ABSTRACT
Levamisole is an antihelminth drug and a common cocaine contaminant, present in an estimated 71% of cocaine samples in the US. Levamisole-contaminated cocaine has been linked to an ANCA-associated vasculitis with cutaneous, renal, and pulmonary manifestations. We report the case of a 46 year old woman with known cocaine exposure who presents with recurrent, large purpuric and maculopapular rash of the extremities and face and review existing cases of levamisole/cocaine-associated ANCA vasculitis, We summarize the clinical presentation, treatment, and outcomes of levamisole induced vasculitis. There is emerging research on pathogenesis relating to neutrophil extracellular traps (NETs). We review studies implicating role of NETs in the pathogenesis of levamisole induced vasculitis. Further research to explore the use of NETs as therapeutic targets in drug induced vasculitis is needed.
KEYWORDS: Anti-neutrophil cytoplasmic antibody, cocaine, levimasole
1. Introduction
Levamisole is an anthelminthic drug with immunostimulatory properties. Historically when used therapeutically, it has been shown to cause agranulocytosis, particularly neutropenia, liver toxicity, gastrointestinal distress, and vasculitic purpura and necrosis of the ears [1]. Since the 2000s, levamisole has been shown to be a popular contaminant in cocaine. According to the Drug Enforcement Administration (DEA)’s Cocaine Signature Program data, by 2009, approximately 71% of tested cocaine samples in the US contained levamisole. Concurrently, increasing cases of levamisole-related adverse events, particularly agranulocytosis and vasculitis, have been reported among cocaine users [2]. Cocaine itself is also associated with autoimmunity formation, and it is conceivable that exposure to both drugs may be contributing to these diseases. There is emerging data on the role of neutrophil extracellular traps (NETs) in the pathogenesis of levamisole induced vasculitis. In this review, we discuss a case with clinical characteristics of levamisole induced cutaneous vasculitis and provide a literature review and recent updates on pathogenesis of levamisole induced vasculitis.
Figure 5.
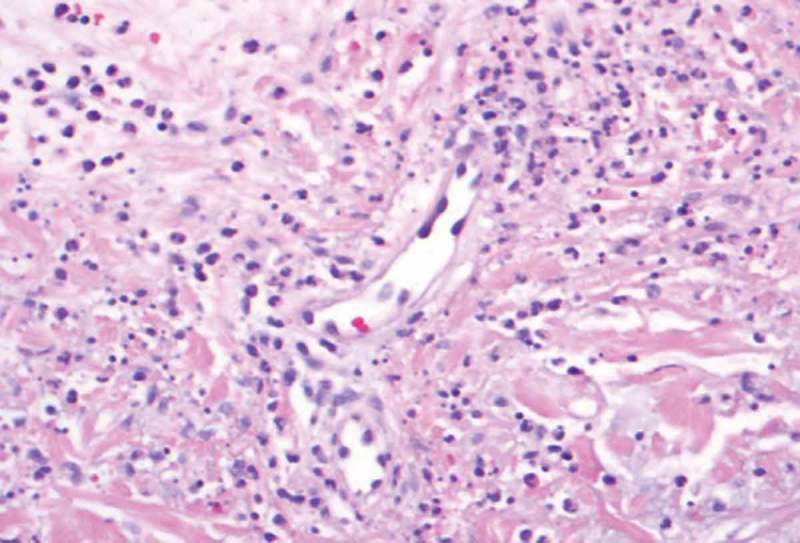
40X. Neutrophils and nuclear dust in the blood vessel walls causing destruction of the blood vessel walls.
2. Case presentation
The patient is a 46 year old woman with a history of polysubstance abuse, suspected rheumatoid arthritis, asthma, and untreated hepatitis C who presented to the ED with a painful hemorrhagic rash. The rash began 4 days prior to admission as tender erythematous patches on both thighs, then turned deep purple and became blood-filled bullae. She also reported diffuse arthralgias and epigastric pain, but no fevers, chills, or additional constitutional symptoms. Notably, two years prior, she had had a similar but milder presentation after cocaine exposure at an outside hospital, where she improved with pulse steroids. She denied drug use, but urine toxicology was positive for cocaine and cannabinoids. Physical exam was notable for 25 × 15 cm palpable purpura on the anterior surfaces of bilateral thighs, with focal areas of blistering and skin tearing (Figures 1 and 2). There were also smaller lesions on the right lateral shin and dorsal surfaces of bilateral hands (Figure 1). She was hypotensive on presentation and was admitted to the MICU for concern for Stevens Johnson Syndrome. Admission labs were notable for a normocytic anemia of 8.9 with a white blood cell count of 3.69 with an absolute lymphocyte count of 920 and normal platelet count. Serum creatinine was 1.9 mg/dL (last known value was 1.0 mg/dL 7 years prior). ANA titer was 1:80. P-ANCA was positive; C-ANCA was negative. MPO ELISA was positive. C3 and C4 were both decreased at 42 and 7 mg/dL respectively (ref: 79–152, 12–42). Cardiolipin IgM was positive at 42 mpl units (ref < 20) and IgG and IgA were negative. RF and Ro, La, SCL-70, RNP, smith antibodies were all negative. Cryofibrinogen screen was negative, as was an extended infectious work up. She was diagnosed with levamisole induced cutaneous vasculitis. Due to the extent of her wounds, she was transferred to the Burn Wound Unit and later received debridement, excision and grafting of her lower extremity wounds. Medically, she received prednisone, wound care, and pain management with good recovery; her AKI resolved with fluids. She declined biopsy at this visit. One year later, she presented again with purpura and bullae of the hands and feet, as well as a reticulo-papular rash affecting bilateral arms and posterior thighs and ulceration of the lip and tongue. During this hospitalization she received a punch biopsy of a lesion on her right wrist, which showed small vessel necrotizing leukocytoclastic vasculitis (Figures 4 and 5). Although testing for levamisole was not performed, based on the clinical presentation, serology and skin pathology, a diagnosis of levamisole adulterated cocaine vasculitis was made. She was treated with stress dose methylprednisolone and transitioned to oral prednisone dose of 20mg per day, in addition to wound care. She recovered and was discharged with plan to taper her prednisone.
Figure 1.
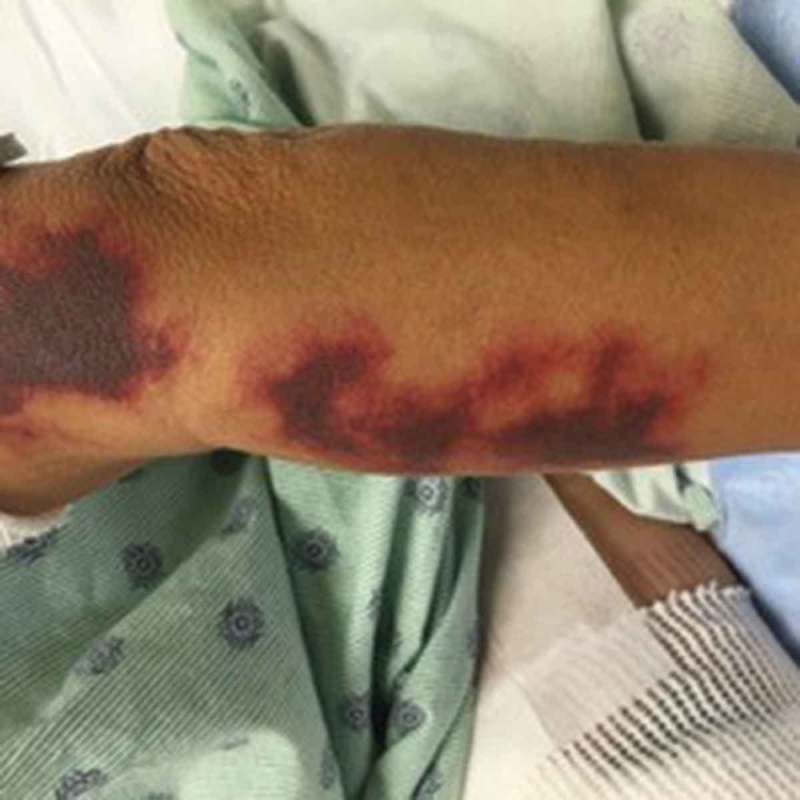
Retiform purpura in the arms.
Figure 2.
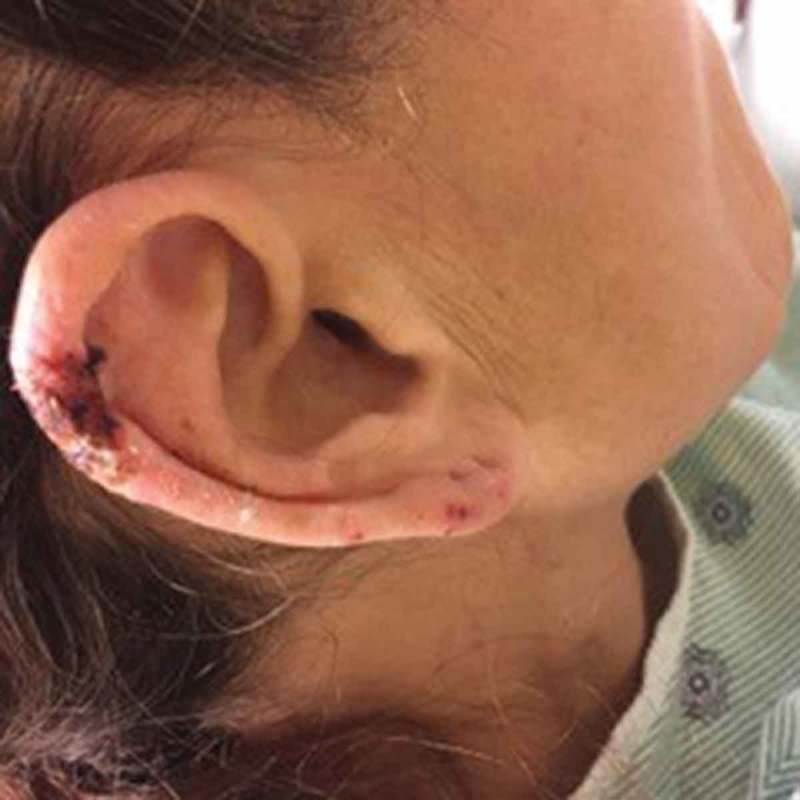
Purpura involving the ear.
Figure 4.
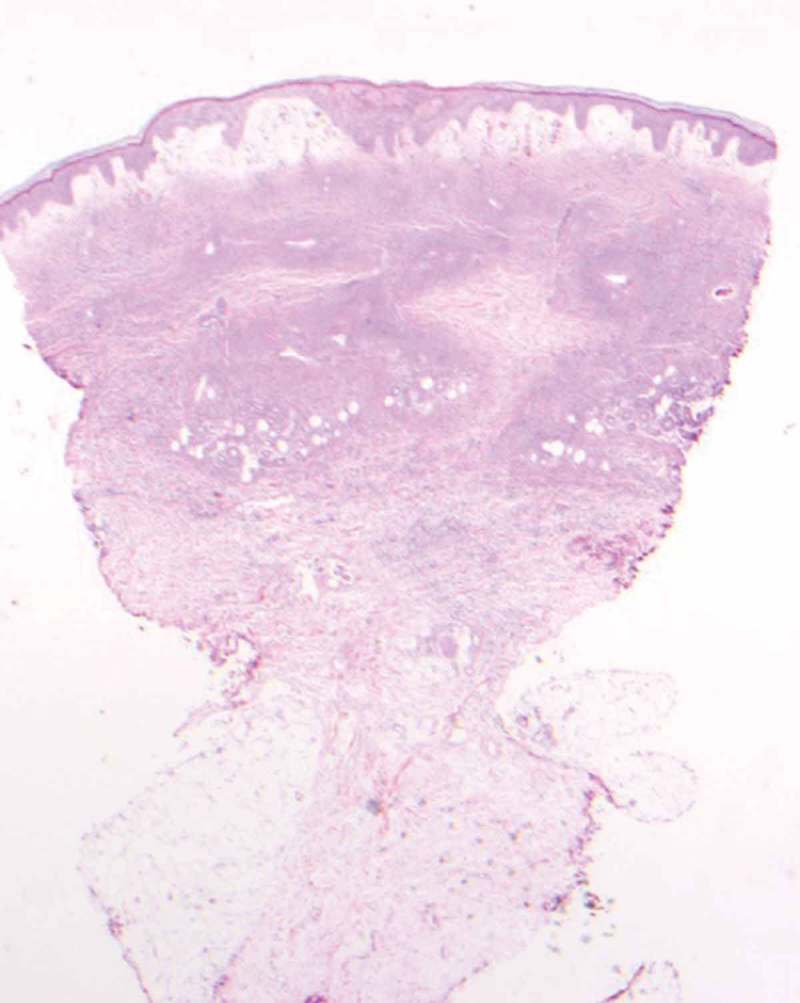
2X. Sections show a punch biopsy of skin. There is subepidermal edema with erythrocyte extravasation, Many superficial and deep dermal small vascular channels are surrounded by neutrophils and nuclear dust.
3. Discussion and review of the literature
Our case highlights the classic presentation of levamisole induced cutaneous vasculitis which is characterized clinically by vasculitic skin rash and serologically by positive ANA, anti-cardiolipin antibody and MPO ANCA in the setting of active cocaine use. Our patient responded to corticosteroids. We provide a literature review of levamisole induced vasculitis and discuss recent studies that provide insight into the pathogenesis of levamisole induced vasculitis.
3.1. Initial diagnosis and differential
The most common initial presentation of levamisole/cocaine-associated vasculitis is purpura, for which there is a broad differential. In the acute setting, it is particularly important to exclude other absolute emergencies such as meningococcal sepsis, thrombotic disorders (e.g., disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, warfarin-induced necrosis, antiphospholipid syndrome, and others) and embolic disease, hematologic malignancies, paraproteinemias, Stevens Johnson Syndrome, etc. Once the differential has been narrowed and vasculitis is suspected, tests for autoantibodies, particularly ANCAs, should be performed. Fundamentally the diagnosis is histopathologic, usually through skin or renal biopsy demonstrating small vessel vasculitis. As a small vessel vasculitis, the disease mimics its cousins in this class, particularly the ANCA-associated vasculitides, such as granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and microscopic polyangiitis. Differentiation from these other diseases is based on a history of active cocaine use, especially if a temporal relationship can be established with flares, although this is difficult given most patients continue to use cocaine regularly.
3.2. Clinical presentation of levamisole adulterated cocaine associated vasculitis
In the last few years, many cases of levamisole/cocaine-associated vasculitis have been reported. Over 90% of reported cases present with various cutaneous manifestations, including most commonly purpura, as well as necrosis, abscesses, and bullae [3,4]. The classic skin rash is retiform purpura which consists of branching purpuric lesions caused by blockage of blood flow in the dermal and subcutaneous vasculature. A classic association is purpura of the ears (Figure 3), but the most commonly affected are the lower extremities (Figure 2). In addition to cutaneous findings, arthralgias (31% −83%) and constitutional symptoms (72%) including fever, night sweats, and weight loss are common [5,6].
Figure 3.
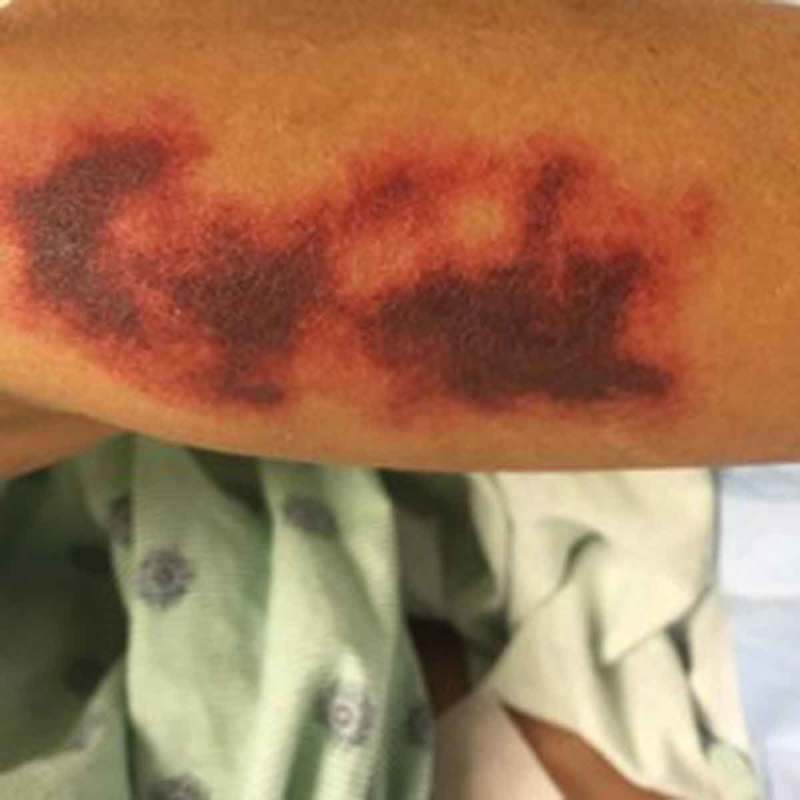
Retiform purpura in the leg.
Renal manifestations affect a minority of patients, but have been consistently reported. In McGrath’s series of 30 patients, 8 had abnormal urine dipsticks, of whom 2 had severe renal impairment [5]. In a 2017 review, Neel et al discussed 17 cases of biopsy-proven pauci-immune glomerulonephritis linked to levamisole/cocaine exposure, spanning 2011–2017 [7].
Relatively few patients present with pulmonary disease. A 2011 case series of 5 patients described pulmonary involvement in 3 patients, including bronchitis and bronchiolitis, desquamating interstitial pneumonia and hypersensitivity pneumonitis, and subcentimeter pulmonary nodules [8]. In the series described by McGrath et al, three of thirty patients had pulmonary hemorrhage [5]. No patients in either of the above case series presented with known concomitant pulmonary and renal disease. Carlson et al reported a patient with alveolar hemorrhage and glomerulonephritis who went on to remain dialysis dependent despite aggressive immunosuppressive therapy [9].
3.3. Laboratory findings
The vast majority of patients are positive for p-ANCA (88% – 100%) and MPO (66% – 100%) [5,6]. A large subset (50% in McGrath et al) are also PR3 positive; indeed in the review by Gulati et al. of 61 patients with levamisole-induced vasculitis, 66% were PR3 positive compared to only 56% MPO positive, t [3,5]. In the review by Neel et al. of 17 patients with biopsy-proven pauci-immune GN, 16 patients were positive for anti-MPO and one was not reported, and a similar 50% were positive for PR3 [7]. Autoantibody to other neutrophil antigens like elastase has been described. Neutrophil elastase shares epitopes with PR3 and may account for the false positive PR3 ANCA due to cross reactivity (ref 9).This dual positivity for PR3 ANCA and MPO ANCA is a characteristic feature of drug induced vasculitis. In addition, patients are often positive for other autoantigens, including ANA, lupus anticoagulant, anti-cardiolipin, anti-dsDNA, and others. Complement levels are low in some patients. Levamisole detection is not routinely performed in samples and is difficult with standard techniques due to short plasma half-life. Reports of detecting levamisole using liquid chromatography/mass spectrometry and using hair analysis have been described in the literature.
3.4. Treatment and outcome
Reported treatment for levamisole vasculitis varies by clinical presentation. In the case series by Pearson et al, which mostly comprised of patients with cutaneous findings and no known end-organ damage, 60% of patients who improved were treated with systemic corticosteroids and 40% were treated with wound care and antibiotics when appropriate. The authors noted their data did not support that steroids improved the disease course, but that perhaps were justifiable in cases of ‘striking’ inflammation [6]. Similarly, in the mostly cutaneous cases reviewed by Gulati et al, 43% were treated with immunosuppressants and 57% were not, and lesions resolved with drug discontinuation alone. Authors noted that cocaine continuation was associated with 44% relapse [3]. The decision of whether or not to use immunosuppression is an important one given that many patients present with concomitant neutropenia.
In cases with organ failure, immunosuppression is the mainstay of treatment. We identified 22 cases of levamisole-associated biopsy-proven pauci-immune glomerulonephritis, including 18 mentioned in the review of Neel et al, and several other recent cases [3–5,7,9–21]. 21 of 22 cases received immunosuppressive therapy; the remaining patient was anuric and received urgent HD. Of 20 cases whose frontline therapy was specified, 13 received corticosteroids with cyclophosphamide, 3 received corticosteroids with rituximab, 3 received corticosteroids alone and 1 received cyclophosphamide alone. 4 of the 20 patients also received plasmapheresis due to severity of disease. When specified, the vast majority of patients continued on oral prednisone with or without taper at discharge, with a few converting to azathioprine. Follow-up was sparse, but of 21 cases for whom outpatient follow up was available, 15 improved or had stable disease, while 6 were dialysis dependent. The median presenting creatinine was 11.05 mg/dL (range 6.6 to 20.83) for these 6 patients who went on to become dialysis dependent, and 3.25 mg/dL (range 0.8 to 7.09) for the 13 stable/improved patients for whom it was available. The majority of patients continued to use cocaine. Treatment is similar for pulmonary involvement; in cases of alveolar hemorrhage plasmapheresis may be indicated [3–5,7,9–21].
3.5. Pathophysiology
The exact mechanism behind the development of ANCAs remains under investigation. Under normal circumstances, the target antigens of ANCAs – most commonly myeloperoxidase (MPO) and serine proteinase 3 (PR3) are contained within cytoplasmic granules of neutrophils and thus have limited exposure to the immune system. Over the last decade, neutrophil extracellular traps (NETs) have been under increasing scrutiny as an important part of the pathogenesis of not only ANCA-associated vasculitis, but other autoimmune diseases including systemic lupus erythematosus, psoriasis, rheumatoid arthritis and chronic lung disease [22,23]. In a unique process of programmed cell death, activated neutrophils extrude NETs containing dsDNA, histones as well as granule contents such as MPO, PR3, elastase, cathepsin G, lactoferrin, and other proteins [24]. It has been suggested that the NETosis process both generates and is induced by ANCAs.
NETs were initially reported in biopsies of renal glomeruli of AAV patients and have been found also in skin lesions in subsequent studies [25,26]. In addition, it has been shown that patients with AAV have higher levels of circulating NETs as well as certain components of NETs, although the extent to which these levels correlate with disease activity remains under investigation [27,28]. NETs are implicated in inflammation in a variety of ways. They are shown to induce direct endothelial damage. They activate the alternative complement pathway. They are a major component of thrombi and are hypothesized to provide structural support to thrombi formation as well as activate the coagulation cascade in history- and tissue-factor-dependent manners, among other mechanisms [29–31]. The relationship between NETosis and ANCAs appear to be bidirectional. It has been shown that NET components, particularly relevantly MPO and PR3, induce ANCA and autoimmunity in mice [24,32]. On the other hand, ANCA IgGs, immune complexes, and sera from AAV patients have been shown to induce NETosis, suggesting a vicious cycle [32].
Focusing particularly on levamisole/cocaine associated vasculitis, Lood et al. showed that both cocaine and levamisole could induce in vitro release of NET-associated neutrophil elastase, a known ANCA antigen in cocaine users, in neutrophils obtained from healthy donors. They also showed that IgG from individuals with levamisole/cocaine-associated vasculitis potentiate this drug-induced NETosis and bound to NET-released elastase [33]. Carmona-Rivera et al further investigated the mechanism underlying this process, showing that levamisole-induced NETosis was dependent on M3 muscarinic receptors as well as the activation of NOX, Akt, and RAF/MEK/ERK pathways. In addition, NET directed antibodies were found in the sera of patients using levamisole-contaminated cocaine with or without clinical symptoms of vasculitis, and NET formation was identified in affected tissue of skin biopsies of patients with levamisole/cocaine-associated vasculitis [34]. Similar studies demonstrating drug-induced NET structural impairment and subsequent ANCA production and symptom induction have also been performed for propylthiouracil, another medication known for MPO ANCA-associated vasculitis [35]. NETs are being explored as potential therapeutic targets and several approaches are now being used to block the effects of NETs on vascular damage in experimental models [32].
4. Conclusion
Levamisole/cocaine-induced vasculitis has become an increasingly well-recognized disease entity over the last decade. Here, we presented a classic case with prominent, recurrent cutaneous manifestations and reviewed the literature to date, including emerging research on the role of NETosis in the pathogenesis of this condition.
5. Proposed criteria for diagnosis of levamisole associated ANCA vasculitis
| History of ingestion | High index of suspicion in setting associated cocaine/drug use |
| Constitutional symptoms | Fever, night sweats, weight loss and arthralgia |
| Skin manifestations | Purpura (retiform classically), necrosis, abscesses and bullae |
| Renal manifestations | Microscopic hematuria, proteinuria, elevated creatinine, features of pauci immune glomerulonephritis of renal biopsy |
| Pulmonary manifestations | Bronchitis, bronchiolitis, interstitial pneumonia, nodules and hemorrhage |
| Serology | Low complements, ANCA (MPO/PR3), ANA, dsDNA, lupus anticoagulant, |
The above criteria are not absolute and the aforementioned features might occur in isolation or in varied combinations
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Nolan AL, Jen K.. Pathologic manifestations of levamisole-adulterated cocaine exposure. Diagnostic Pathology. 2015. May 6;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Impact of Drugs on Society - National Drug Threat Assessment 2010 [Internet] ; 2010updated February; cited 2017 November25 Available from: https://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm.
- [3].Gulati S, Donato A. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012July;34(1):7–10. [DOI] [PubMed] [Google Scholar]
- [4].Systemic Levamisole-Induced Vasculitis in a Cocaine User without Cutaneous Findings: A Consideration in Diagnosis [Internet].: Hindawi ; 2015cited 2017 October26 Available from: https://www.hindawi.com/journals/crim/2015/547023/. [DOI] [PMC free article] [PubMed]
- [5].McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clinical Journal of the American Society of Nephrology. 2011December1;6(12):2799–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: A review of the literature - eScholarship. Dermatology Online Journal. 2012July1;18(7):1. [PubMed] [Google Scholar]
- [7].Néel A, Agard C, Hamidou M. Vasculitides induced by cocaine and/or levamisole. Joint Bone Spine. 2018 Jan;85(1):9–14. [DOI] [PubMed] [Google Scholar]
- [8].Ullrich K, Koval R, Koval E, et al. Five consecutive cases of a cutaneous vasculopathy in users of levamisole-adulterated cocaine. JCR: Journal of Clinical Rheumatology. 2011June;17(4):193–196. [DOI] [PubMed] [Google Scholar]
- [9].Carlson AQ, Tuot DS, Jen K, et al. Pauci-immune glomerulonephritis in individuals with disease associated with levamisole-adulterated cocaine: a series of 4 cases. Medicine. 2014October;93(17):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garg L, Gupta S, Swami A, et al. Levamisole/Cocaine induced systemic vasculitis and immune complex glomerulonephritis. Case Reports in Nephrology. 2015;2015:e372413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shiue Z, McNicholas B, Cormack F, et al. Antineutrophil cytoplasmic antibody mediated glomerulonephritis associated with levamisole-adulterated cocaine. Clinical Nephrology - Case Studies. 2015January1;3(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roca-Argente L, Moll-Guillen J, Espí-Reig J, et al. Membranous glomerulonephritis and cellular crescents induced by levamisole-adulterated cocaine abuse: a case report. Annals of Translational Medicine. 2015October30;3(18):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chawdhary K, Parke A. Connecticut medicine. Connecticut Medicine. 2015Jun-Jul;79(6):343–346. [PubMed] [Google Scholar]
- [14].Liu YJ, Mutnuri S, Siddiqui SB, et al. Levamisole-adulterated cocaine nephrotoxicity ultrastructural features. Am J Clin Pathol. 2016May1;145(5):720–726. [DOI] [PubMed] [Google Scholar]
- [15].Veronese FV, Dode RSO, Friderichs M, et al. Cocaine/levamisole-induced systemic vasculitis with retiform purpura and pauci-immune glomerulonephritis. Brazilian Journal of Medical and Biological Research = Revista Brasileira De Pesquisas Médicas E biológicas/Sociedade Brasileira De Biofísica … [Et Al.]. 2016;49(5):e5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moinuddin I, Madhrira M, Bracamonte E, et al. Membranous nephropathy with crescents associated with levamisole-induced MPO-ANCA vasculitis. Pathology - Research and Practice. 2016July1;212(7):650–653. [DOI] [PubMed] [Google Scholar]
- [17].van der Veer T, Pennings E, Tervaert JWC, et al. Levamisole-contaminated cocaine: a hairy affair. BMJ Case Reports. 2015August26;2015:bcr2015210970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sirvent AE, Enríquez R, Andrada E, et al. Glomerulonefritis necrosante en el síndrome por consumo de cocaína y levamisol. Nefrología. 2016January1,;36(1):76–78. [DOI] [PubMed] [Google Scholar]
- [19].Carrara C, Emili S, Lin M, et al. Necrotizing and crescentic glomerulonephritis with membranous nephropathy in a patient exposed to levamisole-adulterated cocaine. Clin Kidney J. 2016April;9:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Collister D, Sathianathan C, Ryz K, et al. ANCA associated vasculitis secondary to levamisole-adultered cocaine with associated membranous nephropathy: a case series. American Journal of Nephrology. 2017;45(3):209–216. [DOI] [PubMed] [Google Scholar]
- [21].Olives TD, Kornas RL, Fujisawa R, et al. Unexpected complication of cocaine-associated anti-neutrophil cytoplasmic antibody vasculitis related to persistent in-hospital cocaine use. Journal of Addiction Medicine. 2017April1;11(2):157–160. [DOI] [PubMed] [Google Scholar]
- [22].English JCI. Granulomatous disorders of adult skin, an issue of dermatologic clinics. Philadelphia (PA): Elsevier Health Sciences; 2015. [Google Scholar]
- [23].Al-Hussain T, Hussein MH, Conca W, et al. Pathophysiology of ANCA-associated vasculitis. Advances in Anatomic Pathology. 2017July;24(4):226. [DOI] [PubMed] [Google Scholar]
- [24].Sangaletti S, Tripodo C, Chiodoni C, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012October11,;120(15):3007–3018. [DOI] [PubMed] [Google Scholar]
- [25].Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nature Medicine. 2009June;15(6):623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abreu-Velez AM, Smith JG, Howard MS. Presence of neutrophil extracellular traps and antineutrophil cytoplasmic antibodies associated with vasculitides. N Am J Med Sci. 2009November;1(6):309–313. [PMC free article] [PubMed] [Google Scholar]
- [27].Wang H, Sha L, Ma T, et al. Circulating level of neutrophil extracellular traps is not a useful biomarker for assessing disease activity in antineutrophil cytoplasmic antibody-associated vasculitis: e0148197. PLoS ONE. 2016February1;11(2):e0148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Söderberg D, Segelmark M. Neutrophil extracellular traps in ANCA-associated vasculitis. Frontiers in Immunology. 2016;7(UNSP 256):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rao AN, Kazzaz NM, Knight JS. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? 世界心脏病学杂志: 英文版(电子版). 2015;7(12):829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Annals of the Rheumatic Diseases. 2014October19,;73(10):1854–1863. [DOI] [PubMed] [Google Scholar]
- [31].Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine. 2017March1,;23(3):279–287. [DOI] [PubMed] [Google Scholar]
- [32].Söderberg D, Segelmark M. Neutrophil extracellular traps in vasculitis, friend or foe? Current Opinion in Rheumatology. 2018 Jan;30(1):16–23. [DOI] [PubMed] [Google Scholar]
- [33].Lood C, Hughes GC. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford). 2017April1;56(4):638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carmona-Rivera C, Purmalek MM, Moore E, et al. A role for muscarinic receptors in neutrophil extracellular trap formation and levamisole-induced autoimmunity. JCI Insight. 2017;2(3):e89780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakazawa D, Tomaru U, Suzuki A, et al. Abnormal conformation and impaired degradation of propylthiouracil‐induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis & Rheumatism. 2012November;64(11):3779–3787. [DOI] [PubMed] [Google Scholar]


