ABSTRACT
Acute Generalized Exanthematous Pustulosis (AGEP) is a rare drug reaction manifesting as pustular lesions with surrounding erythema following exposure. The disease is often self-limited and treatment is supportive. It may present in an atypical variant with vesicles that desquamate into erosions, which classifies the disease as an AGEP/SJS Overlap. This overlap syndrome can carry a substantial mortality rate and necessitate elevation in the level of care. Hydroxychloroquine has been implicated in cases of AGEP, and we present a case of AGEP/SJS overlap attributed to this common medication. Given the prevalence of drug eruptions, it is critical for the physicians to recognize and not overlook this rare and potentially fatal dermatological emergency.
KEYWORDS: AGEP, SJS/TEN overlap, Plaquenil, hydroxychloroquine, acute generalized exanthematous pustulosis, sero-negative arthritis
1. Introduction
Dermatological eruptions are among the most common complications a clinician manages when prescribing treatments to patients, and have been reported to occur in as many a 3–16% of all hospitalized patients [1,2]. These eruptions are known to occur with frequently prescribed medications, and often resolve following withdrawal of the offending agent. Severe eruptions, including certain dermatological emergencies, are far rarer. Two such conditions, Stevens – Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), are well known among clinicians. A lesser known one, Acute Generalized Exanthematous Pustolosis (AGEP), is equally important for the internist to recognize as it may follow a course similar to that of SJS or TEN, in a syndrome termed ‘AGEP Overlap’ [3].
2. Case scenario
A 71-year-old woman with a past medical history of rheumatoid factor-negative arthritis presented to the emergency room with a three-day history of a pustular rash, fourteen days after starting Hydroxychloroquine. It began on her upper chest, and quickly spread to cover her entire chest, abdomen, back, shoulders, neck, scalp and under bilateral breasts. The lesions were intensely pruritic and with scale, but without odor, blood, or drainage. Skin in the breast folds was excoriated and tender. The patient denied any changes in her diet, detergents or soaps, as well as recent travel, sick contacts or exposures to animals, ticks or other vectors. She had no history of drug reactions, environmental allergies or history of anaphylaxis.
Physical Examination demonstrated widespread erythroderma with pustular and scaling lesions across her chest (Figure 1), back, shoulders (Figure 2), upper extremities, neck (Figure 3) and head. Sloughing and excoriations under her bilateral breasts (Figure 4) and lesions involving her hard palate were also noted. Non-blanching purpura were noted on her bilateral lower extremities (Figure 5). The total estimated involved body surface area was near thirty percent, however, estimated surface area with desquamation was around one percent. Her vital signs were stable and she was afebrile. She had no signs of respiratory distress, no audible stridor and no tongue swelling. Laboratory investigations demonstrated leukocytosis with a WBC of 20.8 10E3/uL and Eosinophilia with a count of 1.86 10E3/uL. The patient was initially treated for suspected AGEP with supportive measures including intravenous fluid resuscitation and inpatient medical-surgical ward nursing care. Her Hydroxychloroquine was discontinued. Within 12 hours, the rash expanded to include larger areas of sloughing, and expansive palate lesions. Her vitals remained stable throughout this evolution. This changed prompted intravenous corticosteroids and anti-histamines. With progressive sloughing and mucous membrane sensitivity, the patient was transferred to the regional inpatient burn care unit. There, punch biopsy revealed ‘neutrophilic pustule formation with mild acantholysis, basilar epidermal spongiosis, rare dyskeratotic keratinocytes, superficial dermal perivascular chronic inflammation with neutrophils and rare eosinophils, and mild papillary dermal edema suggestive of AGEP.’ She was treated for a total of ten days in the burn unit, and survived to discharge. At a six-month interval follow-up, she had no long term sequelae, and remained off of Hydroxychloroquine.
Figure 1.
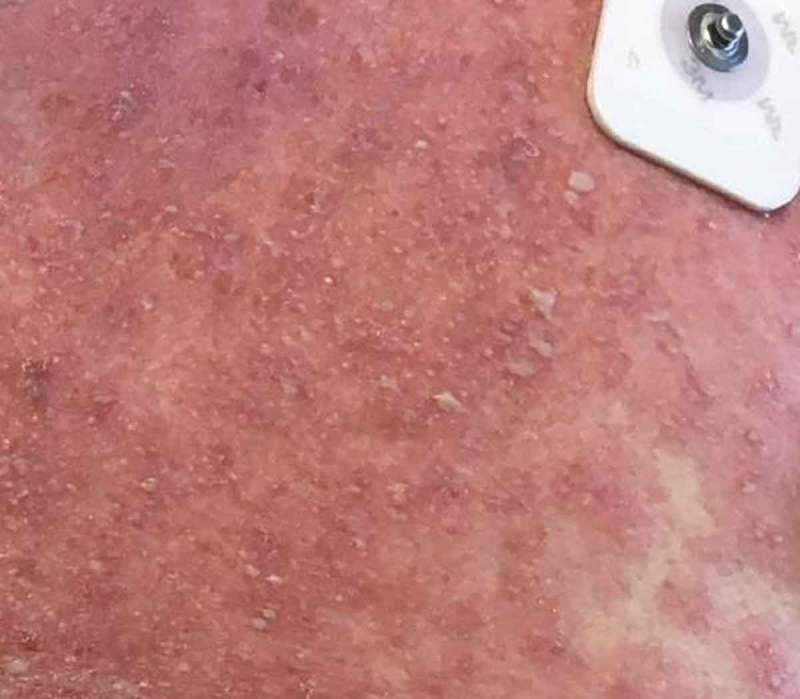
Right chest.
Figure 2.
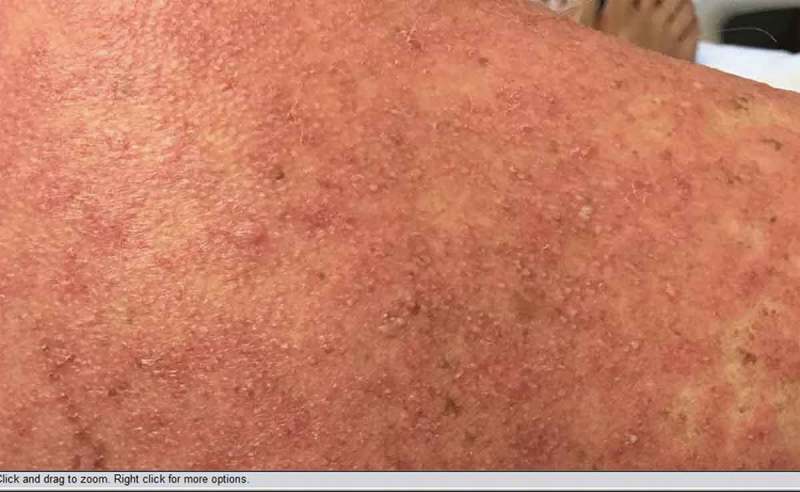
Right shoulder.
Figure 3.
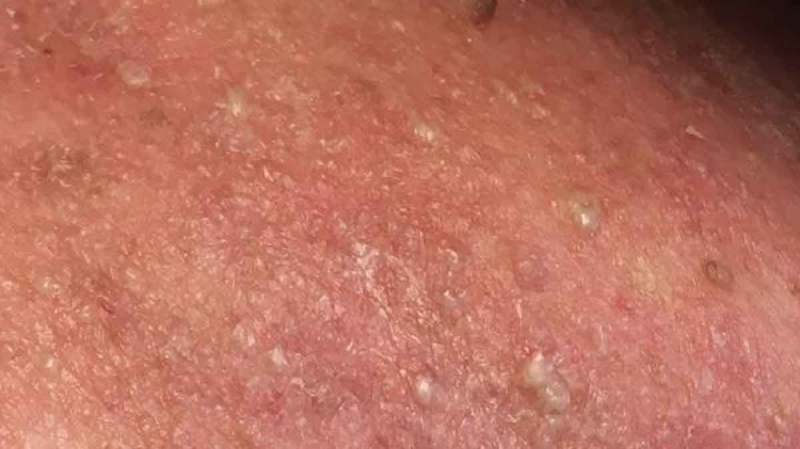
Right neck.
Figure 4.
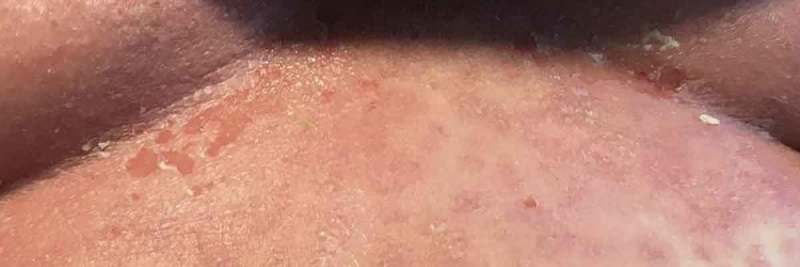
Under bilateral breasts.
Figure 5.
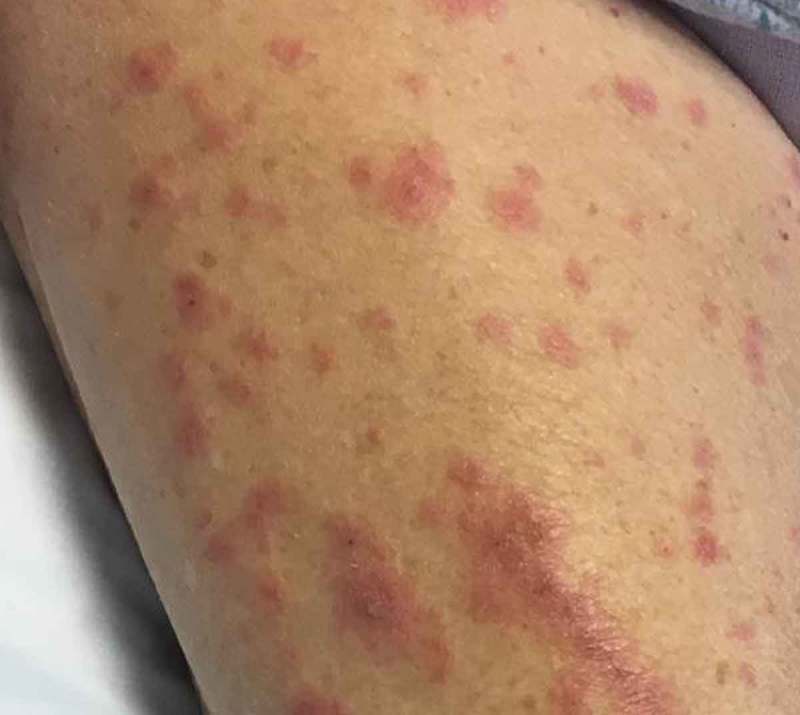
Right thigh.
3. Discussion
AGEP is a rare dermatological drug eruption that typically presents with the rapid development of innumerable, non-follicular pustules overlying widespread erythroderma, and rapidly spreads in a diffuse or patchy distribution [4,5]. This drug reaction tends to occur soon after administration of an offending agent, often one day after a culprit antibiotic or eleven days after drugs of other classes [6]. AGEP in association with administration of Hydroxychloroquine has been infrequently reported in the literature, with a total of twenty-one cases following a PubMed search using the terms ‘Hydroxychloroquine’ and ‘Pustolosis.’ Typically, this disease process is self-limited and requires basic supportive measures [7–9]. Rarely, AGEP can present in an atypical fashion with the development of blisters and vesicles that can coalesce, desquamate and form superficial erosions in an AGEP/SJS Overlap Syndrome [3,10,11]. This overlap syndrome can follow a course similar to that seen with SJS/TEN, which includes severe fluid loss, thermal dysregulation and secondary skin infections [12].
SJS and TEN are two entities on a spectrum of presentations of the same disease process, and are the two most well-known, highly mortal dermatological emergencies. These drug reactions are characterized by extensive epidermal sloughing and necrosis, with SJS involving less than 10% of total BSA and TEN involving greater than 30% [13,14]. The mortality associated with SJS and TEN are also on a continuum, with typical mortality rates approaching 10% to 30%, respectively [15,16]. Treatment typically involves aggressive supportive care including fluid and electrolyte repletion, temperature management, pain control and prevention of secondary skin infections. This is typically accomplished in burn care unit.
4. Conclusion
AGEP Overlap is a complicated and rare dermatological drug eruption that follows a course similar to that of SJS and TEN. Because of the complications that arise with these atypical lesions, the mortality associated with AGEP Overlap Syndrome can be as high as 5%, which is similar to the mortality rates seen in SJS [17]. Treatment parallels that of SJS/TEN, and transfer to a burn care unit may improve morbidity and mortality [18]. Given the frequency of drug eruptions, along with the implied morbidity and mortality of this condition, clinicians must promptly recognize and initiate treatment for an AGEP Overlap Syndrome, particularly in patients newly started on Hydroxychloroquine.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Nayak S, Acharjya B.. Adverse cutaneous drug reaction. Indian J Dermatol. 2008;53(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician J. 2003;68(9):1781–1791. [PubMed] [Google Scholar]
- [3].Azib S, Florin V, Fourrier F, et al. Severe acute generalized exanthematous pustulosis with blistering mimicking toxic epidermal necrolysis, associated with a primary mumps infection. Clin Exp Dermatol. 2014;39(6):723–725. [DOI] [PubMed] [Google Scholar]
- [4].Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustolosis: A clinical reaction pattern. J Cutan Pathol. 2001;28(3):113. [DOI] [PubMed] [Google Scholar]
- [5].Speeckaert M, Speeckaert R, Lambert J, et al. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol. 2010;20(4):425–433. [DOI] [PubMed] [Google Scholar]
- [6].Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157(5):989. [DOI] [PubMed] [Google Scholar]
- [7].Bailey K, McKee D, Wismer J, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: first case report in Canada and review of the literature. J Cutan Med Surg. 2013;17(6):414–418. [DOI] [PubMed] [Google Scholar]
- [8].Charfi O, Kastalli S, Sahnoun R, et al. Hydroxychloroquine-induced acute generalized exanthematous pustulosis with positive patch-testing. Indian J Pharmacol. 2015;47(6):693–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park JJ, Yun SJ, Lee JB, et al. A case of hydroxychloroquine induced acute generalized exanthematous pustolosis confirmed by accidental oral provocation. Ann Dermatol. 2010;22(1):102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine. Clin Rheumatol. 2009;28(12):1449–1452. [DOI] [PubMed] [Google Scholar]
- [11].Peermohamed S, Haber RM. Acute generalized exanthematous pustulosis simulating toxic epidermal necrolysis: a case report and review of the literature. Arch Dermatol. 2011;147(6):697–701. [DOI] [PubMed] [Google Scholar]
- [12].Kostopoulous TC, Krishna SM, Brinster NK, et al. Acute generalized exanthematous pustulosis: atypical presentations and outcomes. J Eur Acad Dermatol Venereol. 2015;29(2):209–214. [DOI] [PubMed] [Google Scholar]
- [13].Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92. [PubMed] [Google Scholar]
- [14].Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol. 1997;24(11):726. [DOI] [PubMed] [Google Scholar]
- [15].Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197–1204. [DOI] [PubMed] [Google Scholar]
- [16].Mahar PD, Wasiak J, Hii B, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centers. Burns. 2014;40(7):1245. [DOI] [PubMed] [Google Scholar]
- [17].Vassallo C, Derlino F, Brazzelli V, et al. Acute generalized exanthematous pustulosis: report of five cases and systematic review of clinical and histopathological findings. Ital J Dermatol Venereol. 2014;149(3):281–290. [PubMed] [Google Scholar]
- [18].Pomahac B, Lim J, Liu A. A case report of generalized pustulosis with systemic manifestations requiring burn intensive care unit admission. J Burn Care Res. 2008;29(6):1004–1008. [DOI] [PubMed] [Google Scholar]


