Abstract
Effects of a tannic acid blend (ByPro; Silvateam USA, Ontario, CA) added to steam-flaked corn–based fishing diets on beef cattle growth performance, carcass characteristics, nutrient digestibility, fecal N volatilization, and meat lipid oxidation were evaluated. Steers (n = 144; 349 ± 25 kg initial BW) were blocked by initial BW and assigned randomly to 1 of 3 treatments with 12 pens/treatment and 4 steers/pen and fed ad libitum. Treatments included a control (CON; no ByPro) and ByPro fed at 30 or 60 g DM/steer daily (30-ByPro and 60-ByPro, respectively). Pen fecal samples were collected 7 d after cattle were shipped to slaughter for estimation of N volatilization. Strip loins were aged for 21 d for evaluation of color and antioxidant activity. Intake quadratically increased (P = 0.05) from d 0 to 35, whereas linear trends were observed for increased DMI from d 0 to 105 and d 0 to slaughter (P = 0.07 and P = 0.06, respectively), resulting in a 3.7% greater overall DMI for 60-ByPro than for CON. No differences were detected for carcass-adjusted ADG (P = 0.65) or G:F (P = 0.17). Carcass characteristics including HCW (P = 0.52), fat thickness (P = 0.32), LM area (P = 0.57), quality grade (P = 0.44), yield grade (P = 0.29), and percentage of condemned livers (P = 0.13) were not affected by treatments. Apparent total tract digestibility of starch linearly decreased tendency (P = 0.03) with increasing ByPro dose, whereas tends for a linear decrease (P = 0.09) in CP and a quadratic increase (P = 0.09) in OM digestibility were observed. No effects of treatment (P ≥ 0.39) were noted for fecal N volatilization. An increase (P < 0.01) in metmyoglobin in strip loin steaks was observed with ByPro inclusion. Oxymyoglobin decreased (P < 0.01) as display day progressed, except on d 5, at which time CON and 30-ByPro steaks had lower proportions than 60-ByPro steaks. Only subtle changes in discoloration ratio and deoxymyoglobin were observed, whereas no effects (P ≥ 0.43) for pH or thiobarbituric acid reactive substances were noted. Feeding ByPro increased DMI during the first half of the feeding period without negatively affecting gain efficiency; however, fecal N retention was not altered by ByPro. ByPro did not negatively affect meat quality or carcass characteristics, and it did not seem to affect retail meat antioxidant activity.
Keywords: digestibility, feedlot cattle, lipid oxidation, tannic acid
INTRODUCTION
Tannins are found in many plants such as alfalfa and sorghum that are commonly included in ruminant diets (Frutos et al., 2004). They are a group of water-soluble, polyphenolic compounds that can complex with proteins, starches, and some vitamins and minerals. At a moderate pH, such as the ruminal environment, complexes are likely to be formed, whereas dissociation can occur at the lower pH of the abomasum and initial portion of duodenum (Porter, 1992). The tannins ability to precipitate proteins and other nutrients, if correctly dosed, could possibly increase the digestibility and efficiency of nutrient utilization, because they might escape ruminal digestion, thereby decreasing ruminal losses, and become available for digestion and absorption in the small intestine. Barajas et al. (2012a,b) and Rivera-Méndez et al. (2017) reported that a tannic acid blend (ByPro; Silvateam USA, Ontario, CA) increased ADG by 0.15 kg when included in a corn-based diet fed to yearling bulls and Holstein steers, respectively.
The ability of tannin to bind protein (Hagerman, 1992; McAllister et al., 2005) could also decrease fecal N volatilization, because some tannin–protein complexes might not completely dissociate during digestion. Tannins also could confer antioxidant activity as a result of their polyphenolic and flavonoid structure (Pennington and Fisher, 2009). Cyanidin, a form of tannin, has an oxygen radical absorbance capacity of approximately 4,500 μmol trolox equivalents/mmol (oxygen radical absorbance capacity), whereas ascorbic acid has an oxygen radical absorbance capacity of between 500 and 1,000 μmol trolox equivalents/mmol (Velasco and Williams, 2011), which suggests other tannins could have the ability to induce a similar effect. As a result of these potential biological actions, our objective was to evaluate the effects of the dose of a tannic acid blend (ByPro) on growth performance, carcass characteristics, apparent total tract nutrient digestibility, fecal N volatilization, and meat lipid oxidation in finishing beef steers fed a steam-flaked corn–based diet.
MATERIALS AND METHODS
All procedures involving live animals were approved by the Texas Tech University Animal Care and Use Committee (protocol number 13033-04).
Cattle Receiving and Processing
Medium- to large-framed beef steers (n = 162; British × Continental) were received on May 23 and 24, 2013, at the Texas Tech University Burnett Center near Idalou, TX. On arrival, the cattle were randomly allocated into receiving pens, individually identified with a unique ear tag, treated with an internal and external parasiticide (Dectomax; Zoetis Animal Health, Florham Park, NJ), vaccinated for respiratory disease pathogens (Bovi-Shield Gold 4; Zoetis Animal Health), and metaphylactically treated with tilmicosin phosphate (Micotil 300; Elanco Animal Health, Greenfield, IN). Before starting the study, steers were limit-fed (1.8% of BW) a 65% concentrate receiving diet (Table 1). After 7 d, cattle the received a clostridial and Pasteurella vaccination (One Shot Ultra 7; Zoetis Animal Health) and were treated for internal parasites (Safe-Guard; Merck Animal Health, Summit, NJ), implanted (Component TE-IS; Elanco Animal Health, Indianapolis, IN), and individually weighed. Based on this unshrunk BW, 18 steers (lightest/heaviest BW, poor temperament, and physical or health issues) were eliminated from the study pool. The remaining 144 steers (349 ± 25 kg initial BW) were stratified by BW and assigned to BW blocks (12 blocks). Steers were then physically sorted into the blocks and randomly assigned within BW strata to 1 of 3 pens (4 steers/pen; concrete, partially slotted floor; 3 m wide by 6 m deep; 2.5 m linear bunk space). Dietary treatments were randomly assigned to pens within each BW block. Steers were kept under observation with the same dietary regimen for 5 d after being placed in pens until initial BW measurements were taken, which corresponded to study initiation. Immediately after the initial BW measurement, a 75% concentrate step-up diet was fed followed by a second step-up diet (85% concentrate), until steers were finally transitioned to the 92% concentrate finishing diets. The 2 step-up diets contained the respective tannic acid (ByPro) treatments and were each fed for 4 d (Table 1).
Table 1.
Dietary ingredients and calculated nutritional composition of receiving and step-up diets fed to steers
| Adaptation diet | |||
|---|---|---|---|
| Item | Receiving(65% concentrate) | Step-up 2 (75% concentrate) | Step-up 3(85% concentrate) |
| Days on feed | 17 | 4 | 4 |
| Period included in the study | No | Yes | Yes |
| Ingredient, % DM | |||
| Steam-flaked corn (330 g/L) | 38.72 | 47.32 | 57.05 |
| Wet corn gluten feed (Sweet Bran1) | 20.00 | 20.00 | 20.00 |
| Alfalfa hay | 17.50 | 12.50 | 7.50 |
| Cottonseed hulls | 17.50 | 12.50 | 7.50 |
| Yellow grease | 3.00 | 3.00 | 3.00 |
| Supplement2 | 2.00 | 2.00 | 2.00 |
| Urea | 0.38 | 0.48 | 0.60 |
| Limestone | 0.90 | 1.20 | 1.35 |
| Premix3 | 0 | 1.00 | 1.00 |
| Calculated composition,4 DM basis | |||
| DM, % as-is basis | 82.1 | 80.1 | 79.7 |
| CP | 13.5 | 13.5 | 13.5 |
| Ca | 0.61 | 0.64 | 0.61 |
| P | 0.36 | 0.36 | 0.36 |
| K | 0.98 | 0.89 | 0.79 |
| S | 0.21 | 0.21 | 0.21 |
| NEm, Mcal/kg | 1.81 | 1.93 | 2.06 |
Cargill Corn Milling, Blair, NE.
Supplement contained (DM basis) 67.7538% carrier (cottonseed meal), 0.5% antioxidant (Endox; Kemin Industries, Inc., Des Moines, IA), 3.76% urea, 10% potassium chloride, 15% sodium chloride, 0.0022% cobalt carbonate, 0.1965% copper sulfate, 0.0833% iron sulfate, 0.0031% ethylenediamine dihydroiodide, 0.167% manganous oxide, 0.125%, 0.9859% zinc sulfate, 0.0099% vitamin A (1,000,000 IU/g), and 0.157% vitamin E (500 IU/g) and provided (dietary) 30 mg/kg of monensin (0.75% Rumensin-90 in supplement; Elanco Animal Health, Indianapolis, IN) and 9 mg/kg of tylosin (0.5063% Tylan-40 in supplement; Elanco Animal Health).
Premix contained the following: cottonseed meal for the control diet, 0.33% tannic acid (ByPro; Silvateam USA, Ontario, CA) for the 30 g DM of ByPro/steer daily diet, and 0.66% tannic acid (ByPro) for the 60 g DM of ByPro/steer daily diet. Cottonseed meal was used as the carrier for the ByPro premixes.
Calculated composition using tabular values (NRC, 1996).
Experimental Design and Dietary Treatments
Treatments consisted of 1) control (CON), no tannic acid blend (premix added to the diet contained only carrier [cottonseed meal]); 2) tannic acid to provide an estimated intake of 30 g DM of ByPro/steer daily (30-ByPro); and 3) tannic acid to provide an estimated intake of 60 g DM of ByPro/steer daily (60-ByPro). Dietary treatments were based on a series of research trials by Barajas et al. (2011a, 2012a,b), which included 30 g DM/animal daily of ByPro. The 3 dietary treatments were arranged in a randomized complete block design. Pen (4 steers/pen) was the experimental unit, with 1 pen per treatment within each BW block (12 blocks), resulting in 12 pens/treatment and 36 experimental units. Ingredients and dietary analyzed nutritional composition of the 3 treatment diets are provided in Table 2. Each treatment diet contained the same mineral and vitamin supplement to meet or exceed NRC (1996) requirements, monensin (33 mg/kg, DM basis), and tylosin (9 mg/kg, DM basis). The ByPro premix mixtures were prepared in batches to supply quantities to last 15 to 20 d. Desired ByPro intake (0, 30, and 60 g of DM/animal daily) was divided by the assumed DMI of 9,070 g to calculate the percentage inclusion of ByPro in dietary premixes (0, 0.33, and 0.66% of dietary DM, respectively; Tables 1 and 2).
Table 2.
Dietary ingredient inclusion and analyzed nutritional composition of finishing diets containing tannic acid (ByPro1) fed to steers
| Finishing diet2 | |||
|---|---|---|---|
| Item | Control | 30-ByPro | 60-ByPro |
| Ingredient, % DM | |||
| Steam-flaked corn | 63.72 | 63.72 | 63.72 |
| Wet corn gluten feed (Sweet Bran3) | 20.00 | 20.00 | 20.00 |
| Alfalfa | 4.00 | 4.00 | 4.00 |
| Cottonseed hulls | 4.00 | 4.00 | 4.00 |
| Yellow grease | 3.00 | 3.00 | 3.00 |
| Supplement4 | 2.00 | 2.00 | 2.00 |
| Urea | 0.68 | 0.68 | 0.68 |
| Limestone | 1.60 | 1.60 | 1.60 |
| Premix5 | 1.00 | 1.00 | 1.00 |
| Analyzed composition,6 % DM | |||
| DM, % | 78.7 | 78.4 | 78.1 |
| CP | 13.5 | 13.2 | 13.6 |
| NDF | 20.8 | 22.3 | 21.1 |
| ADF | 11.9 | 12.3 | 11.4 |
| EE7 | 5.1 | 5.4 | 5.2 |
| Ash | 5.7 | 5.7 | 5.6 |
| Ca | 0.65 | 0.74 | 0.69 |
| P | 0.38 | 0.43 | 0.43 |
| K | 0.74 | 0.85 | 0.82 |
| S | 0.17 | 0.19 | 0.18 |
| NEm,8 Mcal/kg | 2.14 | 2.14 | 2.13 |
| NEg,8 Mcal/kg | 1.47 | 1.46 | 1.46 |
Silvateam USA, Ontario, CA.
30-ByPro = 30 g DM of ByPro/steer daily; 60-ByPro = 60 g DM of ByPro/steer daily.
Cargill Corn Milling, Blair, NE.
Supplement contained (DM basis) 67.7538% carrier (cottonseed meal), 0.5% antioxidant (Endox; Kemin Industries, Inc., Des Moines, IA), 3.76% urea, 10% potassium chloride, 15% sodium chloride, 0.0022% cobalt carbonate, 0.1965% copper sulfate, 0.0833% iron sulfate, 0.0031% ethylenediamine dihydroiodide, 0.167% manganous oxide, 0.125% selenium premix (0.2% Se), 0.9859% zinc sulfate, 0.0099% vitamin A (1,000,000 IU/g), and 0.157% vitamin E (500 IU/g) and provided (dietary) 30 mg/kg of monensin (0.75% Rumensin-90 in supplement; Elanco Animal Health, Indianapolis, IN) and 9 mg/kg of tylosin (0.5063% Tylan-40 in supplement; Elanco Animal Health).
Premix contained cottonseed meal for the control diet, 0.33% tannic acid (ByPro) for the 30-ByPro diet, and 0.66% tannic acid (ByPro) for the 60-Bypro diet. Cottonseed meal was used as the carrier for the ByPro premixes.
Analyzed composition from a commercial laboratory (Servi-Tech Laboratories, Amarillo, TX) with the exception of CP, which was analyzed using the Kjeldahl method.
EE = ether extract.
Calculated composition using tabular values (NRC, 1996).
Management, Feeding, and Weighing Procedures
Standard procedures for feed mixing and delivery at the Burnett Center were used throughout the study. All low-moisture ingredients from diets were mixed in a 1.27 m3–capacity paddle mixer. The Burnett Center feed milling system is operated by a computer-controlled batching system. Once the total amount of low-moisture ingredients for a given treatment was mixed, the feed was conveyed to a Roto-Mix 84-8 (Roto-Mix, Dodge City, KS) mixer/delivery wagon, where the high-moisture ingredient (wet corn gluten feed) was mixed. Diet samples were obtained on a weekly basis for all treatment diets. Samples were collected as feed was discharged into the bunk by the Roto-Mix 84-8 unit or taken directly from the feed bunk soon after feed has been delivered. Subsamples from the same dietary treatment were composited to approximately 400 g. One portion of the sample was stored frozen for subsequent determination of nutritional composition (dried at 60°C for 48 h in a forced-air drying oven), whereas another portion was dried at 100°C for 24 h (forced-air drying oven) to determine the DM content of the diet. Frozen samples were composited by treatment diet for each 35-d period of the study. Samples (60°C) were ground to pass a 1-mm screen in a Wiley mill (Thomas Scientific, Swedesboro, NJ), and overall composites were analyzed for DM, ash, CP, NDF, ADF, Ca, P, K, S, and ether extract (Table 2) by a commercial laboratory (Servi-Tech Laboratories, Amarillo, TX) by AOAC International (2005) methods, with the exception of CP, which was analyzed using the Kjeldahl method (method 976.05; AOAC, 1995) in the Texas Tech University Ruminant Nutrition Laboratory (Lubbock, TX).
Feed bunks were inspected visually (read) once daily at approximately 0700 to 0800 h. Feed was allotted to pens so that 0 to 0.5 kg of feed remained in the bunk at the time of bunk reading. All cattle were fed once daily in the morning. At the time that bunks were read, a computer-generated feed bunk sheet provided the delivery amounts for the previous 3 d, so the amount to feed the pen for the current day was based on the previous pattern of consumption and any feed remaining in the bunk. When feed allotted to a pen needed to be decreased, the decrease was based on a visual estimate of feed remaining in the bunk at the time of the bunk reading. When feed to a pen was decreased, the bunk reading process on the following day took into account the feed offered on the previous day plus the estimated feed remaining in the bunk on the previous day. The weekly dietary DM values (100°C) during intermediate periods of the study were used in conjunction with feed bunk orts samples to determine the feed intake by the animals in each pen.
Initial BW (individual) was taken on d 0 (scale was validated with 454 kg of certified weights). Intermediate BW measurements were obtained on a pen basis (scale readability ±2.3 kg; scale validated with 907 kg of certified weights before use) using a platform scale at 35-d intervals throughout the study (d 35, 105, and 140). Individual BW measurements were taken on d 70 at when cattle were reimplanted (Component TE-S; Elanco Animal Health) and at shipment for slaughter. On d 70, steers also received an external parasiticide (Ultra Boss Permethrin; Schering-Plough Animal Health, Kenilworth, NJ). All scales were validated with at least 454 kg of certified weights before use.
Slaughter and Carcass Measurements
Cattle in the various BW blocks were shipped to a commercial slaughter facility (Tyson Fresh Meats, Amarillo, TX) when approximately 65% or more animals in a given block had sufficient visual finish to grade USDA Choice. The heaviest 3 blocks of steers were shipped after 121 d on feed and the next 5 heaviest blocks after 156 d on feed, with the remaining 4 lightest blocks shipped after 175 d on feed. Personnel from West Texas A&M University (Canyon, TX) collected HCW and liver abscess data, and the in-plant camera system was used to determine yield grade, quality grade, marbling score, LM area, and 12th-rib fat. Liver condemnations were classified for liver abscesses using methods described by Brink et al. (1990). Dressing percent was calculated by dividing HCW by nonshrunk final BW. Carcass-adjusted BW was calculated from HCW divided by the average dressing percent across treatments (62.14%) and adjusted by a 4% shrink. Carcass-adjusted ADG was calculated from carcass-adjusted final shrunk BW, initial BW, and days on feed, and carcass-adjusted G:F was calculated as carcass-adjusted ADG divided by the average DMI for the experimental period.
Carcasses from the first (n = 32) and second (n = 30) slaughter group grading USDA Select and Choice (n = 6 Select in slaughter group 2 only; n = 56 Choice) were identified by personnel from the West Texas A&M Beef Carcass Research Center (Canyon, TX). One strip loin from each carcass was identified and collected during fabrication approximately 48 h after slaughter. Following fabrication, strip loins were individually vacuum packaged and boxed for shipment to Texas Tech University (Lubbock, TX). Strip loins were maintained at 2 to 4°C until 7 d postmortem, when they were further processed.
Apparent Digestibility Evaluation
Starting on d 90, a 5-d digestibility experiment was conducted. Samples of the experimental diets and feed bunk refusals were collected daily from d 90 through 96. Two samples were taken, 1 for analysis of DM and the other was frozen for nutritional analyses. Fecal samples from at least 2 animals per pen (typically collected from all 4 animals) were collected from the concrete pen floor immediately after defecation. Collection of fecal samples occurred twice daily at approximately 0800 and 1600 h on d 92 through 96. Fecal samples were frozen and individually stored after each collection day. On the last day of collection, samples were sorted by pen and day of collection and thawed overnight. The next morning, samples were homogenized inside each collection bag and then composited by pen (100 ± 0.5 g of wet sample), generating a total of 36 samples. Composite fecal samples (500 g) were dried in forced-air oven at 55°C for 72 h. Feed samples (composited by treatment; 3 samples) and feed refusals (composited by pen; 36 feed refusals) were dried at 55°C for 48 h. Feed, refusals, and fecal samples were ground in a Wiley mill (Thomas Scientific, Swedesboro, NJ) to pass a 1-mm screen. Daily refusals from the digestibility evaluation were composited by percentage of weight (10%) recovered from the bunk from each morning before feeding, homogenized, and then subsampled following procedures previously described. Acid-insoluble ash was used as internal maker to estimate total fecal output and consequent apparent nutrient digestibility coefficients (Van Keulen and Young, 1977). Apparent total tract digestion of DM, OM, CP, and starch were calculated as follows: 100 − 100 × [(AIA concentration in feed/AIA concentration in feces) × (nutrient concentration in feces/nutrient concentration in feed)]. Nutrient concentrations were corrected for orts (i.e., orts-corrected quantity of nutrient consumed divided by orts-corrected quantity of DM consumed).
Fecal Nitrogen Volatilization
After animals were shipped to the slaughter facility, feces on the pen surface were allowed to air-dry for 7 d, after which approximately 500 g of feces (as-is basis) were collected from the driest surface area of the pens. Samples were ground in a Wiley mill to pass a 1-mm screen and then stored for further analyses. Fecal N volatilization was calculated as described by Cole et al. (2006) as follows: N volatilization (% of intake) = (N:P ratio of diet − N:P ratio of manure)/(N:P ratio of diet), N volatilization (g/d) = [(N volatilization (% of intake))/100] × N intake (g/d), and N volatilization (kg/steer) = [(N volatilization (g/steer daily)) × days on feed]/1,000.
Laboratory Analyses of Diets, Orts, Feces, and Pen Surface Samples
Diets composited by period were sent to a commercial laboratory (Servi-Tech Laboratories) for chemical analyses, and results are shown in Table 2. Samples of diets, orts, and feces collected during the digestion and were analyzed for AIA, total starch, N, OM, NDF, and P. Pen surface air-dried fecal samples from the N volatilization phase were analyzed only for N and P. Total starch and P contents were analyzed by a commercial laboratory (Servi-Tech Laboratories). For N determination, approximately 0.5 ± 0.005 g of ground feed and orts and 0.3 ± 0.005 g of fecal samples were analyzed using the combustion method (model TruMac N [Leco, St Joseph, MI]; method 4.2.10 [AOAC, 1997]). Ash content was determined by burning each sample in a muffle furnace at 600°C for 4 h and used to determine OM (method 942.05; AOAC, 1995). Acid-insoluble ash was analyzed as described by Van Keulen and Young (1977) using the 2 N HCl method, as follows: 5 g of sample (diets, feces, and orts) were weighed and dried (forced-air oven) for 2 h at 135°C. Dry matter content was calculated followed by ash content (450°C overnight). Ash was combined with 100 mL of 2 N HCl in a 600-mL beaker and boiled for 5 min under a fume hood. Contents were transferred (100°C deionized water) onto ashless filter paper. Filters were ashed overnight at 450°C, and residue after incineration (AIA) was gravimetrically estimated.
Fiber content (NDF and ADF) was determined according to Van Soest et al. (1991) with the modification proposed in the Ankom device manual (ANKOM Technology Method; ANKON Technology Corp., Macedon, NY) using sodium sulfite and α-amylase and correcting for ash.
Strip Loin Analyses
Proximate Composition
At the time of subprimal processing, the anterior portion (approximately first anterior 5.1 cm) of each subprimal was reserved for analysis of proximate composition. Samples were individually packaged and frozen (−20°C) until later analyses. Expanded polystyrene trays containing meat samples were overwrapped with polyvinyl chloride (PVC) film (MAPAC L; oxygen transmission rate = 21,700 cm3 of O2 per m2 per 24 h; Borden Packaging and Industrial Products, North Andover, MA) using a heat-sealing overwrap machine (Heat Sealing Equipment Co., Cleveland, OH). Before analyses, sections were thawed, trimmed of external fat, and ground using a commercially available grinder (Kitchen Aid with grinder adaptor, model KP26M1XER Professional 600; KitchenAid, Benton Harbor, MI) to obtain approximately 200 g of sample. Compositional analysis of moisture, fat, protein, and collagen was conducted using an AOAC-approved (method 2007.04 was conducted as described by Anderson, 2007) near-infrared spectrophotometer (FOSS Food Scan 78800; FOSS NIRSystems, Inc., Laurel, MD).
Raw Color Analysis
Following removal of a section for proximate analysis, a 2.54-cm thick steak was removed for evaluation of retail display color. Single steaks were placed on black, expanded polystyrene trays (Cryovac Ltd., Duncan, SC). Trays were overwrapped with PVC film (MAPAC L; oxygen transmission rate = 21,700 cm3 of O2 per m2 per 24 h; Borden Packaging and Industrial Products) using a heat-sealing overwrap machine (Heat Sealing Equipment Co.). Immediately following packaging, trays were placed in coffin-style retail display cases (model H1; Hussmann Corp., Bridgeton, MO) under continuous fluorescent lighting (1,900 lux) using high-output bulbs with a color temperature rating of 3,500° K and a color rendering index of 70. Packages were kept in the retail case for 7 d at 0 to 2°C. Each day for 7 d, instrument color (CIE L*, a*, and b*) of strip steak packages was measured through the packaging film at 3 different locations across the package surface using a portable spectrophotometer (Hunter Miniscan XE Plus, model MSXP-4500C; Hunter Associates Laboratory, Inc., Reston, VA) with illuminant A, a standard observer angle of 10°, and 2.54-cm aperture (CIE, 1978). Instrument calibration was completed before use at each sampling period using black and white tiles covered with PVC packaging film (AMSA, 2012). The CIE L*, a*, and b* values were used to calculate hue angle (tan−1 a*/b*) and saturation index[(a*2 + b*2)1/2] for each sample. The observations (3 observations) were averaged before statistical analyses.
Reflectance values for calculation of relative pigment proportions (% oxymyoglobin, deoxymyoglobin, and metmyoglobin) were obtained using methods developed by Krzywicki (1979) as described by the American Meat Science Association (2012). Briefly, the portable spectrophotometer previously described was used to measure reflectance at 470, 525, 575, 580, 610, 630, 680, 700, and 730 nm on 3 locations on the loaf. The reflex attenuance of each wavelength was obtained by calculating the logarithm of the reciprocal of the reflectance. The reflex attenuance values for 470, 525, 575, and 730 nm were used to calculate the relative proportion (%) of metmyoglobin, deoxymyoglobin, and oxymyoglobin using formulas summarized by the American Meat Science Association (2012). Furthermore, a discoloration:redness ratio was calculated from the reflectance values at 580 and 630 nm as described by the American Meat Science Association (2012). This ratio is indicative of the relative proportion of oxymyoglobin, the predominant pigment at 630 nm, to metmyoglobin, which is predominant at 580 nm. Therefore, values closer to 1.0 are indicative of discoloration.
Lipid Oxidation and pH
Samples for evaluation of thiobarbituric acid reactive substances (TBARS) were obtained at the time of subprimal portioning. At portioning, a sample was obtained from the anterior end of the subprimal, frozen in liquid nitrogen, placed in an individually labeled sealable plastic sample bag, and stored (−80°C) until later analyses. Immediately before analysis, samples were homogenized using a commercial blender and liquid nitrogen. Approximately 10 g of the homogenate was added to a 50-mL centrifuge tube, and analysis of TBARS proceeded as described by Luque et al. (2011). Samples were evaluated in duplicate and averaged before statistical analysis. Meat pH was measured using a 50-g sample as described by Luque et al. (2011).
Statistical Analyses
Growth performance and apparent total tract nutrient digestibility data were analyzed with pen as the experimental unit in a randomized complete block design using the GLIMMIX procedures of SAS (SAS Inst., Inc., Cary, NC). The model included the fixed effect of treatment and random effect of block. Least squares mean differences were adjusted using the Tukey test, and bias of degrees of freedom was adjusted using the Kenward–Roger method. Carcass data (USDA quality grade and liver scores) were entered on an individual animal basis, with the model including the same effects as previously described. Because of the nature of carcass data distribution (binomial), the link function of the GLIMMIX procedure of SAS was used for data analysis of treatment effects, and the inverse link function was used for reporting of responses. Preplanned linear and quadratic contrasts for ByPro inclusion (0, 30, and 60 g DM of ByPro/steer daily) were evaluated. Data from evaluations of meat quality traits were analyzed using a linear mixed model (GLIMMIX). ByPro treatments, retail display day (where appropriate), and their interaction served as independent variables of interest. Slaughter group (2 groups) was used as random variable, whereas carcass quality grade was incorporated as a covariate. Least squares means, generated for independent variables and their interaction (where appropriate), were separated using the PDIFF option and differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Growth Performance
Feedlot performance data are shown in Table 3. Cumulative data are shown only for d 0 to 35, d 0 to 70, d 0 to 105, and d 0 to 140 because of the different days on feed for the 3 slaughter groups after d 121.
Table 3.
Effects of ByPro1 (tannic acid) dose (g/steer daily) in steam-flaked corn–based diets on beef cattle growth performance
| ByPro dose | P-value3 | |||||
|---|---|---|---|---|---|---|
| Item | Control | 30 g | 60 g | SEM2 | Linear | Quadratic |
| Average days on feed | 154 | 154 | 154 | – | – | – |
| Initial BW, kg | 349 | 349 | 349 | 7.51 | 0.38 | 0.33 |
| FsBW,4 kg | 598 | 599 | 601 | 6.09 | 0.72 | 0.90 |
| Adj.FsBW,5 kg | 598 | 602 | 599 | 5.91 | 0.87 | 0.53 |
| DMI, kg/d | ||||||
| d 0 to 35 | 8.99 | 9.24 | 9.14 | 0.08 | 0.14 | 0.05 |
| d 0 to 70 | 9.57 | 9.82 | 9.82 | 0.15 | 0.2 | 0.47 |
| d 0 to 105 | 9.71 | 9.99 | 10.09 | 0.18 | 0.07 | 0.63 |
| d 0 to 140 | 9.82 | 9.99 | 10.02 | 0.15 | 0.38 | 0.73 |
| d 0 to end | 9.90 | 10.16 | 10.27 | 0.16 | 0.06 | 0.65 |
| ADG, kg | ||||||
| d 0 to 35 | 2.07 | 2.14 | 2.13 | 0.05 | 0.44 | 0.33 |
| d 0 to 70 | 1.82 | 1.90 | 1.90 | 0.05 | 0.16 | 0.53 |
| d 0 to 105 | 1.87 | 1.90 | 1.94 | 0.04 | 0.17 | 0.89 |
| d 0 to 140 | 1.84 | 1.86 | 1.87 | 0.03 | 0.52 | 0.96 |
| d 0 to end | 1.62 | 1.64 | 1.65 | 0.04 | 0.51 | 0.98 |
| Adj. d 0 to end6 | 1.62 | 1.66 | 1.64 | 0.04 | 0.65 | 0.38 |
| G:F | ||||||
| d 0 to 35 | 0.23 | 0.232 | 0.233 | 0.005 | 0.75 | 0.99 |
| d 0 to 70 | 0.190 | 0.193 | 0.194 | 0.004 | 0.45 | 0.81 |
| d 0 to 105 | 0.193 | 0.19 | 0.192 | 0.003 | 0.84 | 0.50 |
| d 0 to 140 | 0.187 | 0.186 | 0.187 | 0.003 | 0.87 | 0.76 |
| d 0 to end | 0.164 | 0.161 | 0.161 | 0.002 | 0.33 | 0.71 |
| Adj. d 0 to end6 | 0.163 | 0.163 | 0.159 | 0.002 | 0.17 | 0.51 |
Silvateam USA, Ontario, CA.
n = 12.
P-values for the linear and quadratic effects of ByPro dose.
FsBW = final shrunk BW. A 4% shrink was applied to final live BW.
Adj.FsBW = carcass-adjusted FsBW, calculated from HCW divided by the average dressing percent across treatments (62.14%) and adjusted by a 4% shrink.
Adj. = carcass-adjusted; carcass-adjusted ADG and G:F from Adj.FsBW, initial BW, and days on feed.
Dry matter intake quadratically increased (P = 0.05) from d 0 to 35 with inclusion of ByPro. From d 0 to 105 (P = 0.07) and from d 0 to the end, DMI tended (P = 0.06) to linearly increase; overall DMI was 3.7% greater for steers fed 60-ByPro than for CON steers. No effects were observed for ADG during the current study (P ≥ 0.16), and ByPro inclusion did not affect overall (d 0 to end) carcass-adjusted ADG (P = 0.65) or G:F (P ≥ 0.17). The G:F for d 0 to 35 (P = 0.95), d 0 to 70 (P = 0.72), d 0 to 105 (P = 0.78), d 0 to 140 (P = 0.94), and d 0 to the end also did not differ among treatments (P = 0.58). With few numerical differences, current ADG results did not follow those of Barajas et al. (2012a). Cattle from current study were castrated and implanted, whereas Barajas et al. (2012a) fed intact males. A lower roughage concentration (8 vs. 11.3%) and the use of steam-flaked corn in our study instead of ground sorghum also could have played a role in the different findings between studies. A longer feeding period (156 vs. 56 d) and overall heavier final BW (600 vs. 456 kg) were also important differences between the current study and the work of Barajas et al. (2012a). Moreover, the sorghum grain fed in the study by Barajas et al. (2012b) could have affected the overall dietary tannin concentration, because sorghums might also contribute dietary tannins. Based on the DMI of bulls in Barajas et al. (2012a), bulls could have been consuming as much as 10.2 g more tannin per kilogram of DM daily than what was estimated by the authors (considering possible contributions from the supplement and grain sorghum; assuming average tannin concentration in sorghum grain [average 0.55%, range of 0.1 to 1.2%] as reported by Sedghi et al., 2012).
The findings in the current study also conflicted with the findings of another study performed by Barajas et al. (2012b), in which yearling bulls were fed dry-ground corn–based diets with a tannin inclusion level of 0.33% of dietary DM. These authors noted an increase in ADG of 0.187 kg/d, and bulls fed the tannin blend also finished 14.7 kg heavier than bulls fed the control (no tannin blend) diet. In contrast to our study, Barajas et al. (2012b) fed the bulls for an 84-d finishing period compared with an average of 156 d for steers in our study. Diets also differed in ingredient composition; Barajas et al. (2012b) fed a ground corn–based diet with an average of 36.6% inclusion of ground corn over the trial. Moreover, for the first 21 d, an average of 27.7% corn silage was included in the diets. Barajas et al. (2012b) also used only plant-based protein sources (canola meal and dry distiller's grains) with no urea added. Their diets contained dry distiller's grains with solubles (average of 12.59%, DM basis) and corn straw as a roughage source (average of 11.09%, DM basis).
The results of an additional study by Barajas et al. (2011a) investigating the length time of inclusion of ByPro on performance of finishing bulls indicated that feeding bulls a 96% concentrate, dry distiller's grain with solubles, and ground corn–based diet for 0, 68, and 100% of 102 d yielded an increase in final BW (15 kg) for cattle fed the entire finishing phase. Cattle in the study conducted by Barajas et al. (2011a) were Bos indicus × Bos taurus bulls that were fed in Mexico. There were 20 bulls in each treatment group (366 kg average initial BW), separated into pens of 5 each. The authors also observed improvements of 0.155 kg/d for ADG and 10.9 kg of HCW for cattle fed the longest amount of time compared with the controls. Dry matter intake decreased by 0.62 kg/d, resulting in G:F being increased by 0.015 when ByPro was fed 68 and 100% of the feeding period compared with animals that were not fed ByPro. In contrast to the results of Barajas et al. (2011a), ADG and G:F in our study were not affected, although DMI tended to increase with ByPro.
A study that evaluated the influence of continuously feeding 28.14 g/animal daily of ByPro in ground corn–based diets to finishing bulls (B. indicus × B. taurus; 184 kg average initial BW) for a 226-d finishing trial (Barajas et al., 2011b) resulted in an increase in final BW by nearly 37 kg and an increase in ADG of 0.162 kg/d. Diets in this study contained 37.7% corn straw for the first 28 d, 30.4% corn straw from d 29 to 105, 18.1% corn straw from d 106 to 133, and 14.0% corn straw from d 134 to the end of the trial. In contrast, steers in the current study received a 12.5% inclusion of alfalfa hay and cottonseed hulls for the first 4 d of the finishing period, 7.5% alfalfa and cottonseed hulls for d 5 to 8, and then only 4.0% alfalfa and cottonseed hulls for the remainder of the feeding trail. This major difference in roughage concentration between the study of Barajas et al. (2011b) and the current study could explain the differences in performance observed between the studies. The ADG in the study of Barajas et al. (2011b) was 1.36 and 1.53 kg/d for control and treated cattle, respectively, resulting in an increase in ADG of 0.25 kg/d for ByPro cattle vs. the controls. For the current study, ADG was 1.62, 1.66, and 1.64 kg/d for the CON, 30-ByPro, and 60-ByPro diets, respectively. Major differences in roughage concentration, protein source, and length of feeding period between current study and studies conducted by Barajas et al. (2011a, 2012a,b) could account for the inconsistent responses noted among these reports. Perhaps the protein precipitation effect induced by tannins would have a greater effect when true protein sources are used rather than NPN, combined with a lower availability of carbohydrate sources. Ruminal microbiota could also adapt to effects of tannins, minimizing the treatment effect, which partially justifies improvements in growth performance in the relatively shorter-term studies of Barajas et al. (2011a,b, 2012a,b) and Rivera-Méndez et al. (2017) as well as the numerical advantage observed for the ByPro treatments in ADG from d 0 to 70 and from d 0 to 105 in the present study. These differences coupled with greater DMI observed for steers fed ByPro during the first half of the current study suggest a greater potential for use of ByPro in earlier stages of the feeding phase and perhaps in diets with less extensively processed grain rather than the steam-flaked corn fed in the current study.
Despite the greater intake and only slightly greater gain until d 105, the feed efficiency for steers fed ByPro in our study was not negatively affected, again suggesting the opportunity to use ByPro during earlier portions of the feeding period. This hypothesis is supported by the fact that positive gain effects of ByPro on bulls were observed when animals were slaughtered earlier in terms of BW and days on feed compared with the current study (Barajas et al., 2011a, 2012a,b).
Carcass Characteristics
Carcass measurements are shown in Table 4. Carcass characteristics including HCW (P = 0.52), 12th-rib fat thickness (P = 0.32), LM area (P = 0.57), and yield grade (P = 0.29) were not affected by dietary inclusion of ByPro. No differences in quality grade (P = 0.44) were observed among the 3 treatments, with cattle grading, on average, 87% Premium Choice and Choice. Despite slight numerical differences, dressing percent was not affected by dietary treatments (quadratic, P = 0.19) nor were KPH (P = 0.12) or liver scores (P = 0.13). Although not statistically significant (quadratic, P = 0.13), the liver score for steers fed 30-ByPro were 9.5 percentage units lower than the average of both CON and 60-ByBro treatments (17.8%).
Table 4.
Effects of ByPro1 (tannic acid) dose (g/steer daily) in steam-flaked corn–based diets on beef cattle carcass characteristics
| ByPro dose | P-value3 | |||||
|---|---|---|---|---|---|---|
| Item | Control | 30 g | 60 g | SEM2 | Linear | Quadratic |
| HCW, kg | 387 | 390 | 387 | 3.82 | 0.86 | 0.52 |
| Dressing percent4 | 62.08 | 62.42 | 61.91 | 0.28 | 0.66 | 0.19 |
| 12th-rib fat, cm | 1.48 | 1.55 | 1.46 | 0.10 | 0.87 | 0.43 |
| LM area, cm2 | 93.4 | 92.7 | 94.6 | 2.20 | 0.57 | 0.58 |
| Marbling score5 | 48.4 | 47.8 | 48.7 | 1.18 | 0.87 | 0.55 |
| KPH | 2.0 | 2.1 | 2.1 | 0.03 | 0.38 | 0.12 |
| Calculated yield grade | 3.0 | 3.1 | 2.9 | 0.18 | 0.73 | 0.29 |
| Quality grade6 | ||||||
| Premium Choice, % | 32.0 | 40.1 | 35.1 | 8.09 | 0.76 | 0.44 |
| Choice, % | 52.0 | 48.8 | 52.1 | 8.33 | 0.99 | 0.72 |
| Upper Choice,7 % | 85.5 | 90.3 | 88.6 | 5.58 | 0.62 | 0.55 |
| Select, % | 14.5 | 9.7 | 11.4 | 5.58 | 0.54 | 0.61 |
| Liver score | ||||||
| Total,8 % | 17.0 | 8.3 | 18.5 | 5.9 | 0.85 | 0.13 |
| Abscessed (A+), % | 4.3 | 4.2 | 6.2 | 3.6 | 0.68 | 0.80 |
| Abscessed (A), % | 7.7 | 3.7 | 11.3 | 4.8 | 0.5 | 0.17 |
Silvateam USA, Ontario, CA.
n = 12.
P-values for the linear and quadratic effects of ByPro dose.
Dressing percent was calculated using nonshrunk final BW/HCW.
30 = sight; 40 = small; 50 = modest; 60 = moderate; 70 = slightly abundant.
Quality grade, as determined by USDA personnel.
Includes Prime, Premium Choice, and Choice.
Includes A+, A, A−, and condemned or flukes.
In contrast to our findings, Camacho et al. (2011) observed that feeding ByPro at an inclusion rate of 0.32% of DM to B. indicus × B. taurus bull calves (n = 20; 184 kg initial BW) resulted in a 36.8-kg increase in final BW for the calves fed the tannin-containing diet compared with the controls. In this same study, HCW was increased by 25.4 kg vs. the controls and LM area also increased (3.45 cm2 greater than controls) when the tannin blend ByPro was added to ground corn–based diets fed to bull calves. Similar to the current study, Camacho et al. (2011) observed that inclusion of ByPro did not affect dressing percent, 12th-rib fat, KPH, or marbling score. Diets fed by Camacho et al. (2011) were based on dry-ground corn, canola meal, and dry distiller's grains with solubles, which are substantially different from the steam-flaked corn–based diets with wet corn gluten feed that were fed in the current study. Moreover, no extra NPN source was fed by Camacho et al. (2011), in contrast to the inclusion of urea in the current study.
Krueger et al. (2010) observed a 9.3-kg increase in HCW for steers fed either chestnut or mimosa tannins compared with the controls, which contrasts the absence of effects on HCW observed in the current study. Nonetheless, similar to the present study, Krueger et al. (2010) did not detect differences in carcass characteristics among treatments. Krueger et al. (2010) fed sorghum hay at an inclusion of 7.1% (DM basis), which probably did not influence tannin concentration in diets, because tannins are more concentrated in the grain fraction of sorghum (Van Soest, 1994). In addition, for the Krueger et al. (2010) study, the amount of tannins fed was greater than in the present study (108 vs. 30 or 60 g/animal daily, respectively). Moreover, cattle were slaughtered at a lighter weight, with HCW averaging 280 kg compared with an average HCW of 388 kg in our study. The smaller yield and lower quality grades reported by Krueger et al. (2010) compared with current study also reflects fewer days on feed, which has been relatively consistent in the limited number of studies that have evaluated tannin blends.
Nutrient Intake and Digestibility
Nutrient digestibility data are presented in Table 5. Dietary concentrations of AIA averaged 1.80, 1.69, and 1.84% for the CON, 30-ByPro, and 60-ByPro diets, respectively, whereas fecal AIA concentrations averaged 7.08, 7.13, and 7.06% for the CON, 30-ByPro, and 60-ByPro diets, respectively (data not shown). Individual diet AIA concentration was used for estimation of total fecal output and consequent apparent total tract nutrient digestibility calculations. Although AIA concentration slightly varied among diets, if the average AIA concentration of the 3 diets is used instead of individual dietary values, interpretation of data is not affected. Intake of DM (P = 0.07) and OM (P = 0.08) tended to linearly increase with increasing dietary inclusion of ByPro. No differences (P ≥ 0.17) were observed for starch, NDF, or CP intake. A linear increase in fecal output of starch (P = 0.03) and CP (P = 0.04) was noted when steers were fed increasing levels of ByPro. Digestibility coefficients for apparent total tract digestibility of the CON diet were consistent with previous studies evaluating steam-flaked corn–based beef cattle finishing diets with inclusions of wet corn gluten feed similar to that in the present study (Sindt et al., 2003; Domby et al., 2014). Apparent total tract digestibility of OM (P = 0.09) tended to quadratically increase with an increasing dose of ByPro by roughly 2 percentage units. A linear decrease (P = 0.03) in starch digestibility (1 percentage unit) was observed with ByPro inclusion, and a linear (P = 0.09) tendency for decreased CP digestibility with ByPro inclusion was observed, although only for the 60-ByPro diet. Although not statistically significant (linear, P = 0.36, and quadratic, P = 0.41), NDF digestibility was 5.80 and 4.52 percentage units greater for cattle fed 30-ByPro and 60-ByPro, respectively, compared with cattle fed the CON. Greater DMI when ByPro was fed during the initial feeding phase and a tendency for greater OM digestibility, coupled with a slight decrease in starch digestibility confirms potential compensation in total tract fiber digestibility were observed, even though only numerical differences in NDF digestibility were observed, in the current study. Previously mentioned studies showing improved growth performance of cattle fed ByPro were conducted with diets containing greater roughage inclusion than in the present study. Further investigation of relationships among tannic acid blends and dietary fibrous ingredients might reveal a greater potential for the use of tannins in beef cattle diets.
Table 5.
Effects of ByPro1 (tannic acid) dose (g/steer daily) in steam-flaked corn–based finishing diets on beef cattle apparent total tract digestibility
| ByPro dose | P-value3 | |||||
|---|---|---|---|---|---|---|
| Item | Control | 30 g | 60 g | SEM2 | Linear | Quadratic |
| Intake,4 kg/d | ||||||
| DM | 9.46 | 9.78 | 10.01 | 0.289 | 0.07 | 0.86 |
| OM | 8.83 | 9.16 | 9.33 | 0.269 | 0.08 | 0.73 |
| Starch | 4.42 | 4.62 | 4.57 | 0.132 | 0.29 | 0.27 |
| CP | 1.25 | 1.24 | 1.31 | 0.038 | 0.30 | 0.20 |
| NDF | 2.09 | 2.16 | 2.18 | 0.062 | 0.17 | 0.60 |
| Fecal output, kg/d | ||||||
| DM | 2.46 | 2.36 | 2.65 | 0.158 | 0.25 | 0.17 |
| OM | 2.29 | 2.14 | 2.42 | 0.161 | 0.41 | 0.13 |
| Starch | 0.08 | 0.10 | 0.13 | 0.020 | 0.03 | 0.55 |
| CP | 0.38 | 0.37 | 0.44 | 0.026 | 0.04 | 0.09 |
| NDF | 1.28 | 1.20 | 1.24 | 0.112 | 0.71 | 0.54 |
| Apparent total tract digestibility, % | ||||||
| DM | 73.9 | 75.7 | 73.4 | 1.58 | 0.75 | 0.14 |
| OM | 74.0 | 76.5 | 73.5 | 1.71 | 0.94 | 0.09 |
| Starch | 98.2 | 98.0 | 97.2 | 0.42 | 0.03 | 0.54 |
| CP | 69.9 | 70.1 | 66.4 | 2.02 | 0.09 | 0.26 |
| NDF | 38.5 | 44.3 | 43.0 | 4.87 | 0.36 | 0.41 |
| Diet composition, %, DM basis | ||||||
| DM, % as-is basis | 78.2 | 78.3 | 78.4 | – | – | – |
| OM | 93.4 | 93.7 | 93.2 | – | – | – |
| Starch | 46.7 | 47.3 | 45.6 | – | – | – |
| CP | 13.4 | 12.7 | 13.2 | – | – | – |
| NDF | 23.6 | 23.6 | 23.3 | – | – | |
Silvateam USA, Ontario, CA.
n = 12.
P-values for the linear and quadratic effects of ByPro dose.
Intake during the digestibility measurement period of the experiment (d 90 through 96 of the feeding period).
The tendency for decreased apparent CP digestibility observed in the present study is similar to the findings of Jayanegara and Palupi (2010), who evaluated in vitro and in vivo results from 19 studies, culminating in the finding that CP digestibility linearly decreased with inclusion of dietary tannins. The authors also observed a consistent decrease in OM digestibility, whereas a quadratic tendency was noted for increased OM digestibility in the current study. When ByPro was fed at 60 g/animal daily, starch was the only nutrient that showed a significant decrease in apparent digestibility. Despite the fact that ruminal starch and protein digestion are related (NASEM, 2016), and, therefore, a potential decrease in CP digestibility could negatively affect starch ruminal digestion, the remaining starch could potentially be digested at the intestinal level. It has been also hypothesized that when tannic acid complexes with starch components, the extent to which starch is dissociated in the acidic pH of the abomasum and initial part of the duodenum is variable, thereby limiting the availability of starch for digestion and absorption. Animals seem to have the ability to develop a tolerance to tannins with prolonged exposure (Van Soest, 1994). Digestibility might be influenced to a greater extent at the earlier stages of feeding, which would coincide with the increase in DMI with ByPro during the beginning of the feeding period observed in the present study.
Fecal Nitrogen Volatilization
There were no differences observed in fecal N volatilization as a percentage of intake, grams per steer daily, and total kilograms per steer (Table 6). Volatilization of N in feces in the current study, for both treatment and CON groups, was similar to volatilized amounts observed in the diets containing similar RDP concentrations (average 8.93% RDP in the current study vs. 8.15% in steam-flaked corn–based finishing diets fed by Cole et al., 2006). Cole et al. (2006) observed an increase in fecal N volatilization as the percentage of CP from NPN sources increased. The percentage of dietary NPN in our study was similar to the highest inclusion rate of NPN in the study of Cole et al. (2006). It could be inferred that the amount of true protein vs. NPN affects the ability of tannins to bind to protein structures and not to NPN sources. It might also be possible that the level of tannins included in the diets in the present study was simply not high enough to create any sort a difference in its ability to completely complex with proteins and to maintain that complex in the feces.
Table 6.
Effects of ByPro1 (tannic acid) dose (g/steer daily) on fecal N volatilization
| ByPro inclusion | P-value3 | |||||
|---|---|---|---|---|---|---|
| N volatilization | Control | 30 g | 60 g | SEM2 | Linear | Quadratic |
| Percent of intake | 55.2 | 55.4 | 51.6 | 3.74 | 0.91 | 0.39 |
| Grams/steer daily | 111 | 110 | 106 | 7.21 | 0.56 | 0.81 |
| Kilograms/steer | 17.0 | 16.7 | 16.5 | 4.81 | 0.69 | 0.96 |
Silvateam USA, Ontario, CA.
n = 12.
P-values for the linear and quadratic effects of ByPro dose.
Meat Analyses
Historically, consumers use lean color at retail as their primary indicator of meat freshness and exhibit a preference for a bright cherry-red lean color (McMillin, 2008). As such, the maintenance of desirable lean color and the incorporation of strategies that promote its stability are advantageous to the meat industry. Overall, feeding ByPro had little influence on objective evaluations of the lean color of overwrap-packaged beef strip loin steaks during retail display. Instrument-based assessment of lean color lightness (L*), redness (a*), yellowness (b*), and hue did not differ (P ≥ 0.16) among treatments (Table 7). Saturation values, however, which are indicative of color depth and intensity, tended (P = 0.07) to be greater in steaks from cattle fed the CON or 30-ByPro diets.
Table 7.
Instrument color values (L*, a*, b*, hue angle, and saturation), discoloration ratio, and relative pigment proportions (% oxymyoglobin, deoxymyoglobin, and metmyoglobin) of overwrapped packaged longissimus lumborum steaks during retail display as influenced by dose (0, 30, and 60 g/steer daily) of ByPro1 (tannic acid) in steam-flaked corn–based diets of finishing beef cattle
| ByPro inclusion | |||||
|---|---|---|---|---|---|
| Item | Control | 30 g | 60 g | SEM2 | P-value3 |
| Instrument color | |||||
| L* | 42.4 | 39.8 | 39.7 | 3.50 | 0.33 |
| a* | 27.2 | 26.4 | 26.5 | 2.70 | 0.41 |
| b* | 23.6 | 23.1 | 23.1 | 2.21 | 0.16 |
| Hue angle4 | 41.4 | 41.7 | 41.4 | 0.65 | 0.48 |
| Saturation5 | 36.1 | 35.2 | 34.9 | 3.36 | 0.07 |
| Pigment proportion6 | |||||
| Discoloration ratio | 6.7 | 9.5 | 6.6 | 4.46 | 0.60 |
| Metmyoglobin | 19.5b | 20.5ab | 23.3a | 4.88 | <0.01 |
| Deoxymyoglobin | 17.3 | 16.6 | 16.1 | 2.37 | 0.17 |
a,bLeast squares means in a row with different superscripts differ (P < 0.05).
Silvateam USA, Ontario, CA.
n = 10 (for each display day).
P-value for the effect of treatment.
Hue angle = tan−1 b*/a*.
Saturation index = (a*2 + b*2)1/2.
Relative pigment proportions (%; oxymyoglobin, metmyoglobin, and deoxymyoglobin) were calculated using reflectance values obtained using a portable spectrophotometer (Hunter Miniscan XE Plus, model MSXP-4500C; Hunter Associates Laboratory, Inc., Reston, VA) and formulas outlined by the American Meat Science Association (2012).
The bright cherry-red lean color that consumers desire is a result of oxymyoglobin formation (Mancini and Hunt, 2005). Conversely, consumers discriminate against the presence of brown or discolored lean, which is a result of the oxidation of oxymyoglobin to metmyoglobin. In the current study, steaks from cattle fed the 60-ByPro diet had a greater (P = 0.002) proportion of metmyoglobin than steaks from cattle fed the CON diet, which was not different from steaks of cattle fed the 30-ByPro diet. These results correspond to the tendency for decreased saturation values, also indicative of discolored lean, in steaks from cattle fed the 60-ByPro diet (Table 7). The effects of retail display day (7 different days) on the instrument color values, discoloration ratio, and relative pigment proportions, regardless of dietary treatment, followed expected patterns (Table 8).
Table 8.
The effects of retail display day on the instrument color values (L*, a*, b*, hue angle, and saturation), discoloration ratio, and relative pigment proportions (% deoxymyoglobin and metmyoglobin) of overwrapped packaged longissimus lumborum steaks from cattle fed steam-flaked corn–based diets with ByPro1 (tannic acid) fed at 0, 30, or 60 g/steer daily
| Retail display day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM2 | P-value3 |
| Instrument color | ||||||||||
| L* | 41.47 | 43.06 | 41.14 | 38.71 | 39.60 | 39.68 | 45.72 | 35.64 | 2.99 | 0.18 |
| a* | 36.62a | 30.50b | 29.22bc | 28.90c | 25.20d | 22.89e | 19.63f | 20.80f | 2.73 | <0.01 |
| b* | 30.20a | 24.28b | 24.43b | 24.86b | 21.98c | 20.80d | 18.62e | 20.77d | 2.22 | <0.01 |
| Hue angle4 | 39.36de | 38.43e | 39.99d | 40.78cd | 41.55c | 42.88b | 43.54b | 45.35a | 0.71 | <0.01 |
| Saturation5 | 47.50a | 39.00b | 38.12b | 38.13b | 33.49c | 30.98d | 26.42e | 29.58d | 3.38 | <0.01 |
| Pigment proportion6 | ||||||||||
| Discoloration ratio | 29.06a | 6.50b | 6.06b | 6.87b | 4.60b | 3.80b | 2.87b | 0.80b | 6.44 | <0.01 |
| Metmyoglobin | 4.81f | 13.00e | 15.79e | 16.13e | 23.85d | 29.37c | 34.82b | 40.61a | 4.96 | <0.01 |
| Deoxymyoglobin | 34.00a | 20.87b | 20.00b | 17.18c | 13.81d | 10.59e | 8.54f | 7.77f | 2.41 | <0.01 |
a–fLeast squares means in a row with different superscripts differ (P < 0.05).
Silvateam USA, Ontario, CA.
n = 10.
P-value for the effect of retail display day.
Hue angle = tan−1 b*/a*.
Saturation index = (a*2 + b*2)1/2.
Relative pigment proportions (%; metmyoglobin and deoxymyoglobin) were calculated using reflectance values obtained using a portable spectrophotometer (Hunter Miniscan XEPlus, model MSXP-4500C; Hunter Associates Laboratory, Inc., Reston, VA) and formulas outlined by the American Meat Science Association (2012).
Retail display length did not influence lean color lightness (L*; P = 0.18); however, lean redness (a*), yellowness (b*), and saturation values all decreased as display progressed. These results correspond to previous evaluations of beef lean color, which have documented color deterioration in overwrapped beef steaks during retail display (Jeremiah and Gibson, 2001; Mancini and Hunt, 2005). Likewise, the proportion of metmyoglobin rose (P < 0.01) as retail display progressed from 0 to 7 d. After 7 d of retail display, approximately 40% of the lean surface was in the metmyoglobin state.
The ByPro treatment and retail display day interacted (P < 0.01, SEM 3.40) to influence oxymyoglobin proportions during retail display (Fig. 1). As expected, oxymyoglobin proportions decreased during retail display for all treatments. No differences as a result of ByPro inclusion were noted until d 5 through 7 of retail display, when steaks from the 60-ByPro and CON treatments exhibited greater (P < 0.05) oxymyoglobin proportions than steaks from the 30-ByPro treatment. Quadratic effects of dietary tannins on quality indicators such as TBARS have been also observed in other studies (Liu et al., 2009).
Figure 1.
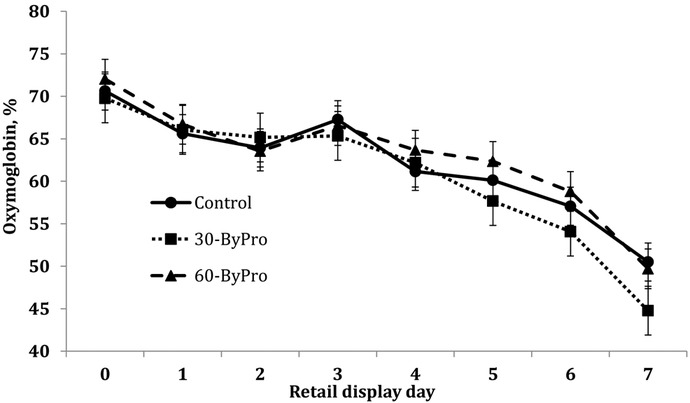
The effect of tannic acid blend (ByPro; Silvateam USA, Ontario, CA) on strip loin steaks' oxymyoglobin from cattle fed steam-flaked corn–based diets. Retail display length interacted (P < 0.01, SEM 3.40, n = 29) to influence the proportion of oxymyoglobin present on the lean surface on d 5 through 7, where oxymyoglobin proportions from control (no tannic acid) and 60 g DM of ByPro/steer daily (60-ByPro) were greater (P = 0.01) than those from 30 g DM of ByPro/steer daily (30-ByPro).
As shown in Table 9, ByPro inclusion did not influence the pH (P = 0.78) or formation of lipid oxidation byproducts (as indicated by TBARS) in beef strip loins aged for 21 d. Although dietary inclusion of ingredients with antioxidant activity has previously been shown to offer protection from lipid oxidation (Formanek et al., 2001; Georgantelis et al., 2007), no measureable protection from oxidation was observed in the current study, although further evaluation of a larger dose range might be warranted. When observed, the antioxidant effects attributed to the feeding of dietary tannins have been associated with protein oxidation rather than lipid oxidation. However, lipid oxidation and protein oxidation are closely related, especially in traditionally packaged beef subjected to fluorescent retail lighting. Lipid oxidation byproducts have been shown to accelerate protein oxidation in meat. The heme moiety associated with the protein pigment responsible for meat color is subject to oxidation when lipid peroxyradicals are present, and oxidation results in discoloration. Because meat discoloration can be associated with both process (lipid and protein oxidation) and because of the influence of lipid oxidation on protein oxidation, it is prudent to include lipid oxidation measures to explain variation in meat color.
Table 9.
Thiobarbituric acid reactive substances (TBARS; mg malondialdehyde/kg of meat) in 21-d aged beef strip loins from cattle fed steam-flaked corn–based diets with ByPro1 (tannic acid) fed at 0, 30, or 60 g/steer daily
| ByPro inclusion | |||||
|---|---|---|---|---|---|
| Variable | Control | 30 g | 60 g | SEM2 | P-value3 |
| TBARS | 0.3041 | 0.3186 | 0.3176 | 0.013 | 0.43 |
| pH | 5.427 | 5.393 | 5.380 | 0.124 | 0.77 |
Silvateam USA, Ontario, CA.
n = 10.
P-value for the effect of retail display day.
Conclusions
The tannic acid blend (ByPro) evaluated in the current study seemed to positively influence DMI at the beginning of the finishing phase when added to steam-flaked corn–based finishing diets. Effects of ByPro on growth performance should be further investigated for steers fed growing diets with a greater roughage concentration. Carcass characteristics were not greatly affected by ByPro inclusion. Although fiber digestion was not affected by treatments, further investigation is needed to evaluate effects of ByPro on dietary situations in which the fiber content is greater than in the current study. Feeding ByPro at 30 or 60 g/steer daily did not alter fecal N volatilization, and ByPro did not seem to greatly influence the shelf life of overwrap-packaged steaks. Greater doses of ByPro than used in the present study might be required to elicit antioxidant activity and extend the shelf life in meat.
Footnotes
Supported by funds provided by Silvateam USA, Ontario, CA.
LITERATURE CITED
- American Meat Science Association (AMSA) 2012. AMSA meat color measurement guidelines. AMSA, Champaign, IL. [Google Scholar]
- Anderson S. 2007. Determination of fat, moisture, and protein in meat and meat products by using the FOSS FoodScan™ near-infrared spectrophotometer with FOSS artificial neural network: Calibration model and associated database: Collaborative study. J. AOAC Int. 90:1073–1083. [PubMed] [Google Scholar]
- AOAC 1995. Official method of analysis. 16th ed.AOAC Int., Arlington, VA. [Google Scholar]
- AOAC 1997. Official methods of analysis. 16th ed., 3rd rev AOAC Int., Arlington, VA. [Google Scholar]
- AOAC International 2005. Official methods of analysis. 18th ed.Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Barajas R., Cervantes B. J., Arechiga S. C., Espino M. A., Flores L. R., Camacho A., Romo J. A. 2011a. Effect of length feeding additional tannins-extract on feedlot-performance of finishing-bulls. J. Anim. Sci. 89(E-Suppl. 1):615 (Abstr.)21036930 [Google Scholar]
- Barajas R., Cervantes B. J., Camacho A., Verdugo M., Espino M. A., Flores L. R., Romo J. A., Velaquez E. A., Lomeli J. J. 2011b. Influence of addition of tannins-extract in low concentration of dietary dry matter on feedlot-performance of bulls. J. Anim. Sci. 89(E-Suppl. 1):615 (Abstr.)21036930 [Google Scholar]
- Barajas R., Cervantes B. J., Espino M. A., Camacho A., Verdugo M., Flores L. R., Arechiga S. C., Lomeli J. J., Romo J. A. 2012a. Influence of tannins extract addition on feedlot-performance of bulls fed sorghum-based diets. J. Anim. Sci. 90(Suppl. 3):372–373. (Abstr.)23365383 [Google Scholar]
- Barajas R., Cervantes B. J., Espino M. A., Camacho A., Verdugo M., Flores L. R., Loneli J. J., Romo J. A. 2012b. Effect of tannins extract supplementation on feedlot performance and plasma urea nitrogen of yearling bulls fed dry-ground corn-based diets containing corn-DDG and cane molasses. J. Anim. Sci. 90(Suppl. 3):600 (Abstr.) [Google Scholar]
- Brink D. R., Lowry S. R., Stock R. A., Parrott J. C. 1990. Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. J. Anim. Sci. 68:1201–1207. doi: 10.2527/1990.6851201x [DOI] [PubMed] [Google Scholar]
- Camacho A., Cervantes B. J., Espino M. A., Verdugo M., Flores L. R., Romo J. A., Barajas R. 2011. Influence of addition of tannins-extract in low concentration of dietary dry matter on carcass characteristics of bull-calves. J. Anim. Sci. 89(E-Suppl. 1):615 (Abstr.)21036930 [Google Scholar]
- CIE 1978. Recommendations on uniform color spaces—Color equations, psychometric color terms. Supplement no. 2 to CIE Publ. No. 15 (E-1.3.L). Comission Internationale de L'Eclairage. Recommendations on Uniform Color Spaces, Color-Equations, Psychometric Color Terms; CIE Publication: Paris, France, 1978; Volume 15. p. 1–21 (E-1.3.L). [Google Scholar]
- Cole N. A., Defoor P. J., Galyean M. L., Duff G. C., Gleghorn J. F. 2006. Effects of phase-feeding of crude protein on performance, carcass characteristics, serum urea nitrogen concentrations, and manure nitrogen of finishing beef steers. J. Anim. Sci. 84:3421–3432. doi: 10.2527/jas.2006-150 [DOI] [PubMed] [Google Scholar]
- Domby E. M., Anele U. Y., Gautam K. K., Hergenreder J. E., Pepper-Yowell A. R., Galyean M. L. 2014. Interactive effects of bulk density of steam-flaked corn and concentration of Sweet Bran on feedlot cattle performance, carcass characteristics, and apparent total tract nutrient digestibility. J. Anim. Sci. 92:1133–1143. doi: 10.2527/jas.2013-7038 [DOI] [PubMed] [Google Scholar]
- Formanek Z., Kerry J. P., Higgins F. M., Buckley D. J., Morrissey P. A., Farkas J. 2001. Addition of synthetic and natural antioxidants to alpha-tocopheryl acetate supplemented beef patties: Effects of antioxidants and packaging on lipid oxidation. Meat Sci. 58:337–341. doi: 10.1016/S0309-1740(00)00149-2 [DOI] [PubMed] [Google Scholar]
- Frutos P., Hervas G., Giraldez F. J., Mantecon A. R. 2004. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2:191–202. doi: 10.5424/sjar/2004022-73 [DOI] [Google Scholar]
- Georgantelis D., Georgios B., Katikou P., Ambrosiadis I., Fletouris D. J. 2007. Effect of rosemary extract, chitosan and alpha-tocopherol on lipid oxidation and color stability during frozen storage of beef burgers. Meat Sci. 75:256–264. doi: 10.1016/j.meatsci.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Hagerman A. E. 1992. Tannin-protein interactions. In: Ho C. T., Lee C. Y., Huang M. T. editors, Phenolic compounds in food and their effects on health. I. Analysis, occurrence, and chemistry. Am. Chem. Soc. Symp. Ser. No. 506. Am. Chem. Soc., Washington, DC: p. 236–247. [Google Scholar]
- Jayanegara A., Palupi E. 2010. Condensed tannin effects on nitrogen digestion in ruminants: A meta-analysis from in vitro and in vivo studies. Media Peternakan 33:176–181. doi: 10.5398/medpet.2010.33.3.176 [DOI] [Google Scholar]
- Jeremiah L., Gibson L. 2001. The influence on storage temperature and storage time on color stability, retail properties and case-life of retail-ready beef. Food Res. Int. 34:815–826. doi: 10.1016/S0963-9969(01)00104-1 [DOI] [Google Scholar]
- Krueger W. K., Gutierrez-Banuelos H., Carstens G. E., Min B. R., Pinchak W. E., Gomez R. R., Anderson R. C., Krueger N. A., Forbes T. D. A. 2010. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 159:1–9. doi: 10.1016/j.anifeedsci.2010.05.003 [DOI] [Google Scholar]
- Krzywicki K. 1979. Assessment of relative content of myoglobin, oxymyoglobin, and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. doi: 10.1016/0309-1740(79)90019-6 [DOI] [PubMed] [Google Scholar]
- Liu H. W., Francesco G., Gasco L., Brugiapaglia A., Lussiana C., Guo K. J., Tong J. M., Zoccarato I. 2009. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 83:678–683. doi: 10.1016/j.meatsci.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Luque L. D., Johnson B. J., Martin J. N., Miller M. F., Hodgen J. M., Hutcheson J. P., Nichols W. T., Streeter M. N., Yates D. A., Allen D. M., Brooks J. C. 2011. Zilpaterol hydrochloride supplementation has no effect on the shelf life of ground beef. J. Anim. Sci. 89:817–825. doi: 10.2527/jas.2010-3317 [DOI] [PubMed] [Google Scholar]
- Mancini R. A., Hunt M. C. 2005. Current research in meat color. Meat Sci. 71:100–121. doi: 10.1016/j.meatsci.2005.03.003 [DOI] [PubMed] [Google Scholar]
- McAllister T. A., Martinez T., Bae H. D., Muir A. D., Yanke L. J., Gones G. A. 2005. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 31:2049–2068. [DOI] [PubMed] [Google Scholar]
- McMillin K. W. 2008. Where is MAP going? A review and potential future for modified atmosphere packaging of meat. Meat Sci. 80:43–65. doi: 10.1016/j.meatsci.2008.05.028 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM) 2016. Nutrient requirements of beef cattle. 8th rev ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 1996. Nutrient requirements of beef cattle. 7th ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pennington J. A. T., Fisher R. A. 2009. Classification of fruits and vegetables. J. Food Compos. Anal. 22(Suppl):S23–S31. doi: 10.1016/j.jfca.2008.11.012 [DOI] [Google Scholar]
- Porter L. J. 1992. Structure and chemical properties of the condensed tannins. In: Hemingway R. W., Laks P. E. editors, Plant polyphenols. Plenum Press, New York: p. 245–258. [Google Scholar]
- Rivera-Méndez C., Plascencia A., Torrentera N., Zinn R. A. 2017. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 45(1):199–203. doi: 10.1080/09712119.2016.1141776 [DOI] [Google Scholar]
- Sedghi M., Golian A., Soleimani-Roodi P., Ahmadi A., Aami-Azghadi M. 2012. Relationship between color and tannin content in sorghum grain: Application of image analysis and artificial neural network. Rev. Bras. Cienc. Avic. 14:57–62. doi: 10.1590/S1516-635X2012000100010 [DOI] [Google Scholar]
- Sindt J. J., Drouillard J. S., Titgemeyer E. C., Montgomery S. P., Coetzer C. M., Farran T. B., Pike J. N., Higgins J. J., Ethington R. T. 2003. Wet corn gluten feed and alfalfa hay combinations in steam-flaked corn finishing cattle diets. J. Anim. Sci. 81:3121–3129. doi: 10.2527/2003.81123121x [DOI] [PubMed] [Google Scholar]
- Van Keulen J., Young B. A. 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44:282–287. doi: 10.2527/jas1977.442282x [DOI] [Google Scholar]
- Van Soest P. J. 1994. Nutritional ecology of the ruminant. 2nd ed.Cornell Univ. Press, Ithaca, NY. [Google Scholar]
- Van Soest P. J., Robertson J. B., Lewis B. A. 1991. Methods for dietary fiber neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Velasco V., Williams P. 2011. Improving meat quality through natural antioxidants. Chilean J. Agric. Res. 71(2):313–322. doi: 10.4067/S0718-58392011000200017 [DOI] [Google Scholar]


