Abstract
Background:
Children with sickle cell disease (SCD) often have infections, growth deficits, and impaired immunity, problems that also are observed in individuals with a vitamin A deficiency (plasma retinol concentration <20 μg/dL). The goal of this study was to investigate the association between vitamin A, health status, and the in vitro immune function of children with SCD.
Methods:
Fifty-nine children (40 SS, 11 SC, and 8 Sβthalassemia [Sβthal] hemoglobin genotypes) 9 months to 18 years old were investigated for plasma levels of retinol, retinol binding protein, C-reactive protein, alpha-1-acid glycoprotein, lymphocyte proliferation, and interleukin (IL)-2 activity in supernatant of phytohemagglutinin-treated lymphocytes.
Results:
The plasma retinol concentrations of children with SCD (mean 57.6 μg/dL, range 4.6-116 μg/dL) were not different from those of 21 normal individuals (mean 62 μg/dL, range 28.7-162 μg/dL). Plasma retinol concentrations did not vary by hemoglobin genotype but were lower in boys than in girls (P < 0.05) and were also lower in children with inflammation (P = 0.1). Seven children (11.9%) (6 HbSS, 1 HbSβ0thal) were vitamin A–deficient, and 9 children (15.3%) had suboptimal vitamin A status (plasma retinol concentration of 20-29 μg/dL). Children with vitamin A deficiency had slightly lower height (P = 0.09) and weight mean percentiles, lymphocyte proliferative responses, and IL-2 activity (P > 0.1), but higher means of C-reactive protein (P = 0.05), pain crisis episodes and inflammation (P = 0.1), and health scores (P > 0.1) than children who were not vitamin A–deficient. Lymphocyte proliferative responses negatively correlated with health score, pain crisis episodes, and blood units received, but positively correlated with retinol binding protein (P < 0.05 to P = 0.1).
Conclusion:
Identification and correction of suboptimal vitamin A status in children with SCD may improve immunity and attenuate certain health complications associated with this disease.
Keywords: Anemia–sickle cell, infection, inflammation, interleukin-2, lymphocyte proliferation, vitamin A
INTRODUCTION
The role of vitamin A in morbidity and mortality resulting from infectious illnesses in humans and laboratory animals has been well established.1,2 Vitamin A deficiency (plasma retinol <20 μg/dL) impairs antibody responses to T cell–dependent antigens and immunoglobulin (Ig) M switching to IgA, decreases the percentage of Th2+ cells, impairs T cell homing in the gut (one of the main routes of pathogen entry into the body), increases gut permeability to pathogens and antigens, and increases susceptibility to infections.3-7 Increased susceptibility to infection reduces growth rate, either through nutrient loss or by reduced food intake. Vitamin A deficiency also induces a proinflammatory response with elevated blood levels of type 1 cytokines such as tumor necrosis factor-alpha (TNF-α) and interferon gamma but low antiinflammatory cytokines such as interleukin (IL)-4 and IL-10.2,3
In a previous study involving 34 children with sickle cell disease (SCD), we observed that almost one in every two children had retinol binding protein <30 mg/L.8 In contrast, only 20% of children had a transferrin concentration <2 g/L, and no child had albumin levels <35 g/L. The data suggested that suboptimal vitamin A status (as suggested by low plasma retinol binding protein concentration) rather than general protein energy malnutrition might have been responsible for the low retinol binding protein concentrations.
Growth retardation (low body weight for age, height for age, body fat, and delayed skeletal maturation) is common in children with SCD.9-12 The mechanisms of growth retardation are likely multifactorial and may involve deficits in protein, energy, and micronutrients such as zinc and vitamin A.13-16
Although numerous studies have been conducted on the effect of deficits or supplementation of certain micronutrients, specifically zinc, on in vitro immune function and infection rates in children with SCD, there is a paucity of data on vitamin A status in the same population.13-15 This study was designed to investigate (1) vitamin A status in children with SCD who present to Children’s Hospital of New Orleans; (2) whether suboptimal vitamin A status is associated with poor clinical status and growth deficits and with altered in vitro immune responses of peripheral blood mononuclear cells in these patients; (3) whether hemoglobin genotypes, inflammation, and/or sex are confounding factors in impaired in vitro lymphocyte function associated with suboptimal vitamin A status in this population.
METHODS
Study Population and Design
The study was approved by the Louisiana State University Health Sciences Center (LSUHSC) Institutional Review Board and Children’s Hospital of New Orleans (Protocol #1789). Children whose data are included in this analysis were enrolled in a prospective study on the association between nutritional status and lymphocyte proliferation and certain complications usually associated with SCD. Inclusion criteria were (1) patients were registered at Children’s Hospital of New Orleans and received primary healthcare services related to SCD from the LSUHSC hematology/oncology physicians; (2) age 0.5 to 18 years; (3) boys and girls with HbSS, HbSC, and/or HbSβthalassemia (HbSβthal) genotypes; and (4) patients who had not received bone marrow transplantation to correct hemoglobinopathy, splenectomy, or other major surgery related and unrelated to SCD. Patients who did not fulfill the above criteria were excluded. Prior to 1997, hydroxyurea was not prescribed to children with SCD at Children’s Hospital of New Orleans, and none of the patients had received this drug.
A letter of invitation to participate in the study was sent to parents and guardians of all potential patients that briefly described the goal of the study. Additionally, parents/guardians were briefed during clinic visits. Among the 90 children who signed a consent form, 31 children were excluded from the analysis because of missing data on at least 3 measurements (markers of inflammation, biochemical markers of nutritional status, or plasma retinol concentration).
Blood Drawing and Chart Review
During clinic visits, blood samples (2-4 mL) were drawn in heparinized vacutainers under stable conditions from 59 children with SCD who were 9 months to 18 years old. Two hundred microliters (200 μL) were removed for measurement of hemoglobin, hematocrit, and white blood cell counts by standard techniques. The remaining blood samples were centrifuged at 2,000 rpm at room temperature for 10 minutes. Plasma was aspirated and aliquoted in 500 μL volumes and immediately frozen at −70°C until used for various measurements. Plasma retinol was measured by fluorescence spectrometry. For quality control, plasma retinol also was measured in frozen plasma samples obtained from individuals without SCD (7 LSUHSC laboratory employees [4 males and 3 females, age range 38-46 years, mean 43 years] and 14 children [8 males and 6 females, age range 3-18 years, mean 8.5 years]).
Information on infections, pain crises, anthropometry weight, height, age, hemoglobin genotype, and blood transfusions was obtained from patients’ charts for the period January 1994 through December 1996. To rule out the possibility that abnormal plasma retinol levels were attributable to inflammation, C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP) were measured by radial immunodiffusion, and TNF-α was measured by enzyme-linked immunosorbent assay. Inflammation was considered present when AGP and/or CRP were >1 g/L and >10 mg/L, respectively. Retinol binding protein was also measured by radial immunodiffusion because its plasma levels are reduced by vitamin A deficiency independently of inflammation and overt protein-energy malnutrition, and levels <30 mg/L are considered abnormal.17,18
Growth deficits were present when either weight or height was below the 10th percentile. Health scores were defined as 0 (no pain crisis episodes, infections, hospital admissions, or blood transfusions during the study period) or ≥1 (the sum of pain crisis episodes, infections, hospital admissions, and blood transfusions received during the study period).
Lymphocyte Proliferation
Mononuclear cells were isolated by density gradient centrifugation on Ficoll-Hypaque from blood samples collected in heparinized blood. Cell viability was determined by trypan blue exclusion test. Lymphocyte proliferation was assessed by incubating 0.20 × 106 viable cells per 200 μL of culture medium in the presence of 0-15 μg/mL phytohemagglutinin (PHA) at 37°C, 5% CO2 in a humidified atmosphere. Per liter of RPMI-1640, the culture medium contained 100 mL heat-inactivated human AB serum, 5 × 104 units penicillin, 50 mg streptomycin, 1 mmol sodium pyruvate, 0.1 mmol nonessential amino acids, 2 mmol L-glutamine, and 50 μmol β-mercaptoethanol. After 48 hours of incubation, cultures (made in triplicate) were pulsed with 1 μCi 3H-thymidine and further incubated for 24 hours under identical conditions. The rate of cell proliferation was assessed by estimating the amount of radioactivity uptake (incorporated into DNA expressed as counts per minute) in a liquid scintillation counter as we previously reported.19
Interleukin-2 Study
Macrocultures containing 2 × 106 viable cells were mixed with 0-15 μg PHA in a total volume of 1 mL, transferred to round bottom 12 × 75 mm culture tubes, and incubated in identical conditions as microcultures. After 48 hours, the tubes were centrifuged at 400 times gravity for 10 minutes. The supernatant was collected, aliquoted in 500 μL samples, and immediately frozen at −70°C. The IL-2 activity in the supernatant was assessed by the growth of the IL-2–dependent CTLL-2 cell line as described in the literature.20
Statistical Analysis
Descriptive status, analysis of variance, Pearson correlation coefficient, and chi-square test were performed with MYSTAT, second edition (SYSTAT Software, Inc.).21 Vitamin A status, IL-2 concentrations, and lymphocyte proliferative responses to PHA were analyzed as a function of sex, retinol binding protein, inflammation, and health scores. Multiple regression analysis was computed with IL-2 and/or lymphocyte proliferation as dependent variables and health status (plasma retinol concentration, weight, height, infection, blood transfusion, acute phase proteins, retinol binding protein, hemoglobin genotype, and hematologic measurements) as independent variables. The level of significance was set at P < 0.05.
RESULTS
Assessment of Clinical Status
The mean ± SEM age of the 59 study subjects was 7.63 ± 0.59 years. The cohort included 34 boys and 25 girls. As shown in Table 1, 67.8% of patients had HbSS genotype. Children with HbSC genotype were younger than those with HbSS and HbSβthal genotypes (P < 0.05). Of the 8 SCD children with β-thalassemia, 3 had HbSβ0thal and 5 had HbSβ+thal genotypes. Mean concentrations of plasma retinol were not different among the 3 groups of hemoglobin genotypes, and they also were not significantly different from those of the laboratory controls or the 14 blood samples that were obtained from children without SCD (Table 2).
Table 1. Demographic Characteristics of the Study Population.
| Hemoglobin Genotype | |||||
|---|---|---|---|---|---|
| HbSS | HbSC | HbSβthal | Total | Mean Age, years, ± SEM | |
| Boys | 23 | 7 | 4 | 34 | 7.63 ± 0.71 |
| Girls | 17 | 4 | 4 | 25 | 7.63 ± 1.00 |
| Total | 40 | 11 | 8 | 59 | 7.63 ± 0.59 |
| Mean age, years, ± SEM | 8.20 ± 0.66b | 5.03 ± 1.24a | 8.34 ± 2.02b | 7.63 ± 0.59 | |
Note: Two boys and one girl had HbSβ0 genotype. The mean ± SEM ages in years of children with HbSβ0 (n = 3) and HbSβ+ (n = 5) were 9.17 ± 2.41 and 7.85 ± 5.43, respectively (and were not significantly different). Children with HbSC genotype were younger than those with HbSS or HbSβthal (a<b; P < 0.05).
Table 2. Plasma Retinol Levels in Patients and Controls.
| Group | n | Mean (± SEM) Plasma Retinol Level, μg/dL | Plasma Retinol Range, μg/dL |
|---|---|---|---|
| Children with HbSS | 40 | 57.1 ± 4.7 | 6-116 |
| Children with HbSC | 11 | 60.9 ± 5.7 | 39-87 |
| Children with HbSβthal | 8 | 55.5 ± 12.8 | 4.6-109 |
| Children in the control group | 14 | 59.0 ± 5.88 | 28.7-119 |
| Laboratory employees | 7 | 70.0 ± 17.39 | 43.0-162 |
| All patients | 59 | 57.6 ± 3.7 | 4.6-116 |
| All controls | 21 | 62 ± 6.5 | 28.7-162 |
Note: No significant differences were observed between patients and the combined controls and/or by hemoglobin genotype.
Seven of the 59 (11.9%) children (age range, 5.5-13.58 years) had plasma retinol concentrations below normal (<20 μg/dL), and 9 other children (15.3%; 5 boys with HbSS genotype, 2 girls with HbSS genotype, and 2 boys with HbSβ+thal) had suboptimal plasma retinol concentrations of 20-29 μg/dL.22-24 None of the plasma samples obtained from laboratory employees or from children without SCD had plasma retinol concentrations below normal. Children with plasma retinol concentrations below normal tended to have lower means for height percentile (P = 0.09) and weight percentile (P > 0.1) but higher means of pain crisis episodes (P = 0.05), CRP (P = 0.05), and inflammation scores (P = 0.07) (Table 3). However, all other measurements showed no significant differences between the 2 groups of children with SCD.
Table 3. Mean (± SEM) Age and Clinical Status of Children With Sickle Cell Disease as a Function of Plasma Retinol Concentration.
| Variable | Plasma Retinol <20 μg/dL (n = 7)a | Plasma Retinol ≥20 μg/dL (n = 52)a | P Value | Plasma Retinol <20 μg/dL (n = 5 boys with HbSS) | Plasma Retinol ≥20 μg/dL (n = 18 boys with HbSS) | P Value |
|---|---|---|---|---|---|---|
| Age, years | 8.81 ± 1.1 | 7.47 ± 0.65 | 8.87 ± 1.51 | 7.22 ± 0.80 | ||
| Weight, kg | 26.83 ± 2.82 | 25.54 ± 2.33 | 25.38 ± 3.35 | 22.53 ± 2.25 | ||
| Weight percentile | 28.21 ± 10.77 | 37.72 ± 4.62 | 20.50 ± 9.25 | 23.06 ± 5.89 | ||
| Height, cm | 131.5 ± 6.22 | 119.26 ± 4.45 | 128.8 ± 7.52 | 118.64 ± 6.99 | ||
| Height percentile | 22.5 ± 5.82 | 40.28 ± 4.87 | 0.09 | 25.07 ± 6.61 | 26.31 ± 7.46 | |
| Pain crises | 1.57 ± 0.43 | 0.72 ± 0.19 | 0.05 | 1.40 ± 0.51 | 0.82 ± 0.23 | |
| Units of blood | 0.71 ± 0.71 | 1.17 ± 0.33 | 1.00 ± 0.998 | 1.35 ± 0.64 | ||
| Infections | 0.57 ± 0.297 | 0.58 ± 0.13 | 0.60 ± 0.399 | 0.81 ± 0.28 | ||
| Hospitalizations | 1.86 ± 0.96 | 1.43 ± 0.27 | 1.20 ± 0.582 | 2.07 ± 0.59 | ||
| Hematocrit, % | 26.5 ± 1.87 | 27.78 ± 0.69 | 25.10 ± 2.28 | 24.22 ± 0.78 | ||
| Hemoglobin, g/dL | 8.50 ± 0.54 | 8.79 ± 0.2 | 8.22 ± 0.64 | 7.74 ± 0.18 | ||
| White blood cells × 106/mL | 12.10 ± 0.97 | 12.69 ± 0.75 | 11.57 ± 1.11 | 14.57 ± 1.26 | ||
| C-reactive protein, mg/L | 18.27 ± 12.8 | 5.50 ± 1.64 | 0.05 | 25.58 ± 17.23 | 8.38 ± 4.24 | 0.08 |
| Alpha-1-acid glycoprotein, g/L | 071 ± 0.19 | 0.69 ± 0.04 | 0.83 ± 0.22 | 0.66 ± 0.08 | ||
| Retinol binding protein, mg/L | 19.30 ± 4.06 | 21.56 ± 1.14 | 14.72 ± 2.76 | 19.22 ± 1.81 | ||
| Tumor necrosis factor-α, pg/mL | 32.71 ± 19.7 | 50.61 ± 13.64 | 33.74 ± 28.05 | 69.73 ± 28.62 | ||
| Inflammation score | 0.71 ± 0.29 | 0.35 ± 0.08 | 0.07 | 0.80 ± 0.37 | 0.33 ± 0.16 | 0.1 |
| Health scoreb | 5.43 ± 1.67 | 3.84 ± 0.61 | 5.20 ± 1.83 | 4.83 ± 1.09 |
All hemoglobin phenotypes included.
Health score is the sum of the number of episodes of pain crisis, infection, hospital admission, and/or blood units received.
Among the 7 patients with plasma retinol levels below normal, 5 boys and 1 girl had HbSS genotype, and the seventh child was a boy with HbSβ0thal genotype. When the 5 boys with plasma retinol concentrations below normal and HbSS genotype were compared with 18 boys having the same hemoglobin genotype but with plasma retinol within the normal range, we again observed the following trend: higher mean CRP levels (P = 0.08) and inflammatory score (P = 0.1). No significant differences in the means of hematologic measurements and TNF-α concentrations were observed among vitamin A–deficient and vitamin A–adequate children.
Because only 1 girl had plasma retinol below normal, statistical analysis of various measurements could not be performed as a function of sex and vitamin A status. However, in the overall study population, girls had higher mean plasma retinol concentrations than boys (Table 4, P < 0.05). The means for weight and height percentiles tended to be higher in girls than boys, but mean infection episodes, inflammation scores, CRP, and TNF-α concentrations were lower in girls than boys, although differences were not statistically significant (Table 4). Although inflammation was associated with slightly lower mean plasma retinol concentration (P = 0.1), it did not significantly affect other health indices (Table 5, P > 0.1).
Table 4. Mean (± SEM) Age and Clinical Status of Boys and Girls With Sickle Cell Disease Independent of Vitamin A Status.
| Variable | Boys (n = 34) | Girls (n = 25) |
|---|---|---|
| Age, years | 6.62 ± 0.71 | 7.63 ± 1.01 |
| Plasma retinol, μg/dL | 49.8 ± 4.90 | 68.1 ± 5.1a |
| Weight, kg | 25.78 ± 2.65 | 25.64 ± 3.26 |
| Weight percentile | 34.05 ± 5.59 | 39.7 ± 6.60 |
| Height, cm | 121.32 ± 4.76 | 119.86 ± 7.27 |
| Height percentile | 35.76 ± 5.95 | 41.66 ± 6.65 |
| Pain crises | 0.72 ± 0.16 | 1.00 ± 0.38 |
| Units of blood | 0.91 ± 0.37 | 1.41 ± 0.49 |
| Infections | 0.66 ± 0.16 | 0.50 ± 0.18 |
| Hospitalizations | 1.43 ± 0.33 | 1.57 ± 0.47 |
| Hematocrit, % | 26.98 ± 0.93 | 28.48 ± 0.87 |
| Hemoglobin, g/dL | 8.58 ± 0.32 | 9.00 ± 0.36 |
| White blood cells × 106/mL | 13.30 ± 0.84 | 11.72 ± 1.06 |
| C-reactive protein, mg/L | 8.85 ± 0.39 | 4.444 ± 1.2 |
| Alpha-1-acid glycoprotein, g/L | 0.689 ± 0.06 | 0.689 ± 0.05 |
| Retinol binding protein, mg/L | 21.02 ± 1.52 | 21.63 ± 1.64 |
| Tumor necrosis factor alpha, pg/mL | 59.4 ± 17.89 | 32.55 ± 14.04 |
| Inflammation score | 0.412 ± 0.12 | 0.36 ± 0.098 |
| Health scoreb | 3.82 ± 0.65 | 4.35 ± 1.05 |
P < 0.05; girls had higher mean plasma retinol concentrations than boys.
Health score is the sum of the number of episodes of pain crisis, infection, hospital admission, and/or blood units received.
Table 5. Mean (± SEM) Age and Clinical Status of Children with Sickle Cell Disease as a Function of Inflammation.
| Variable | Without Inflammation (n = 40) | With Inflammationa (n = 19) |
|---|---|---|
| Age, years | 8.354 ± 0.753 | 6.09 ± 0.811b |
| Plasma retinol, μg/dL | 60.9 ± 7.0 | 50.90 ± 4.3c |
| Weight, kg | 28.39 ± 2.58 | 18.864 ± 2.21 |
| Weight percentile | 38.08 ± 5.16 | 32.393 ± 7.63 |
| Height, cm | 125.64 ± 4.60 | 107.23 ± 7.14 |
| Height percentile | 39.54 ± 5.24 | 34.64 ± 8.42 |
| Pain crises | 0.892 ± 0.31 | 0.688 ± 0.24 |
| Units of blood | 1.333 ± 0.398 | 0.563 ± 0.33 |
| Infections | 0.526 ± 0.15 | 0.688 ± 0.18 |
| Hospitalizations | 1.60 ± 0.35 | 1.25 ± 0.43 |
| Hematocrit, % | 27.45 ± 0.81 | 28.00 ± 1.08 |
| Hemoglobin, g/dL | 8.81 ± 0.31 | 8.673 ± 0.39 |
| White blood cells × 106/mL | 12.364 ± 0.79 | 13.15 ± 1.23 |
| Health scored | 4.154 ± 0.77 | 3.78 ± 0.72 |
Inflammation is defined as C-reactive protein >10 mg/L and/or α1-acid glycoprotein >1.0 g/L.
P < 0.05.
P = 0.1.
Health score is the sum of the number of episodes of pain crisis, infection, hospital admission, and/or blood units received.
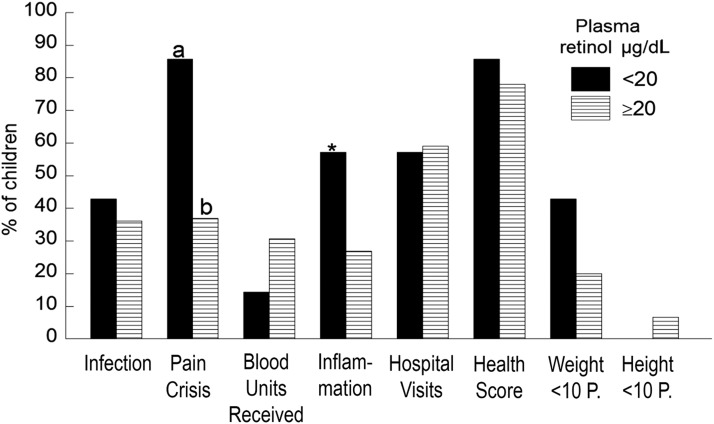
As shown in Figure 1, a higher percentage of children with plasma retinol below normal had at least one episode of pain crisis (P < 0.05), had inflammation (P = 0.1), or had weight below the 10th percentile (P > 0.1) compared to children with concentrations within the normal range. However, the percentages of children with SCD who had white blood cell counts >15 × 106 mL did not differ by plasma retinol concentrations (data not shown).
Figure 1. Percentage of children with sickle cell disease who had any of the listed health problems as a function of plasma retinol concentration below normal (<20 μg/dL) and within the normal range (≥20 μg/dL). A higher percentage of children with plasma retinol <20 μg/dL vs children with levels ≥20 μg/dL had pain crisis episodes (chi-square test, a>b, P < 0.05) or inflammation (*P = 0.1). For all other measurements, no significant differences were detected.
Lymphocyte Proliferation as Function of Plasma Retinol Levels, Inflammation, and Health Scores
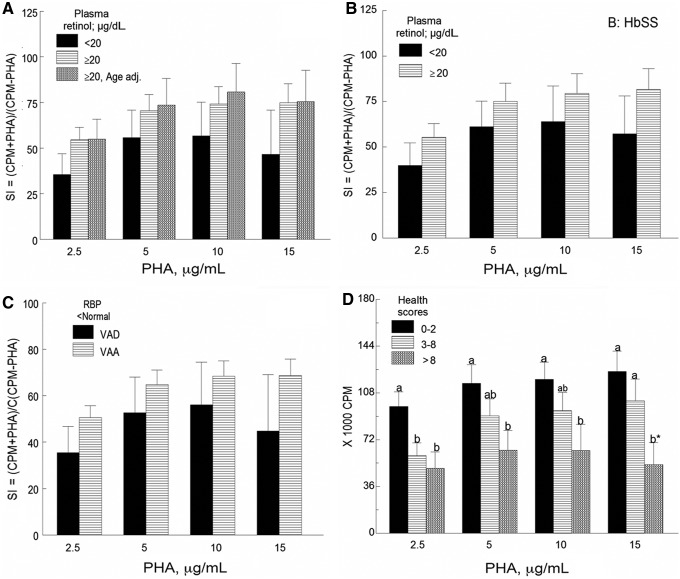
At all PHA concentration levels tested, the rate of DNA synthesis (Figure 2A) tended to be lower (mean decrease 29.3%) in children with plasma retinol concentrations below normal compared to those with levels within the normal range in both the age-adjusted and age-unadjusted analyses (P > 0.1). The same trend was observed when the analysis included only boys with HbSS genotype with plasma retinol levels <20 μg/dL vs ≥20 μg/dL (Figure 2B, P > 0.1). The mean stimulation indexes of cell cultures from children with plasma retinol concentrations and retinol binding protein below normal were 18.2% lower than those of children with normal concentrations of plasma retinol but retinol binding protein below normal level (Figure 2C, P > 0.1). Mean lymphocyte proliferative responses of peripheral blood mononuclear cells obtained from children with inflammation were 12% lower than those of children without inflammation, although differences were not statistically significant (graph not shown). A dose-dependent decrease (18.2% to 57.6%) in the rate of DNA synthesis or 3H-thymidine uptake expressed as counts per minute in PHA-treated cells, corrected for baseline counts per minute, was seen with increased health scores (Figure 2D, P < 0.05 to P = 0.1).
Figure 2. Lymphocyte proliferative responses of peripheral blood mononuclear cells to phytohemagglutinin (PHA) (A) as a function of plasma retinol concentration (n = 7 VAD and n = 52 VAA children); (B) in boys with HbSS and VAD (n = 5) and boys with HbSS and VAA (n = 18); (C) in children with retinol binding protein (RBP) and plasma retinol below normal (n = 6) and children with normal plasma retinol but RBP below normal (n = 31); and (D) as a function of health scores (n = 29, n = 20, and n = 8 for health scores 0-2, 3-8, and >8, respectively). For 2A, 2B, and 2C, no significant differences in mean stimulation indexes (SI) were found between children with plasma retinol levels <20 μg/dL and those with levels ≥20 μg/dL. For 2D, for each PHA concentration (2.5, 5, 10, or 15 μg/mL), a dose-dependent decrease in the rate of DNA synthesis was seen with increased health scores (P < 0.05 to P = 0.1). Values are mean ± SEM. CPM, counts per minute; VAA, vitamin A–adequate, VAD, vitamin A–deficient.
Interleukin-2 Concentration as a Function of Plasma Retinol, Sex, Inflammation, and Health Scores
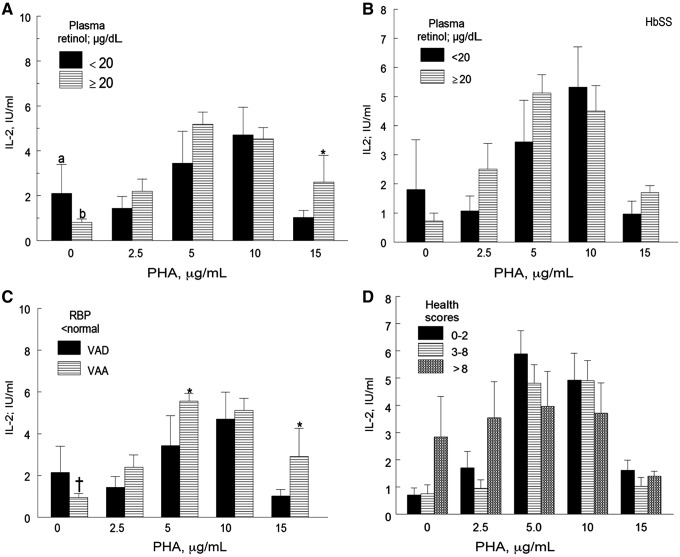
In nonactivated cultures, the mean IL-2 concentrations were higher in cultures of peripheral blood mononuclear cells obtained from children with plasma retinol below normal compared to those with normal concentrations (Figure 3A, P < 0.05). In cells treated with 2.5, 5, and 10 μg/mL PHA, the mean IL-2 concentrations were 11%-26.6% lower in children with plasma retinol concentrations below normal than in those with normal concentrations (P > 0.1).
Figure 3. Interleukin-2 (IL-2) concentrations in phytohemagglutinin (PHA)-treated and untreated peripheral blood mononuclear cells in children with sickle cell disease (A) as a function of plasma retinol levels (n = 52 children with normal plasma retinol levels and n = 7 children with deficient plasma retinol levels); (B) in boys with HbSS and plasma retinol below normal (n = 5) and boys with normal plasma retinol (n = 18); (C) in children with retinol binding protein (RBP) and plasma retinol below normal (n = 6) and children with normal plasma retinol but RBP below normal (n = 31); and (D) as a function of health scores (n = 29, n = 20, and n = 8 for health scores 0-2, 3-8, and >8, respectively). For 3A and 3C (PHA 0 μg/mL), the mean IL-2 concentrations of children with plasma retinol <20 μg/dL were higher than those with plasma retinol ≥20 mg/dL (a>b, P < 0.05 in 3A; †P = 0.052 in 3C). For PHA 5 and/or 15 μg/mL, the mean IL-2 concentrations of children with plasma retinol <20 μg/dL were lower than those of children with plasma retinol ≥20 μg/dL (*P < 0.1 but >0.05 in 3C). The means of IL-2 activity between children plasma retinol <20 μg/dL and those with levels ≥20 μg/dL (3B) and as a function of health scores (3D) were not statistically different. Values are mean ± SEM. VAA, vitamin A–adequate, VAD, vitamin A–deficient.
Contrary to what we expected, the mean IL-2 activity of cells treated with 15 μg/mL PHA was marginally higher for children with plasma retinol below normal than in children with plasma retinol ≥20 μg/mL.
Mean IL-2 concentrations in PHA-treated cultures from boys with HbSS genotype and plasma retinol below normal levels were 10.6% lower than those of boys with HbSS and normal plasma retinol concentrations; however, the differences were not statistically significant (Figure 3B).
In nonactivated cells, the mean IL-2 concentration of nonactivated cells obtained from children with plasma retinol and retinol binding protein below normal levels tended to be higher than that obtained from children with plasma retinol and retinol binding protein within the normal range (Figure 3C, P = 0.05). However, as expected, the mean IL-2 concentrations of PHA-treated cells obtained from children with plasma retinol and retinol binding protein below normal levels were 8.6%-27.6% lower than those of children with normal plasma retinol but retinol binding protein below normal (Figure 3C, P = 0.1 for 5 μg/mL and 15 μg/mL PHA). When plasma retinol concentrations were not taken into account, we observed slightly higher (mean ± SD) IL-2 activity in cells incubated with 5 μg/mL PHA from girls (6.43 ± 2.712 U/mL) than from boys (4.478 ± 2.25 U/mL, P > 0.1). IL-2 concentration in the supernatant of PHA-activated cultures was not significantly affected by inflammation (graph not shown). When cells were activated with 5, 10, and 15 μg/mL PHA, there was a small decrease in the mean IL-2 activity (13.3%-36.5%) with increasing health scores (Figure 3D).
Clinical Status and In Vitro Immunity in Children With β-Thalassemia
Despite the small sample size, data for selected measurements were also analyzed as a function of β-thalassemia subgroups. Children with HbSβ0thal genotype (n = 3) had a slightly higher mean (± SEM) CRP concentration (3.0 ± 1.70 mg/L) than children with HbSβ+thal genotype (n = 5) (0.62 ± 0.62 mg/L, P = 0.08). Mean (± SEM) plasma retinol concentrations were not significantly different between children with HbSβ0thal (53.53 ± 0.30 μg/dL) and those with HbSβ+thal genotype (56.60 ± 13.50 μg/dL). Also, no significant difference was observed between both groups with all other measurements (data not shown).
Correlation Coefficients and Multiple Regression Analysis
Lymphocyte proliferative responses to PHA positively and significantly correlated with retinol binding protein concentrations, negatively correlated with health scores, pain crises, and units of blood received (Table 6, P < 0.05), and nonsignificantly correlated with infection episodes. Although the same trend was observed between IL-2 concentrations in the supernatants of PHA-treated cultures and measures of clinical status, none of the correlations was statistically significant (data not shown). Plasma retinol concentrations positively correlated with weight percentile (r = 0.181), height percentile (r = 0.222), IL-2 concentration (5 μg/mL PHA only, r = 0.184), and number of blood units received (r = 0.329, P < 0.05). Plasma retinol concentrations negatively correlated with inflammation scores (r = –0.235, P < 0.05) and nonsignificantly with the number of pain crisis episodes (r = –0.170) and hospitalizations (r = –0.127). Multiple regression analysis with IL-2 and/or lymphocyte proliferative responses to PHA as dependent variables did not show any strong association with anthropometric measurements, inflammation, hematologic measurements, or sex.
Table 6. Correlation Coefficients (r) Between Indicators of Clinical Status and Lymphocyte Proliferative Responses to Phytohemagglutinin (PHA) (corrected for baseline 3H-thymidine uptake) in Children With Sickle Cell Disease.
| Variable | PHA 2.5 μg/mL | PHA 5 μg/mL | PHA 10 μg/mL | PHA 15 μg/mL |
|---|---|---|---|---|
| Retinol binding protein | 0.242a | 0.307a | 0.349a | 0.367a |
| Health scoreb | −0.319a | −0.272a | −0.287a | −0.326a |
| Pain crises | −0.252a | −0.182 | −0.195 | −0.195 |
| Units of blood | −0.195 | −0.243a | −0.266a | −0322a |
| Infections | −0.151 | −0.138 | −0.172 | −0.042 |
The (r) value is significantly different from zero (P < 0.05).
Health score is the sum of the number of episodes of pain crisis, infection, hospital admission, and/or blood units received.
DISCUSSION
Our study reveals a few important observations: (1) no child <5 years of age had a plasma retinol concentration below normal (<20 μg/dL); (2) vitamin A deficiency was observed in 7/59 children (nearly 12%), a percentage that is significantly higher than that observed (0.6%-1.5%, 95% confidence interval, mean of 1%) in American children of the same age range23,24; (3) while the weight and height mean percentiles were <50th percentile, severe growth deficits (weight or height <3rd percentile) were not common in the overall population. Nearly 43% (3/7) of the children with plasma retinol below normal, compared with 22% of those with normal plasma retinol concentrations, had weight below the 10th percentile. However, while no child with plasma retinol concentration below normal had height below the 10th percentile, nearly 7% of those with adequate plasma retinol levels did, and only 2 children in the overall population had height below the 3rd percentile. The lack of a high prevalence of severe growth deficits could be attributed to excellent healthcare and disease management and may also suggest that either macronutrient intake or utilization were adequate.
Although differences were small and not statistically significant, the overall health status (weight percentile, height percentile, and inflammation scores) appeared to be worse in boys than in girls. Boys also had lower mean plasma retinol levels and IL-2 activity in the supernatant of PHA-treated cells than girls. Although the experimental design did not allow us to identify the mechanisms of these differences, published data about adults without SCD have clearly demonstrated significant sex differences in the risk of infections and in immune responses to vaccines.25,26 Women have lower incidences and severity of many viral, bacterial, and protozoan infections than men, and they also mount stronger humoral and cell-mediated immune responses to infection.26 In a 2013 study, Sankaran-Walters et al reported that women had a higher degree of immune activation of CD4+ and CD8+ blood- and gut-associated lymphocytes than men, and women also produced higher mean Th1-associated cytokines (TNF-α, IL-1β, and IL-17) that are required to recover from respiratory infections but lower Th2-associated cytokines (IL-4 and IL-10).26 Sex differences that may increase the risk of certain types of viral and bacterial infections start in infancy or prepubertal periods, with higher incidences and mortality rates in boys than in girls 0-14 years of age.25 At least in adults, certain measurements of SCD severity vary by sex. In one study, the prevalence and severity of pain crises were reported to be lower in females than in males; and the mean age at death was higher in females (29.1 years) than in males (26.2 years).27 In our study, the mean number of pain crises did not vary by sex, and the mean number of infections was only slightly and nonsignificantly lower in girls than in boys. However, as expected, the mean concentrations of CRP, TNF-α, and inflammatory scores were higher in boys than in girls. These factors may explain in part the lower mean plasma retinol levels in boys than in girls (Table 4). Because of the paucity of data on differences in immune responses in boys vs girls with SCD or adult male and female patients with SCD, this is an area of interest for future studies.
We observed a positive and significant correlation between lymphocyte proliferative responses to PHA and retinol binding protein (a surrogate marker of vitamin A status, especially in the absence of inflammation or overt protein-energy malnutrition) but a negative correlation with health scores, blood units received, pain crises, and infections. Plasma retinol positively correlated with IL-2 concentrations in culture activated with 5 μg/mL PHA. From the current data, we are unable to state which came first: inadequate vitamin A status affecting health scores (pain crises, inflammation assessed by CRP) and in vitro immune function or the opposite. In either case, pain crisis episodes are usually associated with inflammation and reduced nutrient intake (including vitamin A), and these factors may in turn affect immune function.
Several studies have been conducted on vitamin A status in children with SCD and its influence on health status or the role of vitamin A status in lymphocyte proliferation and cytokine secretion in individuals and laboratory animals without SCD.1-3,16,28-32 The negative effect of suboptimal vitamin A status on immunity has been clearly demonstrated. In 2 studies conducted on blood lymphocytes obtained from healthy adult subjects without SCD, the addition of vitamin A (in the form of retinoic acid) to culture medium resulted in increased IL-2 secretion, lymphocyte proliferation, and dendritic cell maturation.33,34 However, to our knowledge, ours is the first study in which in vitro immune function (lymphocyte proliferation, IL-2 secretion) and plasma levels of TNF-α were investigated as a function of vitamin A status in children with SCD. The small differences persisted after taking into account hemoglobin genotype and sex (comparing boys with HbSS and plasma retinol below normal concentration and those with levels within the normal range). The high spontaneous IL-2 secretion observed in PHA-untreated cultures may suggest that lymphocytes from vitamin A–deficient children are activated in vivo as a result of underlying problems such as continuous free radical formation and hemolysis. One other important observation is that 6/7 children with plasma retinol concentrations below normal were children with HbSS genotype, consistent with the fact that patients with this hemoglobin genotype tend to have a more severe disease than those with other genotypes (for example, HbSC or HbSβ+thal).
We did not investigate the mechanisms of impaired lymphocyte proliferation in the current study. However, we can speculate that these mechanisms could be related to differences in the number of immunocompetent T lymphocytes between vitamin A–deficient and vitamin A–adequate children or to impaired gene expression of IL-2, IL-2 receptors, or other factors that regulate lymphocyte proliferative responses to mitogens and antigens.3,33,34 Low percentages of CD4+ and CD8+ T cells previously have been reported in patients without SCD but with vitamin A deficiency, and they were corrected after the addition of vitamin A supplements.35
Vitamin A, through its metabolite all-trans retinoic acid, has been shown to modulate adaptive immunity through various mechanisms such as regulation of T cell differentiation and activation, secretion of proinflammatory and antiinflammatory cytokines, expression of cell receptors, and proteins such as cyclin D3 that regulate T cell proliferation.36 Retinoic acid has also been shown to downregulate apoptosis of activated human lymphocytes in vitro and to regulate the expression of nuclear factors of activated human T cells that control the production and/or secretion of IL-2.34,37 Blocking of retinoic acid receptor alpha abrogated the retinoic acid effect on IL-2 secretion and cell proliferation.38 This finding implies that any of these mechanisms could be responsible for the reduced lymphocyte proliferation and/or IL-2 secretion in activated lymphocytes from children with SCD and below-normal plasma retinol concentration.
The observed concentrations of plasma retinol in our study were higher than those reported by other investigators who have studied American and non-American children with SCD.16,22,29 Three possible explanations could account for the difference: (1) methods used for plasma retinol assay, (2) use of vitamin supplements, and (3) differences in the population. In our study, plasma retinol was assayed by fluorometric methods, whereas other investigators have used high-performance liquid chromatography. Additionally, Dougherty et al included only children with HbSS genotype in their study.22 Because micronutrient (multivitamin-mineral) supplements are commonly used in the United States, this practice may, in part, explain the high plasma retinol concentrations in our studied population.
Our study has several limitations: (1) the small sample size, (2) cross-sectional nature of the study, (3) lack of information on vitamin A intake, and (4) lack of data on the mechanisms of impaired lymphocyte proliferation and/or IL-2 secretion. These issues will be addressed in a future study.
CONCLUSION
Our study suggests that approximately 12% of the researched children with SCD had plasma retinol concentrations below normal. The affected children had poorer in vitro immune function and health compared to those with an adequate vitamin A status. Considering the negative effects of poor immunity on infection, a predisposing factor for pain crisis, our data suggest that routine measurement of plasma retinol should be performed in children with SCD, especially those >5 years of age.
ACKNOWLEDGMENTS
This work was supported by general research funds from the Louisiana State University Health Sciences Center, Department of Pediatrics, Division of Hematology/Oncology and the Sickle Cell Center of Southern Louisiana, New Orleans. The authors have no conflict of interest (no direct financial benefits in the subject matter of this article) to report.
We thank all parents who allowed their children to be a part of the study; the nurses who coordinated blood drawing; Mr Gerald Lane for assisting in the isolation of peripheral blood mononuclear cells, in vitro cell cultures, and measurements of acute phase proteins; and Ms Leone Coe for her very important feedback and for editing the manuscript. Special thanks go to Dr Lolie Yu for her useful suggestions at different phases of the study, including the preparation of the manuscript.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Jason J, Archibald LK, Nwanyanwu OC,et al. . Vitamin A levels and immunity in humans. Clin Diagn Lab Immunol. 2002. May;9(3):616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008. September;8(9):685-689. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bono MR, Tejon G, Flores-Santibañez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016. June 13;8(6). doi: 10.3390/nu8060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MH, Taparowsky EJ, Kim CH. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity. 2015. July 21;43(1):107-119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman DR, De Calisto J, Simmons NL,et al. . Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol. 2011. August 15;187(4):1877-1883. doi: 10.4049/jimmunol.1101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czarnewski P, Das S, Parigi SM, Villablanca EJ. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients. 2017. January 13;9(1). doi: 10.3390/nu9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CC, Noelle RJ. Seeing through the dark: new insights into the immune regulatory functions of vitamin A. Eur J Immunol. 2015. May;45(5):1287-1295. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warrier RP, Kuvibidila S, Gordon L, Humbert J. Transport proteins and acute phase reactant proteins in children with sickle cell anemia. J Natl Med Assoc. 1994. January;86(1):33-39. [PMC free article] [PubMed] [Google Scholar]
- 9.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007. May;61(5 Pt 1):607-613. [DOI] [PubMed] [Google Scholar]
- 10.Bennett EL. Understanding growth failure in children with homozygous sickle-cell disease. J Pediatr Oncol Nurs. 2011. Mar-Apr;28(2):67-74. doi: 10.1177/1043454210382421. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sagladi AW, Cipolotti R, Fijnvandraat K, Brabin BJ. Growth and nutritional status of children with homozygous sickle cell disease. Ann Trop Paediatr. 2008. September;28(3):165-189. doi: 10.1179/146532808X335624. [DOI] [PubMed] [Google Scholar]
- 12.Singhal A, Thomas P, Cook R, Wierenga K, Serjeant G. Delayed adolescent growth in homozygous sickle cell disease. Arch Dis Child. 1994. November;71(5):404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyacinth HI, Gee BE, Hibbert JM. The role of nutrition in sickle cell disease. Nutr Metab Insights. 2010. January 1;3:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad AS, Beck FW, Kaplan J,et al. . Effect of zinc supplementation on incidence of infections and hospital admissions in sickle cell disease (SCD). Am J Hematol. 1999. July;61(3):194-202. [DOI] [PubMed] [Google Scholar]
- 15.Zemel BS, Kawchak DA, Fung EB, Ohene-Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. Am J Clin Nutr. 2002. February;75(2):300-307. [DOI] [PubMed] [Google Scholar]
- 16.Behera S, Dixit S, Bulliyya G, Kar SK. Vitamin A status and hematological values in sickle cell disorder cases. Indian J Med Sci. 2012. Jul-Aug;66(7-8):169-174. doi: 10.4103/0019-5359.114180. [DOI] [PubMed] [Google Scholar]
- 17.Gamble MV, Ramakrishnan R, Palafox NA, Briand K, Berglund L, Blaner WS. Retinol binding protein as a surrogate measure for serum retinol: studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am J Clin Nutr. 2001. March;73(3):594-601. [DOI] [PubMed] [Google Scholar]
- 18.Larson LM, Addo OY, Sandalinas F,et al. . Accounting for the influence of inflammation on retinol-binding protein in a population survey of Liberian preschool-age children. Matern Child Nutr. 2017. April;13(2). doi: 10.1111/mcn.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuvibidila SR, Gardner R, Velez M, Yu L. Iron deficiency, but not underfeeding reduces the secretion of interferon-gamma by mitogen-activated murine spleen cells. Cytokine. 2010. December;52(3):230-237. doi: 10.1016/j.cyto.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Hank JA, Surfus J, Gan J,et al. . Distinct clinical and laboratory activity of two recombinant interleukin-2 preparations. Clin Cancer Res. 1999. February;5(2):281-289. [PubMed] [Google Scholar]
- 21.Munro HB. Differences among group means: one way analysis of variance. In: Munro HB, Page IB, eds. Statistical Methods for Health Care Research. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1993:99-128. [Google Scholar]
- 22.Dougherty KA, Schall JI, Kawchak DA,et al. . No improvement in suboptimal vitamin A status with a randomized, double-blind, placebo-controlled trial of vitamin A supplementation in children with sickle cell disease. Am J Clin Nutr. 2012. October;96(4):932-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Sciences; 2012:100 https://www.cdc.gov/nutritionreport/pdf/nutrition_book_complete508_final.pdf. Accessed September8, 2018. [Google Scholar]
- 24.Ballew C, Bowman BA, Sowell AL, Gillespie C. Serum retinol distributions in residents of the United States: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2001. March;73(3):586-593. [DOI] [PubMed] [Google Scholar]
- 25.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014. July 15;209 Suppl 3: S120-S126. doi: 10.1093/infdis/jiu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankaran-Walters S, Macal M, Grishina I,et al. . Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013. May 7;4(1):10. doi: 10.1186/2042-6410-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rami Helvaci M, Ayyildiz O, Gundogdu M. Gender differences in severity of sickle cell diseases in non-smokers. Pak J Med Sci. 2013. July;29(4):1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright D, Reid M, Stennett R. Antioxidant intake in sickle cell disease. Intern J Clin Nutr. 2014;2(3):53-59. doi: 10.12691/ijcn-2-3-2. [DOI] [Google Scholar]
- 29.Schall JI, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA. Vitamin A status, hospitalizations, and other outcomes in young children with sickle cell disease. J Pediatr. 2004. July;145(1):99-106. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994. February 15;152(4):1515-1522. [PubMed] [Google Scholar]
- 31.Nauss KM, Mark DA, Suskind RM. The effect of vitamin A deficiency on the in vitro cellular immune response of rats. J Nutr. 1979. October;109(10):1815-1823. [DOI] [PubMed] [Google Scholar]
- 32.Bessler H, Wyshelesky G, Osovsky M, Prober V, Sirota L. A comparison of the effect of vitamin A on cytokine secretion by mononuclear cells of preterm newborns and adults. Neonatology. 2007;91(3):196-202. [DOI] [PubMed] [Google Scholar]
- 33.Ertesvag A, Engedal N, Naderi S, Blomhoff HK. Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. J Immunol. 2002. November 15;169(10):5555-5563. [DOI] [PubMed] [Google Scholar]
- 34.Engedal N, Ertesvag A, Blomhoff HK. Survival of activated human T lymphocytes is promoted by retinoic acid via induction of IL-2. Int Immunol. 2004. March;16(3):443-453. [DOI] [PubMed] [Google Scholar]
- 35.Semba RD, Muhilal, Ward BJ,et al. . Abnormal T-cell subset proportions in vitamin-A-deficient children. Lancet. 1993. January 2;341(8836):5-8. [DOI] [PubMed] [Google Scholar]
- 36.Engedal N, Gjevik T, Rune Blomhoff R, Blomhoff HK. All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. J Immunol. 2006. September 1;177(5):2851-2861. [DOI] [PubMed] [Google Scholar]
- 37.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005. June;5(6):472-484. [DOI] [PubMed] [Google Scholar]
- 38.Hall JA, Cannons JL, Grainger JR,et al. . Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic receptor alpha. Immunity. 2011. March 25;34(3):435-447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]