Abstract
The inclusion of natural antioxidants in the diet, through fresh forages or condensed tannins, might prolong meat shelf life and modify the meat quality. The aim of this study was to evaluate the effect of the dam's feeding system during lactation and the inclusion of quebracho in the fattening concentrate of male lambs on meat color, chemical composition, and lipid oxidation. Dams and their suckling lambs were fed indoors or allowed to graze on alfalfa or sainfoin until lambs reached 42 d old. Thereafter, the weaned lambs were fed concentrates with 5% quebracho or without quebracho until reaching 22 to 24 kg BW. Meat of suckling lambs from dam's fed indoors (Indoor lambs) presented greater intramuscular fat content and lower α-tocopherol content than meat of suckling lambs from dam's fed Alfalfa (Alfalfa lambs) and Sainfoin (Sainfoin lambs; P < 0.01), independent of the fattening diet. Regarding meat color of longissimus thoracis et lumborum muscle, on average, Indoor lambs' meat presented greater lightness, yellowness, and hue angle values than Alfalfa and Sainfoin lambs' meat (P < 0.05). The redness was affected by the interaction between the feeding system during lactation and the time of storage, but, on average, Alfalfa and Sainfoin lambs had greater redness than Indoor lambs (P < 0.05). The lipid oxidation from 5 to 14 d of meat display time observed for Sainfoin lambs was lower than that for Indoor lambs (P < 0.05). The intramuscular fatty acid profile of meat from the Sainfoin and Alfalfa lambs met particularly well the Food and Agriculture Organization of the United Nations recommendation for human health, compared that of meat from the Indoor lambs. The dietary inclusion of quebracho during fattening modified meat α-tocopherol content, oxymyoglobin levels after 8 d of storage, and fatty acid profiles. In conclusion, the results indicate higher importance of the diet during suckling than during the subsequent fattening period on meat quality parameters such as color, lipid oxidation, and fatty acid profile. Dams grazing Sainfoin provide a more stable lamb meat, and it would be cheaper to feed the dams with fresh forages with a high α-tocopherol content than supplementing the concentrate of the lambs with synthetic α-tocopherol.
Keywords: alfalfa, fatty acids, indoor, lipid oxidation, sainfoin
INTRODUCTION
Traditional farming systems under Mediterranean conditions have partially or totally replaced grazing with indoor feeding (Ripoll-Bosch et al., 2014). In these systems, the pregnant ewes are stalled around parturition and they are ad libitum fed a mixed dry ration or hay supplemented with concentrates and remain indoors until the weaning of the lamb (around 45 d). After weaning (12–14 kg), lambs are fed concentrates plus straw ad libitum until 22 to 24 kg BW to obtain a homogeneous product and to provide easy flock management as the herd size grows (Bernués et al., 2011). Nowadays, there is interest to reintroduce grazing to increase farm self-reliance, profitability, and sustainability (Ripoll-Bosch et al., 2014) and to fulfill the demands of consumers for animal products from natural, healthy, and sustainable systems.
Suckling indoors-fed lambs had lower or similar weight gains compared with grazing suckling lambs (Álvarez-Rodríguez et al., 2010; Joy et al., 2012; Ripoll et al., 2013), and meat quality was affected, but only mildly at light slaughter BW (Joy et al., 2012). Grazing alfalfa (Medicago sativa) increased the meat shelf life related to the natural presence of α-tocopherol (Ripoll et al., 2013). Sainfoin (Onobrychis viciifolia) can be included in the ration of lactating ewes because it is a high-quality forage with a chemical composition similar to that of alfalfa except for the content of condensed tannins (CT; Theodoridou et al., 2011). Condensed tannins can improve meat shelf life (Vasta and Luciano, 2011), depending on the type, time, and dose fed (Luciano et al., 2009b, 2011). Another source of CT is quebracho (Schinopsis lorentzii), with high CT content, that can be easily added to the concentrate fed in the fattening period.
The aims of the study were to evaluate 1) the effect of the dam's feeding system (grazing on alfalfa, grazing on sainfoin, or fed indoors) during the suckling period (ewe–lamb pairs) on meat quality after the fattening indoor period and 2) the effect of CT from quebracho during fattening on meat quality in weaned concentrate-fed lambs.
MATERIALS AND METHODS
The experiment was conducted in the facilities of the Centro de Investigación y Tecnología Agroalimentaria (CITA) Research Centre in Zaragoza, Spain (41°3′ N, 0°47′ W, and 216 m above sea level). The Animal Ethics Committee of the CITA approved the experimental procedures, which were in compliance with the normative of the European Union (2010) on the protection of animals used for experimental and other scientific purposes.
Animal Management and Experimental Design
The experiment had a 3 × 2 factorial design, comprising 3 dam feeding systems during lactation (indoors, grazing on alfalfa, and grazing on sainfoin) and the inclusion of quebracho in the fattening concentrate of the weaned lamb (the Control and a commercial concentrate with 5% quebracho [QUE]).
After lambing, 63 single-bearing ewes and their male lambs, from the Rasa Aragonesa breed, were randomly assigned according to the ewe's BW (46.6 ± 1.4 kg) and BCS (2.43 ± 0.07) to 1 of the 3 feeding systems during lactation. Each treatment was divided into 2 replicates. All newborn lambs were weighed, their umbilical cords were disinfected, and they received an injection of 1 mL of a mixture of vitamin E, selenium, vitamin A, and vitamin D3 (chevita GmbH, Pfaffenhofen, Germany).
Indoors
Twenty-one ewe–lamb pairs were housed indoors as is usual in this system. Each replicate was stocked in a pen (n = 10 or 11 pairs/pen; 15 m2/pen). The ewes received a dry total mixed ration ad libitum. The main components of the total mixed ration were barley straw (50%), corn grain (11.6%), barley grain (11.5%), legume grain with 17% CP (9.3%), rapeseed meal (7%), and soybean meal (3.3%) and sugar beet molasses (3.5%), cottonseed (1.4%), calcium carbonate (1.86%), salt (0.23%) and mineral–vitamin corrector (0.2%). The estimated DMI was 1.9 kg DM/d per ewe.
Alfalfa
Twenty-one ewe–lamb pairs were rotationally grazed on alfalfa. The 2 replicates were stocked in separate paddocks (n = 10 or 11 pairs/paddock; 1,005 m2/paddock). The average weekly production of alfalfa was 3,089 ± 511 kg DM/ha, with a minimum of 1,391 ± 7 kg DM/ha and a maximum of 4,314 ± 72 kg DM/ha. The estimated DMI was 1.3 kg DM/d per ewe.
Sainfoin
Twenty-one ewe–lamb pairs were rotationally grazed on sainfoin. The 2 replicates were stocked in separate paddocks (n = 10 or 11 pairs/paddock; 1,005 m2/paddock). The average weekly production of sainfoin was 4,477 ± 900 kg DM/ha, with a minimum of 1,380 kg DM/ha and a maximum of 7,568 ± 40 kg DM/ha. The estimated DMI was 1.9 kg/d per ewe.
During lactation, the lambs had access to the dams' milk and to the dams' ration (forage in the paddock when they were stocked or the total mixed ration when they were indoors). Since the first week after birth, the lambs had free access to concentrate so they could adapt to it, avoiding potential problems in early weaning. The commercial concentrate was offered to lambs only through a creep concentrate feeder. The lambs were weaned when they reached 42 ± 2 d of age. The daily concentrate intake, BW, and age of the lambs per treatment are reported in Table 1.
Table 1.
Dry matter intake, BW, and age of lambs according to the lactation feeding system and the inclusion of quebracho in the fattening concentrate
| Lactation1 | Concentrate2 | |||||
|---|---|---|---|---|---|---|
| Item | Alfalfa | Sainfoin | Indoor | Control | QUE | RMSE3 |
| DMI, g concentrate/d | ||||||
| Lactation period | 30.5 | 33.9 | 102.9 | – | – | 17.10 |
| Fattening period | 818 | 840 | 845 | 900 | 768 | 99.10 |
| BW, kg | ||||||
| At birth | 3.9 | 4.1 | 3.9 | 0.84 | ||
| At weaning | 14.6 | 15.7 | 15.4 | – | – | 2.73 |
| At slaughter | 22.9 | 23.5 | 22.9 | 23.4 | 22.9 | 1.07 |
| Age, d | ||||||
| At weaning | 42 | 41 | 43 | – | – | 4.20 |
| At slaughter | 70 | 69 | 70 | 68 | 71 | 9.66 |
Indoor = ewes fed a total mixed ration; Alfalfa = grazing on alfalfa; Sainfoin = grazing on sainfoin.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
RMSE = root mean square error.
After weaning, lambs were balanced by BW and allocated in a pen, with a total of 12 pens (6 or 5 lambs per pen; 6 m2/pen) in the experiment. The lambs were fed during the fattening period with concentrates plus straw ad libitum. Half of the lambs from each paddock/pen received a commercial concentrate (Control), and the other half received the commercial concentrate with 5% added quebracho (as-fed basis; SYLVAFEED ByPro Q; Adial Nutrition, Girona, Spain; QUE). Water and mineral blocks were always offered ad libitum. Both concentrates were pelleted. The main components of the Control concentrate were corn (35%), soybean meal (23.8%), wheat (20%), and barley (15%). The main ingredients of the QUE treatment were corn (40%), soybean meal (26.3%), wheat (20%), and quebracho (5%). Concentrates were isoenergetic and isoproteic. The chemical compositions of the feedstuffs used in the present study are reported in Table 2. Lambs were slaughtered when they reached the target slaughter weight (22–24 kg BW). The performance of lambs at weaning and slaughter is reported in Table 1.
Table 2.
Chemical and fatty acid composition of the feedstuffs used in the experiment
| Ewe feedstuffs | Lamb concentrates | ||||
|---|---|---|---|---|---|
| Item | TMR1 | Alfalfa | Sainfoin | Control2 | QUE2 |
| Chemical composition, g/kg DM | |||||
| Moisture | 924.0 | 919.5 | 916.0 | 886.1 | 889.1 |
| CP | 109.0 | 189.7 | 161.3 | 195.4 | 210.1 |
| NDF | 427.0 | 454.9 | 451.9 | 177.7 | 176.5 |
| ADF | 200.0 | 310.2 | 352.0 | 43.6 | 41.1 |
| ADL | 25.3 | 66.8 | 82.7 | 6.0 | 6.3 |
| Ash | 47.9 | 90.4 | 78.4 | 54.3 | 53.8 |
| Condensed tannins, cyanidin equivalent | 0.8 | 1.5 | 21.9 | 0.6 | 3.7 |
| α-Tocopherol, µg/g DM | 5.5 | 132.2 | 396.9 | 4.9 | 3.1 |
| Fatty acids, % FAME3 | |||||
| C14:0 | 2.8 | 0.9 | 0.5 | 0.5 | 0.6 |
| C16:0 | 31.2 | 24.7 | 23.4 | 25.2 | 28.5 |
| C16:1n-7 | 0.5 | 0.4 | 0.3 | 0.1 | 0.2 |
| C17:0 | 0.3 | 0.5 | 0.4 | 0.1 | 0.1 |
| C18:0 | 7.5 | 6.6 | 6.6 | 2.9 | 3.4 |
| C18:1n-9 | 23.1 | 3.5 | 3.5 | 29.8 | 30.8 |
| C18:2n-6 | 26.6 | 18.5 | 16.3 | 37.6 | 33.0 |
| C18:3n-3 | 2.8 | 35.6 | 40.8 | 2.4 | 2.0 |
TMR = total mixed ration, which was fed to the Indoor ewes.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
FAME = fatty acid methyl esters. These were the identified total FAME.
Slaughter and Meat Sampling Procedures
When lambs reached the target slaughter weight, they were slaughtered using standard commercial procedures according to Council Regulation (2009) on the protection of animals at the time of killing. A head-only electrical stunning was applied (1.00 A) to lambs, and then they were exsanguinated. After slaughter, the light lamb carcasses were chilled at 4°C for 24 h in total darkness. Then, the carcasses were split along the dorsal line. The rectus abdominis muscle of the left carcass and both carcasses' longissimus thoracis et lumborum (LTL) muscles were collected. The pH of the LTL muscle was measured at the fourth vertebral region with a pH meter equipped with a Crison 507 penetrating electrode (Crison Instruments, S.A., Barcelona, Spain). The LTL muscle from the fourth to the sixth lumbar vertebrae of the left carcass was sliced and packed to determine the chemical composition and α-tocopherol content. The same portion from the right carcass was sliced and packed to analyze the fatty acid composition. The LTL muscles from the 6th to the 13th thoracic vertebrae were sliced into 2.5-cm-thick samples and randomly assigned to 7 display times (0, 2, 5, 7, 9, 12, and 14 d), placed in trays, and wrapped with oxygen-permeable polyvinyl chloride film and kept in darkness at 4°C until being measured for color. The 0-d samples were allowed to bloom also in darkness at 4°C for 1 h before being measured. Immediately after the color measurement, the samples were vacuum-packed and frozen (−20°C) until lipid oxidation analysis.
Instrumental Meat Color and Heme Pigment Estimations
The color of the rectus abdominis and LTL muscles was measured using a Minolta CM-2006d spectrophotometer (Konica Minolta Holdings, Inc., Osaka, Japan) in the CIE L*, a*, and b* color space with a measurement area diameter of 8 mm, including a specular component and a 0% UV, standard illuminant D65 that simulates daylight (color temperature of 6,504 K), observer angle 10°, and zero and white calibration. The integrating sphere has a diameter of 52 mm, and the measurement area was covered with a dust cover CM-A149. The lightness (L*), redness (a*), and yellowness (b*) were recorded. The hue angle (H°) was calculated as H° = tan−1(b*/a*) × 57.29, expressed in degrees, and chroma (C*) was calculated as C* = (a*2 + B*2)1/2.
The color of the rectus abdominis muscle was measured after the fascia covering was removed, and the color of the LTL muscle was measured on the surface. In both muscles, the color was measured at 2 locations randomly selected to obtain a mean value with a representative reading of surface color. The measurements were averaged. To standardize the measurements, a white tile was placed behind the muscles.
The relative contents of metmyoglobin (MMb) and oxymyoglobin (MbO2) were estimated using the (K/S at 572 nm)/(K/S at 525 nm) and (K/S at 610 nm)/(K/S at 525 nm) ratios, where K is the absorption coefficient and S is the scattering coefficient, respectively, as described in Ripoll et al. (2013). Both ratios decrease when the pigment content increases. The vertical axis of the figure was inverted to obtain the correct visual impression when viewing the curves.
Meat Chemical Composition
Meat samples were lyophilized and then minced to determine the DM, CP, intramuscular fat (IMF), and α-tocopherol contents and fatty acid profile. Samples were weighed before and after lyophilization to obtain the DM content. The IMF content was determined following the Ankom procedure (AOCS, 2005) with an XT10 Ankom extractor (Ankom Technology, Madrid, Spain). The content of CP (N × 6.25) was determined following the Dumas procedure (method 984.13; AOAC, 1999) using a nitrogen analyzer (model NA 2100; CE Instruments Ltd., ThermoQuest SA, Barcelona, Spain).
The extraction of α-tocopherol from the feedstuffs was performed according to Val et al. (1994) and in the LTL muscle according to the ISO 12822 (2014). The content of α-tocopherol was determined using ultra-high-resolution liquid chromatography (Waters UHPLC Acquity H-Class System; Waters Corp., Milford, MA) coupled to a fluorescence detector (Waters 2475 Multi λ Fluorescence Detector, working with excitation wavelength = 295 and emission wavelength = 330; Waters Corp.). The quantification was performed as proposed by Chauveau-Duriot et al. (2010).
The lipid oxidation was determined using the thiobarbituric acid-reactive substances method, as described in Ripoll et al. (2013). Fatty acids from the feedstuffs were extracted and derivatized according to the method described by Sukhija and Palmquist (1988; extracted with heptane instead of benzene) and by Lee et al. (2012) in IMF. Fatty acids methyl ester determination was performed using gas chromatography (SCION 436-GC; Bruker, Billerica, MA) equipped with a cyanopropyl capillary column (BR-2560, 100 m × 0.25 mm i.d. × 0.20 µm thickness; Bruker), flame ionization detector, and Compass CDS software (Bruker). Fatty acid quantification was performed as described in the ISO 12966-4 (2015), and identification was performed using the GLC 538 and GLC 463 standard references (Nu-Chek Prep Inc., Elysian, MN). Fatty acids were expressed as a percentage of the total amount of the identified fatty acids. After individual fatty acid determination, the sum of the SFA (C10:0 + C12:0 + C14:0 + C15:0 + C16:0 + C18:0), MUFA (C16:1 + the sum of C18:11t9,t10,t11,t12 + C18:1n-9 + C18:1n-7), PUFA (C18:2n-6 + CLA + C20:4n-6 + C20:5n-3 + C22:5n-3 + C226n-3), n-6 PUFA (C18:2n-6 + C20:4n-6), and n-3 PUFA (C20:5n-3 + C22:5n-3+C226n-3) were calculated. The PUFA:SFA and n-6:n-3 PUFA ratios were calculated.
Statistical Analyses
Data were analyzed with the SAS statistical software (SAS version 9.3; SAS Inst. Inc., Cary, NC). Rectus abdominis color results and pH, DM, CP, IMF, α-tocopherol contents and fatty acid composition of the LTL muscle were analyzed with the GLM procedure for the dam's feeding system during lactation, the inclusion of quebracho in the fattening concentrate, and the interaction as the fixed effects. The color, pigments, and lipid oxidation of the LTL muscle were analyzed with a mixed model (MIXED procedure) using repeated measurements. The dam's feeding system during lactation, the inclusion of quebracho in the fattening concentrate, the meat display time, and the interactions were included as fixed effects, and lamb was included as random effect. The degrees of freedom were adjusted with the correction of Kenward–Roger to account for unequal observations or missing values. To model the error, different variance–covariance matrices were tested, and the one with the lowest Akaike and Bayesian information criteria was chosen. Multiple comparisons among treatments were performed using the Tukey method. The interaction between the dam's feeding system during lactation and the inclusion of quebracho in the fattening concentrate was removed from the model when it was not significant (P > 0.05), and the analyses were repeated. The least squares means and SE were obtained, and differences were considered when P < 0.05. Trends were discussed when 0.10 < P ≤ 0.05.
RESULTS
Meat pH, Chemical Composition, and α-Tocopherol Contents of the Longissimus Thoracis et Lumborum Muscle
The dam's feeding system during lactation did not affect the pH, DM, or CP content in lambs' LTL muscle (Table 3). However, Indoor lambs presented the greatest IMF, Alfalfa lamps intermediate, and Sainfoin lambs the lowest (P < 0.01). In contrast, Indoor lambs had lower α-tocopherol content than the Alfalfa and Sainfoin lambs (P < 0.001), whose content did not differ between them (P < 0.05).
Table 3.
Effect of the feeding system during lactation and the inclusion of quebracho in the concentrate during fattening on the pH and chemical composition of the meat
| Lactation1 | Concentrate2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Indoor | Alfalfa | Sainfoin | Control | QUE | RMSE3 | Lactation | Concentrate |
| pH | 5.5 | 5.6 | 5.6 | 5.6 | 5.6 | 0.03 | 0.420 | 0.640 |
| DM | 24.8 | 24.7 | 24.4 | 24.7 | 24.5 | 0.69 | 0.122 | 0.270 |
| Intramuscular fat, % FM4 | 2.9a | 2.4ab | 2.3b | 2.4 | 2.6 | 0.23 | 0.007 | 0.440 |
| CP, % FM | 18.3 | 18.5 | 18.3 | 18.4 | 18.3 | 0.59 | 0.379 | 0.301 |
| α-Tocopherol, mg/kg FM | 0.4b | 1.0a | 1.26a | 1.0 | 0.8 | 0.33 | 0.001 | 0.019 |
Means within a row with different superscripts significantly differ among feeding system during lactation (P < 0.05).
Indoor = ewes fed a total mixed ration; Alfalfa = grazing on alfalfa; Sainfoin = grazing on sainfoin.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
RMSE = root mean square error.
FM = fresh matter.
The inclusion of quebracho in the fattening concentrate of the lambs did not affect the pH, DM, CP, and IMF contents (P > 0.05) but decreased the α-tocopherol content by 21% compared with the Control lambs (P < 0.05).
Instrumental Color and Heme Pigments of Meat
The dam's feeding system during lactation affected only the redness of the rectus abdominis muscle (P < 0.05), which was greater in Sainfoin lambs, intermediate in Alfalfa lambs, and lower in Indoor lambs (Table 4). The inclusion of quebracho in the fattening concentrate had no effect on the color of the rectus abdominis muscle.
Table 4.
Effect of the feeding system during lactation and the inclusion of quebracho in the fattening concentrate on the color of the rectus abdominis muscle
| Lactation1 | Concentrate2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Indoor | Alfalfa | Sainfoin | Control | QUE | RMSE3 | Lactation | Concentrate |
| Lightness (L*) | 46.9 | 45.9 | 45.3 | 45.8 | 46.3 | 3.16 | 0.24 | 0.57 |
| Redness (a*) | 9.2b | 10.2ab | 10.4a | 10.2 | 9.7 | 1.50 | 0.03 | 0.24 |
| Yellowness (b*) | 8.9 | 8.7 | 8.5 | 8.8 | 8.6 | 1.93 | 0.84 | 0.77 |
| Hue angle | 43.3 | 40.3 | 39.3 | 40.4 | 41.6 | 8.34 | 0.28 | 0.57 |
| Chroma | 13.0 | 13.5 | 13.6 | 13.6 | 13.1 | 1.51 | 0.42 | 0.20 |
Means within a row with different superscripts significantly differ among feeding system during lactation (P < 0.05).
Indoor = ewes fed a total mixed ration; Alfalfa = grazing on alfalfa; Sainfoin = grazing on sainfoin.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
RMSE = Root mean square error.
The color and heme pigment parameters of the LTL muscle were significantly affected by the time of display (P < 0.001). The lightness, yellowness, and hue angle color parameters of the LTL muscle were modified by the dam's feeding system (Table 5). On average, the LTL muscle of the Indoor lambs had greater lightness, yellowness, and hue angle values than those of the Alfalfa and Sainfoin lambs (Table 5). Redness was affected by the interaction between the dam's feeding system during lactation and the display time (P < 0.01; Fig. 1). Redness remained almost steady throughout the display time in the LTL muscle of the Alfalfa and Sainfoin lambs, whereas it was significantly higher during the first day of display (0 d) in the LTL muscle of the Indoor group. On average, the LTL muscles of the Alfalfa and Sainfoin lambs had greater redness than the LTL muscles of the Indoor lambs (P < 0.05).
Table 5.
Effect of the feeding system during lactation and the inclusion of quebracho in the fattening concentrate on the instrumental color, heme pigments, and lipid oxidation in the longissimus thoracis et lumborum muscle
| Lactation1 | Concentrate2 | P-value | |||||
|---|---|---|---|---|---|---|---|
| Item | Indoor | Alfalfa | Sainfoin | Control | QUE | Lactation | Concentrate |
| Lightness (L*) | 42.0a ± 0.37 | 40.4b ± 0.40 | 40.9b ± 0.38 | 41.0 ± 0.31 | 41.2 ± 0.31 | 0.010 | 0.625 |
| Redness (a*)3 | 11.4b ± 0.18 | 12.1ab ± 0.21 | 12.3a ± 0.21 | 12.1 ± 0.17 | 11.7 ± 0.17 | 0.005 | 0.087 |
| Yellowness (b*) | 7.0a ± 0.18 | 6.2b ± 0.19 | 6.4b ± 0.19 | 6.5 ± 0.15 | 6.6 ± 0.15 | 0.016 | 0.595 |
| Hue angle | 31.5a ± 0.92 | 27.2b ± 0.96 | 27.2b ± 0.94 | 28.0 ± 0.77 | 29.3 ± 0.76 | 0.002 | 0.234 |
| Chroma3 | 13.5 ± 0.16 | 13.7 ± 0.17 | 14.0 ± 0.17 | 13.9 ± 0.14 | 13.6 ± 0.14 | 0.177 | 0.149 |
| Metmyoglobin3 | 1.14a ± 0.010 | 1.06b ± 0.010 | 1.08ab ± 0.010 | 1.09 ± 0.008 | 1.08 ± 0.008 | 0.001 | 0.486 |
| Oxymyoglobin3,4 | 0.43a ± 0.005 | 0.42ab ± 0.005 | 0.41b ± 0.005 | 0.41 ± 0.004 | 0.42 ± 0.004 | 0.013 | 0.036 |
| Lipid oxidation3 | 1.4a ± 0.09 | 1.2b ± 0.09 | 0.8c ± 0.09 | 1.2 ± 0.08 | 1.0 ± 0.07 | 0.001 | 0.100 |
Means within a row with different superscripts significantly differ among feeding system during lactation (P < 0.05).
Indoor = ewes fed a total mixed ration; Alfalfa = grazing on alfalfa; Sainfoin = grazing on sainfoin.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
Affected by the interaction between the feeding system during lactation and the display time (P < 0.05; see Fig. 1, 2, and 3).
Affected by the interaction between the inclusion of quebracho in the fattening concentrate and the display time (P < 0.05; see Fig. 2).
Figure 1.
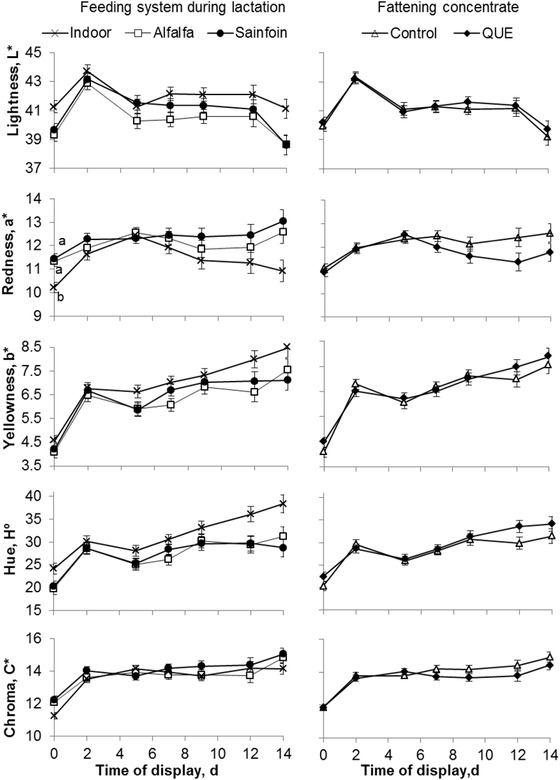
Evolution of the instrumental color of longissimus thoracis et lumborum muscle of light lambs according to the feeding system during lactation and the inclusion of quebracho in the fattening concentrate. a,bWithin a main effect and display time, different letters indicate differences at P < 0.05 between treatments. QUE = commercial concentrate with 5% quebracho.
The interaction between the dam's feeding system during lactation and the display time affected the MMb (P < 0.05) and MbO2 levels (P < 0.01; Fig. 2). The LTL muscles of the Indoor lambs had a different evolution than those of the Alfalfa and Sainfoin lambs, but without a clear effect of the dam's feeding system during lactation. Independent of the storage time, Indoor lambs had lower MMb than did the Alfalfa lambs (P < 0.01) and a lower MbO2 than did the Sainfoin lambs (P < 00.5). In addition, the MbO2 and redness were highly correlated (r = −0.87, P < 0.001).
Figure 2.
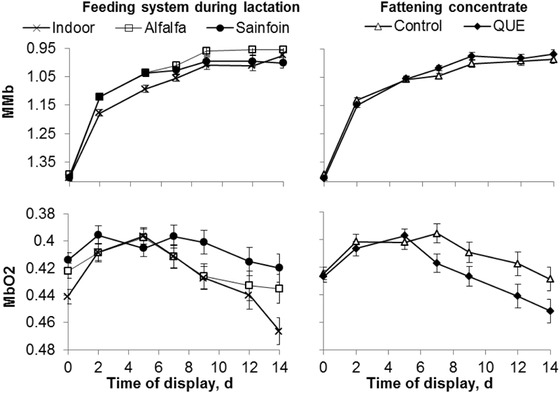
Evolution of metmyoglobin (MMb) and oxymyoglobin (MbO2) of the longissimus thoracis et lumborum muscle of light lambs according to the feeding system during lactation and the inclusion of quebracho in the fattening concentrate. QUE = commercial concentrate with 5% quebracho.
The inclusion of quebracho in the fattening concentrate had no effects on color parameters and MMb content of the LTL muscle (Table 5). However, the interaction of the QUE treatment with the display time affected the MbO2 content (P < 0.05). The MbO2 content increased between 0 and 2 d, peaked at 7 d, and decreased until 14 d of display, although not significantly, in Control lambs. The MbO2 content increased between 0 and 2 d, remained steady until 5 d, and linearly decreased until 14 d in QUE lambs (Fig. 2). On average, the inclusion of quebracho in the fattening concentrate reduced the MbO2 (P < 0.05).
Lipid Oxidation of the Longissimus Thoracis et Lumborum Muscle
Lipid oxidation of the LTL muscle was affected by the interaction between the dam's feeding system during lactation and the display time (P < 0.001; Table 5) but not by the inclusion of quebracho in the fattening concentrate (P > 0.05). Lipid oxidation of the LTL muscle did not differ among feeding systems during lactation until displayed for 7 d, when it was greater for Indoor lambs, intermediate for Alfalfa lambs, and lower for Sainfoin lambs (P < 0.05; Fig. 3). Lipid oxidation similarly increased in the 3 feeding systems between 7 and 12 d of display, being greater in the Indoor and Alfalfa lambs than in Sainfoin lambs (P < 0.05). Between 12 and 14 d of display, the lipid oxidation considerably increased in the Sainfoin lambs, was intermediate in the Indoor lambs, and was low in the Alfalfa lambs (43, 24, and 10%, respectively); consequently, the Alfalfa treatment had an intermediate lipid oxidation, similar to the rest of the treatments (P > 0.05), but the Indoor treatment was still different (P < 0.05) from the Sainfoin treatment.
Figure 3.
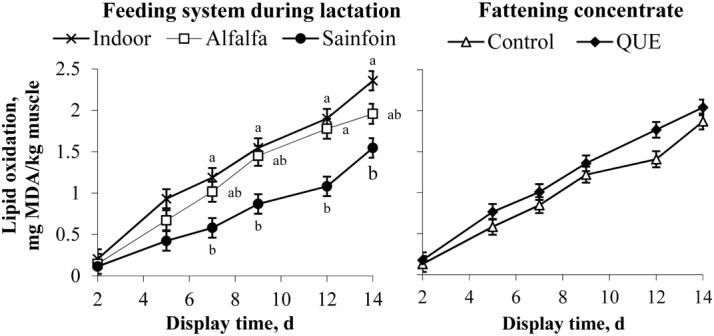
Evolution of the lipid oxidation of the longissimus thoracis et lumborum muscle of light lambs according to the feeding system during lactation and the inclusion of quebracho in the fattening concentrate. a,bWithin a main effect and time of display, different letters indicate differences at P < 0.05 between treatments. QUE = commercial concentrate with 5% quebracho; MDA = malondialdehyde.
Fatty Acid Composition of the Longissimus Thoracis et Lumborum Muscle
The fatty acid profile results are presented in Table 6. The fatty acid profile of the Alfalfa and Sainfoin lambs differed only in the percentages of the individual n-3 PUFA (P < 0.05), which were lower in the Alfalfa lambs than in the Sainfoin lambs. The LTL muscle of the Alfalfa and Sainfoin lambs presented lower C18:1n-9 and greater C18:2n-6, C18:3n-3, CLA, C20:5n-3, and C22:5n-3 percentages than that from the Indoor lambs (P < 0.01). The inclusion of quebracho in the fattening concentrate increased the percentages of C16:0, C18:1, and C18:2n-6 and decreased the percentages of C15:0, C17:0, and C17:1 (P < 0.05).
Table 6.
Effect of the feeding system during lactation and the inclusion of quebracho in the fattening concentrate on fatty acids composition of longissimus thoracis et lumborum muscle of light lambs
| Lactation × Concentrate2,3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indoor | Alfalfa | Sainfoin | P-value | |||||||
| Percent FAME1 | Control | QUE | Control | QUE | Control | QUE | RMSE4 | Lactation | Concentrate | Lactation × Concentrate |
| C10:0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.06 | 0.060 | 0.216 | 0.784 |
| C12:0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | 0.16 | 0.104 | 0.905 | 0.743 |
| C14:0 | 4.2 | 4.6 | 4.3 | 4.2 | 4.8 | 4.8 | 1.04 | 0.217 | 0.663 | 0.661 |
| C15:0 | 0.5bxy | 0.4by | 0.6ax | 0.5axy | 0.6ax | 0.5ax | 0.09 | 0.001 | 0.026 | 0.635 |
| C16:0 | 24.4xy | 25.4x | 24.3xy | 24.8xy | 23.6y | 24.5xy | 1.18 | 0.061 | 0.008 | 0.750 |
| C16:1 | 1.9a | 2.0a | 1.9ab | 1.8ab | 1.8b | 1.8b | 0.26 | 0.035 | 0.972 | 0.463 |
| C17:0 | 1.4xy | 1.0w | 1.5x | 1.1zw | 1.5xy | 1.2yz | 0.17 | 0.056 | 0.001 | 0.164 |
| C17:1 | 0.8x | 0.5z | 0.7xy | 0.5yz | 0.6xyz | 0.5yz | 0.18 | 0.586 | 0.001 | 0.107 |
| C18:0 | 14.3 | 13.8 | 14.5 | 14.0 | 14.4 | 14.4 | 1.05 | 0.589 | 0.185 | 0.645 |
| C18:15 | 4.2xy | 4.9xy | 3.9y | 5.5x | 4.4xy | 4.7xy | 0.99 | 0.865 | 0.002 | 0.114 |
| C18:1n-9 | 36.7ax | 35.4axy | 35.1bxyz | 32.4bz | 32.8byz | 33.3byz | 2.06 | 0.001 | 0.031 | 0.049 |
| C18:1n-7 | 0.4 | 0.3 | 0.4 | 0.3 | 0.3 | 0.4 | 0.19 | 0.181 | 0.367 | 0.209 |
| C18:2n-6 | 6.6by | 7.3bxy | 7.1ay | 8.7ax | 7.5abxy | 7.6abxy | 1.10 | 0.037 | 0.012 | 0.091 |
| C18:3n-3 | 0.5cw | 0.5cw | 1.1bz | 1.1byz | 1.6ax | 1.4axy | 0.27 | 0.001 | 0.653 | 0.235 |
| CLA | 0.4by | 0.4by | 0.6axy | 0.7ax | 0.6axy | 0.6axy | 0.20 | 0.002 | 0.387 | 0.522 |
| C20:4n-6 | 1.8xy | 1.7y | 1.7y | 1.9xy | 2.2x | 1.6y | 0.35 | 0.366 | 0.094 | 0.003 |
| C20:5n-3 | 0.2cz | 0.2cz | 0.4by | 0.4by | 0.8ax | 0.5ay | 0.16 | 0.001 | 0.006 | 0.001 |
| C22:5n-3 | 0.4cz | 0.4cz | 0.6byz | 0.7bxy | 0.9ax | 0.7axy | 0.17 | 0.001 | 0.651 | 0.007 |
| C22:6n-3 | 0.2by | 0.2by | 0.2by | 0.2by | 0.4ax | 0.2ay | 0.09 | 0.001 | 0.010 | 0.031 |
| SFA6 | 45.5 | 46.0 | 45.9 | 45.3 | 45.7 | 46.3 | 1.77 | 0.771 | 0.702 | 0.467 |
| MUFA7 | 44.0ax | 43.1axy | 42.1bxyz | 40.6byz | 40.0bz | 40.7byz | 2.04 | 0.001 | 0.272 | 0.189 |
| PUFA7 | 10.2bz | 10.8bz | 11.8ayz | 13.8ax | 14.1ax | 12.7axy | 1.47 | 0.001 | 0.283 | 0.002 |
| n-6 PUFA8 | 8.5y | 9.0xy | 8.8y | 10.6x | 9.7xy | 9.2xy | 1.35 | 0.062 | 0.092 | 0.031 |
| n-3 PUFA8 | 1.3cz | 1.3cz | 2.3by | 2.5by | 3.8ax | 2.9ay | 0.59 | 0.001 | 0.135 | 0.011 |
| PUFA8:SFA6 ratio | 0.2bz | 0.2bz | 0.3ayz | 0.3ax | 0.3ax | 0.3axy | 0.04 | 0.001 | 0.351 | 0.003 |
| n-6:n-3 ratio | 7.0ax | 7.0ax | 4.2byz | 4.5by | 2.7cz | 3.4cyz | 1.42 | 0.001 | 0.323 | 0.690 |
Means within a row with different superscripts significantly differ among feeding system during lactation (P < 0.05).
Means within a row with different superscripts significantly differ among feeding system during lactation × fattening concentrate (P < 0.05).
FAME = fatty acid methyl esters. These were the identified total FAME.
Indoor = ewes fed a total mixed ration; Alfalfa = grazing on alfalfa; Sainfoin = grazing on sainfoin.
Control = commercial concentrate; QUE = commercial concentrate with 5% quebracho.
RMSE = root mean square error.
Sum of C18:11t9,t10,t11,t12.
Sum of C10:0, C12:0, C14:0, C15:0, C16:0, and C18:0.
Sum of C16:1, C18:11t9,t10,t11,t12, C18:1n-9, and C18:1n-7.
Sum of C18:2n-6, CLA, C20:4n-6; C20:5n-3; C22:5n-3, and C226n-3.
The percentages of C18:1n-9, C20:4n-6, C20:5n-3, C22:5n-3, and C22:6n-3 were affected by the interaction between the dam's feeding system during lactation and the inclusion of quebracho in the fattening concentrate (P < 0.05). The inclusion of quebracho decreased the C20:4n-6, C20:5n-3, C22:6n-3, and n-3 PUFA in the Sainfoin lambs but not in the Alfalfa or Indoor lambs (P < 0.05).
Indoor lambs presented greater MUFA and lower PUFA than the Alfalfa and Sainfoin lambs (P < 0.001). However, the effect of the inclusion of quebracho in the fattening concentrate on total PUFA, n-6 PUFA, and n-3 PUFA depends on the dam's feeding system during lactation (P < 0.01). The inclusion of quebracho increased the total PUFA and n-6 PUFA only in the Alfalfa lambs (P < 0.05) and decreased the n-3 PUFA only in the Sainfoin lambs (P < 0.05). Regardless of the inclusion of quebracho, the Sainfoin lambs had the greatest, the Alfalfa lambs intermediate, and the Indoor lambs the lowest n-3 PUFA (P < 0.001). The n-6:n-3 ratio was greater in the Indoor lambs, intermediate in the Alfalfa lambs, and lower in the Sainfoin lambs (P < 0.001).
DISCUSSION
Sainfoin grazing during lactation was compared with 2 systems that have proven to yield good ADG of lambs in an autochthonous breed specialized in lamb production with low milk production (around 1 kg/d; Jaime and Purroy, 1995). Suckling lambs that grazed sainfoin had similar BW and age at weaning to their counterparts, which suggests that the intake of standard milk was similar among strategies.
In both grazing forage paddocks, the herbage mass was not limited throughout the experiment. The forage availability was always equal to or greater than 2 kg DM/d per ewe, which is above the threshold that limits the voluntary intake (Valderrábano and Folch, 1984). Besides, the forage quality was high throughout the whole grazing period (data not shown), which assured that the energy and CP intake were not limiting the animal performance, as reflected in the BW of the lambs at weaning. Except for the CT content, the concentrate fed to lambs during the fattening period (Control vs. QUE) had a similar chemical composition.
Meat pH, Chemical Composition, and α-Tocopherol Contents
The range of pH values of the LTL muscle was narrow (from 5.54 to 5.57), similar to that reported in light lambs of the same breed (Ripoll et al., 2012) and of other local breeds (Díaz et al., 2002; Carrasco et al., 2009). These pH values correspond to a normal range, ruling out dark-cutting or stress problems (Carrasco et al., 2009).
The effect of the dam's feeding system during lactation was noticeable in the IMF and α-tocopherol contents of the LTL muscle, despite the subsequent fattening period of 28 (±2) d. The greatest IMF content in Indoor lambs could be related to the higher levels of intake in this group and the lower level of exercise (Velasco et al., 2001).
As in the current experiment, the greater α-tocopherol content in the muscle is deposited when ruminants are fed fresh forages or concentrates rich in α-tocopherol (Ripoll et al., 2013; Jose et al., 2016). The present results highlight the importance of the dam's diet during the suckling period on tocopherol content in light lamb muscle, regardless of the type of concentrate fed during the period of fattening. The α-tocopherol from the feedstuff ingested by the dam and deposited into the muscle of the suckling lamb through the milk remains after a fattening period, which lasted 30 d. Most likely, it would be cheaper to feed the dams with fresh forages with high α-tocopherol content than supplementing the concentrate of the lambs with synthetic α-tocopherol.
The lower α-tocopherol content in the muscle presented in the QUE lambs is related to the differences in the composition of the concentrates, which were formulated to be isoenergetic and isoproteic. The variation of ingredients influenced the total of α-tocopherol content in the concentrates (3.1 vs. 4.9 µg/g DM for QUE and Control, respectively), and consequently, the muscle reflected these differences in α-tocopherol intake.
Instrumental Color and Heme Pigments
The greater lightness in the LTL muscle of Indoor lambs compared with that of Alfalfa lambs has been previously reported in light lambs fed Indoor concentrates or grazing alfalfa with their dams until slaughter (Ripoll et al., 2012, 2013) and in grazing weaned lambs (Díaz et al., 2002). Other factors such as pH, IMF, carcass weight, and age at slaughter are responsible for differences in lightness between forage-fed ruminants and concentrate-fed ruminants (Priolo et al., 2001). In the current experiment, pH was similar between the feeding systems, but differences in the IMF as well as in the carcass weight were observed (data not shown).
Grazing animals experienced higher physical activity, resulting in a greater concentration of heme pigments, which involve darker (Renerre, 1986) and more red meat (Carrasco et al., 2009), features that are often rejected by Spanish consumers (Bernués et al., 2012). The redness seems to be more appropriate than lightness when assessing color acceptability (Hopkins, 1996). Khliji et al. (2010) reported that redness values explained the variation of consumer scores better than the lightness values. These authors suggested that when the redness values are equal to or exceed 9.5, consumers will consider the meat color acceptable. The redness of the meat in the present study in all treatments was above this limit. The greater redness observed in the rectus abdominis and in the LTL muscles in lambs from grazing ewes (alfalfa and sainfoin) was also observed in light lambs under the grazing system vs. those stalled indoors (Carrasco et al., 2009). The differences in redness are partially related to the greater intake, mainly through milk, of carotenoids and α-tocopherol (Ripoll et al., 2008) and partially to the physical activity, as discussed above.
Discoloration of lamb meat occurs when the hue angle quickly increases at the end of the display time, and this characteristic is a good descriptor of meat discoloration (Carrasco et al., 2009; Luciano et al., 2009a). In the current experiment, the greater hue angle of Indoor lambs in contrast to Alfalfa and Sainfoin lambs confirms the protective effect of the herbage-based diets against meat discoloration compared with the concentrate-based diets (Luciano et al., 2012). In the present study, the greater MMb of Alfalfa and Sainfoin lambs than Indoor lambs agrees with the greater content of heme pigments in the grazing animals, caused by the physical activity; therefore, these lambs had more heme pigment to be oxidized. The MMb followed the typical, almost logarithmic, evolution previously reported by Ripoll et al. (2013), whereas the deoxymyoglobin (DMb) remained steady (Lagerstedt et al., 2011; Albertí et al., 2014). Hence, MbO2 also increased in the first days and then decreased from approximately the fifth day of display (Ripoll et al., 2013).
In the current experiment, the lack of effect of the inclusion of quebracho in the fattening concentrate on the hue angle of the LTL muscle agrees with the results obtained when 8.9% quebracho was supplemented in the concentrates for a 60-d fattening period (Luciano et al., 2011). The source of CT can influence their effect on meat color. An increase in the lightness of muscle occurs when CT are from other sources, such as those in Acacia cyanophylla (Priolo et al., 2002) or Hedysarum coronarium (Priolo et al., 2005), because the CT reduced the production of hemoglobin (Priolo and Vasta, 2007). However, when Cistus ladanifer was fed to lambs, no effects were observed on the meat color (Francisco et al., 2015). In the present study, the short fattening period and the low dose have impaired the possible effect of the CT from quebracho on the meat color. Additionally, López-Andrés et al. (2013) suggested that dietary quebracho seems not to be degraded or absorbed in gastrointestinal tract, which could also have contributed to the lack of effect observed in the current study.
The increment of MMb of fresh meat is due to the conversion of MbO2 to DMb followed by an oxidation of DMb to MMb. From Day 5 to 7, meat from the QUE treatment had a greater deoxygenation of MbO2 than the Control group. Deoxygenation of MbO2 to DMb is favored under low oxygen partial pressures that occur when the dissolved oxygen in muscle tissue is consumed by enzymatic reactions, including mitochondrial respiration and cytochrome P450 activity. The lower values of MbO2 were related to greater, but not significant, values of MMb in the QUE treatment. The rate of deoxygenation of MbO2 from Day 7 to 14 was similar for the Control and QUE treatments. The inclusion of quebracho seems to protect DMb from oxidation, but further investigations are required.
Lipid Oxidation
The dam's feeding system during lactation had a greater effect on lipid oxidation than the inclusion of quebracho in the fattening concentrate. The threshold of lipid oxidation for acceptable meat was set at 1 mg malondialdehyde/kg of muscle in light lambs in Spain (Ripoll et al., 2011). This threshold was reached after the LTL muscles of the Indoor, Alfalfa, and Sainfoin lambs were stored for 5, 7, and 11 d, respectively. These results highlight the importance of the dams' diets during lactation on the shelf life of the light lamb meat, even though the lambs were finished on concentrates for a short feeding period. The lipid oxidation of the LTL muscle could be partially related to the contents of α-tocopherol (González-Calvo et al., 2015b) and polyphenols (Vasta and Luciano, 2011) in the muscle, as the contents of each in the diet reduced lipid oxidation in the lamb meat (Francisco et al., 2015; Liu et al., 2016). According to Moñino et al. (2008), the polyphenols of the feedstuffs are transferred through the ewe's milk to the lamb. Therefore, polyphenols from sainfoin could have been transferred to the lambs through suckling. Moreover, the CT may protect other antioxidants from oxidation, such as α-tocopherol (Yamamoto et al., 2006; Iglesias et al., 2012). Therefore, the high content of α-tocopherol and CT present in the sainfoin could explain the lower lipid oxidation observed in the lambs of the Sainfoin group in the current experiment.
No effects of CT from quebracho were observed on meat lipid oxidation, in line with Brogna et al. (2014) in lambs fed 80 g/kg of quebracho or in the minced meat from lambs fed 8.9% of quebracho in the concentrate (Luciano et al., 2009b). The different effect of CT from quebracho and from Sainfoin can be partially related to the α-tocopherol content, which was low in the QUE lambs, and to the type of CT. Furthermore, the CT from quebracho was not absorbed in the gastrointestinal tract, although it induced antioxidant effects in animal tissues (López-Andrés et al., 2013).
Fatty Acid Composition of the Longissimus Thoracis et Lumborum Muscle
The dam's feeding system during lactation had more effect on the fatty acid profile of the IMF than the inclusion of quebracho in the fattening concentrate. The meat of the Alfalfa and Sainfoin lambs was healthier, from a human point of view, than that of the Indoor lambs, as the CLA and n-3 PUFA contents were greater. The interest for these fatty acids is related to their possible beneficial effect in reducing the incidence of cancer, diabetes, and atherosclerosis (Pariza et al., 1999; Lee et al., 2005; Park and Pariza, 2007). Sainfoin lambs presented greater n-3 PUFA than Indoor and Alfalfa lambs, probably due to the presence of the CT in the sainfoin (Girard et al., 2016) and, to some extent, the differences in n-3 PUFA level between fresh forages and a total mixed ration. Condensed tannins improve the fatty acid profile in ruminants, thereby reducing the ruminal biohydrogenation and increasing Δ9–desaturase protein expression in the muscle (Vasta et al., 2009; Toral et al., 2016). According to Girard et al. (2016), the levels of 18:3n-3 in IMF appear to be dose-dependent in terms of CT intake and/or chemical structure. In the present study, lambs had access to only forage during the suckling phase, when the intake of solid food was minimal (Álvarez-Rodríguez et al., 2007). After weaning, lambs were stalled and ate only concentrate and straw. Therefore, the grazing period was noticeably shorter than that of the other study of the literature (González-Calvo et al., 2015a). The greater n-3 PUFA of Sainfoin lambs compared with Alfalfa lambs agrees with the results reported by Girard et al. (2016). The n-3 PUFA percentages in the Indoor treatment were similar to those observed by Roche et al. (2012) with the same breed and under similar feeding conditions.
The effects of the inclusion of quebracho on the fatty acid profile, expressed as grams per 100 g muscle, of lamb meat has been studied only with an inclusion of 8.9% quebracho for 60 d (Vasta et al., 2009) and 80 g quebracho/kg for 12 d (Brogna et al., 2014), which was too short a time for differences to manifest. Therefore, their results are not completely comparable with the present results, because of the different time on feed studied and because of the different way the content of fatty acid was expressed. In the current experiment, certain individual fatty acids and total PUFA, n-6 PUFA, and n-3 PUFA were unexpectedly affected by the interaction between feeding system during lactation and inclusion of quebracho in the fattening concentrate. Therefore, the effect of CT from quebracho depends on the dose and time fed but also on the type of feeding received in the lactation period, as reported in the current experiment. Moreover, the effects of CT from quebracho differ also between concentrate- and forage-fed lambs (Vasta et al., 2009). In fact, Vasta et al. (2009) showed no effect of CT from quebracho on total SFA and MUFA content of meat in forage-fed lambs whereas the content of some individual fatty acid and n-3 PUFA and n-6 PUFA differed. In the abovementioned study, the inclusion of quebracho especially affected the fatty acids involved in the biohydrogenation pathway, particularly the C18:2n-6 and C18:1n-7, which partially agrees with the results of the current experiment. The reasons for the discrepancy of the effect of inclusion of quebracho depending on the dams feeding system remain unclear, and further studies should be performed to elucidate them.
Conclusions
The dams' feeding system affected the meat quality of light lambs more than the inclusion of quebracho in the fattening concentrate, highlighting the importance of the lactation period. Rearing lambs with their dams in alfalfa and sainfoin is recommended more than the indoor system, because grazing during lactation increased the meat color stability and improved the meat fatty acid profile, which are important to human consumers. Grazing on sainfoin is especially recommended because this system extended the meat shelf life more than 4 d and resulted in a more appropriate fatty acid profile. The inclusion of 5% quebracho in the fattening concentrate had mild effects on the meat quality. Further studies should be performed to clarify the effect of condensed tannins (type, dose, and intake) and the residual effect of the dams' diet during the lactation period on the meat quality.
LITERATURE CITED
- Albertí P., Beriain M. J., Ripoll G., Sarriés V., Panea B., Mendizabal J. A., Purroy A., Olleta J. L., Sañudo C. 2014. Effect of including linseed in a concentrate fed to young bulls on intramuscular fatty acids and beef color. Meat Sci. 96(3):1258–1265. doi: 10.1016/j.meatsci.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Álvarez-Rodríguez J., Sanz A., Delfa R., Revilla R., Joy M. 2007. Performance and grazing behaviour of Churra Tensina sheep stocked under different management systems during lactation on Spanish mountain pastures. Livest. Sci. 107(2–3):152–161. doi: 10.1016/j.livsci.2006.09.011 [DOI] [Google Scholar]
- Álvarez-Rodríguez J., Sanz A., Ripoll-Bosch R., Joy M. 2010. Do alfalfa grazing and lactation length affect the digestive tract fill of light lambs? Small Rumin. Res. 94(1–3):109–116. doi: 10.1016/j.smallrumres.2010.07.009 [DOI] [Google Scholar]
- American Oil Chemists' Society (AOCS) 2005. AOCS standard procedure Am 5-04: Rapid determination of oil/fat utilizing high temperature solvent extraction. AOCS Press, Urbana, IL. [Google Scholar]
- AOAC 1999. Official methods of analysis. 16th ed.AOAC Int., Washington, DC. [Google Scholar]
- Bernués A., Ripoll G., Panea B. 2012. Consumer segmentation based on convenience orientation and attitudes towards quality attributes of lamb meat. Food Qual. Prefer. 26(2):211–220. doi: 10.1016/j.foodqual.2012.04.008 [DOI] [Google Scholar]
- Bernués A., Ruiz R., Olaizola A., Villalba D., Casasús I. 2011. Sustainability of pasture-based livestock farming systems in the European Mediterranean context: Synergies and trade-offs. Livest. Sci. 139(1–2):44–57. doi: 10.1016/j.livsci.2011.03.018 [DOI] [Google Scholar]
- Brogna D. M. R., Tansawat R., Cornforth D., Ward R., Bella M., Luciano G., Priolo A., Villalba J. 2014. The quality of meat from sheep treated with tannin- and saponin-based remedies as a natural strategy for parasite control. Meat Sci. 96(2, Part A):744–749. doi: 10.1016/j.meatsci.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Carrasco S., Panea B., Ripoll G., Sanz A., Joy M. 2009. Influence of feeding systems on cortisol levels, fat color and instrumental meat quality in light lambs. Meat Sci. 83(1):50–56. doi: 10.1016/j.meatsci.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Chauveau-Duriot B., Doreau M., Nozière P., Graulet B. 2010. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 397(2):777–790. doi: 10.1007/s00216-010-3594-y [DOI] [PubMed] [Google Scholar]
- Regulation Council. 2009. N° 1099/2009 of 24 Septemebr 2009 on the proteccion of animals at the time of killing. O.J. L 303/1 [Google Scholar]
- Díaz M. T., Velasco S., Cañeque V., Lauzurica S., Ruiz De Huidobro F., Pérez C., González J., Manzanares C. 2002. Use of concentrate or pasture for fattening lambs and its effect on carcass and meat quality. Small Rumin. Res. 43(3):257–268. doi: 10.1016/S0921-4488(02)00016-0 [DOI] [Google Scholar]
- European Union 2010. Directive 2010/63/EU of the European Parliament and of the 28 Council of 22 September 2010 on the protection of animals used for 29 scientific purposes. Off. J. Eur. Communities 30(L276):33–79. [Google Scholar]
- Francisco A., Dentinho M. T., Alves S. P., Portugal P. V., Fernandes F., Sengo S., Jerónimo E., Oliveira M. A., Costa P., Sequeira A., Bessa R. J. B., Santos-Silva J. 2015. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Sci. 100:275–282. doi: 10.1016/j.meatsci.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Girard M., Dohme-Meier F., Silacci P., Ampuero Kragten S., Kreuzer M., Bee G. 2016. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 96(6):1923–1933. doi: 10.1002/jsfa.7298 [DOI] [PubMed] [Google Scholar]
- González-Calvo L., Joy M., Blanco M., Dervishi E., Molino F., Sarto P., Ripoll G., Serrano M., Calvo J. H. 2015a. Effect of vitamin E supplementation or alfalfa grazing on fatty acid composition and expression of genes related to lipid metabolism in lambs. J. Anim. Sci. 93(6):3044–3054. doi: 10.2527/jas.2014-8758 [DOI] [PubMed] [Google Scholar]
- González-Calvo L., Ripoll G., Molino F., Calvo J. H., Joy M. 2015b. The relationship between muscle α-tocopherol concentration and meat oxidation in light lambs fed vitamin E supplements prior to slaughter. J. Sci. Food Agric. 95(1):103–110. doi: 10.1002/jsfa.6688 [DOI] [PubMed] [Google Scholar]
- Hopkins D. L. 1996. Assessment of lamb meat color. Meat Focus Int. 5:400–401. [Google Scholar]
- Iglesias J., Pazos M., Torres J. L., Medina I. 2012. Antioxidant mechanism of grape procyanidins in muscle tissues: Redox interactions with endogenous ascorbic acid and α-tocopherol. Food Chem. 134(4):1767–1774. doi: 10.1016/j.foodchem.2012.03.072 [DOI] [PubMed] [Google Scholar]
- ISO 12822 2014. Foodstuffs. Determination of vitamin E by high performance liquid chromatography. Measurement of α- β- ƴ- and Δ tocopherol. [Google Scholar]
- ISO 12966-4 2015. Animal and vegetable fats and oils. Gas chromatography of fatty acid methyl esters. Part 4: Determination by capillary gas chromatograpy. [Google Scholar]
- Jaime C., Purroy A. 1995. Level and quality of protein in rations for lactating ewes. Ann. Zootech. 44(2):135–142. doi: 10.1051/animres:19950204 [DOI] [Google Scholar]
- Jose C. G., Jacob R. H., Pethick D. W., Gardner G. E. 2016. Short term supplementation rates to optimise vitamin E concentration for retail color stability of Australian lamb meat. Meat Sci. 111:101–109. doi: 10.1016/j.meatsci.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Joy M., Sanz A., Ripoll G., Panea B., Ripoll-Bosch R., Blasco I., Alvarez-Rodriguez J. 2012. Does forage type (grazing vs. hay) fed to ewes before and after lambing affect suckling lambs performance, meat quality and consumer purchase intention? Small Rumin. Res. 104(1–3):1–9. doi: 10.1016/j.smallrumres.2011.09.048 [DOI] [Google Scholar]
- Khliji S., van de Ven R., Lamb T. A., Lanza M., Hopkins D. L. 2010. Relationship between consumer ranking of lamb color and objective measures of color. Meat Sci. 85(2):224–229. doi: 10.1016/j.meatsci.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Lagerstedt Å., Lundström K., Lindahl G. 2011. Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. longissimus dorsi steaks after different ageing times. Meat Sci. 87(2):101–106. doi: 10.1016/j.meatsci.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Cho K. H., Lee K. T., Kim M. R. 2005. Antiatherogenic effects of structured lipid containing conjugated linoleic acid in C57BL/6J mice. J. Agric. Food Chem. 53(18):7295–7301. doi: 10.1021/jf050626e [DOI] [PubMed] [Google Scholar]
- Lee M. R. F., Tweed J. K. S., Kim E. J., Scollan N. D. 2012. Beef, chicken and lamb fatty acid analysis – A simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 92:863–866. doi: 10.1016/j.meatsci.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Liu H., Li K., Mingbin L., Zhao J., Xiong B. 2016. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 116:236–242. doi: 10.1016/j.meatsci.2016.02.024 [DOI] [PubMed] [Google Scholar]
- López-Andrés P., Luciano G., Vasta V., Gibson T. M., Biondi L., Priolo A., Mueller-Harvey I. 2013. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 110(4):632–639. doi: 10.1017/S0007114512005703 [DOI] [PubMed] [Google Scholar]
- Luciano G., Biondi L., Pagano R. I., Scerra M., Vasta V., López-Andrés P., Valenti B., Lanza M., Priolo A., Avondo M. 2012. The restriction of grazing duration does not compromise lamb meat color and oxidative stability. Meat Sci. 92(1):30–35. doi: 10.1016/j.meatsci.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Luciano G., Monahan F. J., Vasta V., Biondi L., Lanza M., Priolo A. 2009a. Dietary tannins improve lamb meat color stability. Meat Sci. 81(1):120–125. doi: 10.1016/j.meatsci.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Luciano G., Monahan F. J., Vasta V., Pennisi P., Bella M., Priolo A. 2009b. Lipid and color stability of meat from lambs fed fresh herbage or concentrate. Meat Sci. 82(2):193–199. doi: 10.1016/j.meatsci.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Luciano G., Vasta V., Monahan F. J., López-Andrés P., Biondi L., Lanza M., Priolo A. 2011. Antioxidant status, color stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem. 124(3):1036–1042. doi: 10.1016/j.foodchem.2010.07.070 [DOI] [Google Scholar]
- Moñino I., Martínez C., Sotomayor J. A., Lafuente A., Jordán M. J. 2008. Polyphenols transmission to Segureño lamb meat from ewes' diet supplemented with the distillate from rosemary (Rosmarinus officinalis) leaves. J. Agric. Food Chem. 56(9):3363–3367. doi: 10.1021/jf7036856 [DOI] [PubMed] [Google Scholar]
- Pariza M. W., Park Y., Cook M. E. 1999. Conjugated linoleic acid and the control of cancer and obesity. Toxicol. Sci. 52(2. Suppl)107–110. doi: 10.1093/toxsci/52.2.107 [DOI] [PubMed] [Google Scholar]
- Park Y., Pariza M. W. 2007. Mechanisms of body fat modulation by conjugated linoleic acid (CLA). Food Res. Int. 40(3):311–323. doi: 10.1016/j.foodres.2006.11.002 [DOI] [Google Scholar]
- Priolo A., Bella M., Lanza M., Galofaro V., Biondi L., Barbagallo D., Ben Salem H., Pennisi P. 2005. Carcass and meat quality of lambs fed fresh sulla (Hedysarum coronarium L.) with or without polyethylene glycol or concentrate. Small Rumin. Res. 59:281–288. doi: 10.1016/j.smallrumres.2005.05.012 [DOI] [Google Scholar]
- Priolo A., Ben Salem H., Atti N., Nefzaoui A. 2002. Polyethylene glycol in concentrate or feedblocks to deactivate condensed tannins in Acacia cyanophylla Lindl. foliage 2. Effects on meat quality of Barbarine lambs. Anim. Sci. 75(1):137–140. doi: 10.1017/S1357729800052917 [DOI] [Google Scholar]
- Priolo A., Micol D., Agabriel J. 2001. Effects of grass feeding systems on ruminant meat color and flavour. A review. Anim. Res. 50(3):185–200. doi: 10.1051/animres:2001125 [DOI] [Google Scholar]
- Priolo A., Vasta V. 2007. Effects of tannin-containing diets on small ruminant meat quality. Ital. J. Anim. Sci. 6(Suppl. 1):527–530. [Google Scholar]
- Renerre M. 1986. Influence des facteurs biologiques et technologiques sur la couleur de la viande bovine. (In French.) Bull. Tech. CRZV Theix, INRA 65:41–45. [Google Scholar]
- Ripoll G., Albertí P., Joy M. 2012. Influence of alfalfa grazing-based feeding systems on carcass fat color and meat quality of light lambs. Meat Sci. 90(2):457–464. doi: 10.1016/j.meatsci.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Ripoll G., González-Calvo L., Molino F., Calvo J. H., Joy M. 2013. Effects of finishing period length with vitamin E supplementation and alfalfa grazing on carcass color and the evolution of meat color and the lipid oxidation of light lambs. Meat Sci. 93(4):906–913. doi: 10.1016/j.meatsci.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Ripoll G., Joy M., Muñoz F. 2011. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 87(1):88–93. doi: 10.1016/j.meatsci.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Ripoll G., Joy M., Muñoz F., Albertí P. 2008. Meat and fat color as a tool to trace grass-feeding systems in light lamb production. Meat Sci. 80(2):239–248. doi: 10.1016/j.meatsci.2007.11.025 [DOI] [PubMed] [Google Scholar]
- Ripoll-Bosch R., Joy M., Bernués A. 2014. Role of self-sufficiency, productivity and diversification on the economic sustainability of farming systems with autochthonous sheep breeds in less favoured areas in Southern Europe. Animal 8(8):1229–1237. doi: 10.1017/S1751731113000529 [DOI] [PubMed] [Google Scholar]
- Roche A., Ripoll G., Joy M., Folch J., Panea B., Calvo J. H., Alabart J. L. 2012. Effects of the FecXR allele of BMP15 gene on the birth weight, growth rate and carcass quality of Rasa Aragonesa light lambs. Small Rumin. Res. 108(1–3):45–53. doi: 10.1016/j.smallrumres.2012.06.011 [DOI] [Google Scholar]
- Sukhija P. S., Palmquist D. L. 1988. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 36:1202–1206. doi: 10.1021/jf00084a019 [DOI] [Google Scholar]
- Theodoridou K., Aufrère J., Andueza D., Le Morvan A., Picard F., Stringano E., Pourrat J., Mueller-Harvey I., Baumont R. 2011. Effect of plant development during first and second growth cycle on chemical composition, condensed tannins and nutritive value of three sainfoin (Onobrychis viciifolia) varieties and lucerne. Grass Forage Sci. 66(3):402–414. doi: 10.1111/j.1365-2494.2011.00798.x [DOI] [Google Scholar]
- Toral P. G., Hervás G., Missaoui H., Andrés S., Giráldez F. J., Jellali S., Frutos P. 2016. Effects of a tannin-rich legume (Onobrychis viciifolia) on in vitro ruminal biohydrogenation and fermentation. Span. J. Agric. Res. 14(1):e0602. doi: 10.5424/sjar/2016141-8989 [DOI] [Google Scholar]
- Val J., Monge E., Baker N. R. 1994. Improved HPLC method for rapid analysis of the xanthophyll cycle pigments. J. Chromatogr. Sci. 32(7):286–289. doi: 10.1093/chromsci/32.7.286 [DOI] [Google Scholar]
- Valderrábano J., Folch J. 1984. Producción intensiva de corderos en praderas de regadío. Primeros resultados. (In Spanish.) An. Inst. Nac. Invest. Agrar., Ser.: Ganad. (Spain) 21:23–34. [Google Scholar]
- Vasta V., Luciano G. 2011. The effects of dietary consumption of plants secondary compounds on small ruminants' products quality. Small Rumin. Res. 101(1–3):150–159. doi: 10.1016/j.smallrumres.2011.09.035 [DOI] [Google Scholar]
- Vasta V., Mele M., Serra A., Scerra M., Luciano G., Lanza M., Priolo A. 2009. Metabolic fate of fatty acids involved in ruminal biohydrogenation in sheep fed concentrate or herbage with or without tannins. J. Anim. Sci. 87(8):2674–2684. doi: 10.2527/jas.2008-1761 [DOI] [PubMed] [Google Scholar]
- Velasco S., Cañeque V., Pérez C., Lauzurica S., Díaz M. T., Huidobro F., Manzanares C., González J. 2001. Fatty acid composition of adipose depots of suckling lambs raised under different production systems. Meat Sci. 59(3):325–333. doi: 10.1016/S0309-1740(01)00135-8 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Miyamoto S., Moon J. H., Murota K., Hara Y., Terao J. 2006. Effect of dietary green tea catechin preparation on oxidative stress parameters in large intestinal mucosa of rats. Biosci. Biotechnol. Biochem. 70(1):286–289. doi: 10.1271/bbb.70.286 [DOI] [PubMed] [Google Scholar]


