Abstract
OBJECTIVES:
To determine whether women with surgical menopause have a higher risk of frailty than naturally menopausal women.
DESIGN:
Prospective cohort study with up to 18 years of follow-up.
SETTING:
Four U.S clinical centers.
PARTICIPANTS:
Community-dwelling white women aged 65 and older (mean 71.2 ± 5.2) enrolled in the Study of Osteoporotic Fractures (N=7,699).
MEASUREMENTS:
Surgical menopause was based on participant self-report of having undergone bilateral oophorectomy before menopause. The outcome was incident frailty, classified as robust, prefrail, frail, or death at 4 follow-up interviews, conducted 6 to 18 years after baseline. Information on baseline serum total testosterone concentrations was available for 541 participants.
RESULTS:
At baseline, 12.6% reported surgical meno-pause. Over the follow-up period, 22.0% died, and 10.1% were classified as frail, 39.7% as prefrail, and 28.3% as robust. Surgically menopausal women had significantly lower total serum testosterone levels (13.2 ± 7.8 ng/dL) than naturally menopausal women (21.7 ± 14.8 ng/dL) (p=0.000), although they were not at greater risk of frailty (adjusted odds ratio (aOR)=0.94, 95% confidence interval (CI)=0.72–1.22), prefrailty (aOR=0.96, 95% CI=0.80–1.10), or death (aOR=1.17, 95% CI=0.97–1.42) after adjusting for age, body mass index, and number of instrumental activity of daily living impairments. There was no evidence that oral estrogen use modified these associations.
CONCLUSION:
In postmenopausal women, surgical menopause was not associated with greater risk for frailty than natural menopause, even in the absence of estrogen therapy. Future prospective studies are needed to investigate hormonal mechanisms involved in development of frailty in older postmenopausal women.
Keywords: surgical menopause, postmenopausal, frailty, testosterone
Frailty is becoming increasingly recognized as an important complex syndrome that disproportionately affects older women and increases the risk of disability and mortality.1,2 Testosterone levels decline progressively with age in women, and very low serum testosterone is a risk factor for frailty.3 Bilateral oophorectomy also results in a dramatic decline in production of testosterone.4 Although surgical menopause (premenopausal bilateral oophorectomy) is associated with greater risk of physical dysfunction, osteoporosis, and death,5 whether it increases the long-term risk of frailty in older women is unknown.
Biological and epidemiological studies suggest that surgical menopause may increase frailty risk by decreasing serum testosterone levels. Despite the decline in ovarian production of estrogen during natural menopause, the ovaries continue to produce testosterone.6 By contrast, surgical menopause results in a significant reduction in serum testosterone levels as a result of bilateral oophorectomy. Approximately 1 in 9 women aged 35 to 45 have undergone hysterectomy, with 40% undergoing concurrent bilateral oophorectomy,6,7 resulting in abrupt onset of menopause and decline in serum testosterone and estradiol levels. This abrupt menopause and resulting low testosterone may pre-dispose women to frailty. Surgically menopausal women have poorer physical performance than naturally menopausal women, regardless of estrogen replacement therapy use.6,7 Furthermore, low testosterone is associated with greater risk of frailty in older men8 and with poor physical functioning in older women, and therefore may contribute to the onset of frailty in this population.9
Using data from the Study of Osteoporotic Fractures (SOF), we explored whether older women with a history of surgical menopause would be at higher risk of developing frailty than women with natural menopause and whether adjustment for use of estrogen therapy would attenuate this association.
METHODS
Study Sample
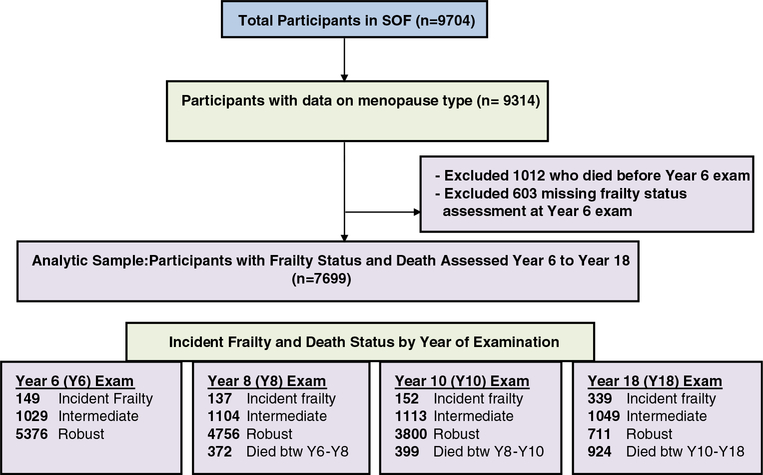
The sample comprised of participants enrolled in the SOF,10 a prospective study of 9,704 community-dwelling white women aged 65 and older recruited between 1986 and 1988 from 4 U.S geographical areas: Baltimore, Maryland; Minneapolis, Minnesota; the Monongahela Valley, Pennsylvania; and Portland, Oregon. Women who had undergone bilateral hip replacement or were unable to walk without assistance were excluded. Interviews and comprehensive clinical assessments were conducted approximately every 2 years from 1986 to 2008 (baseline through Visit 9). At baseline, 9,314 women self-reported their age at menopause; whether they had had 1 or both of their ovaries removed; and if so, at what age. Of these women, 7,699 had data on frailty status assessed at the Year 6 (conducted between 1992 and 1994), Year 8 (1995–96), Year 10 (1997–98), or Year 18 (2004–05) examination; 1,012 died before the first frailty assessment at Year 6. Thus, the present analysis included 7,699 women with data on menopause history, frailty, and vital status over the follow-up period (Figure 1).
Figure 1.
Study of Osteoporotic Fractures participants: 7,699 women with data on exposure (surgical or natural menopause) and outcome (frailty status or death) constituted the analytical sample.
Type of Menopause
At the baseline interview, women reported whether they had ever had a hysterectomy or oophorectomy (unilateral or bilateral), their age at this procedure, and age at menopause (defined as age of last menstrual period for natural menopause and age when bilateral oophorectomy was performed for surgical menopause). Participants were classified as having surgical menopause if both ovaries had been removed before menopause. Participants who had at least 1 ovary after reported age at menopause were classified as having natural menopause (reference group). For secondary analyses, we defined women who had undergone natural menopause as those who reported an age at menopause without previous hysterectomy or oophorectomy to prevent potential misclassification bias from inaccurate reporting of the date and type of surgery.
Frailty
SOF lacked key components of frailty at baseline, but given that the inclusion criteria required women to be relatively healthy, we assumed that no participants were frail at baseline. Frailty status was categorized at the Year 6, 8, 10, and 18 follow-up examinations. Frailty was based on the SOF frailty index, which is a variation on the Cardiovascular Health Study (CHS) frailty index.2 The SOF Frailty Index has been well validated as being comparable with the more complex CHS criteria11–13 and performs similarly to the CHS index in predicting falls, incident functional limitations, fractures, and death.2,14 The SOF index consisted of 3 components: intentional or unintentional weight loss of greater than 5% in the past year, inability to rise from a chair 5 consecutive times without using arms, and perceived low energy level based on an item from the Geriatric Depression Scale.14 Participants who had at least 2 of these criteria were categorized as frail, those with 1 were considered prefrail, and those who met no criteria were defined as robust.
A single outcome variable was created that combined information from all 4 follow-up examinations when frailty status was assessed. First, we prioritized the outcome categories as follows: frailty, death, prefrail, and robust. A participant was considered to have an incident case of frailty at the first follow-up visit at which she met SOF criteria for frailty. For example, if a participant was classified as frail at any of these 4 visits, she was classified as frail. If a participant was not classified as frail at any of these 4 visits but was classified as prefrail at any of these visits, she was classified as prefrail in our outcome variable. If a woman died before Year 18 and was not classified as frail at any of the follow-up visits, she was classified as dead; this included women who were classified as prefrail at a follow-up visit and died before Year 18, under the assumption that they died before the visit at which they might have been classified as frail. Otherwise, women who were not classified as frail or prefrail at any of these visits and survived to Year 18 or were missing information on frailty status at follow-up visits but did not die (had frailty status assessed at Years 6 and 8 but not at subsequent visits) were classified as robust.
Covariables
Covariables included baseline age, years of education, smoking status, body mass index (BMI), and number of instrumental activity of daily living (IADLs, range 0–5) difficulties. Self-reported oral corticosteroid and estrogen use were categorized as never, past, and current. Number of chronic diseases was the sum of 4 diseases: coronary heart disease, diabetes mellitus, stroke, and chronic obstructive pulmonary disease (range 0–4). Usual walking speed (m/s) was calculated as an average of 2 timed walks at usual pace over a 6-m course.15 Self-rated health was categorized as excellent, good, and fair or poor.
Blood Collection and Hormone Measurements
Blood was collected at the baseline examination from all participants after an overnight fast and immediately frozen to −20°C. Within 2 weeks, all samples were shipped to a central repository and stored in liquid nitrogen at −190°C until hormones were assayed. Testosterone assays were available on 541 women who were included in the breast cancer and fracture case-control substudies of SOF but who had not experienced either of these outcomes at the time of blood collection. (Serum testosterone levels were obtained before subjects were identified as cases or controls.) Methodology of testosterone measurements was similar between the 2 substudies for total testosterone but not free testosterone, so only total testosterone was analyzed. Serum concentrations of total testosterone (ng/dL) were measured using radioimmunoassay after extraction and aluminum oxide column chromatography with an interassay coefficient of variation of 6.1% to 13.4%16,17 (Endocrine Science, Calabasas Hill, CA; Corning Nichols Institute, San Juan Capistrano, CA). Overall, mean total testosterone levels did not significantly differ between the 2 substudies (Supplementary Table S1).
Statistical analysis
Characteristics of women who underwent surgical menopause were compared with those of women who underwent natural menopause using the Student t-test for continuous variables and chi-square test for categorical variables. Multinomial logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (CI) for the association between surgical menopause and each level of frailty (prefrail, frail, death) (reference robust). This logistic regression framework18 was used to combine incidence results across the multiple examinations. In secondary analyses, we evaluated the association between serum total testosterone levels and each level of frailty in our subsample. Given the positively skewed distribution of total testosterone levels, these data were normalized using natural logarithm transformation for analysis.
The multivariable model included age and BMI because they were considered clinically important covariates. Using an iterative process, potential confounders were identified and added to the multivariate model if they were independently associated with frailty status (p<.05) and their addition to a model that included only the surgical menopause variable changed the estimate for that variable by 10% or more. Covariates used to define surgical menopause (e.g., age at menopause) were not included in multivariable models. We tested effect modification according to oral estrogen use by adding an interaction term between surgical menopause and estrogen use into the regression model. We also performed stratified analysis of never, past, and current oral estrogen users. All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Sample Characteristics
The mean age of the 7,699 participants was 71.2 ± 5.2;13.8% were using oral estrogen, and 12.6% reported surgical menopause. Surgically menopausal women were significantly younger at menopause than naturally menopausal women, were more likely to have taken estrogen and to have used it for a longer time, and had significantly lower serum total testosterone levels (13.2 ± 8.2 vs 21.7 ± 14.9 ng/dL; p<.01) (Table 1). Baseline characteristics of women excluded from the analyses (n=2,005) did not significantly differ from the analytical sample.
Table 1.
Baseline Characteristics of Study of Osteoporotic Fractures Participants (N = 7,699)
| Characteristic | Natural Menopause, n = 6,731 | Surgical Menopause, n = 968 | P-Value21 |
|---|---|---|---|
| Age, mean ± SD | 71.3 ± 5.0 | 70.6 ± 4.7 | 0.000 |
| Smoking status, n (%) | 0.339 | ||
| Nonsmoker | 4131 (61.6) | 575 (59.5) | |
| Former smoker | 1,962 (29.3) | 292 (30.2) | |
| Current smoker | 614(9.2) | 100 (10.3) | |
| Education, years, mean ± SD | 12.8 ± 2.8 | 12.3 ± 2.7 | 0.000 |
| Body mass index, kg/m2, mean ± SD | 26.4 ± 4.4 | 26.6 ± 4.7 | 0.242 |
| Age at menopause, mean ± SD | 48.7 ± 5.0 | 44.4 ± 7.4 | 0.000 |
| Hysterectomy, n (%) | 1,946 (29.0) | 967 (99.9) | <.01 |
| Number of chronic conditions, mean ± SD32 | 0.2 ± 0.5 | 0.3 ± 0.5 | 0.012 |
| Number of instrumental activity of daily living impairments, mean ± SD | 1.0 ± 2.1 | 1.3 ± 2.3 | 0.002 |
| Walking speed, m/s, mean ± SD | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.513 |
| Oral corticosteroid use, n (%) | 0.913 | ||
| Never | 5,847 (88.3) | 841 (89.7) | |
| Past | 658 (9.9) | 91 (9.6) | |
| Current | 120 (1.8) | 16(1.7) | |
| Oral estrogen use, n (%) | 0.000 | ||
| Never | 4,012 (60.4) | 350 (36.6) | |
| Past | 1,840 (27.7) | 311 (32.5) | |
| Current | 792 (11.9) | 295 (30.9) | |
| Total number of years taking oral estrogen, mean ± SD | 7.7 ± 8.6 | 11.7 ± 10.6 | 0.000 |
| Self-rated health, n (%) | 0.000 | ||
| Excellent | 2,256 (33.5) | 272 (28.1) | |
| Good | 3,497 (52.0) | 512 (52.9) | |
| Fair, poor, very poor | 978 (14.5) | 183 (18.9) | |
| Total testosterone, ng/dL, mean ± SD (n = 541) | 21.7 ± 14.8 | 13.2 ± 7.8 | 0.000 |
T-tests or chi-square tests for comparison of characteristics according to menopause type.
Heart disease, stroke, chronic obstructive pulmonary disease, diabetes.
SD = standard deviation.
Surgical Menopause and Risk of Frailty
Average age at interview at which women were first classified as frail was 79.6. Approximately 10% of women became frail between Years 6 and 18, 39.7% became prefrail, and 28.3% were robust; 22% died before being classified as prefrail or frail. In multinomial logistic regression analyses, surgical menopause was not associated with risk of any level of frailty or death (frailty: adjusted OR (aORs)=0.94, 95% CI=0.72–1.22; prefrailty: aOR=0.96, 95% CI=0.80–1.10); death: aOR=1.17, 95% CI=0.97–1.42) (Table 2).
Table 2.
Association Between Surgical Menopause and Incidence of Frailty (N = 7,699)
| Prefrail | Frail | Died | |||||
|---|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | |||||||
| Exposure | Frail, n (%) | Adjusted Adjusted | Fully Adjusted1 | Adjusted Adjusted | Fully Adjusted1 | Adjusted Adjusted | Fully Adjusted1 |
| Surgical menopause | 90 (9) | 1.02 (0.86–1.21) | 0.96 (0.8–1.1) | 1.00 (0.77–1.29) | 0.94 (0.72–1.22) | 1.20 (0.99–1.45) | 1.17 (0.97–1.42) |
Adjusted for age, body mass index, and number of instrumental activity of daily living impairments.
Natural menopause (frail: n = 687, 10%) is the reference group.
Stratified Analyses According to Oral Estrogen Use
There was no significant association between the interaction between estrogen use and surgical menopause and risk of frailty or death (prefrail, p=0.757 frail, p=0.834; death, p=0.930). Surgical menopause was not associated with frailty risk in women who never used oral estrogen or in current or past users (Supplementary Table S2).
Serum Total Testosterone and Frailty
Total testosterone levels were not associated with any frailty level (Supplementary Table S3). Because of the small sample size, we lacked power to compare effect modification in surgically and naturally menopausal women.
Sensitivity Analyses
Our results did not change when we redefined naturally menopausal women as those with intact uterus and ovaries (Supplementary Table S4), excluded women in the natural menopause group who underwent postmenopausal bilateral oophorectomy(n=362) (Supplementary Table S5), excluded women aged 85 and older, or adjusted for age at meno-pause and duration of estrogen use (data not shown).
DISCUSSION
In a large sample of postmenopausal Caucasian women followed for up to 18 years, those with surgically induced menopause were not at greater risk of developing frailty than naturally menopausal women. Contrary to our hypothesis, our findings suggest that low serum testosterone levels may not be a primary mechanism to explain the higher prevalence of frailty in older postmenopausal women. Furthermore, estrogen use did not modify this relationship. To our knowledge, this is the first population-based prospective cohort study to investigate this association in postmenopausal women and adds to studies of testosterone concentrations and frailty that have been conducted primarily in men.8,19
Women who had previously undergone bilateral oophorectomy had significantly lower serum testosterone levels. It was hypothesized that they would be at greater risk of frailty based on the role of androgens in maintaining muscle mass, bone health, and physical function20,21 and findings that testosterone therapy significantly increased total lean body mass in oophorectomized women.22 Future prospective studies are needed to replicate or refute our findings.
Naturally menopausal women with lower estrogen levels have been found in cross-sectional studies to have less muscle mass and strength,23,24 suggesting a beneficial role of estrogen therapy, but we found no modification according to use of estrogen. Our results contradict a recent study that found that higher endogenous estradiol levels were associated with frailty in older women who had undergone natural menopause.25 It is likely that SOF participants are not representative of current estrogen users because they were enrolled in the late 1980s, before safety concerns about hormone therapy were raised.26
Our results are consistent with previous SOF analyses that found no effect of bilateral oophorectomy before natural menopause27 or postmenopausal bilateral oophorectomy28 on fracture risk, although the long-term health consequences of premenopausal bilateral oophorectomy may not result solely from hormonal changes after surgical menopause but could result from conditions (e.g., cancer, fibroids) that led to this surgery being performed.
Limitations of our study include the sample being primarily older white women without significant physical impairments. Thus, our results may have limited generalizability to other racial or ethnic groups or populations at high risk of disability. The proportion of the surgical menopause group was small (12.6%). We lacked medical records to confirm type of surgery and age at oophorectomy, although misclassification of surgical menopause was unlikely because of the significantly lower serum testosterone concentrations in these women and similar results when restricting the natural menopause group to women with intact ovaries and uterus. Our outcome variable combined data from 4 follow-up examinations to maximize power by using all available data on frailty, although we may have misclassified some participants as robust or prefrail because they were missing later follow-up visits at which they might have been classified as frail, potentially biasing our results toward the null. Covariates such as BMI and IADL impairment were measured at the baseline visit, long after surgical or natural menopause, making it possible that they were on the causal pathway rather than confounders. In addition, total testosterone was measured at a single time point and may not reflect commonly seen diurnal variations. A less sensitive radio-immunoassay was used instead of the gold standard technique of tandem mass spectrometry.29
Strengths of our study included its large, geographically diverse sample, use of the validated SOF frailty index,14 and multinomial modeling to capture all levels of frailty and the competing risk of death concurrently. In conclusion, neither surgically induced menopause nor serum total testosterone concentration was associated with frailty in older women. These findings provide reassurance that long-term risk of frailty is not greater in surgically menopausal women, regardless of estrogen use. Nonetheless, given the low incidence of frailty in our sample and the current debate about prophylactic oophorectomy in women undergoing hysterectomy to prevent ovarian cancer,30 more research is needed to better understand the association between surgical menopause and frailty and potential underlying hormonal mechanisms in older women.
Supplementary Material
ACKNOWLEDGMENTS
Financial Disclosure:
National Institutes of Health provides funding support for the SOF. The National Institute on Aging (NIA) provides support under Grants R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, R01 AG026720, R01 AG18037, and R21 AG050428. Additional funding support was also provided by National Heart, Lung, and Blood Institute Grant K08 HL132122–02
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Table S1: Distribution of Total Testosterone Levels in Study of Osteoporotic Fractures Fracture and Breast Cancer substudies
Table S2: Association Between Surgical Menopause and Incidence of Frailty According to Oral Estrogen Use
Table S3: Association Between Serum Total Testosterone and Incidence of Frailty
Table S4: Association Between Menopause Type and Incidence of Frailty, 5,480 Study of Osteoporotic Fractures Participants
Table S5: Association Between Menopause Type and Incidence of Frailty, 7,737 Study of Osteoporotic Fractures Participants
REFERENCES
- 1.Mitnitski A, Song X, Skoog I et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 2005;53:2184–2189. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3.Carcaillon L, Blanco C, Alonso-Bouzon C et al. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: the Toledo Study for Healthy Aging. PLoS One 2012;7:e32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D et al. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 2000;85:645–651. [DOI] [PubMed] [Google Scholar]
- 5.Shuster LT, Rhodes DJ, Gostout BS et al. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowers M, Zheng H, Tomey K et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab 2007;92:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers M, Tomey K, Jannausch M et al. Physical functioning and meno-pause states. Obstet Gynecol 2007;110: 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyde Z, Flicker L, Almeida OP et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab 2010;95:3165–3172. [DOI] [PubMed] [Google Scholar]
- 9.Morley JE, Kim MJ, Haren MT. Frailty and hormones. Rev Endocr Metab Disord 2005;6:101–108. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Black DM, Nevitt MC et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 1990;263:665–668. [PubMed] [Google Scholar]
- 11.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston Study. J Am Geriatr Soc 2009;57:1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensrud KE, Ewing SK, Taylor BC et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008;168:382–389. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Ewing SK, Cawthon PM et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009;57:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilotta C, Nicolini P, Case A et al. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures (SOF) criteria and adverse health outcomes among community-dwelling older outpatients in Italy. A one-year prospective cohort study. Arch Gerontol Geriatr 2012;54:e23–e28. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Nevitt MC, Yunis C et al. Correlates of impaired function in older women. J Am Geriatr Soc 1994;42:481–489. [DOI] [PubMed] [Google Scholar]
- 16.Stone K, Bauer DC, Black DM et al. Hormonal predictors of bone loss in elderly women: a prospective study. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1998;13:1167–1174. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Ettinger B, Pressman A et al. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol Psychiatry 1998;43:694–700. [DOI] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Lee ML, Belanger AJ et al. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990;9:1501–1515. [DOI] [PubMed] [Google Scholar]
- 19.Eichholzer M, Barbir A, Basaria S et al. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III). Aging Male 2012;15:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings SR, Browner WS, Bauer D et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1998;339:733–738. [DOI] [PubMed] [Google Scholar]
- 21.Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23: 279–302. [DOI] [PubMed] [Google Scholar]
- 22.Floter A, Nathorst-Boos J, Carlstrom K et al. Effects of combined estrogen/-testosterone therapy on bone and body composition in oophorectomized women. Gynecol Endocrinol 2005;20:155–160. [DOI] [PubMed] [Google Scholar]
- 23.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact 2009;9:186–197. [PubMed] [Google Scholar]
- 24.Kurina LM, Gulati M, Everson-Rose SA et al. The effect of menopause on grip and pinch strength: results from the Chicago, Illinois, site of the Study of Women’s Health Across the Nation. Am J Epidemiol 2004;160:484–491. [DOI] [PubMed] [Google Scholar]
- 25.Carcaillon L, Garcia-Garcia FJ, Tresguerres JA et al. Higher levels of endogenous estradiol are associated with frailty in postmenopausal women from the toledo study for healthy aging. J Clin Endocrinol Metab 2012;97:2898–2906. [DOI] [PubMed] [Google Scholar]
- 26.Rossouw JE, Anderson GL, Prentice RL et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288: 321–333. [DOI] [PubMed] [Google Scholar]
- 27.Vesco KK, Marshall LM, Nelson HD et al. Surgical menopause and nonvertebral fracture risk among older US women. Menopause 2012;19:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoniucci DM, Sellmeyer DE, Cauley JA et al. Postmenopausal bilateral oophorectomy is not associated with increased fracture risk in older women. J Bone Miner Res 2005;20:741–747. [DOI] [PubMed] [Google Scholar]
- 29.Bhasin S, Pencina M, Jasuja GK et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framing-ham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 2011;96:2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundsell H, Ekman G, Gullberg B et al. Some aspects of prophylactic oophorectomy and ovarian carcinoma. Ann Chir Gynaecol 1981;70:36–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.