ABSTRACT
The objective of this study was to examine the effect of percent Brahman genetics on Warner–Bratzler shear force (WBSF), desmin and troponin-T (TnT) degradation, hydroxylysyl pyridinoline (HP) crosslink content, and perimysial collagen melting temperature. Steers (n = 131) produced in 2012 and 2013 were harvested at 1.27 cm of visual s.c. back fat thickness. Steers were divided into 4 genetic categories consisting of steers that contained 6/32 or less Brahman genetics, 12/32 Brahman genetics, 14/32 to 18/32 Brahman genetics, and 23/32 to 32/32 Brahman genetics. Twenty-four hours after harvest, a 7.62-cm piece of the longissimus lumborum beginning at the 13th rib was collected and aged for 14 d. Following aging, three 2.54-cm steaks were cut for WBSF, trained sensory panel, and laboratory analyses. Laboratory analyses steaks were used to determine protein degradation, HP crosslink analysis, and perimysial collagen melting temperature. Data were analyzed using a polynomial regression for unequally spaced treatments. As the percent Brahman genetics increased, WBSF increased (linear, P = 0.01). As percent Brahman genetics increased, tenderness score decreased (less tender) and connective tissue score increased (more connective tissue; linear, P = 0.01). As the percentage of Brahman genetics increased, the amount of degraded desmin (38 kDa) and TnT (34 and 30 kDa) decreased (linear, P < 0.03) whereas the amount of immunoreactive 36 kDa TnT increased (linear, P = 0.04). Percent Brahman genetics had no effect (P = 0.14) on HP crosslink content but did tend to increase (P = 0.07) perimysial collagen melting temperature as the percent Brahman increased. The percentage of Brahman genetic influence was positively correlated to WBSF (r = 0.25), 36 kDa immunoreactive TnT (r = 0.26), and perimysial collagen melting temperature (r = 0.25, P = 0.01). Sensory panel tenderness (r = −0.44), juiciness (r = −0.26), and connective tissue scores (r = −0.63); 38 kDa degraded desmin (r = −0.34), 34 (r = −0.36) and 30 kDa degraded TnT (r = −0.29); and HP collagen crosslinks (r = −0.20) were negatively correlated to percent Brahman genetic influence (P < 0.03). Increasing Brahman genetic influence in steers negatively affects tenderness, partially through a reduction in degradation of desmin and TnT. Although HP collagen crosslinks are unaffected by Brahman genetics, a tendency for increased perimysium melting temperature indicates that other collagen-stabilizing crosslinks may be affected.
Keywords: Angus, Brahman, collagen crosslink, proteolysis, tenderness
INTRODUCTION
Tenderness is the most important, but variable, attribute contributing to consumer beef eating satisfaction (Miller et al., 2001). Numerous studies have indicated that steaks from cattle of greater Bos indicus genetic influence were tougher than steaks from Bos taurus cattle (Crouse et al., 1989; Johnson et al., 1990; Pringle et al., 1997). The University of Florida (Gainesville, FL) possesses a unique multibreed herd of cattle that encompasses a spectrum of percentages of Brahman and Angus breeding. This herd serves as an excellent model to study beef palatability, as data collected over 20 yr demonstrated that steaks from cattle with a large Brahman influence were less tender and contained more connective tissue than steaks with less Brahman influence (Elzo et al., 2012). Researchers have attributed decreased tenderness of Brahman steaks to greater postmortem calpastatin activity within the muscle from these animals, which resulted in inhibition of myofibrillar protein degradation (Wheeler et al., 1990; Shackelford et al. 1991; Pringle et al., 1997). Riley et al. (2005) concluded that calpastatin activity explained the difference in tenderness between breeds and that insoluble collagen had the greatest influence on the tenderness of Brahman cattle, which led them to hypothesize that altering insoluble collagen might assist in improving the tenderness of meat from Brahman cattle.
Along with postmortem myofibrillar protein proteolysis and sarcomere length, connective tissue content heavily influences meat tenderness, but its exact contribution has been hard to quantify (for reviews, see Koohmaraie et al., 2002, and Purslow, 2005). Collagen is the major component of intramuscular connective tissue, and formation of crosslinks within the collagen molecule determines its solubility (McCormick, 1999). Using longissimus lumborum (LL) steaks from the same multibreed herd used in this study, Gonzalez et al. (2014) reported an increase in connective tissue and decrease in overall tenderness sensory scores as the percentage of Brahman genetic influence increased. Gonzalez et al. (2014) also reported that the gene expression profile of animals of greater Brahman influence indicated increased collagen crosslinking, which may have negatively influenced the tenderness of those steaks. Therefore, the objective of this study was to examine the effect of Brahman genetics on desmin and troponin-T (TnT) degradation, collagen crosslinking, and meat tenderness of LL steaks.
MATERIALS AND METHODS
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee, and the University of Florida Institutional Review Board approved procedures for use of human subjects in sensory panel evaluations.
Steer Management
Steers (n = 131) in the 2012 and 2013 production cycles were selected from the University of Florida (Gainesville, FL) multibreed herd. An extensive description of the herd and larger genetic study from which the animals used for this experiment were selected was provided by Elzo et al. (2012). Briefly, steers are born from mid December to mid March, kept with their mother on bahiagrass pasture until they are weaned in September, fed a standard corn–protein commercial diet at a contract feeder, and harvested when their pen reached a common visual s.c. back fat thickness of 1.27 cm. Based on the percentage of Angus and Brahman breeding, steers were divided into 4 breed categories: Angus steers that contained 6/32 or less Brahman genetics (n = 21), Brangus steers that contained 12/32 Brahman genetics (n = 34), Half steers contained 14/32 to 18/32 Brahman genetics (n = 57), and Brahman steers contained 23/32 to 32/32 Brahman genetics (n = 19).
Longissimus Lumborum Collection and Processing
Steers were harvested at a commercial abattoir (FPL Food LLC, Augusta, GA). The 2012 cattle were killed approximately every month during June (5 Angus, 2 Brangus, 10 Half, and 5 Brahman), July (1 Angus, 1 Brangus, 2 Half, and 1 Brahman), and August (3Angus, 2 Brangus, 8 Half, and 8 Brahman). The 2013 cattle were killed approximately every month during May (4 Angus, 2 Brangus, 2 Half, and 5 Brahman), June (6 Angus, 6 Brangus, 9 Half, and 13 Brahman), and August (2 Angus, 6 Brangus, 3 Half, and 25 Brahman). Descriptive statistics of the carcass characteristics of steers selected for analyses are presented in Table 1. Following a 24-h chilling period, a 7.62-cm portion of the LL beginning at the 13th rib was removed from the left side of the carcass, vacuum packaged, and aged until 14 d postmortem at 2 ± 1°C. Following aging, three 2.54-cm-thick LL steaks were fabricated from anterior end of each loin portion. Steak 1 was used to measure objective tenderness by Warner–Bratzler shear force (WBSF), steak 2 was used for trained sensory panel evaluation, and steak 3 was used for myofibrillar protein degradation and collagen crosslink analyses. Steaks used for WBSF and sensory analyses were frozen at −40°C until analysis, and steaks designated for laboratory analyses were frozen at −80°C until analysis.
Table 1.
Descriptive statistics of carcass characteristics of steers selected for analyses
| Angus/Brahman genetics1 | |||||
|---|---|---|---|---|---|
| Item | Angus | Brangus | Half | Brahman | SD |
| 2012 | |||||
| No. | 9 | 5 | 20 | 14 | |
| HCW, kg | 333 | 339 | 324 | 308 | 25 |
| Rib eye area, cm2 | 75.7 | 70.6 | 77.0 | 73.4 | 6.5 |
| 12th-rib back fat, cm | 1.3 | 1.3 | 1.5 | 1.2 | 0.5 |
| KPH, % | 2.3 | 2.4 | 2.6 | 2.3 | 0.6 |
| Lean maturity2 | 140 | 142 | 146 | 144 | 9 |
| Bone maturity3 | 140 | 140 | 146 | 139 | 7 |
| Marbling score4 | 524 | 344 | 419 | 350 | 86.7 |
| Quality grade5 | 7.8 | 5.8 | 6.8 | 5.6 | 1.2 |
| Yield grade | 3.5 | 3.8 | 3.4 | 3.2 | 0.6 |
| 2013 | |||||
| No. | 12 | 14 | 14 | 43 | |
| HCW, kg | 347 | 329 | 335 | 307 | 26 |
| Rib eye area, cm2 | 79.1 | 74.9 | 77.2 | 75.2 | 8.1 |
| 12th-rib back fat, cm | 1.5 | 1.3 | 1.4 | 1.3 | 0.3 |
| KPH, % | 2.6 | 2.7 | 2.4 | 2.4 | 0.3 |
| Lean maturity | 139 | 145 | 146 | 146 | 9 |
| Bone maturity | 146 | 149 | 151 | 144 | 10 |
| Marbling score | 597 | 481 | 467 | 390 | 88.4 |
| Quality grade | 8.7 | 7.4 | 7.0 | 6.3 | 1.1 |
| Yield grade | 3.6 | 3.5 | 3.4 | 3.2 | 0.5 |
Steers (n = 131) were classified into 4 categories based on the percentage of Angus and Brahman genetics. The breed groups were steers that contained 6/32 or less Brahman genetics (Angus), steers that contained 12/32 Brahman genetics (Brangus), steers that contained 14/32 to 18/32 Brahman genetics (Half), and steers that contained 23/32 to 32/32 Brahman genetics (Brahman).
100 = A; 200 = B; 300 = C; 400 = D; 500 = E.
100 = A; 200 = B; 300 = C; 400 = D; 500 = E.
100 = practically devoid; 200 = traces; 300 = slight; 400 = small; 500 = modest; 600 = moderate.
4 = standard; 5 = select−; 6 = select+; 7 = choice−; 8 = choice; 9 = choice+; 10 = prime−.
Warner–Bratzler Shear Force and Trained Sensory Panel Analyses
Warner–Bratzler shear force and sensory panel methods were conducted according to guidelines published by the American Meat Science Association (1995). Twenty-four hours prior to cooking, steaks were removed from the freezer and vacuum bag. Frozen steak weights were obtained and steaks were placed on trays with Dri-Loc absorbent pads (Sealed Air Corp., Elmwood Park, NJ) and allowed to thaw at 4 ± 2°C. Following thawing and prior to cooking, thawed steak weight was recorded and thaw loss was calculated ([(frozen steak weight − thawed steak weight)/frozen steak weight] × 100). A thermocouple (OMEGA Engineering, Inc., Stamford, CT) was placed at the geometric center of each steak to monitor internal temperature during cooking using a 1100 Labtech Notebook for Windows 1998 (Computer Boards Inc., Middleboro, MA). Steaks were cooked on preheated Hamilton Beach Indoor/Outdoor open top electric grills (Hamilton Beach Brands, Inc., Washington, NC) to an internal temperature of 35°C, turned once, and cooked until a final temperature of 71°C was reached. After cooking, WBSF steaks were weighed again for cooking loss ([(thawed steak weight − cooked steak weight)/thawed steak weight] × 100), placed on trays, and chilled at 4 ± 2°C for 24 h. Following chilling, six 1.27-cm-diameter cores were collected from each steak parallel to the muscle fibers. Each core was sheared once through the center, perpendicular to the orientation of the muscle fibers, using an Instron Universal Testing Machine (Instron Corp., Canton, MA) equipped with a Warner–Bratzler shear blade set at a crosshead speed of 200 mm/min.
Steak thawing and cooking procedures for sensory panel analysis were followed as described above. Following cooking, steaks were cut into 1.27- by 1.27- by 2.54-cm cubes, and 2 cubes of each sample were presented to each panelist of an 8-member trained sensory panel. Panelists evaluated 6 samples per session in a positive-pressure, ventilated room with cubicles equipped with red incandescent lighting. Panelists evaluated each sample for overall tenderness, connective tissue amount, juiciness, and beef-flavor intensity using 8-point scales (1 = extremely tough, abundant, extremely dry, and extremely bland, respectively, and 8 = extremely tender, none, extremely juicy, and extremely intense, respectively,). Panelists also evaluated samples for any off-flavors using a 6-point scale (1 = extreme off-flavor and 6 = no off-flavor).
Desmin and Troponin-T Western Blot Analysis
Sample preparation for analysis of desmin and TnT was conducted as described by Huff-Lonergan et al. (1996) and modified by Lonergan et al. (2001). Briefly, 0.2 g of sample was homogenized in 5 mL of whole muscle protein extraction buffer (10 mM sodium phosphate and 2% SDS, pH 7.0). The homogenate was centrifuged at 1,500 × g for 15 min at 20°C, and protein concentrations were quantified using a Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Using the protein concentrations, samples were mixed with sample loading/tracking dye so that 45 and 30 µg of protein were loaded into the gel lanes for desmin and TnT, respectively.
Protein separation and western blotting of desmin and TnT were conducted according to procedures described by Melody et al. (2004) with modifications described by Phelps et al. (2015). Protein isolates were separated with 8- by 10-cm 10% polyacrylamide separating gels with 5% polyacrylamide stackers at a constant amperage (40 mA). Proteins were transferred to nitrocellulose membranes (88018; Thermo Scientific) using a TE77X Semi-dry Transfer Unit (Hoefer, Inc., Holliston, MA) at a constant amperage (140 mA) for 1.5 h.
Membranes were blocked with 5% nonfat dry milk (NFDM) in 10 mM Tris, pH 8.0; 150 mM NaCl; and 0.1% Tween-20 (TBS-T) for 1 h at room temperature to block nonspecific antigen binding sites. Desmin blots were incubated for 20 h at 4°C with a whole antiserum rabbit anti-desmin antibody (D8281; Sigma-Aldrich Corporation, St. Louis, MO) diluted 1:15,000 in 1% NFDM and TBS-T. Troponin-T blots were incubated for 20 h at 4°C with a mouse monoclonal anti-TnT antibody (T6277; Sigma-Aldrich Corporation) diluted 1:30,000 in 5% NFDM and TBS-T. Desmin blots were incubated with an anti-rabbit, horseradish peroxidase–linked secondary antibody (7074; Cell Signaling Technology, Inc., Danvers, MA) diluted 1:2,000 in 5% NFDM and TBS-T for 1 h at room temperature. Troponin-T blots were incubated with an anti-mouse, horseradish peroxidase–linked secondary antibody (7076; Cell Signaling Technology, Inc.) diluted 1:20,000 in 5% NFDM and TBS-T for 1 h at room temperature. Membranes were developed using an enhanced chemiluminescence kit (ECL Plus; GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and imaged using a ChemiDoc-It 415 Imaging System (UVP, LLC, Upland, CA). Band intensities were measured using VisionWorksLS Image Acquisition and Analysis Software (UVP, LLC). Intact and degraded forms of desmin measured were immunoreactive bands located at 55 and 38 kDa, respectively. Intact and degraded forms of TnT measured were immunoreactive bands located at 40 (intact), 36, 34, and 30 kDa (degraded), respectively. Bands were normalized to a pooled sample on each blot.
Collagen Crosslinking Analysis
Samples were extracted by procedures described by Smith and Judge (1991) for analysis of the collagen crosslink hydroxylysyl pyridinoline (HP), with modifications. Muscle samples were minced and ground using a coffee grinder (Mr. Coffee; Sunbeam Products, Inc., Pittsburgh, PA) before 0.5 g was dried at 60°C. One hundred milligrams of dried sample was mixed with 2 mL of 6 M HCl and hydrolyzed for 18 h in a 105°C oven. Hydrolysates were allowed to cool in darkness for 30 min and then filtered through Whatman number 1 filter paper (GE Healthcare Bio-Sciences Corp.). After filtration, the pH of samples was adjusted to 7.0 ± 0.1 and the final sample volume was adjusted to 10 mL with ultrapure water. The HP concentration of each sample was measured using a commercial ELISA kit (Microvue PYD; Quidel Corp., San Diego, CA) strictly following the kit instructions. The amount of HP is expressed as nanograms of HP per gram of wet tissue.
Differential Scanning Calorimetry
Extraction of the perimysial connective fraction was conducted according to procedures described by Light and Champion (1984) with modifications. Briefly, 1 g of diced sample was weighed and half (0.5 g) of the sample was homogenized in 1 mL of 0.05 M CaCl2. The homogenate was filtered through a 1-mm2 mesh screen. The remaining 0.5 g of sample was homogenized and filtered in a similar manner. Material that did not pass through the mesh screen from the two 0.5-g extractions was combined and homogenized in 1 mL of the same CaCl2 solution and refiltered a total of 3 times. At the end of the extraction, any nonfiltered material was deemed the perimysial fraction and was lyophilized before being subjected to differential scanning calorimetry (DSC) procedures described by Li et al. (2008) with modifications. Five milligrams of sample was weighed into aluminum pans and sealed. Samples were scanned from 0 to 100°C with a 5°C/min temperature increase using a Shimadzu 201-52943 DSC (Shimadzu Scientific Instruments, Kyoto, Japan). The maximum transition temperature was estimated using TA-60WS software (Shimadzu Scientific Instruments).
Statistical Analyses
All data were analyzed as a generalized randomized complete block design using the MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC). The fixed effect was the breed category and the random effect was the year born. Steer was the experimental unit, with the shape of the response to increasing the percentage of Brahman characterized using polynomial regression for unequally spaced treatments. Differences were considered significant at P ≤ 0.05 and regarded as tendencies at 0.05 > P ≤ 0.10. Logarithmic transformation was applied to TnT for degraded 34 kDa and immunoreactive 36 kDa to minimize major deviations from the normal distribution. Pearson's correlation coefficients between percent Brahman genetics and LL steak WBSF, trained sensory panel scores, intact and degraded forms of desmin and TnT, and collagen characteristics were evaluated for correlation using PROC CORR of SAS.
RESULTS
As the percentage of Brahman genetics increased, strip loin steak thaw loss increased (linear, P = 0.01; Table 2), but there was no effect (P > 0.14) on cook loss. As the percentage of Brahman genetics increased, WBSF increased (linear, P = 0.01). Furthermore, as the percentage of Brahman genetics increased, sensory panel scores of strip loin steak tenderness, connective tissue, and juiciness decreased (linear, P = 0.01). Brahman genetics had no effect on beef flavor or off-flavor scores (P > 0.35).
Table 2.
Effect of Angus and Brahman genetics on objective and subjective measures of longissimus lumborum steak tenderness and palatability
| Angus/Brahman genetics1 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | Angus | Brangus | Half | Brahman | SEM | Linear | Quadratic |
| Objective measures | |||||||
| Thaw loss, % | 1.07 | 1.99 | 1.51 | 2.14 | 0.70 | 0.01 | 0.44 |
| Cooking loss, % | 15.73 | 13.80 | 15.39 | 14.21 | 1.43 | 0.14 | 0.54 |
| Shear force, N | 37.22 | 38.23 | 40.78 | 43.46 | 2.47 | 0.01 | 0.86 |
| Subjective measures2 | |||||||
| Tenderness | 6.30 | 5.69 | 6.05 | 5.30 | 0.18 | 0.01 | 0.81 |
| Juiciness | 6.27 | 5.92 | 5.99 | 5.84 | 0.12 | 0.01 | 0.22 |
| Connective tissue | 6.76 | 6.49 | 6.64 | 6.11 | 0.21 | 0.01 | 0.49 |
| Beef flavor | 5.75 | 5.70 | 5.87 | 5.68 | 0.09 | 0.56 | 0.35 |
| Off-flavor | 5.86 | 5.85 | 5.85 | 5.87 | 0.05 | 0.84 | 0.73 |
Steers (n = 131) were classified into 4 categories based on the percentage of Angus and Brahman genetics. The breed groups were steers that contained 6/32 or less Brahman genetics (Angus), steers that contained 12/32 Brahman genetics (Brangus), steers that contained 14/32 to 18/32 Brahman genetics (Half), and steers that contained 23/32 to 32/32 Brahman genetics (Brahman).
Tenderness, juiciness, beef flavor, and connective tissue: 1 = extremely tough, extremely dry, extremely bland, and abundant, respectively, and 8 = extremely tender, extremely juicy, extremely intense, and none, respectively,). Off-flavor: 1 = extremely intense and 6 = none).
Increasing the percentage of Brahman genetics did not affect the amount of intact desmin (P > 0.51; Table 3) but decreased the amount of 38 kDa desmin (linear, P = 0.01). Steaks from steers with a greater percentage of Brahman genetics had a decreased (quadratic, P = 0.04) amount of intact TnT and a decreased amount of 34 and 30 kDa TnT degradation bands (linear, P < 0.03). In contrast to these results, increasing Brahman genetics increased (linear, P = 0.04) the intensity of the 36 kDa immunoreactive TnT band.
Table 3.
Effect of Angus and Brahman genetics on longissimus lumborum steak desmin and troponin-T protein degradation, collagen crosslinking, and melting temperature
| Angus/Brahman genetics1 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | Angus | Brangus | Half | Brahman | SEM | Linear | Quadratic |
| Desmin form2 | |||||||
| Intact, 55 kDa | 0.96 | 0.86 | 0.81 | 1.05 | 0.34 | 0.51 | 0.11 |
| Degraded, 38 kDa | 1.42 | 1.27 | 0.97 | 0.76 | 0.69 | 0.01 | 0.89 |
| Troponin-T form2 | |||||||
| Intact, 40 kDa | 1.30 | 0.94 | 1.01 | 1.11 | 0.44 | 0.34 | 0.04 |
| Immunoreactive, 36 kDa3 | 0.79 | 0.94 | 0.93 | 2.03 | 0.53 | 0.04 | 0.47 |
| Degraded, 34 kDa3 | 0.74 | 0.70 | 0.46 | 0.19 | 0.41 | 0.01 | 0.30 |
| Degraded, 30 kDa | 1.40 | 1.54 | 1.39 | 0.94 | 0.78 | 0.03 | 0.13 |
| Collagen characteristics | |||||||
| HP crosslink4 | 1,644 | 1,719 | 1,747 | 1,408 | 156 | 0.14 | 0.14 |
| Melting temperature5 | 55.2 | 49.6 | 57.4 | 62.1 | 4.90 | 0.07 | 0.17 |
Steers (n = 131) were classified into 4 categories based on the percentage of Angus and Brahman genetics. The breed groups were steers that contained 6/32 or less Brahman genetics (Angus), steers that contained 12/32 Brahman genetics (Brangus), steers that contained 14/32 to 18/32 Brahman genetics (Half), and steers that contained 23/32 to 32/32 Brahman genetics (Brahman).
Data measured in arbitrary units.
Log transformation was used to normalize the data.
Hydroxylysyl pyridinoline (HP) crosslink measured in nanograms per gram tissue.
Perimysium collagen peak differential scanning calorimetry melting temperature in °C.
There was no effect (P > 0.14) of Brahman genetics on the amount of the HP collagen crosslink. In contrast, as Brahman genetics increased, peak transitional temperature of perimysial collagen from steaks tended (linear, P = 0.07) to increase.
Pearson's correlations coefficients between the percentage of Brahman genetics and characteristics of LL steaks are displayed in Table 4. Warner–Bratzler shear force was positively correlated (r = 0.25, P < 0.01) with the percentage of Brahman genetics. Sensory panel tenderness, juiciness, and connective tissue score were negatively correlated (r = −0.44, r = −0.26, and r = −0.63, respectively) but beef flavor was not correlated (P = 0.17) with the percentage of Brahman genetics (P < 0.01). Intact desmin tended (P = 0.06) to be positively correlated (r = 0.18) and degraded 38 kDa desmin was negatively correlated (r = −0.34, P < 0.01) with the percentage of Brahman genetics. Intact TnT was not correlated (P = 0.56) with the percentage of Brahman genetics, but 36 kDa immunoreactive TnT was positively correlated (r = 0.26) and the 34 and 30 kDa bands were negatively correlated (r = −0.34 and r = −0.29, respectively) with the percentage of Brahman genetics (P < 0.01). Finally, the HP collagen crosslink was negatively correlated (r = −0.20, P = 0.03) with the percentage of Brahman genetics, whereas the transitional temperature of perimysial collagen was positively correlated (r = 0.25) with the percentage of Brahman genetics (P < 0.01).
Table 4.
Pearson's correlation coefficients between percent Brahman genetics and longissimus lumborum steak Warner–Bratzler shear force, trained sensory panel scores, intact and degraded forms of desmin and troponin-T, and collagen characteristics
| Item | Correlation coefficient | P-value |
|---|---|---|
| Warner–Bratzler shear force | 0.25 | 0.01 |
| Trained sensory panel scores | ||
| Tenderness | −0.44 | <0.01 |
| Juiciness | −0.26 | 0.01 |
| Connective tissue | −0.63 | <0.01 |
| Beef flavor | −0.12 | 0.17 |
| Desmin form | ||
| Intact, 55 kDa | 0.18 | 0.06 |
| Degraded, 38 kDa | −0.34 | <0.01 |
| Troponin-T form | ||
| Intact, 40 kDa | 0.06 | 0.56 |
| Immunoreactive, 36 kDa | 0.26 | 0.01 |
| Degraded, 34 kDa | −0.36 | 0.01 |
| Degraded, 30 kDa | −0.29 | 0.01 |
| Collagen characteristics | ||
| HP crosslink1 | −0.20 | 0.03 |
| Melting temperature2 | 0.25 | 0.01 |
Hydroxylysyl pyridinoline (HP) crosslink.
Perimysium collagen peak differential scanning calorimetry melting temperature.
DISCUSSION
Brahman (B. indicus)-influenced cattle are commonly used in warm, subtropical-like climates of the Southeastern United States because they are adapted to be more efficient than B. taurus–type cattle in these conditions. Although these cattle are more efficient, they produce less-tender meat, which is, therefore, less palatable than meat from B. taurus–influenced cattle. Numerous studies reported that steaks from B. indicus–influenced cattle are less tender than those from B. taurus cattle when measured both objectively and subjectively (Koch et al., 1982; Crouse et al., 1988; Pringle et al., 1997); however, the magnitude of the negative effect on WBSF seems to be related to the percentage of Brahman genetic influence.
Crouse et al. (1989) reported that steaks from steers with 25, 50, and 100% Brahman genetic influence had WBSF values 15, 24, and 25% greater, respectively, than steaks from steers with 0% Brahman influence. The authors also reported that sensory panel overall tenderness and connective tissues scores linearly decreased as the percent Brahman genetic influence increased, indicating less-tender steaks with more connective tissue. Johnson et al. (1990) reported that it took 75% Brahman genetic influence to produce steaks with WBSF that differed from 100% Angus steaks. Stolowski et al. (2006) found that steaks from 7 muscles of three-fourths Brahman cattle decreased in WBSF value over 42 d of aging; however, WBSF values of steaks from cattle with greater than one-half Brahman genetics plateaued at Day 28 of aging and did not improve in tenderness thereafter. Most recently, Aroeira et al. (2016) found that B. indicus LM steaks were tougher than B. taurus steaks throughout a 21-d aging study and that freezing did not improve the tenderness of B. indicus steaks. Therefore, although the literature indicates that increasing the percentage of Brahman influence negatively affects tenderness, the exact biological contributors are unknown.
The Angus–Brahman multibreed herd was established at the University of Florida in 1989 to study the genetic influence these breeds have on numerous carcass and meat quality characteristics. Elzo et al. (2012) summarized WBSF and trained panel scores of meat produced from 1989 through 2009 and found that as the percentage of Brahman genetic influence increased, WBSF increased by up to 20% when comparing 100% Angus and 100% Brahman steaks. Additionally, trained sensory panel tenderness and connective tissues scores decreased by as much as 20 (less tender) and 16% (more connective tissue), respectively, when Brahman genetic influence was increased. Gonzalez et al. (2014) also examined LL WBSF and trained panel palatability scores from this herd and found the same trends described by Elzo et al. (2012), but differences between breeds did not occur until the animals had 50% or greater of Brahman influence. In the current study, as the percentage of Brahman breeding increased, WBSF linearly increased whereas sensory panel tenderness and connective tissue amount linearly decreased. Pringle et al. (1997) reported strong Brahman genetic influences on these meat characteristics, but the effects reported were quadratic as the percentage of Brahman influence increased. Despite the lack of agreement in the shape of the response between the current study and that of Pringle et al. (1997), the 2-yr population selected for the current study is representative of the herd historical data presented by Elzo et al. (2012). Therefore, this population was a good population in which to study the protein degradation and collagen effects on the measures described above.
The calpain proteolytic system of enzymes has been widely accepted as the major contributors of postmortem proteolysis. Specifically, calpain-1 and -2 have been identified as the enzymes responsible for improvements in tenderness during postmortem cold storage (for review, see Huff-Lonergan et al. [2010]). Geesink et al. (2006) indicated that calpain-1 is mostly responsible for postmortem proteolysis, whereas Colle and Doumit (2017) reported that calpain-2 was activated in LL and semimembranosus steaks during extended aging (up to 42 d). Additionally, Colle et al. (2018) reported calpain-2 can contribute to early proteolysis, but calcium chloride injection or freezing of the meat was required. The endogenous inhibitor of the calpains, calpastatin, can have a negative effect on the extent of postmortem proteolysis. Cruzen et al. (2014) demonstrated the inhibitory ability of calpastatin by showing increased calpastatin activity and decreased protein degradation and tenderness in meat from mature/older cattle. Hence, the activity of these enzymes largely determines meat tenderness.
In both Angus and Brahman cattle, selection for calpain and calpastatin activity has been identified as a means to improve meat palatability. Historically, the inherent mechanism identified for decreased tenderness in Brahman cattle was increased calpastatin activity (Wheeler et al., 1990; Shackelford et al., 1991; Pringle et al., 1997). Café et al. (2010) found that selecting Brahman cattle for favorable calpastatin genes could improve tenderness to a level detectable by consumers, whereas Tait et al. (2014) found that calpastatin genes affect variation and mean tenderness values in an Angus population. A 31% increase in calpastatin activity was noted between 0 and 100% Brahman–influenced strip loin steaks (Pringle et al., 1997). Shackelford et al. (1991) found that Brahman cattle possessed an elevated calpastatin activity of 20% compared with Angus–Hereford cattle. Whipple et al. (1990) also reported that B. indicus cattle possessed greater calpastatin activity than B. taurus cattle at 24 h postmortem. In contrast to these studies, Stolowski et al. (2006) found that calpastatin activity increased in the gluteus medius and semimembranosus as the percentage of Brahman genetics increased but that there was no breed effect on the LM. Despite this finding and due to the fact that calpain and calpastatin activity were not measured in the current study (due to limited samples available for analyses), it is hypothesized that differences in tenderness between the breeds groups in the current study was due to differences in calpain proteolytic system activity.
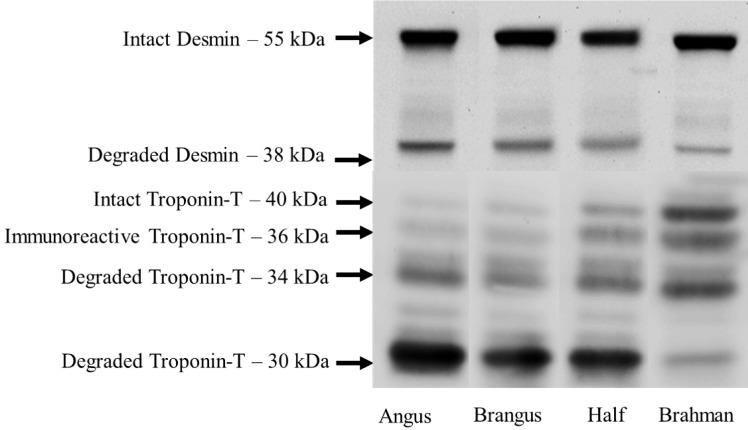
Although studies have focused on the calpain proteolytic system effect on reduced Brahman steak tenderness, few studies have focused on the proteins degraded by these enzymes. Desmin and TnT degradation have been commonly used as proteins associated with calpain-mediated postmortem proteolysis. Numerous studies report that amounts of intact and degraded myofibrillar proteins are associated with meat tenderness (Olson and Parrish, 1977; Huff-Lonergan et al., 1996; Melody et al., 2004). Cruzen et al. (2014) saw less degraded TnT (30 kDa subunit) and Rhee et al. (2004) saw less degraded desmin in the tougher triceps brachi muscle compared with the LM and semimembranosus. Ho et al. (1997) found that Brahman/Simmental–crossed cattle did not differ in the rate of desmin and TnT degradation compared with Angus/Jersey–crossed cattle. Whipple et al. (1990) found that desmin was degraded by 14 d of postmortem aging in B. taurus cattle but was still present in cattle with a high percentage of Brahman influence. In the current study, as the percentage of Brahman genetic influence increased, there were linear decreases in the appearance of all degraded bands of both desmin and TnT. In contrast, the immunoreactive 36 kDa TnT subunit increased with Brahman influence (Fig. 1). It is hypothesized that this subunit is an isoform of intact TnT; therefore, the increase in the 36 kDa subunit may have indicated that the ability to reduce this subunit to the smaller degradation products was inhibited or delayed as the Brahman percentage increased. Oddly, there were no linear or quadratic responses for intact desmin and the shape of the genetic response to the presence of intact TnT was quadratic. The shape of the intact TnT response could be due to incomplete separation of the 2 isoforms of the protein reported by Muroya et al. (2007) or that B. indicus cattle have a greater abundance of contractile proteins, including TnT (Rodrigues et al., 2017). Although it is unknown if B. indicus cattle also possess a greater abundance of desmin, which could be the reason for a lack of a linear or quadratic response, Phelps et al. (2016) used the same method used here to examine desmin and TnT degradation. In their study, Phelps et al. (2016) found that between d −7 and −14 postmortem, intact TnT rapidly disappeared whereas intact desmin did not change but degraded desmin increased. Therefore, because samples were collected at d −14 postmortem, this could be the reason for the lack of response in intact desmin but a linear response in the degraded portion. When observing the relationship between Brahman genetics and the intensity of degradation product bands, all correlations were significant, therefore strengthening the fact that increasing the Brahman genetic influence reduced postmortem myofibrillar proteolysis and contributed to less-tender steaks.
Figure 1.
Representative western blots of desmin and troponin-T degradation of longissimus lumborum steaks from steers that contained 6/32 or less Brahman genetics (Angus), steers that contained 12/32 Brahman genetics (Brangus), steers that contained 14/32 to 18/32 Brahman genetics (Half), and steers that contained 23/32 to 32/32 Brahman genetics (Brahman).
With carcass and subprimal aging, it becomes apparent that calpastatin activity does not fully explain the reduction in tenderness. Riley et al. (2005) found that insoluble collagen content affected tenderness more than calpastatin activity as Brahman steaks were aged longer. Ebarb et al. (2016) also reported that as LL beef steaks were aged longer, the correlation between muscle fiber size and WBSF decreased, possibly due to postmortem proteolysis. Additionally, the correlation between insoluble collagen content and WBSF increased. Cruzen et al. (2014) concluded that despite the semimembranosus having similar calpain and calpastatin activity and amount of degraded TnT compared with the LM, connective tissue contributed to the differences in tenderness between the muscles. Stolowski et al. (2006) found that total collagen content was not affected by percent Brahman genetics; however, a decrease in collagen solubility was seen only in the semitendinosus of cattle with greater than 50% Brahman genetics.
McCormick (1994) reported that as the amount of heat-stable trivalent crosslinks are increased, the toughness of meat increases. This is due to the collagen not being able to gelatinize during the heating process (Light et al., 1985). In a prior study using this herd, Gonzalez et al. (2014) found that genes associated with collagen crosslinking were significantly correlated with WBSF and sensory panel tenderness and connective tissue scores. In the current study, there were no effects of percent Brahman influence on the amount of HP crosslinks; however, the melting temperature of the collagen tended to linearly increase as the Brahman percentage increased. Additionally, HP crosslink content and melting temperature were also correlated. The fact that there was a negative correlation for HP crosslink content but no linear or quadratic Brahman genetic response could be due to the method of HP quantification and the possibility that Brahman steers could have more collagen. However, data from the same herd presented by Gonzalez et al. (2014) indicated there were no differences between the breed classifications for insoluble, soluble, and total collagen content. Therefore, the method of quantification was most likely not the reason for the divergence in the data.
Although no effects were seen in HP crosslink content, melting temperature, as measured with DSC, of extracted perimysial collagen tended to increase with increased percent Brahman genetics. Differential scanning calorimetry has been used to demonstrate reductions in thermal transition temperature due to weakening of connective tissue (Christensen et al., 2013). Chang et al. (2015) found that melting temperature of perimysial collagen decreased due to sonication, but no changes were seen in measured insoluble collagen content. More importantly, melting temperature of perimysial collagen was correlated to WBSF whereas insoluble collagen content was not, thus illustrating that the ultrastructure of collagen may have a greater effect on tenderness than global measures associated with collagen solubility. In muscle, 3 trivalent crosslinks alter the solubility of collagen, HP, lysylpryridinoline, and Ehrlich chromagen (Eyre et al., 1984). In the current study, only HP was measured, which leaves the effect of Brahman genetics on the other 2 crosslinks unknown. Roy et al. (2015) demonstrated that the presence of the 3 trivalent crosslinks was differentially affected based on breed, muscle, and use of growth promotants. Therefore, the unexpected result for HP crosslink content but increase in melting temperature may indicate that the other 2 trivalent crosslinks may have been affected by percent Brahman influence to increase melting temperature.
In summary, these data continue to explore the negative influence of Brahman genetics on LL tenderness. As expected, data indicated that as the percentage of Brahman genetics increased, WBSF increased and sensory panel scores decreased for tenderness and connective tissue amount. Decreased Brahman steak tenderness was due to reduced degradation of desmin and TnT. Although Gonzalez et al. (2014) detected increased mRNA expression of crosslinking genes from animals produced by this herd, HP crosslink content was not influenced by Brahman genetics. Despite a lack of response, melting temperature of perimysial collagen tended to increase as the percentage of Brahman genetics increased, thus indicating that genetic alterations to the other 2 trivalent crosslinks could have strengthened the collagen molecule to increase melting temperature, increase the amount of connective tissue detected by panelists, and ultimately produce tougher steaks. Future research should focus on the effect Brahman genetics has on these 2 crosslinks.
FOOTNOTES
Contribution number 18-101-J of the Kansas Agricultural Experiment Station, Manhattan, KS 66506.
LITERATURE CITED
- American Meat Science Association 1995. Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. Natl. Livest. Meat Board, Chicago, IL. [Google Scholar]
- Aroeira C. N., Filho R. A. T., Fontes P. R., Gomide L. A. M., Ramos A. L. S., Ladeira M. M., Ramos E. M. 2016. Freezing, thawing and aging effects on beef tenderness from Bos indicus and Bos taurus cattle. Meat Sci. 116:118–125. doi: 10.1016/j.meatsci.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Café L. M., McIntyre B. L., Robinson D. L., Geesink G. H., Barendse W., Pethick D. W., Thompson J. M., Greenwood P. L. 2010. Production and processing studies on calpain-system gene markers for tenderness in Brahman cattle: 2. Objective meat quality. J. Anim. Sci. 88:3059–3069. doi: 10.2527/jas.2009-2679 [DOI] [PubMed] [Google Scholar]
- Chang H. J., Wang Q., Tang C. H., Zhou G. H. 2015. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J. Food Qual. 38:256–267. doi: 10.1111/jfq.12141 [DOI] [Google Scholar]
- Christensen L., Ertbjerg P., Løje H., Risbo J., van den Berg F. W. J., Christensen M. 2013. Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Sci. 93:787–795. doi: 10.1016/j.meatsci.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Colle M. J., Doumit M. E. 2017. Effect of aging on calpain-1 and -2 activity in beef longissimus lumborum and semimembranosus muscles. Meat Sci. 131:142–145. doi: 10.1016/j.meatsci.2017.05.014 [DOI] [PubMed] [Google Scholar]
- Colle M. J., Nasados J. A., Rogers J. M., Kerby D. M., Colle M. M., Van Buren J. B., Richard R. P., Murdoch G. K., Williams C. J., Doumit M. E. 2018. Strategies to improve beef tenderness by activating calpain-2 earlier postmortem. Meat Sci. 135:36–41. doi: 10.1016/j.meatsci.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Crouse J. D., Cundiff L. V., Koch R. M., Koohmaraie M., Seideman S. C. 1989. Comparisons of Bos indicus and Bos taurus inheritance for carcass beef characteristics and meat palatability. J. Anim. Sci. 67:2661–2668. doi: 10.2527/jas1989.67102661x [DOI] [Google Scholar]
- Crouse J. D., Seideman S. C., Cundiff L. V. 1988. The effect of carcass electrical stimulation on meat obtained from Bos indicus and Bos taurus cattle. J. Food Qual. 10:407–416. doi: 10.1111/j.1745-4557.1988.tb00300.x [DOI] [Google Scholar]
- Cruzen S. M., Paulino P. V. R., Lonergan S. M., Huff-Lonergan E. 2014. Postmortem proteolysis in three muscles from growing and mature beef cattle. Meat Sci. 96:854–861. doi: 10.1016/j.meatsci.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Ebarb S. M., Drouillard J. S., Maddock-Carlin K. R., Phelps K. J., Vaughn M. A., Burnett D. D., Paulk C. B., Grieger D. M., Gonzalez J. M. 2016. Effect of growth-promoting technologies on longissimus lumborum muscle fiber morphometrics, collagen solubility, and cooked meat tenderness. J. Anim. Sci. 94:869–881. doi: 10.2527/jas.2015-9888 [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Johnson D. D., Wasdin J. G., Driver J. D. 2012. Carcass and meat palatability breed differences and heterosis effects in an Angus-Brahman multibreed population. Meat Sci. 90:87–92. doi: 10.1016/j.meatsci.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Koob T. J., Vanness K. P. 1984. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem. 137:380–388. doi: 10.1016/0003-2697(84)90101-5 [DOI] [PubMed] [Google Scholar]
- Geesink G. H., Kuchay S., Chishti A. H., Koohmaraie M. 2006. μ-Calpain is essential for postmortem proteolysis of muscle proteins. J. Anim. Sci. 84:2834–2840. doi: 10.2527/jas.2006-122 [DOI] [PubMed] [Google Scholar]
- Gonzalez J. M., Johnson D. D., Elzo M. A., White M. C., Stelzleni A. M., Johnson S. E. 2014. Effect of Brahman genetic influence on collagen enzymatic crosslinking gene expression and meat tenderness. Anim. Biotechnol. 25:165–178. doi: 10.1080/10495398.2013.846862 [DOI] [PubMed] [Google Scholar]
- Ho C. Y., Stromer M. H., Rouse G., Robson R. M. 1997. Effects of electrical stimulation and postmortem storage on changes in titin, nebulin, desmin, troponin-T, and muscle ultrastructure in Bos indicus crossbred cattle. J. Anim. Sci. 75:366–376. doi: 10.2527/1997.752366x [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E., Mitsuhashi T., Beekman D. D., Parrish F. C., Jr, Olson D. G., Robson R. M. 1996. Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J. Anim. Sci. 74:993–1008. doi: 10.2527/1996.745993x [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E., Zhang W., Lonergan S. M. 2010. Biochemistry of postmortem muscle – Lessons on mechanisms of meat tenderization. Meat Sci. 86:184–195. doi: 10.1016/j.meatsci.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Johnson D. D., Huffman R. D., Williams S. E., Hargrove D. D. 1990. Effects of percentage Brahman and Angus breeding age-season of feeding and slaughter end point on meat palatability and muscle characteristics. J. Anim. Sci. 68:1980–1986. doi: 10.2527/1990.6871980x [DOI] [PubMed] [Google Scholar]
- Koch R. M., Dikeman M. E., Crouse J. D. 1982. Characterization of biological types of cattle (Cycle III). III. Carcass composition, quality and palatability. J. Anim. Sci. 54:35–45. doi: 10.2527/jas1982.54135x [DOI] [Google Scholar]
- Koohmaraie M., Kent M. P., Shackelford S. D., Veiseth E., Wheeler T. L. 2002. Meat tenderness and muscle growth: Is there any relationship? Meat Sci. 62:345–352. doi: 10.1016/S0309-1740(02)00127-4 [DOI] [PubMed] [Google Scholar]
- Li C., Zhou G., Xu X. 2008. Changes of meat quality characteristics and intramuscular connective tissue of beef semitendinosus muscle during postmortem aging for Chinese Yellow bulls. Int. J. Food Sci. Technol. 43:838–845. doi: 10.1111/j.1365-2621.2007.01524.x [DOI] [Google Scholar]
- Light N., Champion A. E. 1984. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem. J. 219:1017–1026. doi: 10.1042/bj2191017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N., Champion A. E., Voyle C., Bailey A. J. 1985. The role of epimysial, perimysial and endomysial collagen in determining texture in six bovine muscles. Meat Sci. 13:137–149. doi: 10.1016/0309-1740(85)90054-3 [DOI] [PubMed] [Google Scholar]
- Lonergan S. M., Huff-Lonergan E., Rowe L. J., Kuhlers D. L., Jungst S. B. 2001. Selection for lean growth efficiency in Duroc pigs influences pork quality. J. Anim. Sci. 79:2075–2085. doi: 10.2527/2001.7982075x [DOI] [PubMed] [Google Scholar]
- McCormick R. J. 1994. The flexibility of the collagen compartment of muscle. Meat Sci. 36:79–91. doi: 10.1016/0309-1740(94)90035-3 [DOI] [PubMed] [Google Scholar]
- McCormick R. J. 1999. Extracellular modifications to muscle collagen implications for meat quality. Poult. Sci. 78:785–791. doi: 10.1093/ps/78.5.785 [DOI] [PubMed] [Google Scholar]
- Melody J. L., Lonergan S. M., Rowe L. J., Huiatt T. W., Mayes M. S., Huff-Lonergan E. 2004. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 82:1195–1205. doi: 10.2527/2004.8241195x [DOI] [PubMed] [Google Scholar]
- Miller M. F., Carr M. A., Ramsey C. B., Crockett K. L., Hoover L. C. 2001. Consumer thresholds for establishing the value of beef tenderness. J. Anim. Sci. 79:3062–3068. doi: 10.2527/2001.79123062x [DOI] [PubMed] [Google Scholar]
- Muroya S., Ohnishi-Kameyama M., Oe M., Nakajima I., Chikuni K. 2007. Postmortem changes in bovine troponin-T isoforms on two-dimensional electrophoretic gel analyzed using mass spectrometry and western blotting: The limited fragmentation into basic poly peptides. Meat Sci. 75:506–514. doi: 10.1016/j.meatsci.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Olson D. G., Parrish F. C. 1977. Relationship of myofibril fragmentation index to measures of beefsteak tenderness. J. Food Sci. 42:506–509. doi: 10.1111/j.1365-2621.1977.tb01533.x [DOI] [Google Scholar]
- Phelps K. J., Drouillard J. S., Jennings J. S., Depenbusch B. E., Van Bibber-Krueger C. L., Miller K. A., Vaughn M. A., Burnett D. D., Gonzalez J. M. 2015. Effects of the PN Beef Program diet for feedlot cattle on feedlot performance and carcass characteristics. J. Anim. Sci. 93:1298–1308. doi: 10.2527/jas.2014-8661 [DOI] [PubMed] [Google Scholar]
- Phelps K. J., Drouillard J. S., Silva M. B., Miranda L. D. F., Ebarb S. M., Van Bibber-Krueger C. L., O'Quinn T. G., Gonzalez J. M. 2016. Effect of extended postmortem aging and steak location on myofibrillar protein degradation and Warner-Bratzler shear force of beef M. semitendinosus steaks. J. Anim. Sci. 94:412–423. doi: 10.2527/jas.2015-9862 [DOI] [PubMed] [Google Scholar]
- Pringle T. D., Williams S. E., Lamb B. S., Johnson D. D., West R. L. 1997. Carcass characteristics, the calpain proteinase system, and aged tenderness of Angus and Brahman crossbred steers. J. Anim. Sci. 75:2955–2961. doi: 10.2527/1997.75112955x [DOI] [PubMed] [Google Scholar]
- Purslow P. P. 2005. Review: Intramuscular connective tissue and its role in meat quality. Meat Sci. 70:435–447. doi: 10.1016/j.meatsci.2004.06.028 [DOI] [PubMed] [Google Scholar]
- Rhee M. S., Wheeler T. L., Shackelford S. D., Koohmaraie M. 2004. Variation in palatability and biochemical traits within and among eleven beef muscles. J. Anim. Sci. 82:534–550. doi: 10.2527/2004.822534x [DOI] [PubMed] [Google Scholar]
- Riley D. G., Johnson D. D., Chase C. C., Jr, West R. L., Coleman S. W., Olson T. A., Hammond A. C. 2005. Factors influencing tenderness in steaks from Brahman cattle. Meat Sci. 70:347–356. doi: 10.1016/j.meatsci.2005.01.022 [DOI] [PubMed] [Google Scholar]
- Rodrigues R. T. S., Chizzotti M. L., Vital C. E., Baracat-Pereira M. C., Barros E., Busato K. C., Gomes R. A., Laderia M. M., da Silva Martins T. 2017. Differences in beef quality between Angus (Bos taurus taurus) and Nellore (Bos taurus indicus) cattle through a proteomic and phosphoproteomic approach. PLoS One 12:e0170294. doi: 10.1371/journal.pone.0170294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B. C., Sedgewick G., Aalhus J. L., Basarab J. A., Bruce H. L. 2015. Modification of mature non-reducible collagen cross-link concentrations in bovine m. gluteus medius and semitendinosus with steer age at slaughter, breed cross and growth promotants. Meat Sci. 110:109–117. doi: 10.1016/j.meatsci.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Shackelford S. D., Koohmaraie M., Miller M. F., Crouse J. D., Reagan J. O. 1991. An evaluation of tenderness of the longissimus muscle of Angus by Hereford versus Brahman crossbred heifers. J. Anim. Sci. 69:171–177. doi: 10.2527/1991.691171x [DOI] [PubMed] [Google Scholar]
- Smith S. H., Judge M. D. 1991. Relationship between pyridinoline concentration and thermal stability of bovine intramuscular collagen. J. Anim. Sci. 69:1989–1993. doi: 10.2527/1991.6951989x [DOI] [PubMed] [Google Scholar]
- Stolowski G. D., Baird B. E., Miller R. K., Savell J. W., Sams A. R., Taylor J. F., Sanders J. O., Smith S. B. 2006. Factors influencing the variation in tenderness of seven major beef muscles from three Angus and Brahman breed crosses. Meat Sci. 73:475–483. doi: 10.1016/j.meatsci.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Tait R. G., Jr, Shackelford S. D., Wheeler T. L., King D. A., Casas E., Thallman R. M., Smith T. P. L., Bennett G. L. 2014. μ-Calpain, calpastatin, and growth hormone receptor genetic effects on preweaning performance, carcass quality traits, and residual variance of tenderness in Angus cattle selected to increase minor haplotype and allele frequencies. J. Anim. Sci. 92:456–466. doi: 10.2527/jas.2013-7075 [DOI] [PubMed] [Google Scholar]
- Wheeler T. L., Savell J. W., Cross H. R., Lunt D. K., Smith S. B. 1990. Mechanisms associated with the variation in tenderness of meat from Brahman and Hereford cattle. J. Anim. Sci. 68:4206–4220. doi: 10.2527/1990.68124206x [DOI] [PubMed] [Google Scholar]
- Whipple G., Koohmaraie M., Dikeman M. E., Crouse J. D., Hunt M. C., Klemm R. D. 1990. Evaluation of attributes that affect longissimus muscle tenderness in Bos taurus and Bos indicus cattle. J. Anim. Sci. 68:2716–2728. doi: 10.2527/1990.6892716x [DOI] [PubMed] [Google Scholar]