ABSTRACT
In vitro DM disappearance (IVDMD) and gas production can be used to rapidly estimate apparent total tract digestibility of DM and GE in feed ingredients used in swine diets. However, the accuracy of the system in estimating ME among sources feed ingredients with high content of dietary fiber is not clear. Objectives of this study were 1) to measure IVDMD of feed ingredients with high insoluble fiber content and determine and compare in vitro gas production kinetics from fiber fermentation among wheat straw (WS; 16 sources; 69.0–83.4% NDF), soybean hulls (SBH; 16 sources; 60.9–67.7% NDF), and corn distiller's dried grains with solubles (DDGS; 16 sources; 28.8–44.0% NDF); and 2) to estimate ME contributions resulting from gas production of DDGS. Each 2-g sample was hydrolyzed for 2 h with pepsin and for a subsequent 4 h with pancreatin. Hydrolyzed residues were filtered, washed, dried, weighed, pooled within the same sample, and used for subsequent fermentation using swine fecal inocula. Volume of gas produced was recorded at 11 time points during 72 h of incubation. Parameters of gas production kinetics were calculated using a nonlinear monophasic model, and differences among ingredients were compared using a mixed model. The IVDMD from simulated gastric and small intestinal hydrolysis (IVDMDh) in DDGS (55.7%) was greater (P < 0.05) than that in SBH (19.7%), which was greater (P < 0.05) than that in WS (14.5%). In vitro DM digestibility from simulated large intestine fermentation (IVDMDf) of SBH (68.5%) was greater (P < 0.05) than that of DDGS (52.7%), which was greater than that of WS (41.8%). In vitro DM digestibility from simulated total tract digestion (IVDMDt) was greatest (P < 0.01) in DDGS (79.2%) followed by SBH (74.8%), and both were greater than that in WS (50.2%). The asymptotic gas production (mL/g substrate) was greater (P < 0.05) for SBH (293) than for DDGS (208) and WS (53). There were differences (P < 0.01) in IVDMDh among sources of WS, SBH, and DDGS, whereas IVDMDf and IVDMDt were different (P < 0.01) among sources of SBH but not among sources of DDGS or WS. There were no differences in asymptotic gas production among sources of WS, SBH, or DDGS. In conclusion, the modified 3-step procedure allowed for characterizing the variability of DM digestibility and asymptotic gas production resulting from residue fermentation among WS, SBH, and DDGS and among sources of each ingredient.
Keywords: corn distiller's dried grains with solubles, in vitro asymptotic gas production, in vitro dry matter digestibility, metabolizable energy, soybean hulls, wheat straw
INTRODUCTION
Dynamic estimation of ME content is essential to improve accuracy in diet formulation when using high-fiber ingredients such as corn distiller's dried grains with solubles (DDGS; Kerr et al., 2013; Urriola et al., 2014). Many published ME prediction equations include fiber content as a necessary input because there is considerable variability in total dietary fiber (TDF; 28.6 to 34.9%). There are also differences in apparent total tract digestibility (ATTD; 29.3 to 57%; Urriola et al., 2010). Therefore, further improvement in the accuracy of ME prediction equations for DDGS may be possible by using in vitro procedures to determine the ATTD of TDF and gas production resulting from hindgut fermentation of DDGS (Anguita et al., 2006; NRC, 2012).
A modified, 3-step in vitro procedure has been used to measure IVDMD and total gas production of various feed ingredients for swine (Bindelle et al., 2007; Jha et al., 2015). Although fermentation and gas production has been compared among feed ingredients with variable soluble dietary fiber (SDF) content, no studies have evaluated this for ingredients with high insoluble dietary fiber (IDF) content. Because IDF is typically less fermentable than SDF, fermentation and gas production differences among high-IDF ingredients may be less than previously reported (Jaworski et al., 2015). Likewise, the technique has not been used to compare different sources of the same ingredient. The hypothesis of the present study was that high-fiber ingredients, and sources within each ingredient, differ in in vitro DM digestibility as well as fermentation characteristics and contributions to ME content. Therefore, the first objective of this study was to determine the extent of in vitro enzymatic hydrolysis and fermentation among various sources of wheat straw (WS), soybean hulls (SBH), and corn DDGS. The second objective was to estimate the contribution of energy from gas production among sources of DDGS.
MATERIALS AND METHODS
This study was designed to determine IVDMD and gas production among 3 sources of feed ingredients containing relatively high IDF content. Although not commonly used in practical swine diets, WS and SBH were selected as model ingredients because they contain a high concentration of IDF. The samples of corn DDGS were obtained from Kerr et al. (2013) and used for further comparisons of in vitro and in vivo data.
Sample Collection
A total of 48 samples were collected between May and July 2013, including 16 sources of WS, 16 sources of SBH, and 16 sources of corn DDGS (Table 1). Corn DDGS samples were obtained from a previous study (Kerr et al., 2013) and Highwater Ethanol, LLC (Lamberton, MN).
Table 1.
Analyzed composition of wheat straw (WS), soybean hulls (SBH), and corn distiller's dried grains with solubles (DDGS), DM basis
| Item | Parameter | WS1 | SBH1 | Corn DDGS1 |
|---|---|---|---|---|
| TDF2 | Mean, % | 90.8 | 78.9 | 34.4 |
| Range, % | 81.8–99.8 | 74.6–82.1 | 30.8–44.1 | |
| SD, % | 4.4 | 1.9 | 3.2 | |
| NDF | Mean, % | 76.5 | 65.5 | 34.3 |
| Range, % | 69.0–83.4 | 60.9–67.7 | 28.8–44.0 | |
| SD, % | 3.7 | 1.8 | 4.0 | |
| NDF:TDF ratio | Mean, % | 84.1 | 83.0 | 100.6 |
| Range, % | 81.7–86.6 | 80.6–85.6 | 69.2–127.3 | |
| SD, % | 1.4 | 1.2 | 13.7 | |
| ADF | Mean, % | 54.9 | 49.6 | 11.2 |
| Range, % | 51.5–59.7 | 47.8–51.9 | 8.6–15.0 | |
| SD, % | 1.8 | 1.2 | 2.0 | |
| Hemicellulose3 | Mean, % | 21.6 | 15.9 | 23.1 |
| Range, % | 15.1–30.2 | 12.3–19.0 | 15.5–30.0 | |
| SD, % | 3.7 | 1.4 | 3.3 |
Data represent 16 sources each of WS, SBH, and corn DDGS. All the analysis is single analysis.
TDF = total dietary fiber.
Calculated as NDF − ADF.
Enzymatic Hydrolysis
All samples were ground to pass through a 1-mm mesh screen. The first 2 steps of the pepsin and pancreatin hydrolysis followed the procedures developed by Boisen and Fernández (1997), and subsequent steps followed modifications by Jha et al. (2011a,b). Briefly, 2 g of each sample (6 batches, with 1 replicate per batch) were weighed into a 500-mL conical flask and incubated at 39°C in a water bath. One hundred milliliters of phosphate buffer solution (0.1 M 7:1 KH2PO4:Na2HPO4, pH 6.0) and 40 mL 0.2 M HCl solution (pH 2.0) were added to each replicate. The pH was adjusted to 2.0 by adding 1 M HCl or 1 M NaOH. Two milliliters of 5 mg/mL chloramphenicol (C0378; Sigma-Aldrich Corp., St. Louis, MO) solution (dissolved in ethanol) was added to prevent bacterial growth during hydrolysis. Each replicate was treated with 4 mL of 100 mg/mL fresh porcine pepsin (P7000, 421 pepsin units/mg solids; Sigma-Aldrich Corp.) solution (dissolved in 0.2 M HCl) at 39°C and incubated in a water bath for 2 h, and all the flasks were shaken gently by hand for 5 s every 15 min. Subsequently, 40 mL of 0.2 M phosphate buffer (7:1 KH2PO4:Na2HPO4, pH 6.8) and 20 mL of 0.6 M NaOH were added to each flask. The pH was adjusted to 6.8 with 1 M HCl or 1 M NaOH, and 4 mL of 100 mg/mL fresh porcine pancreatin (P1750, 4 times the specifications of the United States Pharmacopeia; Sigma-Aldrich Corp.) solution (dissolved in 0.2 M phosphate buffer) was added. The hydrolysis continued for 4 h under the same conditions as used for pepsin hydrolysis.
After enzymatic hydrolysis, residues were collected by filtration (40 µm filter paper; VWR International, Radnor, PA); washed with distilled water, ethanol (2 × 20 mL, 95%), and acetone (2 × 20 mL, 99.5%); dried for 72 h at 55°C; and weighed for determination of IVDMD. To obtain sufficient residues for the subsequent in vitro fermentation, 4 to 8 replicates of the enzymatic hydrolysis procedure were conducted depending on the amount of residue remaining (Table 2).
Table 2.
Number of in vitro batches and replicates of the modified 3-step procedure for wheat straw (WS), soybean hulls (SBH), corn distiller's dried grains with solubles (DDGS), and blanks
| 2-step enzymatic hydrolysis | Fermentation | |||
|---|---|---|---|---|
| Item | Batch1 | Replicates per batch | Batch | Replicates per batch |
| WS | 4–6 | 1 | 3 | 2 |
| SBH | 5–6 | 1 | 3 | 2 |
| DDGS | 6–8 | 1 | 3 | 2 |
| Blank | 3 | 2 | ||
The number of in vitro batches was determined by the amount of residue leftover from each sample, with the goal of obtaining sufficient amount of residue for the following fermentation step.
In Vitro Fermentation
The rate and amount of in vitro fermentation of the hydrolyzed residues was assessed using a cumulative gas production technique (Bindelle et al., 2007, 2009; Jha et al., 2015). Hydrolyzed residues from enzymatic hydrolysis of the same sample were pooled for in vitro fermentation. Blank inocula without substrates were used as controls. Samples were analyzed in 3 batches, with 2 replicates each of blanks and hydrolyzed residues of WS, SBH, and DDGS per batch (Table 2). A greater number of batches were conducted for DDGS and SBH samples to obtain sufficient residue for subsequent analyses. About 0.2 g of each mixed hydrolyzed residue was weighed, and all blanks along with hydrolyzed residues of WS, SBH, and DDGS were incubated at 39°C in a 125-mL rubber-stoppered serum bottle with 30 mL buffer solution (included macro- and microminerals; Menke and Steingass, 1988) and fecal inoculum. Fecal inoculum was obtained from 5 growing pigs (19 to 21 wk of age and 68.5 to 83.4 kg BW; Hampshire × Yorkshire) from the University of Minnesota St. Paul Campus Swine Teaching and Research Facility. Pigs had the same genetic background and were fed a standard commercial corn–soybean meal diet without antibiotics (Maverick Nutrition Inc., Austin, MN). Fecal samples were randomly collected from 3 out of the 5 pigs immediately after the pigs defecated. Feces were immediately pooled and placed in air tight plastic bags after collection, all air was removed, and bags were sealed, kept at 39°C, and delivered to the laboratory within 30 min after collection. The inoculum was prepared by diluting blended feces in an inoculation solution composed of distilled water (474 mL/L), trace mineral solution (0.12 mL/L containing 132 g/L of CaCl2, 100 g/L of MnCl3·4H2O, 10 g/L of CoCl2·6H2O, and 80 g/L of FeCl3·6H2O), in vitro buffer solution (237 mL/L containing 4.0 g/L of NH4HCO3 and 35 g/L of NaHCO3), macromineral solution (237 mL/L composed of 5.7 g/L of Na2HPO4, 6.2 g/L of KH2PO4, 0.583 g/L of MgSO4·7H2O, and 2.22 g/L of NaCl), and resazurin (blue dye, 0.1% wt/vol solution; 1.22 mL/L) and filtered through 4 layers of cheesecloth. The final inoculum concentration was 0.05 g feces/mL of buffer. Thirty milliliters of inoculum was transferred into bottles containing the hydrolyzed residues, and the bottles were sealed with rubber stoppers before being placed in a 39°C water bath for incubation. Anaerobiosis was maintained in the inoculation solution by the addition of a reducing solution (47.5 mL/L of distilled water, 2 mL/L of 1 M NaOH, and 335 mg/L of Na2S) and CO2 (Jha et al., 2011a,b, 2015).
Gas produced during fermentation was measured at 2, 5, 8, 12, 16, 20, 24, 30, 36, 48, and 72 h through an inverted 25-mL burette with its stopcock end attached to vacuum and its open end submerged into a 39°C water bath. Before assembling the burette apparatus, the headspace volume of the burette was determined. To measure gas volume at each time point, the inverted burette was filled with water to remove the air and then the serum bottle was quickly transferred from the incubation water bath to the water bath with the measuring burette and a 20-gauge needle was inserted through the rubber stopper. At each gas measurement time point, the operator opened the valve to release all the gas into the burette and immediately recorded the volume displaced by the gas produced in the bottle using burette calibration marks. Once the measurement was recorded, the bottles were immediately transferred back into the incubating water bath. After in vitro fermentation, residues were collected by filtration, washed, dried, and weighed following the same procedures described for the hydrolyzed residues.
Physicochemical Analysis
All feed ingredient samples were analyzed at the University of Missouri Agricultural Experiment Station Chemical Laboratories (Columbia, MO). Chemical analyses were performed according to standard AOAC International (Horwitz, 2006) procedures using the following methods: DM (method 930.15), ADF (method 973.18), NDF (method 2002.04), and TDF (method 991.43), and the concentration of hemicellulose was calculated by percent NDF − percent ADF.
Calculations
Hemicellulose.
Hemicellulose (%) = NDF (%) − ADF (%).
Total Feces Needed to Prepare the Inoculum Per Batch.
Feces (g) = 30 mL × number of samples × number of replicates per batch × 0.05 g/mL.
Gas Volume Released at Each Time Point.
in which V is expressed in milliliters, Vh is the volume of the burette headspace, and Vr is the reading volume record.
in which 0 < V < Vh and Vr was measured the same as the headspace volume.
in which V > Vh + 25, and V needed to be measured at least 2 times. Although V rarely exceeded burette capacity, it did occur for a few samples. Under the conditions when the observer noted that the gas volume was approaching the open end of the burette and more gas was being produced, the observer stopped the valve and recorded the volume as Vr1. Then the burette was refilled with water and subsequent gas volume produced was measured and recorded as Vr2. The same process continued for the nth time until all gas produced was measured and recorded.
In Vitro DM Digestibility from Simulated Gastric and Small Intestinal Hydrolysis.
The IVDMD from simulated gastric and small intestinal hydrolysis (IVDMDh; %) was calculated as follows:
IVDMDh = [(dry weight of the sample before hydrolysis – dry weight of residues)/dry weight of the sample before hydrolysis] × 100.
In Vitro DM Digestibility from Simulated Large Intestine Fermentation.
The IVDMD from simulated large intestine fermentation (IVDMDf; %) was calculated as follows:
IVDMDf = [(dry weight of hydrolyzed residues – dry weight of the residues after fermentation)/dry weight of hydrolyzed residues] × 100.
In Vitro DM Digestibility from Simulated Total Tract Digestion.
The in vitro DM digestibility from simulated total tract digestion (IVDMDt; %) was calculated as follows:
Kinetics of Gas Production.
Gas accumulation curves recorded during the 72 h of fermentation were modified according to a monophasic model (Groot et al., 1996): G = A/[1 + (BC/tC)], in which G (mL/g DM substrate) denotes the amount of gas produced per gram of DM incubated, A (mL/g DM) represents the asymptotic gas production, B (h) is the time after incubation at which half of the asymptotic amount of gas has been produced, C is a constant determining the sharpness of the switching characteristic of the profile, and t is the incubation time.
When calculating IVDMDh, IVDMDt, and accumulated gas production volume, all data were corrected by subtracting blank values from observed values.
Volatile Fatty Acids and Energy Production.
The amount of VFA and energy production was calculated from referenced VFA production (Jha et al., 2015). Because there is no reference VFA data from the modified 3-step procedure of WS and SBH, we were able to calculate VFA and energy production for only corn DDGS samples.
Volatile fatty acid production (mmol) = (A/200 mL) × VFAr (mmol), in which 200 mL represents the maximum gas production volume and the VFAr is referenced VFA production (Jha et al., 2015), where acetate was 3.92 mmol, propionate was 1.61 mmol, and butyrate was 0.57 mmol per gram DM of fermented substrate.
Total energy production from VFA (kcal) = (acetate (mmol)) × 209.6 cal/mmol + (propionate (mmol)) × 366.4 cal/mmol + (butyrate (mmol)) × 522.2 cal/mmol, in which 209.6, 366.4, and 522.2 cal/mmol represent the amount of energy produced from acetate, propionate, and butyrate, respectively (Christensen et al., 1999).
For corn DDGS, the total energy produced from VFA was calculated using the following equation: Energy (cal/g DM) = total energy production from VFA (kcal) × (1 − IVDMDh).
Statistical Analyses
The kinetics of gas production parameters were modeled using PROC NLIN of SAS 9.3 (SAS Inst. Inc., Cary, NC). The IVDMD and fitted gas production kinetic parameters were analyzed using PROC MIXED of SAS 9.3.
Comparisons among the 3 high-fiber ingredients (WS, SBH, and corn DDGS) as well as the comparisons within the 16 sources nested under each ingredient were analyzed using the following linear additive model:
in which y was the parameter to be tested (IVDMDh, IVDMDf, and gas production kinetic parameters A, B, and C), µ was the overall population mean, τi was the effect of the ith (i = 1, 2, 3) ingredient, αj(i) was the effect of the jth (j = 1, 2, 3, …, 16) source nested under each ingredient, βk was the effect of batch (k = 6 for IVDMDh and k = 3 for IVDMDf, IVDMDt, A, B, and C), and Eijk = experiment error. Ingredient (n = 3) and source (n = 16) nested under each ingredient were fixed factors, and the batch was a random factor. The least squares means within 16 sources nested under each ingredient were analyzed by the slice effect. Differences were considered significant when P ≤ 0.05 and a trend when 0.05 < P < 0.1.
Correlations between NDF, ADF, or TDF and IVDMD and asymptotic gas production were analyzed using PROC CORR in SAS 9.3. Data were separated into 4 sets of variables: 16 sources of each high-fiber ingredient (WS, SBH, and DDGS) and all 48 sources of the 3 high-fiber ingredients (WS + SBH + DDGS).
RESULTS AND DISCUSSION
Variability in Fiber Composition among Ingredients
The concentration of TDF was greatest in WS, followed by SBH, and was lowest for DDGS (Table 1). Among the sources of each ingredient, the concentration of TDF varied less among sources of WS (CV = 4.9) and SBH (CV = 2.5) than among sources of DDGS (CV = 9.3). The concentration of NDF was less than TDF for WS and SBH but not for DDGS. The ratio between NDF and TDF was 0.84 and 0.83 for WS and SBH and was 1.00 for DDGS. In SBH, hemicelluloses accounted for a smaller fraction of NDF than in DDGS.
Variability in In vitro DM digestibility among Ingredients
The IVDMDh of corn DDGS was greater (P < 0.01) than that of SBH, which was greater (P < 0.01) than that of WS (Table 3). The IVDMDf of SBH was greater (P < 0.01) than that of DDGS, which was greater (P < 0.01) than that of WS. Therefore, the IVDMDt of DDGS was greater (P < 0.01) than that of SBH, which was also greater (P < 0.01) than that of WS. There were also differences (P < 0.01) in IVDMDh within sources of WS, SBH, and DDGS, and the CV for IVDMDh among sources of WS and SBH was greater than that of DDGS. There were no differences in IVDMDf within sources of WS or within sources DDGS, but there were differences among sources of SBH (P = 0.01).
Table 3.
Variability in IVDMD of wheat straw (WS), soybean hulls (SBH), and corn distiller's dried grains with solubles (DDGS)1
| Item | Parameter | WS | SBH | Corn DDGS | P-value2 |
|---|---|---|---|---|---|
| IVDMDh3 % | Mean, % | 14.5a | 19.7b | 55.7c | <0.01 |
| Range, % | 11.2–18.3 | 16.7–23.0 | 45.3–63.2 | ||
| SD,% | 1.5 | 4.1 | 3.5 | ||
| CV, % | 10.5 | 20.9 | 6.3 | ||
| P-value4 | <0.01 | <0.01 | <0.01 | ||
| IVDMDf5 % | Mean, % | 41.8a | 68.5c | 52.7b | <0.01 |
| Range, % | 36.8–48.0 | 49.0–83.2 | 41.4–64.2 | ||
| SD,% | 3.5 | 8.2 | 5.9 | ||
| CV, % | 8.4 | 11.9 | 11.3 | ||
| P-value | 0.98 | 0.01 | 0.41 | ||
| IVDMDt6 % | Mean, % | 50.2a | 74.8b | 79.2c | <0.01 |
| Range, % | 44.6–56.3 | 59.7–86.7 | 76.0–83.5 | ||
| SD,% | 3.3 | 6.4 | 2.0 | ||
| CV, % | 6.5 | 8.5 | 2.5 | ||
| P-value | 0.86 | <0.01 | 1.00 |
Means within rows with different superscripts differ (P < 0.05).
Data represent 16 sources each of WS, SBH, and corn DDGS.
Refers to the comparison of the least squares mean value among WS, SBH, and corn DDGS.
IVDMDh = in vitro DM digestibility from simulated gastric and small intestinal hydrolysis.
Refers to the comparison of the least squares mean value within 16 sources of WS, SBH, or DDGS.
IVDMDf = in vitro DM digestibility from simulated large intestine fermentation.
IVDMDt = in vitro DM digestibility from simulated total tract digestion.
Kinetics of Gas Production During In Vitro Fermentation
The asymptotic gas production of SBH was greater (P < 0.01) than that of DDGS, which was greater (P < 0.01) than that of WS (Table 4). Consistent with this observation, time to half asymptote (B) was reached faster (P < 0.01) among samples of SBH than DDGS and WS, but there were no differences between WS and DDGS.
Table 4.
Differences in fitted kinetic parameters on the gas accumulation recorded for wheat straw (WS), soybean hulls (SBH), and corn distiller's dried grains with solubles (DDGS) during in vitro fermentation
| Item | Parameter | WS1 | SBH1 | Corn DDGS1 | P-value2 |
|---|---|---|---|---|---|
| A,3 mL/g substrate | Mean | 53.0c | 293.0a | 208.0b | <0.01 |
| Range | 32.0–80.0 | 262–324 | 183–246 | ||
| SD | 13.0 | 15.0 | 21.0 | ||
| CV, % | 24.3 | 5.2 | 9.8 | ||
| P-value4 | 1.00 | 0.74 | 0.37 | ||
| B,5 h | Mean | 22.3a | 14.5b | 24.4a | <0.01 |
| Range | 12.1–40.2 | 13.3–15.9 | 17.9–33.8 | ||
| SD | 7.8 | 0.7 | 4.5 | ||
| CV, % | 35.1 | 4.5 | 18.5 | ||
| P-value | <0.01 | 1.00 | 0.40 | ||
| C,6 dimensionless | Mean | 1.87c | 2.54a | 1.30b | <0.01 |
| Range | 1.14–3.40 | 2.36–2.74 | 1.10–1.58 | ||
| SD | 0.62 | 0.1 | 0.17 | ||
| CV, % | 33.3 | 4.1 | 12.8 | ||
| P-value | 0.01 | 1.00 | 1.00 |
Means within rows with different superscripts differ (P ≤ 0.05).
Data represent 16 sources each of WS, SBH, and corn DDGS.
Refers to the comparison among WS, SBH, and corn DDGS.
A = asymptotic gas production, expressed as milliliters per gram of DM of substrate.
Refers to the comparison within 16 sources of WS, SBH, or corn DDGS.
B = the time (h) after incubation at which half of the asymptotic amount of gas has been produced.
C = a constant that determines the sharpness of the switching characteristic of the gas profile.
Correlations of Fiber Composition, In vitro DM digestibility, and Asymptotic Gas Production
A greater concentration of fiber in each ingredient resulted in less IVDMDh, as measured by TDF (r = −0.99), NDF (r = −0.99), or ADF (r = −0.99; Table 5). However, the concentration of ADF and NDF, but not TDF, was negatively correlated with IVDMDh in DDGS. The concentrations of TDF, NDF, and ADF were not correlated with disappearance of DM during in vitro fermentation (IVDMDf) or asymptotic gas production, but the correlations of IVDMDf and asymptotic gas production with concentrations of dietary fiber depended on the specific type of ingredient analyzed. For example, IVDMDf was not correlated with concentration of TDF, ADF, or NDF of individual ingredients (WS, SBH, or DDGS). The asymptotic gas production for IVDMDf was positively correlated with asymptotic gas production of WS + SBH + DDGS.
Table 5.
Correlations among fiber composition, in vitro DM digestibility (IVDMD), and asymptotic gas production in 3 high-fiber ingredients: wheat straw (WS), soybean hulls (SBH), and corn distiller's dried grains with solubles (DDGS)1
| Item | Ingredients | TDF2 | NDF | ADF | IVDMDf,3 % |
|---|---|---|---|---|---|
| IVDMDh4 | WS | −0.79* | −0.82* | −0.42 | |
| SBH | −0.65* | −0.74* | −0.78* | ||
| Corn DDGS | −0.22 | −0.72* | −0.73* | ||
| WS + SBH + corn DDGS | −0.98* | −0.98* | −0.99* | ||
| IVDMDf | WS | −0.08 | −0.04 | 0.20 | |
| SBH | 0.23 | 0.11 | 0.02 | ||
| Corn DDGS | −0.14 | 0.41 | 0.48† | ||
| WS + SBH + corn DDGS | −0.07 | −0.11 | 0.00 | ||
| A5 | WS | −0.15 | −0.14 | 0.15 | 0.27 |
| SBH | 0.26 | 0.25 | 0.44† | −0.01 | |
| Corn DDGS | −0.14 | 0.41 | 0.48† | −0.64 | |
| WS + SBH + corn DDGS | −0.34* | −0.40* | −0.27† | 0.84* |
Pearson correlation coefficients (r).
TDF = total dietary fiber.
IVDMDf = in vitro DM digestibility from simulated large intestine fermentation.
IVDMDh = in vitro DM digestibility from simulated gastric and small intestinal hydrolysis.
A = asymptotic gas production, expressed as milliliters per gram of DM of substrate.
*Significant correlation (P ≤ 0.05).
†Correlation trend (0.05 < P < 0.10).
Variability of VFA and Energy Production of Distiller's Dried Grains with Solubles
Calculated VFA production ranged from 5.6 to 7.5 mmol/g DM of fermented DDGS, with a CV of 9.5%. The energy calculated from the VFA produced was 17.2 to 27.3% of DE (NRC, 2012; Kerr et al., 2013) and 18.3 to 28.9% of ME (NRC, 2012; Kerr et al., 2013) in DDGS samples, with a CV of 14.1%.
The hypothesis of this experiment was that in vitro hydrolysis and fermentation of DM in ingredients with insoluble fiber content are different not only among ingredients but also within sources of the same ingredient. Data from this experiment confirmed the hypothesis that in vitro digestibility of DM at the hydrolysis step differs among feed ingredients, which is partially due to differences in the concentration of fiber. In contrast to IVDMDh, IVDMDf was not dependent on the concentration of fiber in ingredients. Ingredients such as WS and SBH have a high concentration of fiber, but fermentability of this fiber is much greater in SBH than in WS and is intermediate in DDGS. This observation suggests that factors (e.g., sugar composition, structural composition, concentration of lignin) other than fiber concentration affect fiber fermentability (Gutierrez et al., 2014).
The value of fitted kinetic parameters or in vitro DM digestibility of high-fiber ingredients appears to depend on the type of feed ingredient being evaluated. Among sources of WS, there was a smaller CV of IVDMDf than the CV that was observed among sources of SBH or DDGS.
Fiber Solubility
There are different analytical methods to measure dietary fiber. Neutral detergent fiber represents the sum of cellulose, hemicelluloses, and lignin, whereas ADF represents the sum of cellulose and lignin, but neither of these methods includes soluble fiber fractions such as pectins, gums, and glucans (Grieshop et al., 2001). However, both soluble and insoluble fiber fractions can be measured using the TDF procedure (method 991.43; Horwitz, 2006). According to studies reported in the literature, fiber composition in WS, SBH, and DDGS is mainly insoluble. However, WS, SBH, and DDGS vary in their relative concentrations of insoluble fiber, where WS contains 98 to 99% IDF (Panthapulakkal et al., 2006; Alemdar and Sain, 2008), corn DDGS contains 95 to 100% IDF (Urriola et al., 2010), and both of these sources contain more IDF than SBH (83 to 94%; Cole et al., 1999). It has been suggested that the greater the concentration of IDF in an ingredient source, the less fermentable the fiber will be. The ATTD of SDF is greater than that of IDF among sources of corn DDGS (Urriola et al., 2010). The greater amount of SDF in SBH may contribute to the greater IVDMDf than that observed in DDGS or WS. However, even at the greatest reported concentration, SDF in SBH made up only about 20% of the total fiber content. Consequently, we speculate that a significant portion of IDF in SBH should be fermentable to achieve the observed IVDMDf. In contrast, SDF represents less than 10% of the total fiber in corn DDGS (Urriola et al., 2010), and a significant portion of IDF in corn DDGS must be fermentable to achieve the observed IVDMDf. These observations suggest that the concentration of fiber in a feed ingredient or subsequent analysis of IDF and SDF concentrations are insufficient to predict fermentability of fiber in the large intestine of pigs and that other measurements such as viscosity, water binding capacity, and extent of fermentation are necessary.
Physical Structure and Lignin Content of Fiber
The extent of fiber fermentation is quite variable among ingredients and depends on the accessibility of fiber to the microbial population in the hindgut (Oakenfull, 2001). Lignification of fiber is an important structural factor that affects fiber accessibility to fermentation (Jung et al., 1997). The concentration of lignin has a negative effect on nutrient digestibility because it is not digested in the gastrointestinal tract and its structure traps other nutrients by preventing access to and contact by endogenous digestive enzymes (Jung et al., 1997). Therefore, we speculate that differences in lignin concentration may partially explain differences in fiber fermentability among ingredients with relatively high IDF concentrations such as WS, DDGS, or SBH. The concentration of lignin in WS (15.9%; Tamaki and Mazza, 2011) is greater than in SBH (2.6% lignin; Matkovic et al., 2010) and corn DDGS (2.6% lignin; NRC, 2012). As a result, the greater concentration of lignin in WS may partially explain the lesser IVDMDf in WS compared with that in DDGS or SBH but not the difference in IVDMDf between SBH and DDGS.
Volatile Fatty Acids and Energy Produced from Dietary Fiber Fermentation
Volatile fatty acids produced during fermentation are metabolized to supplying energy to the host (Bergman, 1990). The average supply of energy from VFA, which contributes toward meeting the maintenance energy requirement of pigs, has been reported to be 15 to 24% (Dierick et al., 1989; Yen et al., 1991). To test the extent that VFA can contribute to ME, we used data on VFA production during in vitro fermentation because we used experimental conditions comparable to those reported by Jha et al. (2015) for corn DDGS fermentation. Specifically, the NDF content in corn DDGS used in our experiment was 34% compared with 32% NDF in the corn DDGS source evaluated by Jha et al. (2015). As a result, our IVDMDh was comparable (55.7%) to the value (59.6%) reported by Jha et al. (2015). Likewise, the IVDMDf (52.7%) and asymptotic gas production (208 mL/g DM) of corn DDGS in our study were similar to the IVDMDf (53.4%) and asymptotic gas production (200 mL/g DM) reported by Jha et al. (2015).
From our data, we calculated the amount of energy produced per gram of DM in DDGS and the ratio of energy produced from VFA relative to DE and ME in DDGS (Table 6). The range of the VFA to DE or ME ratios observed in our study are similar to those observed in other studies (Anguita et al., 2006; Iyayi and Adeola, 2015). In vitro studies have shown that diets containing 24% nonstarch polysaccharides from barley and sugar beet pulp contributed 17.6% of the energy available for DE (Anguita et al., 2006) and diets containing 15% NDF from wheat bran contributed 24.7% of available energy from fermentation to DE (Iyayi and Adeola, 2015). Therefore, energy produced from fiber fermentation of high-fiber ingredients in the large intestine can contribute a significant portion of DE.
Table 6.
Variability of VFA and energy production during in vitro fermentation of corn distiller's dried grains with solubles (DDGS) using pig fecal inocula
| Item | Mean | Range | SD |
|---|---|---|---|
| VFA production, mmol/g DM of fermented substrate1 | 6.4 | 5.6–7.5 | 0.6 |
| Energy production, cal/g DM of fermented substrate2 | 1,781.4 | 1,561.7–2,106.3 | 169.6 |
| Energy production from corn DDGS, cal/g DM | 789.0 | 627.6–962.1 | 106.3 |
| Energy from VFA: DE3 of corn DDGS, % | 21.7 | 17.2–27.3 | 3.1 |
| Energy from VFA: ME3 of corn DDGS, % | 23.1 | 18.3–28.9 | 3.3 |
Fermented substrate was the hydrolyzed residue from pepsin and pancreatin hydrolysis (Jha et al., 2015).
Fermented substrate was the hydrolyzed residue from pepsin and pancreatin hydrolysis (Christensen et al., 1999).
DE and ME values from 12 samples were previously determined by Kerr et al. (2013), and another 4 samples were based on published values from the Nutrient Requirements of Swine (NRC, 2012) for corn DDGS containing > 6% and < 9% ether extract (3,396 kcal ME/kg and 3,582 kcal DE/kg) and corn DDGS containing > 10% ether extract (3,434 kcal ME/kg and 3,620 kcal DE/kg).
There are several factors that affect the proportion of energy that fiber fermentation contributes to DE. These factors include 1) the extent of fermentation or overall degradation of fiber, 2) the composition of VFA from fermentation, and 3) DM disappearance in the large intestine, which not only includes dietary fiber but also includes fermentable protein. Data from this study show that the greatest source of variation in the contribution of hindgut fermentation toward DE is due to the extent of fiber fermentation. This is observed in the large difference in gas production and in vitro disappearance of DM during fermentation among WS, DDGS, and SBH and is consistent with results reported in previous studies (Anguita et al., 2006; Jha et al., 2011b). However, the accuracy of using this modified 3-step procedure to estimate energy contributions to ME needs further refinement by conducting studies where the actual production of VFA and CP disappearance during in vitro fermentation are measured.
Necessity of Dynamic Prediction of Digestible Dietary Fiber
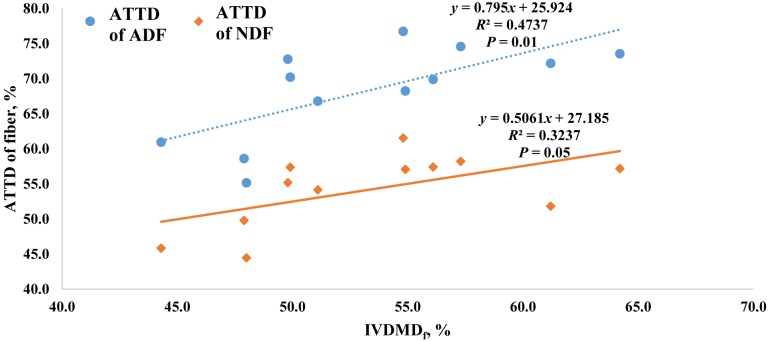
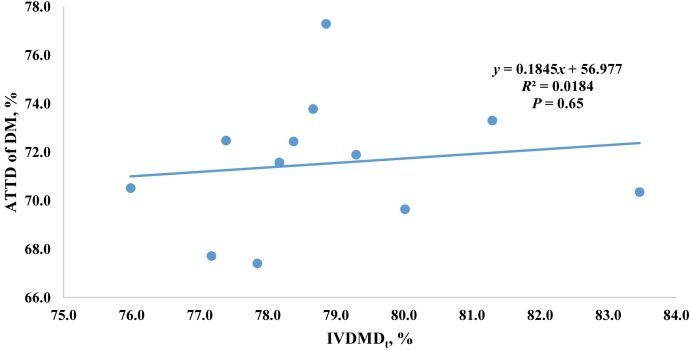
When developing energy prediction equations, the chemical composition (e.g., NDF, ADF, TDF) and concentrations only partially represent the proportion of the nutrients digested and absorbed in the intestine (NRC, 2012). However, in vivo animal nutrient digestibility experiments are time consuming and expensive. Therefore, using the modified 3-step in vitro digestibility procedure is a promising method that could replace the need for in vivo nutrient digestibility studies to provide inputs in energy prediction equations for high-fiber ingredients if these inputs are similar to in vivo digestibility estimates. Jaworski et al. (2015) reported that the total concentration of nonstarch polysaccharides was correlated to in vitro ATTD of DM in corn, wheat, sorghum, and corresponding coproducts. However, less is known about the accuracy of using this procedure to predict in vivo ATTD of DM and fiber among sources of corn DDGS. Therefore, we conducted a regression analysis of ATTD of NDF and ADF using 12 DDGS samples from Kerr et al. (2013) relative to IVDMDf (Fig. 1) as well as a regression of ATTD of DM (Kerr et al., 2013) with IVDMDt (Fig. 2). Both ATTD of NDF and ATTD of ADF were correlated with in vitro DM digestibility from the large intestine (IVDMDf), but the R2 were less than desired to obtain accurate prediction. These relatively low R2 may be attributed to the lack of accurate prediction of ATTD of DM by using IVDMD from the current study.
Figure 1.
Prediction of the apparent total tract digestibility (ATTD) of ADF or NDF among 12 sources of corn distiller's dried grains with solubles from in vitro DM disappearance during fermentation with fecal inoculum from growing pigs (in vitro DM digestibility from simulated large intestine fermentation [IVDMDf]).
Figure 2.
Prediction of the apparent total tract digestibility (ATTD) of DM among 12 sources of corn distiller's dried grains with solubles from in vitro DM disappearance during fermentation with fecal inoculum from growing pigs (in vitro DM digestibility from simulated large intestine fermentation [IVDMDf]).
In conclusion, use of the modified 3-step procedure allowed for characterizing the variability of DM digestibility and asymptotic gas production resulting from residue fermentation among feed ingredients with a high concentration of IDF such as WS, SBH, and DDGS as well as among sources of each ingredient. The accuracy of the procedure in determining DM digestibility of other high-fiber feed ingredients, as well as the accuracy in predicting ME among fibrous feed ingredients, requires further investigation.
FOOTNOTES
Financial support was provided by the National Pork Board (number 13-014).
LITERATURE CITED
- Alemdar A., Sain M. 2008. Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls. Bioresour. Technol. 99:1664–1671. doi: 10.1016/j.biortech.2007.04.029 [DOI] [PubMed] [Google Scholar]
- Anguita M., Canibe N., Pérez J. F., Jensen B. B. 2006. Influence of the amount of dietary fiber on the available energy from hindgut fermentation in growing pigs: Use of cannulated pigs and in vitro fermentation. J. Anim. Sci. 84:2766–2778. doi: 10.2527/jas.2005-212 [DOI] [PubMed] [Google Scholar]
- Bergman E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. [DOI] [PubMed] [Google Scholar]
- Bindelle J., Buldgen A., Delacollette M., Wavreille J., Agneessens R., Destain J. P., Leterme P. 2009. Influence of source and concentrations of dietary fiber on in vivo nitrogen excretion pathways in pigs as reflected by in vitro fermentation and nitrogen incorporation by fecal bacteria. J. Anim. Sci. 87:583–593. doi: 10.2527/jas.2007-0717 [DOI] [PubMed] [Google Scholar]
- Bindelle J., Buldgen A., Lambotte D., Wavreille J., Leterme P. 2007. Effect of pig faecal donor and of pig diet composition on in vitro fermentation of sugar beet pulp. Anim. Feed Sci. Technol. 132:212–226. doi: 10.1016/j.anifeedsci.2006.03.010 [DOI] [Google Scholar]
- Boisen S., Fernández J. A. 1997. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 68:277–286. doi: 10.1016/S0377-8401(97)00058-8 [DOI] [Google Scholar]
- Christensen D. N., Bach Knudsen K. E., Wolstrup J., Jensen B. B. 1999. Integration of ileum cannulated pigs and in vitro fermentation to quantify the effect of diet composition on the amount of short-chain fatty acids available from fermentation in the large intestine. J. Sci. Food Agric. 79:755–762. doi: 10.1002/(SICI)1097-0010(199904)79:5<755::AID-JSFA248>3.0.CO;2–2 [DOI] [Google Scholar]
- Cole J. T., Fahey G. C., Jr, Merchen N. R., Patil A. R., Murray S. M., Hussein H. S., Brent J. L., Jr 1999. Soybean hulls as a dietary fiber source for dogs. J. Anim. Sci. 77:917–924. [DOI] [PubMed] [Google Scholar]
- Dierick N. A., Vervaeke I. J., Demeyer D. I., Decuypere J. A. 1989. Approach to the energetic importance of fibre digestion in pigs. I. Importance of fermentation in the overall energy supply. Anim. Feed Sci. Technol. 23:141–167. doi: 10.1016/0377-8401(89)90095-3 [DOI] [Google Scholar]
- Grieshop C. M., Reese D. E., Fahey G. C., Jr 2001. Nonstarch polysaccharides and oligosaccharides in swine nutrition. In: Lewis A. J., Southern L. L. editors, Swine nutrition. 2nd ed.CRC Press, Boca Raton, Florida: p. 107–130. [Google Scholar]
- Groot J. C. J., Cone J. W., Williams B. A., Debersaques F. M. A., Lantinga E. A. 1996. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 64:77–89. doi: 10.1016/S0377-8401(96)01012-7 [DOI] [Google Scholar]
- Gutierrez N. A., Serão N. V. L., Kerr B. J., Zijlstra R. T., Patience J. F. 2014. Relationships among dietary fiber components and the digestibility of energy, dietary fiber, and amino acids and energy content of nine corn coproducts fed to growing pigs. J. Anim. Sci. 92:4505–4517. doi: 10.2527/jas.2013-7265 [DOI] [PubMed] [Google Scholar]
- Horwitz W. editor. 2006. Official methods of analysis of AOAC International. 18th ed.AOAC Int., Gaithersburg, MD. [Google Scholar]
- Iyayi E. A., Adeola O. 2015. Quantification of short-chain fatty acids and energy production from hindgut fermentation in cannulated pigs fed graded levels of wheat bran. J. Anim. Sci. 93:4781–4787. doi: 10.2527/jas.2015-9081 [DOI] [PubMed] [Google Scholar]
- Jaworski N. W., Lærke H. N., Bach Knudsen K. E., Stein H. H. 2015. Carbohydrate composition and in vitro dry matter digestibility of dry matter and non-starch polysaccharides in corn, sorghum, and wheat and co-products from these grains. J. Anim. Sci. 93:1103–1113. doi: 10.2527/jas.2014-8147 [DOI] [PubMed] [Google Scholar]
- Jha R., Bindelle J., Rossnagel B., Van Kessel A., Leterme P. 2011a. In vitro evaluation of the fermentation characteristics of the carbohydrate fractions of hulless barley and other cereals in the gastrointestinal tract of pigs. Anim. Feed Sci. Technol. 163:185–193. doi: 10.1016/j.anifeedsci.2010.10.006 [DOI] [Google Scholar]
- Jha R., Bindelle J., Van Kessel A., Leterme P. 2011b. In vitro fibre fermentation of feed ingredients with varying fermentable carbohydrate and protein levels and protein synthesis by colonic bacteria isolated from pigs. Anim. Feed Sci. Technol. 165:191–200. doi: 10.1016/j.anifeedsci.2010.10.002 [DOI] [Google Scholar]
- Jha R., Woyengo T. A., Li J., Bedford M. R., Vasanthan T., Zijlstra R. T. 2015. Enzymes enhance degradation of the fiber-starch-protein matrix of distillers dried grains with solubles as revealed by a porcine in vitro fermentation model and microscopy. J. Anim. Sci. 93:1039–1051. doi: 10.2527/jas.2014-7910 [DOI] [PubMed] [Google Scholar]
- Jung H. G., Mertens D. R., Payne A. J. 1997. Correlation of acid detergent lignin and Klason lignin with digestibility of forage dry matter and neutral detergent fiber. J. Dairy Sci. 80:1622–1628. doi: 10.3168/jds.S0022-0302(97)76093-4 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Dozier W. A., III, Shurson G. C. 2013. Effects of reduced-oil corn distillers dried grains with solubles composition on digestible and metabolizable energy value and prediction in growing pigs. J. Anim. Sci. 91:3231–3243. doi: 10.2527/jas.2013-6252 [DOI] [PubMed] [Google Scholar]
- Matkovic K., Mehta R., van der Kamp J., Jones J., McCleary B., Topping D. 2010. Interaction of insoluble oat fibre, dough conditioners and other dough constituents in optimizing bread formula with high whole grain and fibre content. In: van der Kamp J. W. editor, Dietary fibre: New frontiers for food and health. Wageningen Academic Publishers, Wageningen, the Netherlands: p. 135–150. [Google Scholar]
- Menke K. H., Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28:7–55. [Google Scholar]
- NRC 2012. Nutrient requirements of swine.11th rev. ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Oakenfull D. 2001. Physicochemical properties of dietary fiber: Overview. In: Cho S. S., Dreher M. L. editors, Handbook of dietary fiber. Marcel Dekker, Inc., New York, NY: p. 195–206. doi: 10.1201/9780203904220.pt2 [DOI] [Google Scholar]
- Panthapulakkal S., Zereshkian A., Sain M. 2006. Preparation and characterization of wheat straw fibers for reinforcing application in injection molded thermoplastic composites. Bioresour. Technol. 97:265–272. doi: 10.1016/j.biortech.2005.02.043 [DOI] [PubMed] [Google Scholar]
- Tamaki Y., Mazza G. 2011. Rapid determination of lignin content of straw using Fourier transform mid-infrared spectroscopy. J. Agric. Food Chem. 59:504–512. doi: 10.1021/jf1036678 [DOI] [PubMed] [Google Scholar]
- Urriola P. E., Li M., Kerr B. J., Shurson G. C. 2014. Evaluation of prediction equations to estimate gross, digestible, and metabolizable energy content of maize dried distillers' grains with solubles (DDGS) for swine based on chemical composition. Anim. Feed Sci. Technol. 198:196–202. doi: 10.1016/j.anifeedsci.2014.09.006 [DOI] [Google Scholar]
- Urriola P. E., Shurson G. C., Stein H. H. 2010. Digestibility of dietary fiber in distillers' co-products fed to growing pigs. J. Anim. Sci. 88:2373–2381. doi: 10.2527/jas.2009-2227 [DOI] [PubMed] [Google Scholar]
- Yen J. T., Nienaber J. A., Hill D. A., Pond W. G. 1991. Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J. Anim. Sci. 69:2001–2012. doi: 10.2527/1991.6952001x [DOI] [PubMed] [Google Scholar]