Summary
When activated by WASP family proteins, Arp2/3 complex nucleates branched actin filaments important for processes like cellular motility and endocytosis [1]. WASP-mediated activation of Arp2/3 complex requires a preformed actin filament, ensuring that activation by WASP creates branched instead of linear filaments. However, this biochemical requirement also means that assembly of branched actin networks must be primed with an initial seed filament [2–4]. We recently described a class of activators called WISH/DIP/SPIN90 (WDS) proteins, that unlike WASP, activate Arp2/3 complex without a preformed filament [4]. While this property may allow WDS proteins to serve as seed filament generators, it is unknown if actin filaments nucleated by WDS-activated Arp2/3 complex can activate WASP-bound Arp2/3 complex. Further, despite their potential importance as branched actin network initiators, little is known about how WDS proteins turn on Arp2/3 complex. Here we use two color single molecule TIRF microscopy to show that Dip1, the S. pombe WDS protein [5], co-opts features of branching nucleation to activate Arp2/3 complex. Specifically, it activates Arp2/3 complex to nucleate linear filaments analogous to the branch created by WASP-mediated activation. The barbed ends of Dip1-Arp2/3 nucleated filaments are free to elongate and their pointed ends remain anchored to Dip1-bound Arp2/3 complex. The linear filaments nucleated by Dip1-bound Arp2/3 complex activate WASP-bound Arp2/3 complex as potently as spontaneously nucleated or branched actin filaments. These observations provide important insights into the regulation of Arp2/3 complex by its activators and the molecular basis for initiation of branched actin networks.
Keywords: Arp2/3 complex, Dip1, actin, S. pombe, endocytosis, Wsp1, WASP
eTOC Blurb
Balzer et. al. use multi-color single molecule TIRF microscopy to show actin filaments nucleated by Dip1-bound Arp2/3 complex stimulate branching nucleation by another Arp2/3 complex activator, WASP. Their data support a model in which Dip1 provides the first actin filaments to kickstart assembly of branched actin networks.
Results
Dip1 co-opts features of branching nucleation to create linear filaments.
Unlike WASP, WDS proteins activate Arp2/3 complex without preformed actin filaments, suggesting they trigger nucleation using a mechanism different from WASP [4]. Several other observations support this hypothesis. For instance, WDS proteins lack the canonical Arp2/3 complex interacting region of WASP, the CA (Central, Acidic) segment, and instead use a C-terminal armadillo repeat motif domain to bind and activate Arp2/3 complex [4,6]. In addition, WASP uses its conserved V region to recruit monomers directly to Arp2/3 complex for activation [7,8], while WDS proteins lack V regions and do not bind to actin monomers [4]. Finally, WASP and WDS proteins bind to different sites on Arp2/3 complex [6,9–13]. However, despite these differences, some experiments indicate potentially overlapping activation mechanisms for these two nucleation promoting factors (NPFs). For instance, both WDS and WASP family proteins stimulate movement of Arp2 and Arp3 into or near a short pitch dimer (filament-like) arrangement, a conformational change required for activation [4,14–17]. How WDS proteins use a set of molecular features both common to and distinct from WASP to switch on Arp2/3 complex is currently unclear, and is critical to understanding this class of actin regulators.
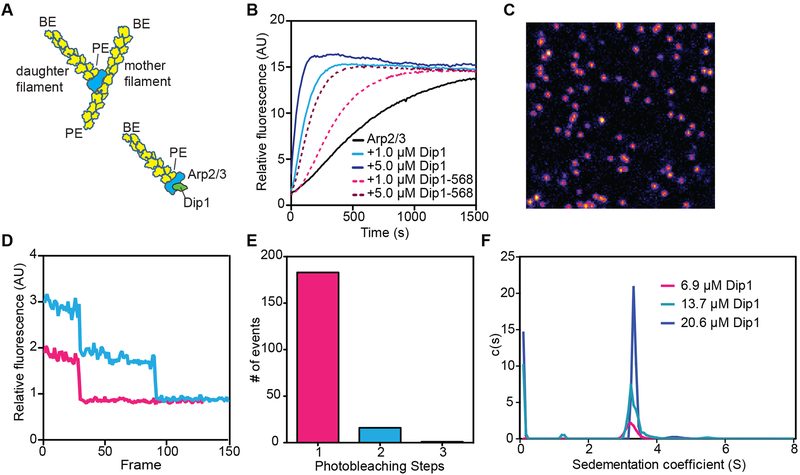
During WASP-mediated activation of Arp2/3 complex, the nucleated filament (daughter filament) elongates from the barbed ends of Arp2 and Arp3, while the pointed end of the Arps bind to the sides of a pre-existing (mother) filament (Figure 1A) [18]. Therefore, we reasoned that like WASP, Dip1 could activate Arp2/3 complex to nucleate filaments that remain anchored by their pointed ends to the complex, with their barbed ends free to elongate (Figure 1A). In this mechanism, linear filaments generated by Dip1-Arp2/3 complex are analogous to branches generated by WASP-activated Arp2/3 complex. To test this model, we fluorescently labeled Dip1 with Alexa Fluor 568 to visualize its influence on actin polymerization in single molecule TIRF microscopy assays. Dip1 labeled with Alexa Fluor 568 on an engineered N-terminal cysteine (568-Dip1) showed decreased activity compared to unlabeled Dip1, but significantly accelerated actin assembly in the presence of Arp2/3 complex, indicating the labeled protein retained activity (Figure 1B). Dip1 at multiple concentrations sedimented as a single peak by analytical ultracentrifugation and lysine biotinylated 568-Dip1 bound to coverslips photobleached predominantly in single steps (Figure 1C–F and Table S1). Together these data suggest Dip1 is a monomer at low and high concentrations and 568-Dip1 is suitable for simple single-molecule imaging.
Figure 1: Model of Dip1-mediated Arp2/3 complex activation and characterization of Alexa Fluor 568 labeled Dip1.
A. Cartoon of a branched filament nucleated by WASP-activated Arp2/3 complex (top) showing resulting filament polarities (barbed end, BE; pointed end, PE). Bottom half of panel shows a model of Dip1-mediated activation of Arp2/3 complex. In this model, the linear filament nucleated by Dip1-bound Arp2/3 complex is analogous to the daughter filament nucleated during branching nucleation. B. Time course of polymerization of 3 μM 15% pyrene-labeled actin in the presence of 50 nM S. pombe Arp2/3 complex (SpArp2/3 complex) and either unlabeled or Alexa Fluor 568 labeled Dip1 (568-Dip1). C. Representative image of 568-Dip1 molecules bound to a coverslip and visualized by TIRF microscopy. D. Examples of fluorescence intensities of single 568-Dip1 puncta over time. E. Quantification of events in which 568-Dip1 photobleached in one versus multiple steps. F. Plot of c(S) versus (S) for sedimentation velocity analytical ultracentrifugation of three concentrations of unlabeled Dip1. Dip1 sediments predominantly or entirely as a monomer at all three concentrations. See also Table S1.
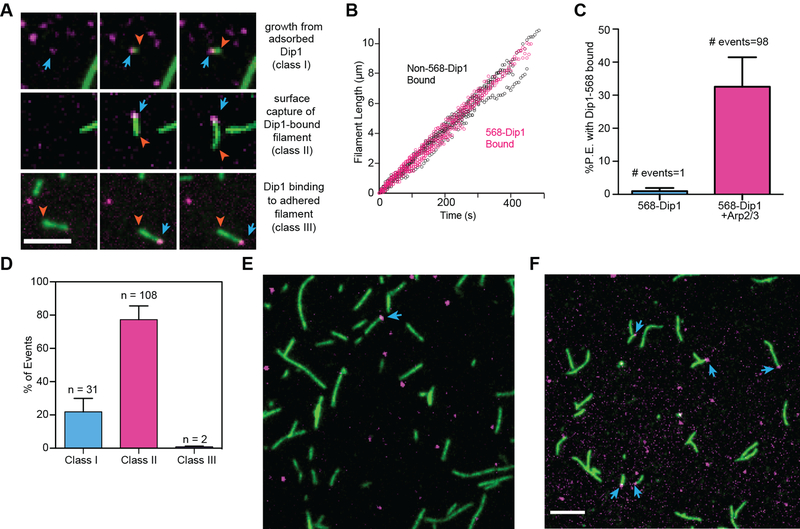
In actin polymerization reactions containing (non-biotinylated) 568-Dip1, Oregon Green actin and unlabeled Arp2/3 complex, we observed Dip1 molecules bound to one end of actin filaments that adhered to the imaging surface (Figure 2A). The free ends of Dip1-bound filaments elongated at the rate expected for free barbed ends at 1 μ M actin [19], indicating Dip1 molecules bind the pointed end (Figure 2B). Dip1 only bound to filament ends in reactions containing Arp2/3 complex (Figure 2C). Therefore, we conclude that Dip1 binds actin filament pointed ends indirectly through Arp2/3 complex. Together, these data demonstrate that the linear actin filament generated by Dip1-activated Arp2/3 complex is analogous to the branch created by WASP-mediated activation.
Figure 2: Dip1 co-opts features of branching nucleation to create linear filaments.
A. TIRF microscopy images of actin polymerization reactions containing 6 nM 568-Dip1, 1.5 μM 33% Oregon Green labeled actin and 500 nM SpArp2/3 complex. Top, middle and bottom row show three different classes of events. Blue arrows show Dip1 bound to filament ends, red arrow heads mark elongating filament ends. Scale bar: 5 μm. B. Plot of filament length versus time for free filaments or filaments with bound Dip1. A total of 3 Non-568-Dip1 bound and 3 568-Dip1 filaments were measured. C. Quantification of the percentage of pointed ends with 568-Dip1 bound in actin polymerization reactions containing 6 nM 568-Dip1 and 1.5 μM 33% Oregon Green labeled actin with or without 500 nM SpArp2/3 complex. Error bars: standard error from 4 reactions. We speculate that the single event observed in the absence of Arp2/3 complex is coincident colocalization of a filament end with a surface adsorbed molecule of 568-Dip1. D. Quantification of three classes of events in which Dip1 is observed on the ends of filaments for the conditions described in B. Error bars show standard error from 4 reactions. E. TIRF microscopy image from a reaction in which 1.5 μM 33 % Oregon Green actin filaments were sheared and flowed into the imaging chamber before adding 6 nM 568-Dip1 and 500 nM SpArp2/3 complex. The total percentage of pointed ends bound to 568-Dip1 is indicated in the upper right corner (of 362 total pointed ends observed). F. TIRF microscopy image showing the same sized field of view as F with conditions described in B, in which Dip1 activates Arp2/3 complex to nucleate linear filaments. The total percentage of pointed ends bound to 568-Dip1 is indicated in the upper right corner (of 242 total pointed ends observed). Scale bar: 5 μm. See also Videos S1–S3.
Three distinct classes of events produced actin filaments with Dip1 bound at their pointed ends (Figure 2A,D). In class I Dip1 filament binding events (31 out of 141 observations), Dip1 non-specifically adsorbed to the surface and an actin filament appeared to nucleate from the Dip1 punctum (Figure 2A,D, Video S1). While we cannot eliminate the possibility that these events represent capture of a spontaneously nucleated actin filament by surface-adsorbed Dip1, our observations argue against this interpretation (see below). Therefore, we interpret these events as Dip1-Arp2/3 mediated nucleation of linear filaments. In a second, more frequent class of events (class II, 108 of 141), actin filaments “pre-loaded” with Dip1 were observed when they landed on the imaging surface (Figure 2A,D, Video S2). These events could represent surface capture of filaments that were nucleated by Dip1 and Arp2/3 complex in the reaction chamber above the zone of TIRF illumination. Alternatively, they might be spontaneously nucleated actin filaments that bound Dip1 and Arp2/3 complex at their pointed ends before landing on the imaging surface. Two observations argue against the latter explanation. First, association of Dip1 and Arp2/3 complex with pre-existing pointed ends is rare, as we observed very few instances in which Dip1-Arp2/3 bound to free pointed ends of surface-captured filaments (class III events, 2 out of 141 events, Figure 2A,D, Video S3). Second, in a separate experiment, we asked whether Dip1 binds pointed ends generated by shearing spontaneously nucleated filaments in the presence of Arp2/3 complex. Dip1 rarely bound pointed ends of spontaneously nucleated and Arp2/3-capped sheared filaments (<1% of pointed ends) (Figure 2E), but was frequently observed (33 % of pointed ends) bound in reactions in which the Dip1-Arp2/3 complex assembly nucleated linear filaments (Figure 2F). Therefore, our data show that Dip1 associates strongly with Arp2/3 complex on a filament pointed end only if it has cooperated with the complex to nucleate that filament.
Actin filaments nucleated by Dip1 and Arp2/3 complex activate WASP-bound Arp2/3 complex
Dip1 possesses a key biochemical property that could allow it to seed branched actin network assembly: it activates Arp2/3 complex without requiring a preformed filament [4]. In cells, deletion of Dip1 stalls WASP (called Wsp1 in S. pombe) at endocytic sites and decreases the rate at which new endocytic actin networks are initiated, leading to a significant decrease in the total number of cortical actin puncta [5]. Together, these observations led us to propose that that actin filaments nucleated by Dip1-activated Arp2/3 complex might stimulate WASP-mediated activation of the complex to initiate branched network assembly. However, it is not clear whether filaments nucleated by Dip1-activated Arp2/3 complex can trigger activation of WASP-bound Arp2/3 complex. Importantly, several recent studies show that the mode by which an actin filament is nucleated can strongly influence its interactions with other actin regulators. For instance, filaments nucleated by the fission yeast formin Cdc12 preferentially bind the fission yeast tropomyosin Cdc8 [20]. Likewise, actin filaments nucleated by WASP-activated Arp2/3 complex are preferentially excluded from interactions with tropomyosin [21]. These and other experiments suggest the identity of protein(s) bound to the filament end may influence the conformation of interior actin filament subunits [22]. While the precise mechanism of Dip1-mediated activation of the complex is still unclear, a similar allosteric mechanism could influence the ability of Dip1-Arp2/3 complex nucleated filaments to activate WASP-bound Arp2/3 complex. Therefore, it is important to directly test whether filaments generated by Dip1-activated Arp2/3 complex can seed branched actin network initiation.
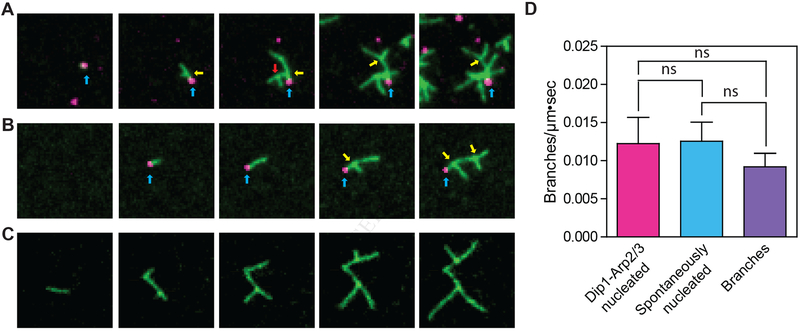
The experiments we describe above demonstrate that 568-Dip1 molecules mark actin filaments nucleated by Dip1-Arp2/3 complex (Figure 2A), allowing us to distinguish them from spontaneously nucleated filaments. Therefore, our single molecule TIRF experiments provide an opportunity to directly test whether linear filaments nucleated by Dip1-activated Arp2/3 complex can activate WASP-bound Arp2/3 complex. In an assay containing Arp2/3 complex, 568-Dip1, Oregon Green 488 actin and the Arp2/3 complex-activating fragment of Wsp1, Wsp1-VCA, we observed multiple events in which new actin filaments appeared to nucleate from 568-Dip1 puncta non-specifically adsorbed to the surface (Figure 3A). As these linear filaments elongated, we frequently observed branched filaments growing from their sides (Figure 3A). We also observed branches growing from Dip1 bound filaments that landed on the imaging surface after nucleation (Figure 3B, Video S4). Together, these experiments demonstrate that linear filaments nucleated and bound by Dip1 and Arp2/3 complex stimulate branching nucleation by WASP-bound Arp2/3 complex. Under the conditions of these reactions, we observed branching nucleation not only from Dip1-bound filaments, but also from free unbound filaments and from pre-existing branches (Figure 3A–C, Video S5). Therefore, we asked if each source of filaments is equally potent in activating WASP-bound Arp2/3 complex. We found that branches nucleated at the same rate from all three filament sources (Figure 3D).
Figure 3: Filaments nucleated by Dip1-bound Arp2/3 complex activate Wsp1-bound Arp2/3 complex to seed branched network assembly.
A, B. TIRF microscopy images of Dip1-Arp2/3 nucleated filaments seeding branching nucleation by Wsp1 and Arp2/3 complex. The reaction contained 6 nM 568-Dip1, 250 nM SpArp2/3 complex, 250 nM GST-Wsp1-VCA and 1.5 μM 33% Oregon Green actin. Blue arrows point to Dip1 molecules bound to pointed ends. Yellow arrows point to actin filament branches that grew from Dip1-Arp2/3 nucleated filaments. The red arrow points to a branch that nucleated from another branch. Panels A and B show growth of filaments from surface adsorbed Dip1 (class I event) or from a surface captured Dip1-bound filament (class II event), respectively. C. TIRF images of branches growing from spontaneously nucleated filaments used for quantification in D. The reaction contained 250 nM SpArp2/3 complex, 250 nM GST-Wsp1-VCA and 1.5 μM Oregon Green actin. White arrows point to branches. Scale bar is 1 μm in A,B and C. D. Quantification of the rate of branching on three sources of filaments: spontaneously nucleated filaments, pre-existing branches and Dip1-Arp2/3 nucleated seed filaments. Error bars: standard error from 3 separate reactions. See also Videos S4 and S5.
Discussion
Our data show that the actin filament nucleated by Dip1 and Arp2/3 complex is analogous to the branched filament created by WASP-mediated activation of the complex (Figure 1A). That Dip1 co-opts features of branching nucleation has implications for understanding multiple aspects of Arp2/3 complex function. For instance, because Arp2/3 complex stays anchored on pointed ends during and after Dip1-mediated activation in this mechanism, the pointed ends of filaments nucleated by Dip1-Arp2/3 complex are protected from depolymerization. Therefore, specific mechanisms may be required to stimulate dissociation of Arp2/3 complex from the pointed ends of filaments nucleated by Dip1-activated Arp2/3 complex. GMF is an ADFH (actin depolymerization factor homology) family protein recently shown to bind directly to Arp2/3 complex to dissociate branches [23–25]. GMF may also have a role in dissociating Arp2/3 complex from the ends of linear filaments nucleated by Dip1-activated Arp2/3 complex.
Multiple NPFs or putative NPFs are present at endocytic sites in S. pombe, including, Myo1, Wsp1, Dip1, and Pan1 [5,26,27]. While few experiments address how NPFs coordinately regulate the complex, our results here, together with previous work [4], show that Wsp1 and Dip1 use distinct mechanisms to activate a common mode of nucleation by Arp2/3 complex. If these mechanisms can work in concert, these two classes of NPFs could synergistically activate the complex, potentially influencing the rates of network seeding or propagation of branching, or both. Quantitative biochemical studies of the influence of coordinated regulation of Arp2/3 complex by these two NPFs will be important to understand the kinetics of branched actin initiation and propagation in cells.
Here we demonstrate that actin filaments nucleated by Dip1-activated Arp2/3 complex stimulate branching nucleation by WASP-bound Arp2/3 complex to seed assembly of branched actin networks. Several lines of evidence suggest this seeding function of WDS proteins is broadly conserved. First, diverse WDS proteins activate the complex with similar biochemical properties and share a conserved Arp2/3 complex activating domain [4,6]. We anticipate that seeds generated by any WDS family protein will activate WASP-bound Arp2/3 complexes, as we observed here for Dip1. Second, previous studies suggest other WDS family proteins are important for actin assembly and may be involved in initiation of branched actin filament networks. For instance, deletion of the budding yeast homologue of Dip1, Ldb17, causes endocytosis defects, decreasing the number of endocytic actin patches and increasing their size [28]. This phenotype is identical to the dip1Δ phenotype in fission yeast [5], and is consistent with a model in which Ldb17 initiates new patches by providing preformed filaments to activate WASP-bound Arp2/3 complex. Less is known about the in vivo function of the mammalian WDS protein, SPIN90, but experiments suggest that it interacts with endocytic proteins, and contributes to uptake of at least one endocytic cargo, EGFR [29]. In addition to its roles in endocytosis, some studies suggest SPIN90 may play a role in assembly of actin in lamellipodia. Specifically, one group showed that knockdown of SPIN90 in COS-7 cells prevents PDGF-induced ruffling [30]. Whether SPIN90 provides seed filaments to initiate branched actin assembly in lamellipodia is an important open question. In addition, it will be important to determine the relative contributions of WDS proteins in seeding compared to other potential mechanisms, which include seeding by formin-nucleated filaments [31], Arp2/3-independent filament nucleation by NPF proteins [32], and capture of filaments generated by cofilin-mediated severing [33].
STAR Methods:
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brad Nolen (bnolen@uoregon.edu).
Experimental Model and Subject Details
S. pombe strain TP150 was cultured at 30°C in YE5S media until the cells entered the exponential phase, at which point they were harvested for protein purification.
Method Details
Experimental design details:
All the data in Figure 1 are single replicates with the exception of panel E, where 200 binding events were counted from 2 separate movies. All puncta within each field of view were analyzed. No statistics were used in any of the analyses in Figure 1. In Figure 2,B, three filaments lengths were measured for each condition (568-Dip1 bound vs non-bound) from a single TIRF movie. Filaments were selected for measurement only if their growth was unobstructed by other filaments in the field and they remained adhered to the surface. For the 568-Dip1 bound filaments that were tracked, 568-Dip1 remained bound to the end of the filament for the duration of measurement, which was greater than 400 seconds in all cases. In Figure 2C, 106 total filaments were counted in the absence of Arp2/3 and 242 were counted in the presence of Arp2/3 complex. In both cases, counts were made from a total of four replicate movies. All filaments in each selected frame of a movie were counted. In panel 2D, 242 total filaments were counted from a total of four replicate movies to determine the percentage of each class of 568-Dip1 binding events. All filaments in a selected frame of the movie were counted. The bars represent the average 568-Dip1 binding percentage for each binding class from the four replicates with standard error for each class. In Figure 3D, to determine the branching density of the three sources of seed filaments, all the filaments in three replicate movies were measured and counted. The bars represent the average branching rate from the three replicates with standard error for each seed filament source. A two-tailed T test assuming unequal variances was used to determine if the branching rates were significantly different. No other statistical methods, sample size estimations, strategies for randomization, stratification, or blinding were used in the experiments or their analyses in this study.
Protein Expression, Purification, and Labeling:
To generate a Dip1 construct for site specific labeling with a cysteine reactive fluorescent dye, the six endogenous cysteines were mutated to alanine by amplifying pGV67-SpDip1 [4] with non-overlapping 5’-phosphoryated primers encoding the mutation. The N-terminal Not1 restriction site, used to generate the GST-TEV-Dip1 expression vector, codes for a cysteine that was exploited for labeling. For expression and labeling of mutant protein, BL21-CodonPlus(DE3)RIL E. coli transformed with the pGV67 Dip1 expression vector was grown in 5 mL of LB plus 100 μg/mL ampicillin and 35 μg/mL chloramphenicol overnight at 37°C. One milliliter of this culture was used to inoculate 50 mL of LB plus ampicillin and chloramphenicol that was grown until turbid at 37°C with shaking at 180 rpm. Ten milliliters of this culture were used to inoculate 1L of LB plus ampicillin and chloramphenicol. These cultures were grown to an O.D.600 of 0.6–0.7, induced with 0.4 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG), and grown overnight at 22 °C for 12–14 hours. To each 1L culture ethylenediaminetetraacetic acid (EDTA) and phenylmethanesulfonyl fluoride (PMSF) were added to 2 mM and 0.5 mM, respectively. The cultures were pelleted at 4000 rpm for 20 minutes in the Fiberlite F8B rotor at 4°C. Cells were resuspended in 100 mL of lysis buffer; 20 mM Tris pH 8.0, 140 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, plus 2 protease inhibitor tablets (Roche). The resuspended cells were lysed by sonication on ice with intermittent pulses to keep the temperature below 10°C. The lysate was clarified by centrifugation in a JA-20 rotor at 18,000 rpm for 30 minutes, and the soluble fraction was loaded on a 10mL glutathione sepharose column equilibrated in GST-binding buffer (20 mM Tris pH 8, 140 mM NaCl, 2 mM EDTA, 1 mM DTT). Protein was eluted with 30 mL of elution buffer (20 mM Tris pH 8.0, 140 mM NaCl and 50 mM glutathione adjusted to pH 8.0). Peak fractions were pooled and TEV protease was added at a 25:1 ratio (by mass). The reaction mix was dialyzed overnight at 4 °C against 20 mM Tris p H 8.0, 50 mM NaCl and 1 mM dithiothreitol. The sample was loaded onto a 6 ml Resource Q column at pH 8.0 and eluted with a gradient of 50 mM to 500 mM NaCl. Protein was then concentrated in an Amicon-Ultra concentration device before loading on a Superdex 200 HiLoad 16/60 gel filtration column and eluted in a buffer containing 20 mM HEPES pH 7.0 and 50 mM NaCl. Peak fractions were pooled and concentrated to ~ 40 μM for labeling. A 10 mM solution of Alexa Fluor 568 C5 Maleimide was prepared by dissolving in DMSO according to manufacturer’s protocol. Protein was labeled by the dropwise addition of a 10–40 molar ratio of dye:protein while stirring at 4°C. After 12–16 hours, the reaction was dialyzed against 20 mM Tris pH 8.0, 50 mM NaCl, and 1 mM dithiothreitol for 24 hours at 4 °C with buffer exchanges after 4hr and 8hrs. Labeled protein sample was loaded on a 5mL Hi-Trap desalting column and peak fractions were pooled and flash frozen in liquid nitrogen. The concentration of 568-Dip1 and the percentage labeled was calculated by measuring the absorbance at 575 nm and 280 nm. The concentration of Alexa Fluor 568 was calculated using the extinction coefficient, ε, 91,900 M−1 cm−1. The concentration of Dip1 was calculated using Beer’s law with the following correction factor for contribution from Alexa Fluor 568: [Dip1] = (Abs280 – (Abs575 * 0.403))/ε, where, ε, the extinction coefficient of Dip1 was estimated based on amino acid content at 36,330 M−1 cm−1. Biotinylated 568-Dip1 was prepared by incubating a 15 fold molar excess of EZ-Link-NHS-PEG12-biotin (Thermofisher) for 9 hours in labeling buffer (20 mM Imidazole pH 7.5, 50 mM NaCl) at 4 °C. The reaction was quenched by dialysis overnight in 20 mM Tris pH 8.0 and 50 mM NaCl.
To prepare lysates for S. pombe Arp2/3 complex purification, 10 mL of a turbid culture of S. pombe (TP150 strain) cells was added to each 1L of YE5S in a 2.8 L flask. These cultures were grown for ~12 hrs at 30°C with shaking. All subsequent steps were carried out at 4 °C. EDTA and PMSF were added to 2mM and 0.5 mM, respectively, and the cells were harvested by centrifugation. The pellet was resuspended in 2 mL of lysis buffer (20mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA, 1mM DTT) per gram of wet cell pellet, plus 6 protease inhibitor tablets per liter of lysis buffer. Cells were lysed in a microfluidizer (Microfluidics Model M-110EH-30 Microfluidizer Processor) at 23 kPSI for 5 to 6 passes. After lysis 0.5 mM PMSF was added and the lysate was spun down in a JA-10 (Beckman) rotor at 9,000 rpm for 25 minutes. The supernatant was transferred to prechilled 70 mL polycarbonate centrifuge tubes (Beckman Coulter # 355655) and spun at 34,000 rpm for 75 minutes at 4°C in a Fiberlite F37L rotor (Thermo-Scientific). The supernatant was filtered through cheesecloth into a graduated cylinder and the volume was measured. Under heavy stirring, 0.243 g of ammonium sulfate per mL of supernatant was added over approximately 30 minutes. The solution stirred for an additional 30 minutes, then pelleted in the Fiberlite F37L rotor at 34,000 rpm for 90 minutes. The pellet was resuspended in 50 mL of PKME (25 mM PIPES, 50 mM KCl, 1 mM EGTA, 3 mM MgCl2, 1 mM DTT and 0.1 mM ATP) and dialyzed against 8 L PKME overnight in 50,000 MWCO dialysis tubing. The dialysate was spun down at 34,000 rpm for 90 minutes in the Fiberlite F37L rotor. Ten milliliters of GS4B beads equilibrated in GST binding buffer (20 mM Tris pH 8, 140 mM NaCl, 1 mM EDTA, and 1 mM DTT) were charged with 15 mg of GST-N-WASP-VCA to make a GST-VCA affinity column. Additional binding buffer was added until no protein was detectable in the flow through by Bradford assay. The column was then equilibrated in PKME pH 7.0 and then supernatant was loaded at 1 mL per min before washing the column with additional PKME (~45 mL). The column was washed with PKME +150 mM KCl until no protein was detected in the flow through by Bradford assay (~30 mL). Protein was eluted with PKME + 1 M NaCl into ~2 mL fractions until no protein was detected by a Bradford assay (~30mL). Fractions containing Arp2/3 complex were pooled and dialyzed against 2 L of QA buffer (10 mM PIPES, 25 mM NaCl, 0.25 mM EGTA, 0.25 mM MgCl2, pH 6.8 w/KOH) in 50,000 MWCO dialysis tubing overnight. The complex was then purified by ion exchange chromatography on an FPLC using a 1mL MonoQ column with a linear gradient of QA buffer to 100% QB buffer (10 mM PIPES, 500 nM NaCl, 0.25 mM EGTA, 0.25 mM MgCl2, pH 6.8 w/KOH) over 40 column volumes with a flow rate of 0.5 mL per minute. Fractions containing Arp2/3 complex were pooled and dialyzed against Tris pH 8.0, 50 mM NaCl and 1 mM DTT in 50,000 MWCO dialysis tubing overnight. The dialysate was concentrated to 1.5 mL in a 30,000 MWCO concentrator tube (Sartorius Vivaspin Turbo 15 #VS15T21) using the Fiberlite F13B rotor at 2,500 rpm for 5 to 10 minute cycles. Between each cycle the solution was mixed by gentle pipetting. The concentrated sample was loaded on a Superdex 200 size exclusion column in Tris pH 8, 50mM NaCl, and 1mM DTT. Eluted fractions with pure Arp2/3 complex were concentrated as described above and the final concentration determined by measuring the absorbance at 290 nm(E290 =139,030 M−1cm−1) before flash freezing.
To purify GST-Wsp1-VCA, 5 mL of LB plus 100 μg/mL ampicillin and 35 μg/mL chloramphenicol was inoculated with BL21(DE3)-RIL cells transformed with a pGv67-GST-Wsp1-VCA plasmid and grown overnight at 37 °C. One milliliter of this culture was used to inoculate 50 mL of LB plus ampicillin and chloramphenicol and was grown until turbid at 37 °C with shaking at 180 rpm. Ten mL of this culture was used to inoculate each 1L of LB plus ampicillin and chloramphenicol. These cultures were grown at 37°C to an OD600 of 0.4 to 0.6 and induced with 400 μL of 1 M IPTG per liter of culture. The cultures were grown at 22 °C for 12–14 hrs. To each 1L culture, EDTA and PMSF were added to 2 mM and 0.5 mM, respectively, before pelleting at 4000 rpm for 20 minutes at 4°C in the Fiberlite F8B roto r. The pellet was resuspended in 100 mL of lysis buffer (20 mM Tris pH 8, 140 mM NaCl, 1 mM DTT, 0.5 mM PMSF) plus 2 protease inhibitor tablets. The cells were lysed by sonication on ice with intermittent pulses to keep the temperature below 10 °C. The lys ate was then spun down for 45 min at 18,000 rpm in a JA-20 rotor at 4°C. The clarified lysate was l oaded onto a column containing 10 mL of GS4B beads equilibrated in GST-binding buffer (20 mM Tris pH 8, 140 mM NaCl, 2 mM EDTA, 1 mM DTT) and washed with 7 column volumes of GST-binding buffer. Protein was eluted from the column with 30 mL of GST-elution buffer pH adjusted to 8.0 with NaOH (20 mM Tris pH 8.0, 100 mM NaCl, 1 mM DTT, 50 mM reduced L-glutathione). The elution was dialyzed overnight at 4 °C in 2 L of 20 mM Tris pH 8.0, 50 mM NaCl, and 1 mM DTT in 3500 MWCO dialysis tubing. The dialysate was then loaded onto a Source30Q column on an FPLC equilibrated in QA buffer (20 mM Tris pH 8.0, 100 mM NaCl, 1 mM DTT). GST-Wsp1-VCA was eluted from the column over a 20 column volume gradient to 100% QB buffer (20 mM Tris pH 8.0, 500 mM NaCl, 1 mM DTT). Fractions containing GST-Wsp1-VCA were concentrated to 1.5 mL and flowed over an Superdex 75 size exclusion column equilibrated in 20 mM Tris pH 8.0, 150 mM NaCl, and 1 mM DTT. Pure fractions of GST-Wsp1-VCA were pooled and concentrated to desired volume using a 3500 MWCO spin concentrator tube (Sartorius Vivaspin Turbo 15 #VS15T91) in the Fiberlite F13B rotor at 2,500 rpm for 5 to 10 minute cycles at 4°C. An extinction coefficient of 5,500M−1cm−1 was used to determine protein concentration.
Biotin-inactivated myosin was prepared by reacting 2 mg of myosin with 5 μ L of 250 mM EZ-Link-Maleimide-PEG11-Biotin dissolved in DMSO. The labeling reaction was carried out in 500 μL reaction buffer (20 mM HEPES, 500 mM KCl, 5 mM EDTA pH 8.0, 1 μM ATP and 1 mM MgCl2) on ice for 6 hours. The Biotin-myosin was then dialyzed into 0.5 L of storage buffer (20 mM Imidazole pH 7.0, 500 mM KCl, 5 mM EDTA pH 8.0, 1 mM DTT and 50% glycerol) using a 3500 MWCO dialysis thimble (Thermofisher Slide-A-Lyzer MINI dialysis unit 0069550).
Analytical Ultracentrifugation:
Dip1 was diluted to 6.9, 13.7, and 20.6 μM to a final buffer concentration of 20 mM Tris pH 8.0, 50 mM NaCl, and 1 mM TCEP. Cells were inserted into a Beckman An50 Ti rotor and spun at 50,000 rpm at 20 °C in a Beckman XL-I analytical ultracentrifuge. Se dimentation was monitored using interference optics and the resulting radial interference scans were fit using a non-interacting continuous c(S) distribution model in SEDFIT [34]. The frictional ratio was set to 1.2 for the initial fit of the data without using the nonlinear regression method and was then optimized using nonlinear regression algorithms to improve the fit. The fits were regularized using a confidence level of 0.95 and were considered satisfactory if the root mean squared deviation was less than 0.01 and the residuals were random and less than 2% of the signal. The final parameters, including the fitted values the sedimentation coefficient and the frictional ratios are shown in Table S1.
TIRF microscopy slide preparation:
TIRF flow chambers were constructed and reactions setup as previously described with slight modifications [19]. Coverslips (24 × 60 # 1.5) were cleaned in Coplin jars by sonicating in acetone followed by 1 M KOH for 25 min each, with a deionized water rinse between each sonication step. Coverslips were then rinsed twice with methanol and aminosilanized by incubating in 1% APTES (Sigma), 5 % acetic acid in methanol solution for 10 min before sonicating for 5 min, and then incubating for an additional 15 min. Coverslips were then rinsed with 2 volumes of methanol followed by thorough flushing with deionized water. After air drying, TIRF chambers were created by sandwiching cleaned coverslips and a glass microscope slide using double-sided tape to create an ~14 μL, 0.5 cm wide chamber. Chambers were passivated by incubating chambers for 4–5 hours in 300 mg/mL methoxy PEG succinimidyl succinate, MW5000 (JenKem) containing 1–3% biotin-PEG NHS ester, MW5000 (JenKem) dissolved in 0.1 M NaHCO3 pH 8.3. Excess PEG was washed away with 0.1 M NaHCO3 pH 8.3 and chambers were stored in deionized water for less than 1 week. All cleaning steps were carried out at room temperature. Immediately prior to imaging, chambers were incubated for 8 minutes with 1 μM NeutrAvidin (ThermoFisher) followed by 8 minutes with 50–150 nM biotin inactivated myosin (Cytoskeleton, Inc), both prepared in 50 mM Tris pH 7.5, 600 mM NaCl. Chambers were washed 2 times with 20 mg/mL BSA in 50 mM Tris pH 7.5, 600 mM NaCl followed by 2 washes with 20 mg/mL BSA in 50 mM Tris pH 7.5, 150 mM NaCl. Chambers were finally pre-incubated with TIRF buffer (10 mM Imidazole pH 7.0, 1 mM MgCl2, 1 mM EGTA, 50 mM KCl, 100 mM DTT, 0.2 mM ATP, 25 mM Glucose, 0.5 % Metylcellulose (400 cP at 2%), 0.02 mg/mL Catalase (Sigma) and 0.1 mg/mL Glucose Oxidase (MP Biomedicals)).
Actin Polymerization Reactions in TIRF chambers:
In a typical reaction, 1 μL of 2.5 mM MgCl2 and 10 mM EGTA was mixed with 5 μL of 9 μM 33% Oregon Green actin and incubated for 2 minutes. Four microliters of the actin solution was then added to 16 μL of a solution containing 1.25x TIRF buffer and any other proteins. Reactions were imaged on a Nikon TE2000 inverted microscope equipped with 100× 1.49 numerical aperture TIRF objective, 50 mW 488 nm and 561 nm Sapphire continuous wave solid state laser lines (Coherent), a dual band TIRF (zt488/561rpc) filter cube (Chroma C143315), and a 1x −1.5x intermediate magnification module. Images were collected using an 512×512 pixel EM-CCD camera (iXon3, Andor). For two color reactions, typical imaging conditions were 50 ms exposures with the 488 nm laser and 50 to 100 ms exposures with the 561 nm laser at 1 s intervals. For photobleaching measurements of 568-Dip1, 150 ms exposures with the 561 nm laser and 500 ms intervals were used. The concentration of 568-Dip1 was kept in the low nanomolar range in all assays to prevent high backgrounds of non-specifically adsorbed 568-Dip1 from obscuring Dip1 filament binding events.
Pyrene Actin Polymerization Assay:
In a typical reaction, 2 μL of 10X ME buffer (5 mM MgCl2, 20 mM EGTA) was added to 20 μL of 15% pyrene labeled actin and allowed to incubate for 2 minutes in 96 well flat bottom black polystyrene assay plates (Corning 3686). To initiate the reaction, 78 μL of buffer containing all other proteins was added to the actin, bringing the final buffer concentration in the reaction to 10 mM Imidazole pH 7.0, 50 mM KCL, 1 mM EGTA, 1 mM MgCl2, 200 μM ATP and 1 mM DTT. Polymerization of actin was monitored using a TECAN Safire 2 plate reader by exciting the pyrene actin at 365 nm and monitoring the emission at 407 nm.
Pointed End Binding Assay:
A solution of 7.5 μM 33% Oregon Green actin was pre-incubated for 2 minutes with 0.5 mM MgCl2 and 2 mM EGTA to exchange the calcium in G-actin storage buffer for magnesium. The actin was then diluted in TIRF buffer and reacted for 20 minutes. Filaments were sheared by drawing solution two times through a 30-gauge needle on a 3 mL syringe. The reaction was diluted 10-fold in TIRF buffer containing 6 nM 568-Dip1, 500 nM S. pombe Arp2/3 complex, and 0.1 μM 33% Oregon Green actin. Control reactions did not contain Arp2/3 complex. Binding to pointed ends was assayed by equilibrating the final mixture for 2 minutes and then imaging by TIRF microscopy in flow chambers as described above.
TIRF Microscopy Image Analysis:
Images were prepared in Image J. Background was subtracted with a 10-pixel rolling ball radius for the 561 channel and a 15-pixel rolling ball radius for the 488 channel. The total actin polymer was calculated using a custom image processing script run in Matlab (Mathworks), described as follows. For each frame, pixels corresponding to filament fluorescence were identified using image segmentation followed by morphological area opening to remove non-filament small fluorescent objects. The final pixel number value was converted to micrometers (1px = 106.7 nm) to yield the total length of actin filaments in the image frame. To measure the number of Dip1 pointed end binding events (Figure 3) and calculate the branching rates from different filament sources (Figure 3), reactions were analyzed up to 120 seconds after imaging was initiated. A custom ImageJ plugin was used to measure the lengths of actin filaments over time (Figure 3, plugin was a gift from Jeff Kuhn).
Quantification and Statistical Analysis
The number of replicates and meaning of error bars can be found in the figure legends. The significance of data in Figure 3D was calculated using a two-tailed T test assuming unequal variances.
Supplementary Material
Video S1: TIRF microscopy video showing an actin filament (green) growing from a Dip1 molecule (magenta) non-specifically adsorbed the coverslip surface (class I event), Related to Figure 2. Reaction contains: 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S2: TIRF microscopy video showing an actin filament pre-bound to Dip1 landing on the coverslip surface (class II event), Related to Figure 2. Dip1 is shown in magenta and actin is shown in green. Reaction contains 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S3: TIRF microscopy video showing Dip1 (magenta) binding to the pointed end of a surface-captured actin filament (class III event), Related to Figure 2. Reaction contains 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S4: TIRF microscopy video showing an actin filament (green) nucleated by Dip1-bound Arp2/3 (magenta) serving as the mother filament for branching nucleation, Related to Figure 3. Reaction contains 250 nM Arp2/3 complex, 150 nM GST-Wsp1-VCA, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM.
Video S5: Same conditions as Video 4, but with a larger field of view, Related to Figure 3.
Highlights.
Dip1 co-opts aspects of branching nucleation to activate Arp2/3 complex
Dip1 binds the pointed ends of actin filaments indirectly through Arp2/3 complex
Actin filaments nucleated by Dip1-Arp2/3 complex activate WASP-bound Arp2/3 complex
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01 GM092917 and R01 GM127440 (B.J.N), T32 GM007759 (to C.J.B, A.R.W and L.A.H.) and by the American Heart Association, grants #18PRE33960110 (C.J.B) and #15PRE25900011 (A.R.W). We thank Jeff Kuhn for providing the filament analysis plugin for ImageJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
“The authors declare no competing interests.”
References
- 1.Rotty JD, Wu C, and Bear JE (2013). New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 14, 7–12. [DOI] [PubMed] [Google Scholar]
- 2.Achard V, Martiel J-L, Michelot A, Guérin C, Reymann A-C, Blanchoin L, and Boujemaa-Paterski R (2010). A “Primer”-Based Mechanism Underlies Branched Actin Filament Network Formation and Motility. Current Biology 20, 423–428. [DOI] [PubMed] [Google Scholar]
- 3.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, and Pollard TD (1999). Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 96, 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner AR, Luan Q, Liu S-L, and Nolen BJ (2013). Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Curr. Biol 23, 1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu R, and Chang F (2011). Characterization of dip1p reveals a switch in Arp2/3-dependent actin assembly for fission yeast endocytosis. Curr Biol 21, 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luan Q, Liu S-L, Luke A Helgeson, and Nolen, B.J. Structure of the nucleation promoting factor SPIN90 bound to the actin filament nucleator Arp2/3 complex. EMBO J. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchand J-B, Kaiser DA, Pollard TD, and Higgs HN (2001). Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nature Cell Biology 3, 76–82. [DOI] [PubMed] [Google Scholar]
- 8.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, and Kirschner MW (1999). The Interaction between N-WASP and the Arp2/3 Complex Links Cdc42-Dependent Signals to Actin Assembly. Cell 97, 221–231. [DOI] [PubMed] [Google Scholar]
- 9.Luan Q, Zelter A, MacCoss MJ, Davis TN, and Nolen BJ (2018). Identification of Wiskott-Aldrich syndrome protein (WASP) binding sites on the branched actin filament nucleator Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 115, E1409–E1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ti S-C, Jurgenson CT, Nolen BJ, and Pollard TD (2011). Structural and biochemical characterization of two binding sites for nucleation-promoting factor WASp-VCA on Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 108, E463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padrick SB, Doolittle LK, Brautigam CA, King DS, and Rosen MK (2011). Arp2/3 complex is bound and activated by two WASP proteins. Proc. Natl. Acad. Sci. U.S.A. 108, E472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodnick-Smith M, Liu S-L, Balzer CJ, Luan Q, and Nolen BJ (2016). Identification of an ATP-controlled allosteric switch that controls actin filament nucleation by Arp2/3 complex. Nat Commun 7, 12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boczkowska M, Rebowski G, Kast DJ, and Dominguez R (2014). Structural analysis of the transitional state of Arp2/3 complex activation by two actin-bound WCAs. Nat Commun 5, 3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodnick-Smith M, Luan Q, Liu S-L, and Nolen BJ (2016). Role and structural mechanism of WASP-triggered conformational changes in branched actin filament nucleation by Arp2/3 complex. PNAS 113, E3834–E3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X-P, Rouiller I, Slaughter BD, Egile C, Kim E, Unruh JR, Fan X, Pollard TD, Li R, Hanein D, et al. (2012). Three-dimensional reconstructions of Arp2/3 complex with bound nucleation promoting factors. EMBO J. 31, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez Espinoza, Sofia Metskas, Ann Lauren, Chou Steven Z., Rhoades Elizabeth, and Pollard Thomas D. (2018). Conformational changes in Arp2/3 complex induced by ATP, WASp-VCA and actin filaments. PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetrick B, Han MS, Helgeson LA, and Nolen BJ (2013). Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol 20, 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouiller I, Xu X-P, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, and Hanein D (2008). The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 180, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn JR, and Pollard TD (2005). Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J 88, 1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skau CT, Neidt EM, and Kovar DR (2009). Role of Tropomyosin in Formin-mediated Contractile Ring Assembly in Fission Yeast. Mol Biol Cell 20, 2160–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao JY, Goins LM, Petek NA, and Mullins RD (2015). Arp2/3 complex and cofilin modulate binding of tropomyosin to branched actin networks. Curr. Biol 25, 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp G, Bugyi B, Ujfalusi Z, Barkó S, Hild G, Somogyi B, and Nyitrai M (2006). Conformational changes in actin filaments induced by formin binding to the barbed end. Biophys. J 91, 2564–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luan Q, and Nolen BJ (2013). Structural basis for regulation of Arp2/3 complex by GMF. Nat Struct Mol Biol 20, 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ydenberg CA, Padrick SB, Sweeney MO, Gandhi M, Sokolova O, and Goode BL (2013). GMF severs actin-Arp2/3 complex branch junctions by a cofilin-like mechanism. Curr Biol 23, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M, Smith BA, Bovellan M, Paavilainen V, Daugherty-Clarke K, Gelles J, Lappalainen P, and Goode BL (2010). GMF is a cofilin homolog that binds Arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Curr. Biol 20, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirotkin V, Beltzner CC, Marchand J-B, and Pollard TD (2005). Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. The Journal of Cell Biology 170, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovar DR, Sirotkin V, and Lord M (2011). Three’s company: The fission yeast actin cytoskeleton. Trends Cell Biol 21, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burston HE, Maldonado-Báez L, Davey M, Montpetit B, Schluter C, Wendland B, and Conibear E (2009). Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol 185, 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh H, Kim H, Chung K-H, Hong NH, Shin B, Park WJ, Jun Y, Rhee S, and Song WK (2013). SPIN90 Knockdown Attenuates the Formation and Movement of Endosomal Vesicles in the Early Stages of Epidermal Growth Factor Receptor Endocytosis . PLoS One 8 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3858329/ [Accessed August 7, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DJ, Kim SH, Lim CS, Choi KY, Park CS, Sung BH, Yeo MG, Chang S, Kim J-K, and Song WK (2006). Interaction of SPIN90 with the Arp2/3 Complex Mediates Lamellipodia and Actin Comet Tail Formation. J. Biol. Chem 281, 617–625. [DOI] [PubMed] [Google Scholar]
- 31.Isogai T, van der Kammen R, Leyton-Puig D, Kedziora KM, Jalink K, and Innocenti M (2015). Initiation of lamellipodia and ruffles involves cooperation between mDia1 and the Arp2/3 complex. J. Cell. Sci 128, 3796–3810. [DOI] [PubMed] [Google Scholar]
- 32.Urbanek AN, Smith AP, Allwood EG, Booth WI, and Ayscough KR (2013). A Novel Actin-Binding Motif in Las17/WASP Nucleates Actin Filaments Independently of Arp2/3. Current Biology 23, 196–203. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, and Pollard T (2013). Actin filament severing by cofilin dismantles actin patches and produces mother filaments for new patches. Curr Biol 23, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuck P (2000). Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J 78, 1606–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapust RB, Tözsér J, Fox JD, Anderson DE, Cherry S, Copeland TD, and Waugh DS (2001). Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 14, 993–1000. [DOI] [PubMed] [Google Scholar]
- 36.Liu S-L, May JR, Helgeson LA, and Nolen BJ (2013). Insertions within the actin core of actin-related protein 3 (Arp3) modulate branching nucleation by Arp2/3 complex. J. Biol. Chem 288, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of Image Analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: TIRF microscopy video showing an actin filament (green) growing from a Dip1 molecule (magenta) non-specifically adsorbed the coverslip surface (class I event), Related to Figure 2. Reaction contains: 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S2: TIRF microscopy video showing an actin filament pre-bound to Dip1 landing on the coverslip surface (class II event), Related to Figure 2. Dip1 is shown in magenta and actin is shown in green. Reaction contains 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S3: TIRF microscopy video showing Dip1 (magenta) binding to the pointed end of a surface-captured actin filament (class III event), Related to Figure 2. Reaction contains 500 nM Arp2/3 complex, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM. (15 fps)
Video S4: TIRF microscopy video showing an actin filament (green) nucleated by Dip1-bound Arp2/3 (magenta) serving as the mother filament for branching nucleation, Related to Figure 3. Reaction contains 250 nM Arp2/3 complex, 150 nM GST-Wsp1-VCA, 6 nM Alexa568-Dip1 and 1.5 μM 33% Oregon Green labeled actin. Scale bar: 2 μM.
Video S5: Same conditions as Video 4, but with a larger field of view, Related to Figure 3.