ABSTRACT
Although peripubertal mammary development represents only a small fraction of the total mass of mammary parenchyma present in the udder at the end of gestation and into lactation, there is increasing evidence that the tissue foundations created in early life can affect future mammary development and function. Studies on expression of estrogen and progesterone receptors seem to confirm the relevance of these steroids in prepubertal mammary development, but connections with other growth factors, hormones, and local tissue factors remain elusive. Enhanced preweaning feeding in the bovine appears to enhance the capacity of mammary tissue to response to mammogenic stimulation. This suggests the possibility that improved early nutrition might allow for creation of stem or progenitor cell populations to better support the massive ductal growth and lobulo-alveolar development during gestation. Increasing evidence that immune cells are involved in mammary development suggests there are unexpected and poorly understood connections between the immune system and mammary development. This is nearly unexplored in ruminants. Development of new tools to identify, isolate, and characterize cell populations within the developing bovine mammary gland offer the possibility of identifying and perhaps altering populations of mammary stem cells or selected progenitor cells to modulate mammary development and, possibly, mammary function.
Keywords: ESR1, mammary stem cells, prepubertal, progesterone
INTRODUCTION
Dairy physiologists have long questioned the relevance of neonatal and peripubertal mammary growth in calves and heifers for future development and milk production. Several very early studies (Swett, 1927; Swett and Matthews, 1934) sought to define relationships between mammary scores (palpation of parenchymal tissue) or mammary anatomy and future milk production. Since this time, studies generally concentrated on possible effects of altered mammary development through changes in feeding, diet formulation, or endocrine manipulation during preweaning, weaning to puberty, or puberty to gestation on subsequent development or lactation. At a minimum, it is evident that the mammary tissue foundation produced in the neonate and young calf provides the underpinning for subsequent mammary growth and, ultimately, lactation.
Analogous to findings demonstrating the effects of fetal and neonatal development on future physiology, metabolism, and health (Plagemann et al., 2012; Roberts et al., 2016), it is logical to suggest that neonatal mammary development likely acts to modify future growth and function of the mammary gland. Studies over the past 2 decades have assessed management of replacement heifers and how these feeding, housing, and care decisions influence the quality of herd replacements. For some time, a major goal was to shorten the time needed to transition replacement heifers into the lactating herd. Because puberty is highly correlated with BW (Sejrsen, 1994), changes in feeding schemes to increase BW gain can produce heifers that reach puberty at earlier ages. However, as previously reviewed (Sejrsen and Purup, 1997; Zanton and Heinrichs, 2005), greater prepubertal gains can also decrease first lactation performance. Reductions in development of mammary parenchyma and corresponding increased mass of the mammary fat pad occur with excessive prepubertal BW gain. Suggested mechanisms for reduced performance include failed cell proliferation, failed cellular differentiation, alterations in mammary stem cells, and premature puberty so that there is a reduction in the length of the peripubertal allometric growth phase and, likely, fewer estrus cycles prior to conception. The comprehensive studies by Meyer et al. (2006a,b) provide compelling evidence that the reduction in the usual length of the allometric growth period prior to onset of puberty, in excessively fed heifers, limits udder development.
To add further complexity, as summarized by Khan et al. (2011), enhanced feeding of calves prior to weaning correlates with increased future milk production (Soberon et al., 2012). We (Geiger et al., 2016a,b) showed that enhanced feeding increased mammary development in calves compared with restricted-fed controls (Fig. 1). Demonstration of divergent effects of feeding rate before weaning vs. after weaning illustrates the malleable nature of mammary development as well as how little we truly understand about links between early management of calves and heifers and future performance (Capuco and Akers, 2010).
Figure 1.
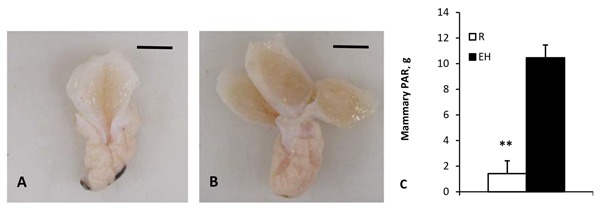
Parenchymal from heifer calves. Panel A shows trimmed dissected mammary parenchyma from a heifer calf on restricted diet at time of weaning. Note reduced parenchymal tissue compared with trimmed dissected mammary parenchyma from an enhanced-fed heifer calf at time of weaning (Panel B). Reference bars indicated 1 cm. Panel C provides quantitative comparison of parenchymal tissue in restricted- vs. enhanced-fed calves (n = 8 per treatment). Data adapted from Geiger et al. (2016b; with permission). ** P ≤ 0.01. PAR = parenchymal; R = restricted fed; EH = enhanced fed.
Work primarily with nursing piglets has demonstrated that early colostrum feeding has dramatic impacts on the subsequent development of the reproductive tract and ultimately the reproductive success of the gilts. As reviewed by Bartol et al. (2017), these studies led to development of the “lactocrine” hypothesis, which is the concept that biologically active agents (growth factors, hormones, bioactive peptides, etc.) in mammary secretions program postnatal uterine development (Bartol et al., 2008). Taking into account that mammary development, like the reproductive tract development, also occurs primarily postnatally, it should not be unexpected that early colostrum and milk feeding influence future mammary development. These concepts have been easier to explore in litter-bearing species (because of reduced costs, similarity of littermates, etc.). Regardless, such studies underscore the seemingly forgotten idea that mammary secretions evolved not just to provide nutrition to the suckling young but also to provide protection and, likely, signaling molecules to promote growth and development (Capuco and Akers, 2010). In addition, increased appreciation of the milk microbiome in establishment of the gut microbiome and modulation of immune responses (Rautava, 2016) further emphasizes the relevance of mammary secretions to neonatal development. A recent report (Wilson et al., 2017) showed that preweaned restricted-fed calves have impaired endometrial gland development and alterations in growth factor–related signaling molecules. This suggests that the level of nutrition or components in milk replacer can affect reproductive tract development in calves.
The reasonable conclusion from published literature is that both preweaning and postweaning nutrition and management can influence mammary development and health, immune competency, physiology, or gene activity to modify future productivity (Khan et al., 2011). The goal of this review is to describe some of the developmental changes in the peripubertal bovine mammary gland induced by endocrine and nutritional manipulation during the peripubertal period and to provide some discussion of possible hypotheses to explain impacts on future performance.
HORMONAL CONTROL OF PREPUBERTAL MAMMARY DEVELOPMENT
It is not unexpected that many of the impacts of nutrition on peripubertal mammary development involve changes in concentrations of hormones and growth factors and their receptors. As reviewed (Sejrsen and Purup, 1997; Purup et al., 2000; Vestergaard et al., 2003), changes in feeding rate or diet produces changes in GH, IGF-I, IGF-I binding proteins, etc., that can influence mammary cell proliferation and development both systemically and locally (Akers et al., 2000). In several studies, we evaluated the effects of pubertal ovariectomy on mammary development. On the surface, it is intuitive that the ovary would be important for mammary development, but it is important to appreciate that in these studies, the ovariectomy occurred well before puberty. Several points seem clear. The earlier the ovariectomy and the longer the interval between ovariectomy and tissue collection, the greater the negative effects on overall mammary growth (Berry et al., 2003; Velayudhan et al., 2012). Likewise, as noted with negative impacts of prepubertal overfeeding, local mammary tissue IGF-I is reduced and secretion of some inhibitory IGF-I binding proteins is increased in response to prepubertal ovariectomy (Berry et al., 2003). As demonstrated in somewhat older prepubertal heifers, ovariectomy reduced mammary development and acute mammary cell proliferation (Purup et al., 1993b, 1995). Furthermore, exogenous GH was not able to stimulate mammary growth in ovariectomized heifers. In addition, mammary explants from ovariectomized heifers were less sensitive to IGF-I as measured by direct receptor binding (Purup et al., 1995), but explants from both intact and ovariectomized heifers showed increased proliferative responses to graded concentrations of added IGF-I (Purup et al., 1993a). Although Purup et al. (1993b) noted small but significant differences in circulating concentrations of estradiol in intact vs. ovariectomized heifers, in subsequent experiments using younger animals, we showed no difference in concentrations of estradiol despite decreased mammary development after ovariectomy (Velayudhan et al., 2015).
In addition to the IGF-I axis, it can be suggested that ovarian steroids mediate prepubertal mammary development. For example, exogenous estradiol stimulates mammary growth in prepubertal ruminants, regardless of ovarian status (Woodward et al., 1993; Ellis et al., 1998), and there is a dose-related response when estradiol is added to heifer mammary explants (Purup et al., 1993b). However, Woodward et al. (1994) reported that MAC-T cells, an immortalized bovine mammary cell line (Huynh et al., 1991), proliferated in a dose-responsive manner to added fetal bovine serum, tissue extracts, insulin, or IGF-I, but the cells were unresponsive to epidermal growth factor (EGF), bovine growth hormone (bGH), prolactin, estradiol, or progesterone (P4). Specific to the estrogen response, this suggests that MAC-T cells are either estrogen receptor negative or that such monocultures lack a cohort of other cells required for a local proliferative response to estrogen. For example, Capuco et al. (2002) showed that actively proliferating bovine mammary epithelial cells do not express estrogen receptor α (ESR1; i.e., 5-bromo-2-deoxyuridine [BrdU]–positive cells were ESR1 negative). This suggests that ESR1 signaling in mammary tissue induces local tissue mediators that stimulate proliferation in neighboring cells rather than directly stimulating cell division in receptor-positive cells. Perhaps ESR1 stimulation alters local production of IGF-I axis molecules that in turn promote an increase in mammary development. However, this is almost certainly a simplistic and naïve view of effects of estrogen on the developing mammary gland. Li and Capuco (2008) analyzed transcript profiles from prepubertal heifers either ovariectomized or intact with or without short-term treatment (54 h) with exogenous estrogen. They noted that the expression of 2,344 genes was altered by estrogen, with 1,016 genes changed by estrogen regardless of tissue (mammary fat pad vs. parenchyma) or ovarian status. Functional classes of genes impacted included those associated with cell-to-cell signaling, cell growth and proliferation, cell movement, and cell morphology. In parenchymal tissue, estrogen impacts phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and G protein receptor signaling pathways and, in the mammary fat pad, estrogen altered cyclic adenosine monophosphate–mediated and IGF-I signaling pathways. This suggests mammary tissue responses to estrogen are complex.
A combination of GH and estrogen are known to be key mammogenic hormones driving peripubertal mammary duct development (Tucker, 2000). However, it is also clear that direct effects of both GH and estrogen on mammary epithelial cell proliferation were difficult to demonstrate. Estrogen induces production of multiple growth factors in a variety of normal and malignant tissues (Sirbasku, 1978). Moreover, a plethora of growth factors (e.g., IGF-I, fibroblast growth factors, EGF, and TGF-β) can stimulate proliferation of mammary cells. A relationship between estrogen stimulation and the IGF-I axis is probably most well developed in rodent models (Kleinberg and Ruan, 2008), but evidence in ruminants also supports this concept (see above). Berryhill et al. (2016) discuss many of the nuances associated with estrogen-dependent and -independent stimulation of mammary cell proliferation in their recent review.
At least in ruminants, the conventional thought has been that P4 is primarily important in lobulo-alveolar formation during gestation. Progesterone receptors are present in bovine mammary cells of prepubertal heifers but are lost following ovariectomy (Velayudhan et al., 2015). It is difficult to envision that expression of the receptors would not be physiologically relevant; however, treatment of heifers with exogenous P4 had no significant effect on mammary cell proliferation (Woodward et al., 1993). Although results are mixed (see Berryhill et al., 2016, for review), exogenous P4 was shown to stimulate ductal growth in male and female C3H mice (Skarda et al., 1989), stimulate appearance of terminal endbuds in BALB/c mice (Atwood et al., 2000), and increase cell proliferation in ovariectomized mice (Aupperlee et al., 2013). Progesterone can also act in concert with IGF-I to increase ductal development (Ruan et al., 2005). As Berryhill et al. (2016) describe for rodents, it is likely that in addition to estrogen, P4 and prolactin are involved in pubertal mammary ductal development, but how this translates to farm animals is largely unexplored. For example, Horigan et al. (2009) evaluated the effects of combinations of prolactin and ovarian steroids on mammary growth in ovariectomized gilts. They reported positive proliferative responses to estrogen, estrogen + P4, and estrogen + prolactin but not P4 + prolactin. Both ESR1 and PG receptors are expressed in mammary epithelial cells at multiple stages of development (i.e., calves, nonpregnant and pregnant heifers, and the lactation and dry period); however, mRNA expression and immunochemical localization for both receptors is greatest for calves (Connor et al., 2005).
We have recently studied the effects of prepubertal ovariectomy and treatment with the antiestrogen tamoxifen on mammary development, cell proliferation, and expression of ESR1 and progesterone receptor (PGR) in prepubertal heifers (Tucker et al., 2016). Briefly, calves were given tamoxifen or a placebo from 28 d of age until slaughter at 120 d of age. As with ovariectomy, overall mammary parenchymal growth measured as either dissected mass or DNA was approximately 50% lower in tamoxifen-treated calves. Interestingly, at slaughter, the number of Ki67-positive epithelial cells was greater in tamoxifen-treated calves, significantly so in the tissue region closest to the mammary fat pad. Treatment did not affect the location of ESR1- or PGR-positive cells within the epithelial layer. Overall, about 40% of cells were positive for ESR1, but the proportion did not differ by treatment. Most striking, using multispectral imaging, was the 6.2-fold lower expression of ESR1 among epithelial cells that were ESR1 positive. There was also reduced expression of ESR1 mRNA. In contrast to the response to ovariectomy (Velayudhan et al., 2015), the proportion of PGR-expressing cells was similar (37%) in tamoxifen- and placebo-treated heifers. Especially evident in placebo-treated heifers was that most ESR1-positive cells also expressed PGR (Fig. 2). However, the degree of PGR expression among positive cells was 42% greater in tamoxifen-treated heifers.
Figure 2.
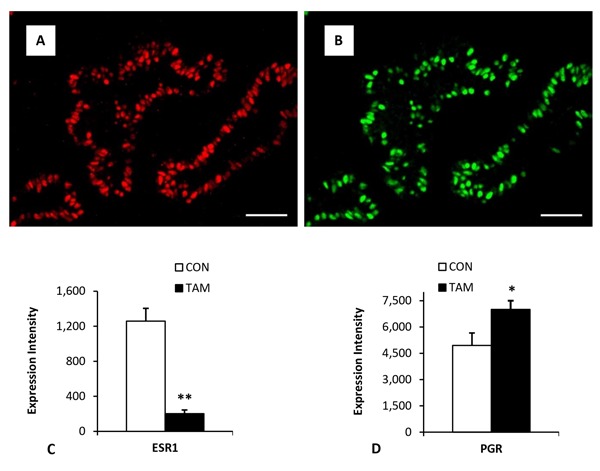
Mammary expression of steroid receptors. Panel A illustrates expression of estrogen receptor α (ESR1) and panel B illustrates expression of progesterone receptor (PGR) in the same mammary tissue section from a prepubertal heifer. Note that a majority of the cells that express ESR1 also express PGR. Panel C shows the dramatic decrease in the level of expression (per positive cell) of ESR1 in prepubertal heifers treated with tamoxifen. Panel D illustrates a modest but significant increase in the intensity of PRG expression in tamoxifen-treated heifers. Data adapted from Tucker et al. (2016; with permission). *P ≤ 0.05; **P ≤ 0.01. CON = placebo treated; TAM = tamoxifen treated.
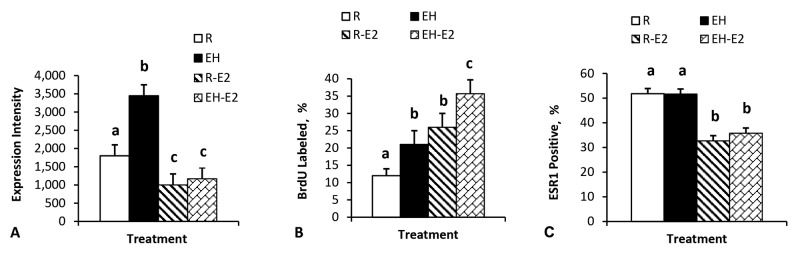
We recently reported (Geiger et al., 2017) the impact of preweaning diet on the expression of ESR1 and PGR in mammary tissue of heifer calves and corresponding rates of cell proliferation. Our hypothesis was that mammary tissue from enhanced-fed calves would be more responsive to mammogenic hormones. Essentially, improved nutrition would better prepare the mammary gland to respond to signals that induce peripubertal mammary development. Our earlier reports (Geiger et al., 2016a,b) showed that enhanced preweaning feeding increased mammary growth and the response to exogenous estradiol.
Figure 3 illustrates differences in ESR1 expression intensity among epithelial cells positive for ESR1, the proportion of ESR1-positive cells, and corresponding rates of cell proliferation of epithelial cells within terminal ductal structures. Capuco et al. (2002) showed that epithelial cells within the distal regions of the terminal ductal units were very highly proliferative and that nearly all actively proliferating cells (BrdU positive) were ESR1 negative. Our data showed increased intensity of ESR1 expression in ESR1-positive cells in enhanced-fed calves compared with restricted-fed calves. Administration of estradiol decreased the intensity of ESR1 expression as well as the proportion of epithelial cells that express ESR1. Cell proliferation increased in enhanced-fed calves compared with restricted-fed calves, and administration of estradiol increased cell proliferation in both dietary groups. Given that ESR1 cells are nonproliferating, the greater rate of BrdU incorporation in estradiol-treated calves may reflect, in part, the greater availability of proliferation-competent epithelial cells in estradiol-treated calves compared with placebo-treated calves. Regardless, these data suggest that changes in expression of ESR1 and/or PGR are likely important in regulation of prepubertal mammary development related to endocrine or nutritional manipulation in prepubertal calves.
Figure 3.
Mammary cell expression of ESR1 in restricted-fed calves vs. enhanced-fed calves at the time of weaning (8 wk) and following 2 wk of treatment with estradiol after weaning. Panel A shows increased expression of ESR1 in cells positive for ESR1 in enhanced-fed calves and reduced expression in both dietary groups following treatment with estradiol. Panel B shows the proportion of 5-bromo-2-deoxyuridine (BrdU)–labeled cells within terminal ductal structures. Proliferation is greater in enhanced-fed calves and further increased proliferation after estradiol in both dietary treatments. Panel C shows the percentage of epithelial cells expressing ESR1. Dietary treatment did not change the proportion of ESR1-positive cells but administration of estradiol reduced the percentage of ESR1-positive cells. In all graphs, bars with different superscripts are significantly different (P ≤ 0.05). Data adapted from Geiger et al. (2017; with permission). R = restricted fed; EH = enhanced fed; R-E2 = restricted fed + estradiol; EH-E2 = enhanced fed + estradiol. a–cMeans with different superscripts differ (P < 0.05).
OTHER FACTORS AFFECTING PERIPUBERTAL MAMMARY DEVELOPMENT
A number of recent rodent studies (Coussens and Pollard, 2011; Need et al., 2014; Brady et al., 2016) provide compelling evidence that a number of immune cells, particularly macrophages, eosinophils, and mast cells, are involved in the regulation of ductal elongation and development. These studies suggest these populations of immune cells act to modify the stromal environment (Unsworth et al., 2014) by localizing along the elongating endbuds and ductal structures. For example, macrophages appear to align in association with collagen fibrils along the neck of the endbuds (Ingman et al., 2006), mast cells align near the bulbous endbud structure (Lilla and Werb, 2010), and eosinophils align near ductal branch points (Reed and Schwertfeger, 2010). Evidence that is more direct comes from experiments where local immune cells were eliminated, leading to markedly impaired mammary development and then recovery after a bone marrow transplant (Gouon-Evans et al., 2000).
Information specific to immune cells and early bovine mammary development is minimal. However, we (Beaudry et al., 2016) have evaluated differences in the distribution of macrophages, mast cells, and eosinophils in bovine mammary tissue associated with age, ovariectomy, and estrogen treatment. Immune cells, in general, were not randomly distributed in the mammary stroma but were most often closely adjacent to epithelial structures. Many of these immune cells can produce cytokines and growth factors capable of stimulating mammary epithelial cells. Immune cells can also respond to many mammary active agents. Figure 4 illustrates a cluster of immune cells in the stroma surrounding developing mammary ducts from a prepubertal calf. In this example, cells are expressing the IGF-I receptor. Expectedly, there is abundant receptor expression within the mammary epithelial cells, but especially striking is the very pronounced expression by clusters of immune cells within the stromal tissue. Such findings suggest there are multiple interactions among populations of immune cells in the mammary tissue and the control of mammary development and function. A recent report (Bruno et al., 2017) clearly demonstrates the ability of the mammary extracellular matrix to redirect the differentiation of testicular and embryonic stem cells to create functional mammary glands. This result reinforces the significance of the local tissue environment in the control of mammary development and function irrespective of hormones and growth factors.
Figure 4.
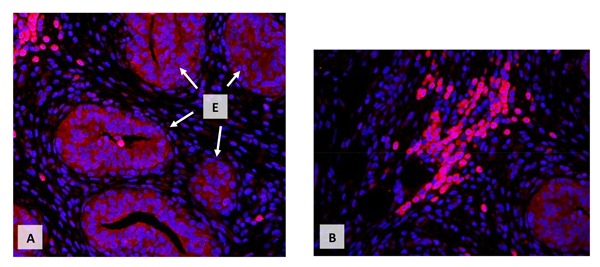
Immunocytochemical localization of IGF-1 receptor in mammary tissue of a prepubertal heifer. In the upper panel, E and arrows indicate consistent expression of IGF-I receptor (red) in the cytoplasm (cell membrane) in clusters of ductal structures. The upper left shows intense receptor expression in a group of immune cells. Panel B shows another grouping of intensely stained immune cells as well as a less intensely stained ductal structure (lower right). In both panels, red indicates IGF-I receptor expression and blue 4',6-diamidino-2-phenylindole staining of cell nuclei (R.M. Akers, unpublished data).
Lastly, a discussion of mammary development would not be comprehensive without some consideration of mammary stem cells (MaSC). Certainly, there has been greater progress in identification of MaSC in rodents than in farm animals. Indeed, the “gold standard” for identification of MaSC has been the capacity of even a single isolated epithelial cell (Kordon and Smith, 1998; Shackleton et al., 2006) to regenerate the mammary gland when transplanted into cleared (native epithelium removed) mammary fat pad in the murine mammary gland. This and related murine studies demonstrated that MaSC are not limited to rudimentary mammary structures but are positioned throughout the developing mammary ductal system and within alveolar structures. True MaSC undergo 2 types of cell division, asymmetric and symmetric. With symmetric division, the result is the creation of 2 daughter stem cells and the expansion of the stem cell population. With asymmetric division, there is self-renewal of the stem cells and production of progenitor cells. These “common” progenitor cells give rise to daughter cells that are the progenitors for the ductal, luminal, and myoepithelial cells. A key feature is that the farther removed from the MaSC the new cell is, the more committed and functionally differentiated it becomes.
Regardless of the hurdles (as reviewed by Capuco and Ellis [2005] and Capuco et al. [2012]), there have been a number of studies seeking to identify stem and progenitor cells in the bovine mammary gland. For example, Ellis and Capuco (2002) quantified proportions of lightly stained, intermediate, and darkly stained epithelial cells in growing bovine mammary glands. The population (approximately 10% of the total) of lightly stained epithelial cells in tissue sections accounted for about 50% of the proliferating cells. They concluded that this population was likely a mixture of stem cells and progenitor cells. Capuco (2007) subsequently described the identification and quantitation of putative bovine MaSC based on long-term labeling of DNA with BrdU (i.e., label-retaining epithelial cells [LREC] that do not express ESR1). Estimates of the percentage of heavily labeled cells corresponded with expected regional differences in the developing udder. Values also corresponded with proportions of MaSC estimated in murine mammary glands. Choudhary et al. (2013) used laser capture microdissection and gene expression to evaluate the transcriptomes of basally located LREC compared with LREC embedded within the epithelium. They also did comparisons between LREC and control cells isolated from the ductal epithelium. They found 592 genes differentially expressed between basal LREC and basally located control cells as well as 110 genes differentially expressed between LREC embedded within the epithelium and other control epithelial cells also embedded within epithelium. Possible MaSC biomarkers included hepatocyte nuclear factor 4 α (HNF4α). Overall, results support the idea that basally located ESR1-negative LREC are likely true bovine MaSC whereas LREC positioned within the epithelial layer are possibly common progenitor cells (see Fig. 5). In related studies, these researchers (Capuco et al., 2009; Choudhary and Capuco, 2012) showed that intramammary infusions of xanthosine increased the population of MaSC/progenitor cells.
Figure 5.
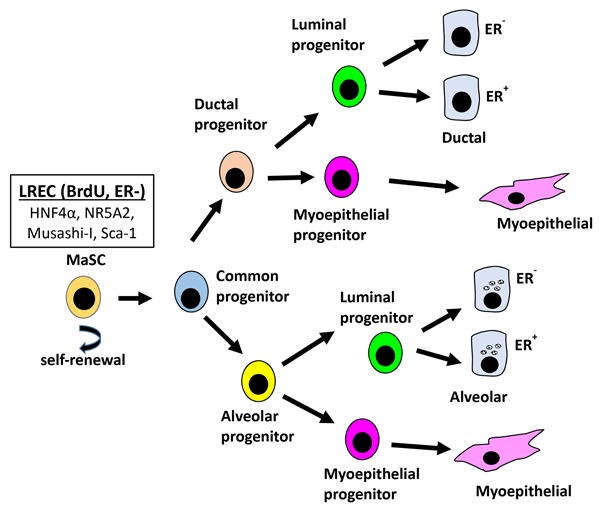
Proposed mammary cell hierarchy adapted from Capuco et al. (2012). LREC = label-retaining epithelial cells; BrdU = 5-bromo-2-deoxyuridine; ER− = ESR1 negative;ER+ = ESR1 positive; HNF4α = hepatocyte nuclear factor 4 α; NR5A2 = nuclear receptor subfamily 5, group A, member 2; Sca-1 = stem cell antigen-1; MaSC = mammary stem cells.
Recent studies have used enzymatic digestion of mammary tissue and cell sorting with panels of antibodies to separate populations of mammary epithelial cells in attempts to better define MaSC as well as various progenitor cells (Motyl et al., 2011; Rauner and Barash, 2012). Use of a myriad of cluster of differentiation (CD) proteins as markers has allowed for identification and segregation of multiple populations of epithelial cells believed to represent authentic MaSC and various progenitor cells. Subsequent approaches to characterize these cells have included tabulation of morphological responses (e.g., formation of colonies with duct-like or alveolar-like structures following transplantation into the cleared fat pads of immunocompromised nude mice). Appearance of specific phenotypes has allowed the putative identifications of ductal, alveolar, and myoepithelial progenitor cells and bovine MaSC (Rauner and Barash, 2016). There is quantitation of numbers of these cell classes over the lactation cycle (Perruchot et al., 2016). Others have estimated the effects of prepubertal nutrition (Daniels et al., 2009), ovariectomy (Ellis et al., 2012), or treatment with antiestrogens (Tucker et al., 2016) on populations of putative bovine MaSC through counting of LREC. Possible populations of bovine MaSC were also estimated by counting the number of cells expressing HNF4α in water buffalo (Choudhary et al., 2016). Similarly, Colitti and Farinacci (2009) estimated the number of MaSC in ovine mammary gland by counting the number of Musashi-I expressing cells across different stages of development. Specific to myoepithelial cells and their progenitors, Ellis and colleagues (Ballagh et al., 2008; Safayi et al., 2012; Tucker et al., 2016) showed that smooth muscle actin or common acute lymphoblastic leukemia antigen are good cytoplasmic markers and that transformation-related protein 63 is an excellent nuclear marker for these cells. Figure 5 provides a summary of a proposed hierarchy of mammary epithelial cell development based on the review from Capuco et al. (2012) and references cited above.
Table 1 provides a summary of cells involved in this hierarchy and various markers believed to be associated with some of these cells. It is likely that some of the cells may share markers. There is also inconsistency in the descriptive characterization of cell populations within the mammary gland (i.e., basal vs. luminal, luminal progenitors vs. lumino-ductal progenitors, etc.). Therefore, there are conflicting results because of the absence of a standard classification for the various cells. This means that absolute identification is likely to require detection of a “set” or a complex of markers to improve confidence. Lastly, it must be considered that responses of presumptive bovine MaSC or progenitors in culture or following transplantation into mice do not reproduce the bovine mammary gland and its function. The recent report by Bruno et al. (2017) demonstrating that the extracellular matrix isolated from the mammary gland can induce embryonic or testicular cells to acquire a mammary phenotype illustrates the significance of tissue environment in regulation of mammary morphogenesis and function.
Table 1.
Summary of possible markers for epithelial cell development in the bovine mammary gland
| Hierarchy member1 | Presumptive marker or markers2 | Reference |
|---|---|---|
| MaSC | LREC (BrdU and ER−), Musashi-1, Sca-1, HNF4α, NR5A2, Pale staining, and CD24med/CD49fpos | Capuco et al. (2012), Colitti and Farinacci (2009), Choudhary et al. (2013), Motyl et al. (2011), Perruchot et al. (2016), and Rauner and Barash (2012, 2016) |
| Common progenitor | CD24high/CD49fneg | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Alveolar progenitor | CD24high/CD49fneg and CD24+/CD49f+ | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Ductal progenitor | CD24neg/CD49fpos | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Luminal progenitor | CD24high/CD49fneg and CD24−/EpCAM+ | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Myoepithelial progenitor | CD24neg/CD49fpos, CD24+/CD10−, P63, P40, CALLA, CD10, and SMA | Capuco et al. (2012), Ellis et al. (2012, Perruchot et al. (2016), Rauner and Barash (2012, 2016), and Safayi et al. (2012) |
| Ductal epithelium | CD24neg/CD49fpos | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Alveolar epithelium | CD24med/CD49fneg | Perruchot et al. (2016) and Rauner and Barash (2012, 2016) |
| Myoepithelial cell | P63, CALLA, CD10, and SMA | Ellis et al. (2012) and Safayi et al. (2012) |
Adapted from Capuco et al. (2012). MaSC = mammary stem cells.
Definitions for markers: LREC = label-retaining epithelial cell (i.e., long-term retention of bromodeoxyuridine); BrdU = 5-bromo-2-deoxyuridine; ER− = ESR1 negative. Musashi-1 is a RNA-binding protein. Sca-1 = stem cell antigen-1, initially associated with hematopoietic cell lineages; HNF4α = hepatocyte nuclear factor 4 alpha (or NR2A1 [nuclear receptor subfamily 2, group A, member 1]), a transcription factor; NR5A2 = nuclear receptor subfamily 5, group A, member 2. Pale staining refers to mammary epithelial cells with minimal organelles and reduced general staining believed to include MaSC. CD = cluster of differentiation; these proteins are cell surface proteins (markers) most often associated with immune cells. Variants of these proteins (CD24, CD49f, etc.) and patterns of expression have been associated with presumptive MaSC and progenitor cells in the bovine. EpCAM = epithelial cell adhesion glycoprotein; P63 = transformation related protein P63; P40 = isoform of P63, δNp63; CD10 or CALLA = common acute lymphoblastic leukemia antigen, a presumptive marker for cytoplasm of myoepithelial cells and precursors; SMA = smooth muscle actin, a classic marker of smooth muscle and mature myoepithelial cells.
In summary, despite many years of study evaluating the effects of hormones and growth factors, diet and management, genetics, and other factors on regulation of peripubertal mammary development and associated expression of genes and proteins in ruminants, understanding remains incomplete. However, new and useful imaging tools (Ellis et al., 2012) and the capacity to identify and study distinct cell populations within the growing mammary gland continue to provide opportunities and unexpected approaches to decipher the keys that control mammary development and ultimately function.
FOOTNOTES
Based on a presentation at the Biology of Lactation in Farm Animals Symposium entitled “The biology of lactation – From genes to cells to milk” held at the 2017 ASAS–CSAS Annual Meeting, July 8, 2017, Baltimore, MD.
I acknowledge support from multiple grants with colleagues including National Resources Inventory USDA grants 2006-35206-15599 and 2009-35208-05778. More recently I am thankful for grant support from USDA – National Institute of Food and Agriculture – Agriculture and Food Research Initiative, 2016-67015-24575, Impact of Pre-Weaning Nutrition on Endocrine Induction of Mammary Development in Dairy Heifers awarded to R. M. Akers and 2016-67011-24703 (Predoctoral Fellowship to A. J. Geiger).
LITERATURE CITED
- Akers R. M., McFadden T. B., Purup S., Vestergaard M., Sejrsen K., Capuco A. V. 2000. Local IGF-I axis in peripubertal ruminant mammary development. J. Mammary Gland Biol. Neoplasia 5:43–51. doi: 10.1023/A:1009563115612 [DOI] [PubMed] [Google Scholar]
- Atwood C. S., Hovey R. C., Glover J. P., Chepko G., Ginsburg E., Robison W. G., Vonderhaar B. K. 2000. Progesterone induces side-branching of the ductal epithelium in the mammary glands of prepubertal mice. J. Endocrinol. 167:39–52. doi: 10.1677/joe.0.1670039 [DOI] [PubMed] [Google Scholar]
- Aupperlee M. D., Liepparndt J. R., Bennett J. M., Schwartz R. C., Haslam S. Z. 2013. Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res. 15:R44. doi: 10.1186/bcr3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartol F. F., Wiley A. A., Bagnell C. A. 2008. Epigenetic programing of the porcine endometrial function and the lactocrine hypothesis. Reprod. Domest. Anim. 43(Suppl. 2):273–279. doi: 10.1111/j.1439-0531.2008.01174.x [DOI] [PubMed] [Google Scholar]
- Bartol F. F., Wiley A. A., George A. F., Miller D. J., Bagnell C. A. 2017. Postnatal reproductive development and the lactocrine hypothesis. J. Anim. Sci. 95:2200–2210. doi: 10.2527/jas.2016.1144 [DOI] [PubMed] [Google Scholar]
- Ballagh K., Korn N., Riggs L., Pratt S. L., Dessuage F., Akers R. M., Ellis S. 2008. Hot topic: Prepubertal ovariectomy alters the development of myoepithelial cells in the bovine mammary gland. J. Dairy Sci. 91:2992–2995. doi: 10.3168/jds.2008-1191 [DOI] [PubMed] [Google Scholar]
- Beaudry K. L., Parsons C. L. M., Ellis S. E., Akers R. M. 2016. Localization and quantitation of macrophages, mast cells, and eosinophils in the developing bovine mammary gland. J. Dairy Sci. 99:796–804. doi: 10.3168/jds.2015-9972 [DOI] [PubMed] [Google Scholar]
- Berry S. D. K., Howard R. D., Jobst P. M., Jiang H., Akers R. M. 2003. Interactions between the ovary and the local IGF-I axis modulate mammary development in prepubertal heifers. J. Endod. 177:295–304. doi: 10.1677/joe.0.1770295 [DOI] [PubMed] [Google Scholar]
- Berryhill G. E., Trott J. F., Hovey R. C. 2016. Mammary gland development – It's not just about estrogen. J. Dairy Sci. 99:875–883. doi: 10.3168/jds.2015-10105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N. J., Chuntova P., Schwertfeger K. L. 2016. Macrophages: Regulators of the inflammatory microenvironment during mammary gland development and breast cancer. Mediators Inflammation 2016: 4549676. doi: 10.1155/2016/4549676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R. D., Fleming J. M., George A. L., Boulanger C. A., Schedin P., Smith G. H. 2017. Mammary extracellular matrix directs differentiation of testicular and embryonic stem cells to form functional mammary glands in vivo. Sci. Rep. 7:40196. doi: 10.1038/srep40196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuco A. V. 2007. Identification of putative bovine mammary epithelial stem cells by their retention of labeled DNA strands. Exp. Biol. Med. 232:1381–1390. doi: 10.3181/0703-RM-58 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Akers R. M. 2010. Management and environmental influences on mammary gland development and milk production. In: Greenwood P. L., Bell A. W., Vercoe P. E., Viljoen G. J. editors, Managing the prenatal environment to enhance livestock productivity. Springer Science+Business Media B. V., Dordrecht, the Netherlands: p. 259–292. [Google Scholar]
- Capuco A. V., Choudhary R. K., Daniels K. M., Li R. W., Evock-Clover C. M. 2012. Bovine mammary stem cells: Cell biology meets production agriculture. Animal 6:382–393. doi: 10.1017/S1751731111002369 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Ellis S. 2005. Bovine mammary progenitor cells: Current concepts and future directions. J. Mammary Gland Biol. Neoplasia 10:5–15. doi: 10.1007/s10911-005-2536-3 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Ellis S., Wood D. L., Akers R. M., Garrett W. 2002. Postnatal mammary ductal growth: Three-dimensional imaging of cell proliferation, effects of estrogen treatment, and expression of steroid receptors in prepubertal calves. Tissue Cell 34:143–154. doi: 10.1016/S0040-8166(02)00024-1 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Evock-Clover C. M., Minuti A., Wood D. L. 2009. In vivo expansion of the mammary stem/progenitor cell population by xanthosine infusion. Exp. Biol. Med. 234:475–482. doi: 10.3181/0811-RM-320 [DOI] [PubMed] [Google Scholar]
- Choudhary R. K., Capuco A. V. 2012. In vitro expansion of the mammary stem/progenitor cell population by xanthosine treatment. BMC Cell Biol. 13:14. doi: 10.1186/1471-2121-13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary R. K., Choudhary S., Kaur H., Pathak D. 2016. Expression of putative stem cell marker, hepatocyte nuclear factor 4 alpha, in mammary gland of water buffalo. Anim. Biotechnol. 27:182–189. doi: 10.1080/10495398.2016.1164179 [DOI] [PubMed] [Google Scholar]
- Choudhary R. K., Li R. W., Evock-Clover C. M., Capuco A. V. 2013. Comparison of the transcriptomes of long-term label retaining cells and control cells microdissected from mammary epithelium: An initial study to characterize potential stem/progenitor cells. Front. Oncol. 3:1–18. doi: 10.3389/fonc.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colitti M., Farinacci M. 2009. Expression of a putative stem cell marker, Musashi 1, in mammary glands of ewes. J. Mol. Histol. 40:139–149. doi: 10.1007/s10735-009-9224-3 [DOI] [PubMed] [Google Scholar]
- Connor E. E., Wood D. L., Sonstegard T. S., da Mota A. F., Bennett G. L., Williams J. L., Capuco A. V. 2005. Chromosomal mapping and quantitative analysis of estrogen-related receptor alpha-1, estrogen receptors alpha and beta and progesterone receptor in the bovine mammary gland. J. Endocrinol. 185:593–603. doi: 10.1677/joe.1.06139 [DOI] [PubMed] [Google Scholar]
- Coussens L. M., Pollard J. W. 2011. Leukocytes in mammary development and cancer. Cold Spring Harb. Perspect. Biol. 3:a003285. doi: 10.1101/cshperspect.a003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels K. M., Capuco A. V., McGilliard M. L., James R. E., Akers R. M. 2009. Effects of milk replacer formulation on measures of mammary growth and composition in Holstein heifers. J. Dairy Sci. 92:5937–5950. doi: 10.3168/jds.2008-1959 [DOI] [PubMed] [Google Scholar]
- Ellis S., Akers R. M., Capuco A. V. 2012. Triennial Lactation Symposium: Bovine mammary epithelial cell lineages and parenchymal development. J. Anim. Sci. 90:1666–1673. doi: 10.2527/jas.2011-4671 [DOI] [PubMed] [Google Scholar]
- Ellis S., Capuco A. V. 2002. Cell proliferation in bovine mammary epithelium: Identification of the primary proliferative cell population. Tissue Cell 34:155–163. doi: 10.1016/S0040-8166(02)00025-3 [DOI] [PubMed] [Google Scholar]
- Ellis S. E., McFadden T. B., Akers R. M. 1998. Prepubertal ovine mammary development is unaffected by ovariectomy. Domest. Anim. Endocrinol. 15:217–225. doi: 10.1016/S0739-7240(98)00009-5 [DOI] [PubMed] [Google Scholar]
- Geiger A. J., Parsons C. L. M., Akers R. M. 2016a. Feeding a higher plane of nutrition and providing exogenous estrogen increases mammary development in Holstein heifer calves. J. Dairy Sci. 99:7642–7653. doi: 10.3168/jds.2016-11283 [DOI] [PubMed] [Google Scholar]
- Geiger A. J., Parsons C. L. M., Akers R. M. 2017. Feeding an enhanced diet to Holstein heifers during the preweaning period alters steroid receptor expression and increases cellular proliferation. J. Dairy Sci. 100(10):8534–8543. doi: 10.3168/jds.2017-12791 [DOI] [PubMed] [Google Scholar]
- Geiger A. J., Parsons C. L. M., James R. E., Akers R. M. 2016b. Growth, intake, and health of Holstein heifer calves fed an enhanced preweaning diet with or without postweaning exogenous estrogen. J. Dairy Sci. 99:3995–4004. doi: 10.3168/jds.2015-10405 [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V., Rothenberg M. E., Pollard J. W. 2000. Post-natal mammary development requires macrophages and eosinophils. Development 127:2269–2282. [DOI] [PubMed] [Google Scholar]
- Horigan K. C., Trott J. F., Barndollar A. S., Scudder J. M., Blauwiekel R. M., Hovey R. C. 2009. Hormone interactions confer specific proliferative and histomorphogenic responses in the porcine mammary gland. Domest. Anim. Endocrinol. 37:124–138. doi: 10.1016/j.domaniend.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Huynh H. T., Robitaille G., Turner J. D. 1991. Establishment of bovine mammary epithelial cells (MAC-T): An in vitro model for bovine lactation. Exp. Cell Res. 197:191–199. doi: 10.1016/0014-4827(91)90422-Q [DOI] [PubMed] [Google Scholar]
- Ingman W. V., Wyckoff J., Gouon-Evans V., Condeelis J., Pollard J. W. 2006. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn. 235:3222–3229. doi: 10.1002/dvdy.20972 [DOI] [PubMed] [Google Scholar]
- Khan M. A., Weary D. M., von Keyserlingk M. A. G. 2011. Invited review: Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 94:1071–1081. doi: 10.3168/jds.2010-3733 [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Ruan W. 2008. IGF-I, GH, and sex steroid effects in normal mammary development. J. Mammary Gland Biol. Neoplasia 13:353–360. doi: 10.1007/s10911-008-9103-7 [DOI] [PubMed] [Google Scholar]
- Kordon E. C., Smith G. H. 1998. An entire functional mammary gland may comprise the progeny from a single cell. Development 125:1921–1930. [DOI] [PubMed] [Google Scholar]
- Li R. W., Capuco A. V. 2008. Canonical pathways and networks regulated by estrogen in the bovine mammary gland. Funct. Integr. Genomics 8:55–68. doi: 10.1007/s10142-007-0055-6 [DOI] [PubMed] [Google Scholar]
- Lilla J. N., Werb Z. 2010. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev. Biol. 337:124–133. doi: 10.1016/j.ydbio.2009.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. J., Capuco A. V., Ross D. A., Lintault L. M., Van Amburgh M. E. 2006a. Developmental and nutritional regulation of the prepubertal heifer mammary gland: I. Parenchyma and fat pad mass and composition. J. Dairy Sci. 89:4289–4297. doi: 10.3168/jds.S0022-0302(06)72475-4 [DOI] [PubMed] [Google Scholar]
- Meyer M. J., Capuco A. V., Ross D. A., Lintault L. M., Van Amburgh M. E. 2006b. Developmental and nutritional regulation of the prepubertal heifer mammary gland: II. Epithelial cell proliferation, parenchymal accretion rate, and allometric growth. J. Dairy Sci. 89:4298–4304. doi: 10.3168/jds.S0022-0302(06)72476-6 [DOI] [PubMed] [Google Scholar]
- Motyl T., Bierla J. B., Kozlowski M., Gajewska M., Gajkowska B., Koronkiewicz M. 2011. Identification, quantitation and transcriptional profile of potential stem cells in bovine mammary gland. Livest. Sci. 136:136–149. doi: 10.1016/j.livsci.2010.08.011 [DOI] [Google Scholar]
- Need E. F., Atashgaran V., Ingman W. V., Dasari P. 2014. Hormonal regulation of the immune microenvironment in the mammary gland. J. Mammary Gland Biol. Neoplasia 19:229–239. doi: 10.1007/s10911-014-9324-x [DOI] [PubMed] [Google Scholar]
- Perruchot M.-H., Arévalo-Turrubiarte M., Dufreneix F., Finot L., Lollivier V., Chanat E., Mayeur F., Dessauge F. 2016. Mammary epithelial cell hierarchy in the dairy cow throughout lactation. Stem Cells Dev. 25:1407–1418. doi: 10.1089/scd.2016.0098 [DOI] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Schellong K., Schulz S., Stupin J. H. 2012. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res., Clin. Endocrinol. Metab. 26:641–653. doi: 10.1016/j.beem.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Purup S., Sejrsen K., Akers R. M. 1993a. Influence of estradiol on insulin-like growth factor I (IGF-I) stimulation of DNA synthesis in vitro in mammary gland explants from intact and ovariectomized prepubertal heifers. Livest. Prod. Sci. 35:182 (Abstr.) [Google Scholar]
- Purup S., Sejrsen K., Akers R. M. 1995. Effect of GH and ovariectomy on mammary tissue sensitivity to IGF-I in prepubertal heifers. J. Endod. 144:153–158. doi: 10.1677/joe.0.1440153 [DOI] [PubMed] [Google Scholar]
- Purup S., Sejrsen K., Foldager J., Akers R. M. 1993b. Effect of exogenous bovine growth hormone and ovariectomy on prepubertal mammary growth, serum hormones and acute in-vitro proliferation response of mammary explants from Holstein heifers. J. Endod. 139:19–26. doi: 10.1677/joe.0.1390019 [DOI] [PubMed] [Google Scholar]
- Purup S., Vestergaard M., Weber M. S., Plaut K., Akers R. M., Sejrsen K. 2000. Local regulation of pubertal mammary growth in heifers. J. Anim. Sci. 78(Suppl. 3):36–47. doi: 10.2527/2000.78suppl_336x [DOI] [Google Scholar]
- Rauner G., Barash I. 2012. Cell hierarchy and lineage commitment in the bovine mammary gland. PLoS One 7(1):e30113. doi: 10.1371/journal.pone.0030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner G., Barash I. 2016. Enrichment for repopulating cells and identification of differentiation markers in the bovine mammary gland. J. Mammary Gland Biol. Neoplasia 21:41–49. doi: 10.1007/s10911-015-9348-x [DOI] [PubMed] [Google Scholar]
- Rautava S. 2016. Early microbial contact, the breast milk microbiome and child health. J. Dev. Orig. Health Dis. 7:5–14. doi: 10.1017/S2040174415001233 [DOI] [PubMed] [Google Scholar]
- Reed J. R., Schwertfeger K. L. 2010. Immune cell location and function during post-natal mammary gland development. J. Mammary Gland Biol. Neoplasia 15:329–339. doi: 10.1007/s10911-010-9188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. J., Funston R. N., Grings E. E., Petersen M. K. 2016. Beef heifer development and lifetime productivity in rangeland-based production systems. J. Anim. Sci. 94:2705–2715. doi: 10.2527/jas.2016-0435 [DOI] [PubMed] [Google Scholar]
- Ruan W., Monaco M. E., Kleinberg D. L. 2005. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology 146:1170–1178. doi: 10.1210/en.2004-1360 [DOI] [PubMed] [Google Scholar]
- Safayi S., Korn N., Bertram A., Akers R. M., Capuco A. V., Pratt S. L., Ellis S. 2012. Myoepithelial cell differentiation markers in prepubertal bovine mammary gland: Effect of ovariectomy. J. Dairy Sci. 95:2965–2976. doi: 10.3168/jds.2011-4690 [DOI] [PubMed] [Google Scholar]
- Sejrsen K. 1994. Relationships between nutrition, puberty and mammary development in cattle. Proc. Nutr. Soc. 53:103–111. doi: 10.1079/PNS19940014 [DOI] [PubMed] [Google Scholar]
- Sejrsen K., Purup S. 1997. Influence of prepubertal feeding level on milk yield potential of dairy heifers: A review. J. Anim. Sci. 75:828–835. doi: 10.2527/1997.753828x [DOI] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simposon K. J., Stingl J., Smyth G. K., Asselin-Labat M. L., Wu L., Lindeman G. J., Visvader J. E. 2006. Generation of a functional mammary gland from a single stem cell. Nature 439:84–88. doi: 10.1038/nature04372 [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. 1978. Estrogen induction of growth factors specific for hormone-responsive mammary, pituitary, and kidney tumor cells. Proc. Natl. Acad. Sci. USA 75(8):3786–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarda J., Fremrova V., Bezecny I. 1989. Progesterone alone is responsible for stimulation of the growth of ducts and of mammary alveolar structures in mice. Endocrinol. Exp. 23:17–28. [PubMed] [Google Scholar]
- Soberon F., Raffrenato E., Everett R. W., Van Amburgh M. E. 2012. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 95:783–793. [DOI] [PubMed] [Google Scholar]
- Swett W. W. 1927. Relationship of conformation and anatomy of the dairy cow to her milk and butterfat producing capacity. J. Dairy Sci. 10:1–14. doi: 10.3168/jds.S0022-0302(27)93810-7 [DOI] [Google Scholar]
- Swett W. W., Matthews C. A. 1934. Dairy cow's udder studies to establish development standards. In: USDA yearbook of agriculture. United States Government Printing Office, Washington, DC: p. 175–181. [Google Scholar]
- Tucker H. A. 2000. Hormones, mammary growth, and lactation: A 41-year perspective. J. Dairy Sci. 83:874–884. doi: 10.3168/jds.S0022-0302(00)74951-4 [DOI] [PubMed] [Google Scholar]
- Tucker H. L. M., Parsons C. L. M., Ellis S., Rhoads M. L., Akers R. M. 2016. Tamoxifen impairs prepubertal mammary development and alters expression of estrogen receptor α (ESR1) and progesterone receptors (PGR). Domest. Anim. Endocrinol. 54:95–105. doi: 10.1016/j.domaniend.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Unsworth A., Anderson R., Britt K. 2014. Stromal fibroblasts and the immune microenvironment: Partners in mammary gland biology and neoplasia. J. Mammary Gland Biol. Neoplasia 19:169–182. doi: 10.1007/s10911-014-9326-8 [DOI] [PubMed] [Google Scholar]
- Velayudhan B. T., Huderson B. P., Ellis S. E., Parsons C. L., Hovey R. C., Rowson A. R., Akers R. M. 2015. Ovariectomy in young prepubertal dairy heifers causes complete suppression of mammary progesterone receptors. Domest. Anim. Endocrinol. 51:8–18. doi: 10.1016/j.domaniend.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Velayudhan B. T., Huderson B. P., McGilliard M. L., Jiang H., Ellis S. E., Akers R. M. 2012. Effect of staged ovariectomy on measures of mammary growth and development of in prepubertal heifers. Animal 6:941–951. doi: 10.1017/S1751731111002333 [DOI] [PubMed] [Google Scholar]
- Vestergaard M., Purup S., Frystyhk J., Løvendahl P., Sorensen M. T., Riis P. M., Flint D. J., Sejrsen K. 2003. Effects of growth hormone and feeding level on endocrine measurements, hormone receptors, muscle growth and performance of prepubertal heifers. J. Anim. Sci. 81:2189–2198. doi: 10.2527/2003.8192189x [DOI] [PubMed] [Google Scholar]
- Wilson M. L., McCoski S. R., Geiger A. J., Akers R. M., Johnson S. E., Akers R. M. 2017. The influence of postnatal nutrition on reproductive tract and endometrial gland development in dairy calves. J. Dairy Sci. 100:3243–3256. doi: 10.3168/jds.2016-11880 [DOI] [PubMed] [Google Scholar]
- Woodward W. L., Akers R. M., Turner J. D. 1994. Lack of mitogenic response to EGR, pituitary and ovarian hormones in bovine mammary epithelial cells. Endocrine 2:529–535. [Google Scholar]
- Woodward W. L., Beal W. E., Akers R. M. 1993. Cell interactions in initiation of mammary epithelial proliferation by oestradiol and progesterone in prepubertal heifers. J. Endod. 136:149–157. doi: 10.1677/joe.0.1360149 [DOI] [PubMed] [Google Scholar]
- Zanton G. I., Heinrichs A. J. 2005. Meta-Analysis to assess effect of prepubertal average daily gain of Holstein heifers on first-lactation production. J. Dairy Sci. 88:3860–3867. [DOI] [PubMed] [Google Scholar]