Abstract
The aim of this study was to evaluate the effect of 2 climatic environments (temperate [TEMP] vs. tropical humid [TROP]) on production and thermoregulation traits in growing pigs. A backcross design involving Large White (LW; heat sensitive) and Creole (CR; heat tolerant) pigs was studied. The same 10 F1 LW × CR boars were mated with related LW sows in each environment. A total of 1,298 backcross pigs (n = 634 pigs from 11 batches for the TEMP environment and n = 664 pigs from 12 batches for the TROP environment) were phenotyped on BW (every 15 d from wk 11 to 23 of age), voluntary feed intake (ADFI, from wk 11 to 23), backfat thickness (BFT; at wk 19 and 23), skin temperature (ST; at wk 19 and 23), and rectal temperature (RT; at wk 19, 21, and 23). The feed conversion ratio was computed for the whole test period (11 to 23 wk). The calculation of the temperature–humidity index showed an average difference of 2.4°C between the TEMP and TROP environments. The ADG and ADFI were higher in the TEMP environment than in the TROP environment (834 vs. 754 g/d and 2.20 vs. 1.80 kg/d, respectively; P < 0.001). Body temperatures were higher in the TROP environment than in the TEMP environment (35.9 vs. 34.8°C for ST and 39.5 vs. 39.3°C for RT, respectively; P < 0.001). Most of the studied traits (i.e., BW, BFT, ADG, ADFI, and RT) were affected by sire family × environment interactions (P < 0.05), resulting in “robust” and “sensitive” families. Our results show a family dependency in the relationships between heat resistance and robustness, suggesting the possibility of finding genotypes with high production and low heat sensitivity. Further research is needed to confirm the genetic × environment interaction and to detect QTL related to heat tolerance.
Keywords: genetic × environment interaction, growing pig, heat stress, temperate, thermoregulation, tropical
INTRODUCTION
Enough food is currently produced for the global population, when ignoring food waste (FAO, 2012). Increasing production is nevertheless important, because 60% more food would be needed by 2050 to ensure food security (Porter et al., 2014). Meat consumption has decreased in the Northern Hemisphere (Thornton, 2010), but the global demand for livestock products should be twice that of today, particularly in developing countries (Delgado et al., 1999). More than 50% of the world pig production occurs in tropical and subtropical regions (FAOSTAT, 2015), particularly in East and Southeast Asia (P.R. China, Vietnam, etc.) and in South America and the southern portion of North America (Brazil, Mexico, etc.). These areas would continue to support the future growth of production (Bruinsma, 2003). However, heat stress (HS) is one of the main limiting factors of pig production (Renaudeau et al., 2012). Meanwhile, the climate is changing. The global temperature is expected to increase in the 21st century (Core Writing Team et al., 2014). It can be suggested that HS-related issues will increase in the future, with negative effects on animal health, welfare, reproduction, and production and, therefore, the economic viability of pig production. Heat stress has led to a global yearly economic loss around US$300 million in the U.S. pig industry (St-Pierre et al., 2003). Differences in tolerance to HS have been observed in several livestock species (Gourdine et al., 2006; Hayes et al., 2009; Mignon-Grasteau et al., 2015), suggesting the existence of a genetic determinism of heat tolerance, but little has been published in pigs. In 2011, the INRA initiated a backcross design based on a tropical local breed (Creole [CR]) and a productive temperate breed (Large White [LW]). The related progeny of backcross growing pigs were tested in a temperate (TEMP) or a tropical humid (TROP) environment with the same procedure to evaluate production traits and thermoregulatory responses. In this article, we present the phenotypic responses to the 2 different environments, focusing on the interactions between the sire family and the environment (G×E).
MATERIALS AND METHODS
All measurements and observations on animals were performed in accordance with the current law on animal experimentation and ethics (CE2012-9 from the Animal Care and Use Committee of Poitou-Charentes and 69-2012-2 from the Animal Care and Use Committee of French West Indies and Guyana) under the direction of Y. Billon and J. Fleury ( INRA-PTEA; authorization number by the French Ministry of Agriculture and Fisheries: 17015 and 971-2011-03 7704, respectively).
Experimental Design
Data were collected from April 2013 to October 2014 in the closed facilities of the INRA experimental unit located in the TEMP area (INRA-Génétique, Expérimentation et Systèmes Innovants, Poitou-Charentes, France; 46° N, 0.45° W) and in the semiopened facilities of the INRA experimental farm located in the Tropical Platform for Animal Experimentation (INRA-Plateforme Tropicale d'Expérimentation sur l'Animal,, Guadeloupe, French West Indies; 16° N, 61° W). To produce F1 animals (Fig. 1), 10 purebred LW dams were inseminated with 5 tropical boars from the CR breed, which is known to be less productive but more heat tolerant than the LW breed (Renaudeau et al., 2007). At the same time, herds of related LW sows were organized in the 2 farms using AI of related sows with same purebred LW boars to homogenize the genetic background of the dams (Fig. 1). Finally, backcross (BC) pigs were produced in each environment from the same 10 F1 (LW × CR) boars. In the TEMP environment, 634 BC pigs from 60 LW sows were reared in 11 contemporary groups, whereas the corresponding number in the TROP environment was 664 from 70 LW sows in 12 contemporary groups. Each F1 boar produced, on average, 65 ± 9 offspring per environment (Fig. 1). Pigs were weaned at 4 wk (wk 4) of age (26.9 ± 1.7 d). After weaning (wk 4), the pigs were raised for 6 wk in pens of 22 animals in the TEMP environment and pens of 12 animals in the TROP environment. At that moment, a preliminary choice was performed to avoid new mixing and to facilitate social interactions during test weeks: 64 pigs from 8 litters (with, on average, 4 castrated males and 4 females by litter) were divided into groups to balance by sire line and by sex. At wk 10, 60 of these 64 pigs were penned in 6 homogeneous groups of 10 pigs of the same sex per contemporary group (30 females and 30 castrated males) and the pigs were moved to growing pens equipped with electronic feeders. After 1 wk of adaptation, the test period started at wk 11 and ended at wk 23. Each pen was equipped with nipple drinkers. Animals had free access to water and were fed ad libitum with commercial diets presented as pellets and formulated to meet the nutritional requirements of growing pigs according to standard recommendations (Noblet et al., 2004). Feed samples were collected and pooled for each contemporary group at the beginning and at the end of the test period for chemical analyses of DM (AOAC Method N° 934.01), ash, OM (AOAC Method N° 942.05), CP (AOAC Method N° 976.06), starch (AOAC Method N° 920.40), NDF, and GE (AOAC, 2004).
Figure 1.
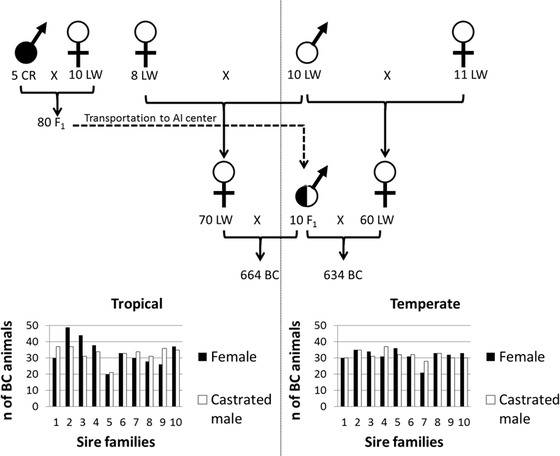
Backcross design with the number of tested female and castrated male backcross pigs per sire family (10 F1 sire family) in temperate and tropical environments. ♂ = male; ♀ = female; CR = Creole; LW = Large White; BC = backcross.
Data Recording
Room ambient temperature (T) and relative humidity (RH) were recorded during the whole duration of the test period. In the TEMP conditions, these climatic parameters were obtained every 5 min in the growing room of the closed experimental facilities using a stand-alone USB data logger (EL-USB-2+; DATAQ Instruments, Inc., Akron, OH) located in the center of the room. In the TROP conditions, the semiopen facilities were equipped with a Campbell weather station (Campbell Scientific Ltd., Shepshed, UK) continuously recording ambient T and RH (1 measurement every 30 min) in each room of the experimental farm.
All pigs were weighed 1 wk before the beginning of the performance test and on Monday every 2 wk from wk 11 (BW at 11 wk of age [BW11]) to 23 (BW at 23 wk of age [BW23]). At wk 19 and 23, the backfat thicknesses (BFT; BFT at 19 wk of age [BFT19] and BFT at 23 wk of age [BFT23]) were evaluated as the average of 6 ultrasonic measurements (Agroscan; ECM Echo Control Medical, Angoulême, France) taken at the shoulder, mid back (P2 site), and loin at a position directly above the point of the elbow, last rib, and last lumbar vertebra locations, taken 5 cm off the midline on each side of the pig. For technical reasons, feed intake was recorded using electronic feeders (ACEMA 128; SKIOLD Acemo, Pontivy, France) during 2-wk periods at only wk 11 through 12, wk 15 through 16, and wk 19 through 20 (defined as period 1) for half of the pigs or only during wk 13 through 14, wk 17 through 18, and wk 21 through 22 (defined as period 2) for the other half, as recommended by Schulze et al. (2001). During the remaining test weeks, pigs had free access to a conventional feed dispenser. Pigs switched from one feeding system to the other on the Monday after weighing.
Rectal temperature (RT) was measured at wk 19, 21, and 23, and skin temperature (ST) was measured at wk 19 and 23. Digital thermometers (Microlife Corp., Paris, France) were used to measure RT, and ST was measured on the back at P2 site using a skin surface thermocouple probe (type K, model 88002K-IEC; Omega Engineering Inc., Stamford, CT) connected to a microprocessor-based handheld thermometer (model HH-21; Omega Engineering Inc.). The RT and ST measurements were performed on unrestrained animals and with a minimum of stress during the weighing events in the morning.
Calculation and Statistical Analyses
A temperature–humidity index (THI) was calculated for each day based on the following formula proposed by the National Oceanic and Atmospheric Administration (NOAA, 1976; cited by Zumbach et al. [2008]): THI = T − (0.55 − 0.0055 × RH) × (T − 14.5), in which T is the average daily T (°C) and RH is the average daily RH (%).
Average daily gains between BW11 and BW23 and for each 2-wk interval from wk 11 to 23 (ADGi–i+2; for i = 13, 15, 17, 19, and 21) were computed. Average daily feed intake was calculated from data collected by the electronic feed dispensers by averaging daily feed intake records of the 6 wk available for each pig in either period 1 or period 2. Schulze et al. (2001) recommended to exclude the 2 first days of record for obtaining reliable feed intake information. In a preliminary analysis, it has been shown that only the exclusion of the first day is needed because readaptation to electronic feeders was not necessary. Consequently, ADFI was estimated by averaging daily feed intake with exclusion of the days of switching between electronic feeders and conventional feed dispenser. Residual feed intake (RFI) was calculated for each animal as the deviation between ADFI and ADFI predicted by a regression of ADFI on ADG between 11 and 23 wk of age [ADG11–23], BFT23, and the average metabolic BW during the test period (AMW11–23). The average metabolic BW was estimated for each animal using the following formula from Noblet et al. (1999):
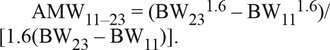 |
The feed conversion ratio (FCR) was estimated by dividing ADFI on ADG. Relative BFT gain between wk 19 and 23 (BFTg19–23) was calculated as (BFT23 − BFT19)/BFT19. The gradient between RT and ST was calculated at wk 19 and 23 as the difference between RT and ST.
Within each environment, extreme values were checked for all traits by detecting animals for which the value of the considered trait was distant from the mean by more than 3 SD (i.e., outliers). For an animal with outliers, the biological consistency of these values with the other records of the animal was evaluated before definitely excluding these outliers from the analysis. The highest number of excluded phenotypes was about 12 for ADFI and FCR. Data on production traits (BW11, BW23, ADG, ADGi–i+2, ADFI, RFI, FCR, BFT19, BFT23, and BFTg19–23) and on thermoregulation traits (RT at 19 wk of age [RT19], RT at 21 wk of age [RT21], RT at 23 wk of age [RT23], ST at 19 wk of age [ST19], and ST at 23 wk of age [ST23] and gradients between RT and ST) were analyzed using linear models (GLM procedure; SAS version 9.4; SAS Inst. Inc., Cary, NC) with the fixed effects of the environment (TEMP vs. TROP), sex (female vs. castrated male), batch within environment (11 in the TEMP environment and 12 in the TROP environment), sire family (10 families), and their interactions as main effects. For all variables in which feed intake was used (ADFI and FCR), the effect of recording period (2 periods) was used as fixed effect. Least squares means of the effects were computed, and the differences between the level effects were tested with a Tukey test. Residuals from these linear models (excluding the effect of sire family [G]) were used to compute Spearman correlations between the traits within environment and between environments for the same trait, and Fisher's Z transformations were performed to compare correlations. Longitudinal records (BFT, RT, and ST) were analyzed using mixed linear models (MIXED procedure; SAS version 9.4) with the same fixed effects as the previous linear models, adding the effect of the week (2 for BFT and ST and 3 for RT). The random effect of the animal was included to account for repeated measurements on the same pig. The G×E interactions were assessed by studying the least squares means from the previous linear models when the effect of the interaction was significant (P < 0.05).
Two different statistical analyses for assessment of robustness and sensitivity between the 10 sire families were performed. In the first analysis, sire families were ranked on the least squares mean differences of their offspring between the TEMP and TROP environments on traits for which G×E interactions were found significant, and scores were attributed to the sire families from 1 (i.e., the lowest least squares mean differences) to 10 (i.e., the highest least squares mean differences). In the second analysis, a principal component analysis (PCA) was performed using the FactoMineR package (Lê et al., 2008) in R version 3.3.2 (R Core Team, 2016). The PCA was done with the 12 variables (BW23, ADG11–23, ADFI, RFI, FCR, BFT19, BFT23, RT19, RT21, RT23, ST19, and ST23) as active variables and the BC pigs as individuals, ignoring the environment (E) and the sire family they belonged to. The variables G, E, and G×E were included in the PCA analysis as illustrative variables to obtain their coordinates on the different principal components.
RESULTS
Environmental Characteristics
Climate
The difference in average daily T between the TEMP and the TROP environments was only about 1.1°C (Table 1), but the corresponding difference in the average daily THI was about 2.4°C, due to the high contrast in RH between environments (+23.3% in the TROP environment).
Table 1.
Climatic characteristics1 of the production environments
| Environment | ||
|---|---|---|
| Climatic parameters | Temperate | Tropical |
| Daily ambient temperature, °C | ||
| Minimum | 20.5 | 22.2 |
| Maximum | 27.7 | 28.9 |
| Mean | 25.2 | 26.3 |
| Daily relative humidity, % | ||
| Minimum | 46.2 | 75.3 |
| Maximum | 76.3 | 93.6 |
| Mean | 61.3 | 84.6 |
| Daily THI,2 °C | ||
| Minimum | 19.2 | 21.7 |
| Maximum | 25.4 | 28.0 |
| Mean | 22.9 | 25.3 |
Average values obtained from daily climatic parameters measured indoor from April 2013 to October 2014 in the tropical humid environment (n = 13,248 hourly temperatures and relative humidities) and from March 2013 to September 2014 (n = 12,504 hourly temperatures and relative humidities).
THI = temperature–humidity index, which combines ambient temperature, relative humidity, and their interaction. The THI was calculated using the following formula: THI (°C) = ambient temperature − (0.55 − 0.0055 × relative humidity) × (ambient temperature − 14.5) (NOAA, 1976; cited in Zumbach et al. [2008]).
Figure 2 shows the daily or hourly variation of the T, RH, and THI in the TEMP and TROP experimental facilities. In TEMP indoor conditions, mean daily T were almost constant whereas the mean daily RH fluctuated more, with the highest values recorded in July (i.e., 68%) and the lowest in March (i.e., 51.7%). Consequently, the mean daily THI fluctuated during the test period between 22.8 and 24.4°C. In TROP conditions where T and RH were not controlled by a climatizing system, the conditions in the semiopen pig experimental building followed the outside climatic changes. Hence, the lowest and the highest mean daily T were measured in March (i.e., 24.3°C) and in August (i.e., 27.7°C), respectively. Mean RH was the lowest in March (i.e., 82.1%) and the highest in October (i.e., 87.7%). As a consequence, the THI was maximum in October (26.8°C) and minimum in March (23.2°C). The hourly T was similar, irrespective of the considered time in the TEMP environment, with values slightly higher than 25°C between 1100 and 2400 h. Similarly to the monthly variations in the TEMP environment, the mean hourly RH fluctuated more than the mean hourly T. In TEMP conditions, RH was highest at about 1100 h and lowest at about 0500 h. The daily T and RH variations in the TEMP environment resulted in limited daily variations of the THI between 22.4°C at 0500 h and 23.4°C at 1600 h. In the TROP environment, the daily variation in T (+17% between lowest and highest values) was higher than that in RH (+8% between lowest and highest values). Consequently, the daily variation of the THI and T are similar, with the minimum and maximum values reached at 0500 and 1200 h, respectively.
Figure 2.
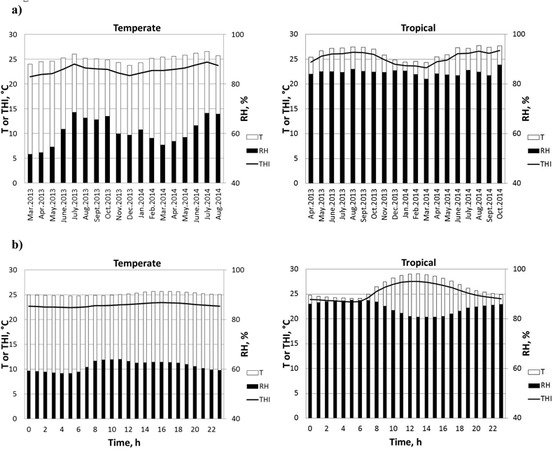
Climatic variation in the pig building facilities according to the production environment (temperate or tropical): (a) daily variation and (b) hourly climatic fluctuation. T = ambient temperature; RH = relative humidity; THI = temperature–humidity index, calculated according to the following formula: THI (°C) = T − (0.55 − 0.0055 × RH) × (T − 14.5), proposed by the National Oceanic and Atmospheric Administration (NOAA, 1976; cited by Zumbach et al. [2008]).
Feed Composition
The chemical composition of the diets is shown in Table 2. The DM content of the diet was 88.0%, irrespective of the environment. Chemical composition and nutritional values of diets were rather similar in both climatic environments.
Table 2.
Chemical and nutritional levels of the commercial diets used in temperate (n = 15) and tropical (n = 19) environments
| Environment | ||
|---|---|---|
| Item | Temperate1 | Tropical |
| Analyzed chemical composition, % of DM | ||
| DM | 88.0 ± 0.4 | 88.0 ± 0.4 |
| CP | 15.9 ± 0.6 | 16.4 ± 0.5 |
| Ash | 5.5 ± 0.2 | 6.0 ± 0.3 |
| Crude fiber | 2.6 ± 0.2 | 4.3 ± 0.3 |
| NDF | 11.4 | 14.8 |
| Fat | 2.3 | 3.8 |
| Starch | 46.3 | 42.7 |
| Energy value,2 MJ/kg | ||
| GE | 16.09 | 16.33 |
| DE | 13.87 | 13.53 |
In the temperate environment, samples were pooled for the determination of energy value, NDF, fat, and starch contents; in the tropical environment, samples were pooled for the determination of NDF.
Values calculated according to Le Goff and Noblet (2001).
Phenotypic Variation of Traits Within Environment
Table 3 shows the descriptive statistics of the main traits studied. Irrespective of the environment, the highest variation for a trait was found for BW23. For production traits, higher CV values were found in the TROP environment. For thermoregulatory traits, CV values were higher in the TEMP environment than in the TROP environment. Between the TEMP and the TROP environments, the highest differences in CV were found for BW11 and BW23.
Table 3.
Means (SD) and CV of the traits recorded according to the production environments
| Environment | ||||||
|---|---|---|---|---|---|---|
| Temperate | Tropical | |||||
| Trait1 | No. | Mean (SD) | CV, % | No. | Mean (SD) | CV, % |
| BW11, kg | 634 | 31.3 (4.2) | 13.4 | 664 | 21.1 (4.6) | 21.8 |
| BW23, kg | 633 | 103.4 (9.9) | 9.6 | 664 | 85.4 (11.4) | 13.3 |
| ADFI, kg/d | 626 | 2.21 (0.46) | 20.9 | 661 | 1.78 (0.42) | 23.5 |
| BFT19, mm | 632 | 16.6 (3.0) | 18.1 | 664 | 12.1 (2.3) | 19.0 |
| BFT23, mm | 628 | 20.6 (3.7) | 18.0 | 664 | 15.6 (3.0) | 19.2 |
| ST19, °C | 634 | 35.1 (0.9) | 2.6 | 664 | 36.1 (0.8) | 2.2 |
| ST23, °C | 633 | 34.5 (1.0) | 2.9 | 664 | 35.7 (0.8) | 2.2 |
| RT19, °C | 631 | 39.5 (0.4) | 1.0 | 663 | 39.7 (0.4) | 1.0 |
| RT21, °C | 633 | 39.3 (0.4) | 1.0 | 664 | 39.5 (0.3) | 0.8 |
| RT23, °C | 628 | 39. 3 (0.4) | 1.0 | 664 | 39.4 (0.3) | 0.8 |
BW11 = BW at 11 wk of age; BW23 = BW at 23 wk of age; BFT19 = backfat thickness at 19 wk of age; BFT23 = backfat thickness at 23 wk of age; ST19 = skin temperature at 19 wk of age; ST23 = skin temperature at 23 wk of age; RT19 = rectal temperature at 19 wk of age; RT21 = rectal temperature at 21 wk of age; RT23 = rectal temperature at 23 wk of age.
Environment Effects
All production performances were influenced by the production environment, except ADG between 21 and 23 wk of age (Table 4). Pigs reared in the TROP environment were younger than TEMP BC pigs, at wk 19 and 23. Higher BW were observed in BC pigs reared in the TEMP environment than in those reared in the TROP environment (+10 kg at the beginning of the test [P < 0.001] and +18 kg at the end of the test [P < 0.001]). Except for the periods of wk 19 to 21 and wk 21 to 23, TEMP BC pigs had higher ADG than TROP BC pigs, with the greatest differences observed during the period between wk 13 and 14 (+192 g/d; P < 0.001). Pigs in TROP had greater ADG than TEMP pigs between wk 19 and 21 (+83 g/d; P < 0.001). The overall growth performance was affected by the production environment, with a reduction of 10% in ADG11–23 related to a reduction of 20% in ADFI for TROP pigs compared with TEMP pigs. The FCR was significantly lower in TROP pigs than in TEMP pigs (−7%; P < 0.05). Irrespective of the week, BFT was greater in TEMP pigs than in TROP pigs (P < 0.01). Relative BFT gain was greater in TROP pigs than in TEMP pigs (+32 vs. +26%; P < 0.01).
Table 4.
Least squares means effect of production environment and sex on production traits
| Environment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperate | Tropical | |||||||||
| Trait1 | Female | Castrated male | Female | Castrated male | No. | RSD2 | Significant effects3 | |||
| Age at wk 11, d | 75a | 75a | 73b | 73b | 1,298 | 1 | E**, G**, and G×E** | |||
| Age at wk 13, d | 161a | 161a | 158b | 158b | 1,298 | 1.3 | E**, G**, and G×E** | |||
| BW11, kg | 30.9a | 31.6a | 20.7b | 21.2b | 1,298 | 4.0 | E**, G**, sex**, and G×E** | |||
| BW23, kg | 101.3a | 105.6b | 82.5c | 87.2d | 1,297 | 9.5 | E**, G**, sex**, and G×E** | |||
| ADFI, kg/d | 2.11a | 2.30b | 1.70c | 1.84d | 1,287 | 0.37 | E**, G**, sex**, Pe**, and G×E** | |||
| RFI, g/d | 2 | 37 | −15 | −25 | 1,283 | 321 | E*, G**, and Pe** | |||
| ADG, g/d | ||||||||||
| From wk 11 to 13 | 688a | 728b | 572c | 592c | 1,291 | 151 | E**, G**, sex**, and G×E** | |||
| From wk 13 to 15 | 869a | 907b | 662c | 733d | 1,296 | 138 | E**, G**, sex**, E × sex*, and G×E** | |||
| From wk 15 to 17 | 850a | 968b | 749c | 788d | 1,295 | 152 | E**, G**, sex**, E × sex*, and G×E** | |||
| From wk 17 to 19 | 971a | 987a | 823b | 907c | 1,294 | 173 | E**, G**, sex**, E × sex*, and G×E** | |||
| From wk 19 to 21 | 768a | 810b | 824b | 870c | 1,281 | 186 | E**, G**, sex**, and G×E** | |||
| From wk 21 to 23 | 793a | 777a | 756a | 794a | 1,284 | 199 | G** and E × sex* | |||
| From wk 11 to 23 | 813a | 854b | 729c | 778d | 1,297 | 85 | E**, G**, sex**, and G×E** | |||
| FCR, kg feed/kg BW gain | 2.61a | 2.70b | 2.45c | 2.47c | 1,286 | 0.43 | E**, G**, sex*, Pe**, and G×E* | |||
| BFT19, mm | 15.7a | 17.5b | 11.4c | 12.7d | 1,296 | 2.3 | E**, G**, sex**, E × sex*, and G×E** | |||
| BFT23, mm | 19.4a | 21.8b | 14.7c | 16.5d | 1,292 | 2.8 | E**, G**, sex**, and G×E** | |||
| BFTg19–23, % | 25.6a | 26.4a | 31.7b | 31.9b | 1,256 | 14.8 | E** and G* | |||
Least squares means within a row with different superscripts significantly differ (P < 0.05).
BW11 = BW at 11 wk of age; BW23 = BW at 23 wk of age; RFI = residual feed intake; FCR = feed conversion ratio; BFT19 = backfat thickness at 19 wk of age; BFT23 = backfat thickness at 23 wk of age; BFTg19–23 = relative backfat thickness gain between wk 19 and 23.
RSD = residual SD.
E = environment; G = sire family; Pe = period of feed intake recordings. Batch within environment was significant for all traits and is not reported in the table.
P < 0.05; **P < 0.01.
Irrespective of the week, ST was greater in TROP pigs than in TEMP pigs (36.1 vs. 35.1°C at wk 19 [P < 0.01] and 35.7 vs. 34.5°C at wk 23 [P < 0.01]; Table 5). Similarly to ST, RT was greater in TROP pigs than in TEMP pigs by about +0.2°C, irrespective of the week (P < 0.01).
Table 5.
Least squares means effect of production environment and sex on thermoregulation traits
| Environment | |||||||
|---|---|---|---|---|---|---|---|
| Temperate | Tropical | ||||||
| Trait1 | Female | Castrated male | Female | Castrated male | No. | RSD2 | Significant effects3 |
| Skin temperature, °C | |||||||
| ST19 | 35.2a | 35.0b | 36.1c | 36.1c | 1,294 | 0.7 | E** |
| ST23 | 34.5a | 34.5a | 35.7b | 35.7b | 1,297 | 0.7 | E** and G** |
| Rectal temperature, °C | |||||||
| RT19 | 39.4a | 39.5a | 39.6b | 39.7c | 1,294 | 0.3 | E**, G**, and sex** |
| RT21 | 39.3a | 39.4b | 39.4b | 39.6c | 1,297 | 0.4 | E**, G*, and sex** |
| RT23 | 39.2a | 39.3b | 39.4b | 39.5c | 1,291 | 0.3 | E**, G**, and sex** |
| Gradient RT–ST, °C | 4.3a | 4.5b | 3.5c | 3.6c | 1,294 | 0.7 | E**, G**, and sex** |
| RT19–ST19, °C | 4.3a | 4.5b | 3.5c | 3.6c | 1,294 | 0.7 | E**, G**, and sex** |
| RT23–ST23, °C | 4.7a | 4.9b | 3.7c | 3.8d | 1,290 | 0.7 | E**, G**, and sex** |
a–dLeast squares means within a row with different superscripts significantly differ (P < 0.05).
ST19 = skin temperature at 19 wk of age; ST23 = skin temperature at 23 wk of age; RT19 = rectal temperature at 19 wk of age; RT21 = rectal temperature at 21 wk of age; RT23 = rectal temperature at 23 wk of age; RT = rectal temperature; ST = skin temperature.
RSD = residual SD.
E = environment; G = sire family. Batch within environment was significant for all traits and is not reported in the table.
P < 0.05; ** P < 0.01.
The residual correlations between production and thermoregulation traits according to the environment are presented in Table 6. Irrespective of the considered environment (TEMP or TROP), significant positive correlations between production traits were found, except for FCR. In the TEMP environment, the residual correlation between FCR and BFT23 was higher than in the TROP environment (r = 0.28 vs. r = 0.14, respectively; P < 0.05). In both the TEMP and the TROP environments, positive residual correlation was found between RT23 and ST23, with higher values in the TROP environment than in the TEMP environment (r = +0.34 vs. r = +0.11, respectively; P < 0.001). Residual correlations between production and thermoregulation traits were not significantly different from 0, except for RT23 and BFT23 (r = −0.07) and ST23 and ADG11–23 (r = −0.09) in the TEMP environment and ST23 and BFT23 (r = −0.10) in the TROP environment (P < 0.05).
Table 6.
Spearman residual correlation between production and thermoregulation traits1; above the diagonal: correlations between traits measured in temperate environment; below the diagonal: correlations between traits measured in tropical environment; on the diagonal: correlation between temperate and tropical environment for the same trait2
| Trait | BW23 | BFT23 | ADFI | ADG11–23 | FCR | RT23 | ST23 |
|---|---|---|---|---|---|---|---|
| BW23 | 0.05b | 0.38a | 0.47a | 0.90a | −0.07a | 0.05 | 0.05 |
| BFT23 | 0.48a | 0.11b | 0.46a | 0.34a | 0.28a | −0.07a | −0.03 |
| ADFI | 0.52a | 0.45a | 0.21b | 0.47a | 0.76a | 0.02 | 0.06 |
| ADG11–23 | 0.93a | 0.47a | 0.54a | 0.26b | −0.13a | 0.05 | 0.09a |
| FCR | −0.12a | 0.14a | 0.68a | −0.15a | 0.62ab | −0.01 | 0.01 |
| RT23 | 0.00 | −0.03 | −0.02 | 0.01 | −0.05 | 0.32b | 0.11a |
| ST23 | −0.03 | −0.10a | 0.02 | 0.03 | −0.02 | 0.34a | 0.79ab |
a,bMeans within a row with different superscripts significantly differ (P < 0.05).
BW23 = BW at 23 wk of age; ADG11–23 = ADG between 11 and 23 wk of age; BFT23 = backfat thickness at 23 wk of age; FCR = feed conversion ratio; ST23 = skin temperature at 23 wk of age; RT23 = rectal temperature at 23 wk of age
Spearman rank correlations between temperate and tropical least squares mean values (1 value per sire family by environment).
It should be pointed out that differences between females and castrated males were also observed in most of traits (except for ST and ages). Focus was not put on the effects of sex, because the most important features in the present study are the differences between the TEMP and TROP environments and the G×E interactions.
Sire Family × Environment Interaction Effects
The effect of the interaction between the sire family and the production environment (G×E) was significant for the majority of the studied traits, except for ADG between 15 and 17 wk of age (P = 0.06), ADG between 17 and 19 wk of age (P = 0.19), ADG between 19 and 21 wk of age (P = 0.16), FCR (P = 0.08), BFTg19–23 (P = 0.15), and body temperatures (P = 0.91 for ST19, P = 0.84 for ST at 21 wk of age, P = 0.25 for RT19, P = 0.11 for RT21, and P = 0.36 for RT23; Tables 4 and 5). However, when taking into account the measurements on RT at wk 19, 21, and 23 as the same trait, the effect of sire family × G×E interaction became significant (P < 0.05), showing that the average RT of BC pigs was affected by the G×E interaction.
Based on the least squares mean estimates from the linear models, we considered that the most robust sire family for a given trait is the family for which the least squares mean difference for the sire family between the TEMP environment and the TROP environment is the lowest. In contrast, the most sensitive sire family was defined as the family for which this difference is the highest. The differences of least squares means of ADG11–23 and RT were not significant for the most robust families, families 1, 2, and 7 (P > 0.47). The differences of least squares means of all studied traits of Table 6 are significant for the most sensitive families, families 6, 5, and 10 (P < 0.037). With the impact of environment (chronic HS in the TROP environment), some traits (such as BW23, BFT23, or ADFI) change with environment, but the slope differs according to the sire family. Spearman rank correlations between families in the different environments for the 10 sire families are presented in Table 6 (on the diagonal). All correlations between TEMP and TROP least squares mean values significantly differ from 1, indicating that G×E interactions exist. However, only correlations between the TEMP and TROP environments for FCR and ST are significantly different from 0, indicating that the G×E interactions for BW23, BFT23, ADFI, ADG11–23, and RT are moderate to strong. Differences in the ranking of sire families in the 2 environments are presented in Fig. 3a (for BW23 and BFT23), Fig. 3b (for ADG11–23 and ADFI), and Fig. 4 (for RT). A difference of 12.3 kg for BW23 between the TEMP environment and the TROP environment was observed (Fig. 3a) for the most robust sire family (family 7, with a BW23 of 101.5 kg in the TEMP environment). The corresponding value for the most sensitive family (family 3; least squares means of 105.9 kg in the TEMP environment) was 22.5 kg. The most robust families for BW23 (families 7, 2, and 1) were also the 3 most robust for BFT23, as shown by Fig. 3. These families had an average contrast for BFT23 of 3.4 mm between the TEMP environment and the TROP environment. For ADFI, similarly to BW23 and BFT23, it seems that there is a general rule: the higher ADFI is in the TEMP environment, the more sensitive the family is (i.e., the higher the difference between the TEMP and the TROP environments is; Fig. 3b). For the average RT from wk 19, 21, and 23 (Fig. 4), there are few rerankings. The trend was that the increase of RT between TEMP and TROP was lower (+0.09 vs. +0.25°C; P < 0.001) for the sire families of BC pigs with the highest RT in TEMP (39.4 °C; families 7, 1, and 9) than the sire families with the lowest RT in TEMP (39.3°C; families 8 and 6).
Figure 3.
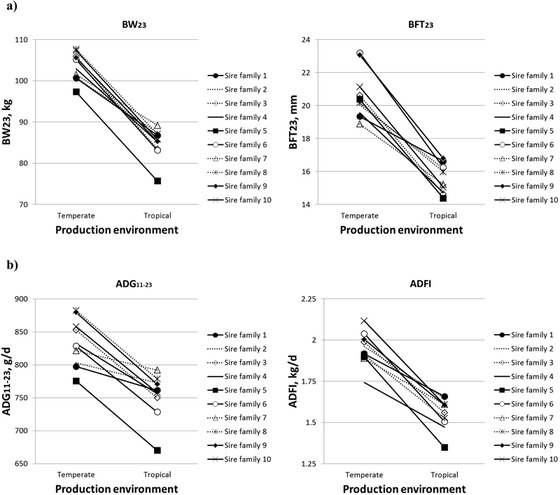
Effect of sire family × production environment (temperate vs. tropical) interactions on (a) BW and backfat thickness (BFT) at the end of test period (wk 23; BW at 23 wk of age [BW23] and BFT at 23 wk of age [BFT23]]) and (b) growth rate (ADG between 11 and 23 wk of age [ADG11–23]) and daily feed intake (ADFI) during the overall test period (least squares means from a linear model analysis applied to each trait).
Figure 4.
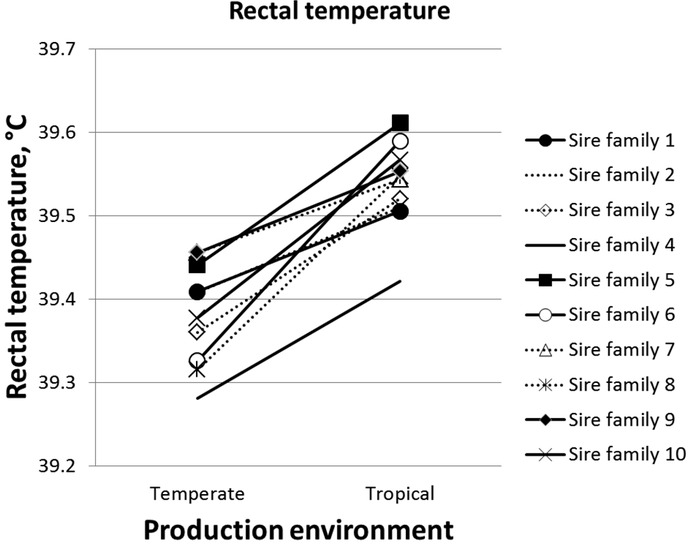
Effect of sire family × production environment (temperate vs. tropical) interactions on the rectal temperature measured from wk 19 to 23 (least squares means from a mixed model analysis).
Figure 5a represents the score of the 10 sire families for each trait where significant interaction between the sire family and the production environment was found (BW23, BFT23, ADG11–23, ADFI, and average RT at test wk 19, 21, and 23). For a studied trait, the score 1 (and, inversely, the score 10) was attributed to the family that had the lowest (and, inversely, the highest) least squares mean difference between the TEMP and the TROP environments. Hence, in Fig. 5a, the closer to the center the sire family's pentagon is, the more robust the family is. Based on the 5 traits studied, it was found that the families 7, 1, and 2 were the most robust sire families. In contrast, families 6, 5, and 10 were found to be the most sensitive. Figure 5b represents the position of the G×E groups (20 groups) on the first principal component. The PCA showed that the first principal component explained 35% of the total variation and it contrasts production variables (BW23, BFT19, BFT23, ADFI, and ADG11–23; r > 0.74) to thermoregulation traits (ST23 and ST19 [r < −0.51] and RT19, RT23, and RT21 [r < −0.15], P < 0.01):
Figure 5.
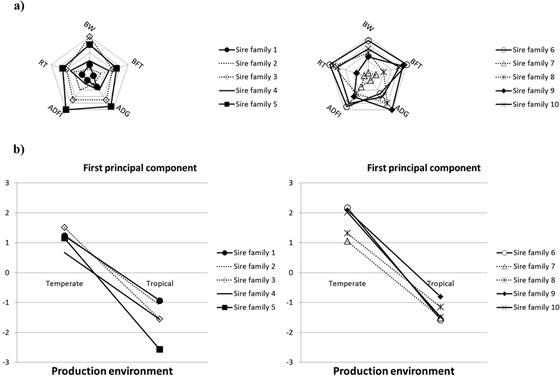
(a) Assessment of robustness and sensitivity in the 10 sire families according to their score (from 1, the most robust, to 10, the most sensitive) on least squares means differences of sire families between temperate and tropical environments on BW and backfat thickness (BFT) at wk 23, growth rate (ADG) and ADFI during the test period, and rectal temperature (RT) measured from wk 19 to 23. The closer to the center the sire family pentagon is, the more robust it is. (b) Assessment of robustness and sensitivity in the 10 sire families according to the coordinates of the 10 sire families in temperate and tropical environments on the first principal component from a principal component analysis with the 12 variables (BW at 23 wk of age [BW23], ADG between 11 and 23 wk of age [ADG11–23], ADFI, residual feed intake [RFI], feed conversion ratio [FCR], BFT at 19 wk of age [BFT19], BFT at 23 wk of age [BFT23], RT at 19 wk of age [RT19], RT at 21 wk of age [RT21], RT at 23 wk of age [RT23], skin temperature at 19 wk of age [ST19], and skin temperature at 23 wk of age [ST23]).
 |
The categorical variables G×E, E, and G allow us to characterize the first principal component (r = 0.55, r = 0.50, and r = 0.02, respectively; P < 0.01). Hence, the same trend as in Fig. 5a was found in Fig. 5b, except for family 7. Indeed, based on the decline of the slope between the TEMP and TROP environments from the coordinates on the first principal component of a given sire family, it was also found that families 1 and 2 were the most robust sire families and families 6, 5, and 10 were the most sensitive. Family 7, which was found to be the first robust family in Fig. 5a , was in the fifth position with the PCA.
DISCUSSION
To the best of our knowledge, designed studies in growing pigs such as ours are scarce in the literature (Misztal, 2017). Our experimental design, in which a large number of contemporary half-sib pigs (more than 600 animals per environment) were reared either in thermoneutral conditions (TEMP) or in tropical HS conditions (TROP), contributes to deciphering the heat tolerance in pigs. A PCA completed by a hierarchical classification was performed (results not shown) on contemporary groups of BC pigs as individuals (without a priori on the TEMP or TROP origin of the group) and climatic parameters (minimum, maximum, and mean of the T, RH, and THI) as explanatory variables. Two clusters were discriminated corresponding to the TEMP batches for the first cluster and the TROP batches for the second cluster. These results confirmed that the TEMP and the TROP environments can be considered 2 different climatic conditions.
It is admitted that the upper limit of the thermoneutral zone, in which no extra energy is used to maintain thermoregulation, is approximately 25°C for growing pigs (Renaudeau et al., 2008). In our study, the average T was 25.2 ± 0.9°C in the TEMP environment and 26.3 ± 1.3°C in the TROP environment. Consequently, we can consider that in the current study, TEMP BC pigs were reared close to the thermoneutral conditions most of the time. In contrast, with a higher average T of 1.1°C (+4%) in the TROP environment, their half-sib TROP pigs were heat stressed most of the time, which suggests that TROP pigs experienced chronic HS. It is well known that high RH accentuates the adverse effect of HS (Huynh et al., 2005). The RH reported in our study was higher in the TROP environment than in the TEMP environment, which may have reduced the TROP pig's ability to dissipate heat by evaporation and magnified the HS effect. Based on the THI, which takes into account the effect of temperature, humidity, and their interaction, the current study shows that TROP pigs actually experienced average ambient conditions 2.4°C (+10%) greater than the TEMP pigs. It should be noticed that the THI used in the present study was formulated to monitor discomfort from temperature and humidity in dairy cattle. This index has been used in studies on HS of pigs (Zumbach et al., 2008; Gourdine et al., 2017). To our knowledge, there is no THI equation directly formulated for pig production performance. Consequently, the THI used in our study must be interpreted in a relative rather than an absolute manner.
Effect of the Production Environment
Our experimental design allows quantifying the effects of 2 different production environments on pigs highly genetically related and reared in conditions close to the commercial production ones (i.e., building and feed). To our knowledge, there are limited data available in the literature for that. However, the effect of increased T (in controlled conditions) on the performance of growing pigs has been extensively described (see the meta-analysis of Renaudeau et al. [2011]). Even if pigs in our study were BC animals partly from a low production level breed (CR), the growth performance of TEMP pigs was in the range of performance values (the higher fraction) that can be found in French commercial conditions (between 779 and 837 g/d; IFIP, 2016). Concerning the performance of TROP pigs, our data set on growth rate, BFT, or age around 90 kg was in the range of values reported by the review of Akanno et al. (2013) from international pig breeds in the tropics (793 ± 29 g/d, 18.6 ± 1.5 mm, and 157 ± 9 d, respectively). The results of the present experiment on growth performance confirm those generally obtained in the literature, with a lower ADG and ADFI in heat-stressed pigs than pigs in a thermal comfort situation (Le Dividich et al., 1998). It should be pointed out that TROP pigs were younger than TEMP pigs. This is mainly due to higher gestation duration in TROP LW dams (114.6 ± 1.5 d) than in TEMP LW dams (114.1 ± 1.2 d), leading to piglets' weaning age of 1 d less in TROP BC pigs (26.5 d) than in TEMP BC pigs (27.4 d). Consequently, the differences between TROP and TEMP pigs could be underestimated without taking into account the age (e.g., as a covariable) in the analysis. However, we have considered that the age differences were caused by the environment. Besides the overall ADG, in our study, the profiles for ADG as a function of age (or BW) are not the same between the TEMP environment and the TROP environment, with higher ADG between test wk 19 and 21 for TROP pigs than for TEMP pigs, suggesting that the near-plateau zone of the growth curve was achieved latter in the TROP environment than in the TEMP environment. The overall ADG (g/d) relative reduction value between the TEMP and the TROP environments was about 10% and was consistent with the estimated values of 9.4% calculated with equation proposed by the meta-analysis of Renaudeau et al. (2011):
in which T is the T (°C) and W is the mean BW during the experiment (kg).
According to our results, the reduction in ADFI between TEMP and TROP pigs was two times higher than the reduction in ADG (20.0 and 9.6%, respectively). However, the ADFI (g/d) relative reduction obtained in our study was higher than the 15% reduction estimated by using Renaudeau et al.'s (2011) equation on ADFI:
The discrepancy is explained by a higher overestimation of TROP pig ADFI (2.06 kg/d from the equation vs. 1.82 kg/d in our study) than of TEMP pig ADFI (2.43 kg/d from the equation vs. 2.23 kg/d in our study), which could be related to a more stressful conditions in the TROP environment due to high RH (on average, 84.6%) that was not taken into account in the ADFI equation from the meta-analysis. Indeed, according to Granier et al. (1998), with RH above 80%, the HS effect on ADFI is accentuated because of the lower ability of pig to lose heat under high ambient RH. It was reported that HS poorly affected FCR, which remains nearly constant (Renaudeau et al., 2011), except for high T level (Renaudeau et al., 2008). In our study, FCR was found higher in TEMP pigs than in TROP pigs (2.65 vs. 2.46 kg/kg). The higher FCR (kg/kg) values observed in the TEMP environment than in the TROP environment were, however, in agreement with the estimated values obtained with FCR equation from Renaudeau et al. (2011; 2.50 vs. 2.28 kg/kg for TEMP and TROP pigs, respectively):
in which CP is the CP content (g/100 g). Our finding suggests that TEMP pigs were less efficient in using feed for growth than TROP pigs. Quiniou et al. (1996, 2013) showed that FCR can be improved by moderate feed restriction (around 90% of the ad libitum). In our study, if we consider that TEMP pigs were fed ad libitum, ADFI of TROP pigs (expressed in g/kg BW0.60 per d) represents, on average, 90% of the TEMP pig ad libitum intake. Consequently, it can be safely suggested that 1) HS that occurred in our TROP conditions was not too severe to increase FCR and 2) even if TROP pigs had free access to feed, they were in restricted feed conditions due to HS, resulting in lower FCR than TEMP pigs. It can be also hypothesized that better FCR and reduced BFT can be obtained for TEMP pigs if feed restriction (around 90%) is applied, but probably with a negative impact on the carcass weight.
Concerning thermoregulation traits, as expected in our study, ST of TROP pigs were greater by, on average, +1°C, influenced by a greater THI of about +2.4°C in TROP conditions than in TEMP conditions. As reported by Collin et al. (2002), an increase in ST is the consequence of enhanced heat exchange between the skin and the environment. However, RT was found higher in TROP pigs than in TEMP pigs and the gradient between RT and ST was found lower, suggesting that TROP pigs were partly unable to lose the entire heat load by sensible pathways (Renaudeau et al., 2007).
Moderate to strong negative correlations between body temperatures and production traits have been reported, but with large SE (Renaudeau et al., 2004; Gourdine et al., 2017). In the present study, no significant correlations between RT or ST and production traits were reported, expected for RT23 with BFT23 and ST23 with ADG11–23 in TEMP conditions (r = −0.07 and r = −0.09, respectively) and RT23 with BFT23 and ST23 with BFT23 in TROP conditions (r = −0.10). It can be suggested that either the number of animals or the pertinence of thermoregulation traits (1 measurement within a day) was limited to estimate accurate correlations or there is a limited association between production and thermoregulation traits in our test conditions.
Effect of the Interaction Between Sire Family and Production Environment
Only a few studies on genetic × environment (G×E) interactions are available in pigs (Lewis and Bunter, 2011; Bloemhof et al., 2013) compared with broilers (N'Dri et al., 2007; Mignon-Grasteau et al., 2015), dairy cattle (Kolmodin et al., 2003; Hayes et al., 2009), and beef cattle (Cardoso and Tempelman, 2012; Santana et al., 2013). This is probably due to the lack of accurate genetic relationships between TEMP and TROP environments and the lack of a large number of observations. With climate change, understanding and taking into account G×E interactions is crucial for future pig production systems (Rauw and Gomez-Raya, 2015). In this study, we first chose to analyze G×E interactions for production and thermoregulation traits by testing the interactions between sire family of BC pigs and the production environment. It has been reported that high G×E interactions may modify the breeding values hierarchy between animals showing different individual sensitivity to HS. In lactating sows, Lewis and Bunter (2011) reported high genetic correlations between seasons and suggested that separated specification of traits by environment would not be necessary. On the other hand, Bloemhof et al. (2013) found sensitive and robust sires in regard to the effect of T on reproductive performance of their daughters.
In our study, where both production and functional traits have been studied, we can ask what a robust animal should be. Regarding production traits, in our study, the effect of TROP environment on production traits of pigs differs according to the sire family. Among the 5 most sensitive families for BW23 (i.e., families 3, 6, 5, 10, and 8), 4 of them were in the top 5 in the TEMP environment. However, when comparing the top 5 in the TEMP environment with the top 5 in the TROP environment for BW23, 2 families (i.e., 8 and 10) were among the best in both environments. Consequently, the criteria to discriminate robustness between families should be chosen with caution, and it should reflect the ability to combine a high production potential with resilience to stressors (Knap, 2005). Further analyses are needed to evaluate more finely the components of robustness and resilience in our data. To our knowledge, little has been published on the effect of G×E interactions on physiological indicators, such as RT. It is generally admitted that pigs with increased RT experience lower production performance (Renaudeau et al., 2004). Based only on our results on residual correlations, it can be argued that there is poor association between RT and production performance. However, residual correlations estimated within family (results not presented) showed a variety of associations from a significant negative correlation between RT23 and ADFI (e.g., the “robust” family 1; r = −0.19) to a positive correlation (e.g., the sensitive family 9; r = 0.16). As reviewed by Renaudeau et al. (2004), an antagonism between heat resistance (as defined by the ability to maintain or to slowly increase inner temperature) and heat robustness (as defined by the ability to maintain production in spite of HS) is generally suggested. Our results show a family dependency in the relationship between heat resistance and robustness, suggesting the possibility of finding families with high production and low HS sensitivity. As pointed out by Rauw and Gomez-Raya (2015), such genotypes would help to make selection for robustness easier.
Conclusions
To our knowledge, this is the first study on G×E interactions on the performance of growing pigs by comparing genetically linked animals reared either in chronic HS or thermal comfort environments. Based on our results on phenotypic differences between BC pigs from different sire families, it can be suggested that there could be strong G×E in heat tolerance, as the best sire families for production traits in thermoneutrality could not be the best in heat conditions. However, further studies are needed to finely describe the components of heat tolerance and robustness to HS. Advances in high-density technologies now offer the opportunity to characterize and integrate fine phenotypes (transcriptomics and metabolomics) and to provide genomic tools for heat tolerance selection. In terms of breeding objectives, different choices can be envisaged, such as selecting for pigs adapted to specific environments of production or selecting for robust pigs able to perform in most conditions of production (Knap, 2005). However, standards for phenotypes discriminating heat tolerance are needed before the implementation of breeding schemes for heat tolerance.
Footnotes
The financial support of the French National Agency of Research (ANR PigHeaT and ANR-12-ADAP-0015), the financial support of the Department of Animal Genetics of INRA (AO2012 PhenoHeaT), the financial support of EU-funds (FEDER, FSE, and FEADER), and the Region Guadeloupe (including the AGROECODIV project) are gratefully acknowledged. Roseline Rosé was supported by a doctoral fellowship from the Region Guadeloupe and the Department of Animal Genetics of INRA.
LITERATURE CITED
- Akanno E. C., Schenkel F. S., Quinton V. M., Friendship R. M., Robinson J. A. B. 2013. Meta-analysis of genetic parameter estimates for reproduction, growth and carcass traits of pigs in the tropics. Livest. Sci. 152:101–113. doi: 10.1016/j.livsci.2012.07.021 [DOI] [Google Scholar]
- AOAC 2004. Official methods of analysis. 18th ed.AOAC Int., Arlington, VA. [Google Scholar]
- Bloemhof S., Mathur P. K., Knol E. F., van der Waaij E. H. 2013. Effect of daily environmental temperature on farrowing rate and total born in dam line sows. J. Anim. Sci. 91:2667–2679. doi: 10.2527/jas.2012-5902 [DOI] [PubMed] [Google Scholar]
- Bruinsma J. editor. 2003. World agriculture: Towards 2015/2030. An FAO perspective. Earthsan, London, UK, and Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- Cardoso F. F., Tempelman R. J. 2012. Linear reaction norm models for genetic merit prediction of Angus cattle under genotype by environment interaction. J. Anim. Sci. 90:2130–2141. doi: 10.2527/jas.2011-4333 [DOI] [PubMed] [Google Scholar]
- Collin A., Vaz M. J., Le Dividich J. 2002. Effects of high temperature on body temperature and hormonal adjustments in piglets. Reprod. Nutr. Dev. 42:45–53. doi: 10.1051/rnd:2002005 [DOI] [PubMed] [Google Scholar]
- Core Writing Team Pachauri R. K., Meyer L. A. editors. 2014. Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change, Geneva, Switzerland. [Google Scholar]
- Delgado C., Rosegrant M., Steinfeld H., Siméon E., Courbois C. 1999. Livestock to 2020: The next food revolution. Vision initiative food, agriculture, and the environment discussion. Paper 28. International Food Policy Research Institute, Washington, DC. [Google Scholar]
- FAOSTAT 2015. FAOSTAT domains – Animal production. Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/faostat/en/#data/QL. (Accessed 21 June 2016.) [Google Scholar]
- Food and Agricultural Organization of the United Nations (FAO) 2012. The state of food insecurity in the world: Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. FAO, the International Fund for Agricultural Development (IFAD), and the World Food Programme (WFP), Rome, Italy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdine J. L., Bidanel J. P., Noblet J., Renaudeau D. 2006. Effects of breed and season on performance of lactating sows in a tropical humid climate. J. Anim. Sci. 84:360–369. doi: 10.2527/2006.842360x [DOI] [PubMed] [Google Scholar]
- Gourdine J. L., Mandonnet N., Giorgi M., Renaudeau D. 2017. Genetic parameters for thermoregulation and production traits in lactating sows reared in tropical climate. Animal 11:365–374. doi: 10.1017/S175173111600135X [DOI] [PubMed] [Google Scholar]
- Granier R., Massabie P., Bouby A. 1998. Effect of the humidity level of ambient air (temperature 28°C) on the growth performance of growing-finishing pigs. (In French.) Journ. Rech. Porcine Fr. 30:331–336. [Google Scholar]
- Hayes B. J., Bowman P. J., Chamberlain A. J., Savin K., van Tassell C. P., Sonstegard T. S., Goddard M. E. 2009. A validated genome wide association study to breed cattle adapted to an environment altered by climate change. PLoS ONE 4(8):e6676. doi: 10.1371/journal.pone.0006676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T. T. T., Aarnink A. J. A., Verstegen M. W. A., Gerrits W. J. J., Heetkamp M. J. W., Kemp B., Canh T. T. 2005. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 83:1385–1396. doi: 10.2527/2005.8361385x [DOI] [PubMed] [Google Scholar]
- Institut Français de l'Industrie Porcine (IFIP) 2016. Gestion technico-économique: Résultats par region – Post-sevreurs-engraisseurs – Période du 01/01/15 au 31/12/15. (In French.) IFIP-Gestion Technico-Economique, Paris, France. [Google Scholar]
- Knap P. W. 2005. Breeding robust pigs. Aust. J. Exp. Agric. 45:763–773. doi: 10.1071/EA05041 [DOI] [Google Scholar]
- Kolmodin R., Strandberg E., Jorjani H., Danell B. 2003. Selection in presence of genotype by environment interaction: Response in environmental sensitivity. Anim. Sci. 76:375–385. doi: 10.1017/S1357729800058604 [DOI] [Google Scholar]
- Lê S., Josse J., Husson F. 2008. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 25:1–18. doi: 10.18637/jss.v025.i01 [DOI] [Google Scholar]
- Le Dividich J., Noblet J., Herpin P., van Milgen J., Quiniou N., Wiseman J., Varley M. A., Chadwick J. P. 1998. Thermoregulation, progress in pig science. Nottingham Univ. Press, Nottingham, UK. [Google Scholar]
- Le Goff G., Noblet J. 2001. Comparative total tract digestibility of dietary energy and nutrients in growing pigs and adult sows. J. Anim. Sci. 79:2418–2427. doi: 10.2527/2001.7992418x [DOI] [PubMed] [Google Scholar]
- Lewis C. R. G., Bunter K. L. 2011. Effects of seasonality and ambient temperature on genetic parameters for production and reproductive traits in pigs. Anim. Prod. Sci. 51:615–626. doi: 10.1071/AN10265 [DOI] [Google Scholar]
- Mignon-Grasteau S., Moreri U., Narcy A., Rousseau X., Rodenburg T. B., Tixier-Boichard M., Zerjal T. 2015. Robustness to chronic heat stress in laying hens: A meta-analysis. Poult. Sci. 94:586–600. doi: 10.3382/ps/pev028 [DOI] [PubMed] [Google Scholar]
- Misztal I. 2017. Breeding and genetics symposium: Resilience and lessons from studies in genetics of heat stress. J. Anim. Sci. 95:1780–1787. doi: 10.2527/jas.2016.0953 [DOI] [PubMed] [Google Scholar]
- N'Dri A. L., Sellier N., Tixier-Boichard M., Beaumont C., Mignon-Grasteau S. 2007. Genotype by environment interactions in relation to growth traits in slow growing chickens. Genet. Sel. Evol. 39:513–528. doi: 10.1186/1297-9686-39-5-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA) 1976. Livestock hot weather stress. US Dept. Commerce, Natl. Weather Serv. Central Reg., Reg. Operations Manual Lett C-31–76. US Govt. Printing Office, Washington, DC. [Google Scholar]
- Noblet J., Karege C., Dubois S., van Milgen J. 1999. Metabolic utilization of energy and maintenance requirements in growing pigs: Effects of sex and genotype. J. Anim. Sci. 77:1208–1216. doi: 10.2527/1999.7751208x [DOI] [PubMed] [Google Scholar]
- Noblet J., Sève B., Jondreville C. 2004. Nutritional values for pigs. In: Sauvant D., Perez J. M., Tran G. editors, Tables of composition and nutritional value of feed materials: Pigs, poultry, cattle, sheep, goats, rabbits, horses, fish. INRA Editions, Paris, France: p. 25–35. [Google Scholar]
- Porter J. R., Xie L., Challinor A. J., Cochrane K., Howden S. M., Iqbal M. M., Lobell D. B., Travasso M. I. 2014. Food security and food production systems. In: Field C. B., Barros V. R., Dokken D. J., Mach K. J., Mastrandrea M. D., Bilir T. E., Chatterjee M., Ebi K. L., Estrada Y. O., Genova R. C., Girma B., Kissel E. S., Levy A. N., MacCracken S., Mastrandrea P. R., White L. L. editors, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY: p. 485–533. [Google Scholar]
- Quiniou N., Dourmad J. Y., Noblet J. 1996. Effect of energy intake on the performance of different types of pig from 45 to 100 kg body weight.1. Protein and lipid deposition. Anim. Sci. 63:277–288. doi: 10.1017/S1357729800014831 [DOI] [Google Scholar]
- Quiniou N., Vautier B., Salaün Y., Van Milgen J., Brossard L. 2013. Modélisation de l'effet de la stratégie alimentaire et du contexte de prix des matières premières sur les performances moyennes, leur variabilité et les rejets azotés à l'échelle d'une population de porcs. (In French.) Journ. Rech. Porcine Fr. 45:155–160. [Google Scholar]
- Rauw W. M., Gomez-Raya L. 2015. Genotype by environment interaction and breeding for robustness in livestock. Front. Genet. 6:310. doi: 10.3389/fgene.2015.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org. (Accessed 18 June 2016.) [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J., Collier R. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Gourdine J.-L., Anais C. 2007. Thermoregulatory responses to high ambient temperature in growing pigs: Effects of temperature level and breed. In: Misr Society of agricultural Engeneering, editors, Animal housing in hot climates: A multidisciplinary view. workshop of the International Commission of agricultural Engeneering section II, April1-4 2007,Cairo, Egypt: p. 34–38. [Google Scholar]
- Renaudeau D., Gourdine J.-L., St-Pierre N. R. 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Kerdoncuff M., Anais C., Gourdine J. L. 2008. Effect of temperature level on thermal acclimation in Large White growing pigs. Animal 2:1619–1626. doi: 10.1017/S1751731108002814 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Mandonnet N., Tixier-Boichard M., Noblet J., Bidanel J. P. 2004. Atténuer les effets de la chaleur sur les performances des porcs: La voie génétique. (In French.) INRA Prod. Anim. 17:93–108. [Google Scholar]
- Santana M. L., Eler J. P., Cardoso F. F., Albuquerque L. G., Ferraz J. B. S. 2013. Phenotypic plasticity of composite beef cattle performance using reaction norms model with unknown covariate. Animal 7:202–210. doi: 10.1017/S1751731112001711 [DOI] [PubMed] [Google Scholar]
- Schulze V., Roehe R., Looft H., Kalm E. 2001. Effects of continuous and periodic feeding by electronic feeders on accuracy of measuring feed intake information and their genetic association with growth performances. J. Anim. Breed. Genet. 118:403–416. doi: 10.1046/j.1439-0388.2001.00158.x [DOI] [Google Scholar]
- St-Pierre N. R., Cobanov B., Schnitkey G. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86(E. Suppl):.E52–E77. [Google Scholar]
- Thornton P. K. 2010. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365:2853–2867. doi: 10.1098/rstb.2010.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbach B., Misztal I., Tsuruta S., Sanchez J. P., Azain M., Herring W., Holl J., Long T., Culbertson M. 2008. Genetic components of heat stress in finishing pigs: Development of a heat load function. J. Anim. Sci. 86:2082–2088. doi: 10.2527/jas.2007-0523 [DOI] [PubMed] [Google Scholar]


