Abstract
Background:
Tooth agenesis, the congenital absence of one or more teeth, can be diagnosed in children in the first decade of life. Tooth agenesis is a phenotypic feature of conditions such as ectodermal dysplasia, cleft lip, cleft palate, Down syndrome, and Van der Woude syndrome. Tooth agenesis can also be nonsyndromic. Studies have shown an association between the genetic determinants of nonsyndromic tooth agenesis and neoplasms in adulthood.
Methods:
This review of the implications of tooth agenesis as a risk indicator for neoplasms in adulthood is based on a search of PubMed to identify published case series, case reports, and review articles. The reference articles were manually searched. The search was limited to articles published in the English language.
Results:
Neoplasms reported in patients with tooth agenesis include colorectal neoplasms and epithelial ovarian cancer, as well as family histories of breast cancer, prostate cancer, and cancers of the brain and nervous system.
Conclusion:
Although odontogenesis and tumorigenesis may seem to be unrelated processes, the clinical association between the two highlights the overlap of genetic determinants and molecular pathways. Tooth agenesis can be diagnosed during childhood and should be considered a marker for risk of neoplasms in adulthood. Healthcare providers should identify tooth agenesis and provide appropriate anticipatory guidance.
Keywords: Anodontia, carcinogenesis, odontogenesis, oligodontia-colorectal cancer syndrome
INTRODUCTION
Tooth agenesis, one of the most common developmental dental anomalies in humans, is the congenital absence of one or more teeth.1 It is diagnosed by dental examination and radiographic assessment of the oral cavity. Tooth agenesis can affect both primary and permanent dentitions, although most case reports and genetic and molecular studies evaluate agenesis of permanent teeth.1 The reported incidence of tooth agenesis is 3%-10% depending on the population being studied.2-4 The incidence is higher in females, and 60% of individuals exhibit unilateral tooth agenesis.3 Third molars are the most commonly missing permanent teeth; 23% is the often-cited incidence rate.3 The next most prevalent missing teeth are mandibular second premolars, followed by maxillary lateral incisors.5 While a high proportion of tooth agenesis cases tend to manifest unilaterally, agenesis of maxillary lateral incisors usually occurs bilaterally.5
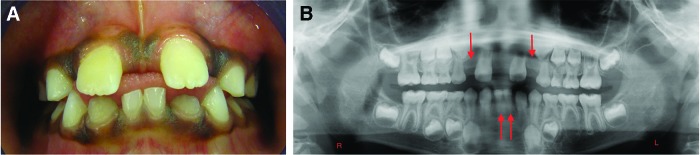
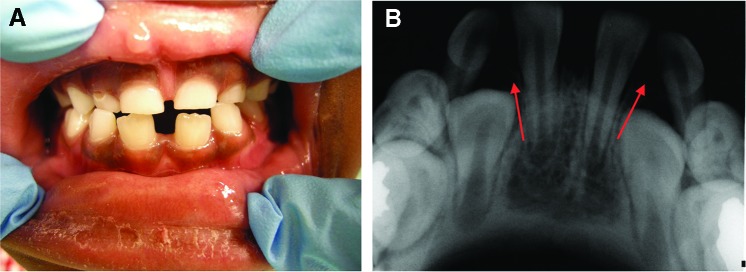
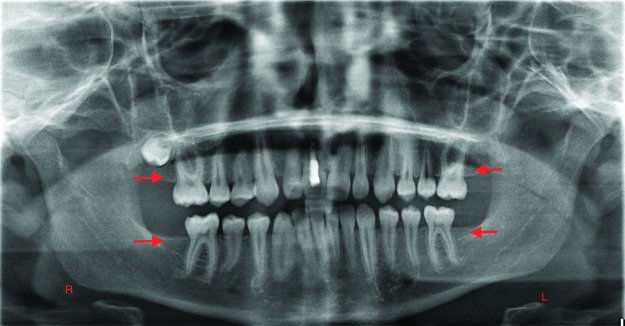
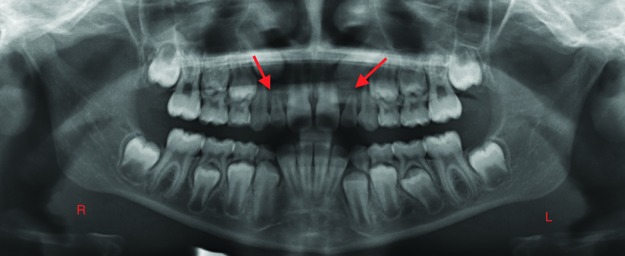
The presence of unusual spacing in a child’s dentition during a well-check visit should lead the pediatrician and the dentist to suspect tooth agenesis. Figure 1A shows a large midline diastema between the maxillary incisors of a 9-year-old male. His mandibular incisors also exhibit spacing and overretained primary central incisors. The patient’s panoramic radiograph confirms agenesis of his maxillary permanent lateral incisors (the clinical midline diastema) and the mandibular central incisors, as well as the mandibular left second premolar (Figure 1B). Figure 2A shows the spacing in the mandibular anterior region of a 7-year-old female who has erupted her permanent mandibular central incisors, but the lateral incisors are not present clinically. The intraoral periapical radiograph of this region confirms the agenesis of the mandibular lateral incisors (Figure 2B).
Figure 1. A. Large midline diastema between the maxillary incisors of a 9-year-old male. The mandibular incisors also exhibit spacing and overretained primary central incisors. B. Panoramic radiograph confirms agenesis of the maxillary permanent lateral incisors (the clinical midline diastema), the mandibular central incisors, and the mandibular left second premolar. Arrows point to the sites of permanent tooth agenesis.
Figure 2. A. Spacing in the mandibular anterior region of a 7-year-old female with permanent mandibular central incisors and clinically missing lateral incisors. B. Intraoral periapical radiograph confirms the agenesis of the mandibular lateral incisors. Arrows point to the sites of permanent tooth agenesis.
This review of the implications of tooth agenesis as a risk indicator for neoplasms in adulthood is based on a contemporary review of literature. A search of PubMed identified case series, case reports, and review articles. The reference articles were manually searched. The literature search was limited to articles published in the English language.
CLASSIFICATION OF TOOTH AGENESIS
Tooth agenesis can be classified based on the number of congenitally missing teeth. Hypodontia refers to the absence of fewer than 6 teeth (not including third molars).5,6 Oligodontia refers to the absence of 6 or more teeth (not including third molars).5,6 Anodontia is the complete absence of teeth.5,6 Hypodontia is more common in the permanent dentition than in the primary dentition.6 Tooth agenesis can also be classified as syndromic and nonsyndromic. Syndromic tooth agenesis is associated with various systemic conditions or syndromes (Table).2,5,7-9 Patients with syndromic tooth agenesis may have other concurrent dental anomalies such as microdontia, short roots, dental impactions, delayed formation of teeth, delayed eruption, transposition of canines and premolars, taurodontism, and enamel hypoplasia.5 Ectodermal dysplasia, cleft lip, cleft palate, Down syndrome, and Van der Woude syndrome are commonly occurring syndromes with tooth agenesis as a feature.5
Table. Common Syndromes and Conditions Manifesting With Tooth Agenesis.
| Syndrome or Condition | Dental Manifestation |
|---|---|
| Ectodermal dysplasia | Hypodontia, oligodontia, anodontia, conical teeth |
| Orofacial digital syndrome | Hypodontia, oligodontia, supernumerary teeth, dental malocclusion |
| Orofacial clefting (cleft lip and/or cleft palate) | Hypodontia, oligodontia |
| Pierre Robin sequence | Hypodontia, microdontia, supernumerary teeth |
| Van der Woude syndrome | Hypodontia, cleft lip, cleft palate |
| Apert syndrome | Hypodontia, enamel hypoplasia |
| Moebius syndrome | Hypodontia |
| Down syndrome | Hypodontia |
| Ellis-van Creveld syndrome | Hypodontia (incisors), conical teeth, taurodontism |
| Witkop tooth and nail syndrome | Hypodontia |
| Soto syndrome | Hypodontia (premolar), enamel hypoplasia |
| Wolf-Hirschhorn syndrome | Hypodontia, taurodontism, microdontia |
In patients with nonsyndromic tooth agenesis, congenitally missing teeth are the only apparent clinical finding. Overall, nonsyndromic tooth agenesis is the most common cause of congenitally missing teeth,8 and it can be sporadic or familial.6 Sporadic cases of tooth agenesis usually present with hypodontia as the only phenotype.8 Familial cases may present with hypodontia as the only clinical feature or may have hypodontia as part of a syndrome.2 In familial cases, the autosomal dominant inheritance pattern is most common, and autosomal recessive inheritance or X-linked inheritance is less common.
Environmental factors can cause sporadic tooth agenesis. Maternal rubella virus infection can lead to tooth agenesis and to other morphologic dental abnormalities.5 Orofacial trauma during odontogenesis may result in tooth agenesis in the region of trauma.5 Exposure to medications such as thalidomide and antineoplastic chemotherapeutic agents and exposure to radiation therapy in the head and neck region during odontogenesis can also lead to tooth agenesis.5
While syndromic tooth agenesis has been studied and quantified in various reports of craniofacial anomalies and syndromes, information is emerging about the genetic determinants of nonsyndromic tooth agenesis and the interrelation of these genetic mutations with other previously unidentified medical conditions.1,3,4,10,11
ODONTOGENESIS
The maxillary and mandibular dental lamina are formed by 6 weeks in utero, and development of the primary teeth commences thereafter, at approximately 8 weeks in utero.7,8 Proliferation of the oral ectoderm within the dental lamina leads to formation of tooth buds. These developing tooth buds contain the genetic determinants for transcription of proteins responsible for initiating signals that eventually regulate the number of teeth that will form.7,8 Failure of initiation within the dental lamina leads to tooth agenesis.8 Developing tooth buds of the permanent teeth form at 10-13 weeks in utero.8
While multiple molecular pathways exist for odontogenesis, the most understood pathways include bone morphogenetic protein, fibroblast growth factor, tumor necrosis factor, sonic hedgehog, and Wnt pathways.2,6,8 While more than 300 genes precisely control odontogenesis, the genes most studied and identified with nonsyndromic tooth agenesis are MSX1, PAX9, and AXIN2.2,6,8
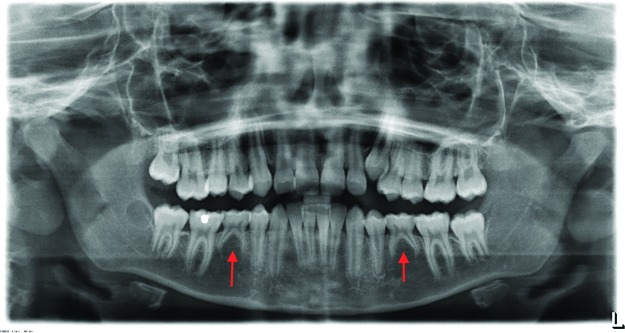
MSX1 is important in epithelial-mesenchymal interactions in early odontogenesis1,3 and was the first gene identified in human nonsyndromic tooth agenesis.2 Mutations in MSX1 are most commonly related to agenesis of the second premolars and third molars.2 Figure 3 is a panoramic radiograph of a 13-year-old with agenesis of the mandibular second premolars. Mutations in MSX1 are also seen in patients with cleft lip, cleft lip palate, nail dystrophy, and Wolf-Hirschhorn syndrome.2
Figure 3. Panoramic radiograph of a 13-year-old with agenesis of mandibular second premolars. Arrows point to the sites of permanent tooth agenesis.
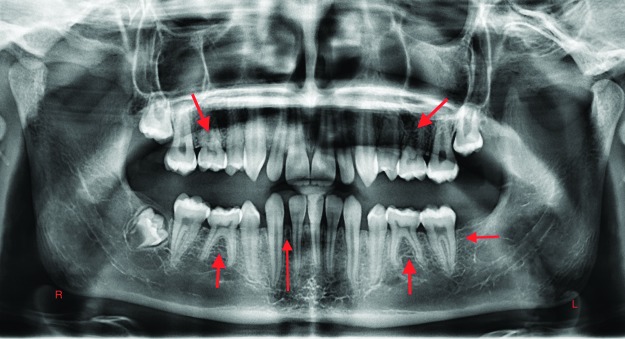
PAX9 is expressed in neural crest-derived mesenchyme, which is involved in odontogenesis.10 Condensation of mesenchyme must occur around the tooth bud epithelium.1,10 PAX9 mutations are likely to manifest as agenesis of second molars or a combination of agenesis of second premolars and mandibular incisors. PAX9 mutations also influence the position and morphology of teeth in affected individuals. Figure 4 is a panoramic radiograph of a 14-year-old with agenesis of the maxillary and mandibular second premolars, mandibular left permanent second molar, and mandibular right lateral incisor. Figure 5 is a panoramic radiograph of a 15-year-old with agenesis of the permanent second molars. PAX9 plays a critical role in fetal development and cancer growth. It is involved in cell proliferation, apoptotic resistance, and cell migration and has been shown to be a prognostic marker of esophageal squamous cell carcinoma.12
Figure 4. Panoramic radiograph of a 14-year-old with agenesis of maxillary and mandibular second premolars, mandibular left permanent second molar, and mandibular right lateral incisor. Arrows point to the sites of permanent tooth agenesis.
Figure 5. Panoramic radiograph of a 15-year-old with agenesis of the permanent second molars. Arrows point to the sites of permanent tooth agenesis.
AXIN2 is a negative regulator of the catenin Wnt signaling pathway and is expressed in dental papilla mesenchyme.5 Mutations in AXIN2 have been associated with tooth agenesis, with the phenotype presenting as a mix of anterior and posterior tooth agenesis.5,13 Figure 6 is a panoramic radiograph of a 9-year-old with agenesis of the maxillary permanent lateral incisors. In an assessment of a Brazilian population, AXIN2 was associated with agenesis of lower incisors.14
Figure 6. Panoramic radiograph of a 9-year-old with agenesis of maxillary permanent lateral incisors. Arrows point to the sites of permanent tooth agenesis.
ODONTOGENESIS AND TUMORIGENESIS
Research in cell biology and human genetics has shown genetic overlap in the occurrence of tooth agenesis and certain human cancers.13,15 Although the process of odontogenesis may seem unrelated to tumorigenesis, various case series have reported an association between tooth agenesis and tumors in humans.13,15,16
In 2004, Lammi et al reported on 4 generations of a Finnish family (12 family members) and described the association between familial tooth agenesis (oligodontia) and colorectal neoplasms.17 Mutations in the AXIN2 gene identified in this familial group were inherited in an autosomal dominant pattern. Colorectal neoplasia (ranging from polyps to colorectal cancer) was found in 6 family members with oligodontia. Neoplasia was not identified in family members with a normal complement of teeth. Through DNA isolation from venous blood and molecular analysis, nonsense and frameshift mutations in the AXIN2 gene were identified. In addition to its role in odontogenesis, the AXIN2 gene regulates the Wnt pathway and thereby controls cellular proliferation, differentiation, and morphogenesis of organ systems.5,13,17 The Lammi et al familial analysis provided evidence of the genetic overlap between colorectal neoplasia and oligodontia linked to AXIN2 mutations.17 In addition to this study, AXIN2 mutations have been identified in several human cancers, including colorectal, skin, gastrointestinal, liver, and ovarian cancer.15,18-20
In 2011, Marvin et al evaluated 3 generations of a family with autosomal dominant oligodontia.21 They detected a nonsense mutation on the AXIN2 gene leading to the phenotype of oligodontia. The family members with the gene mutation and oligodontia also exhibited neoplasms: early onset breast cancer, colorectal cancer, and gastrointestinal adenomas. Family members without oligodontia did not present with neoplasms.21
In 2008, Chalothorn et al reported hypodontia as a marker for epithelial ovarian cancer.22 In their comparison of 50 females with epithelial ovarian cancer and 100 healthy females without epithelial ovarian cancer, 20% of patients with epithelial ovarian cancer had hypodontia compared to 3% of healthy controls, and the difference was statistically significant (P < 0.001). Patients with epithelial ovarian cancer were 8.1 times more likely to have hypodontia than the healthy controls.22
In 2015, Fekonja et al also investigated the occurrence of epithelial ovarian cancer in females with hypodontia.23 In this retrospective study, 150 Slovenian females with epithelial ovarian cancer were compared to age-matched healthy women. The incidence of hypodontia was 19.2% of females with epithelial ovarian cancer vs 6.7% in healthy controls. The difference was statistically significant (P = 0.004) with an odds ratio of 3.32. Further, poorly differentiated tumors were observed in a significantly higher proportion of patients with hypodontia than in patients without hypodontia (P = 0.042). Patients with hypodontia also had a significantly higher incidence of bilateral epithelial ovarian cancer (P = 0.02).23
The correlation between epithelial ovarian cancer and hypodontia is particularly important as epithelial ovarian cancer is considered a fatal malignancy and a silent killer in women.22,23 Ovarian cancer is difficult to diagnose because of the lack of effective screening markers.20 The most effective screening risk factor is a familial history of epithelial ovarian cancer. Tooth agenesis could potentially serve as a noninvasive screening tool to identify patients at risk early in life.22
In 2013, Küchler et al conducted a cross-sectional single-center study in Brazil,14 recruiting 82 patients with tooth agenesis and 328 age-matched controls for the study. Familial history of cancer was collected from all study participants. Patients with tooth agenesis had a significantly higher risk of having a family history of cancer (P = 0.00006, odds ratio 2.7) than the control group.14 Breast cancer was the most commonly reported cancer, followed by prostate cancer and cancers of the brain and nervous system. Individuals with tooth agenesis also had a higher risk of having a family history of female cancers (breast, ovarian, and cervical uterine cancer). The results highlight the potential association between tooth agenesis and neoplasms.14
The genetic etiology of tooth agenesis is an area of academic endeavor that has grown between 2003-2018 because of advances in the understanding of genetic and molecular interactions.5,6,7,24 Genetic mutations in key genes regulating odontogenesis lead to failure of tooth development.5 The same mutations may be associated with the development of neoplasms that are detected later in life.6 The link between tumorigenesis and odontogenesis suggests the plausibility of using hypodontia in childhood as a screening tool or marker for the risk of neoplasms in adulthood.6 The dentist can detect tooth agenesis within the first decade of life and is therefore in a unique position to counsel the child’s family and physician of the dental findings with respect to potential risks of neoplasms later in life.3 Similarly, when pediatricians notice unusual spacing between teeth during well-check visits or elicit a family history of missing teeth, they should ensure that a complete dental assessment for tooth agenesis is performed.
Tooth agenesis has a psychosocial impact, functional limitations, and ongoing dental treatment–related expenses that present challenges to patients and their families. Consequently, dental professionals focus on the management of function and esthetics throughout the growing years of a child with tooth agenesis, and successful dental management usually requires interdisciplinary care involving a pediatric dentist, orthodontist, and prosthodontist. However, in light of the research showing the link between tooth agenesis and cancer, the pediatric dentist should also include a discussion of risk for adult neoplasms in the anticipatory guidance provided to children with hypodontia and their caregivers and forward a summary of dental findings to the child’s primary medical care providers.
The emerging understanding between tooth agenesis and the development of neoplasms in adulthood is based on studies in molecular biology and the genetics of odontogenesis and on clinical reports. These studies have limitations, including sample sizes, ethnicity of study subjects, and incompletely identified genetic markers or molecular processes. Prospective clinical and genetic studies are needed to determine if hypodontia is an unequivocal marker for adult neoplasms.
CONCLUSION
The literature shows a link between tooth agenesis and neoplastic changes that may be one of association rather than of cause and effect. However, this potential association identifies the importance of the oral-systemic health continuum and underscores the importance of collaborative care among all healthcare providers, including dentists and physicians.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kirac D, Eraydin F, Avcilar T,et al. . Effects of PAX9 and MSX1 gene variants to hypodontia, tooth size and the type of congenitally missing teeth. Cell Mol Biol (Noisy-le-grand). 2016. November 30;62(13):78-84. doi: 10.14715/cmb/2016.62.13.14. [DOI] [PubMed] [Google Scholar]
- 2.Ye X, Attaie AB. Genetic basis of nonsyndromic and syndromic tooth agenesis. J Pediatr Genet. 2016. December;5(4):198-208. doi: 10.1055/s-0036-1592421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsson L, Magnusson TE, Thordarson A,et al. . Rare and common variants conferring risk of tooth agenesis. J Dent Res. 2018. May;97(5):515-522. doi: 10.1177/0022034517750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iavazzo C, Papakiritsis M, Gkegkes ID. Hypodontia and ovarian cancer: a systematic review. J Turk Ger Gynecol Assoc. 2016. January 12;17(1):41-44. doi: 10.5152/jtgga.2015.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Coster PJ, Marks LA, Martens LC, Huysseune A. Dental agenesis: genetic and clinical perspectives. J Oral Pathol Med. 2009. January;38(1):1-17. doi: 10.1111/j.1600-0714.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 6.Yin W, Bian Z. The gene network underlying hypodontia. J Dent Res. 2015. July;94(7):878-885. doi: 10.1177/0022034515583999. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni M, Agrawal T, Kheur S. Tooth agenesis: newer concept. J Clin Pediatr Dent. 2011. Fall;36(1):65-69. [DOI] [PubMed] [Google Scholar]
- 8.Juuri E, Balic A. The biology underlying abnormalities of tooth number in humans. J Dent Res. 2017. October;96(11):1248-1256. doi: 10.1177/0022034517720158. [DOI] [PubMed] [Google Scholar]
- 9.Stavropoulos D, Bartzela T, Bronkhorst E, Mohlin B, Hagberg C. Dental agenesis patterns of permanent teeth in Apert syndrome. Eur J Oral Sci. 2011. June;119(3):198-203. doi: 10.1111/j.1600-0722.2011.00821.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonczek O, Balcar VJ, Šerý O. PAX9 gene mutations and tooth agenesis: a review. Clin Genet. 2017. November;92(5):467-476. doi: 10.1111/cge.12986. [DOI] [PubMed] [Google Scholar]
- 11.Vieira AR. Oral clefts and syndromic forms of tooth agenesis as models for genetics of isolated tooth agenesis. J Dent Res. 2003. March;82(3):162-165. doi: 10.1177/154405910308200303. [DOI] [PubMed] [Google Scholar]
- 12.Tan B, Wang J, Song Q,et al. . Prognostic value of PAX9 in patients with esophageal squamous cell carcinoma and its prediction value to radiation sensitivity. Mol Med Rep. 2017. July;16(1):806-816. doi: 10.3892/mmr.2017.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin W, Bian Z. Hypodontia, a prospective predictive marker for tumor? Oral Dis. 2016. May;22(4):265-273. doi: 10.1111/odi.12400. [DOI] [PubMed] [Google Scholar]
- 14.Küchler EC, Lips A, Tannure PN,et al. . Tooth agenesis association with self-reported family history of cancer. J Dent Res. 2013. February;92(2):149-155. doi: 10.1177/0022034512468750. [DOI] [PubMed] [Google Scholar]
- 15.Williams MA, Biguetti C, Romero-Bustillos M,et al. . Colorectal cancer-associated genes are associated with tooth agenesis and may have a role in tooth development. Sci Rep. 2018. February 14;8(1):2979. doi: 10.1038/s41598-018-21368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonds J, Pollan-White S, Xiang L, Mues G, D’Souza R. Is there a link between ovarian cancer and tooth agenesis? Eur J Med Genet. 2014. April;57(5):235-239. doi: 10.1016/j.ejmg.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lammi L, Arte S, Somer M,et al. . Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004. May;74(5):1043-1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001. October;11(5):547-553. [DOI] [PubMed] [Google Scholar]
- 19.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003. June 5;1653(1):1-24. [DOI] [PubMed] [Google Scholar]
- 20.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003. April;129(4):199-221. [DOI] [PubMed] [Google Scholar]
- 21.Marvin ML, Mazzoni SM, Herron CM, Edwards S, Gruber SB, Petty EM. AXIN2-associated autosomal dominant ectodermal dysplasia and neoplastic syndrome. Am J Med Genet A. 2011. April;155A(4):898-902. doi: 10.1002/ajmg.a.33927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalothorn LA, Beeman CS, Ebersole JL,et al. . Hypodontia as a risk marker for epithelial ovarian cancer: a case-controlled study. J Am Dent Assoc. 2008. February;139(2):163-169. [DOI] [PubMed] [Google Scholar]
- 23.Fekonja A, Cretnik A, Zerdoner D, Takac I. Hypodontia phenotype in patients with epithelial ovarian cancer. Radiol Oncol. 2015. March 3;49(1):65-70. doi: 10.2478/raon-2014-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thesleff I. Current understanding of the process of tooth formation: transfer from the laboratory to the clinic. Aust Dent J. 2014. June;59 Suppl 1:48-54. doi: 10.1111/adj.12102. [DOI] [PubMed] [Google Scholar]