uterine arteries used in these myograph experiments were collected from nonpregnant mares. The average dimensions of the uterine artery cross-sections used were 2.59 ± 0.06 mm for length, 0.79 ± 0.03 mm for i.d., and 3.82 ± 0.06 mm for o.d. Exposure of the equine uterine artery to increasing concentrations of serotonin did not result in a contractile response until the 1 × 10−4M addition (P < 0.05; Fig. 4A), whereas exposure to increasing concentrations of norepinephrine produced a significant contractile response at the 1 × 10−5M addition (Fig. 4A). Because there were not sufficient quantities of available equine uterine artery to run the same number of experiments that were conducted with palmar vessels, ergonovine and ergotamine were selected as representative alkaloids from the ergoline and ergopeptine structural classes, respectively (Fig. 1). Interestingly, neither alkaloid was vasoactive in the uterine artery preparations at any concentration evaluated (Fig. 4B). Although there was a slight increase in contractile response, no additions were different for either alkaloid (P > 0.05).
Figure 4.
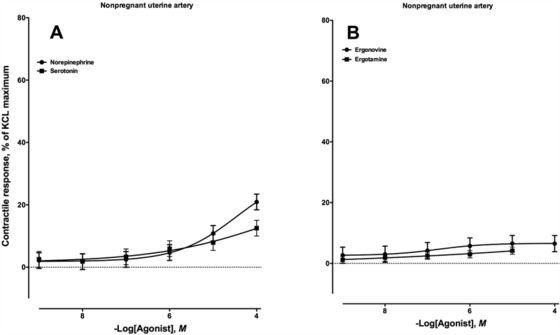
Contractile response of the nonpregnant equine uterine artery to increasing concentrations of (A) norepinephrine (n = 10; −logEC50 = 4.45 ± 0.18, in which EC50 is the effective concentration of the compound required to produce 50% of the observed contractile response) and serotonin (n = 10; −logEC50 = 3.49 ± 5.14) and (B) ergonovine (n = 9; −logEC50 = 6.75 ± 0.06) and ergotamine (n = 10; −logEC50 was not determined).
DISCUSSION
Generally, equine issues related to ergot alkaloid exposure that receive the most attention are more reproductive in nature and typically consist of agalactia, prolonged gestations, abortions, thickened placentas, and increased foal mortality (Cross et al., 1995; Blodgett, 2001). In addition to decreased reproductive performance, ergotism or fescue toxicosis in cattle has also been associated with decreased intakes and ADG, increased body temperatures in warmer climates, sloughing of the hooves in colder climates, and vasoconstriction (Klotz, 2015). Horses that received intravenous infusion of ergovaline, the predominant ergot alkaloid produced by Epichloë coenophiala in tall fescue, exhibited coolness of the extremities, excessive sweating, and prostration (Bony et al., 2001). Furthermore, Webb et al. (2012) and Vivrette et al. (2001) reported that consumption of ergot alkaloids had a significant impact on the ability of horses to recover from exercise in hot conditions, presumably due to peripheral vasoconstriction reducing the efficiency of evaporative cooling. Abney et al. (1993) demonstrated in vitro that ergonovine and ergotamine were vasoactive in the equine dorsal metatarsal artery and lateral saphenous vein. Scanning the distal palmar artery with Doppler ultrasound, McDowell et al. (2013) demonstrated an in vivo vasoconstriction caused by consumption of ground endophyte-infected tall fescue seed in horses. Because receptor populations are not conserved across blood vessel type and location, the current study sought to add to the in vitro knowledge initiated by Abney et al. (1993) by using the vascular region targeted by McDowell et al. (2013) to characterize the vasoactivity of ergot alkaloids.
Palmar Artery and Vein
The pattern of contractile response to increasing concentration of norepinephrine and serotonin in both the palmar artery and the palmar vein indicate the presence of adrenergic and serotonergic receptor populations in these blood vessels. This is similar to other peripheral vessel data that revealed the presence of serotonergic and α-adrenergic receptors in equine digital arteries and veins (Bailey and Elliott, 1998; Zerpa et al., 2010). In the present study, both the palmar artery and the palmar vein exhibited a greater vasoactive response to norepinephrine. This was similar to what Abney et al. (1993) reported for norepinephrine and serotonin using equine lateral saphenous veins and dorsal metatarsal arteries. Furthermore, Abney et al. (1993) evaluated the vasoactivity of ergonovine and ergotamine and saw contractile responses that ranged from approximately 20 to 30% of the norepinephrine maximum. The authors concluded that this was not a sufficient vasoactive response to cause the significant peripheral vasoconstriction in horses that is often observed in cattle (Strickland et al., 2011). The results for ergotamine and ergonovine in the present study agree with the results of Abney et al. (1993), producing only moderate contractile responses that were approximately 20 and 40%, respectively, of the norepinephrine maximum. In further agreement with the conclusions of Abney et al. (1993), these responses are substantially less that what has been reported for ergotamine (43.7%; 1 × 10−5M; Klotz et al., 2007) and ergonovine (68.5%; 1 × 10−4M; Klotz et al., 2010) in a bovine lateral saphenous vein bioassay. If Abney et al. (1993) are correct in their conclusions that ergot alkaloids are minimally responsible for peripheral vasoconstriction in horses, then what was responsible for the significant decreases in lumen diameter, circumference, and blood flow reported by McDowell et al. (2013) in horses consuming endophyte-infected tall fescue seed? One possible answer may be ergovaline. McDowell et al. (2013) fed horses from 0.076 up to 0.713 mg ergovaline/kg BW. Abney et al. (1993) did not evaluate ergovaline. The current study is the first report of ergovaline vasoactivity in equine vasculature and demonstrated that in both the palmar artery and the palmar vein, ergovaline is significantly more active than any other ergopeptine alkaloid evaluated. The maximal response by ergovaline was approached only by that of the ergoline alkaloid ergonovine in the palmar vein. However, ergonovine does not display the sustained contractile response (Pesqueira et al., 2014) that has become a hallmark characteristic of ergopeptide alkaloids in vascular studies in other species (Dyer, 1993; Schöning et al., 2001; Klotz et al., 2007; Pesqueira et al., 2014). Therefore, ergonovine could certainly induce transient instances of vasoconstriction but not the prolonged negative effects associated with fescue toxicosis. Further characterizing the duration of contractile response in equine vasculature is an area where future work is needed.
This study is the first assessment of vasoactivity of lysergic acid, ergocryptine, ergocristine, and ergocornine in equine vasculature. Lysergic acid is found in relatively high concentrations in excreta of horses consuming tall fescue seed (Schultz et al., 2006) and has been proposed as a possible toxin contributing to the observed effects of fescue toxicosis (Hill et al., 2001; Ayers et al., 2009). Like reports in the bovine lateral saphenous vein (Klotz et al., 2006), lysergic acid did not produce a significant contractile response in equine peripheral vasculature, thus limiting the contributions to vasoconstriction that can be attributed to lysergic acid. Although ergocryptine, ergocornine, and ergocristine are not produced in high concentrations by E. coenophiala (Yates and Powell, 1988), they are produced in greater proportions by Claviceps purpurea (Stoll and Hoffmann, 1970). Therefore, these ergopeptide alkaloids have the potential to be consumed by horses grazing contaminated pastures or fed contaminated hay or grains. Results in the current study indicate that these alkaloids are capable of causing moderate vasoconstriction relative to the vasoactivity of ergovaline.
Although it is outside the scope of the data presented in this study, it is tempting to speculate on the interaction of ergot alkaloid consumption and the incidence of laminitis. One hypothesis for pasture-associated laminitis suggests that vasoactive amines derived from the fermentation of fructans in the equine large intestine are initiating factors (Crawford et al., 2007). Many of the vascular receptors for these amines such as serotonin (Katz and Bailey, 2012) have also been demonstrated to interact with ergot alkaloids in other species (Schöning et al., 2001; Klotz et al., 2013). Little work has been done looking at the involvement of ergot alkaloid–induced peripheral vasoconstriction contributing to a reduced blood flow to the extremities and increasing the susceptibility to chronic foot and leg disorders such as laminitis. A study was conducted to evaluate the risk of horses consuming endophyte-infected tall fescue and developing laminitis, and there was an increased risk of horses developing laminitis when exposed to ergot alkaloids in the diet (Rohrbach et al., 1995). Other work has looked at the vasoactivity of various vasoconstrictors in digital arteries and veins collected from healthy horses and those with early laminitis (Baxter et al., 1989). Baxter et al. (1989) reported that the digital blood vessels collected from horses with early laminitis were less responsive to substances such as serotonin that induce vasoconstriction compared with control horses. Interestingly, blood vessels collected from cattle exposed to ergot alkaloids exhibit this same phenomenon of decreased responsiveness or sensitivity to agonists (Klotz et al., 2012, 2013, 2016; Egert et al., 2014). Perhaps this similarity of suppressed vasoactivity in blood vessels collected from horses with early laminitis and those exposed to ergot alkaloids is coincidental. However, given the vasoactivity of ergovaline in the current study combined with the observed in vivo vasoconstriction in horses fed endophyte-infected tall fescue seed of McDowell et al. (2013), the area where ergot alkaloid exposure and incidences of laminitis occur requires further evaluation.
Uterine Artery
Reproductive dysfunction has been the primary disorder associated with equine exposure to ergot alkaloids (Evans, 2002; Cross, 2009); therefore, vasoactivity of ergot alkaloids in reproductive blood vessels was also of interest in the current study. Other than an extensive review of the effects ergot alkaloids can have on the uterus (Saameli, 1978), there is a paucity of data addressing the activity of ergot alkaloids in uterine vasculature. Because only a subset of the horses available for blood vessel collection in the current study was female, the number of compounds that could be evaluated with uterine artery was limited to 4. The biogenic amines norepinephrine and serotonin were selected to assess receptors classes that reside in this vasculature. Ergonovine and ergotamine were selected as representative alkaloids from the ergoline and ergopeptine structural classes, respectively.
Although direct comparisons cannot be made with the palmar artery due to differences in the bioassays used, the uterine artery was very different in both the appearance and the manner of contractile response in comparison with the palmar artery. Relative to the palmar artery, the lumen or the i.d. of the uterine artery was much smaller, as were the contractile responses to the biogenic amines norepinephrine and serotonin. Ergot alkaloids have been shown to have broad binding affinities with adrenergic, serotonergic, and dopaminergic receptors (Pertz and Eich, 1999). The small amount of vasoactivity produced by the highest concentrations of norepinephrine and serotonin suggests a minimal presence of associated receptors. Correspondingly, the absence of ergotamine- or ergonovine-induced vasoactivity, although unexpected, made sense when considering the aforementioned biogenic amine data.
In conclusion, all ergot alkaloids except for lysergic acid were vasoactive in palmar artery and vein preparations. Ergovaline was more vasoactive than any of the other ergot alkaloids evaluated. These data demonstrate that vasoconstriction can occur in equine peripheral vasculature, but horses may exhibit signs of exposure differently than cattle. Conversely, ergotamine and ergonovine were minimally vasoactive in uterine artery preparations, suggesting that effects of ergot alkaloids on fetal development may not be a consequence of restricted blood flow. Future work is needed to delineate specific receptors that are involved in the ergot alkaloid–induced vasoconstriction and the duration of ergot alkaloid effects at these receptors in equine vasculature. Furthermore, information in defining the mechanism of action in equine vascular models would assist in determining if vasoconstrictive effects occur outside of the reproductive maladies associated with fescue toxicosis, and may be currently receiving other diagnoses such as laminitis.
Footnotes
Mention of trade name, proprietary product of specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be available. The USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Abney L. K., Oliver J. W., Reinemeyer C. R. 1993. Vasoconstrictive effects of tall fescue alkaloids on equine vasculature. J. Equine Vet. Sci. 13:334–340. doi: 10.1016/S0737-0806(06)81119-6 [DOI] [Google Scholar]
- Ayers A. W., Hill N. S., Rottinghaus G. E., Stuedemann J. A., Thompson F. N., Purinton P. T., Seman D. H., Dawe D. L., Parks A. H., Ensley D. 2009. Ruminal metabolism and transport of tall fescue ergot alkaloids. Crop Sci. 49:2309–2316. doi: 10.2135/cropsci2009.01.0018 [DOI] [Google Scholar]
- Bailey S. R., Elliott J. 1998. Evidence for different 5-ht1b/1d receptors mediating vasoconstriction of equine digital arteries and veins. Eur. J. Pharmacol. 355:175–187. doi: 10.1016/S0014-2999(98)00520-2 [DOI] [PubMed] [Google Scholar]
- Baxter G. M., Laskey R. E., Tackett R. L., Moore J. N., Allen D. 1989. In vitro reactivity of digital arteries and veins to vasoconstrictive mediators in healthy horses and in horses with early laminitis. Am. J. Vet. Res. 50:508–517. [PubMed] [Google Scholar]
- Blodgett D. J. 2001. Fescue toxicosis. Vet. Clin. North Am. Equine Pract. 17:567–577. doi: 10.1016/S0749-0739(17)30052-4 [DOI] [PubMed] [Google Scholar]
- Bony S., Durix A., Leblond A., Jaussaud P. 2001. Toxicokinetics of ergovaline in the horse after an intravenous administration. Vet. Res. 32:509–513. doi: 10.1051/vetres:2001142 [DOI] [PubMed] [Google Scholar]
- Crawford C., Sepulveda M. F., Elliott J., Harris P. A., Bailey S. R. 2007. Dietary fructan carbohydrate increases amine production in the equine large intestine: Implications for pasture-associated laminitis. J. Anim. Sci. 85:2949–2958. doi: 10.2527/jas.2006-600 [DOI] [PubMed] [Google Scholar]
- Cross D. L. 2009. Toxic effects of the endophyte in horses. In: Fribourg H. A., Hannaway D. B., West C. P. editors, Tall fescue for the twenty-first century. Agron. Monogr. 53. ASA, CSSA, SSSA, Madison, WI: p. 311–325. doi: 10.2134/agronmonogr53.c17 [DOI] [Google Scholar]
- Cross D. L., Redmond L. M., Strickland J. R. 1995. Equine fescue toxicosis: Signs and solutions. J. Anim. Sci. 73:899–908. doi: 10.2527/1995.733899x [DOI] [PubMed] [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–PL228. doi: 10.1016/0024-3205(93)90555-H [DOI] [PubMed] [Google Scholar]
- Egert A. M., Kim D. H., Schrick F. N., Harmon D. L., Klotz J. L. 2014. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 92:1768–1779. doi: 10.2527/jas.2013-7141 [DOI] [PubMed] [Google Scholar]
- Evans T. J. 2002. Endocrine alterations associated with ergopeptine alkaloid exposure during equine pregnancy. Vet. Clin. North Am. Equine Pract. 18:371–378viii. doi: 10.1016/S0749-0739(02)00019-6 [DOI] [PubMed] [Google Scholar]
- Hannaway D. B., Daly C., Halbleib M. D., James D., West C. P., Volenec J. J., Chapman D., Li X., Cao W., Shen J., Shi X., Johnson S. 2009. Development of suitability maps with examples for the United States and China. In: Fribourg H. A., Hannaway D. B., West C. P. editors, Tall fescue for the twenty-first century, Agron. Monogr. 53. ASA, CSSA, SSSA, Madison, WI: p. 33–47. doi: 10.2134/agronmonogr53.c3 [DOI] [Google Scholar]
- Hill N. S., Thompson F. N., Stuedemann J. A., Rottinghaus G. W., Ju H. J., Dawe D. L., Hiatt E. E., 3rd 2001. Ergot alkaloid transport across ruminant gastric tissues. J. Anim. Sci. 79:542–549. doi: 10.2527/2001.792542x [DOI] [PubMed] [Google Scholar]
- Katz L. M., Bailey S. R. 2012. A review of recent advances and current hypotheses on the pathogenesis of acute laminitis. Equine Vet. J.44:752–761. [DOI] [PubMed] [Google Scholar]
- Klotz J. L. 2015. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins (Basel) 7:2801–2821. doi: 10.3390/toxins7082801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., Strickland J. R. 2016. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infected tall fescue. Toxins (Basel) 8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Johnson J. M., Brown K. R., Bush L. P., Strickland J. R. 2013. Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue. J. Anim. Sci. 91:4492–4500. doi: 10.2527/jas.2012-5896 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Barnes A. J. 2014. Isolating and using sections of bovine mesenteric artery and vein as a bioassay to test for vasoactivity in the small intestine. J. Vis. Exp. 92:52020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Brown K. R., Xue Y., Matthews J. C., Boling J. A., Burris W. R., Bush L. P., Strickland J. R. 2012. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 90:682–693. doi: 10.2527/jas.2011-4323 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush L. P., Smith D. L., Shafer W. D., Smith L. L., Arrington B. C., Strickland J. R. 2007. Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 85:2330–2336. doi: 10.2527/jas.2006-803 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush L. P., Smith D. L., Shafer W. D., Smith L. L., Vevoda A. C., Craig A. M., Arrington B. C., Strickland J. R. 2006. Assessment of vasoconstrictive potential of d-lysergic acid using an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 84:3167–3175. doi: 10.2527/jas.2006-038 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., Strickland J. R. 2010. Contractile response of fescue-naive bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids. J. Anim. Sci. 88:408–415. doi: 10.2527/jas.2009-2243 [DOI] [PubMed] [Google Scholar]
- Lyons P. C., Plattner R. D., Bacon C. W. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232:487–489. doi: 10.1126/science.3008328 [DOI] [PubMed] [Google Scholar]
- McDowell K. J., Moore E. S., Parks A. G., Bush L. P., Horohov D. W., Lawrence L. M. 2013. Vasoconstriction in horses caused by endophyte-infected tall fescue seed is detected with Doppler ultrasonography. J. Anim. Sci. 91:1677–1684. doi: 10.2527/jas.2012-5852 [DOI] [PubMed] [Google Scholar]
- Pertz H. H., Eich E. 1999. Ergot alkaloids and their derivatives as ligands for serotoninergic, dopaminergic, and adrenergic receptors. In: Kren V., Cvak L. editors, Ergot: The genus Claviceps. Harwood Academic Publishers, Amsterdam, The Netherlands: p. 411–440. [Google Scholar]
- Pesqueira A., Harmon D. L., Branco A. F., Klotz J. L. 2014. Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. J. Anim. Sci. 92:1213–1218. doi: 10.2527/jas.2013-7142 [DOI] [PubMed] [Google Scholar]
- Rohrbach B. W., Green E. M., Oliver J. W., Schneider J. F. 1995. Aggregate risk study of exposure to endophyte-infected (Acremonium coenophialum) tall fescue as a risk factor for laminitis in horses. Am. J. Vet. Res. 56:22–26. [PubMed] [Google Scholar]
- Saameli K. 1978. Effects on the uterus. In: Berde B., Schild H. O. editors, Ergot alkaloids and related compounds. Springer Berlin Heidelberg, Berlin, Heidelberg: p. 233–319. doi: 10.1007/978-3-642-66775-6_4 [DOI] [Google Scholar]
- Schöning C., Flieger M., Pertz H. H. 2001. Complex interaction of ergovaline with 5-ht2a, 5-ht1b/1d, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 79:2202–2209. doi: 10.2527/2001.7982202x [DOI] [PubMed] [Google Scholar]
- Schultz C. L., Lodge-Ivey S. L., Bush L. P., Craig A. M., Strickland J. R. 2006. Effects of initial and extended exposure to an endophyte-infected tall fescue seed diet on faecal and urinary excretion of ergovaline and lysergic acid in mature geldings. N. Z. Vet. J. 54:178–184. doi: 10.1080/00480169.2006.36692 [DOI] [PubMed] [Google Scholar]
- Stoll A., Hoffmann A. 1970. The chemistry of the ergot alkaloids. Von Nostrand, Reinhold and Company, New York. [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F., Jr, Flythe M. D., Brown K. R. 2011. Board-invited review: St. Anthony's fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi: 10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Vivrette S., Stebbins M. E., Martin O., Dooley K., Cross D. L. 2001. Cardiorespiratory and thermoregulatory effects of endophyte-infected fescue in exercising horses. J. Equine Vet. Sci. 21:65–67. doi: 10.1016/S0737-0806(01)70094-9 [DOI] [Google Scholar]
- Webb G. W., Demster S., Minton K., Webb S. P., Walker E. L., Onyango B. 2012. Effect of ergopeptines associated with tall fescue ingestion on recovery of horses subjected to standardized exercise tests. J. Equine Vet. Sci. 32:788–794. doi: 10.1016/j.jevs.2012.03.015 [DOI] [Google Scholar]
- Yates S. G., Powell R. G. 1988. Analysis of ergopeptine alkaloids in endophyte-infected tall fescue. J. Agric. Food Chem. 36:337–340. doi: 10.1021/jf00080a023 [DOI] [Google Scholar]
- Zerpa H., Berhane Y., Elliott J., Bailey S. R. 2010. Functional role of alpha2-adrenoceptor subtypes in the cooling-enhanced vasoconstriction of isolated cutaneous digital veins of the horse. Eur. J. Pharmacol. 627:194–202. doi: 10.1016/j.ejphar.2009.10.046 [DOI] [PubMed] [Google Scholar]


