Abstract
A total of 239 pigs (initial BW 6.56 ± 0.87 kg, 21 d of age) were used in a 35-d study to investigate the effects of fish meal (FM) and spray dried plasma (SDP) in combination with a bioprocessed soybean meal (SBM) on growth performance and immune responses in weaned pigs. Equal numbers of barrows and gilts were randomly allotted to 1 of 4 dietary treatments (10 pens/treatment) according to initial BW and sex: positive control (CON; corn/SBM diet) containing SDP and FM, the CON with bioprocessed SBM replacing FM (BPSBM+SDP), the CON with bioprocessed SBM replacing SDP (BPSBM+FM), and the CON with bioprocessed SBM replacing both SDP and FM (BPSBM). Experimental diets were fed in Phase I (d 1-7 post-wean) and II (d 8-21) followed by a common Phase III diet (d 22-35); changes in BW and feed disappearance were determined accordingly. Pigs were sensitized against ovalbumin (OVA) and Candida albicans (CAA) on d 7 and 21. Assessment of immune response was based on lymphocyte proliferation in response to mitogens Concanavalin A and phytohemagglutinin (d 14 post-wean), dermal hypersensitivity to OVA and CAA (% increase in local swelling at 2, 6, 24, and 48 h post-injection) on d 28, and primary and secondary anti-OVA IgG at d 21 and 28, respectively. Pigs fed CON were heavier (P < 0.01) than pigs fed BPSBM+FM and BPSBM, and not different from pigs fed BPSBM+SDP, at the end of Phase I and II (6.99, 6.80, 6.52, or 6.60 kg, pooled SEM 0.08, respectively in Phase I or 12.47, 12.18, 11.42, and 11.85 kg, pooled SEM 0.21, in Phase II, respectively). Hypersensitivity to OVA peaked at 2h in pigs fed CON, BPSBM+SDP, and BPSBM+FM or peaked at 6h in pigs fed BPSBM (121.4, 165.6, 139.0, and 144.1%, pooled SEM 22.9, respectively, at 2 h and 86.7, 114.5, 95.0, and 156.8%, pooled SEM 29.4, respectively at 6 h). Peak response to CAA occurred at 2h in all groups (42.6, 55.2, 48.2, and 50.6%, pooled SEM 11.9, respectively, in the CON, BPSBM+SDP, BPSBM+FM, and BPSBM, respectively). There was no difference in hypersensitivity due to experimental diet at any time point. Secondary anti-OVA IgG was 2-fold lower based on optical density values in pigs fed CON compared with BPSBM+FM and BPSBM (0.78 vs. 1.56 and 1.55 optical density at 405 nm, pooled SEM 0.42, respectively). Dietary treatment did not impact lymphocyte proliferation. The bioprocessed SBM is a suitable alternative for FM and/or SDP in Phase I and II nursery diets based on pig growth. The prolonged hypersensitivity to OVA indicate that bioprocessed SBM may have a positive impact on pig immune function and the 2-fold increase in anti-OVA IgG warrants further investigation on the impact of bioprocessed SBM on pig immune function.
Keywords: alternative amino acids, bioprocessed soybean meal, immune response, nursery pig, weaning
INTRODUCTION
Soybean meal (SBM) is a poorly tolerated protein source in the young pig's digestive tract due to antinutritional factors such as glycinin and β-conglycinin, which can decrease performance and induce an immune response (Li et al., 1990, 1991). To address this problem, antibiotics and highly digestible AA sources such as spray-dried plasma (SDP) and fish meal (FM) are incorporated into the diet to help increase feed intake and improve piglet health (Cromwell, 2002; DeRouchey et al., 2010). However, the cost of these products often limits a producer's ability to include them in an economical nursery diet. Bioprocessing or fermentation of plant proteins, specifically SBM, has been shown to reduce antinutritional factors and can be fed to nursery pigs without adversely affecting performance (Kim et al., 2010; Sinn et al., 2016).
One such bioprocessed soybean meal (Prairie AquaTech, Brookings, SD) was evaluated and reported to have comparable CP and AA digestibility relative to FM in 10-kg pigs. Furthermore, pigs fed a diet containing bioprocessed soybean meal had reduced incidence and severity of postweaning diarrhea (Sinn et al., 2016). The impact of feedstuffs on animal performance will continue to be important; however, advances in science and technology are prompting nutritionists to look at more than just performance when considering dietary inclusion of alternative ingredients in the diet. The immature immune system of the weaned pig is attracting more attention because restrictions on antibiotics use in livestock continue. Therefore, the potential for dietary ingredients to boost the innate or adaptive immune response in weaned pigs is of great interest. The objective of this experiment was to assess the effects of bioprocessed soybean meal alone or in combination with SDP or FM in a corn and SBM–based diet on immune response in weaned pigs. We hypothesized that inclusion of bioprocessed soybean meal in nursery pig diets could enhance the innate and adaptive immune response of weaned pigs.
MATERIALS AND METHODS
All procedures used in this experiment were approved by the South Dakota State University Institutional Animal Care and Use Committee (number 15-114A). Pigs used for this trial were the offspring of Landrace × Large White sows mated with Hampshire × German Large White boars and were obtained from a high health commercial piggery (Claremont Hutterite Colony, Castlewood, SD). Pigs were vaccinated against mycoplasma and porcine circovirus (FLEXcombo; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) prior to delivery.
The bioprocessed soybean meal evaluated in this study was provided by Prairie AquaTech and processed by incubating a pasteurized slurry of SBM and water with the fungus Aureobasidium pullulans for 4 to 5 d. After incubation, the slurry is separated by centrifugation, and the solids are recovered and dried (Gibbons and Brown, 2016). The bioprocessed soybean meal contains reduced trypsin inhibitor (68.88 Trypsin inhibitor units/g), raffinose (0.00%), and stachyose (0.02%) compared with conventional SBM (2,596 to 6,090 Trypsin inhibitor units/g, 0.98%, and 3.07%, respectively; Choct et al., 2010; Sinn et al., 2016).
Animals, Diets, and Experimental Design
A total of 239 pigs were weaned at 21 ± 1 d of age (6.56 ± 0.87 kg initial BW) into 48 pens (3 barrows and 3 gilts/pen; n = 10 pens/treatment) for a 35-d study. The study was conducted in 2 blocks, with 120 and 119 pigs in block 1 and 2, respectively. When pigs arrived from the commercial piggery in the second block, there was 1 less pig than expected; therefore, 1 pen in the second block contained 3 barrows and 2 gilts. Each pen (1.8 by 1.3 m) provided a minimum of 0.36 m2/pig and contained 1 nipple drinker and a 3-hole self-feeder to provide ad libitum access to feed and water. At weaning, pigs were randomly allotted to 1 of 4 dietary treatments: 1) a control (CON; corn, SBM, and whey–based diet containing FM and SDP), 2) the CON with bioprocessed soybean meal replacing FM (BPSBM+SDP), 3) the CON with bioprocessed soybean meal replacing SDP (BPSBM+FM), and 4) the CON with bioprocessed soybean meal replacing both FM and SDP (BPSBM). Experimental diets were fed in a 2-phase feeding program where Phase I was fed from 0 to 7 d and Phase II was fed from 8 to 21 d after weaning (Table 1). Phase III consisted of a common corn/SBM diet for 22 to 35 d after weaning. All diets were fed in a meal form and were formulated to meet or exceed NRC requirements for weaned pigs. Subsamples of each diet were collected weekly throughout the trial and stored at −20°C for analysis.
Table 1.
Ingredient composition and nutrient content of control (CON) and experimental diets fed to weaned pigs (as-fed basis)1
| Phase I | Phase II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | BPSBM+SDP2 | BPSBM+FM3 | BPSBM4 | CON | BPSBM+SDP | BPSBM+FM | BPSBM | Phase III |
| Ingredient, % | |||||||||
| Corn | 42.65 | 41.07 | 37.57 | 34.40 | 56.40 | 54.93 | 51.31 | 48.02 | 61.21 |
| BPSBM5 | – | 7.50 | 12.00 | 20.00 | – | 5.00 | 8.00 | 15.00 | – |
| Whey, dried | 25.00 | 25.00 | 25.00 | 25.00 | 10.00 | 10.00 | 10.00 | 10.00 | – |
| Soya oil | 1.25 | 1.45 | 1.00 | 3.40 | 1.00 | 1.50 | 1.40 | 1.80 | 2.10 |
| Select Menhaden fish meal | 7.50 | – | 7.50 | – | 5.00 | – | 5.00 | – | – |
| Blood plasma6 | 6.50 | 6.50 | – | – | 3.00 | 3.00 | – | – | – |
| Soybean meal, dehulled (46.5%) | 15.00 | 15.00 | 15.00 | 15.00 | 22.00 | 22.00 | 22.00 | 22.00 | 33.00 |
| L-Lys HCl | 0.05 | 0.33 | 0.04 | 0.33 | 0.18 | 0.37 | 0.09 | 0.21 | 0.50 |
| DL-Met | 0.19 | 0.19 | 0.14 | 0.17 | 0.17 | 0.15 | 0.10 | 0.10 | 0.13 |
| L-Thr | – | 0.03 | – | 0.08 | – | 0.07 | – | – | 0.18 |
| L-Trp | – | 0.03 | – | 0.07 | – | 0.03 | – | 0.02 | 0.03 |
| Limestone | 0.70 | 1.20 | 0.60 | 1.10 | 1.00 | 1.20 | 0.85 | 1.20 | 1.20 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Monocalcium phosphate | 0.30 | 0.85 | 0.30 | 1.00 | 0.60 | 1.10 | 0.60 | 1.00 | 1.20 |
| Vitamin premix7 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix8 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Zinc oxide | 0.40 | 0.40 | 0.40 | 0.40 | 0.20 | 0.20 | 0.20 | 0.20 | – |
| Formulated content | |||||||||
| Total Lys, % | 1.78 | 1.72 | 1.76 | 1.72 | 1.55 | 1.51 | 1.56 | 1.53 | 1.46 |
| SID9 Lys, % | 1.58 | 1.56 | 1.55 | 1.55 | 1.37 | 1.36 | 1.37 | 1.36 | 1.31 |
| Met + Cys:Lys ratio, % | 0.97 | 0.86 | 0.91 | 0.83 | 0.85 | 0.75 | 0.80 | 0.75 | 0.68 |
| Thr:Lys ratio, % | 1.01 | 0.93 | 0.98 | 0.95 | 0.81 | 0.80 | 0.84 | 0.80 | 0.80 |
| Ile:Lys ratio, % | 1.10 | 1.12 | 0.97 | 1.01 | 0.87 | 0.89 | 0.88 | 0.94 | 0.71 |
| Val:Lys ratio, % | 1.15 | 1.04 | 1.15 | 1.03 | 0.95 | 0.87 | 1.00 | 0.97 | 0.73 |
| Trp:Lys ratio, % | 0.33 | 0.31 | 0.31 | 0.32 | 0.27 | 0.26 | 0.27 | 0.26 | 0.23 |
| DE, kcal/kg | 3,614 | 3,573 | 3,559 | 3,559 | 3,541 | 3,533 | 3,549 | 3,537 | 3,540 |
| Lys:DE ratio, g/Mcal | 4.91 | 4.17 | 3.93 | 3.15 | 4.38 | 3.86 | 3.72 | 3.07 | 4.12 |
| Analyzed composition, % | |||||||||
| DM | 91.89 | 91.70 | 91.85 | 92.98 | 91.13 | 91.00 | 90.78 | 92.03 | 88.77 |
| CP | 22.16 | 23.33 | 25.80 | 25.98 | 23.93 | 21.28 | 22.16 | 25.88 | 21.36 |
| Ash | 6.88 | 6.67 | 6.24 | 6.47 | 6.20 | 5.67 | 6.11 | 6.12 | 5.22 |
| Crude fat | 3.73 | 3.72 | 4.31 | 3.64 | 4.13 | 3.65 | 4.06 | 3.59 | 2.87 |
| Crude fiber | 1.42 | 2.05 | 2.01 | 2.64 | 1.96 | 2.07 | 2.51 | 3.52 | 2.52 |
| Lys | 1.88 | 1.58 | 1.53 | 1.45 | 1.42 | 1.46 | 1.80 | 1.95 | 1.89 |
Experimental diets were fed from d 0 to 7 (Phase I) and from d 8 to 21 (Phase II) after weaning. All pigs received a common Phase III diet from d 22 to 35.
BPSBM+SDP = the CON with bioprocessed soybean meal replacing fish meal.
BPSBM+FM = the CON with bioprocessed soybean meal replacing spray-dried plasma.
BPSBM = the CON with bioprocessed soybean meal replacing both fish meal and spray-dried plasma.
BPSBM = bioprocessed soybean meal (Prairie AquaTech, Brookings, SD).
Appetein (APC, Inc., Ankeny, IA).
Provided, per kilogram of complete diet, 11,002 IU vitamin A supplement, 1,651 IU vitamin D3 supplement, 55.1 IU vitamin E supplement, 0.044 mg vitamin B12 supplement, 4.4 mg menadione as menadione dimethylpyrimidinol bisulfite, 9.91 mg riboflavin supplement, 60.6 mg D-pantothenic acid as d-calcium, 55.1 mg niacin supplement, 1.1 mg folic acid, 3.3 mg pyridoxine as pyridoxine hydrochloride, 3.3 mg thiamine as thiamine mononitrate, and 0.171 mg biotin.
Provided, per kilogram of the complete diet, 165 mg Zn as zinc sulfate, 165 mg Fe as ferrous sulfate, 43.5 mg Mn as manganese sulfate, 16.5 mg Cu as basic copper chloride, 0.36 mg I as ethylenediamine dihydriodide, and 0.3 mg of Se as sodium selenite.
SID = standardized ileal digestiblity.
Pigs were housed in an environmentally controlled room where the initial room temperature was 28 ± 1°C, which was decreased at a rate of 1°C/wk. Animal care followed South Dakota State University protocol including daily monitoring for signs of poor health (i.e., huddling, diarrhea, anorexia, and lethargy). When necessary, veterinary intervention was applied (e.g., Excenel [Zoetis Inc., Kalamazoo, MI] with an intramuscular injection at 1 mL/17 kg once daily for 3 d for diarrhea requiring antibiotic treatment). Individual pigs were removed when veterinary intervention was unsuccessful (i.e., BW loss or no BW gain after d 21 on trial). Average daily gain, ADFI, and G:F were determined by weighing pigs and measuring feed disappearance at 7-d intervals.
Antigen-Specific Immune Response
At d 7 after weaning, 1 pig per pen was selected based on the mean weight of the pen and sensitized with ovalbumin (OVA) and killed Candida albicans (CAA) to assess antibody-mediated immune response and cell-mediated immune response as described by Heriazon et al. (2009) with modifications (Fig. 1). Briefly, pigs were sensitized using 0.5 mg OVA (Grade VII; Sigma-Aldrich Corp., St. Louis, MO), 0.5 mg CAA (crude whole cell; Greer Laboratories, Inc., Lenoir, NC), and 0.5 mg of Quil A (Quillaja saponin; Brenntag Biosector A/S, Frederikssund, Denmark) in 1.0 mL of saline, with an intramuscular injection on d 7 (prime) and 21 (booster) after weaning using a 21-gauge needle. Blood was collected into nonheparinized blood collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) prior to sensitization on d 7 and 21 and again on d 28 to determine presensitization, primary, and secondary anti-OVA IgG as a measure of antibody-mediated immune response. A dermal sensitivity test against OVA and CAA was performed at 28 d after weaning to determine cell-mediated immune response. Pigs received 3 separate 100-µL intradermal injections on the belly: 1) saline, 2) OVA (4 mg/mL in saline), and 3) candin (4 mg/mL in saline; C. albicans purified protein extract; Greer Laboratories, Inc.). Skin thickness on the belly was measured using a skin-fold caliper (model RH15 9LB; Creative Health Products, Inc., Ann Arbor, MI) prior to injection and at 2, 6, 24, and 48 h after injection.
Figure 1.
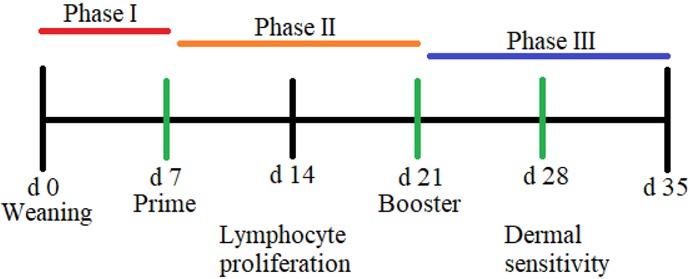
Timeline of sensitization to ovalbumin and Candida albicans, dermal hypersensitivity test, and collection of peripheral blood mononuclear cells for lymphocyte proliferation. Pigs were weaned at 21 ± 1 d of age. Experimental diets were fed in Phase I and II, and a common nursery diet was fed in Phase III.
Anti-Ovalbumin IgG ELISA
Serum antibodies from blood samples taken on d 7, 21, or 28 were determined using an anti-OVA IgG ELISA assay developed at South Dakota State University . A 10-µL serum sample from each pig of the negative control (d 7), primary (d 21), and secondary (d 28) time points were pooled, and the appropriate serum and conjugate dilutions were determined by chessboard titration. Ninety-six–well, high-affinity, flat-bottom polystyrene plates (Corning Inc., Kennebunk, ME) were coated with OVA dissolved in 1 mL carbonate–bicarbonate buffer (pH 9.6) and incubated at 4°C for 24 h. Plates were then washed (100 µL/well) with PBS and 0.05% Tween 20 (Sigma-Aldrich Corp.; washing buffer, pH 7.4) 5 times and blocked with ELISA Ultrablock (200 µL/well; Bio-Rad Laboratories, Inc., Hercules, CA) at room temperature for 1 h and again washed 5 times. Controls and samples were diluted 1:400 and 1:800 and incubated in triplicate for 2 h at room temperature. Plates were washed 5 times, and alkaline phosphatase-conjugated rabbit anti-swine IgG (Sigma-Aldrich Corp., St. Louis, MO) was diluted 1:8,000 in wash buffer and incubated for 1 h at room temperature. After washing the plates 5 times, p-nitrophenyl phosphate disodium (Sigma-Aldrich Corp.) was added and incubated in the dark for 30 min at room temperature. Plates were read on a Spectra MAX 190 plate reader (Molecular Devices, LLC, Sunnyvale, CA) at the 405 nm wavelength, and the optical density values were obtained using SoftMax Pro 6.2.1 Microplate Data Acquisition and Analysis Software (Molecular Devices, LLC).
Peripheral Blood Mononuclear Cell Separation
At 14 d after weaning, a blood sample was collected into sterile vacutainer tubes (Becton, Dickinson and Company) containing K2 EDTA from 1 pig/pen for peripheral blood mononuclear cell (PBMC) separation. Blood was diluted with 15 mL of 1x PBS (pH 7.4) and then gently layered over 15 mL of Histopaque-1077 (Sigma-Aldrich Corp.) into a sterile 50-mL conical vial. Samples were centrifuged at 800 × g for 30 min at room temperature with no brake. The PBMC layer or “white buffy coat” was then carefully collected into a 15-mL conical vial, mixed with 2 mL of water to lyse red blood cell contamination, and then brought to 15 mL with 1x PBS (pH 7.4). Cells were centrifuged at 400 × g for 15 min at room temperature, supernatant was poured off, and cells were resuspended in 1x PBS (pH 7.4). This process was repeated 5 times, and after the fifth wash, cells were suspended in a cell culture medium containing RPMI-1640 (Sigma-Aldrich Corp.) with 10% fetal bovine serum (Sigma-Aldrich Corp.) and 1% PenStrep (100 units/mL penicillin and 100 µg/mL streptomycin; Sigma-Aldrich Corp.). Cells were counted using Trypan blue (Fisher Scientific, Hampton, NH) and a hemocytometer, and cells were diluted to 4 × 106 cells/mL for lymphocyte proliferation.
Lymphocyte Proliferation
Diluted PBMC were plated on Corning Costar 96-well flat-bottom high-affinity plates (Corning Inc.) at a concentration of 2 × 105 cells/mL for a total volume of 100 µL, with 15 wells/sample. Cells were plated in 3 sets of quintuplicate wells for each treatment: 1) cells with no mitogens, which served as a negative control; 2) cells with phytohemagglutinin (PHA-P; 5 µg/mL; Sigma-Aldrich Corp.); and 3) cells with concanavalin A (ConA; 5 µg/mL; Sigma-Aldrich Corp.). Plates were incubated for 96 h at 37°C and 5% CO2. After 96 h, a Roche Applied Biosciences cell proliferation ELISA BrdU kit (colorimetric; catalog number 11 647 229; Roche Applied Biosciences, Mannheim, Germany) was used according to manufacturer's instructions to measure cell proliferation.
Statistical Analysis
Data were analyzed using PROC MIXED of SAS (version 9.3; SAS Inst. Inc., Cary, NC). Pen was the experimental unit for the performance data, and individual pig was the experimental unit for the antibody-mediated immune response, cell-mediated immune response, and lymphocyte proliferation data. Assumption of homogeneity of variances and normality were confirmed prior to ANOVA using PROC GLM in SAS. Least squares means were calculated using the LSMEANS procedure in SAS, and significant differences among treatments were separated using PDIFF option with the Tukey adjustment for the performance data, antibody-mediated immune response, and lymphocyte proliferation. Test results were considered significant where P < 0.05 and tendency toward significance at 0.05 ≥ P ≤ 0.10.
RESULTS
A total of 7 pigs were removed from block 1 and 7 pigs were removed in block 2 for a total removal of 4 from the CON group, 3 from the BPSBM+SDP group, 4 from the BPSBM+FM group, and 3 from the BPSBM group for reasons previously described. There was no difference in initial BW between treatments or by block (Table 2). Pigs fed the CON were heavier (P < 0.01) than pigs fed the BPSBM+FM and BPSBM and not different from pigs fed the BPSBM+SDP at d 7 after weaning. Also, pigs fed the BPSBM+SDP and BPSBM were not different at d 7. Pigs fed the CON remained heavier (P < 0.035) than pigs fed the BPSBM+FM at d 14 and 21 and tended (P = 0.068) to be heavier than pigs fed the BPSBM+FM at d 28. Pigs fed the CON, BPSBM+SDP, or BPSBM were not different at d 14, 21, or 28. There was no difference in BW at d 35 between dietary treatment groups. In Phase I, pigs fed the CON had a greater (P = 0.005) ADG compared with pigs fed the BPSBM+FM and BPSBM but were not different from pigs fed the BPSBM+SDP and pigs fed the BPSBM+SDP were not different from pigs fed the BPSBM. Pigs fed the CON tended to have greater (P = 0.091) ADG than pigs fed the BPSBM+FM or were not different than pigs fed the BPSBM+SDP or BPSBM at the end of Phase II. There was no difference in ADG at the end of Phase III. There was no difference in ADFI at the end of Phase I, II, and III. Similarly, there was no difference in G:F at the end of Phase I and III. However, pigs fed the BPSBM+SDP tended (P = 0.065) to have a greater G:F than pigs fed the BPSBM and were not different from pigs fed the CON or BPSBM+FM at the end of Phase II. There was an effect of block, where initial weight was not different between blocks and pigs in block 1 were heavier than pigs in block 2 at the end of each phase (6.75 ± 0.18 and 6.36 ± 0.18 kg, respectively [P = 0.137], at the beginning; 7.16 ± 0.06 and 6.28 ± 0.06 kg, respectively [P < 0.001], in Phase I; 12.76 ± 0.15 and vs. 11.21 ± 0.15 kg, respectively [P < 0.001], in Phase II; and 22.06 ± 0.26 and 19.38 ± 0.27 kg, respectively [P < 0.001], in Phase III). Pigs in block 1 also had a greater ADG compared with pigs in block 2 at the end of each phase (0.09 ± 0.01 and −0.03 ± 0.01 kg, respectively [P < 0.001], in Phase I; 0.43 ± 0.01 and 0.35 ± 0.01 kg, respectively, [P < 0.001] in Phase II; and 0.67 ± 0.01 and 0.56 ± 0.01 kg, respectively [P < 0.001], in Phase III). Similarly, pigs in block 1 had a greater ADFI compared with pigs in block 2 at the end of each phase (0.15 ± 0.005 and 0.06 ± 0.005 kg, respectively [P < 0.001], in Phase I; 0.53 ± 0.01 and 0.45 ± 0.01 kg, respectively [P < 0.001], in Phase II; and 0.98 ± 0.03 and 0.88 ± 0.03 kg, respectively [P = 0.007], in Phase III). The effect of block on G:F followed a similar pattern (data not shown). However, there was no difference in the pattern of response to diet.
Table 2.
Effect of dietary inclusion of bioprocessed soybean meal (BPSBM) alone or in combination with fish meal (FM) and spray-dried plasma (SDP) on growth performance of weaned pigs1
| Item | CON | BPSBM+SDP | BPSBM+FM | BPSBM | SEM | P-value |
|---|---|---|---|---|---|---|
| BW, kg | ||||||
| d 0 | 6.69 | 6.52 | 6.52 | 6.51 | 0.26 | 0.949 |
| d 7 | 6.99a | 6.80abx | 6.52cy | 6.60bc | 0.08 | 0.001 |
| d 14 | 8.74a | 8.47ab | 7.74b | 8.19ab | 0.24 | 0.035 |
| d 21 | 12.47a | 12.18ax | 11.42by | 11.85ab | 0.21 | 0.011 |
| d 28 | 16.27x | 16.08xy | 15.12y | 15.86xy | 0.31 | 0.068 |
| d 35 | 21.00 | 20.70 | 20.28 | 20.89 | 0.37 | 0.554 |
| ADG, kg/d | ||||||
| Phase I | 0.06a | 0.04ab | 0.02b | 0.01b | 0.01 | 0.005 |
| Phase II | 0.41x | 0.40xy | 0.36y | 0.39xy | 0.02 | 0.091 |
| Phase III | 0.60 | 0.59 | 0.62 | 0.64 | 0.02 | 0.168 |
| ADFI, kg/d | ||||||
| Phase I | 0.12 | 0.11 | 0.10 | 0.11 | 0.01 | 0.129 |
| Phase II | 0.50 | 0.49 | 0.46 | 0.51 | 0.02 | 0.139 |
| Phase III | 0.92 | 0.92 | 0.94 | 0.95 | 0.03 | 0.843 |
| G:F | ||||||
| Phase I | 0.27 | 0.02 | −0.16 | −0.19 | 0.16 | 0.170 |
| Phase II | 0.80ab | 0.83a | 0.80ab | 0.77b | 0.02 | 0.065 |
| Phase III | 0.66 | 0.64 | 0.67 | 0.68 | 0.02 | 0.717 |
Means within a row with different superscripts differ (P < 0.05).
Means within a row with different superscripts differ (P < 0.10).
A total of 239 pigs (6.56 ± 0.87 kg initial BW) in 48 pens (3 barrows and 3 gilts/pen; n = 10 pens/treatment) were used in a 35-d study. Experimental diets were fed from d 0 to 7 (Phase I) and d 8 to 21 (Phase II) after weaning. All pigs received a common Phase III diet from d 22 to 35. CON = control; a corn, soybean meal, whey (25%), FM (7.5%), and SDP (6.5%)–based diet with decreasing inclusion of whey (10%), FM (5%), and SDP (3%) from Phase I to Phase II, respectively. BPSFM+SDP = the CON with BPSBM replacing FM (7.5 and 5% in Phase I and Phase II, respectively). BPSBM+FM = the CON with BPSBM replacing SDP (12 and 8% in Phase I and Phase II, respectively). BPSBM = the CON with BPSBM replacing both FM and SDP (20 and 15% in Phase I and Phase II, respectively).
Immune Response
There were no differences between pigs fed the CON, BPSBM+SDP, BPSBM+FM, or BPSBM in the dermal hypersensitivity response to OVA at 0, 2, 6, 24, or 48 h after injection. The hypersensitivity response to OVA peaked (P < 0.001) at 2 h after injection in pigs fed the CON, BPSBM+SDP, and BPSBM+FM but at 6 h after injection in pigs fed the BPSBM (Fig. 2A). There were no differences in swelling from 6 to 24 h in pigs fed the CON, BPSBM+SDP, and BPSBM+FM, but pigs fed the BPSBM tended (P = 0.053) to have a greater swelling at 6 h compared with 24 h after injection. There were no differences in swelling after injection regardless of dietary treatment. The hypersensitivity response to candin peaked (P < 0.01) at 2 h after injection in pigs fed the CON, BPSBM+SDP, BPSBM+FM, and BPSBM (Fig. 2B). There were no differences in swelling from 6 to 24 h in pigs fed the CON, BPSBM+SDP, and BPSBM+FM, but pigs fed the BPSBM had greater (P = 0.038) swelling at 6 h compared with 24 h after injection. There were no differences between pigs fed the CON, BPSBM+SDP, BPSBM+FM, or BPSBM. Pigs in block 2 had a greater response to OVA at 2 h after injection than pigs in block 1 (132.9 ± 9.25 and 96.8 ± 9.04%, respectively; P = 0.007). Similarly, pigs in block 2 tended to have a greater response to candin at 2 h after injection than pigs in block 1 (51.6 ± 8.25 and 33.0 ± 4.80%, respectively; P = 0.057). At 6 h after injection, pigs in block 2 also had a prolonged response to candin compared with pigs in block 1 (31.6 ± 4.51 and 15.6 ± 4.82%, respectively; P = 0.017). There was no significant treatment × block interaction.
Figure 2.
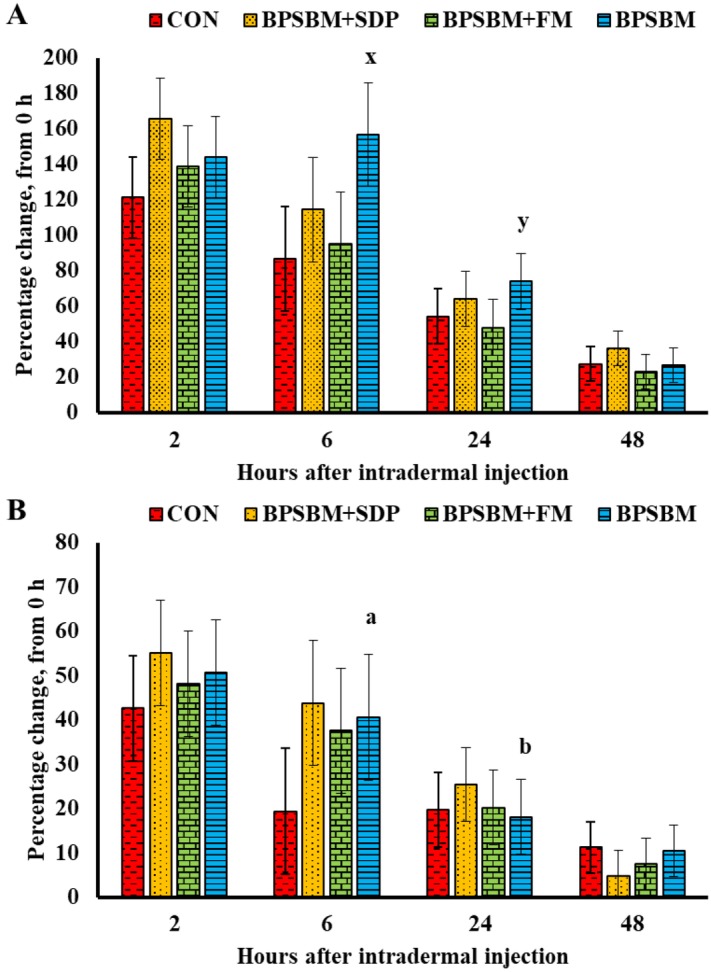
Dermal hypersensitivity to ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO; A) and candin (Greer Laboratories, Inc., Lenoir, NC; B) on the belly of pigs fed diets containing fish meal (FM), spray-dried plasma (SDP), bioprocessed soybean meal, or their combination. Pigs were given an intramuscular injection (0.5 mg Quil A [Brenntag Biosector A/S, Frederikssund, Denmark], 0.5 mg OVA, and 0.5 mg killed Candida albicans in 1 mL saline) at d 7 and 21, and a dermal hypersensitivity test was conducted on d 28. Pigs received 3 intradermal injections: 1) saline (100 µL), 2) OVA (4 mg OVA/mL in 100 µL), and 3) candin (4 mg/mL; C. albicans purified protein extract in 100 µL) on the belly, and skin fold thickness was measured at 0, 2, 6, 24, and 48 h after injection. The percent increase in skin thickness over baseline was calculated. Data represents least squares means and SEM of 10 pigs/dietary treatment. a,bWithin dietary treatment, time points with different superscripts differ (P < 0.05). x,yWithin dietary treatment, time points with different superscripts differ (P < 0.1). CON = control; BPSBM+SDP = the CON with bioprocessed soybean meal replacing FM; BPSBM+FM = the CON with bioprocessed soybean meal replacing SDP; BPSBM = the CON with BPSBM replacing both FM and SDP.
Anti-OVA IgG at 21 and 28 d after sensitization was higher (P = 0.051 and P < 0.001, respectively) compared with that at d 0, indicating a primary and secondary anti-OVA IgG response (Fig. 3). There was no effect of dietary treatment on anti-OVA IgG; however, the 21-d time point is the mean of 5 observations/dietary treatment, rather than 10, due to an error during blood collections in block 1.
Figure 3.
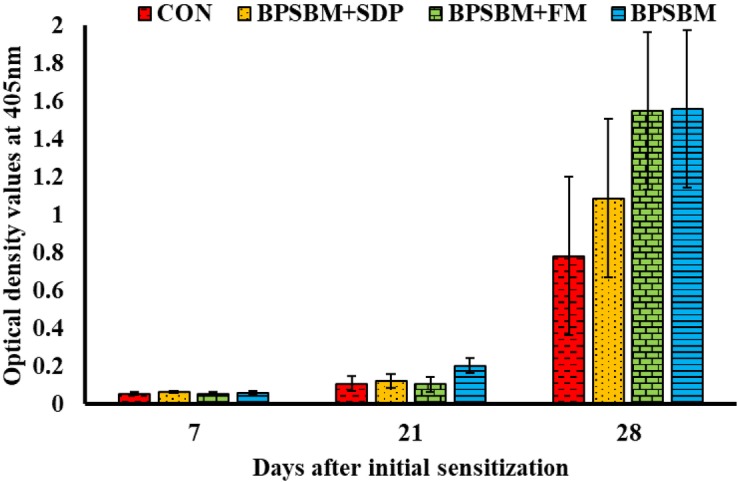
Anti-ovalbumin (OVA) IgG in pigs fed diets containing fish meal (FM), spray-dried plasma (SDP), bioprocessed soybean meal, or their combination. The control (CON) diets contained FM and SDP. Pigs were intramuscularly injected with 0.5 mg Quil A (Brenntag Biosector A/S, Frederikssund, Denmark), 0.5 mg OVA (Sigma-Aldrich, St. Louis, MO), and 0.5 mg killed Candida albicans in 1 mL of saline at d 7 and 21, representing the prime and booster doses. Serum was collected at 7, 21, and 28 d for determination of presensitization, primary, and secondary anti-OVA IgG. Data represents least squares means and SEM of 10 pigs/dietary treatment at d 7 and 21, and 5 pigs/dietary treatments at d 28. BPSBM+SDP = the CON with bioprocessed soybean meal replacing FM; BPSBM+FM = the CON with bioprocessed soybean meal replacing SDP; BPSBM[ = the CON with bioprocessed soybean meal replacing both FM and SDP.
Proliferation of PBMC in response to mitogenic stimulation with PHA-P and ConA was greater than the negative control (1.15 and 2.14, pooled SEM 0.22 ± 0.08 optical density at 370 nm [OD370nm; P = 0.001], respectively, where the negative control had no mitogens added, just cells in cell culture media), confirming the biological effects of PHA-P and ConA at the concentrations used in this study (Fig. 4). Values are based on optical density readings from the ELISA assay. There were significant differences between blocks, where the proliferative response in peripheral blood lymphocytes from pigs in block 2 exposed to PHA-P and ConA was greater than that from pigs in block 1 (1.93 and 2.91 vs. 0.37 and 1.37, pooled SEM 0.11 OD370nm, respectively; P < 0.001). There were no differences between block 1 and 2 for negative control cells (0.23 vs. 0.22, pooled SEM 0.11 OD370nm, respectively; P = 0.192). No difference in proliferation among dietary treatment was detected.
Figure 4.
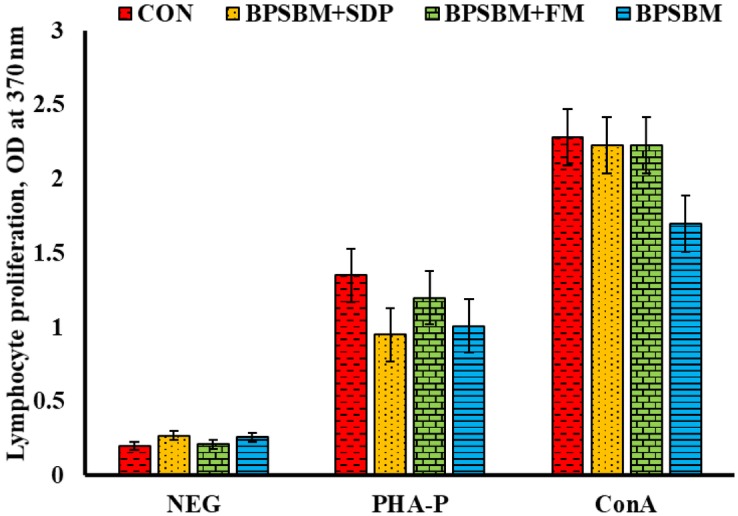
Lymphocyte proliferation in pigs fed diets containing fish meal (FM), spray-dried plasma (SDP), bioprocessed soybean meal (BPSBM), or their combination. The control (CON) diets contained FM and SDP. Proliferation response to phytohemagglutinin (PHA-P) and concanavalin A (ConA) was determined on d 14 after weaning. Data represents least squares means and SEM of 10 pigs/dietary treatment. BPSBM+SDP = the CON with bioprocessed soybean meal replacing FM; BPSBM+FM = the CON with bioprocessed soybean meal replacing SDP; BPSBM = the CON with bioprocessed soybean meal replacing both FM and SDP; OD = optical density; NEG = cells with no mitogenic stimulation.
DISCUSSION
Antinutritional factors in SBM may be detrimental for newly weaned pigs. Further processing of SBM can significantly reduce the levels of antinutritional factors and allow these processed SBM products to be used in weaned pig diets (Min et al., 2004; Sinn et al., 2016) at higher levels with minimal negative impact on growth performance. The aim of this study was to assess nursery pig growth performance and immune response to BPSBM alone or in combination with SDP or FM in early weaning diets. We hypothesized that inclusion of bioprocessed soybean meal in nursery pig diets could enhance the innate and adaptive immune response of weaned pigs. By the end of Phase III, there were no differences in BW among dietary treatment groups despite slower growth in the first 7 d after weaning, confirming that bioprocessed soybean meal could replace SDP or FM with little long-term impact on pig performance. Feed intake during the first week after weaning is typically a challenge, and therefore, many studies often start at 7 d after weaning in an attempt to minimize the effects of weaning and focus on the dietary effects. Jones et al. (2010) observed a similar reduced performance in the early postweaning period in pigs fed diets containing the combination of FM and fermented SBM compared with pigs fed a combination of dried porcine solubles and fermented SBM.
Zhou et al. (2011) reported that feeding enzymatically converted SBM increased the percentage of T lymphocytes and CD4+ and CD8+ cells in peripheral blood of weaned pigs compared with feeding a corn/soy diet. This increase in T lymphocytes is beneficial to a young pig that is heavily reliant on its innate immune system as the primary defense against pathogens. The boost in cellular immunity from an enzymatically converted SBM would suggest that it is reasonable to expect a similar product such as bioprocessed soybean meal to have an impact on immune response. The prolonged hypersensitivity response to OVA in pigs fed BPSBM would indicate an enhanced T cell recruitment, which would benefit a piglet facing an immune challenge. Furthermore, evidence of phytoestrogens and other soy isoflavones found in SBM have been shown to provide immunological benefits (Greiner et al., 2001; Patisaul and Jefferson, 2010), and the presence of estrogen receptors within the gastrointestinal tract (Campbell-Thompson et al., 2001) may positively impact the gut by favoring cell proliferation and digestive function. The removal of antinutritional factors during processing of the soybean meal helps improve digestive function and may provide immunological benefits. To investigate the antigen-specific immune response, dermal hypersensitivity was measured as an estimate of T cell recruitment to the site of inflammation and as an indicator of cell-mediated immune response. The hypersensitivity response to OVA and CAA are T-helper type 2 and T-helper type 1 responses representing immediate and delayed-type hypersensitivity responses, respectively. The peak response to CAA at 2 h in this study differed from the response to CAA reported in cattle and sheep, where delayed hypersensitivity (T-helper type 1; i.e., peak at 24 to 72 h) was reported (You et al., 2008; Heriazon et al., 2009). It is unclear why the dermal response in this study peaked at 2 h after injection rather than 24 to 48 h after injection; however, Kabe et al. (1971) reported an immediate response to aerosol polysaccharide fraction inhalation from CAA in guinea pigs as well as a delayed response 24 h after exposure. Although there was a prolonged response in pigs fed 3 of the experimental diets, peak swelling still clearly occurred at 2 h after injection. The present data indicate that CAA may elicit dual reactions of both immediate and delayed responses, and therefore, an alternative antigen such as Mycobacterium tuberculosis may be needed to elicit a delayed-type dermal hypersensitivity response in pigs.
Although the difference in anti-OVA IgG was not significant, it is important to note the low number of replicates and potential when more replicates are added. An increase in circulating antibodies would be beneficial by potentially allowing the pig to clear an infection faster and more efficiently, thus improving health. This enhancement of the antibody-mediated immune response will need to be evaluated using a practically relevant antigen/vaccination before definite conclusions can be made.
Phytohemagglutinin and ConA are T cell mitogens used to stimulate proliferation in cell culture assays as a measurement of nonspecific cell-mediated immune response. Dietary protein source did not influence PBMC proliferation from mitogenic stimulated cells and negative control cells. The greater proliferation from mitogenic stimulation in block 2, in combination with the lower performance compared with pigs in block 1, indicate that pigs in block 2 were likely experiencing a subclinical challenge. However, responses to diets were not different. This is important because it is often argued that university-run trials are performed in very clean, low-stress environments and may not be representative of what would be observed in the field. The proposed subclinical health challenge that pigs in block 2 underwent would be more representative of a commercial environment. Although this was not the intention, the results are valuable and support the hypothesis that BPSBM can replace both SDP and FM in nursery pig diets. Additional evaluation of the potential for enhanced antibody production with the inclusion of BPSBM following industry-relevant vaccination is warranted.
Footnotes
RSK was funded and supported by NIFA SDSU Agricultural Experiment Station Hatch grant # SD00H547-15
LITERATURE CITED
- Campbell-Thompson M., Lynch I. J., Bhardwaj B. 2001. Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res. 61:632–640. [PubMed] [Google Scholar]
- Choct M., Dersjant-Li Y., McLeish J., Peisker M. 2010. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 23:1386–1398. doi: 10.5713/ajas.2010.90222 [DOI] [Google Scholar]
- Cromwell G. L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7–27. doi: 10.1081/ABIO-120005767 [DOI] [PubMed] [Google Scholar]
- DeRouchey J., Goodband R., Tokach M., Nelssen J., Dritz S. 2010. Nursery swine nutrient recommendations and feeding management. In: Mesinger D. J. editor, National swine nutrition guide. U.S. Pork Center of Excellence, Ames, IA: p. 65–79. [Google Scholar]
- Gibbons W. R., Brown M. L. 2016. Microbial-based process for high-quality protein concentrate. U.S. Patent 9,370,200. Date issued: 21 June. [Google Scholar]
- Greiner L., Stahly T., Stabel T. 2001. The effect of dietary soy genistein on pig growth and viral replication during a viral challenge. J. Anim. Sci. 79:1272–1279. doi: 10.2527/2001.7951272x [DOI] [PubMed] [Google Scholar]
- Heriazon A., Thompson K. A., Wilkie B. N., Mathes-Sears W., Quinton M., Mallard B. A. 2009. Antibody to ovalbumin and delayed-type hypersensitivity to Candida albicans and mycobacteria in lactating Holstein cows using Quil A or Freund's complete adjuvant. Vet. Immunol. Immunopathol. 127:220–227. doi: 10.1016/j.vetimm.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Jones C., DeRouchey J., Nelssen J., Tokach M., Dritz S., Goodband R. 2010. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci. 88:1725–1732. doi: 10.2527/jas.2009-2110 [DOI] [PubMed] [Google Scholar]
- Kabe J., Aoki Y., Miyamoto T. 1971. Antigenicity of fractions from extracts of Candida albicans: The immediate and delayed-type respiratory responses in guinea pigs. J. Allergy Clin. Immunol. 47:59–75. doi: 10.1016/S0091-6749(71)80372-X [DOI] [PubMed] [Google Scholar]
- Kim S., Van Heugten E., Ji F., Lee C., Mateo R. 2010. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88:214–224. doi: 10.2527/jas.2009-1993 [DOI] [PubMed] [Google Scholar]
- Li D. F., Nelssen J. L., Reddy P. G., Blecha F., Hancock J. D., Allee G. L., Goodband R. D., Klemm R. D. 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 68:1790–1799. doi: 10.2527/1990.6861790x [DOI] [PubMed] [Google Scholar]
- Li D. F., Nelssen J. L., Reddy P. G., Blecha F., Klemm R., Goodband R. D. 1991. Interrelationship between hypersensitivity to soybean proteins and growth performance in early-weaned pigs. J. Anim. Sci. 69:4062–4069. doi: 10.2527/1991.69104062x [DOI] [PubMed] [Google Scholar]
- Min B., Hong J., Kwon O., Lee W., Kim Y., Kim I., Cho W., Kim J. 2004. The effect of feeding processed soy protein on the growth performance and apparent ileal digestibility in weanling pigs. Asian-Australas. J. Anim. Sci. 17:1271–1276. doi: 10.5713/ajas.2004.1271 [DOI] [Google Scholar]
- Patisaul H. B., Jefferson W. 2010. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 31:400–419. doi: 10.1016/j.yfrne.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn S., Gibbons W., Brown M., DeRouchey J., Levesque C. 2016. Evaluation of microbially enhanced soybean meal as an alternative to fishmeal in weaned pig diets. Animal 11(5):784–793. doi: 10.1017/S1751731116002020 [DOI] [PubMed] [Google Scholar]
- You Q., Karrow N. A., Quinton M., Mallard B. A., Boermans H. J. 2008. Enhanced cutaneous hypersensitivity reactions are associated with ovine high and low cortisol responsiveness to acute endotoxin challenge. Vet. Dermatol. 19:174–183. doi: 10.1111/j.1365-3164.2008.00664.x [DOI] [PubMed] [Google Scholar]
- Zhou S., Sun Z., Ma L., Yu J., Ma C., Ru Y. 2011. Effect of feeding enzymolytic soybean meal on performance, digestion and immunity of weaned pigs. Asian-Australas. J. Anim. Sci. 24:103–109. doi: 10.5713/ajas.2011.10205 [DOI] [Google Scholar]


