Abstract
Herpesviruses are ubiquitous in animals and cause economic losses concomitant with many diseases. Most of the domestic animal herpesviruses are within the subfamily Alphaherpesvirinae, which includes human herpes simplex virus 1 (HSV-1). Suppression of HSV-1 replication has been reported with α-hydroxytropolones (αHTs), aromatic ring compounds that have broad bioactivity due to potent chelating activity. It is postulated that αHTs inhibit enzymes within the nucleotidyltransferase superfamily (NTS). These enzymes require divalent cations for nucleic acid cleavage activity. Potential targets include the nuclease component of the herpesvirus terminase (pUL15C), a highly conserved NTS-like enzyme that cleaves viral DNA into genomic lengths prior to packaging into capsids. Inhibition of pUL15C activity in biochemical assays by various αHTs previously revealed a spectrum of potencies. Interestingly, the most potent anti-pUL15C αHT inhibited HSV-1 replication to a limited extent in cell culture. The aim of this study was to evaluate three different αHT molecules with varying biochemical anti-pUL15C activity for a capacity to inhibit replication of veterinary herpesviruses (BoHV-1, EHV-1, and FHV-1) and HSV-1. Given the known discordant potencies between anti-pUL15C and HSV-1 replication inhibition, a second objective was to elucidate the mechanism of action of these compounds. The results show that αHTs broadly inhibit herpesviruses, with similar inhibitory effect against HSV-1, BoHV-1, EHV-1, and FHV-1. Based on immunoblotting, Southern blotting, and real-time qPCR, the compounds were found to specifically inhibit viral DNA replication. Thus, αHTs represent a new class of broadly active anti-herpesviral compounds with potential veterinary applications.
Keywords: Herpesvirus, Anti-herpesvirus, α-hydroxytropolones, Nucleotidyltransferase superfamily
1. Introduction
Herpesviruses are a major cause of morbidity, mortality, and economic loss in domestic animal species. Bovine herpesvirus 1 (BoHV-1) is a geographically widespread cause of respiratory disease, conjunctivitis, genital lesions, and abortion in cattle (Biswas et al., 2013). Similarly, equine herpesvirus 1 (EHV-1) is widely prevalent, primarily causing sporadic or epizootic abortions and neonatal death, upper respiratory tract infections and neurological disease in horses (Dunowska, 2014). The effects of herpesviruses extend into companion animals, as disease induced by feline herpesvirus 1 (FHV-1) approaches 100% morbidity in felids, particularly in shelter or cattery populations (Gould, 2011). In addition to their veterinary importance, human herpesvirus infections are considered a worldwide pandemic, and the prevalence is increasing (Koelle and Corey, 2008). Management of veterinary herpesvirus infection usually relies on prevention and control (Dunowska, 2014). For example, clinical trials assessing the efficacy of oral and ophthalmologic anti-FHV-1 therapy in cats with FHV-1 revealed improved clinical signs with limited adverse effects (Fontenelle et al., 2008; Gould, 2011; Thomasy et al., 2011; Thomasy et al., 2016). In people, most anti-herpesviral therapeutics target the viral DNA polymerase, in which acquired mutations and subsequent viral resistance are a major limitation, especially in patients undergoing prolonged therapy (Collins and Darby, 1991; Gable et al., 2014; Krawczyk et al., 2013). Thus, a great need exists for novel anti-herpesviral compounds in both medical and veterinary medicine.
Herpesviruses encode several well-conserved enzymes within the nucleotidyltransferase superfamily (NTS) that contain a ribonuclease H (RNase H)-like fold. In HSV-1, these enzymes include the single stranded DNA binding protein (ICP8), alkaline nuclease (pUL12), DNA polymerase, and the nuclease of the viral terminase (pUL15C) (Boehmer and Lehman, 1997; Bryant et al., 2012; Liu et al., 2006; Schumacher et al., 2012; Selvarajan Sigamani et al., 2013; Yan et al., 2014). Given the enzymes’ similar structure, inhibitors of the HIV RNase and integrase were recently screened for activity against HSV-1 and HSV-2 (Tavis et al., 2014). Tropolones, most notably natural and synthetic α-hydroxytropolones (αHTs), were found to be potent anti-HSV inhibitors (Ireland et al., 2016; Tavis et al., 2014). While tropolones are well known metalloenzyme inhibitors due to the high negative charge character on both the carbonyl and adjacent oxygen at physiological pH, αHTs provide an additional contiguous oxygen that makes them particularly good inhibitors of dinuclear metalloenzymes (Bentley, 2008; Piettre et al., 1997). To investigate the nature of αHT HSV-1 inhibitory activity, one specific HSV-1 NTS-like enzyme, pUL15C of the viral terminase, was assessed in the presence of several synthetic β-thujaplicinol derivatives through the analysis of pUL15C-mediated hydrolysis of short DNA duplexes using dual-probe fluorescence (Masaoka et al., 2016). Interestingly, some of the most potent pUL15C synthetic αHTs inhibited HSV-1 replication only modestly, while those that strongly inhibited HSV-1 replication had poor anti-terminase activity (Ireland et al., 2016; Masaoka et al., 2016). It follows that although αHTs have anti-pUL15C activity in biochemical assays, other essential viral activities are likely responsible for their antiviral effects.
Using αHTs with varying anti-HSV replication and biochemical anti-terminase potency, the goals of this research were to determine whether αHTs have broad herpesviral inhibitory activity against important veterinary herpesviruses, including BoHV-1, EHV-1, and FHV-1, and to determine the mechanism of action of these αHTs using HSV-1 as a model.
2. Materials and methods
2.1. Compound selection
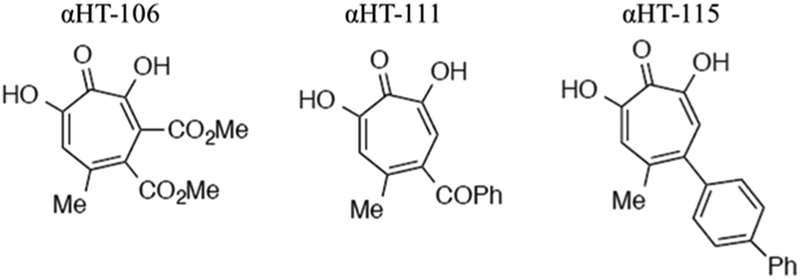
Three αHTs, numbered 106, 111, and 115, were synthesized from kojic acid as previously described (Hirsch et al., 2014; Meck et al., 2012; Williams et al., 2013). The chemical structure of each compound is detailed in Fig. 1. The 50% inhibitory activities (IC50) against pUL15C for the three synthetic αHTs have been previously measured and include 0.18 μM for αHT-106, 5.6 μM for αHT-111, and 49.1 μM for αHT-115 (Ireland et al., 2016; Masaoka et al., 2016). Similarly, the in vitro anti-HSV potencies of the three synthetic αHTs were previously determined. Full suppression of HSV-1 was noted at 50 μM for αHT-106 and at 5 μM for αHT-111. The 50% effective concentration (EC50) against HSV-1 was 0.18 μM for αHT-115 (Ireland et al., 2016; Masaoka et al., 2016). Lastly, the 50% cytotoxic concentration (CC50) for two of the compounds, determined in Vero cells, was previously calculated as > 50 μM for αHT-111 and > 100 μM for αHT-115 (Ireland et al., 2016; Masaoka et al., 2016). All compounds were dissolved in DMSO, aliquoted, and stored at −20 °C.
Fig. 1.
The chemical structure of the three synthetic αHTs compounds used in this study.
2.2. Cells and viruses
African green monkey kidney epithelial cells (CV-1) and bovine kidney epithelial cells (MDBK) were maintained in DMEM growth medium containing 10% newborn calf serum and 100 IU/ml penicillin-0.1 mg/mL streptomycin. Crandell feline kidney epithelial cells (CRFK) were maintained in DMEM growth medium containing 10% fetal bovine serum and 100 IU/ml penicillin-0.1 mg/mL streptomycin. The following strains of virus were used: HSV-1 wild-type strain F, UL15-null mutant virus (Baines et al., 1997), BoHV-1 Cooper strain, EHV-1 strain 10N0148, and FHV-1 strain FH2CS. Virus stocks were grown and titered by plaque assay on permissive cell monolayers. These included CV-1 for HSV-1, MDBK for BoHV-1 and EHV-1, and CRFK for FHV-1. All virus and cell stocks were stored in −80 °C.
2.3. Cytotoxicity assay
One mammalian cell line, MDBK cells, served as a representative sample. MDBK cells were seeded into 12-well plates and incubated in DMEM growth medium as previously described until 80% confluency was reached (~24 h). αHT compounds were added to 1 ml of growth medium to achieve final concentrations of 100 μM, 125 μM, 150 μM, and 175 μM and applied to the cells in duplicate. The cytotoxic agent sodium azide (Sigma-Aldrich) was added to one well (in duplicate) at 300 μM to serve as a positive control. DMSO at the highest concentration served as the negative control. After 24-h incubation, 200 μl of a tetrazolium compound [3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] and an electron coupling reagent (phenazine ethosulfate; PES) (Promega) were added to each well with an additional 2-h incubation. The absorbance at 490 nm was recorded and the survival rate was calculated, using the following equation: Absorbance sample/Absorbance control x 100. The 50% cytotoxic concentration values were calculated using GraphPad Prism. The concentrations of the compounds were log transformed. Non-linear regression, via the four-parameter variable-response log(inhibitor)-versus-response algorithm, was employed to determine the 50% cytotoxic concentration.
2.4. HSV-1, boHV-1, EHV-1, and FHV-1 replication inhibition assays
HSV-1, BoHV-1, and FHV-1 were added to an appropriate permissive cell line (CV-1, MDBK, and CRFK, respectively) at a multiplicity of infection (MOI) of 0.01 for 1 h. EHV-1 was added to a permissive cell line (MDBK) at MOI 0.1 for 1 h. After a 1-h adsorption period, the virus-inoculated medium was removed and the cells were washed once with PBS. Medium was replaced with DMEM containing either DMSO (at the highest concentration) or various concentrations of αHT (10 μM to 50 μM for compounds 106 and 111 or 2 to 10 μM for compound 115) for an additional 47 h. At 48 h post-infection, the cells were assessed by phase-contrast microscopy for cytopathic effect. Samples were frozen at −80 °C, thawed, removed from the dish by scraping, and sonicated to release intracellular virus. Infectious virus was measured by plaque assay on permissive cells, performed once at each concentration for each virus. The activity of the compounds was compared by assessing the degree of viral replication inhibition at 10 μM for the three αHTs.
2.5. Transmission electron microscopy (TEM)
BoHV-1 was added at an MOI of 1.0 PFU/cell to a ~90% confluent monolayer of MDBK cells in a 6-well plate. After 1 h incubation, the virus-inoculum was removed and replaced with DMEM containing one of the following: DMSO (at the highest concentration), 50 μM αHT-106, or 8 μM αHT-115 for an additional 13-h incubation. Fixative was then added to each sample to a final concentration of 2% formaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer pH of 7.4. After 10 min, the cells were removed by scraping, pelleted by centrifugation, and subjected to another round of fixation, resuspension, and centrifugation. The pellet was mixed with an equal amount of 3% agarose and once solidified, cut into cubes and placed in 0.1 M phosphate buffer pH 7.4. The sample was washed with 0.1 M phosphate buffer and 0.08 M glycine for 15 min, followed by fixation in 2% osmium tetroxide in 0.1 M phosphate buffer pH 7.4 in the dark for 1 h. The samples were then subjected to dehydration with 50%, 70%, 80%, 90% ethanol for 15 min each, followed by 100% ethanol for 20 min. The samples were infiltrated with 1:1 ethanol and LR White resin for 2 h, followed by 100% LR White for 2 h. Ultra-thin sections (90 nm) were stained with 2% uranyl acetate and lead citrate, then observed and photographed with a JEOL JEM-1400 transmission electron microscope.
2.6. Immunoblotting
CV1 cells in 6-well plates were infected for 1 h with HSV-1 at an MOI of 5.0; virus-inoculated medium was then removed and replaced with DMEM containing either DMSO (at the highest concentration), or DMSO solubilized 50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115 for an additional 5 or 17 h. The cells were then washed with PBS and solubilized in SDS sample buffer. The solubilized proteins were separated on 12% SDS polyacrylamide gels and transferred to nitrocellulose membranes. The 6 h samples were probed with a monocolonal antibody (mAb) to ICP27 (diluted 1:1000 in PBS plus 1% BSA), while the 18 h samples were probed with a mAb directed against glycoprotein C (gC) that was diluted 1:2000 in PBS with 1% BSA. The bound antibodies were detected by incubation with anti-mouse immunoglobulin conjugated with horseradish peroxidase (HRP) and visualized by enhanced chemiluminescence (ECL; Amersham). For a loading control, the blot was stripped and reprobed with anti-actin antibodies (diluted 1:1000) and developed similarly.
2.7. Absolute quantification of viral DNA using quantitative PCR
CV1 cells were infected with HSV-1(F) at an MOI of 5.0. After 1-h incubation, the virus inoculated medium was removed and replaced with a DMSO vehicle control, 50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115. The samples were incubated for an additional 12 h until DNA extraction (DNeasy). Target primers for UL51 (forward: 5′-GCC AGT CGT TCT AGG TTC AC-3′ and reverse: 5′-GTT AAC GCG CTA CTT CCCG-3′) were used to measure viral DNA, with the iQ SYBR green supermix (Bio-Rad) according to the manufacturer’s directions. Briefly, reactions were performed in a volume of 20 μl, consisting of 50 ng of DNA template, 100 nM of each primer, 7.6 μl H2O, and 10 μl SYBR green mix. The thermal cycling protocol included an initial denaturation for 3 min at 95 °C and 35 cycles consisting of a denaturation step at 95 °C for 10 s and an annealing step at 60 °C for 30 s. Each sample was analyzed in triplicate, and average threshold cycle (CT) values were compared to a standard curve prepared from the pcDNA3 plasmid containing the full length UL51 sequence for absolute quantification (Roller et al., 2014). Statistical significance was assessed using one-way ANOVA with Tukey’s post-hoc analysis (**, p < 0.05).
2.8. Southern blotting
Five 100-mm plates of CV1 cells, one of which was pre-treated with 300 μg/mL of phosphonoacetic acid (PAA; Sigma Aldrich) for 30 min, were infected with HSV-1 (F) at an MOI of 5.0; one 100-mm plate was infected with UL15-null virus at an MOI of 5.0. After the 1-h incubation, virus inoculation media was removed and replaced with DMSO vehicle control (HSV-1 and UL15-null), 300 μg/mL PAA, 50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115). After an additional 17-h incubation, the medium was removed and the cells were washed with phosphate-buffered saline (PBS), scraped, and pelleted by centrifugation at 1,000 x g for 5 min. The cells were lysed, and total cellular DNA was extracted as described previously (Yang et al., 2011). Total DNA (~10 μg) was digested with BamHI, separated on 0.8% agarose gel via electrophoresis, followed by denaturization in 1 M NaOH, and neutralization in Tris buffer. The denatured DNA was transferred to a nylon membrane by capillary action, and the transferred DNA was hybridized with [32P]dCTP-labeled BamHI P fragment of HSV-1(F) DNA. The bound probe was visualized by exposure to X-ray film with intensifying screens at −80 °C.
3. Results
3.1. Compound selection
Three synthetic αHTs were selected that have varying potencies against the nuclease activity of HSV pUL15 as determined by an in vitro dual-probe fluorescence assay (Masaoka et al., 2016). The HSV replication inhibition activity of these three αHTs was also previously evaluated (Ireland et al., 2016). The biochemical anti-terminase and in vitro anti-HSV potencies of the compounds were found to be discordant. The chemical structure of each compound is shown in Fig. 1. The biochemical anti-terminase and in vitro anti-HSV potencies of the three selected compounds are detailed in materials and methods.
3.2. αHTs are minimally toxic in mammalian cell lines and broadly suppress herpesviral replication inhibition
The cytotoxicity of the three αHTs was assessed on MDBK cells using an MTS viability assay. Two of the compounds (αHT-106 and −115) showed minimal cytotoxicity at 24 h with 50% cytotoxic concentration values exceeding 100 μM; αHT-111 had moderate cytotoxi-city at ~78 μM (Table 1). Replication inhibition activity of αHTs was initially assessed with BoHV-1 at concentrations of 0.1, 1, 10 and 50 μM for αHT-106, −111 and −115. Viral yield determined by plaque assay was compared to yield from DMSO-treated cells. No plaques were detected between concentrations of 10 and 50 μM for αHT-106 and −111 and between 1 and 10 μM for αHT-115, indicating greater antiviral potency of αHT-115. Given this initial data, additional replication inhibition assays were performed with αHT concentrations of 10, 20, 30, 40, and 50 μM (αHT-106 and −111) and 2, 4, 6, 8, 10 μM (αHT-115). The log10 suppression values were similar between αHT-106 and −111 (Table 1). In contrast, αHT-115 markedly suppressed viral replication with log10 suppression values ranging between 0.9 and 7.0 at a concentration of 10 μM. Thus, αHT-115 demonstrated more potent anti-viral activity than either αHT-106 or αHT −111.
Table 1.
CC50 and log10 HSV-1, BoHV-1, FHV-1, and EHV-1 suppression values of αHT-106, −111, and −115 at 10 μM. The CC50 values were determined in MDBK cells.
| Log10 Suppression at 10 μMa | |||||
|---|---|---|---|---|---|
| Compound | CC50 (μM)b | HSV-1 | BoHV-1 | FHV-1 | EHV-1 |
| αHT-106 | > 100 | 0.64 | 0.83 | 1.4 | −0.53 |
| αHT-111 | 78 | 1.7 | 0.47 | 1.1 | −0.11 |
| αHT-115 | > 100 | 7.0 | 6.0 | 2.1c | 0.90 |
Determined by plaque assay, comparing the αHT-treated viral titer to DMSO control titer performed once for each virus.
Cytotoxicity assays performed in duplicate.
Value determined at a concentration of 6 μM; suppression at 10 μM was not determined.
3.3. αHTs decrease production of infectious progeny
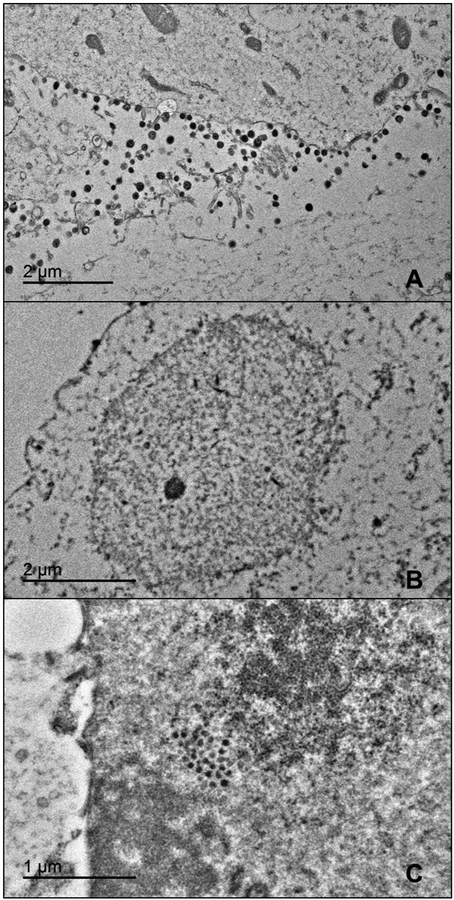
Transmission electron microscopy (TEM) was employed to assess BoHV-1 assembly in the presence of αHTs. MDBK cells were infected with BoHV-1 in the presence of a DMSO vehicle control, 50 μM αHT-106, or 8 μM αHT-115. Given that αHT-111 is considered an intermediate anti-herpesviral and anti-terminase compound, this compound was excluded. Numerous virions and C capsids were observed in the DMSO control BoHV-1 sample (Fig. 2A). Application of both αHT-106 and −115 resulted in a marked decrease in extracellular viral particles and intranuclear capsids (Fig. 2B). We observed a single group of round electron-dense structures of sizes smaller than 120 nm diameter herpesvirus capsids in the nucleus of one αHT-115 treated cell (Fig. 2C). The nature of these particles was of unknown significance and they were not observed elsewhere. The decrease in capsids and virions supported the compounds’ anti-herpesviral activity and suggested either an assembly defect or failure to express viral structural proteins.
Fig. 2.
TEM of MDBK cells inoculated with BoHV-1 and treated with DMSO control (A), 50 μM αHT-106 (B), or 8 μM αHT-115 (C). Note the numerous extracellular virions in the control sample (A). No virions or capsids were observed in the αHT-106 treated (B) and αHT −115 treated samples (not pictured). Clusters of round electron dense intranuclear structures were very rarely present in the αHT-115 sample (C).
3.4. αHTs inhibit expression of true late (γ2) proteins
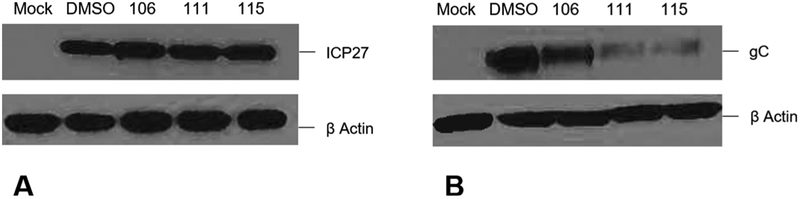
To further elucidate the mechanism of action of αHTs, immunoblotting was performed to assess HSV-1 immediate-early (α genes, represented by ICP27) and late [γ2, represented by glycoprotein C (gC)] protein expression in the presence of αHTs. CV1 cells were inoculated with wild-type HSV-1 and treated with a DMSO vehicle control, 50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115. No change in ICP27 expression was noted between the DMSO control or compound treated samples suggesting normal expression of α gene products (Fig. 3A). However, we observed decreased gC expression in the αHT-treated samples compared to the wild-type HSV-1 control. The decrease in gC expression was more marked in cells treated with αHT-115 and αHT- 111 than αHT-106, and thus mirrored the relative antiviral potencies of these compounds (Fig. 3B). These findings indicate that the effects on protein expression occurred between the expression of immediate-early proteins and that of late proteins. Such a timeframe is consistent with the onset of viral DNA replication.
Fig. 3.
Immunoblotting of α (A) and γ2 (B) protein in mock-infected or αHT-treated HSV-1-infected samples. (A). α protein ICP27 and β-actin, loading control. (B). γ2 protein gC and β-actin, loading control.
3.5. αHTs inhibit DNA replication
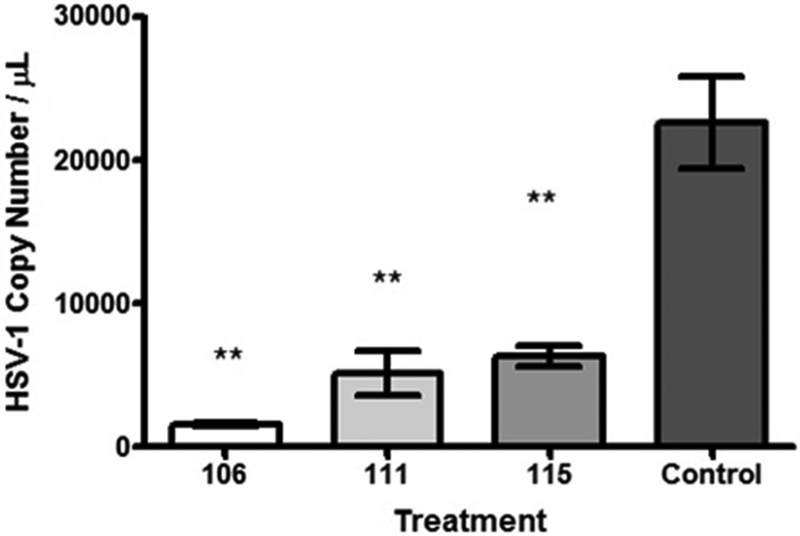
To evaluate whether HTs affected DNA replication, HSV-1 viral DNA produced in the presence or absence of compound was measured using absolute quantification by real time PCR (qPCR) using UL51 specific probes and primers and a standard curve prepared with a plasmid containing the full length UL51 sequence. Significant decreases in HSV-1 viral DNA were observed in αHT-treated samples (50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115) compared to the wild-type HSV-1 control (p < 0.05) (Fig. 4). Specifically, the amount of UL51 target sequences in αHT-treated samples were reduced approximately 72 to 93% compared to the DMSO treated control. All compounds inhibited HSV-1 DNA accumulation to similar extents. These data indicate that αHTs inhibit HSV-1 DNA replication.
Fig. 4.
Real-time qPCR of viral DNA in wild-type HSV-1 control and αHT-treated samples, performed in triplicate. The decrease in HSV-1 DNA copy number is significant, as determined by one-way ANOVA and Tukey’s post-hoc analysis (**, p < 0.05). No significant differences were observed between αHT treated samples (50 μM αHT-106, 30 μM αHT-111, or 8 μM αHT-115).
3.6. Attempted assessment of viral cleavage activity in the presence of αHT
Previous research using an in vitro dual-probe fluorescence assay has shown that the αHTs inhibit nuclease activity of HSV-1 pUL15C to varying extents. Specifically, αHT-106 was more potent than αHT-111, and the effect of αHT-115 was negligible (Masaoka et al., 2016). Although the results of qPCR shown above indicated inhibition of viral DNA replication, a concurrent effect on cleavage via inhibition of the viral terminase could not be excluded.
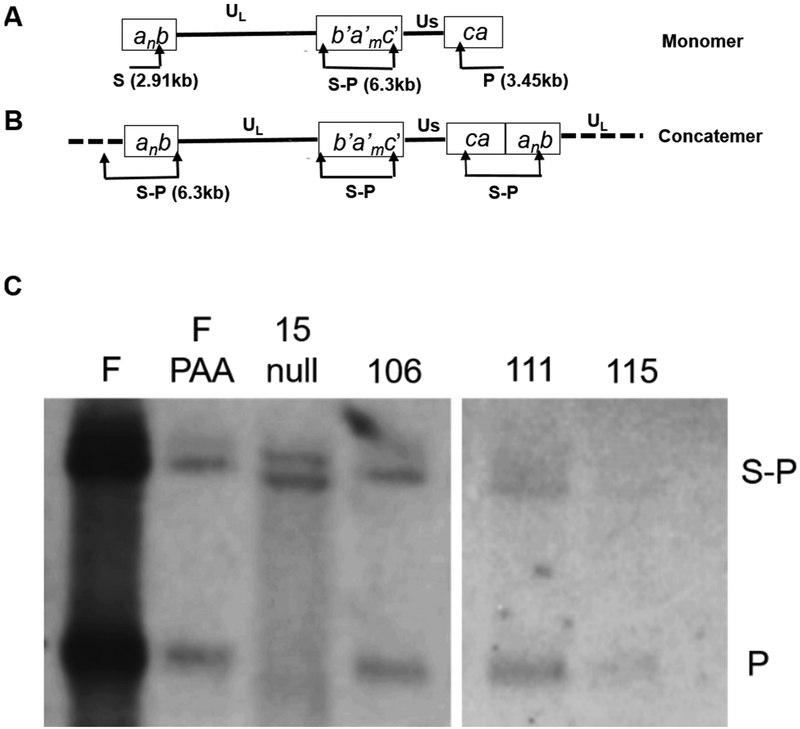
To assess DNA cleavage in the presence and absence of compound treatment, CV1 cells were infected with HSV-1(F) or a UL15-null mutant in the presence of PAA (a known viral DNA synthesis inhibitor), αHT-106, αHT-111, αHT-115, or a DMSO vehicle control. Viral DNA was digested with restriction enzyme, BamHI, separated electrophoretically, and probed with a radiolabeled terminus of the short component (P fragment) (Fig. 5A and B). The junctional S-P fragments were observed in all samples as expected (Fig. 5C). The lane with HSV-1(F) DNA contained a prominent P fragment which was generated by BamHI cleavage at one end, and by nuclease activity of the viral terminase at the other (Zhou et al., 2014). In the PAA treated sample, low levels of DNA were detected and likely represented DNA from virions adsorbed to the cells (input DNA). The BamHI P fragment was observed in this case because packaged virion DNA is a linear molecule. As expected, the UL15-null virus failed to generate the BamHI P fragment because this mutant encodes a defective terminase unable to cleave viral DNA (Yang et al., 2011). DNA levels in cells infected in the presence of the three αHTs were comparable to the PAA control, suggesting that the compounds conferred a marked inhibition of viral DNA replication. The levels of DNA in cells treated with the αHTs were too low to permit meaningful comparison between levels of terminal P fragments and the S-P junction fragments, which is a metric necessary to assess DNA cleavage in cells. Thus, the capacity to verify the previous biochemical findings of anti-terminase activity of αHTs was precluded in this assay by their profound inhibition of viral DNA replication.
Fig. 5.
Viral DNA analysis. (A and B) Schematic diagram of the HSV-1 genome indicating the unique long (UL) and unique short (US) components, and positions of the BamHI P, S, or junctional S-P fragments. The P and S are end fragments unique to terminase-cleaved viral DNA (i.e. the monomer shown in A). (C) Fluorographic image of viral DNA probed with radiolabeled terminus of the short component (P fragment). CV1 cells were infected with HSV-1(F) or UL15-null. The HSV-1(F) infected samples were treated with PAA, αHT-106, αHT-111, αHT-115 or DMSO vehicle-control. The positions of the S-P junctional fragments and BamHI P fragments are indicated. Decreased band intensities representing the junctional and end fragments are noted in the αHT-treated samples and are comparable in level to that of the PAA-treated sample.
4. Discussion
The presented research shows that the αHTs tested have broad anti-herpesviral activity against herpesviruses of veterinary significance and suggests that they act primarily at the level of viral DNA synthesis. αHTs were previously documented to inhibit HSV-1 and −2 replication in cell culture and postulated targets included viral proteins with an RNase H-like domain (Ireland et al., 2016; Tavis et al., 2014). In HSV-1, these targets include DNA polymerase (pUL30), single-stranded DNA binding protein (ICP8, pUL29), alkaline nuclease (pUL12) and the nuclease domain of the viral terminase (pUL15C) (Boehmer and Lehman, 1997; Bryant et al., 2012; Liu et al., 2006; Schumacher et al., 2012; Selvarajan Sigamani et al., 2013; Yan et al., 2014). Both ICP8 and the DNA polymerase are necessary for viral DNA replication (Wu et al., 1988).
αHTs were previously found to inhibit the nuclease domain of the viral terminase in vitro via a dual-probe fluorescence assay. Twenty-one synthetic αHTs had been screened and variable potencies were documented. These potencies were found to negatively correlate with the bulkiness of their modified side chain (Masaoka et al., 2016). Interestingly, the αHTs with potent inhibition of viral replication were relatively poor inhibitors of pUL15C nuclease activity suggesting other HSV targets were being inhibited to confer the anti-replication effects (Ireland et al., 2016; Masaoka et al., 2016).
The three αHTs selected for this research had variable potencies against pUL15C in biochemical assays, ranging from negligible (αHT-115), intermediate (αHT-111), to marked (αHT-106). These compounds were screened for activity against three important veterinary herpesviruses, BoHV-1, EHV-1, and FHV-1 and were also screened for activity against HSV-1 as a control. Two of the compounds (αHT-106 and −115) were non-toxic with a CC50 value > 100 μM, based on an MTS viability assay performed on MDBK cells. The results of the replication inhibition assays were comparable across the different herpesviruses for each compound. The slight differences in log10 suppression values could be attributed to variability between cell types and/or virus. Additionally, the relative potencies of these three αHTs were similar to the previous observations against HSV-1 and −2 with αHT-115 being more potent than αHT-106 and −111 (Ireland et al., 2016). Given that there are no other differences between these compounds we conclude that αHT-115′s biaryl side chain improved its inhibitory effect (Ireland et al., 2016).
To further elucidate the mechanism of action of αHTs against herpesviruses, the effect of αHTs against HSV-1 was assessed with transmission electron microscopy, immunoblotting, real-time qPCR, and southern blotting. The results of these findings were consistent with the inhibition of viral DNA replication. Specifically, despite similar levels of immediate-early protein production in the presence of the compounds, we observed decreased DNA replication dependent true-late (γ2) protein expression in the αHT-treated samples. The results of qPCR confirmed HSV-1 DNA replication inhibition by αHTs, inasmuch as compound treatment reduced UL51 target sequences by 72–93% compared to the wild-type control. The decreased observation of capsids and virions by electron microscopy likely reflected decreased production of late genes, some of which encode capsid proteins. Late gene synthesis is dependent upon viral DNA replication. However, the profound decrease in viral DNA synthesis precluded meaningful assessment of viral DNA cleavage mediated by the terminase, which includes the UL15-encoded nuclease. Therefore, we cannot exclude an additional effect on terminase activity of these compounds in cell culture.
Taken together, these data show that αHTs are broadly effective compounds that primarily target herpesviral DNA replication. As αHTs have potent chelating activity, inhibition of cation-dependent enzyme (s) is likely (Meck et al., 2014). Enzymes with RNase H-like domains require metal binding in their active sites. Three of the four HSV-1 enzymes with RNase H activity or an RNase H-like fold function in viral DNA replication, including DNA polymerase (pUL30 in HSV), the single-stranded DNA binding protein (ICP8, pUL29 in HSV), and the alkaline nuclease (pUL12 in HSV) (Boehmer and Lehman, 1997; Bryant et al., 2012; Liu et al., 2006; Schumacher et al., 2012; Yan et al., 2014). Due to their importance, these enzymes are highly conserved across Herpesviridae. Conservation of their targets might explain the broad anti-herpesvirus activities of αHTs.
The antiviral potency of αHT-115, rivals that of anti-herpesviral compounds currently available on the market. Although not directly compared in this study, the reported EC50 concentrations of acyclovir against HSV-1 range between 0.08 to 55 μM (Zovirax, 2003). While anti-viral therapy is currently of limited use in veterinary medicine, research has shown promising responses with nucleoside analogue therapy in FHV-1 infected cats (Thiry et al., 2009; Thomasy et al., 2011; Thomasy et al., 2016). Specifically, EC50s for the following compounds were calculated in cell culture against FHV-1: 4.3 μM for idoxuridine, 5.2 μM for ganciclovir, 11 μM for cidofovir, 58 μM for acyclovir, and 233 μM for foscarnet (Maggs and Clarke, 2004). Additional research is required to assess the use of αHT-115 in cats infected with FHV-1, as this compound may be a promising therapeutic option in cats.
Information on the in vivo toxicity of synthetic αHTs is limited. However, significant varying side effects in mice given intraperitoneal injections of β-thujaplicinol, a natural tropolone within the αHT family, have been reported. Specifically, at low doses (50–100 mg/kg), the mice experienced depressive effects, including temporary lethargy and hypokinesis. However, at higher doses (150–100 mg/kg), the mice showed stimulant effects including seizures, tachypnea, and eventual death (Meck et al., 2014; Sanders, 1961). Although these data reflect potential side effects of αHTs, the reduced cytotoxicity of derivatives in vitro suggests that these compounds may have fewer toxic effects in vivo. Thus, additional optimization, in vitro cytotoxic research, and eventual in vivo research are needed prior to making conclusions regarding the toxic effects of these compounds.
In conclusion, αHTs inhibit HSV-1 DNA replication and may comprise effective therapeutic agents against different species of herpes-viruses. The veterinary viruses selected in this study (BoHV-1, EHV-1, and FHV-1) are geographically widespread and are a major cause of morbidity and mortality in domestic animals. Furthermore, BoHV-1 and EHV-1 cause economic losses due to abortions and poor performance, either in racing horses or poor growth and milk production in cattle. Current treatment options are limited in veterinary medicine, particularly in equine and bovine medicine. Thus, an efficacious therapeutic option is dire in these industries (Graham, 2013; Lunn et al., 2009). As the activity of the αHT compounds was still in the micromolar range, further optimization of therapeutic activity by alteration of the chemical side chains is needed. Additional studies would be useful to identify the viral target(s) through genetic analysis of viral escape mutants.
References
- Baines JD, Cunningham C, Nalwanga D, Davison A, 1997. The U(L)15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the U(L)15 gene product. J. Virol 71, 2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley R, 2008. A fresh look at natural tropolonoids. Nat. Prod. Rep 25, 118–138. [DOI] [PubMed] [Google Scholar]
- Biswas S, Bandyopadhyay S, Dimri U, Patra PH, 2013. Bovine herpesvirus-1 (BHV-1) – a re-emerging concern in livestock: a revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet. Q 33, 68–81. [DOI] [PubMed] [Google Scholar]
- Boehmer PE, Lehman IR, 1997. Herpes simplex virus DNA replication. Annu. Rev. Biochem 66, 347–384. [DOI] [PubMed] [Google Scholar]
- Bryant KF, Yan Z, Dreyfus DH, Knipe DM, 2012. Identification of a divalent metal cation binding site in herpes simplex virus 1 (HSV-1) ICP8 required for HSV replication. J. Virol 86, 6825–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P, Darby G, 1991. Laboratory studies of herpes simplex virus strains resistant to acyclovir. Rev. Med. Virol 1, 19–28. [Google Scholar]
- Dunowska M, 2014. A review of equid herpesvirus 1 for the veterinary practitioner. Part A: clinical presentation diagnosis and treatment. N. Z. Vet. J 62, 171–178. [DOI] [PubMed] [Google Scholar]
- Fontenelle JP, Powell CC, Veir JK, Radecki SV, Lappin MR, 2008. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. Am. J. Vet. Res 69, 289–293. [DOI] [PubMed] [Google Scholar]
- Gable JE, Acker TM, Craik CS, 2014. Current and potential treatments for ubiquitous but neglected herpesvirus infections. Chem. Rev 114, 11382–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould D, 2011. Feline herpesvirus-1. J. Feline Med. Surg 13, 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DA, 2013. Bovine herpes virus-1 (BoHV-1) in cattle-a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Irish Vet. J 66, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch DR, Cox G, D’Erasmo MP, Shakya T, Meck C, Mohd N, Wright GD, Murelli RP, 2014. Inhibition of the ANT(2)-Ia resistance enzyme and rescue of aminoglycoside antibiotic activity by synthetic alpha-hydroxytropolones. Bioorg. Med. Chem. Lett 24, 4943–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland PJ, Tavis JE, D’Erasmo MP, Hirsch DR, Murelli RP, Cadiz MM, Patel BS, Gupta AK, Edwards TC, Korom M, Moran EA, Morrison LA, 2016. Synthetic alpha-hydroxytropolones inhibit replication of wild-type and acyclovir-resistant herpes simplex viruses. Antimicrob. Agents Chemother. 60, 2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Corey L, 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med 59, 381–395. [DOI] [PubMed] [Google Scholar]
- Krawczyk A, Arndt MAE, Grosse-Hovest L, Weichert W, Giebel B, Dittmer U, Hengel H, Jäger D, Schneweis KE, Eis-Hübinger AM, Roggendorf M, Krauss J, 2013. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci 110, 6760–6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Knafels JD, Chang JS, Waszak GA, Baldwin ET, Deibel MR Jr., Thomsen DR, Homa FL, Wells PA, Tory MC, Poorman RA, Gao H, Qiu X, Seddon AP, 2006. Crystal structure of the herpes simplex virus 1 DNA polymerase. J. Biol. Chem 281, 18193–18200. [DOI] [PubMed] [Google Scholar]
- Lunn DP, Davis-Poynter N, Flaminio MJ, Horohov DW, Osterrieder K, Pusterla N, Townsend HG, 2009. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med 23, 450–461. [DOI] [PubMed] [Google Scholar]
- Maggs DJ, Clarke HE, 2004. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. Am. J. Vet. Res 65, 399–403. [DOI] [PubMed] [Google Scholar]
- Masaoka T, Zhao H, Hirsch DR, D’Erasmo MP, Meck C, Varnado B, Gupta A, Meyers MJ, Baines J, Beutler JA, Murelli RP, Tang L, Le Grice SF, 2016. Characterization of the C-terminal nuclease domain of herpes simplex virus pUL15 as a target of nucleotidyltransferase inhibitors. Biochemistry 55, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck C, Mohd N, Murelli RP, 2012. An oxidopyrylium cyclization/ring-opening route to polysubstituted α-hydroxytropolones. Org. Lett 14, 5988–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck C, D’Erasmo MP, Hirsch DR, Murelli RP, 2014. The biology and synthesis of alpha-hydroxytropolones. MedChemComm 5, 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piettre SR, Ganzhorn A, Hoflack J, Islam K, Hornsperger J-M, 1997. α-Hydroxytropolones: a new class of potent inhibitors of inositol monophosphatase and other bimetallic enzymes. J. Am. Chem. Soc 119, 3201–3204. [Google Scholar]
- Roller RJ, Haugo AC, Yang K, Baines JD, 2014. The herpes simplex virus 1 UL51 gene product has cell type-specific functions in cell-to-cell spread. J. Virol 88, 4058–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders HD, 1961. Some Pharmacological and Microbiological Studies on Beta-hydroxy Thujaplicin. The University of British Columbia, Canada. [Google Scholar]
- Schumacher AJ, Mohni KN, Kan Y, Hendrickson EA, Stark JM, Weller SK, 2012. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog. 8, e1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan Sigamani S, Zhao H, Kamau YN, Baines JD, Tang L, 2013. The structure of the herpes simplex virus DNA-packaging terminase pUL15 nuclease domain suggests an evolutionary lineage among eukaryotic and prokaryotic viruses. J. Virol 87, 7140–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavis JE, Wang H, Tollefson AE, Ying B, Korom M, Cheng X, Cao F, Davis KL, Wold WS, Morrison LA, 2014. Inhibitors of nucleotidyltransferase superfamily enzymes suppress herpes simplex virus replication. Antimicrob. Agents Chemother. 58, 7451–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry E, Addie D, Belak S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Pennisi MG, Radford AD, Truyen U, Horzinek MC, 2009. Feline herpesvirus infection: ABCD guidelines on prevention and management. J. Feline Med. Surg 11, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasy SM, Lim CC, Reilly CM, Kass PH, Lappin MR, Maggs DJ, 2011. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. Am. J. Vet. Res 72, 85–95. [DOI] [PubMed] [Google Scholar]
- Thomasy SM, Shull O, Outerbridge CA, Lim CC, Freeman KS, Strom AR, Kass PH, Maggs DJ, 2016. Oral administration of famciclovir for treatment of spontaneous ocular, respiratory, or dermatologic disease attributed to feline herpesvirus type 1: 59 cases (2006–2013). J. Am. Vet. Med. Assoc 249, 526–538. [DOI] [PubMed] [Google Scholar]
- Williams YD, Meck C, Mohd N, Murelli RP, 2013. Triflic acid-mediated rearrangements of 3-methoxy-8-oxabicyclo[3.2 1]octa-3,6-dien-2-ones: synthesis of methoxytropolones and furans. J. Org. Chem 78, 11707–11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CA, Nelson NJ, McGeoch DJ, Challberg MD, 1988. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J. Virol 62, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Bryant KF, Gregory SM, Angelova M, Dreyfus DH, Zhao XZ, Coen DM, Burke TR Jr., Knipe DM, 2014. HIV integrase inhibitors block replication of alpha-, beta-, and gammaherpesviruses. mBio 5, e01318–01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Wills EG, Baines JD, 2011. A mutation in U(L)15 of herpes simplex virus 1 that reduces packaging of cleaved genomes. J. Virol 85, 11972–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Yang K, Wills E, Tang L, Baines JD, 2014. A mutation in the DNA polymerase accessory factor of herpes simplex virus 1 restores viral DNA replication in the presence of raltegravir. J. Virol 88, 11121–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovirax®, 2003, DSM Pharmaceuticals, Inc., Greenville, NC: [cited 2017; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/18603slr027_zovirax_lbl.pdf]. [Google Scholar]