Abstract
Prostate cancer remains the second most prevalent cancer in men. Its incidence, progression and mortality profiles vary significantly by race and ethnicity, with African-American men having the highest incidence rate and mortality rate in the world. Although these disparities can be partially explained by socioeconomic factors, the underlying molecular causes are complex and require careful research. A considerable amount of literature exists, supporting the association between mitochondrial health and the incidence, aggression and risk of prostate cancer. Genetic alterations in mitochondrial DNA are frequent in prostate cancer; therefore, the resulting mitochondrial dysfunction and metabolic dysregulation may contribute to or indicate oncogenesis. Many of the prominent features of cancer cells are also closely related to mitochondrial functions, such as resistance to apoptosis, excess reactive oxygen species production and altered oxidative phosphorylation. In addition, prostate cancer ethnic disparity is influenced by environmental and lifestyle factors, which involves differences in mitochondrial metabolism and retrograde signaling events.
Introduction
Prostate cancer is the second most diagnosed cancer and the fifth leading cause of cancer death in men worldwide (1). Racial disparities of prostate cancer have long been recognized, but their underlying causes need further research. Although socioeconomic factors could account for such differences to a certain extent, it is now increasingly accepted that such disparity may have genetic and molecular basis. In the United States, black men have the highest incidence and mortality rates from prostate cancer, followed by white and Hispanic men, with Asians and Pacific Islanders having the lowest rates. Furthermore, African-American (AA) men are often diagnosed with more advanced and aggressive prostate cancer compared with any other racial/ethnic group (2). As for prostate cancer mortality, the highest death rate was observed in the Caribbean, whereas the lowest rates were reported in most regions of Asia (3). Although the geographical variation in prostate cancer epidemiology can be partially explained by accessibility of screening tools and medical care, it is also influenced by genetic, lifestyle and other environmental factors. The molecular pathology of prostate cancer is complex. Besides somatic mutations and chromosomal abnormalities, which cause dysregulation of oncogenes and tumor repressor genes, changes in expression of growth factors and their receptors are involved in prostate cancer pathogenesis (4). In addition, metabolic syndrome including obesity and diabetes can also act modify for prostate cancer risk (5). Understanding how each of these different factors influence disease incidence and mortality is key to improving the current prevention, detection and treatment strategies.
With substantial research conducted on nuclear-encoded pathways, an emerging body of work on prostate cancer suggests a role for mitochondrion in this disease. Mitochondria are known as the ‘power plant’ of the cell and play a central role in many cellular functions (6). The circular double-stranded mitochondrial DNA (mtDNA) encodes 13 full-size proteins, all of which are essential components of the mitochondrial oxidative phosphorylation (OXPHOS) system (7). There is considerable cross talk between nucleus and mitochondria. Communication between these two organelles can happen in two ways: retrograde signaling from the mitochondria to the nucleus involving metabolic signals and anterograde signaling from the nucleus to the mitochondria involving cytosolic translated proteins translocated to the mitochondria. Although mitochondrion has its own genome and produces 13 essential electron transport chain proteins, its gene regulation machinery is composed entirely of nuclear-encoded proteins. Mitochondrial functions and metabolism are also vastly influenced by hundreds of nuclear-encoded mitochondrial enzymes. As for retrograde signaling, small molecules such as Ca2+, ATP:ADP ratio and NAD+: NADH ratio can act as secondary messengers and trigger signaling events, hence leading to activation of certain nuclear genes (8).
Mitochondrial functions are closely associated with aging and cancer progression. The first line of evidence supporting a link between cancer and mitochondria is the ‘Warburg effect’, where cancer cells rely heavily on aerobic glycolysis instead of OXPHOS to generate energy (9). This phenomenon could be an adaptation of cancer cells to hypoxic environment and higher demand on proliferation (10). It could also be a result of mitochondrial damage, such as OXPHOS dysfunction caused by mtDNA mutations. Moreover, the mutation rate of mtDNA is higher than that of nuclear DNA and this high mutation rate results in part from the mtDNA’s lack of protective histones and an inefficient DNA repair system (11). Although the causal relationship between cancer and mitochondria has not yet been fully established, mitochondrial impairment is clearly involved in the pathophysiology of prostate cancer (12). The mitochondrial metabolism of human prostate peripheral zone glandular epithelial cells is very unique. Due to the high activity of the zinc uptake transporters in these cells, their mitochondria accumulate substantial amount of zinc. High concentration of zinc can inhibit mitochondrial aconitase and OXPHOS, leading to truncated Krebs cycle and low respiration. In contrast to other tumor cells that utilize inefficient aerobic glycolysis and exhibit impaired OXPHOS, tumorigenesis of prostate peripheral zone glandular epithelial cells is associated with a switch from energy inefficient mitochondrial metabolism to energy efficient mitochondrial metabolism (13). Both large-deletion and point mutation in mtDNA have been found in prostate cancer. Point mutations in mtDNA might influence the activity of certain proteins encoded in mtDNA, depending on the site of these mutations. If the mutation is in a tRNA or an rRNA, it may affect the activity of multiple mitochondrial encoded proteins. If the mutation is in the control region, it may affect replication and transcription. In recent years, research in the field of small open-reading-frames (smORFs) has broadened our understanding about proteomics. mtDNA is a very compact genome, but the mito-smORFs concept adds additional complexity to the information it contains. In fact, it is now recognized that the mitochondrial genome is able to encode more than 13 proteins, as additional peptides can be derived from mitochondrial smORFs existing as ‘nested genes’ (14). The first mitochondrial-derived peptide (MDP) discovered was humanin, which was identified as an important signaling molecule involved in protection from neurodegeneration (15), atherosclerosis (16) and diabetes (17) as well as chemotherapy side effects in cancer models (18). More members of the MDP family, including MOTS-c and small humanin-like peptides (SHLPs), were later characterized as exercise mimetics and insulin sensitizers (19, 20) (Figure 1). Therefore, mutations in mtDNA may not only affect the OXPHOS system but also impair normal cellular homeostasis by changing MDP structure or expression. Cells with a high proportion of large-deletion mutant mtDNA might have characteristics similar to those with depleted mtDNA. Experimentally derived mtDNA-depleted cells (Rho-zero cells) are highly resistant to apoptosis, and some of them display invasive phenotypes (21). These observations suggest that dysregulated mitochondrial function as well as mtDNA are critical for cancer cells in the process of acquiring apoptosis resistance and invasiveness. Taken together, increases in mtDNA mutations together with the depletion of normal mtDNA have been shown to reduce the levels of some or all of the 13 enzymes required for OXPHOS, MDPs important for retrograde signaling, and might affect mitochondrial bioenergetics, redox regulation of cells and Ca2+ homeostasis.
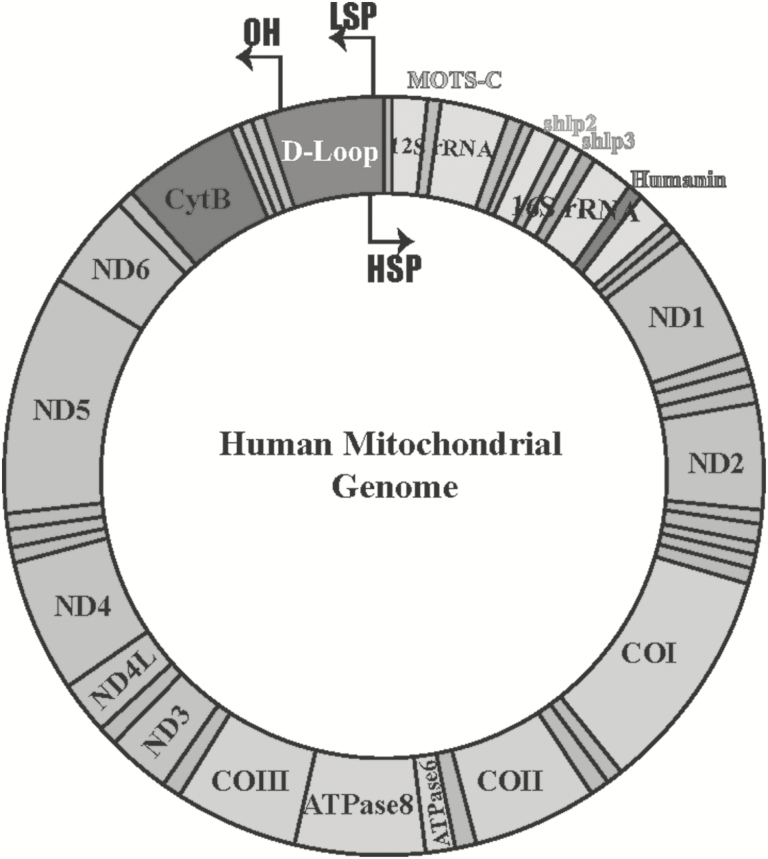
Figure 1.
The human mitochondrial genome. Relative locations of genes are represented as colored blocks including humanin (red), MOTS-c (blue), small humanin-like peptide 2 (SHLP 2) and SHLP3 (orange and green, respectively). Twenty-two tRNAs encoded from mitochondria are represented as teal-colored bands. HSP, heavy strand promoter; LSP, light strand promoter; OH, origin of heavy strand synthesis.
A number of molecular factors specific to tumor cells, including genetic and epigenetic modifications, were found to be associated with prostate cancer racial disparities. Such genetic alterations include single nucleotide polymorphisms (SNPs) as well as insertion/deletions (INDELs). Epigenetic alterations include histone modification and DNA methylation, and overexpression and/or suppression of miRNAs leading to altered tumor microenvironment (22). Chronic inflammation and oxidative stress will trigger biological responses that favor tumor invasion and create a supporting tissue environment for prostate cancer (23). As described earlier, mtDNA mutations and mitochondria integrity are directly related to prostate cancer pathology. It is thus reasonable to speculate that prostate cancer health disparities may have a mitochondrial component. In contrast to the nuclear genome, the mitochondrial genome demonstrates considerable geographic diversity and ethnic specificity, and therefore serves as an important check point for examining race-specific risk (24). Moreover, the compactness of mitochondrial genome enables relatively cheaper and more rapid identification of prostate-cancer-associated mutations and epigenetic modifications. Mitochondrial genetics, energy production/fuel utilization, apoptosis and retrograde signaling all potentially contribute to prostate cancer etiology and perhaps disparities and will be further discussed in detail in this review (Figure 2).
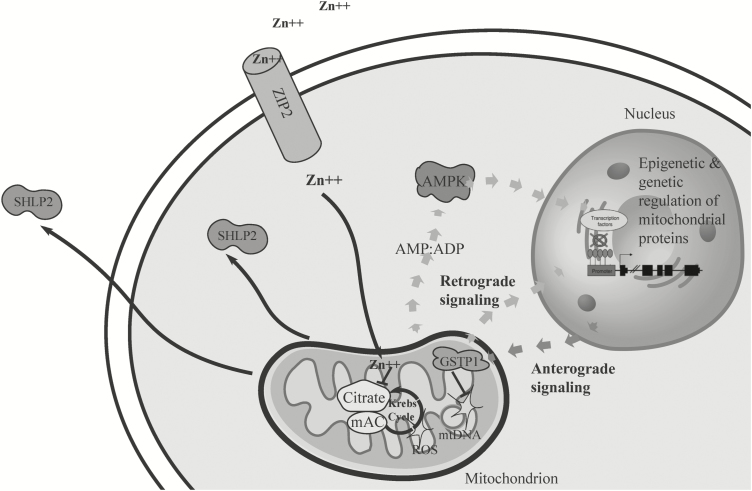
Figure 2.
The cross talk between mitochondrion and nucleus in prostate cancer. Schematic diagram demonstrating how reactive oxygen species (ROS), mitochondrial DNA (mtDNA) damage, mitochondrial metabolism and nuclear gene regulation interact to promote carcinogenesis. ADP, adenosine diphosphate; AMP, adenosine monophosphate; SHLP 2, small humanin-like peptide 2.
Mitochondrial-related genetic alterations in prostate cancer
Structurally, the mtDNA is composed of a coding region packed with protein encoding genes, ribosomal RNAs and transfer RNAs, and a non-coding displacement loop (D-loop) (7). The naked mtDNA is not protected by packaging proteins or the surveillance of efficient repair mechanisms (11). In addition, it is in close proximity to high levels of oxidative stress. Thus, mtDNA somatically mutates at a much higher rate than nuclear DNA, with the D-loop being the most polymorphic region (25–27). Both somatic mutations and active mutagenesis of mtDNA in neoplastic lesions have been observed in prostate cancer. Because each mitochondrion contains 3 to 10 copies of mtDNA and each cell has hundreds of mitochondria, mutations in mtDNA may result in the presence of more than one version of mitochondrial genome within a cell or individual, a state called heteroplasmy (28). These mutations will either lead to inhibition of electron transport chain, increase in mitochondrial reactive oxygen species (ROS) release, or create a more tumorigenic microenvironment. The mitochondrial D-loop is a ‘hotspot’ for mutagenesis, and has two hypervariable regions (29). The origin of replication and two origins of transcription of mtDNA reside in the D-loop; hence, mutations in this control region could have adverse impact on the integrity of mtDNA and expression of mitochondrial proteins and peptides. Genetic alterations in the D-loop have been reported in several prostate cancer studies. Analysis of mitochondrial D-loop sequences was performed using mtDNA isolated from pure populations of prostate cancer cells, benign epithelial gland cells and prostatic intraepithelial neoplasia cells (where available) (30, 31). The specific cell populations were obtained by laser capture microdissection from 16 prostatectomy specimens. The mitochondrial sequence from benign epithelial gland cells from the same individual was used as reference. In 90% of the tumors and/or prostatic intraepithelial neoplasia samples, heteroplasmic and homoplasmic somatic mutations were detected in 34 nucleotide positions, 30 of which were substitutions and 4 of which were INDELs. Two D-loop regions—a mononucleotide sequence repeat and a dinucleotide microsatellite segment—were more susceptible to INDELs. However, the mutations were not correlated with tumor grade or age of the patients. Apart from prostate cancer, other cancer types such as cervical carcinoma and colorectal cancer also reported high frequency of D-loop alterations and mitochondrial microsatellite instability (27–29).
Multiple SNPs and INDELs were also found in the coding region of cancer patients’ mtDNA and are dramatically overrepresented in prostate cancer cases. The coding region of the mitochondrial genome is also susceptible to mutagenesis, and such mutations might be important for prostate cancer risk assessment. For example, mtDNA G10398A polymorphism has been associated with invasive breast cancer in AA women and with a higher risk of prostate cancer in AA men (32–34). Cybrid cells harboring this specific mutation showed elevated ROS production, increased apoptosis resistance and tumorigenicity. G10398A resides in the gene encoding NADH dehydrogenase 3 (ND3), a core component of mitochondrial complex I (32). The G to A polymorphism in ND3 causes the substitution of alanine by threonine in amino acid 114 in the ND3 polypeptide. Oddly, this substitution was accompanied by increased level of 20 kDa complex I subunit and enhanced complex I activity. With an increase in complex I activity and level of complex III activity remained unchanged, the electron transport chain may be in a more reduced state, thereby increasing the retention time of electrons and providing more opportunity for univalent reduction of oxygen, as complexes I and III are the major sources of ROS in cells. Further experiments proved G10398A cybrids were able to evade etoposide-induced apoptosis and demonstrated greater ability to metastasize. The potential mechanism for this is that mtDNA-mutation-induced OXPHOS activity decline will stimulate ROS release; consequently, the elevated oxidative stress will change tumor microenvironment and confer invasiveness. Another independent study also corroborated the mtDNA mutation and ROS observation. The pathogenic mtDNA mutation (np 8993G) was introduced into PC3 cells by cybrid formation. Wild-type and mutant cells were injected into nude mice and allowed to grow. It was found that after 110 days, the tumor volume was more than seven times greater in those animals receiving mutant cells compared with wild-type cells (35). This finding demonstrates the potential in vivo relevance of ROS in prostate tumor growth. Several studies have shown that the mutations in the mitochondrial cytochrome c oxidase subunit 1 (COI) were correlated with prostate cancer. A comprehensive population-based study of mtDNA mutations in prostate cancer was reported by Petros et al. (35). In this study, the entire mitochondrial genome of one individual tumor was first sequenced. In total, 38 substitution mutations were seen, 31 of which were reported polymorphisms and the rest were new mutations. The analysis of 260 prostate cancer patients revealed that 31 (12%) had an inherited mutation in COI compared with <2% of non-cancer controls. They also analyzed a population sample of 1019 European and African mtDNA sequences, and the frequency of COI mutations was found to be 7.8%, which was still lower than the 12% observed in the prostate cancer cohort. The overrepresentation of COI polymorphisms in prostate cancer cases suggests that mutations in the COI gene might be a risk factor for developing prostate cancer (35). Given that COI polymorphisms are more frequent in African mtDNA than in the rest of the world, these SNPs might be genetic determinants for prostate cancer racial disparities. The importance of this mutation analysis is the large sample size, which permitted an epidemiological study of the mtDNA mutations. However, in vitro or in vivo experiments have not yet been conducted, and thus mechanisms could not be easily identified. The presence of somatic mutations in mt-tRNA was also found to be associated with elevated prostate-specific antigen levels in prostate cancer patients (36). Mutations in tRNAs can disrupt mitochondrial gene processing, aminoacylation and translation. The accumulation of non-functional mitochondrial proteins could affect mitochondrial integrity and metabolic fitness, leading to increased leaking of prostate-specific antigen into the blood circulation. All the genetic variations mentioned earlier are summarized in Table I.
Table 1.
A list of mitochondrial-related proteins altered in prostate cancer
| Functions | Modifications in prostate cancer | References | |
|---|---|---|---|
| Mitochondrial-encoded proteins | |||
| D-loop | Replication/transcription origins | Substitutions and INDELs | (30, 31) |
| MT-ND3 | NADH dehydrogenase 3 | Substitutions | (32–34) |
| MT-ATP6 | ATP synthase, translocation of protons and generation of energy | Substitutions | (35) |
| MT-COI | Cytochrome c oxidase I | Substitutions and upregulated expression | (35, 37) |
| MT-tRNAs | Ribosomal functions, protein translation | Substitutions | (36) |
| Nuclear-encoded mitochondrial proteins | |||
| GSTP1 | Antioxidant mechanism | Hypermethylation | (38) |
| ZIP2 | Zinc transporter | Downregulated expression | (39, 40) |
| SOD2 | Antioxidant mechanism | Substitutions | (41) |
| HSP60 | Mitochondrial chaperon, facilitates proper protein folding | Downregulated expression | (42) |
| POLG | mtDNA polymerase | Hypermethylation | (43) |
| PPARGC1A | Mitochondrial biogenesis | Hypermethylation | (44) |
Although the mitochondrial coding region is intronless, smORF can exist as ‘genes within genes’. Previously, silent mutations in the mitochondrial genome were often considered as harmless, as they do not change amino acid composition of the 13 proteins. Nowadays, with the concept of smORF-derived peptides and MDPs fully recognized, these silent mutations may no longer be ‘silent’. They could change MDP structure or even cause truncation of peptides. Humanin is the first MDP identified, and it is encoded within a smORF in the 16S rRNA gene. This 24-amino acid peptide exerts cytoprotective and metabolon-enhancing activity by interacting with IGFBP-3, BAX/BID directly or through receptor-mediated signaling (45). The SNP G2706A is one of the two ancestral mitochondrial mutations that define mitochondrial Haplogroup H. This polymorphism sits in the 16S rRNA and the stop codon of humanin; therefore, it might change the shape of the RNA of the mitochondrial ribosome and the protective peptide humanin. The minor allele (G:G) is predominantly found in Europe, whereas A:A is found in Asia and Africa (46). The racially disparate distribution of this SNP might provide a molecular mechanism for prostate cancer ethnic disparity. The A1382C polymorphism in the MOTS-c coding region is specific to Northeast Asian population, and might be a putative explanation for exceptional longevity of Japanese people (47). Although it might not be directly linked with prostate cancer, it is associated with aging, which is the greatest risk factor for cancer. In a newly reported study, we showed that low circulating levels of the MDP SHLP2 represent a novel biomarker for prostate cancer risk (48). SHLP2 is mitochondrially encoded and also regulates mitochondrial function, and therefore low SHLP2 levels might represent mitochondrial dysfunction (Figure 3). Mitochondrial metabolic transformation is a key event in prostate cancer; lower circulating SHLP2 levels may indicate a microenvironment favoring tumorigenesis and progression. Because SHLP2 has anti-oxidative properties, lower SHLP2 might exacerbate mitochondrial oxidative stress, which could be the major driving force of mitochondrial genome instability and contribute to changes in mitochondrial metabolism. Insulin resistance has been linked to higher risk of prostate cancer, possibly through upregulation of inflammation, higher oxidative burden and unbound testosterone (49). SHLP2 may reduce prostate cancer risk by acting as an insulin sensitizer, which it has been demonstrated to be in mouse models. This study also found that the SHLP2 levels in white men were higher than those of black men, which suggests a possible molecular link between prostate cancer risk and ethnicity. It is speculated that the difference in SHLP2 levels between races could be a manifestation of differences in mitochondrial functions and metabolism. Many environmental and lifestyle factors, such as exercise and diet composition, can lead to this racial disparity. Furthermore, mtDNA of black men may harbor SNPs that repress SHLP2 expression or change its protein structure.
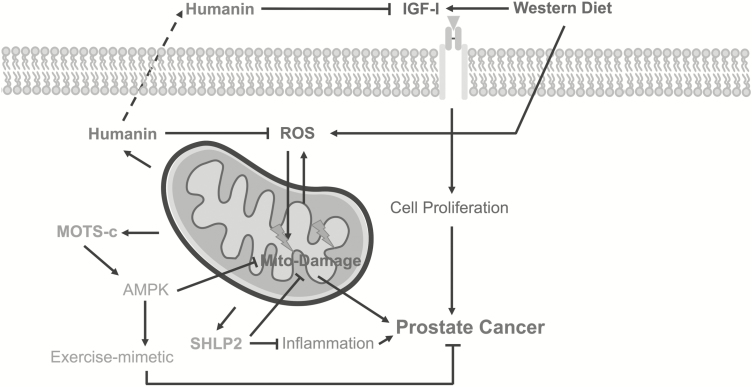
Figure 3.
Mode of action of mitochondrial-derived peptides (MDPs) against prostate cancer. Humanin, MOTS-c and small humanin-like peptide 2 (SHLP 2) are encoded in the mitochondrial genome and participate in various signaling events intracellularly or extracellularly. Humanin can suppress prostate cancer by inhibiting mitochondrial-generated reactive oxygen species (ROS) and reducing the levels of free-circulating insulin-like growth factor-I (IGF-I). MOTS-c exerts antitumor effects via acting as an exercise mimetic, whereas SHLP2 alleviates inflammation and promotes mitochondria health.
Numerous studies have focused on the overall mutational load of mitochondria and mitochondrial haplogroups. Next-generation sequencing of mtDNA from prostate tissue biopsies and matched blood of 115 men revealed a positive correlation between the total burden of acquired mtDNA variation and elevated Gleason score at diagnosis and biochemical relapse (50). Two additional studies also proved that the accumulation of mutations over the entire mitochondrial genome contributes to prostate cancer progression (51). However, whether there is difference in mitochondrial mutational load between ethnic groups is not known. Mitochondrial haplogroups are used to trace the matrilineal inheritance and represent the major phylogenetic branches. Previous studies have suggested that inherited mitochondrial genome variation, which defines population-specific haplogroups, may be associated with prostate cancer risk. Specifically, a study of 221 white North American men showed increased risk in haplogroup U for developing prostate cancer (odds ratio 1.95) (52). Whereas in two other independent studies, common European-derived mtDNA haplogroups are not correlated with increased prostate cancer risk or specimen Gleason score (53). In addition, no association between mitochondrial haplogroups and prostate cancer was found in a Korean population or a Colombian population (37, 54). Apart from population studies, a number of groups have used cybrid models to support the concept that mtDNA haplogroups can influence nuclear gene expression, rates of cell growth and cell behavior. The cellular energetics and gene expression profile were compared between European H cybrids and the African L cybrids in one study (46). It was found that mtDNA haplogroup variants can greatly influence the efficiency of respiration, irrespective the nuclei. The African L cybrids exhibited lower mtDNA copy number but higher mitochondrial gene expression. The L cybrids also have lower ATP turnover rates and lower spare respiratory capacity, which suggests that they may not be able to respond to stress as readily as the H cybrids. The potential association between the African-derived mtDNA haplogroups and aggressive prostate cancer remains to be confirmed, but the cybrid study may provide insight into the mitochondrial determinants of prostate cancer health disparity.
One limitation of current research on mtDNA mutations and prostate cancer is the lack of comprehensive study of the association between mtDNA mutations and tumor grade. Among the studies described previously, most of them fail to mention the association between mtDNA mutations and Gleason grade. As AA men are often diagnosed with more aggressive prostate cancer, it would be ideal to identify a mtDNA mutation that is linked with Gleason grade. Most of the reported mtDNA alterations in prostate cancer to date only involve individuals with Gleason grades 6–7, the most frequent clinical grade at presentation (55). Therefore, future studies need to include more subjects with lower grades and higher grades to ascertain whether there is an association between specific mitochondrial mutations or mutation load and Gleason grade or tumor stage.
Mitochondrial content and proteomic changes in prostate cancer
Mitochondrial genetics is not the only potential molecular determinant of mitochondrial-related prostate cancer health disparity. As mentioned earlier, tumor growth is accompanied by cellular adaptations to hypoxic environment, cell cycle control and excessive proliferation. Mitochondria are key organelles for energy production with a critical role in cell survival and apoptosis. Thus, transformation of normal cells to cancer cells usually involves modulation of mitochondrial protein expression. Mitochondrial proteins consist of mitochondrially translated proteins and nuclear-encoded mitochondrial enzymes. As for the 13 polypeptides and MDPs, their expression is affected not only by mtDNA mutations, but also by the changes in mitochondrial content and transcription/translation machinery. It has been reported that mitochondrial biogenesis and quantity of mitochondria varied between control and prostate cancer patients. Grupp et al.(56) found that the mitochondrial content was tightly linked to pathological features of prostate cancer. A tissue microarray of 11 152 prostate cancer specimens was analyzed in order to evaluate the clinical significance of mitochondria abundance. The mitochondrial content was determined by staining with anti-MTCO2 antibody. The results demonstrated marked increase of mitochondrial content in cancer cells when compared with normal prostate epithelial cells. Moreover, higher numbers of mitochondria were observed with increasing tumor grade and stage, which suggests that increase in mitochondrial content is necessary or supportive for cancer development and progression. The literature also suggests that increase in mtDNA copy number and mitochondrial abundance is required for cancer cell proliferation, and prostate cancer progression is accompanied by the activation of core mitochondrial biogenesis regulators (57). These findings may seem contradictory to the mitochondrial damage theory of prostate cancer, which claims that mitochondrial function decline is a cause of prostate cancer. Nevertheless, there is a possible hypothesis for this discrepancy. Normal prostate epithelial cells accumulate mtDNA damage over time, which may lead to loss of mitochondrial integrity and transformation into cancerous phenotypes. As a result, mitochondrial biogenesis pathways are activated as a compensatory mechanism, thereby increasing the quantity of dysfunctional mitochondria and facilitating cancer cell proliferation. In fact, the upregulation of mtDNA copy number and mitochondrial gene expression in response to DNA damage has been reported in various cancer cases (58). On the contrary, there is evidence supporting a negative association between mitochondrial content and cancer risk. It has been reported that the reduced mtDNA content has been associated with poor prognosis of prostate cancer in AA men (59). Biswas et al. (39) reported that decreased mtDNA content could activate the NF-kB/Rel factors. Activation of NF-kB signaling confers apoptosis resistance and plays pivotal role in cancer malignant transformation. In addition, activation of the AKT pathway by mtDNA deficiency could also inhibit cell apoptosis (40). These results indicate that mitochondrial content and quality have to be maintained at an optimal level. Higher and lower levels of mitochondrial mass might be a risk factor for prostate cancer. As mentioned earlier, different mitochondrial haplogroups demonstrated different levels of mtDNA copy number, which suggests that the difference in mtDNA level between ethnic groups may be a determinant of prostate cancer risk or grade.
The prostate glandular epithelial cells experience drastic metabolic transformation during tumorigenesis, and the transformation must involve biochemical reactions occurred in the mitochondria and regulation at the proteomic level. In other words, proteomic changes could facilitate prostate cancer progression. About 80% of prostate malignancies occur in the peripheral zone of the prostate gland, where the cells are highly specialized secretory cells. Different from cells in the central zone and the rest of the body, peripheral zone cells accumulate substantial amounts of zinc, due to high activity of the zinc uptake transporters (41). The presence of high zinc concentrations in the mitochondria of the peripheral zone cells inhibits the activity of the enzyme mitochondrial aconitase, which prevents the oxidation of citrate through the Krebs cycle. In contrast, malignant cells exhibit marked decrease in zinc and citrate levels. The reduction in mitochondrial zinc levels renders prostate cells energy efficient, as the mitochondrial aconitase is no longer inhibited and production of ATP resumes. Moreover, the low levels of zinc lead to elimination of the proapoptotic effect of zinc. Together with the increased energy production, the suppression of apoptosis promotes proliferation of malignant cells. The zinc homeostasis is tightly regulated by zinc transporters. Recent studies discovered that ZIP2 was abundantly present in normal prostate epithelial cells and in benign prostate hyperplasia, whereas its expression was downregulated in prostate cancer tumor cells. Furthermore, it was found that ZIP2 was significantly lower in AA patients, who generally have higher risk to develop prostate cancer compared with age-matched white individuals. Consistent with this notion, ZIP2 levels were found to be more reduced in tissue samples from AA men compared with Gleason-grade-matched samples from white males (42).
An alternative hypothesis for zinc-depletion-induced malignant transformation is that normal prostate glandular epithelial cells have truncated Krebs cycle and low levels of ROS. These cells may have low oxidative stress tolerance when they encounter this metabolic switch and start to produce more ROS. The mitochondrial antioxidant pathways and enzymes are therefore important for preventing ‘the vicious cycle’ from progressing. Kang et al. (60) studied the association between genetic polymorphisms in three isoforms of superoxide dismutase (SOD) and prostate cancer. Supposedly, dysfunctional antioxidant enzymes are most likely to exacerbate mitochondrial damage and promote cancer development. However, it was found that the Ala variant at SOD2 Ex2 + 24T>C(V16A), despite having higher superoxide scavenging activity, was associated with elevated prostate cancer risk in Caucasians. This finding is incompatible with the original hypothesis. One possible explanation is that higher SOD catalytic activity results in more superoxide to H2O2 conversions. H2O2 accumulation in the cell may also induce DNA damage and hence stimulate carcinogenesis. Stratification by quartiles of dietary and supplemental vitamin E intake demonstrated that smoking and vitamin E intake were modifiers for prostate cancer risk among SOD2 Ala variant carriers; Ala variant carriers who are smokers and have low vitamin E intake are associated with moderately increased risk of prostate cancer. No significant association with prostate cancer was observed for polymorphic variants in SOD3 or SOD1.
Mitochondrial heat-shock proteins (HSPs) and proteases are known to be upregulated to maintain protein folding homeostasis and mitochondrial function. This is mediated by mitochondrial unfolded protein response (UPRmt), a stress response induced by perturbations in the protein-folding environment. It has been reported that the UPRmt is different between cells derived from white and black men with prostate cancer. In fact, gene expression of UPRmt chaperone proteins HSP60 and DNAj was elevated in CA cells compared with AA cells (61). For cancer cells, UPRmt can send proapoptotic signal via MAPK activation (62). As a result, the patients of African descent may have defective apoptosis pathway and more aggressive tumorigenesis. Thus, the differential features of UPRmt might partially explain racial difference in prostate cancer.
Epigenetics and mitochondria in prostate cancer
DNA methylation is the addition of a methyl group to the cytosine within cytosine guanine dinucleotides. DNA methylation at multiple adjacent cytosine guanine dinucleotide sites on promoter region ultimately causes gene silencing by blocking the access of transcriptional factors and/or activators to the target sites. Importantly, most mitochondria proteins are nuclear-encoded proteins. Thus, epigenetic modifications on promoter regions of these nuclear-encoded mitochondrial genes are likely to alter mitochondrial behavior. One study screened 899 mitochondrial-acting nuclear genes and identified 636 genes exhibiting tissue-specific and differentially methylated patterns (63). This study further proved that the tissue-dependent mitochondrial functions were regulated by the methylation status of nuclear mito-genes. Moreover, another study has concluded that DNA methylation levels of the cytosine guanine dinucleotide islands in nuclear-encoded mitochondrial genes were lower than that of non-mitochondrial genes (64). The latter finding was then cross-validated by the data from the HumanMethylation450 BeadChip containing probes that lay within 1000 bp of transcription start sites. Most probes (>5000) at the transcription start sites of mitochondrial proteins showed hypomethylation, whereas 400 probes showed hypermethylation (38). Abundant evidence has accumulated to suggest that epigenetic alterations are significantly different in the prostate tumors of black and white men. Of all the genes examined in prostate tumors so far, glutathione S-transferase Pi 1 (GSTP1) is a mitochondrial antioxidant enzyme (43). Comparison of GSTP1 methylation status with their clinical and pathological outcomes showed that black men carrying GSTP1 hypermethylation were 13.3 times more likely to have prostate cancer, whereas in white men, this ratio was only 3.8 (44). This study suggested that the methylation status of GSTP1 might serve as a putative biomarker for prostate cancer diagnostics in African-descent populations. Apart from epigenetic regulation of mitochondrial enzymes, methylation of mitochondrial proteins responsible for mtDNA replication has also been observed. DNA polymerase gamma (POLG) is involved in the replication of mtDNA and has proofreading functions. It was reported that POLG expression was regulated by the methylation of the POLG gene transcription start site. As an essential gene dictating mtDNA replication, the POLG methylation levels were found to be negatively associated with mtDNA copy number (65). Methylation of the PPARGC1A gene, which is the master regulator of mitochondrial biogenesis, has also been correlated negatively with mtDNA copy number (66). The rationale is relatively straightforward; inhibition of mitochondrial biogenesis via methylation of PPARGC1A will result in suppression of genes promoting mitochondria fission and mtDNA replication, thereby reducing mtDNA copy number. Moreover, evidence gathered from cybrid experiments have revealed that the mitochondria can affect nuclear methylation patterns (67). Given the importance of epigenetics to prostate cancer regulation, and the uniqueness of mitochondria in this disease, more attention and endeavor on this topic are needed to decipher molecular basis of prostate cancer and its ethnic disparity.
Environmental factors in prostate cancer
The most important environmental factors to consider are diet and obesity, as diet is highly variable across ethnicities and obesity has been consistently associated with an increased risk of prostate cancer aggressiveness and mortality. Among the most obvious characteristics of the Western diet are intake of high caloric and fatty foods. Several epidemiological, interventional and animal model studies have not only indicated strong correlations between fat consumption and the rate of prostate cancer mortality (68), but also proved that low-fat diet can slow the rate of LAPC-4 prostate cancer xenograft growth (69). Saturated fat, which is largely fat from animal sources, has been correlated strongly with prostate cancer mortality and increased aggressiveness, although no statistically significant racial differences were observed (70). High intake of dietary fat can contribute to prostate cancer in several different ways, such as influencing androgen signaling, the insulin-like growth factor (IGF) pathway and cell proliferation (71). Although molecular explanation of how fat intake can cause alterations in mitochondria is not fully understood, it is speculated that the fatty acids metabolism plays a critical role (72). The mitochondria are where fatty acids are broken down by beta-oxidation and enter Krebs cycle. The difference in richness of saturated fat in diet may place differential levels of burden on mitochondria. The high levels of fat content may cause mitochondrial damage and decline, which later contributes to prostate cancer initiation. Each ethnic group has different diet preference, thereby possessing different levels of prostate cancer risk.
Exercise is also a key determinant of cancer risk and can affect mitochondrial function (73). Physical activity has been shown to improve patient survival after diagnosis and is linked with reduced risks of cancer. As for prostate cancer, limited evidence has been provided. One study evaluated physical activity of patients suffering from non-metastatic prostate cancer and found out that physical activity improved cancer survival as well as overall survival (74). Another study of 4623 men with localized prostate cancer confirmed the former finding (75). The underlying molecular pathway involves IGF axis, p53 and mitochondrial apoptosis pathway. The serum from men with regular exercise was used to culture LNCaP cells and exhibited higher levels of apoptosis than cells cultured in serum from sedentary controls. The exercise serum stimulated cells also showed higher expression of p53 and Bcl-2 (76).
Exercise decreased serum IGF-I and increased serum IGF binding protein-1 (IGFBP-1), which suggests that IGF-I and IGFBP-1 levels are mediated by diet and exercise. In fact, reduction of IGF-I levels in the circulation has long been proposed as a mechanism of caloric restriction, which is a pro-longevity regimen. The mitochondrial peptide humanin and MOTS-c are caloric restriction and exercise mimetic, respectively. Humanin is a new player in the regulation of the growth hormone–IGF-I axis and interacts with IGFBP-3 to decrease circulating IGF-I levels (77). In other words, decline in mitochondrial integrity and reduction in humanin levels may disrupt the diet-IGF-I pathway. As a result, the suppression of IGF-I by humanin will be altered and the resultant elevated IGF-I levels will promote malignant cell growth (Figure 3). The same rationale applies for exercise-mediated antitumorigenesis effects by MOTS-c (19). Information concerning human genetic polymorphisms in the genetic pathways involved in hormone synthesis, metabolism and action is now accumulating very rapidly. Together with the SNPs found in the MDP coding regions, more comprehensive mitochondrial sequencing studies are required to unravel the roles of MDPs in prostate cancer.
Conclusion
The molecular pathology of prostate cancer is complex and involves multiple elements involving genetic, epigenetic, proteomic and environmental factors, which are all involved in the ethnically disparate disease. Mitochondria are critical organelles for energy metabolism, apoptosis and various essential cellular functions. Mitochondrial function and mito-SNPs may provide partial explanation for the racial disparity of prostate cancer. This review summarizes how mitochondrial genetic polymorphisms and mother mitochondria-related aberrations contribute to the increased incidence, treatment resistance, metastatic potential and tumor recurrence in minority populations, and should direct future endeavors in basic, clinical and translational research on prostate cancer health disparities.
Funding
National Institutes of Health (R01AG034430, DOD PC160353, P50CA92131 and P01AG034906 to P.C.).
Conflict of Interest Statement: P.C. is a consultant and stockholder of CohBar Inc.
Abbreviations
- AA
African American
- COI
cytochrome c oxidase subunit
- D-loop
displacement loop
- GSTP1
glutathione S-transferase Pi 1
- INDELs
insertion/deletions
- MDP
mitochondrial-derived peptide
- mtDNA
mitochondrial DNA
- OXPHOS
oxidative phosphorylation
- POLG
polymerase gamma
- SHLP
small humanin-like peptide
- smORFs
small open-reading-frames
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- UPRmt
mitochondrial unfolded protein response
References
- 1. Zhou C.K., et al. (2016)Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int. J. Cancer, 138, 1388–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimura T. (2012)East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin. J. Cancer, 31, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong M.C., et al. (2016)Global incidence and ortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur. Urol., 70, 862–874. [DOI] [PubMed] [Google Scholar]
- 4. Cazares L.H., et al. (2010)Molecular pathology of prostate cancer. Cancer Biomark., 9, 441–459. [DOI] [PubMed] [Google Scholar]
- 5. Hsing A.W., et al. (2007)Obesity, metabolic syndrome, and prostate cancer. Am. J. Clin. Nutr., 86, s843–s857. [DOI] [PubMed] [Google Scholar]
- 6. Wallace D.C. (1999)Mitochondrial diseases in man and mouse. Science, 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- 7. Anderson S., et al. (1981)Sequence and organization of the human mitochondrial genome. Nature, 290, 457–465. [DOI] [PubMed] [Google Scholar]
- 8. Poyton R.O., et al. (1996)Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem., 65, 563–607. [DOI] [PubMed] [Google Scholar]
- 9. Weinhouse S., et al. (1956)On respiratory impairment in cancer cells. Science, 124, 267–272. [DOI] [PubMed] [Google Scholar]
- 10. Hsu P.P., et al. (2008)Cancer cell metabolism: Warburg and beyond. Cell, 134, 703–707. [DOI] [PubMed] [Google Scholar]
- 11. Neiman M., et al. (2009)The causes of mutation accumulation in mitochondrial genomes. Proc. Biol. Sci., 276, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parr R.L., et al. (2013)Mitochondria, prostate cancer, and biopsy sampling error. Discov. Med., 15, 213–220. [PubMed] [Google Scholar]
- 13. Costello L.C., et al. (2000)The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology, 59, 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yen K., et al. (2013)The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J. Mol. Endocrinol, 50, R11–R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim S.J., et al. (2016)The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget, 7, 46899–46912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bachar A.R., et al. (2010)Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc. Res., 88, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoang P.T., et al. (2010)The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism., 59, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen P. (2014)New role for the mitochondrial peptide humanin: protective agent against chemotherapy-induced side effects. J. Natl. Cancer Inst., 106, dju006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee C., et al. (2015)The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab., 21, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cobb L.J., et al. (2016)Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany. NY), 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moro L., et al. (2009)Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3K/Akt2. Cell Death Differ., 16, 571–583. [DOI] [PubMed] [Google Scholar]
- 22. Shiao S.L., et al. (2016)Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett., 380, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landskron G., et al. (2014)Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res., 2014, 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galtier N., et al. (2009)Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol. Ecol., 18, 4541–4550. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Y.B., et al. (2005)Mutation in D-loop region of mitochondrial DNA in gastric cancer and its significance. World J. Gastroenterol., 11, 3304–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma H., et al. (2005)Mutations in the mitochondrial DNA D-loop region are frequent in cervical cancer. Cancer Cell Int., 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang S.C., et al. (2009)Mitochondrial D-loop mutation is a common event in colorectal cancers with p53 mutations. Int. J. Colorectal Dis., 24, 623–628. [DOI] [PubMed] [Google Scholar]
- 28. Payne B.A., et al. (2013)Universal heteroplasmy of human mitochondrial DNA. Hum. Mol. Genet., 22, 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoneking M. (2000)Hypervariable sites in the mtDNA control region are mutational hotspots. Am. J. Hum. Genet., 67, 1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J.Z., et al. (2002)Extensive somatic mitochondrial mutations in primary prostate cancer using laser capture microdissection. Cancer Res., 62, 6470–6474. [PubMed] [Google Scholar]
- 31. Jerónimo C., et al. (2001)Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene, 20, 5195–5198. [DOI] [PubMed] [Google Scholar]
- 32. Kulawiec M., et al. (2009)mtDNA G10398A variant in African-American women with breast cancer provides resistance to apoptosis and promotes metastasis in mice. J. Hum. Genet., 54, 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Canter J.A., et al. (2005)Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res., 65, 8028–8033. [DOI] [PubMed] [Google Scholar]
- 34. Singh K.K., et al. (2009)Mitochondrial DNA polymorphism and risk of cancer. Methods Mol. Biol., 471, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petros J.A., et al. (2005)mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA, 102, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kloss-Brandstätter A., et al. (2010)Somatic mutations throughout the entire mitochondrial genome are associated with elevated PSA levels in prostate cancer patients. Am. J. Hum. Genet., 87, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim W., et al. (2008)Mitochondrial DNA haplogroup analysis reveals no association between the common genetic lineages and prostate cancer in the Korean population. PLoS One, 3, e2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massie C.E., et al. (2017)The importance of DNA methylation in prostate cancer development. J. Steroid Biochem. Mol. Biol., 166, 1–15. [DOI] [PubMed] [Google Scholar]
- 39. Biswas G., et al. (2003)Mitochondria to nucleus stress signaling: a distinctive mechanism of NFkappaB/Rel activation through calcineurin-mediated inactivation of IkappaBbeta. J. Cell Biol., 161, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelicano H., et al. (2006)Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J. Cell Biol., 175, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Costello L.C., et al. (2004)Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis., 7, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rishi I., et al. (2003)Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol., 11, 253–260. [DOI] [PubMed] [Google Scholar]
- 43. Lee W.H., et al. (1994)Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc. Natl. Acad. Sci. USA, 91, 11733–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gong M., et al. (2012)Genetic polymorphisms of GSTM1, GSTT1, and GSTP1 with prostate cancer risk: a meta-analysis of 57 studies. PLoS One, 7, e50587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ikonen M., et al. (2003)Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. USA, 100, 13042–13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kenney M.C., et al. (2014)Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim. Biophys. Acta, 1842, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fuku N., et al. (2015)The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity?Aging Cell, 14, 921–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiao J., et al. (2017)Low circulating levels of the mitochondrial-peptide hormone SHLP2: novel biomarker for prostate cancer risk. Oncotarget, 8, 94900–94909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsing A.W., et al. (2003)Insulin resistance and prostate cancer risk. J. Natl. Cancer Inst., 95, 67–71. [DOI] [PubMed] [Google Scholar]
- 50. Kalsbeek A.M., et al. (2016)Mutational load of the mitochondrial genome predicts pathological features and biochemical recurrence in prostate cancer. Aging (Albany. NY), 8, 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arnold R.S., et al. (2009)Mitochondrial DNA mutation stimulates prostate cancer growth in bone stromal environment. Prostate, 69, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Booker L.M., et al. (2006)North American white mitochondrial haplogroups in prostate and renal cancer. J. Urol., 175, 468–72; discussion 472. [DOI] [PubMed] [Google Scholar]
- 53. Álvarez-Cubero M.J., et al. (2012)Mitochondrial haplogroups and polymorphisms reveal no association with sporadic prostate cancer in a southern European population. PLoS One, 7, e41201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cano D., et al. (2014)Mitochondrial DNA haplogroups and susceptibility to prostate cancer in a Colombian population. ISRN Oncol., 2014, 530675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khan M.A., et al. (2003)Probability of biochemical recurrence by analysis of pathologic stage, Gleason score, and margin status for localized prostate cancer. Urology, 62, 866–871. [DOI] [PubMed] [Google Scholar]
- 56. Grupp K., et al. (2013)High mitochondria content is associated with prostate cancer disease progression. Mol. Cancer, 12, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reznik E., et al. (2016)Mitochondrial DNA copy number variation across human cancers. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bai R.K., et al. (2005)Simultaneous detection and quantification of mitochondrial DNA deletion(s), depletion, and over-replication in patients with mitochondrial disease. J. Mol. Diagn., 7, 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koochekpour S., et al. (2013)Reduced mitochondrial DNA content associates with poor prognosis of prostate cancer in African American men. PLoS One, 8, e74688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kang D., et al. (2007)Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol. Biomarkers Prev., 16, 1581–1586. [DOI] [PubMed] [Google Scholar]
- 61. Chaudhary A.K., et al. (2016)Mitochondrial dysfunction-mediated apoptosis resistance associates with defective heat shock protein response in African-American men with prostate cancer. Br. J. Cancer, 114, 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rico-Bautista E., et al. (2013)Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling. Oncotarget, 4, 1212–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takasugi M., et al. (2010)DNA methylation status of nuclear-encoded mitochondrial genes underlies the tissue-dependent mitochondrial functions. BMC Genomics, 11, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chinnery P.F., et al. (2012)Epigenetics, epidemiology and mitochondrial DNA diseases. Int. J. Epidemiol., 41, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kelly R.D., et al. (2012)Mitochondrial DNA copy number is regulated in a tissue specific manner by DNA methylation of the nuclear-encoded DNA polymerase gamma A. Nucleic Acids Res., 40, 10124–10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao H., et al. (2017)Epigenetic regulation of an adverse metabolic phenotype in polycystic ovary syndrome: the impact of the leukocyte methylation of PPARGC1A promoter. Fertil. Steril., 107, 467–474.e5. [DOI] [PubMed] [Google Scholar]
- 67. Bellizzi D., et al. (2012)Global DNA methylation levels are modulated by mitochondrial DNA variants. Epigenomics, 4, 17–27. [DOI] [PubMed] [Google Scholar]
- 68. Park S.Y., et al. (2007)Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int. J. Cancer, 121, 1339–1345. [DOI] [PubMed] [Google Scholar]
- 69. Ngo T.H., et al. (2003)Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin. Cancer Res., 9, 2734–2743. [PubMed] [Google Scholar]
- 70. Allott E.H., et al. (2017)Saturated fat intake and prostate cancer aggressiveness: results from the population-based North Carolina-Louisiana Prostate Cancer Project. Prostate Cancer Prostatic Dis., 20, 48–54. [DOI] [PubMed] [Google Scholar]
- 71. Di Sebastiano K.M., et al. (2014)The role of dietary fat throughout the prostate cancer trajectory. Nutrients, 6, 6095–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sadowski M.C., et al. (2014)The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget, 5, 9362–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schadler K.L., et al. (2016)Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget, 7, 65429–65440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kenfield S.A., et al. (2011)Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol., 29, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bonn S.E., et al. (2015)Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol. Biomarkers Prev., 24, 57–64. [DOI] [PubMed] [Google Scholar]
- 76. Barnard R.J., et al. (2007)A mechanism to explain how regular exercise might reduce the risk for clinical prostate cancer. Eur. J. Cancer Prev., 16, 415–421. [DOI] [PubMed] [Google Scholar]
- 77. Xiao J., et al. (2016)Humanin: functional interfaces with IGF-I. Growth Horm. IGF Res., 29, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]