ABSTRACT
The study was to determine whether the expression of genes involved in intestinal water and ion transport would be affected by enterotoxigenic Escherichia coli (ETEC) K88 both in vitro and in vivo. First, 36 male piglets (4 d old) were randomly allotted to either the control or the ETEC K88 group. Each group had 6 replicates with 3 piglets per replicate. All piglets were fed with the same diets for 17 d. On d 15, piglets in the ETEC K88 group were challenged with ETEC K88 (serotype O149:K91:K88ac) at 1 × 108 cfu per pig, whereas those in the control group received the same volume of sterile PBS. After being challenged with ETEC K88 for 72 h (d 18), 1 piglet from each replicate was selected for slaughter to collect samples from the jejunum, ileum, and colon. The mRNA expression and protein abundance of cystic fibrosis transmembrane conductance regulator (CFTR) in the ileum and colon were increased compared with that in the control group (P < 0.05). Furthermore, the mRNA expression of Na-K-Cl cotransporter 1 (NKCC1) in the ileum and colon was increased by ETEC K88 challenge (P < 0.05), whereas in the jejunum, both its mRNA and protein expression were increased by ETEC K88 treatment (P < 0.05). Additionally, an established porcine intestinal epithelial cell line (IPEC-J2) was used to investigate the effect and possible mechanism of ETEC K88 on expression of water channel aquaporins (AQP) and ion transporters. Cells (1.17 × 106 per well) were grown in 6-well plates and treated with ETEC K88 at a multiplicity of infection of 50:1 for 3 h. The mRNA expression of AQP3, AQP11, and Na+/H+ exchanger 3 (NHE3) in IPEC-J2 cells was reduced after ETEC K88 treatment (P < 0.05). Further analyses using western blotting also demonstrated that ETEC K88 decreased the protein expression of AQP3, AQP9, and AQP11 in IPEC-J2 cells (P < 0.05). Moreover, the phosphorylation levels of protein kinase A (PKA) and cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB) were decreased by ETEC K88 challenge (P < 0.05). The results indicate that ETEC K88 challenge induced differential expression of intestinal ion transporters and AQP in young piglets, probably by regulation of the cAMP–PKA signaling pathway. This study might provide new insights about the importance of fluid homeostasis in control of ETEC-induced diarrhea in young piglets.
Keywords: aquaporin, cyclic adenosine monophosphate pathway, diarrhea, enterotoxigenic Escherichia coli K88, ion transporter, piglet
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is one of the most significant causes of morbidity and mortality worldwide to children and young farm animals. The key virulence factors of ETEC are bacterial adhesions (K88, K99, and 987P strains) and enterotoxins (LT, STa, and STb; Nataro and Kaper, 1998; Jin and Zhao, 2000). After colonization in the small intestine and release of enterotoxins, ETEC K88 induces fluid losses (Fairbrother et al., 2005; Guignot et al., 2007) and disturbs ion transport (Eisenhut, 2006), which causes severe watery diarrhea and subsequent growth retardation for neonatal and postweaning piglets, leading to great economic loss in swine production (Fairbrother et al., 2005).
The fast fluid transport and transfer in the gut is facilitated via the transcellular pathways mediated mainly by water channel aquaporins (AQP) and cotransporters but also via paracellular pathways by tight junctions (Zhu et al., 2016). The importance of AQP has attracted increasing attention due to the presence of at least 11 AQP subtypes in the gut (Matsuzaki et al., 2004). Intestinal ion transporters or cotransporters, such as cystic fibrosis transmembrane conductance regulator (CFTR), Na-K-Cl cotransporter (NKCC1), Na+/H+ exchanger 3 (NHE3), and sodium-glucose cotransporter 1 (SGLT1), are also important mediators for regulation of ion homeostasis (Loo et al., 1996; Hamann et al., 2010).
The altered expression of intestinal AQP and ion transporters has been shown to be associated with diarrhea or infection induced by Salmonella (Marchelletta et al., 2013), rotavirus (Cao et al., 2014), and lipopolysaccharide (He et al., 2017). Inhibition of normal absorption and secretion would lead to a rapid loss of intestinal fluids and electrolytes in bacteria toxin–induced diarrhea (Viswanathan et al., 2009). However, their importance in intestinal physiology and functions of piglets remains unclear. Intestinal ion transporters and AQP may be potential targets for the prevention and treatment of diarrhea in human and animals (Ikarashi et al., 2016), which may facilitate the therapeutic targeting of the pathophysiological processes during ETEC-induced diarrhea. Previous study has shown that ETEC K88 challenge increased the incidence of piglet diarrhea (Yang et al., 2014) and impaired tight junction barriers in young piglets (Yang et al., 2014) as well as in porcine intestinal epithelial cells (IPEC-J2; Wu et al., 2016). Whether ETEC K88 might affect the expression of ion transporters and AQP involved in regulating intestinal fluid homeostasis will be determined herein.
MATERIALS AND METHODS
Animal, Diets, and Sample Collection
The present study was performed in accordance with the Chinese guidelines for animal welfare and was approved by the Animal Care and Use Committee of the Guangdong Academy of Agricultural Sciences.
Thirty-six male piglets (Duroc × Landrace × Large White; 4 d old and initial BW of 2.41 ± 0.01 kg) were randomly allotted to either the control or the ETEC K88 group, as described in our previous study (Yang et al., 2014). Each group had 6 replicates (pens) with 3 piglets per replicate. The piglets were separately housed in air-conditioned rooms by group to prevent cross contamination between treatments. The diet (Table 1) was formulated according to the swine nutrient requirements (NRC, 2012) without any in-feed antibiotics supplementation. Diets were freshly mixed with water at a ratio of 1:3 before each feeding. All piglets were fed every 2 h from 0800 to 2400 h and had free access to water. Piglets were fed with the same diets for 17 d. On d 15, piglets in the ETEC K88 group were orally challenged with 10 mL PBS containing approximately 1 × 108 cfu of ETEC K88 (serotype O149:K91:K88ac) obtained from China Institute of Veterinary Drug Control (Beijing, P. R. China), whereas those in the control group received the same volume of sterile PBS.
Table 1.
Composition and nutrient levels of the diet (as fed basis)
| Ingredient | Percentage, % | Nutrient level1 | Content |
|---|---|---|---|
| Whole milk powder | 55.04 | ME, MJ/kg | 18.16 |
| Whey protein concentrate | 20.00 | CP, % | 23.81 |
| Lactose | 10.00 | Ca, % | 1.34 |
| Whey powder | 9.05 | Available P, % | 0.73 |
| Fish meal | 5.00 | Lys, % | 2.25 |
| L-Lys HCl | 0.12 | Met, % | 0.62 |
| DL-Met | 0.07 | Met + Cys, % | 0.98 |
| L-Arg HCl | 0.40 | Thr, % | 1.19 |
| L-Trp | 0.04 | Trp, % | 0.39 |
| Liquid choline chloride | 0.12 | ||
| Vitamin and mineral premix2 | 0.16 | ||
| Total | 100.00 |
Calculated values unless otherwise indicated.
Vitamin and mineral premix supplied per kilogram diet: 10,000 IU vitamin A, 1,500 IU vitamin D3, 50 IU vitamin E, 2.5 mg vitamin K3, 60 μg vitamin B12, 4.5 mg vitamin B1, 12 mg vitamin B2, 60 mg niacin, 36 mg pantothenic acid, 1 mg folic acid, 10 mg vitamin B6, 0.5 mg biotin, 200 mg vitamin C, 100 mg Fe (FeSO4), 6 mg Cu (CuSO4), 4 mg Mn (MnSO4), 100 mg Zn (ZnSO4), 0.3 mg I (CaI2O6), 0.14 mg Co (CoCO4), and 0.3 mg Se (Na2SeO3).
At d 3 after the ETEC K88 challenge, at the peak of infection (d 18), 1 pig from each pen was randomly chosen for slaughter using an intravenous injection of sodium pentobarbital (50 mg/kg BW). The small intestine was separated according to methods previously described (Yang et al., 2014). The middle portions (about 10 cm) of the jejunum, ileum, and colon were longitudinally cut to expose only mucosa samples followed by 3 washes with ice-cold PBS to remove the digesta. Then, mucosa from the jejunum, ileum, and colon was gently scraped with a glass slide, snap-frozen in liquid nitrogen, and then stored at −80°C until use for total mRNA extraction. Moreover, other tissue segments (about 2 cm) were removed from consistent locations of the middle jejunum, ileum, and colon; rinsed with ice-cold PBS; and fixed in 10% neutral formalin at room temperature until analyses for measuring expression and localization of intestinal ion transporters and AQP using immunohistochemistry.
Cell Culture and Treatment with Enterotoxigenic Escherichia coli K88
The ETEC K88 strain was grown in Luria–Bertani broth. After overnight incubation at 37°C with vigorous shaking, ETEC K88 was centrifuged at 5,000 × g for 10 min at 4°C, washed, and resuspended in cold PBS to obtain a final bacterial concentration of approximately 1 × 108 cfu/mL, as described in a previous study (Yang et al., 2014).
The porcine intestinal epithelial cell line (IPEC-J2) was purchased from BioVector NTCC Inc. (Beijing, P. R. China). Cells were grown in Dulbecco's modified
Eagle medium and Ham's F-12 medium (DMEM/F12) supplemented with 5% fetal bovine serum, 1% streptomycin (10,000 μg/mL) and penicillin (10,000 units/mL), insulin–transferrin–selenium (5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL selenium), and 5 ng/mL epidermal growth factor (all from Gibco, Merelbeke, Belgium) and maintained under a humidified atmosphere of 5% CO2 at 37°C. Cells were seeded in 6-well plates at a density of 1.17 × 106 per well and grown until 100% confluence followed by challenge with ETEC K88 at a multiplicity of infection of 50:1 for 3 h, as described in a previous study (Wu et al., 2016). Then, cells were washed twice with PBS, and cell samples were collected for determinations of expression of intestinal ion transporters and AQP at mRNA and protein levels using quantitative PCR and western blotting, respectively.
Isolation of RNA, Synthesis of cDNA Templates, and Quantitative PCR
Total RNA of cells was isolated using Trizol (Invitrogen, Merelbeke, Belgium) according to the manufacturer's protocols. The quality and integrity of total RNA were determined using a NanoDrop ND1000 spectrophotometer (Thermo Fisher Scientific Inc., Walldorf, Germany) at a 260:280 nm ratio of 1.9 to 2.1. The quality of RNA was verified using 1.5% agarose gel electrophoresis with visualization of intact 18S and 28S. The cDNA was synthesized from 1 μg of RNA using the SuperScript II First-Strand Synthesis System for RT-PCR (Invitrogen). The primer sequences were designed using Primer Premier 5.0 software (Palo Alto, CA; Table 2). All primers were checked for their specificity using melting curve analyses and agarose gel electrophoresis. Quantitative real-time PCR (qRT-PCR) reactions for the amplification of targeted genes were performed using SYBR Green PCR Master mix (Takara Biotechnology, Dalian, P. R. China) and a LightCycler 480II System (Roche, Mannheim, Germany). The relative mRNA expression of target genes are expressed relative to β-actin using the 2−ΔΔCT method as described by Livak and Schmittgen (2001).
Table 2.
Sequence, and product size of primers for targeted genes
| Item1 | Primer sequence2 | Product size, bp | Accession no. |
|---|---|---|---|
| AQP1 | F: TGACCTTCGCTATGTGCTTCC | 221 | NM_214454.1 |
| R: GTCCAAGTGTCCAGAGGGGTAG | |||
| AQP3 | F: TGACCTTCGCTATGTGCTTCC | 212 | NM_001110172.1 |
| R: GTCCAAGTGTCCAGAGGGGTAG | |||
| AQP7 | F: CCCGTGCCTCCAAGATGA | 58 | NM_001113438.1 |
| R: CGCATTATTGTTTGCATCTTTGA | |||
| AQP8 | F: GGTGCCATCAACAAGAAGACG | 227 | NM_001112683.1 |
| R: CCGATAAAGAACCTGATGAGCC | |||
| AQP9 | F: TGTCATTGGCCTCCTGATTG | 62 | NM_001112684.1 |
| R: TGGCACAGCCACTGTTCATC | |||
| AQP11 | F: CGTCTTGGAGTTTCTGGCTACC | 229 | NM_001112682.1 |
| R: CCTGTCCCTGACGTGATACTTG | |||
| NHE3 | F: AGCTGGAGATCATAGACCAGGTG | 147 | AF_123280 |
| R: CGGTGAAGAAGATGACGATGAG | |||
| SGLT1 | F: CCCAAATCAGAGCATTCCATTCA | 153 | NM_001164021.1 |
| R: AAGTATGGTGTGGTGGCCGGTT | |||
| NKCC1 | F: CCAATGCTGTTGCAGTTGCT | 264 | XM_005661615.2 |
| R: TGGGCTTCTTGCTCTCCAAG | |||
| CFTR | F: ACTATGGACCCTTCGAGCCT | 123 | NM_001104950.1 |
| R: CGCATTTGGAACCAGCGTAG | |||
| β-actin | F: TCCACCGCAAATGCTTCTAG | 208 | AY550069 |
| R: TGCTGTCACCTTCACCGTTC |
AQP = aquaporin; NHE3 = Na+/H+ exchanger 3; SGLT1 = sodium-glucose cotransporter 1; NKCC1 = Na-K-Cl cotransporter; CFTR = cystic fibrosis transmembrane conductance regulator.
F = forward; R = reverse.
Western Blotting
Cells were lysed for 30 min at 4°C in a lysis buffer with a 1% protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific Inc.). The lysates were then centrifuged at 16,000 × g for 15 min at 4°C to isolate the supernatants. Protein concentrations in the supernatant fluids were determined using a bicinchoninic acid assay (Thermo Fisher Scientific Inc.). Samples were then denatured in boiling water for 10 min with 2-mercaptoethanol (Sigma-Aldrich Corp., St. Louis, MO). Equal amounts of denatured proteins (80 μg per well) were separated on SDS-PAGE. Immunoblots were transferred to a nitrocellulose membrane (Bio-Rad, Irvine, CA) using the Bio-Rad Trans-Blot apparatus and then blocked in 5% nonfat milk containing 1x Tris–Tween–buffered saline (TBST) at room temperature for 2 h. The membrane was incubated with primary antibodies at 4°C overnight with gentle rocking (Table 3). After washing with 1x TBST 3 times, the membranes were washed with 1x TBST and incubated with horseradish peroxidase–linked anti-rabbit or anti-mouse secondary antibodies (Cell Signaling Technology, Inc., Danvers, MA) at room temperature for 2 h. After washing with 1x TBST 6 times for 5 min each time, membrane peroxidase activities were visualized using the SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific Inc.). The immunoreactive proteins were detected on a ChemiDoc XRS imaging system (Bio-Rad) and analyzed using Quantity One software (Bio-Rad). Results were presented as the abundance of each target protein relative to β-actin and normalized to the control group.
Table 3.
Primary antibodies and dilutions used in this study
| Antibody1 | Supplier | Dilution2 |
|---|---|---|
| Rabbit polyclonal to AQP3 | Bioss Antibodies3 | WB, 1:500; IF, 1:200; and IHC, 1:500 |
| Rabbit polyclonal to AQP9 | AVIVA Systems Biology Corp.4 | WB, 1:1,000 and IF, 1:50 |
| Rabbit polyclonal to AQP11 | FabGennix International Inc.5 | WB, 1:500; IF, 1:50; and IHC, 1:500 |
| Mouse monoclonal to NHE3 | Abcam6 | WB, 1:500 and IHC, 1:500 |
| Mouse monoclonal to CFTR | Abcam | IHC, 1:300 |
| Rabbit polyclonal to SGLT1 | Abcam | IHC, 1:500 |
| Rabbit polyclonal to NKCC1 | Cell Signaling Technology, Inc7 | IHC, 1:200 |
| Rabbit polyclonal to PKA (C-α) | Cell Signaling Technology, Inc. | WB, 1:1,000 |
| Rabbit polyclonal to p-PKA (C-α; Thr197) | Cell Signaling Technology, Inc. | WB, 1:1,000 |
| Rabbit polyclonal to PKA (α/β/γ) | Santa Cruz Biotechnology8 | WB, 1:1,000 |
| Rabbit polyclonal to p-PKA (α/β/γ; Thr198) | Santa Cruz Biotechnology | WB, 1:1,000 |
| Rabbit monoclonal to CREB | Cell Signaling Technology, Inc. | WB, 1:1,000 |
| Rabbit monoclonal to p-CREB (Ser133) | Cell Signaling Technology, Inc. | WB, 1:1,000 |
| Mouse monoclonal to β-actin | Abcam | WB, 1:4,000 |
AQP = aquaporin; NHE3 = Na+/H+ exchanger 3; CFTR = cystic fibrosis transmembrane conductance regulator; SGLT1 = sodium-glucose cotransporter 1; NKCC1 = Na-K-Cl cotransporter; PKA = protein kinase A; p-PKA = phosphorylated PKA; CREB = cAMP-response element binding protein; p-CREB = phosphorylated CREB.
IF = immunofluorescence; IHC = immunohistochemistry; WB = western blotting.
Beijing, P. R. China.
San Diego, CA.
Frisco, TX.
Cambridge, MA.
Beverly, MA.
Dallas, TX.
Immunohistochemistry
Intestinal tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin blocks. Five-micrometer sections were mounted on glass slides, deparaffinized in xylene, and gradually hydrated with graded ethanol. The sections were prepared for antigen retrieval using boiling 0.01 M sodium citrate buffer. Methanol and a 1% H2O2 solution were used to incubate slides for 15 min to block endogenous peroxidase activity. Immunoreactive proteins were visualized in sections using VECTASTAIN Elite ABC kits (Vector Laboratories, Youngstown, OH) following the manufacturer's instructions. The sections were individually stained at 4°C overnight with primary antibodies (Table 3) against CFTR (Abcam, Cambridge, MA), NHE3 (Abcam), NKCC1 (Cell Signaling Technology, Inc.), SGLT1 (Abcam), AQP3 (Bioss Antibodies, Beijing, P. R. China), and AQP11 (FabGennix International Inc, Frisco, TX). The slides were rinsed in PBS, dehydrated, and stained with 3,3'-diaminobenzidine tetrahydrochloride (Sigma-Aldrich Corp.) as the color substrate. Slides were finally prepared without counterstaining before the cover slips were affixed, and image captures of 3 to 5 areas per section (2 sections per animal) were taken under a fluorescent microscope (Carl Zeiss AG, Heidenheim, Germany). The expression of intestinal ion transporters and AQP was quantified from the staining intensity using ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD) and expressed as the mean optical density.
Immunofluorescence
For the immunofluorescence assay, IPEC-J2 cells with a density of 5 × 104 mL−1 were grown on 4-chamber Lab-Tek Permanox slides (Nalge Nunc International, Rochester, NY) and incubated in 5% CO2 at 37°C to reach 70% confluency and then subjected to ETEC K88 challenge at the same density as mentioned above for 3 h. Treated cells were then washed in PBS and fixed in −20°C methanol for 10 min followed by blocking with 1% BSA. Cells were stained with primary antibodies of AQP3, AQP9, and AQP11 (Table 3) and incubated at 4°C overnight in a humid chamber. After being washing with 0.03% Tween PBS 3 times, cells was stained with Alexa Fluor 594 goat anti-rabbit IgG secondary antibody (Invitrogen) in a humidified chamber at room temperature for 2 h. Slides were overlaid with mounting reagent ProLong Gold Antifade with DAPI (Invitrogen) and a cover glass. Representative images were taken with a Laser Confocal Microscope (Carl Zeiss AG).
Statistical Analysis
Replicate (pen) was considered the experimental unit for statistical analysis. The immunohistochemistry results were quantified with the average mean optical density of the images from each section of replicate. Data were processed using an unpaired 2-tailed Student's t-test for independent samples using SPSS software (version 17.0; SPSS Inc, Chicago, IL) for comparison between the control and ETEC K88 groups. All values are presented as means ± SEM (n = 6). Differences were considered statistically significant at P < 0.05.
RESULTS
Challenge with Enterotoxigenic Escherichia coli Affects the Expression of Intestinal Ion Transporters in Piglets
The mRNA expression and protein abundance of CFTR in the ileum and colon of young piglets were increased by ETEC K88 challenge compared with those in the control group (P < 0.05; Fig. 1). However, the jejunum expression of CFTR in young piglets, either at the mRNA level or protein level, was not affected by ETEC K88 treatment (P > 0.05; Fig. 1).
Figure 1.
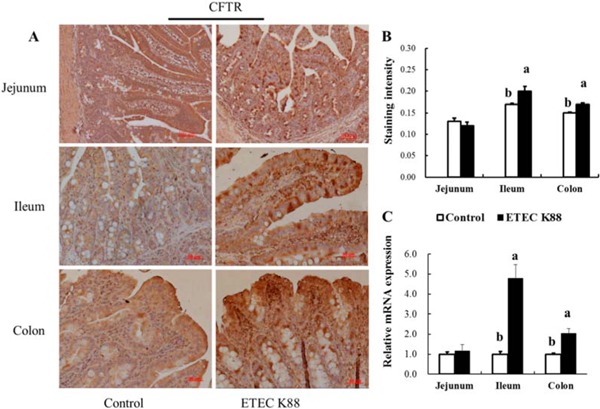
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on intestinal cystic fibrosis transmembrane conductance regulator (CFTR) expression in young piglets. Piglets in the control group were fed the basal diet and challenged with sterile PBS. Piglets in the ETEC K88 group were fed the basal diet and challenged with ETEC K88. The middle portions of the jejunum, ileum, and colon were collected for immunohistochemistry analysis (A). The immunostaining intensity of CFTR (B) in the jejunum, ileum, and colon was quantified by ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD). The relative mRNA expression of CFTR in the jejunum, ileum, and colon was determined by the 2−ΔΔCT method (C). Original magnification 100x. Scale bar = 100 μm. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05).
Furthermore, NKCC1 was expressed mainly in the crypts of the intestines (including the jejunum, ileum, and colon). The NKCC1 mRNA expression and protein expression of NKCC1 in the jejunum, ileum, and colon were increased by ETEC K88 challenge in comparison with the control group (P < 0.05; Fig. 2).
Figure 2.
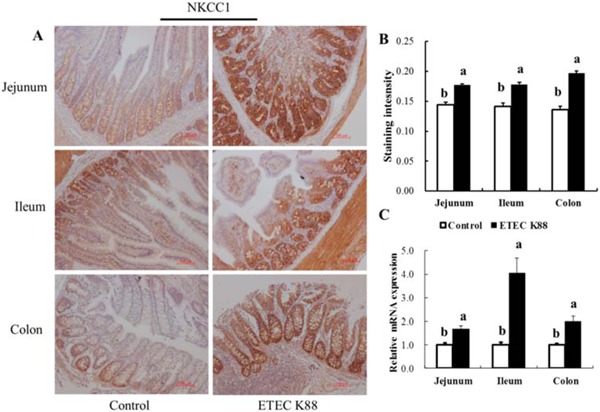
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on intestinal Na-K-Cl cotransporter (NKCC1) expression in young piglets. Piglets in the control group were fed the basal diet and challenged with sterile PBS. Piglets in the ETEC K88 group were fed the basal diet and challenged with ETEC K88. The middle portions of the jejunum, ileum, and colon were collected for immunohistochemistry analysis (A). The immunostaining intensity of NKCC1 (B) in the jejunum, ileum, and colon was quantified by ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD). The relative mRNA expression of NKCC1 in the jejunum, ileum, and colon was determined by the 2−ΔΔCT method (C). Original magnification 100x. Scale bar = 100 μm. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05).
As shown in Fig. 3, NHE3 was mainly distributed in the villous epithelial cells. The expression of NHE3 in the jejunum, ileum, and colon was all decreased by ETEC K88 challenge (P < 0.05). The expression of SGLT1, however, was mainly distributed in the villous epithelium. There was no difference in SGLT1 expression in the jejunum, ileum, and colon between piglets in the ETEC K88 group and piglets in the control group (P > 0.05; Fig. 3).
Figure 3.
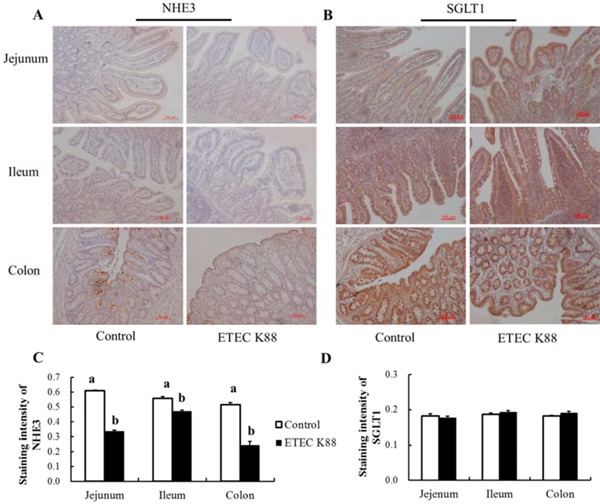
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on intestinal Na+/H+ exchanger 3 (NHE3) and sodium-glucose cotransporter 1 (SGLT1) expression in young piglets. The images of NHE3 (A) and SGLT1 (B) expression in the jejunum, ileum, and colon were captured under a fluorescence microscope (Carl Zeiss AG, Heidenheim, Germany). The immunostaining intensities of NHE3 (C) and SGLT1 (D) were quantified by ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD). Piglets in the control group were fed the basal diet and challenged with sterile PBS. Piglets in the ETEC K88 group were fed the basal diet and challenged with ETEC K88. The middle portions of the jejunum, ileum, and colon were collected for immunohistochemistry. Original magnification 100x. Scale bar = 100 μm. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05).
Challenge with Enterotoxigenic Escherichia coli Affects the Expression of Intestinal Aquaporins in Piglets
AQP3 and AQP11 were abundantly expressed in the gastrointestinal tract of piglets as well as in IPEC-J2 cells (our unpublished data). The protein localizations of AQP3 and AQP11 were mainly distributed in the villus epithelial cells with few in crypt epithelial cells of the intestine (Fig. 4). The immunohistochemistry results showed that the AQP3 protein expression in the jejunum, ileum, and colon as well as AQP11 protein expression in the colon of young piglets was all decreased by ETEC K88 challenge compared with the control group (Fig. 4).
Figure 4.
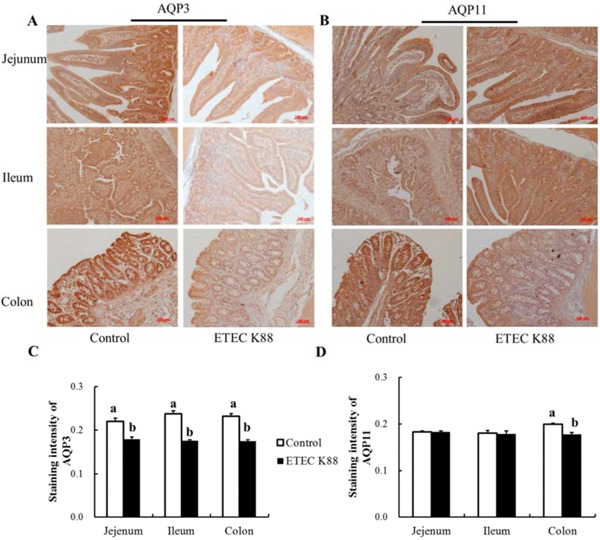
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on aquaporin 3 (AQP3) and AQP11 expression in young piglets. The images of AQP3 (A) and AQP11 (B) expression in the jejunum, ileum, and colon were captured under a fluorescence microscope (Carl Zeiss AG, Heidenheim, Germany). The immunostaining intensities of AQP3 (C) and AQP11 (D) were quantified by ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD). Piglets in the control group were fed the basal diet and challenged with sterile PBS. Piglets in the ETEC K88 group were fed the basal diet and challenged with ETEC K88. The middle portions of the jejunum, ileum, and colon were collected for immunohistochemistry. Original magnification 100x. Scale bar = 100 μm. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05).
Challenge with Enterotoxigenic Escherichia coli Affects the Expression of Aquaporins and Ion Transporters in IPEC-J2 Cells
The mRNA expression of AQP3, AQP11, and NHE3 in IPEC-J2 cells was reduced after ETEC K88 treatment compared with the control group (P < 0.05; Fig. 5). However, there was no difference in gene expression of other transporters (CFTR, NKCC1, SGLT1, AQP1, AQP3, AQP7, AQP8, and AQP9) between the ETEC K88 group and the control group (P > 0.05; Fig. 5).
Figure 5.
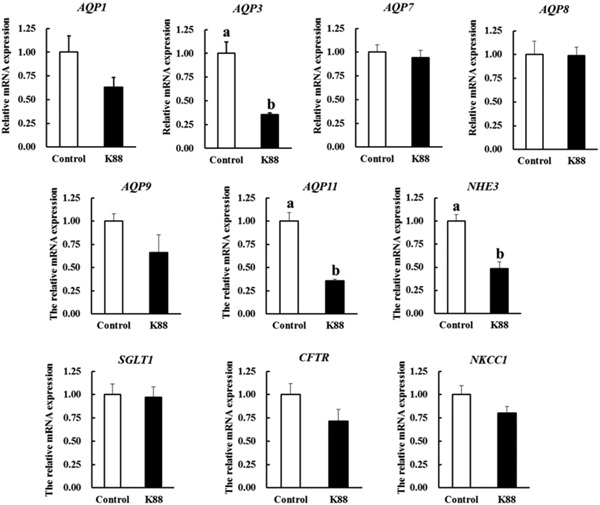
The relative mRNA expression of intestinal ion transporters and aquaporins (AQP) in IPEC-J2 cells. Cells were challenged or not with enterotoxigenic Escherichia coli K88 (K88) for 3 h. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05). CFTR = cystic fibrosis transmembrane conductance regulator; NHE3 = Na+/H+ exchanger 3; NKCC1 = Na-K-Cl cotransporter; SGLT1 = sodium-glucose cotransporter 1.
Furthermore, ETEC K88 decreased the protein expression of AQP3, and AQP11 in IPEC-J2 cells (P < 0.05; Fig. 6). Even though mRNA expression of AQP9 was not affected by ETEC K88 (P > 0.05), the protein expression of AQP9 in IPEC-J2 cells was clearly decreased by ETEC K88 challenge (P < 0.05; Fig. 6).
Figure 6.
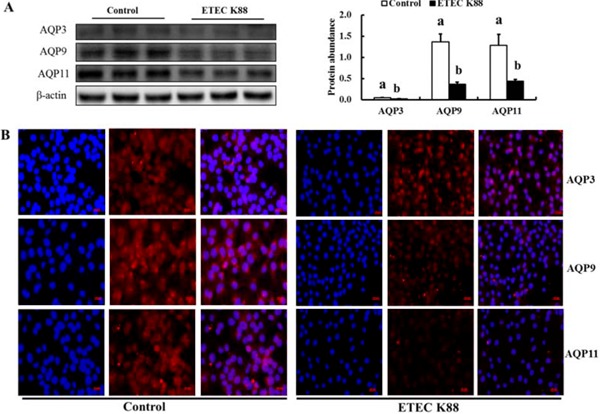
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on the protein expression of intestinal aquaporins (AQP) in IPEC-J2 cells. Cells were challenged or not with ETEC K88 for 3 h. Then, treated cells were collected for western blotting analysis (A). Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05). For immunofluorescence analysis (B), cells after treatment were fixed and incubated with primary antibodies (rabbit polyclonal antibodies to AQP3 [Bioss Antibodies, Beijing, P. R. China], AQP9 [AVIVA Systems Biology Corp, San Diego, CA], AQP11 [FabGennix International Inc, Frisco, TX]; red) followed by incubation with Alexa Fluor 568 goat anti-rabbit secondary antibody (Invitrogen, Merelbeke, Belgium). Nuclei (blue) were stained with 4',6-diamidino-2-phenylindole in ProLong Gold mounting medium (Invitrogen). Representative images (B) were taken with a Laser Confocal Microscope (Carl Zeiss AG, Heidenheim, Germany). Original magnification, 400x.
NHE3 protein expression in IPEC-J2 cells was also decreased by ETEC K88 challenge (Fig. 7). Moreover, the phosphorylation levels of protein kinase A (PKA) and cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB) of the cAMP pathwaywere decreased by ETEC K88 challenge (P < 0.05; Fig. 7), which was in accordance with the decreased expression of AQP3, AQP9, AQP11, and NHE3 in IPEC-J2 cells challenged with ETEC K88.
Figure 7.
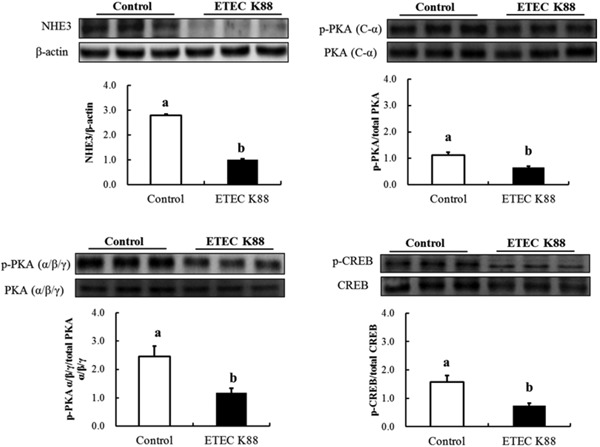
Effect of enterotoxigenic Escherichia coli (ETEC) K88 challenge on the protein abundances of Na+/H+ exchanger 3 (NHE3) and cyclic adenosine monophosphate (cAMP) pathway associated proteins in IPEC-J2 cells. Cells were challenged or not with ETEC K88 for 3 h. Then, treated cells were collected for western blotting analysis. Results are mean ± SEM (n = 6). a,bMeans with different superscripts between columns differ (P < 0.05). CREB = cAMP-response element binding protein; p-CREB = phosphorylated CREB; PKA = protein kinase A; p-PKA = phosphorylated PKA.
DISCUSSION
Enterotoxigenic Escherichia coli is a major cause of diarrhea in neonatal and preweaned piglets (Moon et al., 1999; Dubreuil, 2008; Schroyen et al., 2012) as well as in children less than 5 yr old and the elderly more than 70 yr old (Qadri et al., 2005). It has been well recognized that K88 was the most virulent determinant for ETEC-associated diarrhea in pigs (Zhou et al., 2012). Therefore, the ETEC K88 challenge model has been widely used in animal nutrition to evaluate the impact of postweaning diarrhea and nutrition regulation study for attenuating postweaning diarrhea (Adewole et al., 2016).
To the best of our knowledge, this study provides the first report using an ETEC K88 challenge model to investigate the expression changes of intestinal AQP in weaned piglets. Gut health, especially in young pigs, has a significant benefit to health and performance. The previous studies by others and ours indicated that ETEC K88 inoculation increased the incidence of diarrhea and impaired performance in piglets (Owusu-Asiedu et al., 2003; Yang et al., 2014). However, how this disturbed fluid homeostasis was initiated and the underlying mechanism remained largely unknown. Therefore, the detection of aberrant expression or subcellular alterations of AQP and intestinal ion transporters as well as their regulation may have important application in the diagnostic and therapeutic significance for disease (Guttman and Finlay, 2008; Madeira et al., 2016).
The intestinal epithelium acts as more than a physical barrier; it also functions in the regulation of the absorption of nutrients and fluids as well as electrolyte and water absorption and secretion (Kagnoff, 2014). Diarrheal disorders are almost always associated with changes in fluid and electrolyte movement, especially accompanied with a decrease in absorption and/or an increase in secretion. Moreover, the decreased absorptive surface epithelium, disruption of tight junction barrier function, impaired ion transport, and induction of inflammation have been recognized during ETEC-induced diarrhea (Berkes et al., 2003). We have previously shown that piglets challenged with ETEC K88 displayed impaired intestinal morphology and intestinal permeability and decreased tight junction expression in the jejunum of young piglets (Yang et al., 2014). Here, the present study clearly showed the differential expression of intestinal ion transporters and AQP in young piglets in response to ETEC K88 challenge.
Cholera toxin would cause disorders of intestinal fluid and electrolyte transport and induce great fluid loss into the lumen of the jejunum and ileum, leading to acute diarrhea (Banwell et al., 1970). Moreover, several AQP have been demonstrated to be downregulated under different types of diarrhea (Laforenza et al., 2010; Ikarashi et al., 2012). For example, the water channels AQP3, AQP7, and AQP10 were severely impaired in celiac disease (Laforenza et al., 2010). Accordingly, our results have shown that AQP3 expression in IPEC-J2 cells at both the mRNA and protein levels was decreased by ETEC K88 challenge. It has shown that the protein expression of AQP3, AQP4, and AQP8 in the jejunum and ileum of piglets was decreased by lipopolysaccharide challenge accompanied by an increased diarrhea index (He et al., 2017). Consistently, we also found that that AQP3 protein expression in the jejunum, ileum, and colon was also decreased by ETEC K88 challenge in young piglets.
The inhibition of AQP3 in the colon has been shown to increase the fecal water content and induce diarrhea in mice by regulating the water transport from the luminal side to the vascular side (Ikarashi et al., 2012). Importantly, AQP3 transports not only water but also glycerol, which is essential for ATP production and lipid synthesis that facilitate cell proliferation and wound healing (Verkman et al., 2014). Indeed, AQP3 knock-out mice have been demonstrated to experience impaired wound healing in the colon (Thiagarajah et al., 2007) and disturbed mucosal innate immune responses (Thiagarajah et al., 2017). Collectively, the decreased AQP3 expression observed here may indicate that intestinal AQP3 functions as more than a water channel and also helps regulation of intestinal health of pigs, which requires further investigations in future studies.
The transpeithelial water transfer occurs via both water channels and the intercellular routes. Water can be cotransported across the brush border membrane of the intestine along with Na+ and glucose by SGLT1. This happens when Na+ transport increases the local osmotic pressure in the lateral intercellular spaces, which, in turn, generates osmotic water flow across the epithelium (Loo et al., 1996; Wright and Loo, 2000). The human intestine can absorb at least 5 to 6 L water per day across the brush borders of the enterocytes via SGLT1 (Loo et al., 1996). Even though SGLT1 has been demonstrated to be severely impaired in the duodenal mucosa of celiac disease patients (Laforenza et al., 2010), here we found no difference in SGLT1 expression in the intestine of piglets challenged with ETEC K88. The discrepancy may involve the differences of diarrhea types, the experimental designs, and the species. Another cotransporter of water, NKCC1, has well demonstrated its roles in regulation of cellular water homeostasis in mammalian epithelial cells (Hamann et al., 2010). Here, we also found that the expression of NKCC1 in the jejunum, ileum, and colon of young piglets was increased by ETEC K88 treatment.
Besides water channels, tight junction barriers, and cotransporters, other intestinal ion transporters are also crucial for fluid and electrolyte homeostasis. For example, NHE3 is the Na+/H+ exchanger located on the intestinal brush border membrane and functions in transepithelial Na+ absorption (Yun et al., 1997) and transports Na+ into cells and decreases Cl− release (Schultheis et al., 1998). The genes involved in intestinal ion and water transport were affected in a mouse model of infectious diarrhea (Borenshtein et al., 2008). Another study has shown that NHE3 were severely impaired in patients with celiac disease (Laforenza et al., 2010). In the present study, we showed that NHE3 expression was inhibited by ETEC K88 challenge both in vitro and in vivo. The inhibition of NHE3 leads to a decrease in luminal Na+ absorption and influences the stool hydration and contributes to the development of diarrhea (Schultheis et al., 1998). Therefore, the impaired tight junction barriers shown in our previous study (Yang et al., 2014) combined with the decreased expression of NHE3 observed here might exacerbate the generation of diarrhea in piglets after ETEC challenge, which was in good accordance with previous reports (Clayburgh et al., 2006).
Another important intestinal ion transporter, CFTR, mainly expressed at the apical surfaces of secretory epithelial cells lining the lumen of the gut, mediates electrogenic Cl− transport (Ameen et al., 2000). Previous study in pig alveolar epithelia has also indicated that CFTR was required for maximal transepithelial liquid transport under cAMP stimulation (Li et al., 2012). Notably, CFTR could not transport water directly but through the establishment of cAMP stimulation for regulating Cl− balance or by interaction with other AQP (Huang et al., 2017). When exposed to toxins secreted by the colonization of ETEC, intestinal cAMP pathway are activated, leading to the hyperactivation of the CFTR channel that drives the parallel flows of Na+ and water (Zhang et al., 2012). Therefore, the robust transepithelial secretion of fluid and electrolytes into the intestine lumen overwhelms its reabsorbtion capacity, leading to fluid loss and dehydration (Zhang et al., 2012). Early study has also indicated that weaned piglets depend on the large intestine for reabsorption of fluid and electrolytes, mainly in the colon (Hamilton and Roe, 1977). Accordingly, we found that ETEC K88 challenge altered the CFTR expression in the ileum and colon of piglets. Targeting CFTR inhibition would potentially reduce the intestinal fluid losses in secretary diarrhea caused by bacterial enterotoxins (Ma et al., 2002; Zhang et al., 2012; Cil et al., 2017). However, there was no difference in CFTR expression in the jejunum of piglets as well as in IPEC-J2 cells. This may be due to the fact that IPEC-J2 cells were derived from the jejunum of an unsuckled piglet (Berschneider, 1989).
We further investigated the possible mechanism involved in regulation of gene expression after ETEC inoculation. Our results have shown that the phosphorylation of PKA and CREB was affected by ETEC treatment, which suggested that the cAMP pathway might be involved in the regulation of decreasing intestinal ion transporter (NHE3) and AQP expression (AQP3, AQP9, and AQP11) in IPEC-J2 cells when challenged with ETEC K88. Our results were consistent with previous reports that the water permeability of AQP3 was suppressed by cholera toxin in the human small intestine due to an increase of intracellular cAMP concentration (Hamabata et al., 2002).
Collectively, our study has shown that ETEC induced differential expression of intestinal ion transporters and AQP in weaned piglets. These results may suggest that the fluid homeostasis is essential for regulating intestinal health and control of diarrhea, especially in postweaning piglets.
FOOTNOTES
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (31501967 and 31472112); the Hundred Outstanding Talents Training Program at Guangdong Province, China; the Science and Technology Program of Guangdong Province, China (2015A030310332 and 2016B070701013); the Science and Technology Program of Guangzhou City, China (201607020035); and the Presidential Foundation of Guangdong Academy of Agricultural Sciences, China (201612).
LITERATURE CITED
- Adewole D. I., Kim I. H., Nyachoti C. M. 2016. Gut health of pigs: Challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives – A review. Asian-Australas. J. Anim. Sci. 29:909–924. doi: 10.5713/ajas.15.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen N., Alexis J., Salas P. 2000. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem. Cell Biol. 114:69–75. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B., Mondal A. 1970. Intestinal fluid and electrolyte transport in human cholera. J. Clin. Invest. 49:183–195. doi: 10.1172/JCI106217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes J., Viswanathan V. K., Savkovic S. D., Hecht G. 2003. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451. doi: 10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider H. M. 1989. Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology 96:A41 (Abstr.) [Google Scholar]
- Borenshtein D., Fry R. C., Groff E. B., Nambiar P. R., Carey V. J., Fox J. G., Schauer D. B. 2008. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol. 9:R122. doi: 10.1186/gb-2008-9-8-r122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Yang M., Ou Z., Li D., Geng L., Chen P., Chen H., Gong S. 2014. Involvement of aquaporins in a mouse model of rotavirus diarrhea. Virol. Sin. 29:211–217. doi: 10.1007/s12250-014-3469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cil O., Phuan P. W., Gillespie A. M., Lee S., Tradtrantip L., Yin J., Tse M., Zachos N. C., Lin R., Donowitz M., Verkman A. S. 2017. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins. FASEB J. 31:751–760. doi: 10.1096/fj.201600891R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh D. R., Musch M. W., Leitges M., Fu Y. X., Turner J. R. 2006. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J. Clin. Invest. 116:2682–2694. doi: 10.1172/JCI29218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil J. D. 2008. Escherichia coli STb toxin and colibacillosis: Knowing is half the battle. FEMS Microbiol. Lett. 278:137–145. doi: 10.1111/j.1574-6968.2007.00967.x [DOI] [PubMed] [Google Scholar]
- Eisenhut M. 2006. Changes in ion transport in inflammatory disease. J. Inflamm. (Lond.) 3:5. doi: 10.1186/1476-9255-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother J. M., Nadeau E., Gyles C. L. 2005. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi: 10.1079/AHR2005105 [DOI] [PubMed] [Google Scholar]
- Guignot J., Chaplais C., Coconnier-Polter M. H., Servin A. L. 2007. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 9:204–221. doi: 10.1111/j.1462-5822.2006.00782.x [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Finlay B. B. 2008. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol. 16:535–542. doi: 10.1016/j.tim.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Hamabata T., Liu C., Takeda Y. 2002. Positive and negative regulation of water channel aquaporins in human small intestine by cholera toxin. Microb. Pathog. 32:273–277. doi: 10.1006/mpat.2002.0502 [DOI] [PubMed] [Google Scholar]
- Hamann S., Herrera-Perez J. J., Zeuthen T., Alvarez-Leefmans F. J. 2010. Cotransport of water by the Na+-K+-2Cl(–) cotransporter NKCC1 in mammalian epithelial cells. J. Physiol. 588:4089–4101. doi: 10.1113/jphysiol.2010.194738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. L., Roe W. E. 1977. Electrolyte levels and net fluid and electrolyte movements in the gastrointestinal tract of weanling swine. Can. J. Comp. Med. 41:241–250. [PMC free article] [PubMed] [Google Scholar]
- He L., Huang N., Li H., Tian J., Zhou X., Li T., Yao K., Wu G., Yin Y. 2017. AMPK/alpha-ketoglutarate axis regulates intestinal water and ion homeostasis in young pigs. J. Agric. Food Chem. 65:2287–2298. doi: 10.1021/acs.jafc.7b00324 [DOI] [PubMed] [Google Scholar]
- Huang B., Wang H., Yang B. 2017. Water transport mediated by other membrane proteins. Adv. Exp. Med. Biol. 969:251–261. doi: 10.1007/978-94-024-1057-0_17 [DOI] [PubMed] [Google Scholar]
- Ikarashi N., Kon R., Lizasa T., Suzuki N., Hiruma R., Suenaga K., Toda T., Ishii M., Hoshino M., Ochiai W., Sugiyama K. 2012. Inhibition of aquaporin-3 water channel in the colon induces diarrhea. Biol. Pharm. Bull. 35:957–962. doi: 10.1248/bpb.35.957 [DOI] [PubMed] [Google Scholar]
- Ikarashi N., Kon R., Sugiyama K. 2016. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int. J. Mol. Sci. 17:1172. doi: 10.3390/ijms17071172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. Z., Zhao X. 2000. Intestinal receptors for adhesive fimbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine – A review. Appl. Microbiol. Biotechnol. 54:311–318. doi: 10.1007/s002530000404 [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. 2014. The intestinal epithelium is an integral component of a communications network. J. Clin. Invest. 124:2841–2843. doi: 10.1172/JCI75225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforenza U., Miceli E., Gastaldi G., Scaffino M. F., Ventura U., Fontana J. M., Orsenigo M. N., Corazza G. R. 2010. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 102:457–467. doi: 10.1042/BC20100023 [DOI] [PubMed] [Google Scholar]
- Li X., Comellas A. P., Karp P. H., Ernst S. E., Moninger T. O., Gansemer N. D., Taft P. J., Pezzulo A. A., Rector M. V., Rossen N., Stoltz D. A., McCray P. B., Jr, Welsh M. J., Zabner J. 2012. CFTR is required for maximal transepithelial liquid transport in pig alveolar epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 303:L152–L160. doi: 10.1152/ajplung.00116.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and 2–ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loo D. D., Zeuthen T., Chandy G., Wright E. M. 1996. Cotransport of water by the Na+/glucose cotransporter. Proc. Natl. Acad. Sci. USA 93:13367–13370. doi: 10.1073/pnas.93.23.13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. 2002. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110:1651–1658. doi: 10.1172/JCI0216112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira A., Moura T. F., Soveral G. 2016. Detecting aquaporin function and regulation. Front Chem. 4:3. doi: 10.3389/fchem.2016.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchelletta R. R., Gareau M. G., McCole D. F., Okamoto S., Roel E., Klinkenberg R., Guiney D. G., Fierer J., Barrett K. E. 2013. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonella-induced enteritis. Gastroenterology 145:1358–1368.e1–4. doi: 10.1053/j.gastro.2013.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T., Tajika Y., Ablimit A., Aoki T., Hagiwara H., Takata K. 2004. Aquaporins in the digestive system. Med. Electron Microsc. 37:71–80. doi: 10.1007/s00795-004-0246-3 [DOI] [PubMed] [Google Scholar]
- Moon H. W., Hoffman L. J., Cornick N. A., Booher S. L., Bosworth B. T. 1999. Prevalences of some virulence genes among Escherichia coli isolates from swine presented to a diagnostic laboratory in Iowa. J. Vet. Diagn. Invest. 11:557–560. doi: 10.1177/104063879901100617 [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Owusu-Asiedu A., Nyachoti C. M., Marquardt R. R. 2003. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J. Anim. Sci. 81:1790–1798. doi: 10.2527/2003.8171790x [DOI] [PubMed] [Google Scholar]
- Qadri F., Svennerholm A. M., Faruque A. S., Sack R. B. 2005. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483. doi: 10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroyen M., Stinckens A., Verhelst R., Niewold T., Buys N. 2012. The search for the gene mutations underlying enterotoxigenic Escherichia coli F4ab/ac susceptibility in pigs: A review. Vet. Res.. (Faisalabad) 43:70. doi: 10.1186/1297-9716-43-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis P. J., Clarke L. L., Meneton P., Miller M. L., Soleimani M., Gawenis L. R., Riddle T. M., Duffy J. J., Doetschman T., Wang T., Giebisch G., Aronson P. S., Lorenz J. N., Shull G. E. 1998. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 19:282–285. doi: 10.1038/969 [DOI] [PubMed] [Google Scholar]
- Thiagarajah J. R., Chang J., Goettel J. A., Verkman A. S., Lencer W. I. 2017. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA 114:568–573. doi: 10.1073/pnas.1612921114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah J. R., Zhao D., Verkman A. S. 2007. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 56:1529–1535. doi: 10.1136/gut.2006.104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A. S., Anderson M. O., Papadopoulos M. C. 2014. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 13:259–277. doi: 10.1038/nrd4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V. K., Hodges K., Hecht G. 2009. Enteric infection meets intestinal function: How bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol. 7:110–119. doi: 10.1038/nrmicro2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Loo D. D. 2000. Coupling between Na+, sugar, and water transport across the intestine. Ann. N. Y. Acad. Sci. 915:54–66. doi: 10.1111/j.1749-6632.2000.tb05223.x [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhu C., Chen Z., Zhang W., Ma X., Wang L., Yang X., Jiang Z. 2016. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 172:55–63. doi: 10.1016/j.vetimm.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Yang K. M., Jiang Z. Y., Zheng C. T., Wang L., Yang X. F. 2014. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 92:1496–1503. doi: 10.2527/jas.2013-6619 [DOI] [PubMed] [Google Scholar]
- Yun C. H., Oh S., Zizak M., Steplock D., Tsao S., Tse C. M., Weinman E. J., Donowitz M. 1997. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc. Natl. Acad. Sci. USA 94:3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Fujii N., Naren A. P. 2012. Recent advances and new perspectives in targeting CFTR for therapy of cystic fibrosis and enterotoxin-induced secretory diarrheas. Future Med. Chem. 4:329–345. doi: 10.4155/fmc.12.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Liu Z., Jiang J., Yu Y., Zhang Q. 2012. Differential gene expression profiling of porcine epithelial cells infected with three enterotoxigenic Escherichia coli strains. BMC Genomics 13:330. doi: 10.1186/1471-2164-13-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Chen Z., Jiang Z. 2016. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int. J. Mol. Sci. 17:1399. doi: 10.3390/ijms17091399 [DOI] [PMC free article] [PubMed] [Google Scholar]


