Abstract
The present study was designed to evaluate the effects of dietary betaine on pig performance and serological and hematological indices during thermoneutral and heat-stressed conditions. Individually housed pigs (n = 64; 39.0 ± 1.5 kg BW) were assigned within weight blocks and sex to 1 of 8 treatments. Treatments consisted of 2 environmental conditions (thermoneutral or heat-stressed) and 4 levels of betaine (0, 0.10, 0.15, and 0.20%). Room temperatures followed a daily pattern with a low of 14°C and a high of 21°C for the thermoneutral environment and a low of 28°C and a high of 35°C for the heat-stressed environment. Experimental diets were fed from d −7 (7 d prior to imposing temperature treatments; constant 21°C) until 28. Respiration rate and rectal temperature were measured on d 0, 1, 2, 3, 7, 14, 21, and 28, and blood samples were collected on d 3 and 28. Heat stress reduced (P ≤ 0.008) ADG (0.710 vs. 0.822 kg/d) and ADFI (1.81 vs. 2.27 kg/d) and increased G:F (P = 0.036; 0.391 vs. 0.365). Betaine tended to quadratically increase G:F (P = 0.071; 0.377, 0.391, 0.379, and 0.366 for 0, 0.10, 0.15, and 0.20% betaine, respectively), regardless of environment. Heat stress increased (P ≤ 0.001) respiration rate (48 vs. 23 breaths/30 s) and rectal temperature (39.47 vs. 38.94°C) throughout d 1 to 28. Betaine at 0.10% reduced rectal temperature in heat-stressed pigs but not in control pigs (interaction, P = 0.040). Heat stress increased serum cysteine and triglycerides and reduced Ca, alkaline phosphatase, and lipase, regardless of day of sampling (P ≤ 0.048). Heat stress increased serum creatine phosphokinase (CPK) and K and reduced osmolarity, Na, urea N, methionine, homocysteine, the albumin:globulin ratio, and blood eosinophil count on d 3 but not on d 28 (interaction, P ≤ 0.013). Heat stress increased serum Mg, globulin, creatinine, amylase, and γ-glutamyltranspeptidase and reduced P, the urea N:creatinine ratio, alanine aminotransferase, NEFA, hemoglobin, hematocrit, and red blood cells on d 28 but not on d 3 (interaction, P ≤ 0.034). Betaine increased serum osmolarity and NEFA and reduced CPK and K on d 3 but not on d 28 (interaction, P ≤ 0.060) and increased serum creatinine and reduced amylase on d 28 but not on d 3 (interaction, P ≤ 0.057). Heat stress reduced growth, disturbed ion balance, and increased markers of muscle damage. Betaine had a minor impact on alleviating heat stress with the possible exception of early days of heat exposure. The beneficial effect of betaine was diminished by pig adaptation.
Keywords: betaine, heat stress, pigs
INTRODUCTION
High ambient temperatures account for US$330 million per year in losses for swine producers in the United States (St-Pierre et al., 2003). Heat-stressed pigs have increased mortality and reduced growth, G:F, market weight, and carcass value (Rhoads et al., 2013). During heat stress, pigs adapt by increasing cutaneous vasodilation as a protection mechanism to dissipate metabolic heat, which is compensated by reduced blood flow to the splanchnic organs, causing a shortage of nutrients and oxygen. Reductions in oxygen result in depletion of ATP, accumulation of reactive oxygen species, and disruption of cell membranes, affecting ion homeostasis and adenosine triphosphatase (ATPase) activity (Hall et al., 1999; Cronjé, 2005).
Betaine, or trimethylglycine, is known for its methyl donor and osmolyte properties (Kidd et al., 1997; Craig, 2004). Betaine has been demonstrated to reduce the activity of ion ATPase pumps of the erythrocyte (Moeckel et al., 2002) and maintain ion balance. In addition, betaine prevented liver injury and endotoxin translocation in heat-stressed rats (Wettstein and Haussinger, 1997) and prevented villi damage during coccidiosis infection in poultry (Kettunen et al., 2001; Klasing et al., 2002). Dietary betaine increased growth and reduced hyperthermia of heat-stressed rabbits (Hassan et al., 2011) and increased growth (Farooqi et al., 2005; Sayed and Downing, 2011; He et al., 2015) and reduced mortality (Zulkifli et al., 2004; Awad et al., 2014) in heat-stressed poultry. In pigs, betaine improved the performance of pigs fed low-energy diets (Schrama et al., 2003; Dunshea et al., 2009) due to a reduction in energy requirements for maintenance.
We hypothesized that the osmolyte betaine could ameliorate the negative effects of heat stress. The objective of this study was to evaluate the impact of dietary betaine on pig performance, core body temperature and respiration rate, and serological and hematological indices during thermoneutral and heat-stressed conditions.
MATERIALS AND METHODS
Animal use protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
The experiment was conducted using 64 crossbred ([Landrace × Yorkshire] × [Hampshire × Duroc]) pigs (16 barrows and 48 gilts) with an average initial BW of 39.0 ± 1.5 kg. Pigs were blocked by initial BW and sex and were assigned to 1 of 8 treatments using an experimental animal allotment program (Kim and Lindemann, 2007). Treatments consisted of 2 thermal environments (thermoneutral or heat-stressed) and 4 levels of dietary betaine (0, 0.10, 0.15, and 0.20%; Betafin; Danisco A/S, Marlborough, Wiltshire, UK).
Two identical rooms were used, each equipped with an environmental control system (GT-5124LW Grower Direct; Monitrol Inc., Boucherville, QC, Canada) allowing temperatures to fluctuate over time to mimic those experienced in commercial production systems. One room was used as the thermoneutral environment, and temperatures were set at 15, 14, 15, 15, 17, 18, 19, 20, 21, 20, 17, and 15°C, for 2400, 0200, 0400, 0600, 0800, 1000, 1200, 1400, 1600, 1800, 2000, and 2200 h, respectively. The other room served as the heat-stressed environment, and temperatures were set at 29, 28, 29, 29, 31, 32, 33, 34, 35, 34, 31, and 29°C for 2400, 0200, 0400, 0600, 0800, 1000, 1200, 1400, 1600, 1800, 2000, and 2200 h, respectively. Room temperature was recorded by data recorders (Logtag; MicroDAQ.com Ltd., Contoocook, NH) every 10 min. Two data recorders were placed in each room at approximately the same height as the pigs.
Pigs were housed in individual pens (0.91 by 1.82 m) at the Swine Research Complex (Raleigh, NC) using 32 pens in each of the rooms. Each pen had 1 water nipple drinker and a single space feeder. Feed and water were provided to allow ad libitum access throughout the entire experiment. Each room was equipped with a water meter to measure water disappearance in the room.
Feed was manufactured at the North Carolina State University Feed Mill Educational Unit (Raleigh, NC). Diets were corn–corn dried distiller's grains with solubles–soybean meal based and contained 2.96 g standardized ileal digestible lysine/Mcal ME (Table 1). Diets were formulated to meet or exceed all nutrient concentrations suggested by the NRC (2012). One basal mix was prepared to contain all ingredients, except betaine. This mix was divided into 4 batches, and each batch was mixed with the appropriate amount of betaine to generate the final dietary treatments. Pigs were fed dietary treatments for 7 d (designated as d −7) prior to initiation of temperature treatments. The room temperature during this initial period was set at a constant 21°C. This was followed by the implementation of the 2 thermal environment treatments starting on d 0 and continuing for 28 d.
Table 1.
Composition of experimental diets, as-fed basis1
| Betaine inclusion, % | ||||
|---|---|---|---|---|
| Item | 0 | 0.10 | 0.15 | 0.20 |
| Ingredient, % | ||||
| Corn, yellow dent | 56.38 | 56.33 | 56.30 | 56.27 |
| Corn dried distiller's grains with solubles | 20.05 | 20.03 | 20.02 | 20.01 |
| Soybean meal, 47.5% CP | 17.81 | 17.79 | 17.78 | 17.77 |
| Poultry fat | 2.51 | 2.50 | 2.50 | 2.50 |
| Limestone | 1.46 | 1.46 | 1.46 | 1.46 |
| Monocalcium phosphate, 21% P | 0.53 | 0.53 | 0.53 | 0.53 |
| Salt | 0.50 | 0.50 | 0.50 | 0.50 |
| L-Lysine HCl | 0.42 | 0.42 | 0.42 | 0.42 |
| L-Threonine | 0.10 | 0.10 | 0.10 | 0.10 |
| DL-Methionine | 0.05 | 0.05 | 0.05 | 0.05 |
| Trace mineral premix2 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix3 | 0.03 | 0.03 | 0.03 | 0.03 |
| Phytase4 | 0.01 | 0.01 | 0.01 | 0.01 |
| Betaine5 | 0.00 | 0.10 | 0.15 | 0.20 |
| Calculated composition | ||||
| ME Mcal/kg | 3.41 | 3.41 | 3.41 | 3.41 |
| SID6 Lys:ME, g/Mcal | 2.96 | 2.96 | 2.96 | 2.96 |
| Analyzed composition, as-fed basis | ||||
| CP,7 % | 19.17 | 19.55 | 19.72 | 19.53 |
| Lys,7 % | 1.105 | 1.134 | 1.114 | 1.115 |
| Gly,7 % | 0.756 | 0.777 | 0.762 | 0.745 |
| Met,7 % | 0.353 | 0.352 | 0.347 | 0.345 |
| Betaine,8 % | 0.13 | 0.29 | 0.36 | 0.36 |
| Crude fat,9 % | 6.22 | 6.06 | 6.06 | 6.17 |
| Crude fiber,9 % | 8.79 | 7.97 | 8.07 | 8.16 |
| Ca,9 % | 0.64 | 0.73 | 0.70 | 0.68 |
| P,9 % | 0.54 | 0.53 | 0.52 | 0.53 |
Diets were formulated to meet or exceed all nutrient concentrations suggested by the NRC (2012).
Supplied, per kilogram of complete diet, 16.5 mg Cu as CuSO4, 0.30 mg I as ethylenediamine dihydriodide, 165 mg Fe as FeSO4, 40 mg Mn as MnSO4, 0.30 mg Se as Na2SeO3, and 165 mg Zn as ZnO.
Supplied, per kilogram of complete diet, 8,227 IU vitamin A, 1,172 IU vitamin D3 as D-activated animal sterol, 47.0 IU vitamin E, 0.03 mg vitamin B12, 5.8 mg riboflavin, 35.2 mg niacin, 23.5 mg d-pantothenic acid as calcium pantothenate, 3.8 mg vitamin K as menadione dimethylpyrimidinol bisulfate, 1.7 mg folic acid, and 0.23 mg d-biotin.
Anhydrous betaine (Betafin; Danisco A/S, Marlborough, Wiltshire, UK).
Phytase (Optiphos; Huvepharma, Sofia, Bularia; 2,000 units/g phytase activity).
SID = standardized ileal digestible.
Analyzed by Ajinomoto Heartland, Inc., Chicago, IL.
Analyzed by Eurofins Scientific Inc., Des Moines, IA.
Analyzed by the Agricultural Experiment Station Chemical Laboratories, University of Missouri, Columbia, MO.
Pig BW was measured on d −7, 0, 14, and 28. Feed disappearance was measured from the difference of daily feed additions and feed remaining in the feeder. Feed remaining in the feeders was calculated for d 0, 14, and 28. Measurements were used to calculate pig ADG, ADFI, and G:F. Rectal temperature and respiration rate were measured on d 0, 1, 2, 3, 7, 14, 21, and 28, between 1400 and 1700 h. Measurements on d 0 were collected prior to exposure to thermal environmental treatments. Rectal temperature was measured by using a digital thermometer (M750 Series; GLA Agriculture Electronics, San Luis Obispo, CA). Respiration rate was measured as the number of flank movements per 30 s. On d 3 and 28, blood was collected from the jugular vein by venipuncture into glass vacuum tubes without additive (for serum) and with K3–EDTA (for whole blood). Blood for serum chemistry measurements was centrifuged at 1,000 × g for 20 min at 10°C to collect serum.
Chemical Analysis
The concentration of natural betaine in the final diets was analyzed by Eurofins (Eurofins Scientific Inc., Des Moines, IA) using capillary electrophoresis with UV detection and quantitation by comparison with a standard. Dietary lysine, glycine, and methionine concentrations were measured by Ajinomoto Heartland, Inc. (Chicago, IL) using ion exchange chromatography (method 13903; ISO, 2005). Proximate analysis was conducted by the Agricultural Experiment Station Chemical Laboratories, University of Missouri (Columbia, MO) using AOAC International (AOAC, 2005) procedures. Diets were analyzed for CP (method 990.03), crude fat (method 920.39 (A)), crude fiber (method 978.10), Ca (method 985.01 (A, B, D)), and P (method 966.01).
Serum samples were analyzed for total protein, albumin, globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AlkP), γ-glutamyltranspeptidase (GGTP), urea N, creatinine, glucose, Ca, P, Mg, K, Na, Cl, cholesterol, triglycerides, NEFA, amylase, lipase, creatine phosphokinase (CPK), free cysteine, free methionine, and homocysteine. Whole blood samples were analyzed for white blood cells (WBC), red blood cells (RBC), hemoglobin, hematocrit, platelets, neutrophils, lymphocytes, monocytes, eosinophils, and basophils. All analyses were performed by the Antech Diagnostics laboratory (Lake Success, NY) using an autoanalyzer (Olympus AU 5400; Olympus America Inc., Melville, NY) except for NEFA, cysteine, methionine, and homocysteine. Nonesterified fatty acid concentrations were determined using a commercial kit (NEFA-HR2 kit; Wako Diagnostics, Mountain View, CA) following the manufacturer's protocol. Cysteine, methionine, and homocysteine were measured by HPLC using an autoanalyzer (Hitachi L8900; Hitachi High Technologies America, Inc., Dallas, TX) following the procedures of the Official Journal of the European Communities (European Union Commission, 1998; method L257/16). Osmolarity was calculated using the equation given by Tormey (1997) as follows: serum osmolarity (mOsm per L) = 2[Na (mEq/L) + K (mEq/L)] + [glucose (mg/dL)/18] + [urea N (mg/dL)/2.8].
Statistical Analyses
Data were analyzed using the GLIMMIX procedure of SAS (SAS Inst. Inc., Cary, NC). Degrees of freedom were approximated using the Kenward–Roger method (Kenward and Roger, 1997). The pig was used as the experimental unit. Orthogonal contrast comparisons were conducted to determine linear and quadratic effects of betaine level and effect of days when there were more than 2 time points. Main effects and interactions were considered statistically significant at P ≤ 0.05 and were considered tendencies when 0.05 < P ≤ 0.10. Least squares means were reported with the pooled SEM. Post hoc analysis was conducted using the t-test method.
For pig performance and measurements of rectal temperature and respiration rate on d 0, the following model was used:
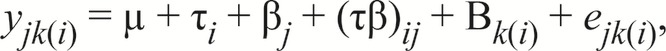 |
in which yjk(i) is the response, µ is the overall mean, τi is the effect due to environment (thermoneutral or heat-stressed), βj is the effect due to betaine level (0, 0.10, 0.15, or 0.20%), (τβ)ij is the effect due to the combination of environment and betaine level, Bk(i) is the random effect due to weight block nested within environment, and ejk(i) is the error, with mean equal to 0 and variance equal to σ2jk(i).
For measurements of rectal temperature and respiration from d 1 to 28 and serology and hematology indices on d 3 and 28, the following model was used:
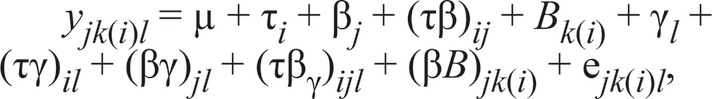 |
in which yjk(i)l is the response; µ is the overall mean; τi is the effect due to thermal environment; βj is the effect due to betaine level; (τβ)ij is the effect due to the combination of environment and betaine level; Bk(i) is the random effect due to weight block nested within environment; γl is the effect due to day (1, 2, 3, 7, 14, 21, and 28 for rectal temperature and respiration rate and 3 or 28 for serology and hematology indices); (τγ)il is the effect due to the combination of environment and day; (βγ)jl is the effect due to the combination of betaine level and day; (τβγ)ijl is the effect due to the combination of environment, betaine level, and day; (βB)jk(i) is the random effect due to among pig variation nested within environment; and ejk(i)l is the error term with mean equal to 0 and variance–covariance matrix equal to σ2jk(i)l, σ2jk(i)l′, cov(yjk(i)l, yjk(i)l′) for rectal temperature and respiration rate and with mean equal to 0 and variance equal to σ2jk(i)l for the serology and hematology indices.
Rectal temperature and respiration rate (d 1 to 28) were analyzed as repeated measurements, and the type of covariance structure was selected based on the lowest Akaike information criteria (Kincaid, 2005). Compound symmetry and unstructured covariance structures were the most suitable for rectal temperature and respiration rate, respectively.
The normality of the residuals was tested using the UNIVARIATE procedure of SAS (Shapiro–Wilk test; Shapiro and Wilk, 1965) and was considered normal at P ≥ 0.10. When the residuals did not have a normal distribution, data were transformed to log normal. Respiration rate, GGTP, creatinine, glucose, triglycerides, NEFA, lipase, CPK, WBC, RBC, neutrophils, lymphocytes, eosinophils, and basophils were transformed to log normal.
RESULTS
Room Temperature
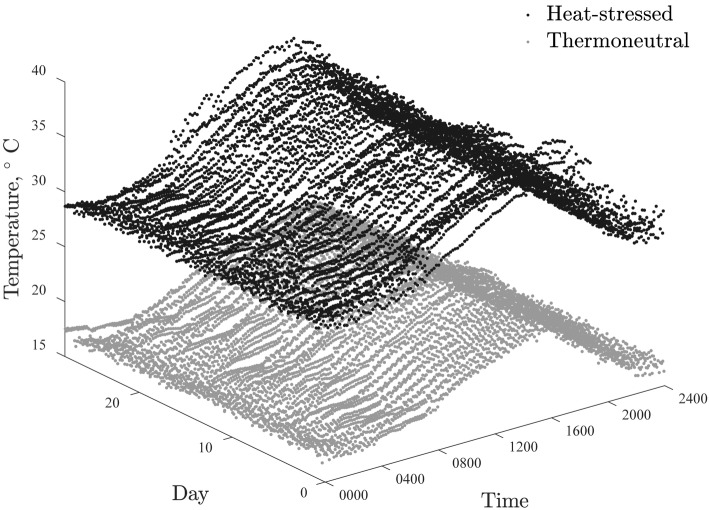
The average room temperature during d −7 to 0 was 21.0 ± 0.7 and 21.6 ± 0.5°C for the rooms used as thermoneutral and heat-stressed environments, respectively (data not shown). During d 0 to 28, the average room temperature was 19.3 ± 2.2 and 31.0 ± 3.1°C for the thermoneutral and heat-stressed rooms, respectively (Fig. 1). Water disappearance was 6.3 and 17.9 L per pig per day from d 0 to 28 for the thermoneutral and heat-stressed environments, respectively.
Figure 1.
Room temperatures of the thermoneutral and heat-stressed environments from d 0 to 28 in intervals of 10 min. Symbols represent individual temperature measurements by temperature data recorders. During d 0 to 28, target temperatures for the thermoneutral environment were 15, 14, 15, 15, 17, 18, 19, 20, 21, 20, 17, and 15°C, for 0000, 0200, 0400, 0600, 0800, 1000, 1200, 1400, 16000, 1800, 2000, and 2200 h, respectively. During d 0 to 28, target temperatures for the heat-stressed environment were 29, 28, 29, 29, 31, 32, 33, 34, 35, 34, 31, and 29°C for 0000, 02000, 0400, 0600, 0800, 1000, 1200, 1400, 1600, 1800, 2000, and 2200 h, respectively. The overall average temperatures during d 0 to 28 were 19.3 ± 2.2 and 31.0 ± 3.1°C for the thermoneutral and heat-stressed rooms, respectively.
Pig Performance
During the course of the experiment, 1 pig was euthanized on d −5 due to a rectal prolapse and 1 pig was removed on d 6 for medical treatment. Both pigs were in the room to be used as the heat-stressed environment, and the pigs were receiving diets containing 0.10 and 0.15% betaine. No differences in growth performance were observed during d −7 to 0 (Table 2). No interactions between environmental temperature and betaine level were observed during d 1 to 28 for growth performance. Heat stress reduced (P ≤ 0.008) ADG and ADFI and increased (P = 0.036) G:F during d 1 to 28. The increase in G:F due to heat stress was more pronounced during d 1 to 14 (P = 0.014). Supplementation of betaine tended to increase G:F in a quadratic manner (P = 0.071), and the greatest response was observed at 0.10% betaine inclusion, regardless of the environmental temperature.
Table 2.
Effects of ambient temperature and dietary betaine on growth performance of pigs1
| Enviroment2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermoneutral | Heat-stressed | P-value | |||||||||
| Betaine inclusion, % | |||||||||||
| Item | 0 | 0.10 | 0.15 | 0.20 | 0 | 0.10 | 0.15 | 0.20 | SEM | Environment | Betaine |
| BW, kg | |||||||||||
| d −7 | 38.8 | 38.8 | 38.7 | 39.0 | 38.5 | 38.7 | 39.6 | 39.9 | 1.487 | 0.860 | 0.443 |
| d 0 | 45.9 | 44.5 | 44.7 | 46.0 | 44.2 | 45.0 | 46.1 | 45.8 | 1.757 | 0.992 | 0.654 |
| d 14 | 57.4 | 56.1 | 56.9 | 58.5 | 54.5 | 55.5 | 55.7 | 56.6 | 2.119 | 0.516 | 0.568 |
| d 28 | 69.1 | 67.1 | 68.0 | 68.9 | 63.3 | 65.5 | 65.6 | 66.2 | 2.398 | 0.282 | 0.810 |
| ADG, kg | |||||||||||
| d −7 to 0 | 1.015 | 0.820 | 0.865 | 0.994 | 0.820 | 0.885 | 0.911 | 0.848 | 0.133 | 0.642 | 0.933 |
| d 1 to 14 | 0.883 | 0.824 | 0.871 | 0.895 | 0.739 | 0.746 | 0.696 | 0.773 | 0.047 | 0.004 | 0.623 |
| d 15 to 28 | 0.840 | 0.787 | 0.791 | 0.743 | 0.625 | 0.712 | 0.711 | 0.682 | 0.043 | 0.020 | 0.678 |
| d 1 to 28 | 0.832 | 0.806 | 0.831 | 0.819 | 0.682 | 0.730 | 0.702 | 0.728 | 0.039 | 0.008 | 0.971 |
| ADFI,3 kg (as-fed basis) | |||||||||||
| d −7 to 0 | 1.77 | 1.75 | 1.77 | 1.78 | 1.83 | 1.69 | 1.73 | 1.75 | 0.106 | 0.896 | 0.848 |
| d 1 to 14 | 2.29 | 2.13 | 2.23 | 2.41 | 1.73 | 1.68 | 1.69 | 1.78 | 0.135 | 0.001 | 0.403 |
| d 15 to 28 | 2.33 | 2.24 | 2.16 | 2.31 | 1.83 | 1.92 | 1.92 | 1.92 | 0.122 | 0.009 | 0.908 |
| d 1 to 28 | 2.28 | 2.19 | 2.28 | 2.36 | 1.78 | 1.80 | 1.81 | 1.85 | 0.131 | 0.002 | 0.783 |
| G:F | |||||||||||
| d −7 to 0 | 0.559 | 0.480 | 0.484 | 0.545 | 0.451 | 0.516 | 0.501 | 0.487 | 0.065 | 0.639 | 0.980 |
| d 1 to 14 | 0.393 | 0.389 | 0.391 | 0.375 | 0.429 | 0.451 | 0.410 | 0.442 | 0.016 | 0.014 | 0.580 |
| d 15 to 28 | 0.366 | 0.355 | 0.348 | 0.327 | 0.343 | 0.375 | 0.374 | 0.358 | 0.021 | 0.396 | 0.701 |
| d 1 to 284 | 0.369 | 0.372 | 0.368 | 0.351 | 0.385 | 0.411 | 0.390 | 0.380 | 0.013 | 0.036 | 0.259 |
Values represent least squares means of 8 individually housed pigs.
The temperature curves were created by temperature set points of 15, 14, 15, 15, 17, 18, 19, 20, 21, 20, 17, and 15°C for thermoneutral conditions and 29, 28, 29, 29, 31, 32, 33, 34, 35, 34, 31, and 29°C for heat-stressed conditions for 0000, 0200, 0400, 0400, 0600, 0800, 1000, 1200, 1400, 1600, 1800, 2000, and 2200 h, respectively.
Dietary treatments were provided 7 d prior to implementation of heat stress (d −7). Environmental treatments started on d 0.
Quadratic effect of betaine level (P = 0.071).
Rectal Temperature and Respiration Rate
Prior to imposing environmental temperature treatments (d 0), rectal temperature and respiration rate did not differ between rooms or due to betaine. Rectal temperature was 39.18 and 39.13°C (pooled SEM 0.05, P = 0.458) for pigs housed in the rooms used for the thermoneutral and heat-stressed environments, respectively. Rectal temperatures were 39.16, 39.09, 39.11, and 39.26°C (pooled SEM 0.07, P = 0.311) for the pigs fed 0, 0.10, 0.15, and 0.20% betaine inclusion, respectively. Respiration rate was 14.8 and 15.9 (pooled SEM 0.7, P = 0.350) flanks movements per 30 s for pigs in the rooms used for the thermoneutral and heat-stressed environments, respectively. Respiration rates were 15.1, 14.9, 15.2, and 16.4 flanks movements per 30 s (pooled SEM 1.0, P = 0.666) for the pigs fed 0, 0.10, 0.15, and 0.20% betaine inclusion, respectively.
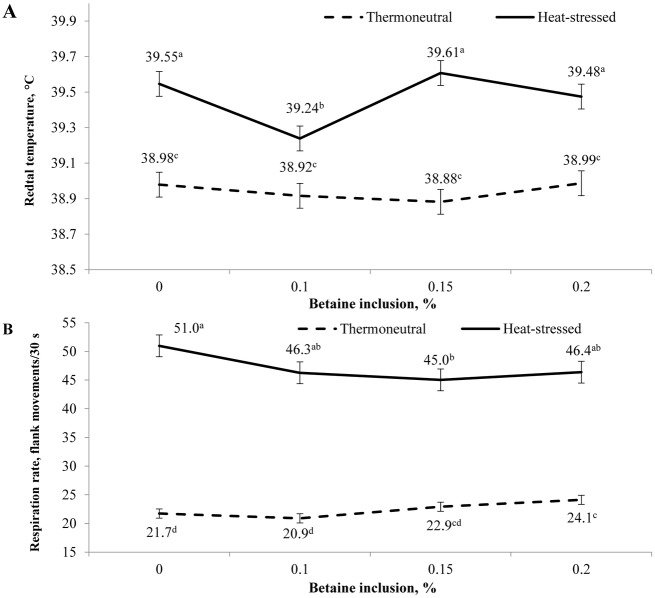
An interaction between environmental temperature and day (P < 0.001) was observed for rectal temperature (Fig. 2A). Exposure to heat stress increased rectal temperature, and this increase was more pronounced during the early phase of heat exposure. Rectal temperature linearly decreased (P ≤ 0.001) as time progressed, regardless of environmental temperature. An interaction between environmental temperature and betaine (P = 0.040) was observed for rectal temperature (Fig. 3A). Betaine supplementation reduced (P < 0.05) rectal temperature of heat-stressed pigs when added at 0.10%. Betaine supplementation did not affect the rectal temperature of pigs housed under thermoneutral conditions.
Figure 2.
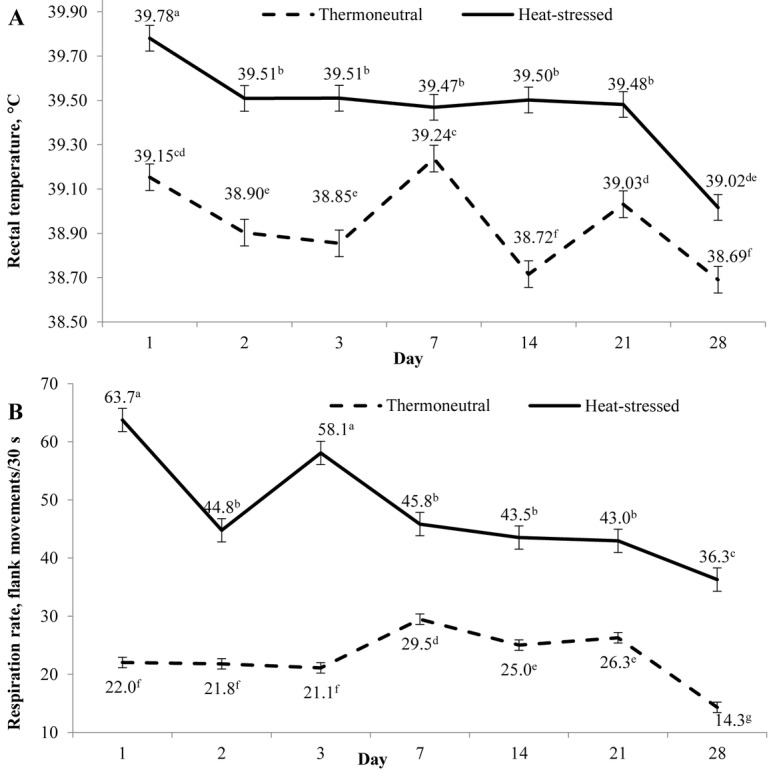
Effect of environmental temperature and day of exposure on rectal temperature and respiration rate on d 1, 2, 3, 7, 14, 21, and 28. Measurements were taken between 1400 and 1700 h. Symbols represent least squares means ± SEM of 32 pigs. a–gMeans with different superscript differ (P ≤ 0.05). (A) An interaction between environmental temperature and day of exposure (P < 0.001) was observed for rectal temperature. Exposure to heat stress increased rectal temperature, and this increase was more evident on d 1 of heat exposure. Rectal temperature linearly decreased (P ≤ 0.001) as time progressed, regardless of the environment. (B) Respiration rate was measured as the number of flank movements per 30 s. An interaction between environmental temperature and day of exposure (P < 0.001) was observed for respiration rate. Exposure to heat stress increased respiration rate, and this increase was more evident on d 1 and 3 of heat exposure. Respiration rate linearly decreased (P ≤ 0.001) as time progressed, regardless of the environment.
Figure 3.
Effect of environmental temperature and betaine supplementation on rectal temperature and respiration rate. Rectal temperature and respiration rate were measured between 1400 and 1700 h and are presented as the mean of measurements taken on d 1, 2, 3, 7, 14, 21, and 28. Symbols represent least squares means ± SEM of 8 pigs and 7 measurements over time (56 observation). a–dMeans with different superscript differ (P ≤ 0.05) (A) An interaction between environmental temperature and betaine (P = 0.040) was observed for rectal temperature. Betaine supplementation reduced rectal temperature of heat-stressed pigs when supplemented at 0.10%. Betaine did not affect pigs housed under thermoneutral conditions. (B) Respiration rate was measured as the number of flank movements per 30 s. An interaction between environmental temperature and betaine (P = 0.027) was observed for respiration rate. Betaine supplementation increased respiration rate in pigs housed in the thermoneutral environment. Betaine reduced respiration rate of pigs housed in the heat-stressed environment.
An interaction between environmental temperature and day (P < 0.001) was observed for respiration rate (Fig. 2B). Exposure to heat stress increased respiration rate, and this increase was more pronounced on d 1 and 3 of heat exposure. Respiration rate linearly decreased (P ≤ 0.001) as time progressed, regardless of the environment. An interaction between environmental temperature and betaine (P = 0.027) was observed for respiration rate (Fig. 3B). Betaine supplementation increased respiration rate of pigs housed under thermoneutral conditions. Betaine reduced respiration rate of pigs housed under heat-stressed conditions, and the lowest value was observed when betaine was added at 0.15%.
Serum Chemistry
Serum chemistry panels were conducted on d 3 and 28 to evaluate the impact of acute and chronic heat stress, respectively. Heat stress significantly impacted many serum chemistry parameters, and the impact of heat stress depended on whether it was measured during the early or late stages of heat stress (Tables 3 and 4).
Table 3.
Effects of dietary supplementation of betaine on serum chemistry of pigs housed under thermoneutral and heat-stressed conditions measured on d 3 and 28 of ambient temperature exposure1
| Environment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermoneutral | Heat-stressed | ||||||||||
| Betaine inclusion, % | |||||||||||
| Item2 | Day | 0 | 0.10 | 0.15 | 0.20 | 0 | 0.10 | 0.15 | 0.20 | SEM | Probability3 |
| P, mg/dL | 3 | 9.35 | 9.00 | 8.90 | 8.90 | 8.75 | 8.25 | 8.36 | 8.61 | 0.199 | E*, D‡, and E × D** |
| 28 | 8.39 | 8.38 | 8.04 | 8.00 | 8.04 | 7.80 | 8.29 | 8.29 | |||
| Ca, mg/ dL | 3 | 10.88 | 10.70 | 10.88 | 10.88 | 10.60 | 10.48 | 10.33 | 10.61 | 0.106 | E** and D‡ |
| 28 | 10.55 | 10.60 | 10.61 | 10.54 | 10.38 | 10.29 | 10.26 | 10.48 | |||
| Mg, mEq/L | 3 | 1.74 | 1.66 | 1.66 | 1.69 | 1.68 | 1.65 | 1.66 | 1.70 | 0.151 | E**, D‡, and E × D‡ |
| 28 | 2.08 | 1.96 | 1.93 | 2.09 | 2.76 | 2.64 | 2.70 | 2.73 | |||
| K, mEq/L | 3 | 6.06 | 5.68 | 5.63 | 5.52 | 7.11 | 6.89 | 6.30 | 6.85 | 0.215 | E‡, D**, E × D†, and B × D* |
| 28 | 5.45 | 5.66 | 5.79 | 5.50 | 6.29 | 6.22 | 6.38 | 6.53 | |||
| Na, mEq/L | 3 | 142.88 | 142.25 | 142.25 | 141.50 | 138.75 | 138.46 | 138.56 | 138.87 | 0.575 | E‡, D**, and E × D‡ |
| 28 | 143.25 | 143.63 | 143.13 | 143.50 | 141.25 | 140.89 | 141.70 | 142.63 | |||
| Na:K ratio | 3 | 24.00 | 25.50 | 25.50 | 25.85 | 19.75 | 20.31 | 22.56 | 20.38 | 0.84 | E‡, D**, and B × D* |
| 28 | 26.38 | 25.63 | 24.75 | 26.13 | 22.63 | 22.60 | 22.56 | 22.00 | |||
| Cl, mEq/L | 3 | 102.00 | 101.75 | 101.88 | 101.38 | 102.00 | 101.82 | 102.46 | 102.00 | 0.653 | |
| 28 | 100.63 | 101.38 | 102.00 | 101.25 | 101.50 | 102.82 | 102.17 | 102.25 | |||
| Urea N, mg/dL | 3 | 16.8 | 14.9 | 14.9 | 15.3 | 13.3 | 11.9 | 12.7 | 13.3 | 1.097 | E × D** and Quad† |
| 28 | 15.3 | 13.1 | 13.6 | 13.6 | 14.1 | 13.2 | 14.3 | 14 | |||
| Creatinine, mg/dL | 3 | 0.790 | 0.849 | 0.774 | 0.731 | 0.923 | 0.910 | 0.877 | 0.927 | 0.037 | E‡, E × B**, and D‡ |
| 28 | 0.912 | 0.936 | 0.911 | 0.908 | 1.110 | 1.079 | 1.080 | 1.208 | |||
| Urea N:creatinine ratio | 3 | 21.2 | 17.5 | 19.2 | 20.9 | 14.4 | 13.0 | 14.5 | 14.3 | 1.387 | E × D‡ |
| 28 | 16.7 | 14.0 | 15.0 | 15.0 | 12.7 | 12.2 | 13.3 | 11.6 | |||
| Osmolarity, mOsm/L | 3 | 309.5 | 307.2 | 306.9 | 305.1 | 302.6 | 299.8 | 300.1 | 302.0 | 1.289 | E‡, D‡, and E × D‡, |
| 28 | 308.2 | 308.6 | 307.9 | 308.2 | 305.4 | 304.3 | 306.9 | 308.9 | E × B* | ||
| Total protein, g/dL | 3 | 5.90 | 5.84 | 5.98 | 5.99 | 5.90 | 6.00 | 5.94 | 5.91 | 0.103 | D* |
| 28 | 6.05 | 5.89 | 5.98 | 6.03 | 6.14 | 6.08 | 6.13 | 5.98 | |||
| Albumin, g/dL | 3 | 3.913 | 3.863 | 3.863 | 3.850 | 3.863 | 3.740 | 3.761 | 3.875 | 0.071 | B*, D‡, Lin†, and Quad* |
| 28 | 4.213 | 3.975 | 4.000 | 4.075 | 4.025 | 3.854 | 3.889 | 3.888 | |||
| Globulin, g/dL | 3 | 2.042 | 1.975 | 2.113 | 2.138 | 2.038 | 2.257 | 2.2 | 2.038 | 0.097 | E*, D†, and E × D** |
| 28 | 1.963 | 1.900 | 1.975 | 1.950 | 2.113 | 2.229 | 2.243 | 2.088 | |||
| Albumin:globulin ratio | 3 | 1.90 | 1.99 | 1.86 | 1.86 | 1.91 | 1.70 | 1.73 | 1.94 | 0.105 | E*, D‡, and E × D‡ |
| 28 | 2.15 | 2.13 | 2.06 | 2.15 | 1.96 | 1.74 | 1.76 | 1.90 | |||
| AST, units/L | 3 | 27.5 | 29.5 | 34 | 32.3 | 43.3 | 38 | 37.7 | 35.9 | 4.165 | E*, D**, and E × D† |
| 28 | 22.8 | 23.1 | 34.3 | 28.3 | 26.8 | 30.3 | 28.4 | 28.1 | |||
| ALT, units/L | 3 | 27.5 | 26.3 | 28.5 | 28.3 | 29.1 | 27.9 | 24.7 | 26.4 | 2.479 | E* and E × D** |
| 28 | 32.1 | 26.4 | 31.4 | 35.4 | 26.4 | 24.3 | 24.6 | 22.8 | |||
| AlkP, units/L | 3 | 165 | 187 | 170 | 176 | 146 | 133 | 130 | 173 | 10.01 | E‡ and D‡ |
| 28 | 152 | 157 | 160 | 158 | 123 | 107 | 121 | 145 | |||
| GGTP, units/L | 3 | 15.3 | 11.6 | 10.5 | 11.1 | 21.8 | 26 | 22.4 | 25.8 | 6.48 | E**, D‡, and E × D* |
| 28 | 37 | 33.7 | 33 | 36.7 | 44.3 | 46.2 | 48.4 | 43.6 | |||
| Amylase, units/L | 3 | 1,523 | 1,563 | 1,364 | 1,594 | 1,388 | 1,345 | 1,462 | 1,458 | 152.8 | D‡ and E × D‡ and B × D† |
| 28 | 1,607 | 1,500 | 1,411 | 1,630 | 1,636 | 1,506 | 1,806 | 1,642 | |||
| Lipase, units/L | 3 | 2.28 | 2.07 | 2.29 | 2.81 | 1.49 | 1.43 | 1.35 | 1.63 | 0.396 | E‡ and D‡ |
| 28 | 2.58 | 2.71 | 3.00 | 2.91 | 1.71 | 2.25 | 2.38 | 2.77 | |||
| CPK, units/L | 3 | 2,277 | 3,622 | 2,392 | 2,474 | 25,6554 | 8,119 | 4,271 | 21,0574 | 1,775 | E‡, E × B†, D‡, and E × D‡ |
| 28 | 1,428 | 1,115 | 2,187 | 1,705 | 1,607 | 1,667 | 1,637 | 3,055 | B × D*, E × B × D†, and Quad† | ||
| Cholesterol, mEq/L | 3 | 109.3 | 106.6 | 104.8 | 105.8 | 106.4 | 102.4 | 113.9 | 116.4 | 4.597 | D‡ |
| 28 | 102.1 | 104.1 | 99.9 | 98.5 | 101.6 | 100 | 107.1 | 106.9 | |||
| Triglycerides, mEq/L | 3 | 34.9 | 32.3 | 27.8 | 30.8 | 36.4 | 33.4 | 33.1 | 34.6 | 3.530 | E* and D† |
| 28 | 29.8 | 29.4 | 24.3 | 23.5 | 32.6 | 32.8 | 29.4 | 40.6 | |||
| NEFA, mEq/L | 3 | 0.081 | 0.087 | 0.098 | 0.091 | 0.078 | 0.081 | 0.082 | 0.092 | 0.010 | E‡, E × D‡, and B × D† |
| 28 | 0.137 | 0.11 | 0.102 | 0.113 | 0.077 | 0.069 | 0.062 | 0.059 | |||
| Glucose, mg/dL | 3 | 100.1 | 107.8 | 104.2 | 98.2 | 109.4 | 104 | 103.4 | 104.9 | 4.438 | D‡ |
| 28 | 96.2 | 96.0 | 93.3 | 96.2 | 95.4 | 97.9 | 99.6 | 99.6 | |||
Values represent least squares means of 8 individually housed pigs.
AST = aspartate aminotransferase; ALT = alanine aminotransferase; AlkP = alkaline phosphatase; GGTP = γ-glutamyltranspeptidase; CPK = creatine phosphokinase.
Effect abbreviations: E = environment; B = betaine; E × B = environment and betaine interaction; D = day; E × D = environment and day interaction; B × D = betaine and day interaction; E × B × D = environment, betaine, and day interaction; Lin = linear effect of betaine; Quad = quadratic effect of betaine.
Individual values were 8,104, 22,373, 27,589, 28,253, 29,400, 34,870, 35,063, and 36,945 for pigs fed 0% betaine and 7,938, 14,003, 14,024, 24,052, 27,488, 28,602, 35,826, and 36,601 for pigs fed 0.2% betaine.
0.10 > P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ‡P ≤ 0.001.
Table 4.
Effects of dietary supplementation of betaine on serum concentrations of methionine, cysteine, and homocysteine in pigs housed under thermoneutral and heat-stressed conditions measured on d 3 and 28 of ambient temperature exposure1
| Environment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermoneutral | Heat-stressed | ||||||||||
| Betaine inclusion, % | |||||||||||
| Item | Day | 0 | 0.10 | 0.15 | 0.20 | 0 | 0.10 | 0.15 | 0.20 | SEM | Probability2 |
| Cysteine, µg/mL | 3 | 141.9 | 119.8 | 146.3 | 141.3 | 162.8 | 145.5 | 141.9 | 162.1 | 16.42 | E* |
| 28 | 105.5 | 111.8 | 125.2 | 124.4 | 159.0 | 146.7 | 136.0 | 161.7 | |||
| Methionine, µg/mL | 3 | 9.40 | 9.57 | 9.37 | 9.25 | 7.53 | 7.61 | 6.52 | 7.75 | 0.6860 | D** and E × D‡ |
| 28 | 7.04 | 6.64 | 7.07 | 6.27 | 7.67 | 8.13 | 7.48 | 8.56 | |||
| Homocysteine, µg/mL | 3 | 0.016 | 0.012 | 0.014 | 0.014 | 0.005 | 0.002 | 0.003 | 0.006 | 0.0047 | E × D* |
| 28 | 0.009 | 0.010 | 0.008 | 0.004 | 0.011 | 0.005 | 0.011 | 0.004 | |||
Values represent least squares means of 8 individually housed pigs.
Effect abbreviations: E = environment; D = day; E × D = environment and day interaction.
P ≤ 0.05; **P ≤ 0.01; ‡P ≤ 0.001.
A tendency for a 3-factor interaction was observed for serum activity of CPK (P = 0.058). Serum activity of CPK was greater in heat-stressed pigs on d 3, and supplementation of betaine alleviated this increase; the lowest CPK activity was observed at 0.15% betaine. Supplementation of betaine did not affect CPK in pigs housed under heat-stressed conditions on d 28 or pigs housed under thermoneutral conditions.
Heat stress reduced serum osmolarity on d 3 but did not affect osmolarity on d 28 (interaction, P < 0.001). In addition, supplementation of betaine reduced osmolarity on d 3 and increased serum osmolarity on d 28 (interaction, P = 0.025).
Serum concentration of creatinine was greater in heat-stressed pigs, and this increase was more pronounced on d 28 (interaction, P = 0.028). In addition, supplementation of betaine increased serum concentrations of creatinine on d 28 but not on d 3 (interaction, P = 0.011). The highest serum concentration of creatinine on d 28 was observed at 0.20% betaine.
Serum activity of amylase was greater in heat-stressed pigs on d 28 (interaction, P < 0.001). In addition, supplementation of betaine at 0.10% decreased serum activity of amylase on d 28, regardless of environmental conditions (interaction, P = 0.057).
Serum concentration of NEFA was lower in heat-stressed pigs, and this reduction was more pronounced on d 28 (interaction, P < 0.001). In addition, supplementation of betaine increased serum concentrations of NEFA on d 3 and reduced it on d 28, regardless of the environment (interaction, P = 0.060).
Serum concentration of K was greater in heat-stressed pigs, and this increase was more pronounced on d 3 (interaction, P = 0.096). In addition, supplementation of betaine decreased serum K concentration on d 3 but not on d 28 (interaction, P = 0.020). The lowest serum K concentration on d 3 was observed at 0.15% betaine.
Serum concentration of albumin was lower in heat-stressed pigs, and this reduction was more pronounced on d 3 (interaction, P = 0.051). In addition, supplementation of betaine reduced serum concentration of albumin in a quadratic manner (P = 0.024). The lowest serum concentration of albumin was observed at 0.10% betaine, regardless of the environmental temperature and day of exposure.
Heat stress increased serum activity of ALT, and this increase was more pronounced on d 3 (interaction, P = 0.097). Heat stress increased serum concentration of Mg and globulin and activity of GGTP, and this increase was more pronounced on d 28 (interaction, P ≤ 0.034). Heat stress reduced serum Na, urea N, methionine, and homocysteine and the albumin:globulin ratio, and this reduction was more pronounced on d 3 (interaction, P ≤ 0.013). Heat stress reduced serum P, the urea N:creatinine ratio, and activity of ALT, and this reduction was more pronounced on d 28 (interaction, P ≤ 0.009).
Heat stress increased serum triglyceride concentrations (P = 0.048). Serum concentrations of triglycerides tended to be lower on d 28, regardless of the environmental temperature (P = 0.053). Heat stress reduced serum Ca concentrations and activity of AlkP (P ≤ 0.002). Serum Ca concentrations and AlkP activity were lower on d 28, regardless of the environment (P < 0.001). Heat stress reduced serum activity of lipase (P = 0.001). Serum activity of lipase was greater on d 28, regardless of the environment (P < 0.001).
Heat stress reduced the serum Na:K ratio (P < 0.001). In addition, supplementation of betaine increased the serum Na:K ratio on d 3 but not on d 28 (P = 0.042). The highest Na:K ratio was observed on d 3 when betaine was supplemented at 0.15%.
Heat stress increased serum concentration of cysteine, regardless of the day or betaine level (P = 0.022). Serum concentration of total protein increased on d 28 (P = 0.001), regardless of the environmental temperature or betaine level. Serum concentration of glucose and cholesterol were reduced on d 28 (P < 0.001), regardless of the environmental temperature or betaine level. Chloride was not affected by the environment, supplementation of betaine, or day of exposure (P ≥ 0.266).
Complete Blood Count
The complete blood count was evaluated on d 3 and 28 to determine the impact of acute and chronic heat stress, respectively (Table 5). A 3-factor interaction was observed for hematocrit percentage and neutrophil counts (P = 0.042). Supplementation of betaine decreased hematocrit percentage and neutrophil counts only in pigs housed under heat-stressed conditions on d 28.
Table 5.
Effects of dietary supplementation of betaine on complete blood counts in pigs housed under thermoneutral and heat-stressed conditions measured on d 3 and 28 of ambient temperature exposure1
| Environment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermoneutral | Heat-stressed | ||||||||||
| Betaine inclusion, % | |||||||||||
| Item2 | Day | 0 | 0.1 | 0.15 | 0.2 | 0 | 0.1 | 0.15 | 0.2 | SEM | Probability3 |
| WBC, 103/µL | 3 | 15.5 | 17.3 | 15.3 | 16 | 14.5 | 17.9 | 15.4 | 15.1 | 0.995 | D‡ |
| 28 | 15.5 | 14.6 | 13.8 | 14.3 | 13.8 | 14.3 | 14.2 | 13.1 | |||
| RBC, 106/µL | 3 | 7.62 | 7.94 | 7.85 | 7.69 | 8.00 | 7.84 | 7.83 | 7.84 | 0.209 | D* and E × D** |
| 28 | 7.96 | 8.11 | 8.14 | 8.15 | 8.00 | 8.01 | 7.70 | 7.61 | |||
| Hemoglobin, g/dL | 3 | 13.11 | 13.69 | 13.19 | 13.11 | 13.8 | 13.44 | 13.65 | 13.3 | 0.335 | E × D** and E × B × D† |
| 28 | 13.79 | 13.61 | 13.99 | 13.55 | 13.4 | 13.62 | 13.23 | 12.88 | |||
| Hematocrit, % | 3 | 41.4 | 43.3 | 42.0 | 41.4 | 42.5 | 42.4 | 43.1 | 42.5 | 1.148 | E × D** and E × B × D* |
| 28 | 42.6 | 43.1 | 43.3 | 43.3 | 42.3 | 43.4 | 41.5 | 40.0 | |||
| Platelet count, 103/µL | 3 | 419 | 390 | 385 | 362 | 380 | 323 | 381 | 340 | 37.28 | B†, D‡, and Lin† |
| 28 | 358 | 306 | 348 | 322 | 377 | 256 | 341 | 262 | |||
| Neutrophils, 103/µL | 3 | 5.18 | 6.49 | 5.36 | 5.34 | 4.65 | 5.95 | 4.00 | 5.28 | 0.529 | D‡ and E × D × B* |
| 28 | 4.23 | 5.60 | 4.52 | 5.25 | 5.00 | 4.30 | 4.08 | 4.14 | |||
| Lymphocytes, 103/µL | 3 | 8.73 | 9.00 | 8.55 | 9.32 | 8.88 | 10.25 | 9.89 | 8.53 | 0.800 | D** |
| 28 | 9.99 | 7.88 | 8.41 | 7.83 | 7.69 | 9.60 | 8.6 | 7.73 | |||
| Monocytes/µL | 3 | 791 | 787 | 765 | 689 | 504 | 798 | 722 | 622 | 81.08 | B†, D*, E × D†, and Quad* |
| 28 | 691 | 720 | 411 | 596 | 657 | 734 | 662 | 538 | |||
| Eosinophils/µL | 3 | 361 | 497 | 451 | 380 | 278 | 461 | 356 | 276 | 73.17 | B**, E × D‡, and Quad** |
| 28 | 330 | 390 | 338 | 220 | 392 | 640 | 442 | 329 | |||
| Basophils/µL | 3 | 172 | 195 | 161 | 177 | 116 | 138 | 158 | 160 | 18.53 | E × D† |
| 28 | 185 | 138 | 122 | 146 | 155 | 161 | 148 | 134 | |||
Values represent least squares means of 8 individually housed pigs.
WBC = white blood cells; RBC = red blood cells.
Effect abbreviations: E = environment; B = betaine; E × B = environment and betaine interaction; D = day; E × D = environment and day interaction; B × D = betaine and day interaction; E × B × D = environment, betaine, and day interaction; Lin = linear effect; Quad = quadratic effect.
0.10 > P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ‡P ≤ 0.001.
Eosinophil count was increased on d 28 when pigs were housed under heat-stressed conditions; conversely, eosinophil count was reduced on d 28 when pigs were housed under thermoneutral conditions (interaction, P = 0.001). In addition, eosinophils quadratically increased with betaine supplementation (P = 0.002), and the highest count of eosinophils was observed when betaine was added at 0.10%, regardless of the environmental temperature or day of measurement.
Hemoglobin decreased on d 28 when pigs were housed under heat-stressed conditions; conversely, hemoglobin increased on d 28 when pigs were housed under thermoneutral conditions (interaction, P = 0.001). The RBC count increased on d 28 when pigs were housed under thermoneutral conditions (interaction, P = 0.003).
Platelet count was reduced on d 28, regardless of the environment (P = 0.001). Supplementation of betaine tended to linearly decrease platelet count (P = 0.077), regardless of the environment or day of measurement. Monocyte count was reduced (P = 0.038) on d 28, regardless of the environmental temperature. Supplementation of betaine quadratically increased the monocyte count (P = 0.036), and the highest monocyte count was observed when betaine was supplemented at 0.10%, regardless of the environmental temperature or day of measurement. Lymphocytes and WBC increased on d 3, regardless of the environmental temperature (P ≤ 0.05). Basophils were not affected by the environmental temperature, supplementation of betaine, or day of exposure (P ≥ 0.196).
DISCUSSION
High environmental temperatures cause significant reductions in profitability in swine production (St-Pierre et al., 2003) because market weight is reduced or housing cost to achieve target weight is increased. Understanding the impact of heat stress on the physiology of the pig can help to evaluate potential dietary strategies to sustain pork production during the summer heat. In the present study, we evaluated the use of natural betaine in diets of growing pigs at incremental levels to determine if the osmoprotectant capacity of betaine could contribute to overcoming the cascade of negative effects caused by heat stress.
Heat tolerance is negatively correlated with BW (Renaudeau et al., 2011). During the course of the experimental period (d 0 to 28), pig BW ranged from 45.3 to 66.7 kg. Temperatures created in the heat-stressed environment were considerably higher than the upper critical temperature of the thermoneutral zone recommended for this range of BW, which is between 23.1 and 22.4°C (Renaudeau et al., 2011). Negative consequences of heat stress were clearly observed, as evidenced by the increase in rectal temperature, respiration rate, and poor growth. The poor growth was mostly associated with a reduction in ADFI. Pigs decrease ADFI as a mechanism to reduce metabolic heat (Renaudeau et al., 2011) that is associated with the activity of eating, digestion, and metabolism (Nienaber et al., 1996).
Renaudeau et al. (2011) fitted polynomial regressions to estimate pig ADFI, ADG, and the feed-to-gain ratio with ambient temperature, BW, and CP in the diet as the independent variables. When equations published by Renaudeau et al. (2011) were used, pigs' ADFI was expected to be reduced by 19%, ADG was expected to be reduced by 24%, and the feed-to-gain ratio was expected to be reduced by 10%. In our experiment, heat stress reduced ADFI by 21% and ADG by 14%, whereas G:F was increased by 7%. The 7% increase in G:F is equivalent to an 8% decrease in the feed-to-gain ratio.
In the present study, betaine supplementation to heat-stressed pigs reduced rectal temperature when included at 0.10% and decreased respiration rate when included at 0.15%, suggesting that betaine supplementation helped to offset hyperthermia. Similar effects were observed in heat-stressed rabbits, where 5 equal-spaced incremental levels of dietary betaine (from 0 to 0.10%) linearly reduced rectal temperature and respiration rate (Hassan et al., 2011). Betaine supplementation (0.05 to 0.15%) to heat-stressed poultry has been shown to increase ADG, ADFI, and G:F (Farooqi et al., 2005; Sayed and Downing, 2011; He et al., 2015) and reduce mortality (Zulkifli et al., 2004; Awad et al., 2014). We observed that betaine supplementation did not affect ADG or ADFI, regardless of inclusion level or environmental temperatures. However, betaine increased G:F when included at 0.10% in pigs housed in the thermoneutral and heat-stressed environments. This increase in G:F appeared to be due to a numeric reduction in ADFI (−90 g/d) when pigs were fed 0.10% betaine in the thermoneutral environment; however, the improvement in G:F in pigs fed 0.10% betaine and housed in the heat-stressed room was due to a numeric increase in ADG (48 g/d). The effect of betaine supplementation under conventional housing (no heat stress) on pig performance was reported in a meta-analysis conducted by Sales (2011). Betaine consistently reduced the amount of feed per unit of gain (12 studies), without affecting ADG (13 studies). The effect of betaine on ADFI (11 studies) was variable but numerically decreased ADFI (Sales, 2011).
Dietary supplementation of betaine to energy-restricted pigs improved growth (Schrama et al., 2003; Dunshea et al., 2009). This effect was associated with a reduction in energy requirements for maintenance (Schrama et al., 2003). Betaine is proposed to reduce the energy requirements for maintenance by reducing the energy demand of osmotic regulation, which will increase energy availability for growth (Schrama et al., 2003). Heat-stressed pigs in the present study had lower energy intake because of reduced ADFI, but they did not respond to a greater extent due to betaine supplementation than pigs housed under thermoneutral conditions.
During heat stress, blood flow increases in the periphery to dissipate metabolic heat. As a compensatory mechanism, blood flow decreases in the splanchnic organs, limiting the supply of oxygen and nutrients. Consequently, shortage of oxygen impairs ATP regeneration (Cronjé, 2005). Depletion of ATP impairs the activity of Na/K ATPase and Ca ATPase, which are essential enzymes regulating ion balance. Moreover, heat stress causes greater water losses due to evaporative cooling, and Na concentrations rise in the extracellular compartment, causing a hypertonic stress (Houpt, 2004). The osmotic pressure is such that water and Na enter the cell, causing cell swelling and rupture of the cell membranes (Cronjé, 2005). Disruption of the cell membrane allows an unregulated Na and Ca influx and K efflux, resulting in increased ion ATPase activity (Cronjé, 2005).
In the present study, pigs exposed to heat stress had lower serum concentrations of Na and higher concentrations of K, especially on d 3 of heat exposure, which may be related to disruptions of the cell membrane. Pigs were able to maintain Na concentrations within normal ranges (135 to 150 mEq/L; Carr, 1998) despite the effect of heat stress. Levels of K were above the normal range (6.7 mEq/L; Carr, 1998) at the beginning of heat stress (d 3), with the exception of pigs fed 0.15% betaine. Nonetheless, exposure to prolonged heat amended Na and K serum concentration, because values of Na and K of heat-stressed pigs were comparable to the thermoneutral counterparts on d 28. Measurements of rectal temperature and respiration rate suggested that the peak of heat stress occurred on d 1. It is possible that a greater ion disturbance could have been observed on d 1.
Elevated values of osmolarity indicate dehydration. In the present study, osmolarity values were considered normal (300 to 315 mOsm/L; Harpur and Popkin, 1965; Waymouth, 1970), regardless of the environment, suggesting that pigs did not experience dehydration. Early exposure to heat stress (d 3) reduced osmolarity, whereas prolonged periods of heat stress (d 28) showed osmolarity values equal to those of pigs housed in the thermoneutral environment. Patience et al. (2005) also reported lower serum osmolarity in heat-stressed (38°C) pigs compared with pigs housed in a thermoneutral environment (20°C). They related this effect to greater water intake or loss of electrolytes in the urine. Although we did not measure individual water consumption, overall water disappearance of the pigs housed in the heat-stress room was 3-fold greater than that of pigs housed in the thermoneutral room.
Multiple organ failures have been observed in the rodent model (Lim et al., 2007) and humans with heat stroke (Bouchama and Knochel, 2002). For instance, heat stroke patients (rectal temperature of about 41 to 43°C) had increased concentrations of plasma ALT by 411%, AST by 590%, and CPK by 89% (Alzeer et al., 1997). Their relative increase in the plasma reflects the magnitude of tissue damage due to thermal injury (Alzeer et al., 1997). High levels of AST and ALT are indicators of liver injury and are generally considered to be biological evidence of liver damage when there is a 3-fold increase in plasma concentrations beyond the upper range (Whalan, 2015). In addition, increased levels of plasma CPK indicate cardiac muscle, skeletal muscle, and brain injury (Whalan, 2015). On d 3, serum AST increased by 32% in pigs housed in the heat-stressed environment compared with pigs housed in the thermoneutral room, whereas ALT was reduced by 2% in heat-stressed pigs. This suggests that there was minimal liver damage due to heat stress. There are few studies that have determined markers of tissue injury in heat-stressed pigs (Hicks et al., 1998; Pearce et al., 2013), and none of them reported changes in plasma ALT, AST, or CPK. In broilers, exposure to heat stress (32°C) increased plasma CPK activity by 54% (Sandercock et al., 2001). In the present study, heat-stressed pigs showed a marked increase in the serum activity of CPK on d 3 of heat exposure, in particular in pigs fed no supplemental betaine (by 853%). Heat-stressed pigs fed 0.15% betaine had the lowest increase in serum activity of CPK (increase was 58%) compared with pigs housed in the thermoneutral room. These findings suggest that betaine may reduce cardiac and skeletal muscle damage due to heat stress, uniquely during the first days of heat exposure because serum activity of CPK on d 28 did not differ from pigs housed under thermoneutral conditions. The physiological and anatomical adaptations that pigs experience during heat stress are not yet fully understood. However, reduced heart size (Heath, 1989; Nienaber et al., 1996; Cruzen et al., 2015) and increased liver size (Li et al., 2015) have been reported in heat-stressed pigs.
Increased serum creatinine and urea N concentrations are indicators of kidney malfunction; however, urea N can be affected by protein intake whereas creatinine is not (Whalan, 2015). In the present experiment, heat-stressed pigs had reduced urea N on d 3 by 17%, but no effect was observed on d 28 compared with pigs in the thermoneutral environment. Clearly, the reduced feed intake due to heat stress is expected to decrease urea N concentrations, and therefore, urea N would not be a reliable indicator to determine kidney malfunction in ad libitum–fed pigs. Heat-stressed pigs had a 20% increase in serum creatinine concentration compared with pigs housed in the thermoneutral environment. Greater accumulation (50%) of creatinine has been reported in heat-stressed pigs (Pearce et al., 2013). The increase in creatinine indicates challenging conditions for the kidneys during heat stress, but concentrations were still within normal ranges (0.8 to 1.7 mg/dL; Carr, 1998).
Greater accumulation of adipose tissue and reduced lean deposition have been observed in pigs reared under heat-stressed conditions (Verstegen et al., 1973; Stahly et al., 1979; Bridges et al., 1998), and this effect has also been observed in broilers (Geraert et al., 1996). Pearce et al. (2013) reported that heat-stressed pig had greater insulin levels, which shifts metabolism toward fat synthesis. This is further supported by reduced serum concentrations of NEFA, an intermediate of fatty acid oxidation, in heat-stressed pigs (Rhoads et al., 2013; Sanz Fernandez et al., 2015). We observed reduced levels of NEFA and increased levels of triglycerides in pigs exposed to heat, which is consistent with previous observations of increased fat deposition.
Red blood cell count, hemoglobin, and hematocrit percentage are positively correlated with dehydration (Whalan, 2015). Waltz et al. (2014) studied the interaction between heat stress (24 vs. 32°C) and water intake (70% allowance of water intake vs. ad libitum water intake) in growing pigs (31 kg of BW). Waltz et al. (2014) observed a small increase in RBC, hemoglobin, and hematocrit percentage in heat-stressed growing pigs, regardless of the water intake. In our experiment, values of RBC, hemoglobin, and hematocrit percentage of heat-stressed pigs were slightly (1%) lower compared with pigs housed in the thermoneutral room. These results are in agreement with osmolarity, suggesting that heat-stressed pigs might compensate the water losses due to evaporative cooling by increasing water intake.
An increase in neutrophil counts can indicate injury of the pancreas, liver, and colon; necrosis of the tissues due to hypoxia; and stress due to cold or heat exposure (Whalan, 2015). However, in our experiment, we observed a reduction of 10% in neutrophil counts due to heat stress. Xiang-hong et al. (2011) also observed a decrease of 7% in neutrophil counts of Bama miniature pigs exposed to 35°C compared with their counterparts housed at 28°C.
Hematology analysis did not reveal major differences due to heat stress. All values were considered in the normal range for growing pigs (Carr, 1998). Interestingly, pigs fed 0.10% betaine showed the highest values for WBC, RBC, hemoglobin, hematocrit, neutrophils, lymphocytes, monocytes, eosinophils, and basophils, regardless of the environment or day. The effect of betaine on hematology indices is unclear, and data have not been previously reported.
Heat stress markedly reduced pig growth performance. Respiration rate and rectal temperature of heat-stressed pigs gradually adapted to high environmental temperatures. Heat stress disrupted serum Na and K balance, especially during the first days of heat stress. Heat-stressed pigs did not demonstrate major organ injury, with the possible exception of cardiac and skeletal muscle during the acute phase of heat stress. Supplementation of betaine alleviated heat stress by improving feed efficiency and reducing rectal temperature. The osmoprotectant capacity of betaine was supported by amending ion balance and preventing cardiac and skeletal muscle injury during the initial days of heat exposure. Therefore, supplementation of betaine had a minor impact on alleviating heat stress, with the possible exception of the early days of heat exposure, which is in contrast to the benefits of betaine reported in studies with growing rabbits and poultry.
Footnotes
Partial funding was provided by The National Pork Board, Des Moines, IA (NPB project number 12-154).
LITERATURE CITED
- Alzeer A. H., El-Hazmi M. A. F., Warsy A. S., Ansari Z. A., Yrkendi M. S. 1997. Serum enzymes in heat stroke: Prognostic implication. Clin. Chem. 43:1182–1187. [PubMed] [Google Scholar]
- AOAC 2005. Official methods of analysis. 18th ed.AOAC Int., Gaithersburg, MD. [Google Scholar]
- Awad A. L., Ibrahim A. F., Fahim H. N., Beshara M. M. 2014. Effect of dietary betaine supplementation on growth performance and carcass traits of Domyati ducklings under summer conditions. Egypt. Poult. Sci. J. 34:1019–1038. [Google Scholar]
- Bouchama A., Knochel J. P. 2002. Heat stroke. N. Engl. J. Med. 346:1978–1988. doi: 10.1056/NEJMra011089 [DOI] [PubMed] [Google Scholar]
- Bridges T. C., Turner L. W., Gates R. S. 1998. Economic evaluation of misting-cooling systems for growing/finishing swine through modeling. Appl. Eng. Agric. 14:425–430. doi: 10.13031/2013.19398 [DOI] [Google Scholar]
- Carr J. 1998. Basic haematology and biochemistry. In: Carr J. editor, Garth pig stockmanship standards. 5M Enterprises Ltd., Sheffield, UK: p. 64. [Google Scholar]
- Craig S. A. 2004. Betaine in human nutrition. Am. J. Clin. Nutr. 80:539–549. [DOI] [PubMed] [Google Scholar]
- Cronjé P. B. 2005. Heat stress in livestock – The role of the gut in its aetiology and a potential role for betaine in its alleviation. Recent Adv. Anim. Nutr. Aust. 15:107–122. [Google Scholar]
- Cruzen S. M., Boddicker R., Johnson T. P. 2015. Carcass composition of market weight pigs subjected to heat stress in utero or during growth. J. Anim. Sci. 93:2587–2596. doi: 10.2527/jas.2014-8347 [DOI] [PubMed] [Google Scholar]
- Dunshea F. R., Cadogan D. J., Partridge G. G. 2009. Dietary betaine and ractopamine combine to increase lean tissue deposition in finisher pigs, particularly gilts. Anim. Prod. Sci. 49:65–70. doi: 10.1071/EA08014 [DOI] [Google Scholar]
- European Union Commission 1998. Commission directive 98/64/EC. OJEC L257, vol. 41:14-28. [Google Scholar]
- Farooqi H. A. G., Khan M. S., Khan M. A., Rabbani M., Pervez K., Khan J. A. 2005. Evaluation of betaine and vitamin C in alleviation of heat stress in broilers. Int. J. Agric. Biol. 5:744–746. [Google Scholar]
- Geraert P. A., Padilha J. C., Guillaumin S. 1996. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: Growth performance, body composition and energy retention. Br. J. Nutr. 75:195–204. [DOI] [PubMed] [Google Scholar]
- Hall D. M., Baumgardner K. R., Oberley T. D., Gisolfi C. V. 1999. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 276:1195–1203. [DOI] [PubMed] [Google Scholar]
- Harpur R. P., Popkin J. S. 1965. Osmolality of blood and intestinal contents in the pig, guinea pig, and Ascaris lumbricoides. Can. J. Biochem. 43:1157–1169. doi: 10.1139/o65-128 [DOI] [PubMed] [Google Scholar]
- Hassan R. A., Ebeid T. A., Abd El-Lateif A. I., Ismail N. B. 2011. Effect of dietary betaine supplementation on growth, carcass and immunity of New Zealand White rabbits under high ambient temperature. Livest. Sci. 135:103–109. doi: 10.1016/j.livsci.2010.06.132 [DOI] [Google Scholar]
- He S., Zhao S., Dai S., Liu D., Bokhari S. G. 2015. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 86:897–903. doi: 10.1111/asj.12372 [DOI] [PubMed] [Google Scholar]
- Heath M. E. 1989. Effects of rearing temperature and level of food intake on organ size and tissue composition in piglets. Can. J. Physiol. Pharmacol. 67:526–532. doi: 10.1139/y89-084 [DOI] [PubMed] [Google Scholar]
- Hicks T. A., McGlone J. J., Whisnant C. S., Kattesh H. G., Norman R. L. 1998. Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. J. Anim. Sci. 76:474–483. [DOI] [PubMed] [Google Scholar]
- Houpt T. R. 2004. Water and electrolytes. In: Reece W. O. editor, Duke's physiology of domestic animals. Cornell Univ. Press, Ithaca, NY: p. 12–25. [Google Scholar]
- International Organization for Standardization (ISO) 2005. ISO 13903:2005(en): Animal feeding stuffs – Determination of amino acids content, https://www.iso.org/obp/ui/#iso:std:iso:13903:ed-1:v1:en. (Accessed 19 February 2016.)
- Kenward M. G., Roger J. H. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi: 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- Kettunen H., Tiihonen K., Peuranen S., Saarinen M. T., Remus J. C. 2001. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidia-infected broiler chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:759–769. doi: 10.1016/S1095-6433(01)00410-X [DOI] [PubMed] [Google Scholar]
- Kidd M. T., Ferket P. R., Garlich J. D. 1997. Nutritional and osmoregulatory functions of betaine. Worlds Poult. Sci. J. 53:125–139. doi: 10.1079/WPS19970013 [DOI] [Google Scholar]
- Kim B. G., Lindemann M. D. 2007. A new spreadsheet method for the experimental animal allotment. J. Anim. Sci. 85(Suppl. 2):112 (Abstr.) [Google Scholar]
- Kincaid C. 2005. Guidelines for selecting the covariance structure in mixed model analysis. Stat. Data Anal. 30:1–8. [Google Scholar]
- Klasing K. C., Adler K. L., Remus J. C., Calvert C. C. 2002. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increases functional properties of phagocytes. J. Nutr. 132:2274–2282. [DOI] [PubMed] [Google Scholar]
- Li Y., Cao Y., Zhou X., Wang F., Shan T., Li Z., Xu W., Li C. 2015. Effects of zinc sulfate pretreatment on heat tolerance of Bama miniature pig under high ambient temperature. J. Anim. Sci. 93:3421–3430. doi: 10.2527/jas.2015-8910 [DOI] [PubMed] [Google Scholar]
- Lim C. L., Wilson G., Brown L., Coombes J. S., Mackinnon L. T. 2007. Pre-existing inflammatory state compromises heat tolerance in rats exposed to heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R186–R194. doi: 10.1152/ajpregu.00921.2005 [DOI] [PubMed] [Google Scholar]
- Moeckel G. W., Shadman R., Fogel J. M., Sadrzadeh S. M. H. 2002. Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sci. 71:2413–2424. doi: 10.1016/S0024-3205(02)02035-0 [DOI] [PubMed] [Google Scholar]
- Nienaber J. A., Hahn G. L., McDonald T. P., Korthals R. L. 1996. Feeding patterns and swine performance in hot environments. Trans. ASAE 39:195–202. doi: 10.13031/2013.27498 [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Patience J. F., Umboh J. F., Chaplin R. K., Nyachoti C. M. 2005. Nutritional and physiological responses of growing pigs exposed to a diurnal pattern of heat stress. Livest. Prod. Sci. 96:205–214. doi: 10.1016/j.livprodsci.2005.01.012 [DOI] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., Baumgard L. H. 2013. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Gourdine J. L., St-Pierre N. R. 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Rhoads R. P., Baumgard L. H., Suagee J. K. 2013. Metabolic priorities during heat stress with an emphasis on skeletal muscle. J. Anim. Sci. 91:2492–2503. doi: 10.2527/jas.2012-6120 [DOI] [PubMed] [Google Scholar]
- Sales J. 2011. A meta-analysis of the effects of dietary betaine supplementation on finishing performance and carcass characteristics of pigs. Anim. Feed Sci. Technol. 165:68–78. doi: 10.1016/j.anifeedsci.2011.02.008 [DOI] [Google Scholar]
- Sandercock D. A., Hunter R. R., Nute G. R., Mitchell M. A., Hocking P. M. 2001. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: Implications for meat quality. Poult. Sci. 80:418–425. doi: 10.1093/ps/80.4.418 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., Baumgard L. H. 2015. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3:e12478. doi: 10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed M. A. M., Downing J. 2011. The effects of water replacement by oral rehydration fluids with or without betaine supplementation on performance, acid-base balance, and water retention of heat-stressed broiler chickens. Poult. Sci. 90:157–167. doi: 10.3382/ps.2009-00594 [DOI] [PubMed] [Google Scholar]
- Schrama J. W., Heetkamp M. J. W., Simmins P. H., Gerrits J. J. 2003. Dietary betaine supplementation affects energy metabolism of pigs. J. Anim. Sci. 81:1202–1209. [DOI] [PubMed] [Google Scholar]
- Shapiro A. S. S., Wilk M. B. 1965. An analysis of variance test for normality (complete samples). Biometrika 52:591–611. doi: 10.1093/biomet/52.3-4.591 [DOI] [Google Scholar]
- Stahly T. S., Cromwell G. L., Aviotti M. P. 1979. The effect of environmental temperature and dietary lysine source and level on the performance and carcass characteristics of growing swine. J. Anim. Sci. 49:1242–1251. doi: 10.2527/jas1979.4951242x [DOI] [Google Scholar]
- St-Pierre N. R., Cobanov B., Schnitkey G. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86(Suppl):E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Tormey W. P. 1997. Are the increasing clinical demands for osmolality measurements and their associated electrolytes appropriate? Ir. J. Med. Sci. 166:75–79. doi: 10.1007/BF02944191 [DOI] [PubMed] [Google Scholar]
- Verstegen M. W., Close W. H., Start I. B., Mount L. E. 1973. The effects of environmental temperature and plane of nutrition on heat loss, energy retention and deposition of protein and fat in groups of growing pigs. Br. J. Nutr. 30:21–35. doi: 10.1079/BJN19730005 [DOI] [PubMed] [Google Scholar]
- Waltz X., Baillot M., Connes P., Bocage B., Ranaudeau D. 2014. Effects of hydration level and heat stress on thermoregulatory responses, hematological and blood rheological properties in growing pigs. PLoS One 9:e102537. doi: 10.1371/journal.pone.0102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymouth C. 1970. Osmolality of mammalian blood and of media for culture of mammalian cells. In Vitro 6:109–127. doi: 10.1007/BF02616113 [DOI] [PubMed] [Google Scholar]
- Wettstein M., Haussinger D. 1997. Cytoprotection by the osmolytes betaine and taurine in ischemia-reoxygenation injury in the perfused rat liver. Hepatology 26:1560–1566. [DOI] [PubMed] [Google Scholar]
- Whalan J. E. 2015. A toxicologist's guide to clinical pathology in animals: Hematology, clinical chemistry, urinalysis. Springer International Publishing AG, Cham, Switzerland. doi: 10.1007/978-3-319-15853-2 [DOI] [Google Scholar]
- Xiang-hong J., Yan-hong Y., Han-jin X., Li-long A., Yingmei X. 2011. Impacts of heat stress on baseline immune measures and a subset of T cells in Bama miniature pigs. Livest. Sci. 135:289–292. doi: 10.1016/j.livsci.2010.07.009 [DOI] [Google Scholar]
- Zulkifli I., Mysahra S. A., Jin L. Z. 2004. Dietary supplementation of betaine (Betafin®) and response to high temperature stress in male broiler chickens. Asian-Australas. J. Anim. Sci. 17:244–249. [Google Scholar]