INTRODUCTION
Glomus tumors are rare mesenchymal neoplasms usually involving the dermis, typically found in a subungual location, and historically hypothesized to arise from cells of the glomus body, an arteriovenous shunt involved in temperature regulation.1,2 The majority of these tumors are benign, although some exhibit more aggressive biologic features and clinical behavior (malignant glomus tumor, or glomangiosarcoma). Others have an intermediate phenotype and are classified as glomus tumors of uncertain malignant potential (GT-UMP).3 Herein, we present a case of an 18-year-old patient with two glomus tumors involving the lateral and posterior cords of the right brachial plexus. The magnetic resonance (MR) imaging, intraoperative and histologic findings, therapeutic approach, and unique response to molecularly targeted therapy are discussed.
CASE REPORT
Clinical History
An 18-year-old female presented with pain in the right shoulder region, insidious in onset, with no inciting event or notable trauma. The pain was localized to the infraclavicular region and occasionally radiated to the elbow. There were no complaints of weakness or dysesthesia in the right upper extremity. Physical examination demonstrated no neurologic deficit.
Imaging
MR imaging of the right upper extremity demonstrated two distinct brachial plexus lesions, one emanating from the distal aspect of the lateral cord and the other from the posterior cord (Fig 1). Both lesions were located in the infraclavicular space contacting the right distal subclavian vascular bundle. The tumors demonstrated no early arterial enhancement but had low apparent diffusion coefficient values4 and were therefore considered indeterminate for malignancy. A mass-like enlargement of the upper trunk (up to 4.4 cm) was also noted, without restricted diffusion. This was believed to represent a peripheral nerve tumor or, alternatively, an acquired or hereditary neuropathy. Finally, there was notable thickening of the right brachial plexus nerve roots without restricted diffusion or enhancement. After extensive discussion with the neurosurgeon, the patient opted for surgical exploration with open biopsy and possible removal of the infraclavicular tumors to provide a histologic diagnosis and guidance on further management.
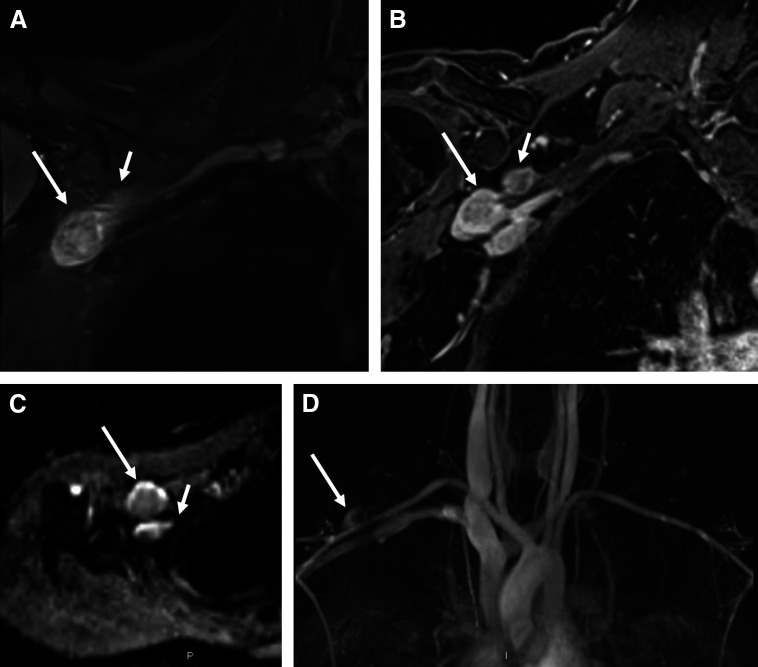
Fig 1.
(A) On coronal fluid-sensitive magnetic resonance sequence through the chest, the right brachial plexus demonstrates asymmetric hyperintensity and thickening of the C5, C6, C7, and T1 nerve roots and upper trunk (not shown) in addition to two discrete masses arising from the distal brachial plexus involving the posterior (short arrow) and lateral (long arrow) cords. The posterior cord mass measures 2.2 × 1.5 × 2.2 cm, and the distal lateral cord measures 2.1 × 1.9 × 3.5 cm. (B) Both masses exhibit enhancement on static post contrast imaging. (C) On diffusion weighted imaging with apparent diffusion coefficient mapping, there is qualitative and quantitative restricted diffusion (apparent diffusion coefficient [ADC] values range from 0.6 to 1 ×10−3 mm2/s in the larger mass and 0.9 to 1 × 10−3 mm2/s in the smaller mass). Qualitatively, a target sign or peripheral elevated signal and central decreased signal is visible on the ADC map. (D) Dynamic magnetic resonance angiography shows late arterial enhancement. Although the anatomic sequences (A and B) and dynamic contrast-enhanced sequences (D) are not worrisome, the low ADC values on diffusion weighted imaging (C) raise the possibility of a hypercellular or potentially malignant neoplasm.
Intraoperative Findings
Open exploration of the right infraclavicular brachial plexus was performed. Both tumors were noted to be intimately involved with the nerve elements. Neurolysis of the lateral cord revealed fascicles outstretched along the surface of the tumor. These were gently mobilized but did not stimulate and were divided, allowing the lesion to be removed en bloc and sent for histopathological analysis.
In the posterior cord region, the axillary nerve was clearly enlarged with tumor. Gentle neurolysis exposed the full extent of enlargement, which revealed that, proximally, the tumor extended into the posterior cord and to the level of the clavicle. Here, nerve and tumor were even more intimately involved, and a clear plane of dissection could not be established. A biopsy was performed, and the epineurium was opened to decompress the lesion. On frozen section, this tumor appeared relatively benign, but given its widespread involvement with the axillary nerve, further aggressive dissection of the remaining tumor was deferred to avoid morbid neurologic deficits.
Histopathology and Molecular Analysis
Histopathologic analysis revealed a well-circumscribed proliferation of relatively monomorphic cells with round nuclei and abundant eosinophilic cytoplasm (Figs 2A and 2B). Immunohistochemical staining revealed diffuse positivity for collagen IV and smooth muscle actin (Fig 2C). Staining for desmin, EMA, CD117, S100, SOX10, synaptophysin, AE1/AE3, and SM31 were negative. MART1 was faint, caldesmon was focal, CD34 highlighted blood vessels, INI-1 expression was intact, and Ki-67 proliferation index was mild to moderate. Electron microscopy demonstrated large cells with well-developed borders, subplasmalemmal densities, and well-developed basal lamina (Fig 2D). The lesions demonstrated features associated with aggressive clinical behavior, including increased mitotic activity, moderate nuclear atypia, and larger size. These findings together led to the diagnosis of malignant glomus tumor per current criteria.3 Molecular genetic analysis using polymerase chain reaction–based sequencing of BRAF exon 15 revealed the BRAF V600E mutation at a mutant allele frequency of 40%. To support this finding, we performed immunohistochemical analysis using antibodies directed against phospho–extracellular signal-regulated kinase (ERK); this revealed variable immunostaining, with both cytoplasmic and nuclear immunoreactivity. In addition to isolated BRAF testing, a conventional cytogenetic analysis of the tumor revealed a normal 46 XX karyotype in all cells examined.
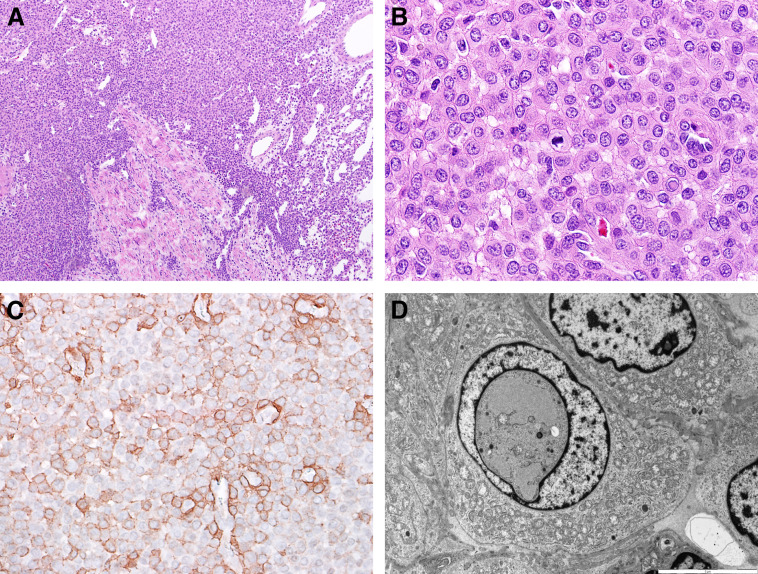
Fig 2.
Representative images of the pathologic specimen. (A) Low-power view of glomus tumor associated with peripheral nerve fascicles, hematoxylin and eosin stain. (B) Tumor cells had ample eosinophilic cytoplasm and evident mitotic activity. (C) Immunohistochemistry showing positive membrane staining for smooth muscle actin. (D) Electron microscopy demonstrated large cells with ample cytoplasm, with patchy increases in mitochondria, well-defined borders, and intranuclear inclusions.
Therapeutic Management
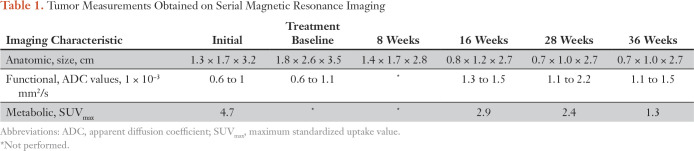
Management of this patient’s malignant tumor was discussed at the Johns Hopkins Hospital multidisciplinary sarcoma tumor board. Surgical resection, although the most accepted approach, was believed to be associated with a high likelihood of surgical morbidity, including loss of neurologic function of the dominant arm. We therefore proposed a trial of molecularly targeted therapy, using the oral RAF inhibitor dabrafenib. The patient started dabrafenib (150 mg per dose twice daily), and 3 weeks later trametinib was added (2 mg daily). MR imaging and 18F-fluorodeoxy-d-glucose positron emission tomography–computed tomography obtained 2 weeks after the addition of trametinib demonstrated interval decrease in tumor size (Table 1). MR imaging at 3 and 6 months after initiating RAF inhibitor therapy showed continued decrease in tumor size (Figs 3 and 4) and at 9 months showed no further change in size or imaging characteristics relative to most recent prior imaging. Currently, our patient continues to receive combination RAF and MEK inhibitor therapy, with monitoring by her local oncologist. Minimal toxicities, including one episode of self-limited pyrexia and mild acneiform eruption, have been reported thus far. She experiences no pain, and the extremity function is normal.
Table 1.
Tumor Measurements Obtained on Serial Magnetic Resonance Imaging
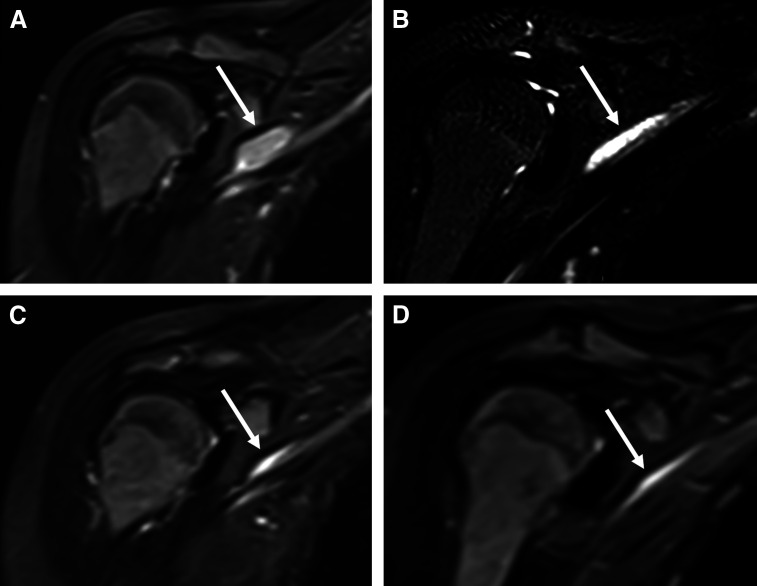
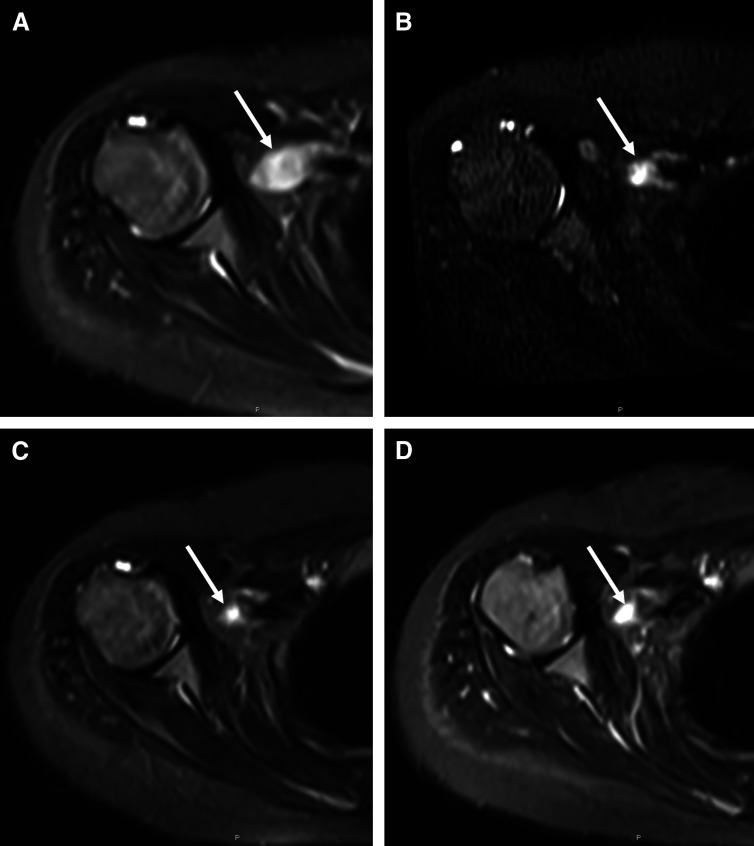
Fig 3.
Sequential coronal fluid-sensitive magnetic resonance images through the chest performed (A) at diagnosis, and after (B) 8 weeks, (C) 16 weeks, and (D) 28 weeks of treatment illustrate the progressive decrease in size of the right distal brachial plexus lesion (arrow) over time.
Fig 4.
Sequential axial fluid-sensitive magnetic resonance images at the level of the right glenohumeral joint performed (A) at diagnosis, and after (B) 8 weeks, (C) 16 weeks, and (D) 28 weeks of treatment illustrate the progressive decrease in size of the right distal brachial plexus lesion (arrow) over time.
DISCUSSION
The RAS-RAF-MEK-ERK signaling pathway is a frequent site of oncogenic mutations, with approximately 30% of human cancer harboring at least one mutation that results in its dysregulation. Activating BRAF mutations are found in approximately 7% of cancers, roughly half of malignant melanomas,5 and in other tumors, including colorectal, ovarian, papillary thyroid, and non–small-cell lung cancers, gliomas, and histiocytic disorders.6-12 The most common mutation, V600E, results in constitutive activation of the ERK pathway.
Small molecule inhibitors of RAF kinase were first studied in clinical trials of patients with melanoma. Vemurafenib demonstrated prolonged progression-free survival and overall survival compared with standard chemotherapy,13,14 and, subsequently, similar improvements in outcomes were shown with dabrafenib.15 Both drugs are now approved by the US Food and Drug Administration for treatment of BRAF V600E-mutated melanoma. To determine whether the benefit of targeted RAF inhibition would extend to nonmelanoma tumors with BRAF V600E, a phase II basket trial explored the use of vemurafenib in patients selected for this mutation. This study demonstrated modest antitumor activity, although not all tumors with BRAF mutation responded, thus enforcing that a single driver oncogene may be associated with a nonuniform response to targeted therapy.16 BRAF mutations also occur rarely in a wide spectrum of cancers, including 1.4% of soft tissue sarcomas.17 A single patient with BRAF-mutated clear cell sarcoma was treated in the vemurafenib basket trial and sustained a partial response.16 The role of RAF inhibitors in other sarcomas, however, has not been explored.
Glomus tumors are mesenchymal neoplasms that are rare and generally benign.18 They can occur as solitary or multiple soft tissue tumors and are most commonly located on the digits, supporting early hypotheses about their origin in the temperature-sensing glomus body. Complete surgical resection is considered the definitive form of treatment, with a high risk for recurrence if incomplete excision is performed. Although molecular genetic studies in glomus tumors are limited, three independent groups have identified BRAF mutations as potential drivers in malignant glomus tumors. In a targeted sequencing analysis, three of 28 glomus tumors were found to harbor BRAF V600E mutations and a single tumor mutation in KRAS.19 A single case of a glomus tumor of the median nerve was found by next-generation sequencing to harbor a BRAF V600E mutation.20 This patient, and those in the retrospective analysis of 28 cases, was treated surgically without consideration for systemic therapy. Finally, in a collection of 102 glomus tumors (56% benign, 15% GT-UMP, and 29% malignant) BRAF V600E mutation was detected in six tumors (6%), all of which were classified as either malignant glomus tumor or GT-UMP (and 0 of 57 benign glomus tumors harbored BRAF mutations).21 These data, although reflective of an overall small sample size, are suggestive of BRAF mutation as a predictor of more aggressive clinical behavior. Interestingly, glomus tumor has previously been associated with neurofibromatosis type 1,22 suggesting a shared terminal event in activation of ERK signaling in these tumors.
Our patient’s response to targeted BRAF inhibition is an extraordinary one and highlights promise for future use in sarcomas with this mutation. This is, to our knowledge, the first case report of a response to molecularly targeted therapy in a BRAF-mutant malignant glomus tumor. This may provide a therapeutic approach for additional patients, particularly those in whom the morbidity associated with surgical resection is considered unacceptable. The authors therefore encourage an expanded sequencing panel that includes, at a minimum, BRAF and other actionable oncogenic variants, in all cases of malignant glomus tumor. Issues surrounding the duration of therapy, however, and other questions, such as a potential impact on fertility and emergence of acquired resistance, remain unguided with the currently available literature and experience.
ACKNOWLEDGMENT
The patient has been provided product via the Novartis Managed Access Program, and the necessary patient consents are in place.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea Cuviello, Anshit Goyal, Allan J. Belzberg, Christine A. Pratilas
Provision of study material or patients: Aviad Zick, Fausto J. Rodriguez, Allan J. Belzberg
Collection and assembly of data: Andrea Cuviello, Aviad Zick, Shivani Ahlawat, Fausto J. Rodriguez, Christine A. Pratilas
Data analysis and interpretation: Andrea Cuviello, Shivani Ahlawat, Fausto J. Rodriguez, Christine A. Pratilas
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Andrea Cuviello
No relationship to disclose
Anshit Goyal
No relationship to disclose
Aviad Zick
Research Funding: Merck (Inst), Eli Lilly (Inst)
Shivani Ahlawat
No relationship to disclose
Fausto J. Rodriguez
Consulting or Advisory Role: AbbVie
Allan J. Belzberg
No relationship to disclose
Christine A. Pratilas
Consulting or Advisory Role: Genentech
REFERENCES
- 1.Shin DK, Kim MS, Kim SW, et al. : A painful glomus tumor on the pulp of the distal phalanx. J Korean Neurosurg Soc 48:185-1872010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheithauer BW, Woodruff JM, Erlandson RA: Tumors of peripheral nervous system, in: Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1999 [Google Scholar]
- 3.Folpe AL, Fanburg-Smith JC, Miettinen M, et al. : Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol 25:1-122001 [DOI] [PubMed] [Google Scholar]
- 4.Demehri S, Belzberg A, Blakeley J, et al. : Conventional and functional MR imaging of peripheral nerve sheath tumors: Initial experience. AJNR Am J Neuroradiol 35:1615-16202014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-9542002 [DOI] [PubMed] [Google Scholar]
- 6.Brose MS, Volpe P, Feldman M, et al. : BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 62:6997-70002002 [PubMed] [Google Scholar]
- 7.Singer G, Oldt R, III, Cohen Y, et al. : Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 95:484-4862003 [DOI] [PubMed] [Google Scholar]
- 8.Kimura ET, Nikiforova MN, Zhu Z, et al. : High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454-14572003 [PubMed] [Google Scholar]
- 9.Ikenoue T, Hikiba Y, Kanai F, et al. : Rapid detection of mutations in the BRAF gene using real-time polymerase chain reaction and melting curve analysis. Cancer Genet Cytogenet 149:68-712004 [DOI] [PubMed] [Google Scholar]
- 10.Pratilas CA, Hanrahan AJ, Halilovic E, et al. : Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res 68:9375-93832008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badalian-Very G, Vergilio JA, Degar BA, et al. : Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 116:1919-19232010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiacci E, Trifonov V, Schiavoni G, et al. : BRAF mutations in hairy-cell leukemia. N Engl J Med 364:2305-23152011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosman JA, Kim KB, Schuchter L, et al. : Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366:707-7142012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McArthur GA, Chapman PB, Robert C, et al. : Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 15:323-3322014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauschild A, Grob JJ, Demidov LV, et al. : Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358-3652012 [DOI] [PubMed] [Google Scholar]
- 16.Hyman DM, Puzanov I, Subbiah V, et al. : Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726-7362015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-7132017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou T, Pan SC, Shieh SJ, et al. : Glomus tumor: Twenty-year experience and literature review. Ann Plast Surg 76:S35-S402016. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 19.Chakrapani A, Warrick A, Nelson D, et al. : BRAF and KRAS mutations in sporadic glomus tumors. Am J Dermatopathol 34:533-5352012 [DOI] [PubMed] [Google Scholar]
- 20.Dahlin LB, Scherman P, Besjakov J, et al. : Intraneural glomus tumor of “uncertain malignant potential” and with BRAF mutation in the median nerve - An unusual case. Clin Neuropathol 36:164-1702017 [DOI] [PubMed] [Google Scholar]
- 21.Karamzadeh Dashti N, Bahrami A, Lee SJ, et al. : BRAF V600E mutations occur in a subset of glomus tumors, and are associated with malignant histologic characteristics. Am J Surg Pathol 41:1532-15412017 [DOI] [PubMed] [Google Scholar]
- 22.Dahlin LB, Müller G, Anagnostaki L, et al. : Glomus tumours in the long finger and in the thumb of a young patient with neurofibromatosis-1 (Nf-1). J Plast Surg Hand Surg 47:238-2402013 [DOI] [PubMed] [Google Scholar]