ABSTRACT
A completely randomized 3 × 3 + 1 factorial experiment was conducted to evaluate the effects of sources and concentrations of Zn on growth performance, nutrient digestibility, serum biochemical endpoints, and fur quality in growing–furring female black mink. One hundred fifty healthy 15-wk-old female mink were randomly allocated to 10 dietary treatments (n = 15/group) for a 60-d trial. Animals in the control group were fed a basal diet, which consisted of mainly corn, soybean oil, meat and bone meal, and fish meal, with no Zn supplementation. Mink in the other 9 treatments were fed the basal diet supplemented with Zn from either zinc sulfate (ZnSO4), zinc glycinate (ZnGly), or Zn pectin oligosaccharides (ZnPOS) at concentrations of either 100, 300, or 900 mg Zn/kg DM. The results showed that mink in the ZnPOS groups had higher ADG than those in the ZnSO4 groups (main effect, P < 0.05). The addition of Zn reduced the G:F (P < 0.05). In addition, CP and crude fat digestibility were linearly increased with Zn supplementation (P < 0.05) and N retention tended to increase with Zn addition (P = 0.08). Dietary Zn supplementation increased the concentration of serum albumin and activity of alkaline phosphatase (P < 0.05). There was a linear effect of dietary Zn on the concentration of tibia Zn and pancreatic Zn (P < 0.05). For fur quality characteristics, the fur density and hair color of mink were improved by dietary Zn concentration (P < 0.05). Compared with ZnSO4 (100%), relative bioavailability values of ZnGly were 115 and 118%, based on tibia and pancreatic Zn, respectively, and relative bioavailability values of ZnPOS were 152 and 142%, respectively. In conclusion, this study demonstrates that Zn supplementation can promote growth and increase nutrient digestibility and fur quality and that ZnPOS is more bioavailable than ZnSO4 and ZnGly in growing–furring female mink.
Keywords: growth performance, mink, nutrient digestibility, tissue zinc concentrations, zinc pectin oligosaccharides
INTRODUCTION
Zinc is an essential trace element in all living animals, and it is involved in growth, energy metabolism, and protein synthesis and various biological activities. Due to its vital role, Zn deficiency impairs cell division, which results in dramatic effects on tissues with a rapid growth rate. These effects include reduced growth of certain tissues, skin lesions, bone abnormalities, and reduced immunity. The Zn requirement for mink is estimated to be between 27 and 60 mg/kg diet (Aulerich et al., 1991). In general, the mink's diet can meet the requirement because it contains a high amount of animal byproducts or fish meal, which are rich in minerals (Liu et al., 2016). Moreover, some studies indicated that Zn supplementation above the requirement can promote growth performance and improve nutrient digestibility in several animal species such as broilers (Liu et al., 2011), pigs (Case and Carlson, 2002), and blue foxes (Liu et al., 2015a). Therefore, it has been a common practice in the animal industry to supplement feed with Zn to increase growth performance. However, the use of high levels of zinc could be detrimental to the environment (Spears and Kegley, 2002). In recent years, organic zinc sources increasingly have been used due to reported better digestibility and bioavailability than inorganic Zn sources, which could reduce the amount of Zn needed to stimulate growth performance and decrease excretion to the environment (Liu et al., 2011; Star et al., 2012; Zhang et al., 2017). For example, Star et al. (2012) reported that the relative biological value of organic Zn (Availa-Zn; Zinpro Corporation, Eden Prairie, MN) was 1.64 compared with zinc sulfate as a reference Zn source (1.00), using tibia Zn content as a response parameter in broilers. Data are lacking regarding the effects of organic Zn on growth performance and fur quality in mink.
Pectin oligosaccharides (POS) are known to have a variety of nutritional and pharmaceutical functions (Ishii et al., 2002). It has been reported that POS can form a complex with minerals by simply mixing it with foods or drinks and promote absorption of minerals into living organisms (Lebel et al., 2014). Research previously conducted in our laboratory (Wang et al., 2016) demonstrated that the supplementation of Zn pectin oligosaccharides (ZnPOS) improved the ADG and increased tissue Zn concentrations in liver and pancreas of broilers. Zinc glycinate (ZnGly), also an organic complex, frequently has been used as a supplemental Zn source (Yu et al., 2010). Supplementation of ZnGly to rat diet improved Zn retention by 30% and ameliorated severity of symptoms of Zn deficiency compared with zinc sulfate in rats (Schlegel and Windisch, 2006).
In this study, we investigated the effects of ZnGly and ZnPOS on nutrient digestibility, tissue Zn deposition and fur quality in mink and estimated the relative bioavailabilities of these organic Zn sources as compared to Zn from zinc sulfate (ZnSO4).
MATERIALS AND METHODS
The experiment was performed at the Teaching and Research Farm of the Institute of Special Economic Animal and Plant Science of the Chinese Academy of Agricultural Sciences (Jilin, P. R. China). The animal protocol for the experiment was reviewed and approved by the Institutional Animal Care and Use Committee at Chinese Academy of Agricultural Sciences (Beijing, P. R. China).
Experimental Design and Management
One hundred fifty 15-wk-old female black mink (0.89 ± 0.07 kg initial BW) were randomly assigned to 9 treatments in a 3 × 3 factorial design and 1 control group based on average age and mean BW. Mink in the control group were fed the basal diet and those in treatment groups were fed the control diet supplemented with either ZnSO4∙7H2O, ZnGly (22% Zn by analysis) or ZnPOS at 100, 300, or 900 mg/kg. The source of ZnPOS that consists of α-(1,4)-linked POS units was obtained from the Feed Research Institute, Chinese Academy of Agricultural Sciences (Beijing, P.R. China), and the content of Zn in the ZnPOS was 7% by analysis. The ZnGly is Zn chelated to glycine, and the content of Zn in the ZnGly was 22% by analysis. The basal diet consisted of corn, fish meal and bone meal, and soybean oil, with no Zn supplementation, and was formulated to meet the Zn requirements of growing mink (Aulerich et al., 1991; Wu et al., 2015b). Zinc content in the control diet was 38 mg/kg by analysis. The actual analyzed Zn concentration in the experimental diets was 127, 319, and 910 mg/kg DM, respectively, for ZnSO4; 136, 329, and 926 mg/kg DM, respectively, for ZnGly; and 123, 340, and 909 mg/kg DM, respectively, for ZnPOS. The composition and chemical analysis of the basal diet are shown in Table 1. Before the initiation of the study, mink were fed for a 7-d adaptation period. The experiment lasted for 60 d from mid September to pelting in December. Animals were individually housed in conventional cages (60 cm long by 40 cm wide by 50 cm high) with additional attached nest boxes (30 cm long by 40 cm wide by 30 cm high) in 2-row sheds. Diets were supplied twice a day at 0730 and 1530 h. Drinking water (<0.01 mg Zn/L by analysis) was available ad libitum from an automatic watering system.
Table 1.
Ingredients and nutrient composition of the basal diet (%; air-dried basis)
| Item | Content |
|---|---|
| Ingredients, % diet | |
| Extruded corn | 27.6 |
| Soybean meal | 24 |
| Corn gluten meal | 2 |
| Fish meal | 18 |
| Bone meat meal | 14.2 |
| Cheese meal | 5 |
| Lysine | 0.3 |
| Methionine | 0.3 |
| NaCl | 0.2 |
| Premix1 | 1 |
| Pectin oligosaccharides2 | 1.1 |
| Glycinate3 | 0.3 |
| Soybean oil | 6 |
| Total | 100 |
| Analyzed composition4 | |
| DM, % diet | 91.3 (2.31) |
| ME, MJ/kg DM | 18.41 (0.56) |
| CP, % DM | 32.72 (0.06) |
| Crude fat, % DM | 15.88 (0.07) |
| Carbohydrates, % DM | 40.16 (0.06) |
| Crude fiber, % DM | 1.79 (0.06) |
| Lysine, % DM | 1.36 (0.05) |
| Methionine, % DM | 0.89 (0.08) |
| Crude ash, % DM | 8.17 (0.09) |
| Zn, mg/kg | 38.11 (0.10) |
| Cu, mg/kg | 56.18 (0.17) |
| Fe, mg/kg | 203.41 (9.15) |
It is a vitamin/mineral premix that contained the following per kilogram of premix composition: 10,000 IU vitamin A palmitate, 6 mg vitamin B1 thiamine hydrochloride, 8 mg vitamin B2 riboflavin, 3 mg vitamin B6 pyridoxine hydrochloride, 2,000 IU vitamin D cholecalciferol, 60 IU vitamin E acetate, 0.1 mg vitamin B12 cobalamin, 1 mg vitamin K menadione, 400 mg vitamin C sodium ascorbate, 40 mg nicotinic acid, 12 mg vitamin B5 niacin, 0.2 mg biotin, 0.8 mg folic acid, 300 mg choline, 82 mg Fe, 120 mg Mn, 1 mg Cu, 0.5 mg I, 0.4 mg Co, and 0.2 mg Se.
Additional amount was added for three pectin oligosaccharides treatments: 100 (1.05% Zn pectin oligosaccharides + 0.05% extruded corn), 300 (0.8% pectin oligosaccharides + 0.3% extruded corn), 900 (0% pectin oligosaccharides + 1.1% extruded corn), and basal diet (1.1% extruded corn).
Additional amount was added to three zinc glycinate treatments: 100 (0.29% zinc glycinate + 0.01% extruded corn), 300 (0.22% zinc glycinate + 0.08% extruded corn), 900 (0% zinc glycinate 900 + 0.3% extruded corn), and basal diet (0.3% extruded corn).
Analyzed composition were analyzed values based on air-dried samples (mean (SEM); n = 3).
Experimental Procedure and Sample Collection
Fasting BW of mink were recorded in the morning before food was provided on d 1, 15, 30, 45, and 60. Feed intake was recorded daily during the entire experiment period. The ADG and feed conversion ratio (G:F) were individually calculated for each mink.
On d 35 of the experiment, 10 mink from each treatment were randomly selected and allocated to metabolism cages for 4 d of a digestive and nitrogen balance trial to study the effects of Zn on the nutrient digestibility. Plastic bottles and trays were used to collect urine and feces, respectively, to avoid metal contamination. According to the volume of urine, 5 drops of methylbenzene and 10 mL 10% H2SO4 per 100 mL urine were added to prevent nitrogen loss. Urine samples were collected into plastic bottles and stored at −20°C until analyzed. Fecal and feed samples were dried to a constant weight in a forced-air drying oven at 65°C and then ground to pass through a 40-mesh sieve. Fecal and feed samples were ground using feed mills that were made of stainless steel. At the end of the experiment, blood samples were taken via the toe clip from mink in the morning after overnight fasting for determining concentrations of serum Zn and serum biochemical parameters. Blood samples were centrifuged at 3,000 × g for 10 min at 4°C, and serum samples were collected and stored at −20°C until being analyzed (Wright and Spears, 2004; Wu et al., 2015c). Liver, pancreas, and the right tibia of each mink were collected at the abattoir and stored at −20°C until being analyzed.
Slaughter Traits and Fur Quality
At the end of the experiment, 10 mink from each treatment were randomly selected, euthanized by electrocution according to the Welfare of Animals Kept for Fur Production (Broom and Fraser, 2007), and pelted immediately after death by skilled workers. Pelt length was measured from the base of the tail to the tip of the nose with a precision of ±0.5 cm. The guard hair length and underfur length were measured using a modified micrometer. Fur density was graded on a scale from 1 to 12 (12 being the best). The color intensity was measured with a colorimetric card and evaluated on a scale ranging from 1 (lightest) to 5 (darkest; Liu et al., 2015a).
Chemical Analysis
The nutrient contents of diets, feces, and urine were analyzed using the methods of the AOAC International (AOAC, 2005). Dry matter of diets and fecal samples was quantified by drying feed or fecal samples at 105°C to a constant weight (method 920.39; AOAC, 2005). Nitrogen was measured using a FOSS Kjeltec 8400 analyzer (Foss, Hillerød, Denmark), and CP was calculated as N × 6.25 (method 984.13; AOAC, 2005). Crude fat in feces and feed was determined using the diethyl ether extraction–submersion method (method 920.39; AOAC, 2005). Crude ash in the diets and feces was determined using an incineration method at 550°C for 4 h (method 942.05; AOAC, 2005). The tibia of each mink was weighed and dried for 48 h at 100°C and then weighed again to determine DM. Bones were then wrapped in filter paper, placed in a side-arm Soxhlet extraction apparatus, extracted with petroleum ether for 48 h, and allowed to air-dry under a hood for 48 h. After lipid extraction, bone sections were dried at 100°C for 18 h and weighed for DM determination. Bone samples were then ashed in a muffle furnace at 600°C for 48 h. Tissue samples were dried at 100°C for 48 h, weighed, and ashed (Gengelbach et al., 1994). Zinc in the feed, bone, and tissues was determined using atomic absorption spectrophotometry (Analytik Jena novAA 400 P; Analytik Jena AG, Jena, Germany; Wu et al., 2015c).
Statistical Analysis
All data are expressed as mean ± SEM and analyzed as a 3 × 3 + 1 factorial experiment based on a completely randomized design using the GLM procedure of SAS (SAS Inst. Inc., Cary, NC). Data were analyzed as repeated measures with a model containing source, concentration, and source × concentration. Data from tibia and pancreas Zn were used to estimate relative Zn bioavailability from ZnPOS and ZnGly, using ZnSO4∙7H2O as the standard source, using multiple linear regression and a slope ratio method (Littell et al., 1997). Linear and quadratic effects due to Zn concentration were determined. A P-value ≤ 0.05 was considered significantly different among means, and P > 0.05 and < 0.10 was considered a trend.
RESULTS
Growth Performance
There were no differences in initial BW (Table 2; P > 0.05). Mink in the ZnPOS groups had higher ADG than those in the ZnSO4 group (main effect, P < 0.05). Mink in the basal diet supplemented with 900 mg Zn/kg from either ZnSO4, ZnGly, or ZnPOS (Zn-900) groups also had higher ADG than those in the basal diet supplemented with 100 mg Zn/kg from either ZnSO4, ZnGly, or ZnPOS (Zn-100) groups (P < 0.05). The addition of Zn reduced the G:F of the mink in the growing–furring period (P < 0.05). There were no concentration × source interactions for any of the endpoints associated with growth performance.
Table 2.
Effects of dietary Zn source and concentration on growth performance in growing–furring mink1
| Item | Initial BW,g | Final BW,g | ADG,g | ADFI,g | F:G2ratio |
|---|---|---|---|---|---|
| Added Zn source and concentration, mg/kg | |||||
| Control | |||||
| 0 | 887.0 | 1,054.8 | 2.48 | 92.75 | 41.01 |
| ZnSO4∙7H2O | |||||
| 100 | 903.9 | 1,122.0 | 2.63 | 83.04 | 28.69 |
| 300 | 889.5 | 1,049.4 | 2.81 | 67.33 | 26.21 |
| 900 | 906.1 | 1,129.3 | 3.00 | 67.39 | 23.74 |
| ZnGly3 | |||||
| 100 | 907.5 | 1,110.0 | 2.89 | 78.28 | 25.43 |
| 300 | 888.1 | 1,118.3 | 3.28 | 62.16 | 20.39 |
| 900 | 886.7 | 1,095.6 | 2.98 | 86.91 | 28.58 |
| ZnPOS4 | |||||
| 100 | 905.6 | 1,114.6 | 2.98 | 77.94 | 29.68 |
| 300 | 895.5 | 1,102.1 | 3.38 | 67.43 | 21.48 |
| 900 | 881.6 | 1,118.2 | 3.38 | 72.76 | 21.94 |
| SEM | 10.3 | 18.1 | 0.11 | 1.28 | 0.26 |
| Zn sources | |||||
| ZnSO4 | 905.4 | 1,102.4b | 2.82b | 72.96 | 26.22 |
| ZnGly | 893.6 | 1,107.9ab | 3.06ab | 75.78 | 24.81 |
| ZnPOS | 890.6 | 1,111.0a | 3.25a | 72.71 | 24.37 |
| SEM | 7.7 | 15.3 | 0.14 | 7.07 | 0.19 |
| Zn concentrations, mg/kg | |||||
| 100 | 906.9 | 1,045.3b | 2.84b | 79.86 | 28.11a |
| 300 | 895.1 | 1,092.5b | 3.12ab | 65.77 | 24.48ab |
| 900 | 890.8 | 1,114.4a | 3.19a | 74.88 | 22.61b |
| SEM | 7.7 | 15.3 | 0.14 | 7.07 | 0.19 |
| Linear | 0.67 | 0.65 | 0.12 | 0.36 | 0.01 |
| Quadratic | 0.86 | 0.74 | 0.26 | 0.13 | 0.03 |
| P-value | |||||
| Sources | 0.88 | 0.03 | 0.04 | 0.23 | 0.09 |
| Concentrations | 0.29 | 0.04 | 0.02 | 0.12 | 0.02 |
| Concentration × source | 0.69 | 0.84 | 0.65 | 0.56 | 0.74 |
Means within a row with different superscripts differ (P < 0.05).
Data are expressed as least squares means with pooled SEM; n = 15 per treatment.
F:G = feed:gain.
ZnGly = zinc glycinate.
ZnPOS = Zn pectin oligosaccharides.
Nutrient Utilization and N Balance
Although the digestibility of DM and ash was not affected by dietary Zn concentrations (P > 0.05), CP and crude fat digestibility was linearly increased by Zn supplementation (P < 0.05; Table 3). Nitrogen intake was not affected by Zn addition (P > 0.05); however, the concentration of fecal N was affected by Zn addition (P < 0.05). Nitrogen retention tended to increase with Zn addition (P = 0.081).
Table 3.
Effects of dietary Zn source and concentration on nutrient digestibility and N balance in growing–furring mink1
| Item | DM,% | CP,% | Fat,% | Ash,% | N intake,g/d | Fecal N,g/d | Urinary N,g/d | N retention,g/d |
|---|---|---|---|---|---|---|---|---|
| Added Zn source and concentration, mg/kg | ||||||||
| Control | ||||||||
| 0 | 63.3 | 56.1 | 84.5 | 26.2 | 4.83 | 2.06 | 2.54 | 0.19 |
| ZnSO4∙7H2O | ||||||||
| 100 | 62.0 | 63.1 | 86.5 | 26.6 | 4.36 | 1.67 | 2.46 | 0.22 |
| 300 | 63.0 | 61.8 | 87.0 | 26.2 | 3.41 | 1.53 | 2.24 | 0.24 |
| 900 | 65.8 | 64.7 | 87.2 | 27.1 | 3.79 | 1.36 | 2.13 | 0.27 |
| ZnGly2 | ||||||||
| 100 | 62.2 | 61.8 | 85.3 | 27.0 | 4.08 | 1.62 | 2.21 | 0.21 |
| 300 | 64.6 | 66.1 | 87.5 | 26.0 | 3.38 | 1.13 | 1.99 | 0.26 |
| 900 | 62.1 | 67.5 | 87.3 | 26.1 | 4.37 | 1.48 | 2.60 | 0.28 |
| ZnPOS3 | ||||||||
| 100 | 64.1 | 63.1 | 86.6 | 27.3 | 3.99 | 1.44 | 2.30 | 0.24 |
| 300 | 63.3 | 64.1 | 88.2 | 26.8 | 3.91 | 1.33 | 2.32 | 0.26 |
| 900 | 64.2 | 67.6 | 88.8 | 26.1 | 3.68 | 1.17 | 2.21 | 0.29 |
| SEM | 0.4 | 1.0 | 1.4 | 0.5 | 0.08 | 0.14 | 0.09 | 0.15 |
| Zn sources | ||||||||
| ZnSO4 | 63.5 | 63.2 | 86.9 | 26.6 | 3.87 | 1.44 | 2.19 | 0.23 |
| ZnGly | 63.0 | 65.0 | 86.7 | 26.4 | 3.89 | 1.39 | 2.26 | 0.24 |
| ZnPOS | 63.9 | 64.9 | 87.9 | 26.8 | 3.86 | 1.38 | 2.21 | 0.26 |
| SEM | 0.3 | 0.7 | 0.5 | 0.4 | 0.08 | 0.06 | 0.08 | 0.09 |
| Zn concentrations, mg/kg | ||||||||
| 100 | 62.8 | 62.7b | 86.2b | 27.0 | 4.14 | 1.58a | 2.33 | 0.23 |
| 300 | 63.7 | 64.2ab | 87.6a | 26.3 | 3.51 | 1.30b | 2.02 | 0.25 |
| 900 | 64.0 | 66.7a | 87.8a | 26.4 | 3.91 | 1.31b | 2.31 | 0.28 |
| SEM | 0.3 | 0.7 | 0.5 | 0.4 | 0.08 | 0.06 | 0.08 | 0.09 |
| Linear | 0.48 | 0.003 | 0.004 | 0.25 | 0.071 | 0.366 | 0.136 | 0.122 |
| Quadratic | 0.72 | 0.11 | 0.10 | 0.78 | 0.044 | 0.167 | 0.180 | 0.261 |
| P-values | ||||||||
| Sources | 0.49 | 0.30 | 0.06 | 0.57 | 0.941 | 0.127 | 0.116 | 0.376 |
| Concentrations | 0.17 | 0.016 | 0.006 | 0.46 | 0.771 | 0.024 | 0.183 | 0.081 |
| Concentration × source | 0.33 | 0.45 | 0.59 | 0.89 | 0.269 | 0.219 | 0.230 | 0.996 |
Means within a row with different superscripts differ (P < 0.05).
Data are expressed as least squares means with pooled SEM; n = 10 per treatment.
ZnGly = zinc glycinate.
ZnPOS = Zn pectin oligosaccharides.
Tissue Mineral Contents
The concentrations of tibia and pancreas Zn in the Zn-900 groups were greater (P < 0.05; Table 4) than those of the Zn-100 groups. The tibia and pancreas Zn concentrations of the mink in the ZnPOS group were greater (P < 0.05) than those in ZnSO4 group. However, Zn supplementation reduced the Cu concentration in the tibia and pancreas (P < 0.05). No significant effects of dietary Zn supplementation on Cu and other mineral concentrations were observed in the liver (P > 0.05).
Table 4.
Effects of dietary Zn source and concentration on tissue mineral contents in growing–furring mink1
| Tibia, mg/kg (DM basis) | Pancreas, mg/kg (DM basis) | Liver, mg/kg (DM basis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Zn | Cu | Fe | Zn | Cu | Fe | Zn | Cu | Fe |
| Added Zn source and concentration, mg/kg | |||||||||
| Control | |||||||||
| 0 | 142.6 | 10.2 | 84.2 | 81.9 | 8.4 | 75.6 | 43.5 | 23.8 | 330.5 |
| ZnSO4∙7H2O | |||||||||
| 100 | 157.8 | 10.7 | 84.7 | 90.9 | 8.1 | 72.3 | 75.9 | 20.0 | 301.8 |
| 300 | 173.6 | 10.1 | 84.1 | 95.6 | 8.3 | 70.6 | 75.8 | 15.9 | 277.2 |
| 900 | 174.8 | 8.6 | 83.6 | 108.4 | 6.5 | 62.3 | 81.2 | 19.2 | 298.0 |
| ZnGly2 | |||||||||
| 100 | 158.6 | 11.0 | 85.0 | 95.1 | 8.7 | 74.6 | 79.2 | 27.0 | 337.4 |
| 300 | 179.4 | 9.4 | 83.8 | 105.6 | 7.3 | 53.6 | 67.5 | 28.6 | 273.1 |
| 900 | 185.2 | 8.8 | 84.1 | 115.2 | 6.5 | 55.7 | 83.9 | 19.5 | 228.2 |
| ZnPOS3 | |||||||||
| 100 | 159.8 | 9.8 | 83.7 | 103.6 | 9.2 | 42.7 | 82.7 | 31.7 | 283.8 |
| 300 | 183.1 | 9.8 | 83.8 | 120.0 | 6.7 | 59.7 | 87.7 | 30.9 | 339.3 |
| 900 | 197.6 | 8.6 | 83.1 | 150.4 | 4.1 | 81.1 | 82.0 | 22.1 | 351.5 |
| SEM | 8.4 | 0.09 | 1.4 | 8.7 | 0.3 | 11.6 | 8.7 | 5.6 | 21.1 |
| Zn sources | |||||||||
| ZnSO4 | 168.9b | 9.7 | 84.1 | 98.8b | 7.6 | 68.1 | 77.3 | 18.3 | 291.9 |
| ZnGly | 174.4ab | 9.7 | 84.1 | 106.9ab | 7.3 | 59.3 | 77.9 | 24.4 | 276.6 |
| ZnPOS | 178.9a | 9.5 | 83.5 | 124.7a | 6.7 | 61.2 | 84.2 | 28.0 | 327.8 |
| SEM | 3.3 | 0.07 | 0.6 | 5.1 | 0.1 | 4.8 | 3.1 | 4.3 | 19.1 |
| Zn concentrations, mg/kg | |||||||||
| 100 | 158.8b | 10.5a | 84.4 | 97.9b | 8.7a | 60.6 | 79.0 | 25.8 | 309.4 |
| 300 | 179.5a | 9.7ab | 83.9 | 107.1ab | 7.4a | 61.4 | 77.7 | 24.9 | 298.2 |
| 900 | 185.0a | 8.6b | 83.4 | 124.7a | 5.7b | 66.4 | 82.5 | 20.3 | 287.9 |
| SEM | 3.3 | 0.07 | 0.6 | 5.1 | 0.1 | 4.8 | 3.1 | 4.3 | 19.1 |
| Linear | 0.04 | 0.06 | 0.20 | 0.03 | 0.02 | 0.16 | 0.12 | 0.71 | 0.44 |
| Quadratic | 0.35 | 0.18 | 0.18 | 0.78 | 0.01 | 0.29 | 0.53 | 0.52 | 0.78 |
| P-values | |||||||||
| Sources | 0.03 | 0.68 | 0.40 | 0.002 | 0.08 | 0.65 | 0.14 | 0.15 | 0.18 |
| Concentrations | 0.01 | 0.04 | 0.14 | 0.004 | 0.001 | 0.83 | 0.40 | 0.44 | 0.67 |
| Concentrations × source | 0.52 | 0.73 | 0.90 | 0.46 | 0.03 | 0.23 | 0.24 | 0.85 | 0.09 |
Means within a row with different superscripts differ (P < 0.05).
Data are expressed as least squares means with pooled SEM; n = 10 per treatment.
ZnGly = zinc glycinate.
ZnPOS = Zn pectin oligosaccharides.
Fur Quality
Zinc supplementation had no effect on guard hair length and underfur length (P > 0.05; Table 5). However, pelt length was longer (P < 0.05) in mink from the Zn-900 group than in mink from the basal diet supplemented with 300 mg Zn/kg from either ZnSO4, ZnGly, or ZnPOS (Zn-300) and Zn-100 groups. Hair color of mink was increased with the increase in dietary Zn concentration (P < 0.05). In addition, Zn concentration had a significant effect on fur density (P < 0.05). Mink fed diets with organic Zn had greater fur density compared to those fed diets with inorganic Zn (P < 0.05).
Table 5.
Effects of dietary Zn source and concentration on fur quality in growing–furring mink1
| Item | Fur length,cm | Guard hair length, cm | Underfur length, cm | Fur density | Color intensity |
|---|---|---|---|---|---|
| Added Zn source and concentration, mg/kg | |||||
| Control | |||||
| 0 | 42.10 | 2.20 | 1.70 | 9.23 | 2.85 |
| ZnSO4∙7H2O | |||||
| 100 | 43.40 | 2.26 | 1.70 | 9.46 | 3.00 |
| 300 | 43.70 | 2.34 | 1.58 | 9.72 | 3.24 |
| 900 | 44.00 | 2.08 | 1.52 | 9.90 | 3.67 |
| ZnGly2 | |||||
| 100 | 42.70 | 2.16 | 1.50 | 9.71 | 3.05 |
| 300 | 43.20 | 2.40 | 1.70 | 9.84 | 3.42 |
| 900 | 43.90 | 2.30 | 1.72 | 9.92 | 3.75 |
| ZnPOS3 | |||||
| 100 | 42.50 | 2.06 | 1.32 | 9.72 | 3.10 |
| 300 | 43.70 | 2.06 | 1.58 | 9.94 | 3.65 |
| 900 | 46.10 | 2.00 | 1.62 | 9.97 | 3.95 |
| SEM | 0.65 | 0.09 | 0.10 | 0.08 | 0.17 |
| Zn sources | |||||
| ZnSO4 | 43.70 | 2.23 | 1.60 | 9.70b | 3.31 |
| ZnGly | 43.26 | 2.28 | 1.64 | 9.82a | 3.40 |
| ZnPOS | 44.10 | 2.12 | 1.51 | 9.87a | 3.57 |
| SEM | 0.4 | 0.07 | 0.07 | 0.06 | 0.11 |
| Zn concentrations, mg/kg | |||||
| 100 | 42.86b | 2.16 | 1.51 | 9.63b | 3.05c |
| 300 | 43.53b | 2.27 | 1.62 | 9.84a | 3.44b |
| 900 | 44.66a | 2.20 | 1.62 | 9.94a | 3.79a |
| SEM | 0.4 | 0.07 | 0.07 | 0.06 | 0.11 |
| Linear | 0.42 | 0.32 | 0.36 | 0.001 | 0.15 |
| Quadratic | 0.56 | 0.23 | 0.14 | 0.001 | 0.46 |
| P-values | |||||
| Sources | 0.10 | 0.19 | 0.23 | 0.01 | 0.05 |
| Concentrations | 0.001 | 0.52 | 0.26 | 0.03 | 0.01 |
| Concentration × source | 0.06 | 0.32 | 0.11 | 0.30 | 0.84 |
Means within a row with different superscripts differ (P < 0.05).
Data are expressed as least squares means with pooled SEM; n = 10 per treatment.
ZnGly = zinc glycinate.
ZnPOS = Zn pectin oligosaccharides.
Relative Bioavailability Values
Bioavailability of ZnGly and ZnPOS relative to ZnSO4 was estimated from tibia Zn concentration and from pancreas Zn concentration using multiple linear regression and a slope ratio method (Fig. 1 and 2). Assuming ZnSO4 is 100% bioavailable, the relative bioavailability values of ZnGly were 115 and 118%, based on tibia Zn and pancreas Zn, respectively, and relative bioavailability values of ZnPOS were 152 and 142%, respectively.
Figure 1.
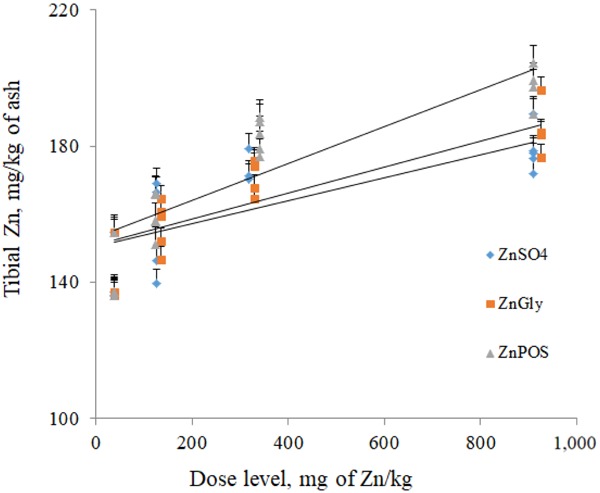
Linear dose response relationship between supplemental Zn sources and tibia Zn content. Relative Zn bioavailability for ZnSO4 is 100%, for zinc glycinate (ZnGly) is 115%, and for zinc pectin oligosaccharides (ZnPOS) is 152%. y = 152.951 + 0.033 × x1 + 0.038 × x2+ 0.050 × x3 (R2 = 0.383). The SE were 8.366, 4.471, and 3.024 for x1, x2, and x3, respectively. Values on the x-axis are based on added values for Zn in the diets.
Figure 2.
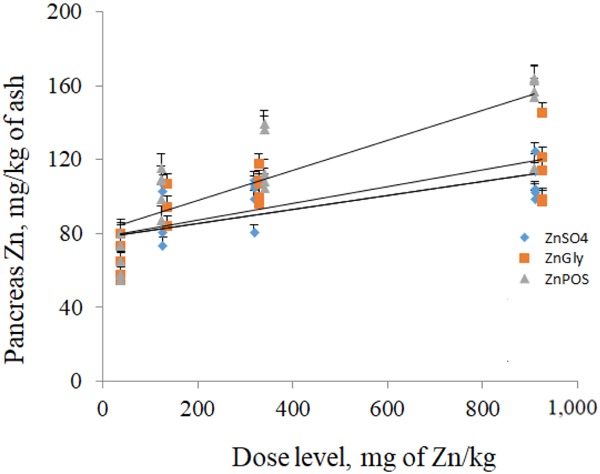
Linear dose response relationship between supplemental Zn sources and pancreas Zn content. Relative Zn bioavailability for ZnSO4 is 100%, for zinc glycinate (ZnGly) is 118%, and for zinc pectin oligosaccharides (ZnPOS) is 142%. y = 83.465 + 0.038 × x1 + 0.045 × x2+ 0.054 × x3 (R2 = 0.501). The SE were 11.127, 6.925, and 4.833 for x1, x2, and x3, respectively. Values on the x-axis are based on added values for Zn in the diets.
DISCUSSION
Results obtained in the present study demonstrate that Zn supplementation can increase ADG and improve the G:F in growing–furring mink. The findings are consistent with results previously observed in poultry (Swinkels et al., 1994; Liu et al., 2011), nursery pigs (Case and Carlson, 2002; Wang et al., 2010), and beef cattle (Malcolm-Callis et al., 2000; Nunnery et al., 2007). Similarly, it was reported that dietary Zn supplementation improved the growth performance of foxes, a carnivorous fur-bearing animal similar to mink (Liu et al., 2015a). The mechanisms responsible for the growth-promoting action of Zn in mink apparently need to be further investigated, especially the function of Zn-containing metalloproteins in mink. It has been reported that Zn plays a vital role in maintaining the structure of metalloproteins such as GH and insulin (Liu et al., 2011). Therefore, Zn may increase nutrient digestibility and utilization by improving blood circulation within the body rather than an enteric effect in the intestinal tract (Case and Carlson, 2002).
In addition, no difference in G:F of the nursery pigs fed diets supplemented with high concentrations of Zn was reported, a finding that is contrary to our results (Smith et al., 1997). This could be due to differences in the concentrations of Zn, animal species, and duration of the trials. For example, Hollis et al. (2005) reported that ADG was increased by Zn addition during the 14- to 28-d period, but there was no improvement in ADG for the initial 14-d period in pigs. He also found that additional Zn at 500 mg/kg was not effective in improving growth in weanling pigs (Hollis et al., 2005).
The improvements in ADG and the feed:gain ratio observed in this experiment might be associated with the digestibility of macronutrients. In support of this, we found that Zn supplementation increased the digestibility of CP and crude fat in mink. Similar results were reported in other species as well (Spears and Kegley, 2002; Zhang et al., 2014). The increase in the digestibility of micronutrients in mink could be due to Zn promoting gut health, as observed in pigs (Case and Carlson, 2002). This would be of significance to mink production, because in fur-bearing animals such as mink, ferret, and foxes, fat plays an important role in meeting energy requirements (Liu et al., 2016). The large amount of fat in feed and the high digestibility of fat provided enough energy against cold weather in winter. The digestibilities of CP, DM, and ash in our study were slightly lower than those reported in other digestive trials with mink (Wu et al., 2014b, 2015b). The differences could be due to variation of feed types and composition of diets. Our results also showed that N retention tended to increase with Zn supplementation. In agreement with our findings, the increasing Zn concentration from 40 to 70 mg/kg DM increased N retention in dry cows (Aliarabi et al., 2015). Increased urinary excretion of N may be an indicator of impaired cellular proliferation (Wallwork and Duerre, 1985; Aliarabi et al., 2015). Likewise, the increased N retention in mink may indicate improved cellular metabolism of nitrogen with Zn supplementation.
There are clear effects of dietary Zn intake on tissue Zn concentrations, but the effects are various among tissues and animals. In the present study, Zn concentrations in the tibia and pancreas of mink fed diets with 900 mg Zn/kg were greater than that found in mink fed diets with 100 mg Zn/kg, which is agreement with the results observed in calves supplemented with 500 mg Zn/kg (Wright and Spears, 2004). However, in this study, Zn concentration in the liver was not affected by dietary Zn concentrations. Similar reports can be found in the literature with pigs (Rincker et al., 2005; Martin et al., 2011; Hill et al., 2014) and foxes (Liu et al., 2015a). Although it has been reported that the deposition of Zn in different tissues is related to the dietary Zn supplementation (Malcolm-Callis et al., 2000), our data indicated that Zn concentrations in the tibia and pancreas would be a good physiological marker of Zn status in this species.
Zinc supplementation decreased Cu concentration in the tibia and pancreas in our study. Similar results also have been obtained in other species such as foxes and pigs (Lebel et al., 2014; Liu et al., 2015a). High concentrations of Zn in the diet inhibit Cu metabolism through competition for binding sites of the protein metallothionein (Towers et al., 1981). It has been reported that moderately high Zn in the diet reduced the tissue Cu concentration in mink (Wu et al., 2015b). No significant difference was found in the tissue Fe concentration in mink supplemented with Zn in our study. Contrary to our results, Zn supplementation had a negative effect on tissue Fe status in humans (Wieringa et al., 2007). However, the high dietary Zn had no effect on tissue Fe concentration in lambs and cows (Hackbart et al., 2010; Aliarabi et al., 2015). The interaction between Zn and Fe has never been reported in mink, and further research is necessary.
Although fur quality of fur-bearing animal is affected by many factors, mineral levels in the diet play a vital role. Other studies (Wu et al., 2014a, 2015a; Liu et al., 2016) have indicated supplementation with minerals such as Cu and Zn supplementation could improve the fur quality of mink and foxes. In the present study, there is was difference in guard hair length and underfur length among Zn treatments, suggesting that Zn concentration in the basal diet is sufficient for fur maturation in mink. In addition, fur density and the color intensity of fur were affected by Zn supplementation in our study, indicating that Zn supplementation may promote the synthesis of hair follicle development and increase the melanin content in hair. Results obtained in the present study agree with a previous study (Lowe et al., 1994) in which dogs supplemented with high Zn content grew more hair. It is also reported that dietary Zn could improve skin quality (Salim et al., 2012). The mechanism responsible for the influence of Zn supplementation on fur quality is less clear, and further research needs to test the effect of Zn supplementation on hair follicle development of mink.
We understand that the amount of Zn supplementation might be considered too high in general practice. But a study of Zn toxicity in the European mink had shown that feeding growing mink conventional diets containing up to 1,500 mg/kg supplemental Zn for 144 d produced no apparent adverse effects (Aulerich et al., 1991). The concentrations were chosen because studies have found that the addition of Zn to diet in excess of the requirement stimulates growth (Case and Carlson, 2002; Liu et al., 2011, 2015a) and that high concentrations of Zn have been used in estimating Zn bioavailability from different sources. Lower concentrations of Zn supplementation than used in the current study can be investigated in future studies.
Conclusions
The results of this study demonstrate that dietary Zn addition improves growth performance by increasing nitrogen retention and improving CP and crude fat digestibility in growing–furring female mink. This study also shows that ZnPOS and ZnGly are more bioavailable compared with inorganic zinc sulfate and that ZnPOS and ZnGly can be used in the mink industry as sources of additional Zn.
FOOTNOTES
The funding for this study was from the Special Fund for Agro-scientific Research in the Public Interest (number 201403047, 2011-G7). The staff of Key Laboratory for Feed Biotechnology of the Ministry of Agriculture is gratefully acknowledged for their valuable help in carrying out these experiments. The authors declare that they have no conflict of interest.
LITERATURE CITED
- Aliarabi H., Fadayifar A., Tabatabaei M. M., Zamani P., Bahari A., Farahavar A., Dezfoulian A. H. 2015. Effect of zinc source on hematological, metabolic parameters and mineral balance in lambs. Biol. Trace Elem. Res. 168:82–90. [DOI] [PubMed] [Google Scholar]
- AOAC 2005. Official methods of analysis. AOAC Int., Arlington, VA [Google Scholar]
- Aulerich R. J., Bursian S. J., Poppenga R. H., Braselton W. E., Mullaney T. P. 1991. Toleration of high concentrations of dietary zinc by mink. J. Vet. Diagn. Invest. 3:232–237. doi: 10.1177/104063879100300309 [DOI] [PubMed] [Google Scholar]
- Broom D. M., Fraser A. F. 2007. Domestic animal behavior and welfare. 4th ed.CABI, Wallingford, UK: p. 308–313. [Google Scholar]
- Case C. L., Carlson M. S. 2002. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 80:1917–1924. doi: 10.2527/2002.8071917x [DOI] [PubMed] [Google Scholar]
- Gengelbach G. P., Ward J. D., Spears J. W. 1994. Effect of dietary copper, iron, and molybdenum on growth and copper status of beef cows and calves. J. Anim. Sci. 72:2722–2727. doi: 10.2527/1994.72102722x [DOI] [PubMed] [Google Scholar]
- Hackbart K. S., Ferreira R. M., Dietsche A. A., Socha M. T., Shaver R. D., Wiltbank M. C., Fricke P. M. 2010. Effect of dietary organic zinc, manganese, copper, and cobalt supplementation on milk production, follicular growth, embryo quality, and tissue mineral concentrations in dairy cows. J. Anim. Sci. 88:3856–3870. doi: 10.2527/jas.2010-3055 [DOI] [PubMed] [Google Scholar]
- Hill G. M., Mahan D. C., Jolliff J. S. 2014. Comparison of organic and inorganic zinc sources to maximize growth and meet the zinc needs of the nursery pig. J. Anim. Sci. 92:1582–1594. doi: 10.2527/jas.2013-6744 [DOI] [PubMed] [Google Scholar]
- Hollis G. R., Carter S. D., Cline T. R., Crenshaw T. D., Cromwell G. L., Hill G. M., Kim S. W., Lewis A. J., Mahan D. C., Miller P. S., Stein H. H., Veum T. L., NCR-42 Committee on Swine Nutrition 2005. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. J. Anim. Sci. 83:2123–2129. doi: 10.2527/2005.8392123x [DOI] [PubMed] [Google Scholar]
- Ishii T., Ichita J., Matsue H., Ono H., Maeda I. 2002. Fluorescent labeling of pectic oligosaccharides with 2-aminobenzamide and enzyme assay for pectin. Carbohydr. Res. 337:1023–1032. doi: 10.1016/S0008-6215(02)00087-3 [DOI] [PubMed] [Google Scholar]
- Lebel A., Matte J. J., Guay F. 2014. Effect of mineral source and mannan oligosaccharide supplements on zinc and copper digestibility in growing pigs. Arch. Anim. Nutr. 68:370–384. doi: 10.1080/1745039X.2014.954357 [DOI] [PubMed] [Google Scholar]
- Littell R. C., Henry P. R., Lewis A. J., Ammerman C. B. 1997. Estimation of relative bioavailability of nutrients using SAS procedures. J. Anim. Sci. 75:2672–2683. doi: 10.2527/1997.75102672x [DOI] [PubMed] [Google Scholar]
- Liu Z., Wu X., Zhang T., Cui H., Guo J., Guo Q., Gao X., Yang F. 2016. Influence of dietary copper concentrations on growth performance, serum lipid profiles, antioxidant defenses, and fur quality in growing–furring male blue foxes (Vulpes lagopus). J. Anim. Sci. 94:1095–1104. doi: 10.2527/jas.2015-9960 [DOI] [PubMed] [Google Scholar]
- Liu Z., Wu X., Zhang T., Guo J., Gao X., Yang F., Xing X. 2015a. Effects of dietary copper and zinc supplementation on growth performance, tissue mineral retention, antioxidant status, and fur quality in growing–furring blue foxes (Alopex lagopus). Biol. Trace Elem. Res. 168:401–410. doi: 10.1007/s12011-015-0376-6 [DOI] [PubMed] [Google Scholar]
- Liu Z. H., Lu L., Li S. F., Zhang L. Y., Xi L., Zhang K. Y., Luo X. G. 2011. Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult. Sci. 90:1782–1790. doi: 10.3382/ps.2010-01215 [DOI] [PubMed] [Google Scholar]
- Lowe J. A., Wiseman J., Cole D. J. 1994. Zinc source influences zinc retention in hair and hair growth in the dog. J. Nutr. 124:2575S–2576S. [DOI] [PubMed] [Google Scholar]
- Malcolm-Callis K. J., Duff G. C., Gunter S. A., Kegley E. B., Vermeire D. A. 2000. Effects of supplemental zinc concentration and source on performance, carcass characteristics, and serum values in finishing beef steers. J. Anim. Sci. 78:2801–2808. doi: 10.2527/2000.78112801x [DOI] [PubMed] [Google Scholar]
- Martin R. E., Mahan D. C., Hill G. M., Link J. E., Jolliff J. S. 2011. Effect of dietary organic microminerals on starter pig performance, tissue mineral concentrations, and liver and plasma enzyme activities. J. Anim. Sci. 89:1042–1055. doi: 10.2527/jas.2009-2384 [DOI] [PubMed] [Google Scholar]
- Nunnery G. A., Vasconcelos J. T., Parsons C. H., Salyer G. B., Defoor P. J., Valdez F. R., Galyean M. L. 2007. Effects of source of supplemental zinc on performance and humoral immunity in beef heifers. J. Anim. Sci. 85:2304–2313. doi: 10.2527/jas.2007-0167 [DOI] [PubMed] [Google Scholar]
- Rincker M. J., Hill G. M., Link J. E., Meyer A. M., Rowntree J. E. 2005. Effects of dietary zinc and iron supplementation on mineral excretion, body composition, and mineral status of nursery pigs. J. Anim. Sci. 83:2762–2774. doi: 10.2527/2005.83122762x [DOI] [PubMed] [Google Scholar]
- Salim H. M., Lee H. R., Jo C., Lee S. K., Lee B. D. 2012. Effect of sex and dietary organic zinc on growth performance, carcass traits, tissue mineral content, and blood parameters of broiler chickens. Biol. Trace Elem. Res. 147:120–129. doi: 10.1007/s12011-011-9282-8 [DOI] [PubMed] [Google Scholar]
- Schlegel P., Windisch W. 2006. Bioavailability of zinc glycinate in comparison with zinc sulphate in the presence of dietary phytate in an animal model with Zn labelled rats. J. Anim. Physiol. Anim. Nutr. 90:216–222. doi: 10.1111/j.1439-0396.2005.00583.x [DOI] [PubMed] [Google Scholar]
- Smith J. W., 2nd, Tokach M. D., Goodband R. D., Nelssen J. L., Richert B. T. 1997. Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. J. Anim. Sci. 75:1861–1866. doi: 10.2527/1997.7571861x [DOI] [PubMed] [Google Scholar]
- Spears J. W., Kegley E. B. 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80:2747–2752. doi: 10.2527/2002.80102747x [DOI] [PubMed] [Google Scholar]
- Star L., van der Klis J. D., Rapp C., Ward T. L. 2012. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 91:3115–3120. doi: 10.3382/ps.2012-02314 [DOI] [PubMed] [Google Scholar]
- Swinkels J. W., Kornegay E. T., Verstegen M. W. 1994. Biology of zinc and biological value of dietary organic zinc complexes and chelates. Nutr. Res. Rev. 7:129–149. [DOI] [PubMed] [Google Scholar]
- Towers N. R., Young P. W., Wright D. E. 1981. Effect of zinc supplementation on bovine plasma copper. N. Z. Vet. J. 29:113–114. doi: 10.1080/00480169.1981.34816 [DOI] [PubMed] [Google Scholar]
- Wallwork J. C., Duerre J. A. 1985. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J. Nutr. 115:252–262. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tang J. W., Ma W. Q., Feng J., Feng J. 2010. Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol. Trace Elem. Res. 133:325–334. doi: 10.1007/s12011-009-8437-3 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yu H., Wu X., Zhang T., Cui H., Wan C., Gao X. 2016. Effects of dietary zinc pectin oligosaccharides chelate supplementation on growth performance, nutrient digestibility and tissue zinc concentrations of broilers. Biol. Trace Elem. Res. 173:475–482. doi: 10.1007/s12011-016-0654-y [DOI] [PubMed] [Google Scholar]
- Wieringa F. T., Berger J., Dijkhuizen M. A., Hidayat A., Ninh N. X., Utomo B., Wasantwisut E., Winichagoon P., SEAMTIZI (South-East Asia Multi-country Trial on Iron and Zinc supplementation in Infants) Study Group 2007. Combined iron and zinc supplementation in infants improved iron and zinc status, but interactions reduced efficacy in a multicountry trial in southeast Asia. J. Nutr. 137:466–471. [DOI] [PubMed] [Google Scholar]
- Wright C. L., Spears J. W. 2004. Effect of zinc source and dietary level on zinc metabolism in Holstein calves. J. Dairy Sci. 87:1085–1091. doi: 10.3168/jds.S0022-0302(04)73254-3 [DOI] [PubMed] [Google Scholar]
- Wu X., Gao X., Yang F. 2015a. Effects of dietary copper on organ indexes, tissular Cu, Zn and Fe deposition and fur quality of growing–furring male mink (Mustela vison). J. Anim. Sci. Technol. 57:6. doi: 10.1186/s40781-015-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Liu Z., Guo J., Wan C., Zhang T., Cui H., Yang F., Gao X. 2015b. Influence of dietary zinc and copper on apparent mineral retention and serum biochemical indicators in young male mink (Mustela vison). Biol. Trace Elem. Res. 165:59–66. doi: 10.1007/s12011-014-0220-4 [DOI] [PubMed] [Google Scholar]
- Wu X., Liu Z., Zhang T., Yang Y., Yang F., Gao X. 2014a. Effects of dietary copper on nutrient digestibility, tissular copper deposition and fur quality of growing–furring mink (Mustela vison). Biol. Trace Elem. Res. 158:166–175. doi: 10.1007/s12011-014-9933-7 [DOI] [PubMed] [Google Scholar]
- Wu X., Zhang T., Liu Z., Zheng J., Guo J., Yang F., Gao X. 2014b. Effects of different sources and levels of copper on growth performance, nutrient digestibility, and elemental balance in young female mink (Mustela vison). Biol. Trace Elem. Res. 160:212–221. doi: 10.1007/s12011-014-0054-0 [DOI] [PubMed] [Google Scholar]
- Wu X. Z., Zhang T. T., Guo J. G., Liu Z., Yang F. H., Gao X. H. 2015c. Copper bioavailability, blood parameters, and nutrient balance in mink. J. Anim. Sci. 93:176–184. doi: 10.2527/jas.2014-8026 [DOI] [PubMed] [Google Scholar]
- Yu Y., Lu L., Wang R. L., Xi L., Luo X. G., Liu B. 2010. Effects of zinc source and phytate on zinc absorption by in situ ligated intestinal loops of broilers. Poult. Sci. 89:2157–2165. doi: 10.3382/ps.2009-00486 [DOI] [PubMed] [Google Scholar]
- Zhang H. B., Wang M. S., Wang Z. S., Zhou A. M., Zhang X. M., Dong X. W., Peng Q. H. 2014. Supplementation dietary zinc levels on growth performance, carcass traits, and intramuscular fat deposition in weaned piglets. Biol. Trace Elem. Res. 161:69–77. doi: 10.1007/s12011-014-0078-5 [DOI] [PubMed] [Google Scholar]
- Zhang Y. N., Zhang H. J., Wang J., Yue H. Y., Qi X. L., Wu S. G., Qi G. H. 2017. Effect of dietary supplementation of organic or inorganic zinc on carbonic anhydrase activity in eggshell formation and quality of aged laying hens. Poult. Sci. 96(7):2176–2183. doi: 10.3382/ps/pew490 [DOI] [PubMed] [Google Scholar]


