This article reports on the effects of esophageal self‐expandable metal stents placement on the risk of esophageal fistula formation and clinical outcomes in patients undergoing concurrent chemoradiation therapy.
Keywords: Esophageal squamous cell carcinoma, Self‐expandable metal stents, Concurrent chemoradiation therapy, Esophageal fistula
Abstract
Background.
The purpose of this study was to review the risks and benefits of concurrent chemoradiation therapy (CCRT) with esophageal self‐expandable metal stents (SEMS) for the treatment of locally advanced esophageal cancer.
Materials and Methods.
Between January 2014 and December 2016, the data from 46 locally advanced esophageal cancer patients who received CCRT at our institution were retrospectively reviewed. Eight patients who received CCRT concomitant with SEMS placement (SEMS plus CCRT group) and thirty‐eight patients who received CCRT without SEMS placement (CCRT group) were identified. The risk of developing esophageal fistula and the overall survival of the two groups were analyzed.
Results.
The rate of esophageal fistula formation during or after CCRT was 87.5% in the SEMS plus CCRT group and 2.6% in the CCRT group. The median doses of radiotherapy in the SEMS plus CCRT group and the CCRT group were 47.5 Gy and 50 Gy, respectively. SEMS combined with CCRT was associated with a greater risk of esophageal fistula formation than CCRT alone (hazard ratio [HR], 72.30; 95% confidence interval [CI], 8.62–606.12; p < .001). The median overall survival times in the SEMS plus CCRT and CCRT groups were 6 months and 16 months, respectively. Overall survival was significantly worse in the SEMS plus CCRT group than in the CCRT group (HR, 5.72; 95% CI, 2.15–15.21; p < .001).
Conclusion.
CCRT concomitant with SEMS for locally advanced esophageal cancer results in earlier life‐threatening morbidity and a higher mortality rate than treatment with CCRT alone. Further prospective and randomized studies are warranted to confirm these observations.
Implications for Practice.
Patients treated with SEMS placement followed by CCRT had higher risk of esophageal fistula formation and inferior overall survival rate compared with patients treated with CCRT alone. SEMS placement should be performed cautiously in patients who are scheduled to receive CCRT with curative intent.
摘要
背景。本次研究的目的是回顾分析同步放化疗(CCRT) 伴随食管自膨式金属支架 (SEMS)用于治疗局部晚期食管癌的风险和益处
材料和方法。2014年1月至2016年12月,回顾性分析了在我院接受CCRT的46名局部晚期食管癌患者的数据。共选择8名接受CCRT 伴随SEMS植入的患者(SEMS+CCRT组)和38名仅接受CCRT、未植入SEMS的患者(CCRT组)。分析了两组患者的食管瘘发生风险和总生存期
结果。在CCRT期间或之后,SEMS+CCRT组和CCRT组的食管瘘发生率分别为87.5%和2.6%。SEMS+CCRT组和CCRT组的中位放疗剂量分别为47.5 Gy 和50 Gy。与单纯CCRT治疗相比,SEMS联合CCRT会导致食管瘘形成风险升高[风险比 (HR), 72.30; 95% 置信区间(CI), 8.62–606.12; p<0.001]。SEMS+CCRT组和 CCRT组的中位总生存期分别为6个月和16个月。SEMS+CCRT组的总生存期显著低于CCRT组(HR, 5.72; 95% CI, 2.15–15.21; p<0.001)
结论。与单独采用CCRT治疗相比,CCRT伴随SEMS用于治疗局部晚期食管癌可导致更早发生危及生命的疾病和更高的死亡率。未来需要展开进一步前瞻性随机研究,以证实这些观察结果。
实践意义:与单独接受CCRT治疗的患者相比,接受SEMS植入伴随CCRT治疗的患者食管瘘形成的风险更高,总生存率也较低。对计划接受CCRT治疗以实现根治目的的患者,应慎用SEMS植入
Introduction
Most esophageal cancer patients suffer from dysphagia. There are several ways to relieve malignant dysphagia in patients with esophageal cancer, such as endoluminal stent placement, surgery, radiotherapy (RT), chemotherapy, and concurrent chemoradiation therapy (CCRT) [1], [2]. Placement of self‐expandable metal stents (SEMS) allows esophageal luminal patency to be easily re‐established and relieves dysphagia in locally advanced esophageal cancer patients [3], [4]. Additionally, the procedural success rate for SEMS insertion was found to be 95%, and the major adverse events included stent migration (1%–33%), severe chest pain (2%–23%), esophageal perforation (3%–9%) and tumor overgrowth (2.5%–36%) [5], [6].
CCRT is considered a neoadjuvant treatment for patients with resectable esophageal tumors [7] and a definitive setting for patients with unresectable locally advanced disease [8]. However, the safety of the combination of SEMS and CCRT or radiotherapy remains controversial. A case report noted the potential risk of lethal aortic pseudoaneurysm and further aortoesophageal fistula following SEMS plus CCRT for esophageal cancer [9]. The reported stent‐related mortality ranged from 0% to 54% in patients treated with CCRT prior to SEMS placement compared with 0%–6% in patients without prior CCRT [6], [10]. Song et al. reported patients who underwent SEMS placement alone or who received radiotherapy before SEMS placement experienced fewer adverse events, such as stent migration and fistula formation, than patients who received radiotherapy after SEMS placement (odds ratios of 0.043 and 0.088, respectively) [11]. Francis et al. found that patients who had received stents during CCRT experienced more acute esophageal toxicity [12].
Nevertheless, a meta‐analysis reported that prior CCRT had no impact on adverse events in stenting [13]. In a randomized trial of 84 inoperable esophageal cancer patients who received SEMS with or without palliative radiotherapy (30 Gy in 10 fractions), no cases of esophageal fistula formation or perforation were observed [14]. From the results of the aforementioned studies regarding patients treated with CCRT followed by SEMS or SEMS plus CCRT, it is difficult to discern whether such life‐threatening adverse events are due to the stents, CCRT effects, the advanced nature of the disease process, or a combination of these factors.
In the current study, we investigated the effects of SEMS placement on the risk of esophageal fistula formation and clinical outcomes in patients undergoing CCRT.
Materials and Methods
Patient Selection
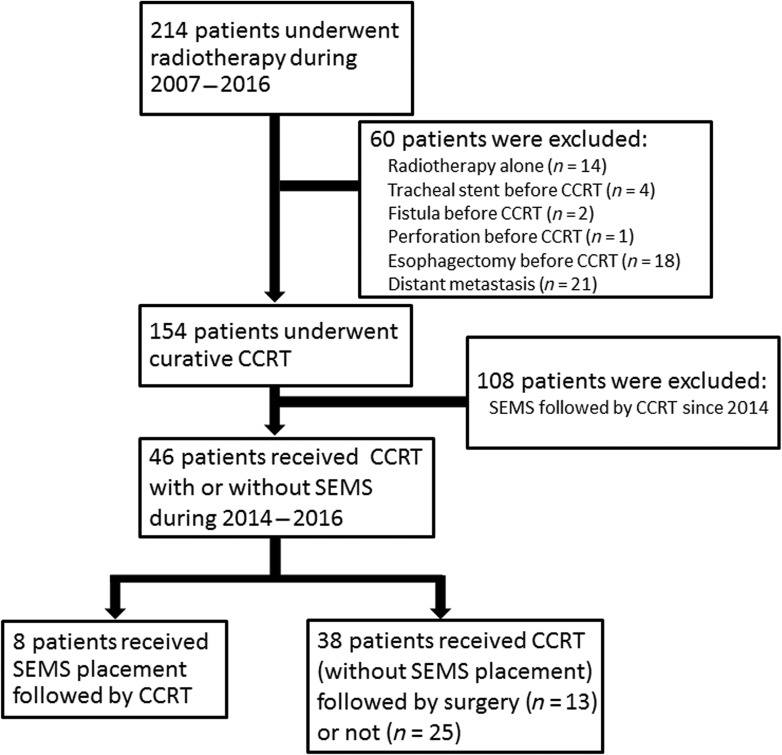
We retrospectively reviewed esophageal cancer patients who received CCRT at the Far Eastern Memorial Hospital between January 2007 and December 2016. Data were collected after receiving approval from the Institutional Review Board of the Far Eastern Memorial Hospital (FEMH‐IRB‐106020‐E). All patients were initially evaluated by a multimodality treatment team consisting of a chest surgeon, a gastroenterologist, a medical oncologist, and a radiation oncologist. Staging investigations included a complete medical history and physical examination, fiberoptic endoscopic evaluation, complete blood cell counts, liver and renal function tests, and computed tomography (CT) of the chest and abdomen with or without positron emission tomography (PET)‐CT. Presence of esophageal fistula was confirmed if CT angiography, contrast‐enhanced CT, or bronchoscopy demonstrated a direct link between the esophagus and the aorta or trachea. Esophageal perforation was diagnosed if contrast‐enhanced CT revealed air in the soft tissue of the mediastinum surrounding the esophagus or abscess cavities adjacent to the esophagus in either the pleural space or the mediastinum [15]. All tumors were staged according to the tumor‐node‐metastasis staging system (American Joint Committee on Cancer Cancer Staging Manual, 7th edition). From January 2007 to December 2016, 214 esophageal cancer patients underwent radiotherapy at our institution. Patients who received radiotherapy alone (n = 14), underwent tracheal stent placement before CCRT (n = 4), exhibited an esophageal fistula or perforation before CCRT (n = 3), underwent an esophagectomy before CCRT (n = 18), or had a distant metastatic disease (n = 21) were excluded. A total of 154 patients with locally advanced esophageal cancer who received CCRT with curative intent were identified. Our hospital has administered the combined modality therapy (SEMS plus CCRT) to patients with esophageal cancer since 2014; therefore, 108 patients who received CCRT between 2007 and 2013 were excluded, and the remaining 46 patients were enrolled. Of the 46 patients, 8 patients who had SEMS concomitant with CCRT were categorized as the SEMS plus CCRT group, and the other 38 patients who received CCRT were identified as the CCRT group (Fig. 1).
Figure 1.
Flowchart of patients identified in the study.
Abbreviations: CCRT, concurrent chemoradiation therapy; SEMS, self‐expandable metal stent.
Placement of Metallic Stent
Every year, approximately 500 esophagogastroduodenoscopies, 300 colonoscopies, and 50 endoscopic retrograde cholangiopancreatographies are performed by two qualified gastrointestinal endoscopists at our hospital. Before the combined SEMS and CCRT therapy was begun (2012–2014), 55 SEMS to release esophageal, biliary, or colorectal obstruction had been successfully placed by the same gastrointestinal endoscopists. Prior to stent insertion, all patients underwent an endoscopy and a complete staging CT scan of the thorax and abdomen. A Wallflex or Ultraflex partially covered SEMS (Boston Scientific, Natick, MA) was used for the patients who underwent SEMS placement. The diameter of the SEMS used in this study was 18/23 mm (diameter of the stent body/flared bilateral ends, respectively) or 23/28 mm, and the stent length ranged from 10 to 15 cm. The stent length was chosen to allow 2–4 cm of stent to lie above and below the end of the stricture site. Flexible esophagoscopy was performed to characterize the lesion. Under fluoroscopic guidance, the proximal and distal extent of the tumor was marked on the skin using radiopaque metal markers. A guidewire was placed through the tumor, and the stent delivery system was inserted over the guidewire. The stent was deployed across the tumor length that was previously delineated by the radiopaque markers under fluoroscopic guidance.
Radiation Therapy
CT‐based, intensity‐modulated radiotherapy with 6‐MV photons (Tomotherapy, Accuray Inc., Madison, WI; Versa HD, Elekta, Crawley, West Sussex, U.K.) was employed at our institution. Target regions and normal structures were contoured using the Pinnacle 3 Treatment Planning System (Philips Healthcare, Madison, WI). The gross tumor volume was defined as the encompassing primary tumor and the metastatic lymph nodes that were found via chest CT or PET‐CT (standard uptake value of >2 to 2.5). The clinical target volume (CTV) included the esophagus of primary tumor expansion 3–5 cm in the superior‐inferior directions and 0.5–1 cm in the lateral and anteroposterior directions. For cervical and upper thoracic squamous cell carcinomas, nodal basins extending superiorly from the lower cervical and supraclavicular region and inferiorly to the subcarinal lymph node basin, inclusive of the upper paraesophageal lymph nodes, were included in the CTV. For lower esophageal squamous cell carcinomas, lymph node basins superiorly from the subcarinal region and inferiorly to the left gastric and common hepatic arteries/celiac lymph nodal basins were generally included [16]. The planning target volume was defined as CTV with a 0.5–0.8 cm expansion margin. Initial fields were treated at a dose of 45 Gy, and an additional 5.4–14.4 Gy was delivered to the reduced fields with an approximate 2‐cm margin encompassing the gross disease. If surgery was feasible, patients stopped the 45 Gy CCRT and underwent surgery 6–8 weeks later.
Chemotherapy
Concurrent chemotherapy consisted of platinum‐based regimens based on our hospital guidelines (cisplatin, 75 mg/m2 3‐hour drip on day 1, and 5‐fluorouracil [5‐FU], 1,000 mg/m2 continuous infusion on days 1–4, repeated every 3 weeks; cisplatin, 35 mg/m2 2‐hour drip on day 1, and leucovorin, 50 mg/m2 30‐minute drip on day 1, and 5‐fluorouracil, 500 mg/m2 30‐minute drip on day 1, repeated every week; cisplatin, 35 mg/m2 2‐hour drip on day 1, repeated every week; or paclitaxel, 80 mg/m2 1‐hour drip on day 1, and cisplatin, 30 mg/m2 2‐hour drip on day 2, repeated every week). Cisplatin could be replaced by carboplatin based on clinical judgment.
Statistical Analysis
Descriptive statistics were used to analyze the patients’ demographic characteristics. Continuous variables were analyzed using Mann‐Whitney U tests. Fisher's exact tests were used to compare categorical variables and to estimate relative risk. Overall survival was defined as the amount of time from the date of cancer diagnosis to the date of death or last follow‐up. The Kaplan‐Meier method was used to estimate overall survival, and log‐rank tests were used to determine significance. We also used univariate and multivariate Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for fistula formation and overall survival. Significance was taken at p < .05 for all tests. Statistical analysis was performed using SPSS software (Version 20.0; IBM Corporation, Armonk, NY). A post hoc power analysis was calculated by G*power 3.1.9.2. The R2 value was calculated by logistic regression with SEMS and covariates of age, pre‐CCRT body weight, tumor location, tumor length, clinical T stage, regional lymph node metastasis, and RT dose.
Results
Patient Characteristics
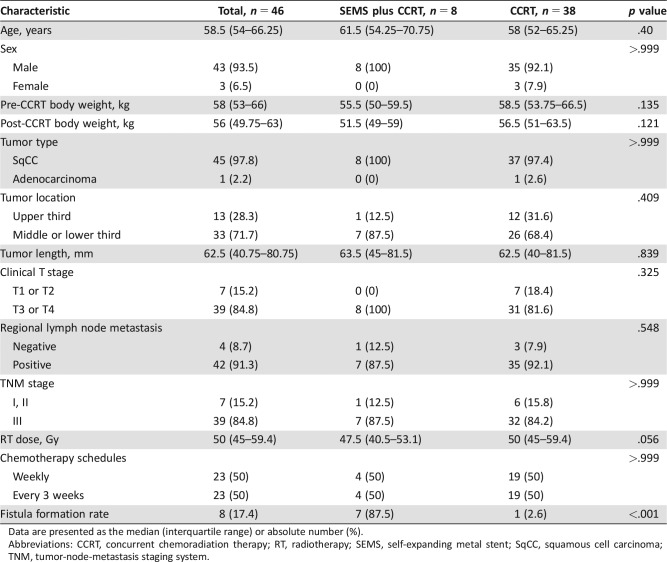
A total of 46 patients were identified; there were 8 patients in the SEMS plus CCRT group and 38 patients who received CCRT without SEMS (13 patients underwent surgery after CCRT). The characteristics of the 46 patients are shown in Table 1, and there were no significant differences between groups. The median age was 58.5 (interquartile range [IQR], 54–66.25) years, and 93.5% of the patients were men. Pre‐CCRT versus post‐CCRT median body weight for the SEMS plus CCRT group and the CCRT group were 55.5 kg versus 51.5 kg and 58.5 kg vs. 56.5 kg, respectively. Only one patient had adenocarcinoma; the other patients were diagnosed with esophageal squamous cell carcinoma. A tumor located in the upper third was found in 28.3% of patients, and 71.7% of patients had a tumor in the middle‐ or lower third of the esophagus. The median tumor length was 62.5 (IQR, 40.75–80.75) mm. T3‐ or T4‐stage cancer was found in 84.8% of patients, and regional lymph node metastasis was found in 91.3% of patients. The characteristics of the patients who underwent CCRT with or without SEMS placement from 2007 to 2016 are listed in supplemental online Table 1. The esophageal fistula formation rates of patients who received CCRT without SEMS during the periods of 2007–2013 and 2014–2016 were 3.7% and 2.6%, respectively.
Table 1. Characteristics of patients who underwent CCRT with or without SEMS placement.
Data are presented as the median (interquartile range) or absolute number (%).
Abbreviations: CCRT, concurrent chemoradiation therapy; RT, radiotherapy; SEMS, self‐expanding metal stent; SqCC, squamous cell carcinoma; TNM, tumor‐node‐metastasis staging system.
Locally Advanced Esophageal Cancer Patients Treated by SEMS plus CCRT with Higher Risk of Esophageal Fistula Formation
Among the 46 patients, esophageal fistula was identified in 8 patients (supplemental online Table 2). Most of the tumors in these patients were located in the middle‐ or lower third of the esophagus (87.5%), and only one tumor was located in the upper third of the esophagus (12.5%). Thus, 87.5% of patients were diagnosed with T3 disease and had lymph node metastasis, and only one patient was diagnosed with T4 disease. The completed radiation dose in the SEMS plus CCRT group was significantly lower than that in the definitive CCRT group (n = 25; median, 47.5 Gy vs. 59.4 Gy, respectively; p = .006). Additionally, there was a trend toward a lower completed radiation dose in the SEMS plus CCRT group than in the CCRT with or without surgery group (n = 38; median, 47.5 Gy vs. 50 Gy, respectively; p = .056). The risk of esophageal fistula formation was higher in the SEMS plus CCRT group than in the CCRT group (87.5% vs. 2.6%, relative risk, 259; p < .001, power = 0.873). The median time to develop esophageal fistula was 3.5 months in the SEMS plus CCRT group, and only one patient developed esophageal fistula formation by 5.5 months in the CCRT group (HR, 72.30; 95% CI, 8.62–606.19; p < .001).
Locally Advanced Esophageal Cancer Patients Treated by SEMS plus CCRT Causing Worse Overall Survival
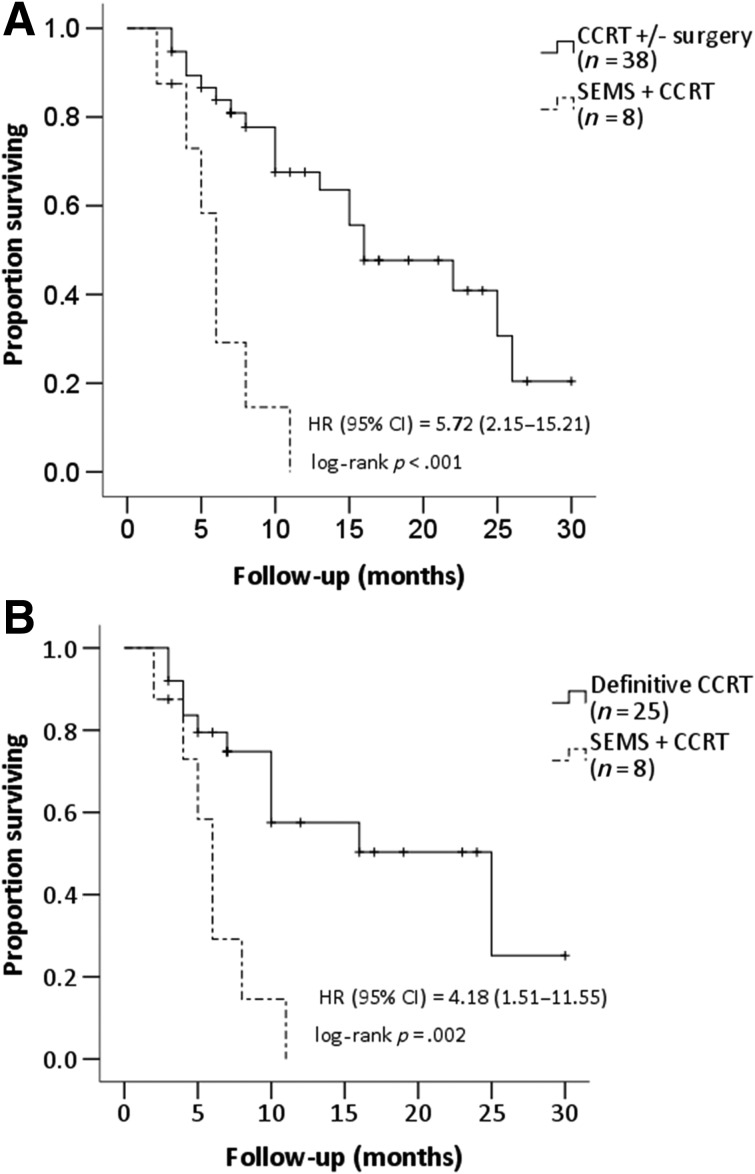
In the CCRT group, 13 patients received a scheduled esophagectomy following CCRT, and 25 patients received definitive CCRT without surgery. No patients in the SEMS plus CCRT group underwent a curative surgery after CCRT. The median overall survival times for the SEMS plus CCRT group and the CCRT group were 6 and 16 months, respectively (HR, 5.72; 95% CI, 2.15–15.21; p < .001; Fig. 2A). Additionally, compared with the definitive CCRT patients (n = 25), those who underwent SEMS concomitant with CCRT suffered from lower overall survival (HR, 4.18; 95% CI, 1.51–11.55; p = .002; Fig. 2B).
Figure 2.
Overall survival among patients with esophageal cancer who underwent SEMS placement followed by CCRT. (A): Patients who received CCRT with or without surgery. (B): Patients who received definitive CCRT without surgery.
Abbreviations: CCRT, concurrent chemoradiation therapy; CI, confidence interval; HR, hazard ratio; SEMS, self‐expandable metal stent.
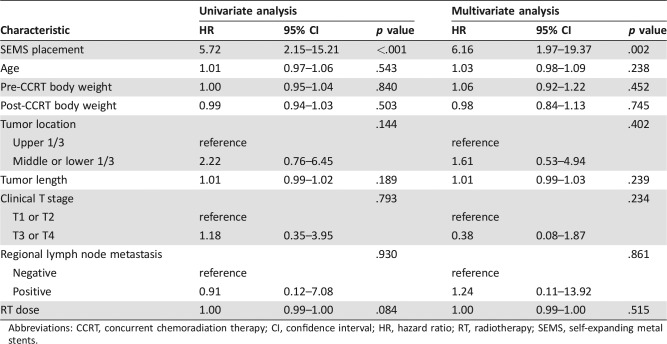
The multivariate Cox proportional hazards model revealed that overall survival was independently associated with SEMS placement (HR, 6.16; 95% CI, 1.97–19.37; p = .002). The age, body weight, tumor location, tumor length, clinical T stage, regional lymph node metastasis, and radiation dose were not related to overall survival in the current study (Table 2).
Table 2. Univariate and multivariate analyses of hazard ratios for overall survival.
Abbreviations: CCRT, concurrent chemoradiation therapy; CI, confidence interval; HR, hazard ratio; RT, radiotherapy; SEMS, self‐expanding metal stents.
Discussion
Esophageal fistula formation or perforation is a life‐threatening adverse event for patients who undergo SEMS placement or SEMS with CCRT. The fistula formation and/or perforation rates for esophageal cancer patients who receive SEMS placement alone or CCRT alone were less than 5.5% and 17.7%, respectively (Table 3). The rate of these life‐threatening adverse events for patients with SEMS placement was 1%–5.5%. Christie et al. reported 100 patients (93% of patients had esophageal cancer) who underwent SEMS placement for dysphagia, and the perforation rate was 1% [17]. In a retrospective analysis of 90 esophageal cancer patients, of whom 68% had adenocarcinoma and 32% had squamous cell carcinoma, the perforation rate for SEMS intubation was 1.1% [18]. In a prospective study in which 231 patients prospectively underwent SEMS placement for middle‐ and lower third esophageal cancer, the perforation rate was 1.3% [19]. In a randomized prospective comparison of self‐expandable plastic stents (SEPS) and SEMS for malignant esophageal dysphagia, the perforation rate was 1.8% for SEMS and 2.1% for SEPS, and the fistula formation rate was 3.7% for SEMS and 2.1% for SEPS [20]. In the current study, no patient suffered perforation or fistula formation within 30 days of SEMS placement.
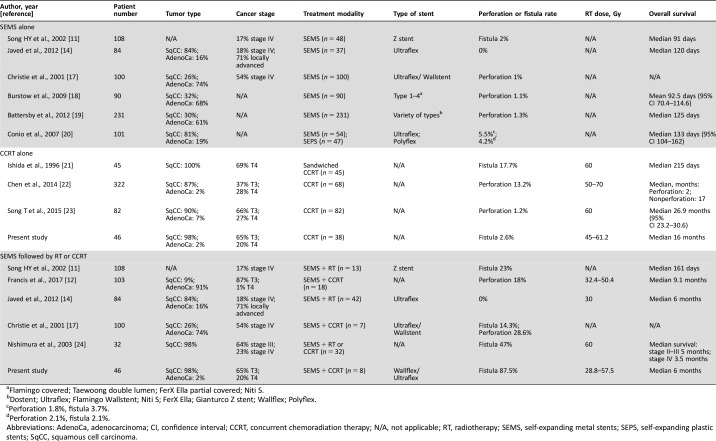
Table 3. Selected publications on esophageal fistula formation or perforation in patients with esophageal cancer treated by stent placement alone, CCRT alone, or stent placement concomitant with CCRT.
Flamingo covered; Taewoong double lumen; FerX Ella partial covered; Niti S.
Dostent; Ultraflex; Flamingo Wallstent; Niti S; FerX Ella; Gianturco Z stent; Wallflex; Polyflex.
Perforation 1.8%, fistula 3.7%.
Perforation 2.1%, fistula 2.1%.
Abbreviations: AdenoCa, adenocarcinoma; CI, confidence interval; CCRT, concurrent chemoradiation therapy; N/A, not applicable; RT, radiotherapy; SEMS, self‐expanding metal stents; SEPS, self‐expanding plastic stents; SqCC, squamous cell carcinoma.
The rate of fistula formation among patients with advanced esophageal squamous cell carcinoma treated by sandwiched cisplatin and 5‐fluorouracil with 60 Gy radiotherapy was 17.7% [21]. Chen et al. retrospectively analyzed 68 esophageal carcinoma patients treated by CCRT with radiation doses of 50–70 Gy, and the perforation rate was 13.2% during or after CCRT [22]. For elderly esophageal cancer patients receiving paclitaxel plus cisplatin concurrent with 60 Gy radiotherapy, the perforation rate was 1.2% [23]. In the current study, the esophageal fistula formation rates of patients who received CCRT without SEMS from 2007 to 2013 and from 2014 to 2016 were 3.7% and 2.6%, respectively (supplemental online Table 1). Altogether, the fistula formation and/or perforation rate for patients who receive platinum‐based chemotherapy concurrent with radiotherapy (45–70 Gy) is 1.2%–17.7%.
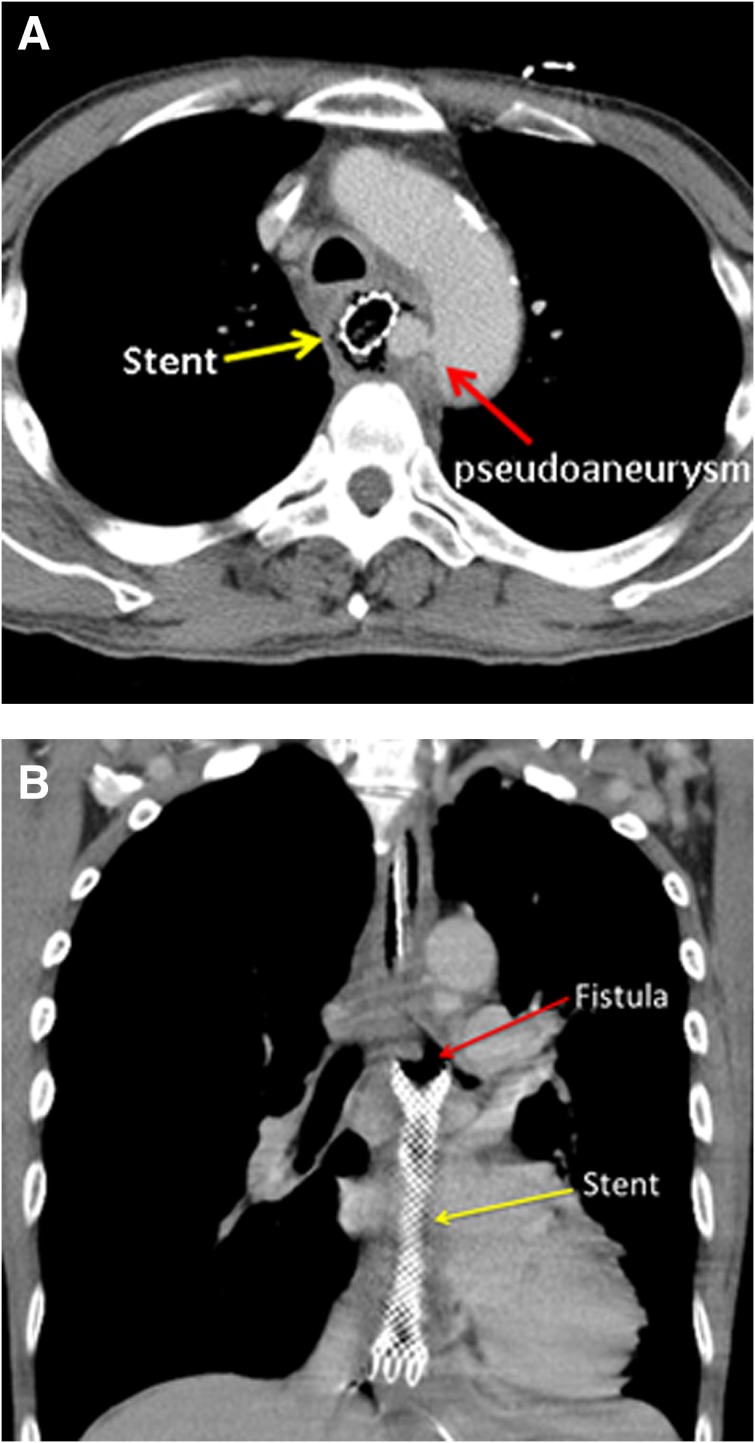
However, in a Japanese study that utilized a questionnaire to analyze 32 esophageal cancer patients who received esophageal stent implantation concurrent with radiotherapy (median 60 Gy) or CCRT due to esophageal stenosis, 47% of patients experienced hematemesis requiring transfusion and/or esophageal fistula formation, and 22% of patients died of hematemesis or esophageal fistula [24]. Christie et al. reported that 28.6% of patients who underwent SEMS placement followed by CCRT developed esophageal perforation, and 14.3% of patients experienced fistula formation [17]. Another study reported 23% esophageal fistula formation in patients who received SEMS placement followed by radiotherapy, whereas only 2% of patients who underwent SEMS placement alone suffered from fistula formation [11]. A recent retrospective study showed an 18% esophageal perforation rate and a 6% hematemesis rate in patients with adenocarcinoma who received SEMS placement during curative CCRT (RT dose of 32.4–50.4 Gy) [12]. In contrast, in a randomized trial of 84 inoperable esophageal cancer patients who underwent SEMS placement with or without palliative radiotherapy (30 Gy), no cases of esophageal fistula formation or perforation were observed [14]. Of the patients treated with SEMS followed by CCRT in the current study, 87.5% of patients developed esophageal fistula during or after CCRT, whereas only 2.6% of patients developed esophageal fistula in the CCRT group. Among the patients with esophageal fistula in the SEMS plus CCRT group, all patients had T3 disease, except one patient who had T4 disease, which was diagnosed according to suspected tracheal invasion by endoscopic ultrasound. However, the patient with T4 disease developed aortic pseudoaneurysm and aortoesophageal fistula, but not tracheoesophageal fistula, after receiving combined modality therapy (Fig. 3A). Additionally, from 2007 to 2016 at our institution, the median time to develop esophageal fistula was 3.5 months in the SEMS plus CCRT group, which was shorter than that in the CCRT alone group (median 5.5 months, supplemental online Table 2). Emami et al. reported a 5% risk of severe adverse events (stricture or perforation) within 5 years after 55 Gy and 60 Gy irradiation of the entire esophagus and one third of the esophagus, respectively [25]. Nevertheless, patients treated by SEMS plus CCRT suffered from fistula at a lower radiation dose (median 50 Gy) than patients who received CCRT alone (59.4 Gy) in the current study. These data reveal that patients treated with SEMS concomitant with CCRT have a higher risk of developing tracheoesophageal fistula or aortoesophageal fistula at a lower irradiation dose and within a shorter interval than those treated with CCRT alone.
Figure 3.
Esophageal fistula formation in patients treated by concurrent chemoradiation therapy with self‐expandable metal stents. (A): The patient with T4 disease developed aortic pseudoaneurysm and aortoesophageal fistula, but not tracheoesophageal fistula, after receiving combined modality therapy. (B): The left main bronchus‐esophageal fistula is located at the subcarinal level at the proximal portion of the esophageal stent.
The survival benefits of SEMS placement followed by radiotherapy in esophageal cancer are controversial. A survival advantage of combined SEMS and radiotherapy was observed compared with SEMS placement alone [11], [14]. The European Society of Gastrointestinal Endoscopy clinical guidelines released in 2016 do not recommend the concurrent use of palliative external radiotherapy and esophageal stent treatment [6]. In a case‐control study on SEMS placement as a bridge to surgery in esophageal cancer, 59% of patients received neoadjuvant chemotherapy or 45 Gy CCRT. The SEMS group was associated with a lower 3‐year overall survival than the control group (25% vs. 44%; p = .023) [26]. Francis et al. also reported that patients who received curative CCRT with stents had a worse median overall survival than patients who did not receive stents (9.1 months vs. 16.8 months; p = .026) [12]. In the current study, the median overall survival was shorter in the SEMS plus CCRT group than in the CCRT group (6 months vs. 16 months; p < .001; Fig. 2A, 2B). Furthermore, esophageal stenting was an independent predictive factor correlated with overall survival, as confirmed by the multivariate Cox proportional hazards model for esophageal cancer patients receiving CCRT (HR, 6.16; 95% CI, 1.97–19.37; p = .002).
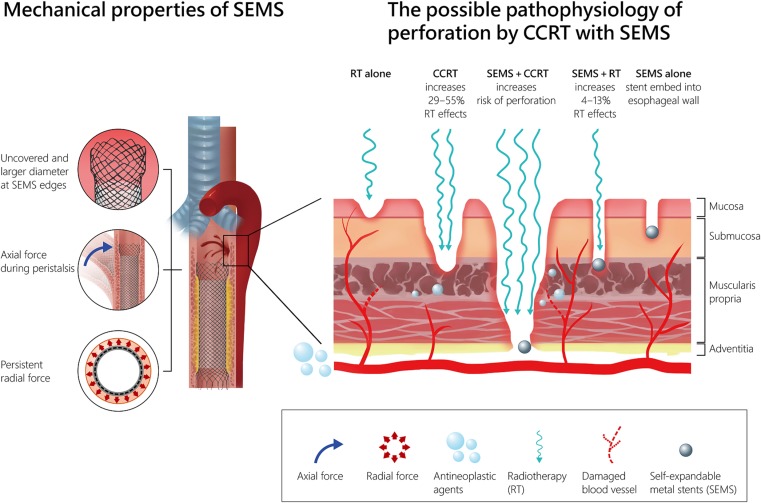
There are multiple factors that may contribute to esophageal wall injury in the setting of SEMS concomitant with CCRT (Fig. 4). The type of esophageal SEMS may play an important role. Wallflex fully covered, Wallflex partially covered, and biodegradable Ella‐BD stents have high axial force and low radial force, whereas Ultraflex and Alimaxx‐ES stents have low axial force and high radial force [27]. The axial force is the force that maintains the stent straight and restricts local bending. The radial force is the force that resists pressure from the esophageal wall to maintain sufficient luminal patency in an esophageal stricture and prevent stent migration. The Wallflex stent, with high axial force that makes it less flexible and more difficult to bend, was used in 87.5% of patients in the current study. Stiff stents are more likely to rub on the esophageal wall, causing friction and pressure ulcerations during esophageal peristalsis. Additionally, uncovered sharp ends of SEMS may cause esophageal wall injury, and larger diameters at the proximal and distal ends may also increase the radial force that exerts pressure on the esophageal wall during esophageal peristalsis, which can result in a deep pressure ulcer and chronic inflammatory reaction [28]. Bick et al. observed that 82% (18/22) of stent‐associated esophagorespiratory fistulas developed at the proximal margin of stents, and all patients who received larger proximal diameter stents (27–28 mm) suffered from fistula formation at the proximal edge of the stent [29]. In the current study, 57.1% of patients developed esophageal fistula at the proximal end of the stent (Fig. 3B), and 75% of these patients were treated with a stent with a larger proximal flare diameter (28 mm).
Figure 4.
The possible mechanism of fistula formation in patients with esophageal cancer treated by SEMS placement concomitant with CCRT.
Abbreviations: CCRT, concurrent chemoradiation therapy; RT, radiotherapy; SEMS, self‐expandable metal stent.
Both radiotherapy and chemotherapy may contribute to the vascular and stromal damage of the esophagus. Irradiation increases the expression of endothelial intercellular adhesion molecule 1, causing polymorphonuclear cell adhesion, increasing apoptosis in microvascular endothelial cells, and damaging the internal elastic lamina of vessels [30], [31]. Platinum‐based chemotherapy also increases the expression of intercellular adhesion molecule 1 in endothelial cells and is involved in the pathophysiological process of cisplatin‐induced vascular toxicity [32]. Moreover, the dose of irradiation was enhanced by platinum‐based chemotherapy with a ratio of 1.29 to 1.55 in an in vitro study [33].
Dose perturbations due to the presence of a metallic esophageal stent during the treatment of esophageal cancer using external beam radiation therapy result in an overdose in the esophageal wall. For the Wallflex esophageal stent combined with radiation, the dose enhancement can range from 4% to 13%, whereas the dose perturbation is negligible for polymer‐based stents and approaches 0% for biodegradable stents [34]. Our study demonstrates that in patients who developed esophageal fistula during or after CCRT, the median radiation doses for patients with SEMS and without SEMS were 50 Gy and 59.4 Gy, respectively. The radiation dose to cause esophageal fistula in the SEMS plus CCRT group is obviously lower than the tolerance dose in patients treated with CCRT alone. In another retrospective analysis of esophageal cancer patients treated with curative CCRT, no patients experienced esophageal perforation in the SEPS group, but 18% of patients experienced esophageal perforation in the SEMS group [12]. This difference can be partially explained by the radiation dose enhancement effect of combined SEMS with CCRT.
The CROSS (Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study) trial recently reported an improved overall survival rate in patients who underwent neoadjuvant CCRT with a carboplatin/paclitaxel‐based regimen and observed esophageal perforation in 1% of patients [7]. Interestingly, Blom et al. reported that patients with esophageal cancer treated with a cisplatin and 5‐FU regimen or a carboplatin/paclitaxel regimen exhibited no differences in 3‐year overall survival rate (p = .725) or between grades 3 and 4 of esophagitis (p = .920) [35]. A randomized trial revealed that no patients developed esophageal fistula or perforation when treated with SEMS and radiotherapy 30 Gy [14]. However, up to 18% of patients developed esophageal fistula after receiving esophageal stent placement plus neoadjuvant CCRT with 32.4–50.4 Gy [12], [36]. In the present study, 37.5% of patients still experienced esophageal fistula under CCRT (cisplatin and 5‐FU regimen) with less than 45 Gy. Therefore, the safety of stenting prior to CCRT in patients who are planned to undergo the curative intent CROSS regimen followed by surgery remains unclear. Nevertheless, by critically evaluating these previously published observations, it is apparent that patients treated with SEMS plus CCRT with a cisplatin‐based regimen have a higher risk of esophageal fistula formation at a lower irradiation dose.
There are some limitations to the present study. First, the sample size of patients who received SEMS before or during CCRT was small, making any statistical conclusions very tentative. Nevertheless, we found a significant association between SEMS placement and esophageal fistula formation. Second, SEMS placement was the only prognostic factor determined as significant by univariate and multivariate analyses in the current study; however, some unobserved covariates might have contributed to the higher fistula formation rate and the worse overall survival. The patients enrolled in the current study were not part of a prospective protocol, such as for performance status, cancer staging, tumor location, or CCRT regimen, leading to potential bias. Patients who were treated with a stent had a higher percentage of distal esophagus tumors (87.5% vs. 68.4%) and T3/T4 lesions (100% vs. 81.6%), and both factors were associated with fistula/perforation, which may cause selection bias and disfavor the SEMS plus CCRT group compared with the CCRT‐only group. Additionally, higher median age and lower pretreatment weight were noted in the SEMS plus CCRT group, which may also contribute to a higher risk of poor prognosis. Third, many types of commercial esophageal stents with a variety of materials and mechanical properties are available; therefore, our study results may not be able to be extrapolated to predict the clinical outcomes of patients who receive other types of esophageal stents combined with CCRT. Further prospective and randomized studies of SEMS combined with curative CCRT are warranted to determine the contribution that SEMS have on the risk of perforation/fistula and survival after controlling for known prognostic variables.
Conclusion
For esophageal cancer patients who are scheduled to receive CCRT, caution should be taken when adopting SEMS concurrent with CCRT. Patients treated with SEMS concomitant with CCRT have a higher risk of tracheoesophageal fistula or aortoesophageal fistula formation at a lower irradiation dose and within a shorter time interval than patients treated with CCRT alone. Additionally, the combination of SEMS and CCRT was associated with a significant decrease in overall survival among patients with locally advanced esophageal carcinoma.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Yueh‐Feng Lu, Chen‐Hsi Hsieh
Provision of study material or patients: Pei‐Wei Shueng, Chen‐Xiong Hsu, Deng‐Yu Kuo, Pei‐Yu Hou, Hsiu‐Ling Chou, Ka‐I Leong, Chen‐Hsi Hsieh
Collection and/or assembly of data: Yueh‐Feng Lu, Chen‐Hsi Hsieh
Data analysis and interpretation: Yueh‐Feng Lu, San‐Fang Chou, Chen‐Hsi Hsieh
Manuscript writing: Yueh‐Feng Lu, Chen‐Shuan Chung, Chao‐Yu Liu, Le‐Jung Wu, Li‐Ying Wang, Chen‐Hsi Hsieh
Final approval of manuscript: Yueh‐Feng Lu, Chen‐Shuan Chung, Chao‐Yu Liu, Pei‐Wei Shueng, Le‐Jung Wu, Chen‐Xiong Hsu, Deng‐Yu Kuo, Pei‐Yu Hou, Hsiu‐Ling Chou, Ka‐I Leong, Cheng‐Hung How, San‐Fang Chou, Li‐Ying Wang, Chen‐Hsi Hsieh
Disclosures
The authors indicated no financial relationships.
References
- 1. Weigel TL, Frumiento C, Gaumintz E. Endoluminal palliation for dysphagia secondary to esophageal carcinoma. Surg Clin North Am 2002;82:747–761. [DOI] [PubMed] [Google Scholar]
- 2. Allum WH, Griffin SM, Watson A et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2002;50(suppl 5):v1–v23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqui AA, Sarkar A, Beltz S et al. Placement of fully covered self‐expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51. [DOI] [PubMed] [Google Scholar]
- 4. Lopes TL, Eloubeidi MA. A pilot study of fully covered self‐expandable metal stents prior to neoadjuvant therapy for locally advanced esophageal cancer. Dis Esophagus 2010;23:309–315. [DOI] [PubMed] [Google Scholar]
- 5. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: A systematic review and meta‐analysis. J Gastrointest Oncol 2014;5:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spaander MC, Baron TH, Siersema PD et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:939–948. [DOI] [PubMed] [Google Scholar]
- 7. Van Hagen P, Hulshof MC, Van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 8. Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–1627. [DOI] [PubMed] [Google Scholar]
- 9. Hou PY, Teng CJ, Chung CS et al. Aortic pseudoaneurysm formation following concurrent chemoradiotherapy and metallic stent insertion in a patient with esophageal cancer. Medicine (Baltimore) 2015;94:e862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lecleire S, Di Fiore F, Ben‐Soussan E et al. Prior chemoradiotherapy is associated with a higher life‐threatening complication rate after palliative insertion of metal stents in patients with oesophageal cancer. Aliment Pharmacol Ther 2006;23:1693–1702. [DOI] [PubMed] [Google Scholar]
- 11. Song HY, Lee DH, Seo TS et al. Retrievable covered nitinol stents: Experiences in 108 patients with malignant esophageal strictures. J Vasc Interv Radiol 2002;13:285–293. [DOI] [PubMed] [Google Scholar]
- 12. Francis SR, Orton A, Thorpe C et al. Toxicity and outcomes in patients with and without esophageal stents in locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 2017;99:884–894. [DOI] [PubMed] [Google Scholar]
- 13. Sgourakis G, Gockel I, Radtke A et al. The use of self‐expanding stents in esophageal and gastroesophageal junction cancer palliation: A meta‐analysis and meta‐regression analysis of outcomes. Dig Dis Sci 2010;55:3018–3030. [DOI] [PubMed] [Google Scholar]
- 14. Javed A, Pal S, Dash NR et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: A randomized trial. J Gastrointest Cancer 2012;43:63–69. [DOI] [PubMed] [Google Scholar]
- 15. Backer CL, LoCicero J, Hartz RS et al. Computed tomography in patients with esophageal perforation. Chest 1990;98:1078–1080. [DOI] [PubMed] [Google Scholar]
- 16. Hazard L, Yang G, McAleer MF et al. Principles and techniques of radiation therapy for esophageal and gastroesophageal junction cancers. J Natl Compr Canc Netw 2008;6:870–878. [DOI] [PubMed] [Google Scholar]
- 17. Christie NA, Buenaventura PO, Fernando HC et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: Short‐term and long‐term follow‐up. Ann Thorac Surg 2001;71:1797–1801. [DOI] [PubMed] [Google Scholar]
- 18. Burstow M, Kelly T, Panchani S et al. Outcome of palliative esophageal stenting for malignant dysphagia: A retrospective analysis. Dis Esophagus 2009;22:519–525. [DOI] [PubMed] [Google Scholar]
- 19. Battersby NJ, Bonney GK, Subar D et al. Outcomes following oesophageal stent insertion for palliation of malignant strictures: A large single centre series. J Surg Oncol 2012;105:60–65. [DOI] [PubMed] [Google Scholar]
- 20. Conio M, Repici A, Battaglia G et al. A randomized prospective comparison of self‐expandable plastic stents and partially covered self‐expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol 2007;102:2667–2677. [DOI] [PubMed] [Google Scholar]
- 21. Ishida K, Iizuka T, Ando N et al. Phase II study of chemoradiotherapy for advanced squamous cell carcinoma of the thoracic esophagus: Nine Japanese institutions trial. Jpn J Clin Oncol 1996;26:310–315. [DOI] [PubMed] [Google Scholar]
- 22. Chen H, Ma XM, Ye M et al. Esophageal perforation during or after conformal radiotherapy for esophageal carcinoma. J Radiat Res 2014;55:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song T, Zhang X, Fang M et al. Concurrent chemoradiotherapy using paclitaxel plus cisplatin in the treatment of elderly patients with esophageal cancer. Onco Targets Ther 2015;8:3087–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishimura Y, Nagata K, Katano S et al. Severe complications in advanced esophageal cancer treated with radiotherapy after intubation of esophageal stents: A questionnaire survey of the Japanese society for esophageal diseases. Int J Radiat Oncol Biol Phys 2003;56:1327–1332. [DOI] [PubMed] [Google Scholar]
- 25. Emami B, Lyman J, Brown A et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 26. Mariette C, Gronnier C, Duhamel A et al. Self‐expanding covered metallic stent as a bridge to surgery in esophageal cancer: Impact on oncologic outcomes. J Am Coll Surg 2015;220:287–296. [DOI] [PubMed] [Google Scholar]
- 27. Hirdes M, Vleggaar F, de Beule M et al. In vitro evaluation of the radial and axial force of self‐expanding esophageal stents. Endoscopy 2013;45:997–1005. [DOI] [PubMed] [Google Scholar]
- 28. Song H, Kim JH, Shin JH et al. A dual‐design expandable colorectal stent for malignant colorectal obstruction: Results of a multicenter study. Endoscopy 2007;39:448–454. [DOI] [PubMed] [Google Scholar]
- 29. Bick BL, Song LM, Buttar NS et al. Stent‐associated esophagorespiratory fistulas: Incidence and risk factors. Gastrointest Endosc 2013;77:181–189. [DOI] [PubMed] [Google Scholar]
- 30. Cervelli T, Panetta D, Navarra T et al. Effects of single and fractionated low‐dose irradiation on vascular endothelial cells. Atherosclerosis 2014;235:510–518. [DOI] [PubMed] [Google Scholar]
- 31. Lindsay S, Kohn HI, Dakin RL et al. Aortic arteriosclerosis in the dog after localized aortic x‐irradiation. Circ Res 1962;10:51–60. [DOI] [PubMed] [Google Scholar]
- 32. Yu M, Han J, Cui P et al. Cisplatin up‐regulates ICAM‐1 expression in endothelial cell via a NF‐kappaB dependent pathway. Cancer Sci 2008;99:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amorino GP, Hamilton VM, Choy H. Enhancement of radiation effects by combined docetaxel and carboplatin treatment in vitro. Radiat Oncol Investig 1999;7:343–352. [DOI] [PubMed] [Google Scholar]
- 34. Abu Dayyeh BK, Vandamme JJ, Miller RC et al. Esophageal self‐expandable stent material and mesh grid density are the major determining factors of external beam radiation dose perturbation: Results from a phantom model. Endoscopy 2013;45:42–47. [DOI] [PubMed] [Google Scholar]
- 35. Blom RL, Sosef MN, Nap M et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus 2014;27:380–387. [DOI] [PubMed] [Google Scholar]
- 36. Jones CM, Griffiths EA. Should oesophageal stents be used before neo‐adjuvant therapy to treat dysphagia in patients awaiting oesophagectomy? Best evidence topic (BET). Int J Surg 2014;12:1172–1180. [DOI] [PubMed] [Google Scholar]