ABSTRACT
Anticipated increases in the world population to 9 billion people will lead to increased demand for food. Dairy products represent one of the most sustainable animal sources of food protein because ruminants can utilize byproduct and forage feeds unsuitable for human consumption. Continued improvements in productivity will depend on deeper understanding of the biology of lactation, including developmental programming of tissues critical to that process. Although prenatal programming of postnatal phenotype is well documented for growth, behavior, and disease, there may also be instances of “programming” that last for a specific physiological stage (e.g., lactation). We distinguish between these 2 terms by the use of developmental programming to describe a permanent effect, whereas the more general term is used to describe nonpermanent impacts on the mammary gland. Despite this complexity, here we review the evidence that exposure to elevated temperature and humidity during late gestation can program reduced yields in the subsequent lactation, largely through effects at the mammary gland. Furthermore, we provide emerging evidence that adult capacity for milk synthesis can be programmed in the calf that dam is carrying by events during fetal life occurring 2 yr before. Specifically, calves born to dams that are heat stressed for the final 6 wk of gestation produce 19% less milk in lactation relative to calves from dams provided with evaporative cooling. Importantly, the increased milk yield in animals derived from dams under evaporative cooling occurred without a greater decline in BW that accompanies negative energy balance during early lactation. Therefore, the increase in milk production suggests an increase in the efficiency of conversion of feed to milk. These data indicate that a brief period of heat stress late in development reduces the physiological efficiency of the cow in a coordinated manner to result in a substantial decline in productivity. It is likely that this programming effect would be observed across genetic lines and result in poor sustainability of milk production. Milk will continue to be an important source of high-quality, human-edible food and technologies that improve the efficiency of production will be critical to enhance sustainability. These data provide compelling support for the concept that programming impacts on the dam and the developing fetus will play a role in optimizing the efficiency of production.
Keywords: dairy cattle, developmental programming, heat stress
INTRODUCTION
Dairy products represent one of the most sustainable animal sources of food protein because ruminants can utilize byproduct and forage feeds unsuitable for human consumption (CAST, 1999). Even as the global population increases, improvements in economic status among large segments of the global population are anticipated to increase per capita demand for animal-based foods (Godfray et al., 2010). Improvements in production efficiency are critical for sustainable intensification of food production. Greater yields of milk per cow translate to less pressure on water supplies, less greenhouse gas production, and less nitrogen and phosphorus output per unit of human-consumable protein (Capper et al., 2009). Based on previous research, management interventions that lead to greater efficiency will reduce the carbon footprint and enhance the sustainability of dairy production (Capper et al., 2009).
It has long been known that heat stress can adversely alter the function of homeotherms such as cattle and other species. Effects of heat stress previously described, including reproductive function (Hansen, 2009), mortality (Crescio et al., 2010), water balance (Beede, 1991), and nutrient intake and utilization (Bernabucci et al., 2010), are ascribed to actions of heat stress on an individual animal. Most often, the effects are associated with physiological perturbations in direct response to the elevated temperature, such as reduced embryo survival, increased water consumption, or decreased DMI during the heat stress. Less well described are those alterations that affect future performance after heat stress abatement, but late gestation is a period when future productivity can be affected in the absence of acute heat stress. Physiologically, lactation represents coordination among major metabolic tissues including the liver, adipose, and digestive tract. In addition, circulatory system dynamics and immune surveillance are altered to support mammary gland function during periods of milk synthesis. Therefore, multiple tissues must be affected to alter milk output to the extent observed in previous studies. Furthermore, we have now demonstrated that deleterious consequences of heat stress can affect the animal's offspring when they mature to adulthood. An important implication is that adverse effects of heat stress can have consequences that extend across at least 1 generation. These mechanisms that underlie the effects of late gestation heat stress are discussed in more detail in the following sections, along with implications for future efficiency of milk production
DRY PERIOD HEAT STRESS EFFECTS ON THE COW
Mammary Development
The dry period, a nonlactating period between successive lactations, is important for the removal of senescent mammary cells and their regrowth before parturition for maximal milk production in the subsequent lactation (Capuco et al., 1997, 2003). Cows that experience heat stress during the dry period have a significant decrease in milk production in the subsequent lactation (summarized in Fig. 1) as a result of compromised mammary growth in the late dry period (Tao et al., 2011). Not only does heat stress during the dry period decrease mammary cell proliferation 2 to 3 wk before parturition compared with cows that were cooled during the dry period (Tao et al., 2011), recent evidence indicates that mammary alveolar number is also reduced (Mejia et al., 2017). However, the underlying biological mechanisms driving the reduction in mammary cell growth by heat stress remain unclear.
Figure 1.
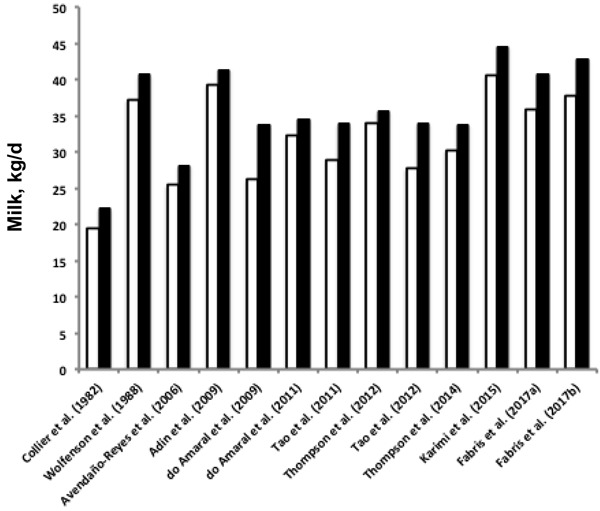
Summary of published studies that examine the effect of heat stress and cooling during the entire dry period on milk production in the subsequent lactation. Open and solid bars represent the daily subsequent milk production (kg/d) of prepartum heat-stressed and cooled cows, respectively. Adapted from Tao and Dahl (2013).
Several possible explanations for the impaired mammary gland development in heat-stressed dry cows warrant consideration. Shortened gestation is a hallmark of cows that are heat stressed during the dry period (Collier et al., 1982; Tao and Dahl, 2013), and it is possible that heat-stressed cows miss a critical time to express full mammary gland development during the dry period. However, cows that experience a 3- to 6-d reduction in gestation length following induced parturition have a decrease in milk production only in early lactation (Beardsley et al., 1976; Bremmer et al., 1999) but not the whole lactation (Schmitt et al., 1975; Bremmer et al., 1999) as observed in heat-stressed dry cows (Tao et al., 2011, 2012). Therefore, just a reduction in total gestation length cannot explain the observed negative effects of late gestation heat stress on milk yield.
Sheep that are heat stressed during pregnancy display poorer placental function relative to normothermic controls (Bell et al., 1987), which leads to a reduction in fetal weight that is correlated with lower placental weight. Of interest, the observed reduction in fetal and placental weight, termed intrauterine growth restriction, occurs absent of any difference in DMI, implicating an effect on nutrient transfer and utilization rather than quantity. Indeed, a number of more recent studies support the concept that overall placental function is impaired in ewes with gestational heat stress (reviewed by Barry et al. [2008]). Even though dry period heat stress occurs later in gestation than that which induces intrauterine growth restriction in sheep, placental function, especially endocrine secretion, is likely altered in cows for the entire dry period and this may impact mammary growth. Of interest, 1 report indicates that circulating progestin concentrations are elevated in heat-stressed dry cows (Collier et al., 1982). In contrast, heat stress reduces concentrations of estrone sulfate (Collier et al., 1982) and placental-associated glycoprotein (Thompson et al., 2013) during the dry period, evidence that placental function is altered with heat stress. Perhaps reduced placental endocrine function limits mammary cell turnover as gestation advances. For example, the increased progestin concentrations under heat stress would seem to be compatible with greater mammary development, but because the most rapid mammary development occurs with elevated progesterone and and estrogen concentrations in combination, the reduction in estrone sulfate may limit the overall growth.
Limited nutrient availability during the dry period could be another potential explanation for compromised mammary growth and the subsequent reduction in milk production. Heat-stressed dry cows also have increased water consumption during the dry period (Tao et al., 2011), which may alter an animal's plasma volume under hyperthermia and adversely affect blood nutrient supply to the mammary gland. However, hydration status is not influenced by heat stress, as suggested by the similar blood hematocrit percentage between heat-stressed and cooled cows during late gestation (Collier et al., 1982).
Similar to lactating dairy cows, dry cows have reduced DMI when exposed to heat stress (do Amaral et al., 2009; Tao et al., 2011, 2012), which may affect the nutrient availability of the mammary gland, potentially limiting its growth during late gestation. Yet in our previous studies (Fig. 2; do Amaral et al., 2011; Tao et al., 2012), although heat-stressed cows consumed less DMI compared with cooled cows, there were no differences in plasma glucose, NEFA, β-hydroxybutyric acid, and insulin during the dry period. Therefore, despite a decrease in feed intake, heat stress actually does not alter the postabsorptive fatty acid and glucose metabolism of the cow at the systemic level during the dry period. Under heat-stress conditions, the blood flow of a lactating cow is redistributed to the periphery as an adaptive strategy to increase the dissipation of body heat; as a consequence, the perfusion of other organs including the mammary gland is reduced (Lough et al., 1990). A similar situation would likely occur in the dry cow. Therefore, the mammary gland of a heat-stressed dry cow is indeed exposed to an environment of limited nutrient delivery, however, that is caused by compromised mammary perfusion under heat stress per se rather than an alteration of the cow's metabolism and systemic nutrient availability. It is still unclear if this is the main driving force behind the impaired mammary growth in heat-stressed cows during the late dry period (Tao et al., 2011), and related cellular events have yet to be uncovered.
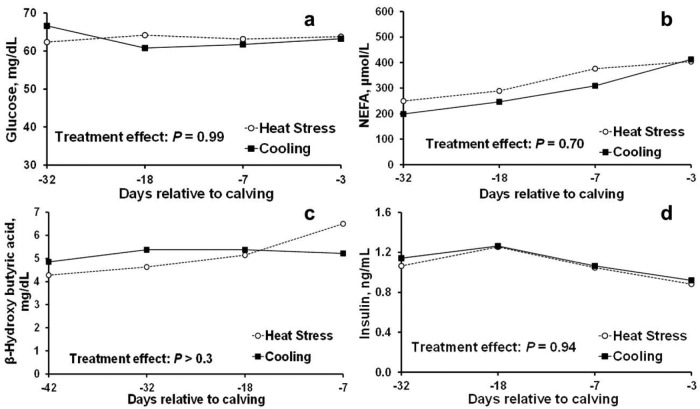
Figure 2.
Plasma glucose (a), NEFA (b), β-hydroxybutyric acid (c), and insulin (d) of heat-stressed (○) and cooled (■) cows during the dry period (adapted from Tao et al. [2012] and do Amaral et al. [2011]). All cows were housed in the same free-stall barn during summer. The stall areas for cooled cows were equipped with active cooling (soakers and fans) but those for heat-stressed cows were not. There were no differences in circulating plasma glucose, NEFA, β-hydroxybutyric acid, and insulin between treatments, suggesting that heat stress did not alter systemic metabolism of the cow during the dry period.
Mammary Epithelial Cell Involution
It is well recognized that mammary involution, which follows milk stasis in the early dry period, involves the 2 intracellular processes, autophagy (Zarzyńska et al., 2007) and apoptosis (Wilde et al., 1997; Sorensen et al., 2006). Involution during the early dry period serves to clear the senescent mammary cells from the previous lactation in preparation for the next, and there are several lines of evidence that emphasize the importance of mammary involution in the early dry period for later milk yield. Our previous studies demonstrated that heat abatement through cooling applied during the entire dry period in summer resulted in an increase in milk production of 5 to 7.5 kg/d in the subsequent lactation relative to cows that were not cooled (do Amaral et al., 2009; Tao et al., 2011, 2012; Thompson et al., 2014; Fabris et al., 2017a,b). In contrast, when active cooling was applied only during the close-up period of the dry period (i.e., the last 2 to 4 wk of gestation), milk production was improved by merely 1.4 kg/d in the next lactation (Urdaz et al., 2006; Gomes et al., 2013). A similar time dependency was also observed for photoperiod, another environmental cue, during the dry period. Cows exposed to a short-day photoperiod during the entire dry period of 42 to 60 d had improved mammary growth in the late dry period (Wall et al., 2005) and produced approximately 3.3 kg/d more milk in the next lactation compared with cows maintained under a long-day photoperiod (Miller et al., 2000; Auchtung et al., 2005; Velasco et al., 2008). However, application of short-day photoperiod to cows only during the last 21 d of the dry period had no effect on the subsequent milk production (Reid et al., 2004). Therefore, disregarding the early dry period in environmental manipulations aimed to improve mammary proliferation can profoundly reduce their impact on the subsequent lactational performance.
The role of mammary cell apoptosis and autophagy during involution on mammary gland development during the dry period has been largely ignored. Programmed cell death, or apoptosis, is a tightly regulated cellular self-destruction mechanism without the induction of inflammation or damage to the surrounding tissue. It is induced by tissue damage or environmental insult and is a trigger for stem and progenitor cell proliferation during tissue regeneration (for review, see Bergmann and Steller [2010]). Mollereau et al. (2013) called this phenomenon “apoptosis-induced compensatory proliferation.” In the mouse, radiation-induced apoptosis of embryonic fibroblasts enhances the proliferation of adjacent stem and progenitor cells in vitro and in vivo (Li et al., 2010). This enhanced cellular proliferation is mediated by PGE2, a downstream effector of caspase-3 released by apoptotic cells (Li et al., 2010; Huang et al., 2011). In cattle, during the dry period, increased apoptosis characterizes mammary gland involution after milk stasis followed by extensive cell proliferation (Capuco et al., 1997; Sorensen et al., 2006), which is consistent with the hypothesis that apoptosis in the early stage of the dry period triggers or enhances subsequent mammary growth.
In addition to apoptosis, autophagy is increased during the early dry period in the mammary gland (Zarzyńska et al., 2007). In contrast to apoptosis, autophagy, which literally means “self-eating,” is a cell survival mechanism, characterized by controlled breakdown and recycling of cellular constituents, such as long-lived proteins and cell organelles. Decreased nutrient availability can be a potent trigger of autophagy (Mizushima et al., 2004), supporting energy supply and cell preservation functions. In addition, extensive autophagy can result in cellular self-destruction and is an essential process during development and tissue remodeling during mammary involution after cessation of milking. In fact, increased autophagy was observed in the mammary gland of cows after milk stasis, possibly induced by the decrease in feed intake following dry off (Zarzyńska et al., 2007). Teplova et al. (2013) recently reported that expression of several autophagy-related genes in the mammary gland of mice was temporarily upregulated after weaning and that defective autophagy delayed mammary gland involution, presumably through decreased engulfment and elimination of apoptotic bodies.
In contrast to the pivotal role of autophagy in mammary involution during the early dry period, its biological significance on mammary growth during the proliferative phase in the late dry period is as yet unclear. Enhanced autophagy inhibits cell proliferation mediated by the cellular mediator Beclin-1 (Wang and Levine, 2010), a known tumor suppressor and essential regulator of autophagy (Yue et al., 2003). For example, an increase in Beclin-1 expression was associated with enhanced autophagy and attenuated cell proliferation in human MCF7 breast carcinoma cells (Liang et al., 1999). In contrast, heterozygous disruption of beclin 1 gene in mice, which lowered Beclin-1 protein expression and decreased autophagy, stimulated mammary cell proliferation (Qu et al., 2003). Therefore, it may be that autophagy plays different roles in mammary development depending on the stage of the dry period. One working hypothesis, illustrated in Fig. 3, is that in the early dry period, the enhanced autophagy is beneficial and facilitates mammary involution, whereas excessive autophagy may be an antiproliferative factor during the extensive mammary growth in the late dry period.
Figure 3.
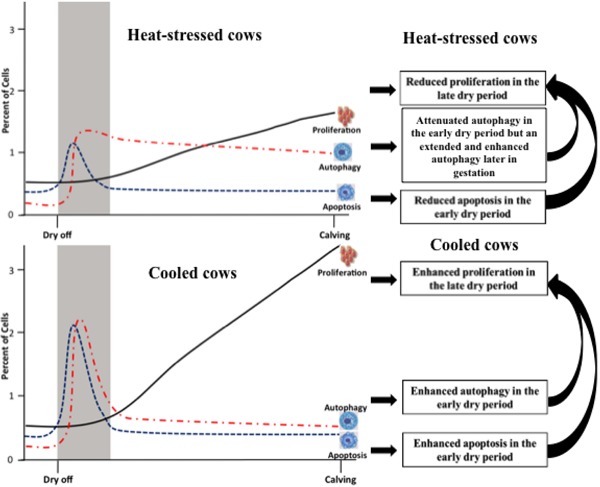
Proposed model of different cellular events in the mammary gland that occur during the dry period of heat-stressed (upper panel) and cooled (lower panel) cows. The shaded area represents mammary involution in the early dry period (approximately 2 wk after dry off). The black solid line, blue dashed line, and red dashed–dotted line represent the course of mammary cell proliferation, apoptosis, and autophagy during the dry period, respectively. Compared with cooling, heat stress may cause an attenuation of autophagy in the early dry period due to lower placental estrone sulfate production and an extended and enhanced autophagic response later in gestation through decreased nutrient supply by lower mammary gland perfusion. The altered pattern of autophagy driven by heat stress may impede cell elimination in the early dry period and cell proliferation late in pregnancy. Additionally, relative to cooled cows, heat-stressed cows may have reduced mammary cell apoptosis in the early dry period through increased cell heat shock protein expression and blood prolactin, which may negatively affect cell turnover in the mammary gland, ultimately compromising lactational performance.
Importantly, environmental heat stress alters both autophagy and apoptosis. One of the most recognized cellular events in response to heat stress is the induction of expression of heat shock proteins (HSP), which act as molecular chaperones to assist protein folding and act to inhibit apoptosis to protect cells from hyperthermia (Kregel, 2002; Lanneau et al., 2007). In primary bovine mammary epithelial cell culture, induction of HSP70 extends thermal tolerance and decreases apoptosis (Collier et al., 2008). Additionally, we have reported that heat stress increases plasma prolactin concentration in dairy cows (do Amaral et al., 2009; Tao et al., 2011), which may also decrease mammary cell apoptosis through downregulation of IGFBP5 gene expression (Accorsi et al., 2002).
Heat stress also affects autophagy. In rat hepatocytes (Oberley et al., 2008), HeLa cells, human embryonic kidney cells 293, COS cells (Nivon et al., 2009), and mouse germ cells (Zhang et al., 2012), autophagy was stimulated by heat stress under in vitro conditions. However, the mammary gland of the late gestation dairy cow may respond differently to heat stress due to a temperature-induced decrease in placental estrone sulfate production (Collier et al., 1982). Estrone sulfate is an estrogenic compound with a long half-life and has been reported to induce autophagy in bovine mammary epithelial cells (Sobolewska et al., 2009). This suggests that heat stress induces a reduction in blood estradiol concentrations, which attenuates mammary cell autophagy and, in turn, impairs mammary involution during the early dry period. However, later in the dry period of heat-stressed cows, the reduction in nutrient availability at the mammary gland from a potentially reduced mammary blood supply relative to cooled cows may stimulate and extend the autophagic response, which further impairs mammary cell proliferation. Taken together, we propose a model whereby heat stress causes a biphasic autophagic response in the dry period that deviates from that in cooled cows and ultimately has a profound impact on subsequent mammary gland proliferation and future milk production (see Fig. 3).
To test the hypothesis that early dry period heat stress drives differences in autophagy in the mammary gland, we collected a series of mammary biopsies during involution from cows exposed to heat stress or cooled during the dry period (Wohlgemuth et al., 2016). In support of our hypothesis, LC3-I and LC3-II protein expression increased in cooled cows during the first week after dry off, whereas there was no change in LC3-I or LC3-II in mammary tissue collected from heat-stressed dry cows, suggesting that autophagy is accelerated with cooling. Perhaps as important to mammary gland remodeling, it was clear that autophagy markers were static throughout the dry period in heat-stressed cows, in contrast to the initial elevation and subsequent decline in cooled dry cows. More recently, we have compared the influence of early dry period cooling with the influence of late dry period cooling on mammary function, specifically milk yield in the next lactation, in an effort to determine the temporal relationship of heat stress and mammary gland regeneration in dry cows (Fabris et al., 2017b). At dry off, cows were exposed to heat stress or cooling for the initial 3 wk of a targeted 6-wk dry period. After 3 wk, half of the animals in each group were switched to the opposite treatment, which resulted in a 2 × 2 factorial arrangement of heat stress or cooling for the entire dry period or the combination of heat stress and cooling during the early or late dry period. In the next lactation, cooled cows produced 5.1 kg/d more milk than the cows that experienced heat stress the entire dry period, and production did not differ among the heat-stressed and both switched groups. Therefore, it appears that heat stress at any time during the dry period reduces subsequent performance. Of interest, the gestation length of all 3 groups that experienced any heat stress in late gestation was reduced relative to the cooled cows, which is further evidence of a linkage between placental function and the ultimate effect on mammary gland proliferation.
In Utero Heat Stress Effects on Calves
Maternal hyperthermia results in significant heat stress for the developing fetus, and this results in substantial impacts at birth but also later in life. In our studies, late gestation heat stress reduced birth weight in bulls and heifers (Tao and Dahl, 2013), confirmation of numerous studies in cattle and other ruminants (Collier et al., 1982; Bell et al., 1987; Wolfenson et al., 1988). As cows are heat stressed in late gestation, so too are fetuses challenged to maintain core temperatures and must accordingly adjust blood flow and nutrient utilization to limit heat production. These adaptations persist into early life when heat-stressed calves have increased circulating insulin and altered glucose clearance, hallmarks of greater peripheral tissue uptake of nutrients and accumulation of adipose tissue (Tao et al., 2014). Calves born to cooled dams are not only heavier but also taller early in life, and those observations are consistent to 1 yr of age (Monteiro et al., 2016). At their first calving, however, there is no difference in BW between heifers born to cooled or heat-stressed dams, indicating that some compensatory weight gain occurs after puberty, and is likely via greater fat accretion in heat-stressed heifers.
It may also be that alterations in maternal colostrum caused by heat stress could affect the function of the calf. Recent data indicate that colostrum quality and specific compounds in milk can affect postnatal outcomes of livestock. Notably, in swine, the “lactocrine hypothesis” posits that relaxin present in milk and ingested by the piglet can affect endometrial development and function later in life (Bartol et al., 2008). Careful dissection of the effects of in utero heat stress on the calf vs. the colostrum revealed that heat-stressed calves are less able to absorb colostral antibodies compared with cooled calves, regardless of the source of colostrum. Furthermore, colostrum does not influence the response to in utero heat stress when tested directly in calves (i.e., there is no difference in antibody uptake from colostrum produced by heat-stressed or cooled cows; Monteiro et al., 2014). Rather, this effect appears to result from altered rates of gut closure following in utero heat stress, wherein apoptosis of jejunal enterocytes is accelerated in calves born to heat-stressed dams, both before and after colostrum ingestion (Ahmed et al., 2016). These observations indicate that colostrum itself does not mediate the impacts of in utero heat stress but that passive immune transfer is another outcome negatively impacted when the dam is heat stressed in late gestation.
Gross deficiencies in nutrient quantity or quality may also produce long-term effects on offspring (Singh et al., 2012). Indeed, nutrient restriction in beef cattle in mid gestation reduces antral follicle count and increases blood pressure (Mossa et al., 2013), evidence of the broad impact of the nutrient restriction on different physiological systems. Bell et al. (1987) reported that heat stress of ewes in mid gestation did not alter the dam's nutrient intake but significantly reduced lamb birth weight as a result of altered nutrient and oxygen supply. Yet although heat stress in late gestation of dairy cows does produce reductions in total caloric intake of the dam, a nutrient restriction of approximately 10% is not consistent with the magnitude of productivity decline observed in the calves in our studies. It is also important to note that dry dairy cows, including those on our studies, are typically fed diets that are well balanced for macro- and micronutrients, so the potential that a specific nutrient deficiency causes the observed responses is unlikely. Of course, placental efficiency of nutrient transfer is likely reduced by heat stress relative to cooling, so there is likely a lower availability to the developing bovine fetus under heat stress.
We have now assessed the effect of in utero heat stress on the calves' performance at maturity, using calves born to cows exposed to heat stress or cooled during the dry period (Monteiro et al., 2016). The lactation phenotype of individual females that were exposed to in utero heat stress or born to cooled dams was expressed about 22 to 24 mo after the exposure to maternal hyperthermia during late fetal life. There was no difference in genetic potential of the 2 groups of calves, as indicated by the overlap of genetic impact of the sires of the heifers used in the study, an estimate known as the predicted transmitting ability (Hill, 1969). That predicted transmitting ability value was 989 ± 98 for the cool progeny, compared with 1,058 ± 112 for the heat stress progeny, suggesting that the response is not affected by relative genetic potential for yield. It is possible, therefore, that maternal heat stress acted to change the developmental program of the mammary or other tissues required for lactation in a persistent manner. Many examples of developmental programming in livestock and other species involve changes to the epigenome (Singh et al., 2012), and we have begun to determine whether this is the case for maternal heat stress. Specifically, we have focused on methylation of cytosines in specific tissues related to metabolism and lactation in bulls and cows, including the liver and mammary gland (Skibiel et al., 2017).
Tissues were collected from bull calves at birth (liver) and mature cows early in their first lactation (mammary gland), and methylation was assessed by bisulfite sequencing. These tissues were from calves born to heat-stressed or cooled dams. Comparing in utero heat-stressed calves with the cooled calves, bull liver showed a significant degree of differential methylation of CpG islands across coding and noncoding regions of the genome (Fig. 4), and a similar pattern of differential methylation was detected in the mammary tissue of mature females (Skibiel et al., 2017). Indeed, heat stress treatment caused similar changes in the methylation pattern across both treatments in a group of approximately 50 genes in both tissues. Therefore, calves that suffered heat stress in utero exhibit differential methylation at birth and maturity, in multiple tissues, and in both genders. This suggests that in utero heat stress programs adaptations that persist after birth, permanently altered the capacity of one or more organs to support lactation. Future studies will focus on the possible transmission of these altered methylation patterns to subsequent generations as well as the possible impact of in utero heat stress on other markers of epigenetic processes.
Figure 4.
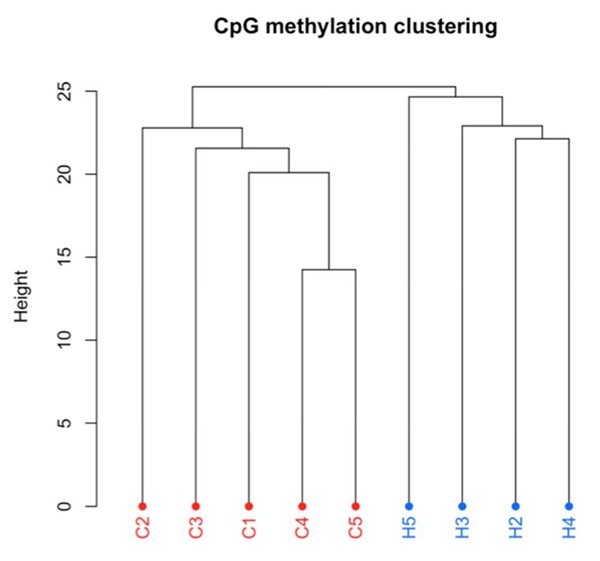
Hierarchical clustering of the DNA methylation profile (CpG islands) in liver tissue samples of bull calves born to dams that were cooled (C; n = 5) or heat stressed (H; n = 4) during late gestation from dry off until calving. Liver samples were collected from calves shortly after birth and before any colostrum was consumed.
CONCLUSIONS
Exposure to heat stress conditions in late gestation limits the progression of mammary development normally associated with the dry period and results in lower yields in the next lactation. The developing calf is also negatively affected by heat stress, as results presented here indicate that establishment of an individual's capacity for milk production starts before birth and that an optimal thermal environment is required to achieve maximum productivity at maturity.
FOOTNOTES
The authors would like to thank the staff of the Dairy Unit of University of Florida for animal care and data collection. In addition, we appreciate the contributions of Francisco Peñagaricano, Izabella Thompson, Stephanie Wohlgemuth, Ana Monteiro, Bahroz Ahmed, Thiago Fabris, Amy Skibiel, Bethany Dado, and numerous undergraduates and student interns for their help in conducting the studies described here. Support for these studies was provided in part by USDA National Institute of Food and Agriculture Tropical and Subtropical Agricultural Research award number 2010-34135-21054 and Agriculture and Food Research Initiative awards number 2010-85122-20623 and number 2015-67015-23409 to G.E. Dahl.
LITERATURE CITED
- Accorsi P. A., Pacioni B., Pezzi C., Forni M., Flint D. J., Seren E. 2002. Role of prolactin, growth hormone and insulin-like growth factor 1 in mammary gland involution in the dairy cow. J. Dairy Sci. 85:507–513. doi: 10.3168/jds.S0022-0302(02)74102-7 [DOI] [PubMed] [Google Scholar]
- Adin G., Gelman A., Solomon R., Flamenbaum I., Nikbachat M., Yosef E., Zenou A., Shamay A., Feuermann Y., Mabjeesh S. J., Miron J. 2009. Effects of cooling dry cows under heat load conditions on mammary gland enzymatic activity, intake of food water, and performance during the dry period and after parturition. Livest. Sci. 124:189–195. doi: 10.1016/j.livsci.2009.01.014 [DOI] [Google Scholar]
- Ahmed B. M. S., Monteiro A. P. A., Younas U., Asar T. O., Liu J.-D., Hayen J., Tao S., Dahl G. E. 2016. Maternal heat stress affects calf passive immunity: Effects on intestinal cell apoptosis. J. Dairy Sci. 98(Suppl. 2):713 (Abstr.) [Google Scholar]
- Auchtung T. L., Rius A. G., Kendall P. E., McFadden T. B., Dahl G. E. 2005. Effects of photoperiod during the dry period on prolactin, prolactin receptor, and milk production of dairy cows. J. Dairy Sci. 88:121–127. doi: 10.3168/jds.S0022-0302(05)72669-2 [DOI] [PubMed] [Google Scholar]
- Avendaño-Reyes L., Alvarez-Valenzuela F. D., Correa-Calderón A., Saucedo-Quintero J. S., Robinson P. H., Fadel J. G. 2006. Effect of cooling Holstein cows during the dry period on postpartum performance under heat stress conditions. Livest. Sci. 281:2535–2547. [Google Scholar]
- Barry J. S., Rozance P. J., Anthony R. V. 2008. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin. Perinatol. 32:225–230. doi: 10.1053/j.semperi.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Bartol F. F., Wiley A. A., Bagnell C. A. 2008. Epigenetic programming of porcine endometrial function and the lactocrine hypothesis. Reprod. Domest. Anim. 43(Suppl. 2):273–279. [DOI] [PubMed] [Google Scholar]
- Beardsley G. L., Muller L. D., Garverick H. A., Ludens F. C., Tucker W. L. 1976. Initiation of parturition in dairy cows with dexamethasone. II. Response to dexamethasone in combination with estradiol benzoate. J. Dairy Sci. 59:241–247. doi: 10.3168/jds.S0022-0302(76)84190-2 [DOI] [PubMed] [Google Scholar]
- Beede D. K. 1991. Mineral and water nutrition. Vet. Clin. North Am.: Food Anim. Pract. 7:373–390. doi: 10.1016/S0749-0720(15)30797-0 [DOI] [PubMed] [Google Scholar]
- Bell A. W., Wilkening R. B., Meschia G. 1987. Some aspects of placental function in chronically heat-stressed ewes. J. Dev. Physiol. 9:17–29. [PubMed] [Google Scholar]
- Bergmann A., Steller H. 2010. Apoptosis, stem cells, and tissue regeneration. Sci. Signal. 3(145):re8. doi: 10.1126/scisignal.3145re8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabucci U., Lacetera N., Baumgard L. H., Rhoads R. P., Ronchi B., Nardone A. 2010. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4:1167–1183. doi: 10.1017/S175173111000090X [DOI] [PubMed] [Google Scholar]
- Bremmer D. R., Christensen J. O., Grummer R. R., Rasmussen F. E., Wiltbank M. C. 1999. Effects of induced parturition and estradiol on feed intake, liver triglyceride concentration, and plasma metabolites of transition dairy cows. J. Dairy Sci. 82:1440–1448. doi: 10.3168/jds.S0022-0302(99)75371-3 [DOI] [PubMed] [Google Scholar]
- Capper J. L., Cady R. A., Bauman D. E. 2009. The environmental impact of dairy production: 1944 compared with 2007. J. Anim. Sci. 87:2160–2167. doi: 10.2527/jas.2009-1781 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Akers R. M., Smith J. J. 1997. Mammary growth in Holstein cows during the dry period: Quantification of nucleic acids and histology. J. Dairy Sci. 80:477–487. doi: 10.3168/jds.S0022-0302(97)75960-5 [DOI] [PubMed] [Google Scholar]
- Capuco A. V., Ellis S. E., Hale S. A., Long E., Erdman R. A., Zhao X., Paape M. J. 2003. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 81:18–31. doi: 10.2527/2003.81suppl_318x [DOI] [PubMed] [Google Scholar]
- Collier R. J., Collier J. L., Rhoads R. P., Baumgard L. H. 2008. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 91:445–454. doi: 10.3168/jds.2007-0540 [DOI] [PubMed] [Google Scholar]
- Collier R. J., Doelger S. G., Head H. H., Thatcher W. W., Wilcox C. J. 1982. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J. Anim. Sci. 54:309–319. doi: 10.2527/jas1982.542309x [DOI] [PubMed] [Google Scholar]
- Council for Agricultural Science and Technology (CAST) 1999. Animal agriculture and global food supply. Task Force Report no. 135. CAST, Ames, IA. [Google Scholar]
- Crescio M. I., Forastiere F., Maurella C., Ingravalle F., Ru G. 2010. Heat-related mortality in dairy cattle: A case crossover study. Prev. Vet. Med. 97:191–197. doi: 10.1016/j.prevetmed.2010.09.004 [DOI] [PubMed] [Google Scholar]
- do Amaral B. C., Connor E. E., Tao S., Hayen M. J., Bubolz J. W., Dahl G. E. 2009. Heat-stress abatement during the dry period: Does cooling improve transition into lactation? J. Dairy Sci. 92:5988–5999. doi: 10.3168/jds.2009-2343 [DOI] [PubMed] [Google Scholar]
- do Amaral B. C., Connor E. E., Tao S., Hayen M. J., Bubolz J. W., Dahl G. E. 2011. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J. Dairy Sci. 94:86–96. doi: 10.3168/jds.2009-3004 [DOI] [PubMed] [Google Scholar]
- Fabris T. F., Laporta J., Corra F. N., Torres Y. M., Kirk D. J., McLean D. J., Chapman J. D., Dahl G. E. 2017a. Effect of nutritional immunomodulation and heat stress during the dry period on subsequent performance of cows. J. Dairy Sci. 100:6733–6742. doi: 10.3168/jds.2016-12313 [DOI] [PubMed] [Google Scholar]
- Fabris T. F., Laporta J., Skibiel A. L., Senn B. D., Corra F. N., Dahl G. E. 2017b. Impact of heat stress during the early and late dry period on subsequent performance in dairy cattle. J. Dairy Sci. 100(Suppl. 2):386 (Abstr.) doi:10.3168/jds.2016-12313 [Google Scholar]
- Godfray H. C., Beddington J. R., Crute I. R., Haddad L., Lawrence D., Muir J. F., Pretty J., Robinson S., Thomas S. M., Toulmin C. 2010. Food security: The challenge of feeding 9 billion people. Science 327:812–818. doi: 10.1126/science.1185383 [DOI] [PubMed] [Google Scholar]
- Gomes G. C., Zuniga J. E., Greco L. F., Sinedino D. P., Ribeiro E. S., Martinez N., Bisinotto R. S., Lima F. S., Karakaya E., Engstrom M. A., Santos J. E. P., Staples C. R. 2013. Effects of evaporative cooling prepartum and vitamin E supplementation on performance of Holstein cows during summer in Florida. J. Anim. Sci. 91(Suppl. 2):242 (Abstr.) [Google Scholar]
- Hansen P. J. 2009. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:3341–3350. doi: 10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. G. 1969. On the theory of artificial selection in finite populations. Genet. Res. 13:143–163. doi: 10.1017/S0016672300002858 [DOI] [PubMed] [Google Scholar]
- Huang Q., Li F., Liu X., Li W., Shi W., Liu F.-F., O'Sullivan B., He Z., Peng Y., Tan A.-C., Zhou L., Shen J., Han G., Wang X.-J., Thorburn J., Thorburn A., Jimeno A., Raben D., Bedford J. S., Li C.-Y. 2011. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17:860–866. doi: 10.1038/nm.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M. T., Ghorbani G. R., Kargar S., Drackley J. K. 2015. Late-gestation heat stress abatement on performance and behavior of Holstein dairy cows. J. Dairy Sci. 98:6865–6875. doi: 10.3168/jds.2014-9281 [DOI] [PubMed] [Google Scholar]
- Kregel K. C. 2002. Invited review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92:2177–2186. doi: 10.1152/japplphysiol.01267.2001 [DOI] [PubMed] [Google Scholar]
- Lanneau D., de Thonel A., Maurel S., Didelot C., Garrido C. 2007. Apoptosis versus cell differentiation: Role of heat shock protein HSP90, HSP70 and HSP27. Prion 1:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Huang Q., Chen J., Peng Y., Roop D. R., Bedford J. S., Li C.-Y. 2010. Apoptotic cells activate the “Phoenix Rising” pathway to promote wound healing and tissue regeneration. Sci. Signal. 3(110):ra13. doi: 10.1126/scisignal.2000634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676. [DOI] [PubMed] [Google Scholar]
- Lough D. S., Beede D. L., Wilcox C. J. 1990. Effects of feed intake and thermal stress on mammary blood flow and other physiological measurements in lactating dairy cows. J. Dairy Sci. 73:325–332. doi: 10.3168/jds.S0022-0302(90)78677-8 [DOI] [PubMed] [Google Scholar]
- Mejia C., Skibiel A. L., Dado-Senn B., Fabris T. F., Sichler V. B., Pinkelton S. A., Dahl G. E., Laporta J. 2017. Exposure of dairy cows to heat stress during late gestation or while in utero affects mammary gland microstructure. J. Dairy Sci. 100(Suppl. 2):167 (Abstr.) [Google Scholar]
- Miller A. R. E., Douglass L. W., Erdman R. A., Dahl G. E. 2000. Effects of photoperiodic manipulation during the dry period of dairy cows. J. Dairy Sci. 83:962–967. doi: 10.3168/jds.S0022-0302(00)74960-5 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15:1101–1111. doi: 10.1091/mbc.E03-09-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B., Perez-Garijo A., Bergmann A., Miura M., Gerlitz O., Ryoo H. D., Steller H., Morata G. 2013. Compensatory proliferation and apoptosis-induced proliferation: A need for clarification. Cell Death Differ. 20:181. doi: 10.1038/cdd.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A. P. A., Tao S., Thompson I. M., Dahl G. E. 2014. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 97:6426–6439. doi: 10.3168/jds.2013-7891 [DOI] [PubMed] [Google Scholar]
- Monteiro A. P. A., Tao S., Thompson I. M. T., Dahl G. E. 2016. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 99:8443–8450. doi: 10.3168/jds.2016-11072 [DOI] [PubMed] [Google Scholar]
- Mossa F., Carter F., Walsh S. W., Kenny D. A., Smith G. W., Ireland J. L., Hildebrandt T. B., Lonergan P., Ireland J. J., Evans A. C. 2013. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88(92):1–9. [DOI] [PubMed] [Google Scholar]
- Nivon M., Richet E., Codogno P., Arrigo A. P., Kretz-Remy C. 2009. Autophagy activation by NFkappaB is essential for cell survival after heat shock. Autophagy 5:766–783. doi: 10.4161/auto.8788 [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Swanlund J. M., Zhang H. J., Kregel K. C. 2008. Aging results in increased autophagy of mitochondria and protein nitration in rat hepatocytes following heat stress. J. Histochem. Cytochem. 56:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. 2003. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112:1809–1820. doi: 10.1172/JCI20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E. D., Wallace R. L., McFadden T. B., Dahl G. E. 2004. Effects of 21-day short day photoperiod treatment during the dry period on dry matter intake and subsequent milk production in cows. J. Dairy Sci. 87(Suppl. 1):424 (Abstr.) [Google Scholar]
- Schmitt D., Garverick H. A., Mather E. C., Sikes J. D., Day B. N., Erb R. E. 1975. Induction of parturition in dairy cattle with dexamethasone and estradiol benzoate. J. Anim. Sci. 40:261–268. doi: 10.2527/jas1975.402261x [DOI] [PubMed] [Google Scholar]
- Singh K., Molenaar A. J., Swanson K. M., Gudex B., Arias J. A., Erdman R. A., Stelwagen K. 2012. Epigenetics: A possible role in acute and transgenerational regulation of dairy cow milk production. Animal 6:375–381. doi: 10.1017/S1751731111002564 [DOI] [PubMed] [Google Scholar]
- Skibiel A. L., Amorín R., Peñagaricano F., Ahmed B. M., Dahl G. E., Laporta J. 2017. Epigenetic effects of in utero exposure to heat stress on the liver and mammary gland of cattle. J. Dairy Sci. 100(Suppl. 2):421 (Abstr.) [Google Scholar]
- Sobolewska A., Gajewska M., Zarzynska J., Gajkowska B., Motyl T. 2009. IGF-1, EGF, and sex steroids regulate autophagy in bovine mammary epithelial cells via the mTOR pathway. Eur. J. Cell Biol. 88:117–130. doi: 10.1016/j.ejcb.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Sorensen M. T., Nørgaard J. V., Theil P. K., Vestergaard M., Sejrsen K. 2006. Cell turnover and activity in mammary tissue during lactation and dry period in dairy cows. J. Dairy Sci. 89:4632–4639. doi: 10.3168/jds.S0022-0302(06)72513-9 [DOI] [PubMed] [Google Scholar]
- Tao S., Bubolz J. W., do Amaral B. C., Thompson I. M., Hayen M. J., Johnson S. E., Dahl G. E. 2011. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 94:5976–5986. doi: 10.3168/jds.2011-4329 [DOI] [PubMed] [Google Scholar]
- Tao S., Dahl G. E. 2013. Heat stress impacts during late gestation on dry cows and their calves. J. Dairy Sci. 96:4079–4093. doi: 10.3168/jds.2012-6278 [DOI] [PubMed] [Google Scholar]
- Tao S., Monteiro A. P. A., Hayen M. J., Dahl G. E. 2014. Short communication: Maternal heat stress during the dry period alters postnatal whole-body insulin response of calves. J. Dairy Sci. 97:897–901. doi: 10.3168/jds.2013-7323 [DOI] [PubMed] [Google Scholar]
- Tao S., Thompson I. M., Monteiro A. P., Hayen M. J., Dahl G. E. 2012. Effects of cooling heat-stressed dairy cows during the dry period on insulin response. J. Dairy Sci. 95:5035–5046. doi: 10.3168/jds.2012-5405 [DOI] [PubMed] [Google Scholar]
- Teplova L., Lozy F., Price S., Singh S., Bernard N., Cardiff R. D., Birge R. B., Karantza V. 2013. ATG proteins mediate efferocytosis and suppress inflammation in mammary involution. Autophagy 9:459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I. M., Tao S., Branen J., Ealy A. D., Dahl G. E. 2013. Environmental regulation of pregnancy-specific protein B concentrations during late pregnancy in dairy cattle. J. Anim. Sci. 91:168–173. doi: 10.2527/jas.2012-5730 [DOI] [PubMed] [Google Scholar]
- Thompson I. M., Dahl G. E. 2012. Dry period seasonal effects on the subsequent lactation. Prof. Anim. Sci. 28:628–631. [AU: [Google Scholar]
- Thompson I. M. T., Tao S., Monteiro A. P., Jeong K. C., Dahl G. E. 2014. Effect of cooling during the dry period on immune response after Streptococcus uberis intramammary infection challenge of dairy cows. J. Dairy Sci. 97:7426–7436. doi: 10.3168/jds.2013-7621 [DOI] [PubMed] [Google Scholar]
- Urdaz J. H., Overton M. W., Moore D. A., Santos J. E. P. 2006. Technical note: Effects of adding shade and fans to a feedbunk sprinkler system for preparturient cows on health and performance. J. Dairy Sci. 89:2000–2006. doi: 10.3168/jds.S0022-0302(06)72267-6 [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Reid E. D., Fried K. K., Gressley T. F., Wallace R. L., Dahl G. E. 2008. Short-day photoperiod increases milk yield in cows with a reduced dry period length. J. Dairy Sci. 91:3467–3473. doi: 10.3168/jds.2008-1028 [DOI] [PubMed] [Google Scholar]
- Wall E. H., Auchtung T. L., Dahl G. E., Ellis S. E., McFadden T. B. 2005. Exposure to short day photoperiod during the dry period enhances mammary growth in dairy cows. J. Dairy Sci. 88:1994–2003. doi: 10.3168/jds.S0022-0302(05)72875-7 [DOI] [PubMed] [Google Scholar]
- Wang C. R., Levine B. 2010. Autophagy in cellular growth control. FEBS Lett. 584:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Addey V. P. C., Li P., Fernig D. G. 1997. Programmed cell death in bovine mammary tissue during lactation and involution. Exp. Physiol. 82:943–953. doi: 10.1113/expphysiol.1997.sp004075 [DOI] [PubMed] [Google Scholar]
- Wohlgemuth S. E., Ramirez-Lee Y., Tao S., Monteiro A. P. A., Ahmed B. M., Dahl G. E. 2016. Short communication: Effect of heat stress on markers of autophagy in the mammary gland during the dry period. J. Dairy Sci. 99:4875–4880. doi: 10.3168/jds.2015-10649 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Flamenbaum I., Berman A. 1988. Dry period heat stress relief effects on prepartum progesterone, calf birth weight, and milk production. J. Dairy Sci. 71:809–818. doi: 10.3168/jds.S0022-0302(88)79621-6 [DOI] [PubMed] [Google Scholar]
- Yue Z., Jin S., Yang C., Levine A. J., Heintz N. 2003. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 100:15077–15082. doi: 10.1073/pnas.2436255100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzyńska J., Gajkowska B., Wojewódzka U., Dymnicki E., Motyl T. 2007. Apoptosis and autophagy in involuting bovine mammary gland is accompanied by up-regulation of TGF-beta1 and suppression of somatotropic pathway. Pol. J. Vet. Sci. 10:1–9. [PubMed] [Google Scholar]
- Zhang M., Jiang M., Bi Y., Zhu H., Zhou Z., Sha J. 2012. Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS One 7:e41412–e41423. [DOI] [PMC free article] [PubMed] [Google Scholar]