Central nervous system metastasis in non‐small cell lung cancer is a therapeutic challenge. This article reports the case of a patient with lung adenocarcinoma, parenchymal brain metastases, and leptomeningeal carcinomatosis successfully treated with immune checkpoint inhibition and stereotactic radiosurgery.
Abstract
Central nervous system metastasis in non‐small cell lung cancer remains a therapeutic challenge and confers a poor prognosis. Here we describe a patient with lung adenocarcinoma, parenchymal brain metastases, and leptomeningeal carcinomatosis who demonstrated a sustained response to programmed death 1 inhibition combined with stereotactic radiosurgery.
Case Report
A 72‐year‐old woman with a 10 pack‐year smoking history presented with chest pain. Chest computed tomography and positron emission tomography scans revealed multiple bilateral pulmonary nodules and metastatic disease in the right adrenal gland and left sixth rib. Brain magnetic resonance imaging (MRI) demonstrated a solitary right frontal lobe metastasis.
Biopsy of a lung nodule revealed poorly differentiated carcinoma, favoring adenocarcinoma. Programmed death‐ligand 1 (PD‐L1) expression by a semiquantitative immunohistochemistry assay (Pathline, Kingston, NY) was “high positive” (>25% distribution and minimum 1+ or 2+ staining intensity). Molecular analysis was negative for epidermal growth factor receptor (EGFR), BRAF, and KRAS mutations and for ROS1 and ALK translocations. She received stereotactic radiosurgery (SRS) to the solitary brain metastasis, then four cycles of carboplatin and pemetrexed with partial response followed by maintenance pemetrexed for seven cycles.
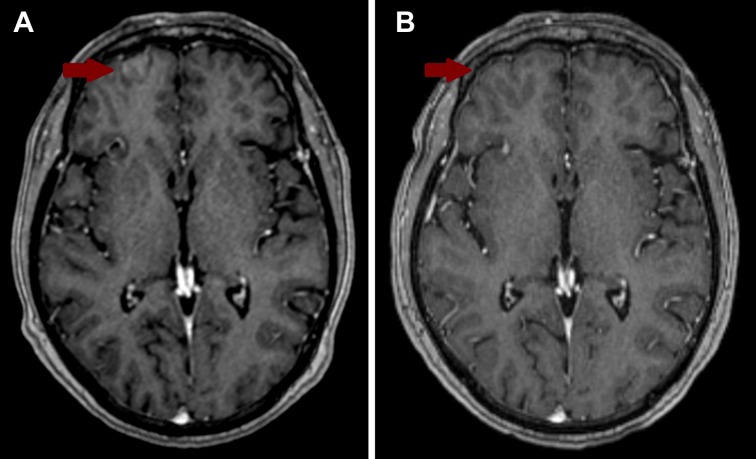
A subsequent brain MRI revealed new focal curvilinear enhancement along the gyri of the right anterior frontal lobe outside of the original radiation field concerning for leptomeningeal carcinomatosis (LC) (Fig. 1A). Cerebrospinal fluid (CSF) analysis showed white blood cells of 1 cells/μL with 71% lymphocytes and 14% neutrophils, 150 red blood cells/μL, glucose 51 mg/dL, and protein 53 mg/dL. Cytology confirmed metastatic adenocarcinoma after pathology consensus conference review. MRI of the cervical, thoracic, and lumbar spine showed no other bulky LC.
Figure 1.
Comparison brain magnetic resonance imaging (MRI) scans. (A) Pre‐treatment image showing a focal area of leptomeningeal enhancement in the right anterior frontal lobe. (B) Post‐treatment image taken 19 months after leptomeningeal carcinomatosis was diagnosed and treated.
Given her high PD‐L1 expression, single focus of leptomeningeal involvement, and asymptomatic disease, SRS combined with anti‐programmed death 1 (PD‐1) therapy was recommended by her multidisciplinary team over whole‐brain radiation therapy (WBRT).
She received SRS to the leptomeningeal focus and three other parenchymal lesions seen on her radiosurgery MRI. She was started on anti‐PD‐1 therapy with pembrolizumab 1 week later. Post‐treatment brain MRI showed significant decrease in size of all four brain lesions and no evidence of LC (Fig. 1B). The patient declined repeat CSF analyses. After 29 cycles (20 months) of pembrolizumab, neuroimaging showed no evidence of LC, resolution of three brain lesions, and significant reduction of the dominant right frontal lobe lesion. She remained radiographically stable and neurologically intact throughout this course.
Discussion
LC occurs in 3.8% of cases of non‐small cell lung cancer (NSCLC), more commonly in lung adenocarcinoma [1]. It is more common in ALK‐rearranged and EGFR‐mutant lung adenocarcinoma, occurring in up to 5% and 9% of cases, respectively [2].
Prognosis is poor for NSCLC patients with LC, with median overall survival between 3.6 and 11 months due to lack of prospectively validated and effective therapies [1], [2]. Response assessment remains challenging, as validated response criteria are still in development. Systemic chemotherapy and intrathecal chemotherapy show improvements in survival of weeks to months across histologies [3]. WBRT is considered the standard of care; however, it has significant cognitive toxicity. As a result, SRS has increasingly replaced WBRT for treatment of parenchymal brain metastases [3], [4].
Immune checkpoint inhibitors have shown promising results in treating solid brain metastases in melanoma and NSCLC, but data are limited in LC. A phase II trial of melanoma and NSCLC patients (PD‐L1 >1% in NSCLC patients) with brain metastases treated with pembrolizumab showed CNS response rates of 22% and 33%, respectively [5]. CheckMate 204 showed an intracranial response rate of 56%, including 19% complete responses with the combination of nivolumab and ipilimumab in patients with melanoma brain metastases, regardless of PD‐L1 expression [6]. Importantly, patients with LC were excluded from these trials.
There is some biologic rationale for efficacy of these agents in LC. It is likely that activated T cells can cross the blood‐brain barrier (BBB) to have antitumor effect in LC [7]. However, the tight junctions between ependymal cells in the choroid plexus may be less permeable to T cells or anti‐PD‐1 monoclonal antibodies reaching the leptomeninges and CSF [8]. The tumor microenvironment can augment BBB permeability to immune cells and therapeutic agents by increasing the composition of desmin+ pericyte subpopulations around the tumor [9].
The presence of functional lymphatics that contain T lymphocytes and dendritic cells in the dural sinuses has altered the “immune privileged” paradigm of the CNS. These lymphatics can drain CSF and carry T cells and antigen‐presenting cells from the CNS into the deep cervical lymph nodes [7]. It is biologically plausible that these T cells can be affected by checkpoint inhibition. Data are awaited from an ongoing phase II trial using pembrolizumab to treat LC in patients with advanced solid tumors (ClinicalTrials.gov Identifier: NCT03091478).
There are only a few case reports and series of immune checkpoint inhibitors for treatment of LC. In one series of five NSCLC patients with CNS metastases treated with nivolumab, two had LC. One patient had four brain metastases treated with SRS 2 months prior to developing local nodular‐type LC. He was started on nivolumab and achieved a durable partial response for 7 months. The second patient achieved stable disease for 10 weeks [10]. Another case report described a patient presenting with auditory hallucinations secondary to LC who achieved a CNS partial response and resolution of auditory hallucinations for 7 months with nivolumab [2]. In all these cases, PD‐L1 expression levels were unknown.
Otsubo et al. recently described a patient with lung adenocarcinoma metastatic to the brain and adrenal glands who developed LC while on pembrolizumab. PD‐L1 expression was 100%, and pembrolizumab was used as upfront therapy with marked systemic and CNS responses initially. Despite a sustained response over six cycles, new LC was detected by neuroimaging and confirmed by CSF sampling. Pembrolizumab was stopped, and the patient was treated with WBRT. Although this report is noteworthy, it represents an isolated case.
Conclusion
To our knowledge, this case demonstrates the longest duration of leptomeningeal response to immune checkpoint inhibition without intrathecal chemotherapy or WBRT. Although follow‐up CSF analysis could not be done (patient declined), the survival of this patient for 20 months after diagnosis of leptomeningeal carcinomatosis with completely normal neurologic function argues against persistence of malignant cells in the CSF and is likely due to anti‐PD‐1 therapy. Anti‐PD‐1 therapy may produce durable responses with limited toxicity for other patients with LC, and results from ongoing studies are eagerly awaited.
Disclosures
The authors indicated no financial relationships.
References
- 1.Umemura S, Tsubouchi K, Yoshioka H et al. Clinical outcome in patients with leptomeningeal metastasis from non‐small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer 2012;77:134–139. [DOI] [PubMed] [Google Scholar]
- 2.Gion M, Remon J, Caramella C et al. Symptomatic leptomeningeal metastasis improvement with nivolumab in advanced non‐small cell lung cancer patient. Lung Cancer 2017;108:72–74. [DOI] [PubMed] [Google Scholar]
- 3.Remon J, Le Rhun E,Besse B. Leptomeningeal carcinomatosis in non‐small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128–137. [DOI] [PubMed] [Google Scholar]
- 4.Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg SB, Gettinger SN, Mahajan A et al. Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: Early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016;17:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawbi HAH, Forsyth PAJ, Algazi AP et al. Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: Results of the phase II study CheckMate 204. JClin Oncol 2017;35(suppl 15):9507a. [Google Scholar]
- 7.Louveau A, Smirnov I, Keyes TJ et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsubo K, Seki N, Nakanishi Y et al. Development of leptomeningeal carcinomatosis during a marked response of brain metastases to pembrolizumab in a patient with non‐small cell lung cancer. Ann Oncol 2018;29:780–781. [DOI] [PubMed] [Google Scholar]
- 9.Lyle LT, Lockman PR, Adkins CE et al. Alterations in pericyte subpopulations are associated with elevated blood–tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res 2016;22:5287–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudnik E, Yust‐Katz S, Nechushtan H et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114–117. [DOI] [PubMed] [Google Scholar]