In the United States, more children die from cancer than from any other disease, and many die in the hospital setting. This article reports how patient‐specific variables might impact location of death for pediatric palliative oncology patients.
Keywords: Pediatric oncology, Palliative care, Palliative oncology, End of life, Location of death
Abstract
Background.
In the U.S., more children die from cancer than from any other disease, and more than one third die in the hospital setting. These data have been replicated even in subpopulations of children with cancer enrolled on a palliative care service. Children with cancer who die in high‐acuity inpatient settings often experience suffering at the end of life, with increased psychosocial morbidities seen in their bereaved parents. Strategies to preemptively identify children with cancer who are more likely to die in high‐acuity inpatient settings have not been explored.
Materials and Methods.
A standardized tool was used to gather demographic, disease, treatment, and end‐of‐life variables for 321 pediatric palliative oncology (PPO) patients treated at an academic pediatric cancer center who died between 2011 and 2015. Multinomial logistic regression was used to predict patient subgroups at increased risk for pediatric intensive care unit (PICU) death.
Results.
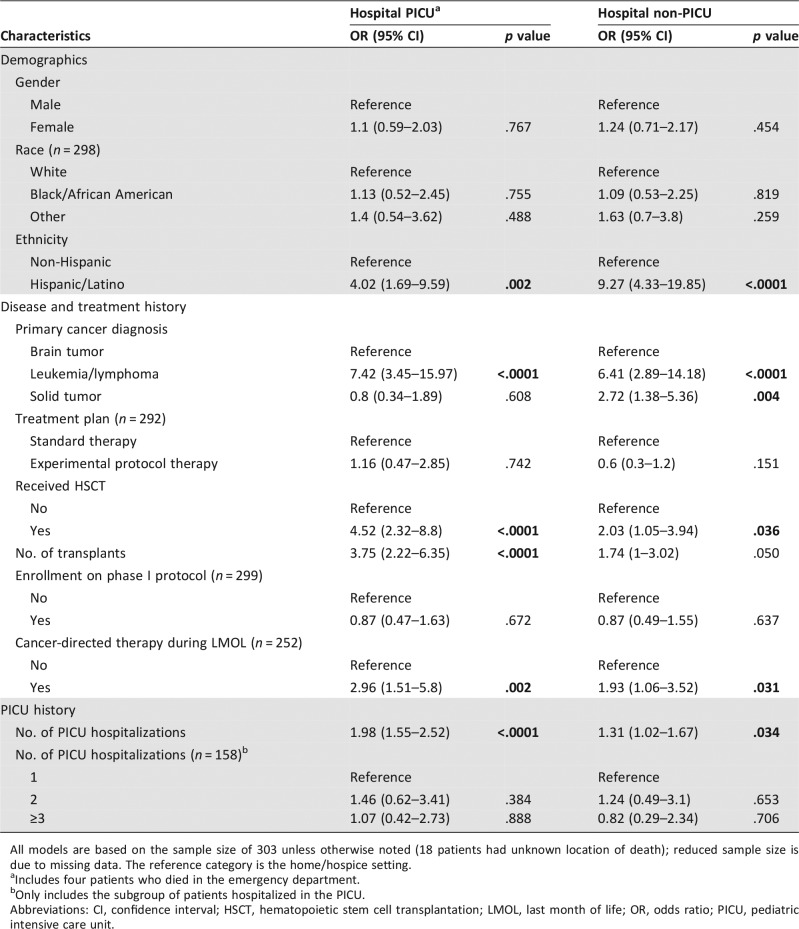
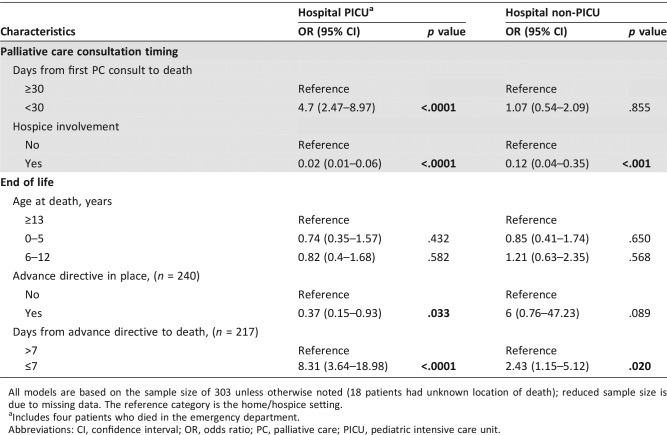
Higher odds of dying in the PICU were found in patients with Hispanic ethnicity (odds ratio [OR], 4.02; p = .002), hematologic malignancy (OR, 7.42; p < .0001), history of hematopoietic stem cell transplant (OR, 4.52; p < .0001), total number of PICU hospitalizations (OR, 1.98; p < .0001), receipt of cancer‐directed therapy during the last month of life (OR, 2.96; p = .002), and palliative care involvement occurring less than 30 days before death (OR, 4.7; p < .0001). Conversely, lower odds of dying in the PICU were found in patients with hospice involvement (OR, 0.02; p < .0001) and documentation of advance directives at the time of death (OR, 0.37; p = .033).
Conclusion.
Certain variables may predict PICU death for PPO patients, including delayed palliative care involvement. Preemptive identification of patients at risk for PICU death affords opportunities to study the effects of earlier palliative care integration and increased discussions around preferred location of death on end‐of‐life outcomes for children with cancer and their families.
Implications for Practice.
Children with cancer who die in high‐acuity inpatient settings often experience a high burden of intensive therapy at the end of life. Strategies to identify patients at higher risk of dying in the pediatric intensive care unit (PICU) have not been explored previously. This study finds that certain variables may predict PICU death for pediatric palliative oncology patients, including delayed palliative care involvement. Preemptive identification of patients at risk for PICU death affords opportunities to study the effects of earlier palliative care integration and increased discussions around preferred location of death on end‐of‐life outcomes for children with cancer and their families.
Introduction
In the U.S., more children die from cancer than from any other disease [1]. These patients suffer significantly throughout their illness trajectory and particularly at the end of life [2], [3]. Integration of palliative care (PC) into the management of children with high‐risk cancer can alleviate physical and emotional symptoms for patients and enhance quality of life for children and families [4], [5]. However, even those children with cancer who receive palliative care services still experience a high burden of intensive therapy and interventions [4], [6].
Approximately one third to one half of children with cancer who do not survive their illness die in the hospital setting [7], [8]. Recently these findings have been replicated in subpopulations of children with cancer enrolled on a palliative care service, in which more than one third of patients died in the hospital and, of those inpatient deaths, nearly half occurred in the pediatric intensive care unit (PICU) [6]. For certain children with cancer, the PICU may offer the best possible setting for a “good death” to occur [9], [10]; however, goal concordance between actual and preferred location of death for patients and families is poorly understood, and significant concerns remain regarding the potential adverse ramifications of PICU death on patient suffering at the end of life (EOL) and bereaved caregiver outcomes.
Recent literature has raised concerns that children with cancer who die in high‐acuity inpatient settings may experience increased suffering at the end of life [4]. Moreover, bereaved parents of children with cancer who die in the inpatient setting experience increased psychosocial morbidities, including depression, anxiety, stress, and complicated grief, compared with bereaved parents of children who die outside of the hospital [11], [12]. Given the potential for adverse sequelae related to PICU death in the context of pediatric palliative oncology (PPO), preemptive identification of those children with cancer enrolled on a palliative care service who are at higher risk for dying in high‐acuity inpatient settings might be helpful to promote earlier discussions around goals of care and preferred location of death prior to the advent of catastrophic illness or disease progression.
Strategies to identify children with cancer who have increased risk of dying in high‐acuity inpatient settings have not been explored previously. At present, little is known about the demographic, disease, treatment, or EOL attributes that may predict location of death for children with cancer. To address this deficit, we conducted a retrospective cohort study of deceased children with cancer enrolled on a PC service at a large academic pediatric cancer center over a 4‐year period. Based on the authors’ collective clinical experience and supplemental review of the medical oncology literature, we hypothesized a priori that pediatric patients with hematologic malignancy, history of hematopoietic stem cell transplant, and late palliative care involvement would have higher odds of dying in the PICU; however, we also explored associations between location of death and other salient demographic, disease, and treatment indicators to look for potential predictors that might preemptively identify subgroups of patients at higher risk for dying in the PICU.
To our knowledge, this is the first study to investigate how patient‐specific variables might affect location of death for PPO patients, with the goal of preemptively identifying subgroups of patients at higher risk for dying in the PICU. Ultimately, we hope that these findings will inform the development and investigation of interventions targeted towards at‐risk subgroups, with the long‐term objective of optimizing the provision of goal concordant care for children with cancer and their families at the EOL.
Materials and Methods
Study Design
This study was conducted at St. Jude Children's Research Hospital (SJCRH). The SJCRH Institutional Review Board reviewed and exempted the study in the context of retrospective analysis of a deceased cohort.
Extensive review of institutional records was conducted to obtain a comprehensive list of all patients with a primary cancer diagnosis who were enrolled on the PC service at SJCRH at the time of their death and whose death occurred between April 1, 2011, and March 31, 2015. This 4‐year window was selected primarily because institution‐wide data became available in the electronic medical record on April 1, 2011, allowing for more thorough and accurate data abstraction, and secondarily because the frequency of requested PC consultations remained stable across this period.
Predicated on review of available data, the cohort was limited to deceased patients with cancer who received PC prior to their death as evidenced by documentation of at least one note in the medical record confirming PC or hospice involvement. For the purpose of this study, hospice involvement was defined as patient enrollment in any community‐based agency that facilitated the provision of hospice services and resources to the patient in the home. In each case, referral to the hospice agency was coordinated by the institutional PC team, with the goal of contracting with any agency able to provide the necessary services in the patient's home location. The decision to focus on a PPO cohort was founded on the fact that 93.0% of patients who died during the study window received some degree of PC; thus, deceased PPO patients served as a relative surrogate for all patients with cancer who died during the 4‐year study window. Given the very small number of patients who died without PC involvement at this institution, it was not statistically feasible to draw meaningful comparisons between patients who died with and without PC involvement.
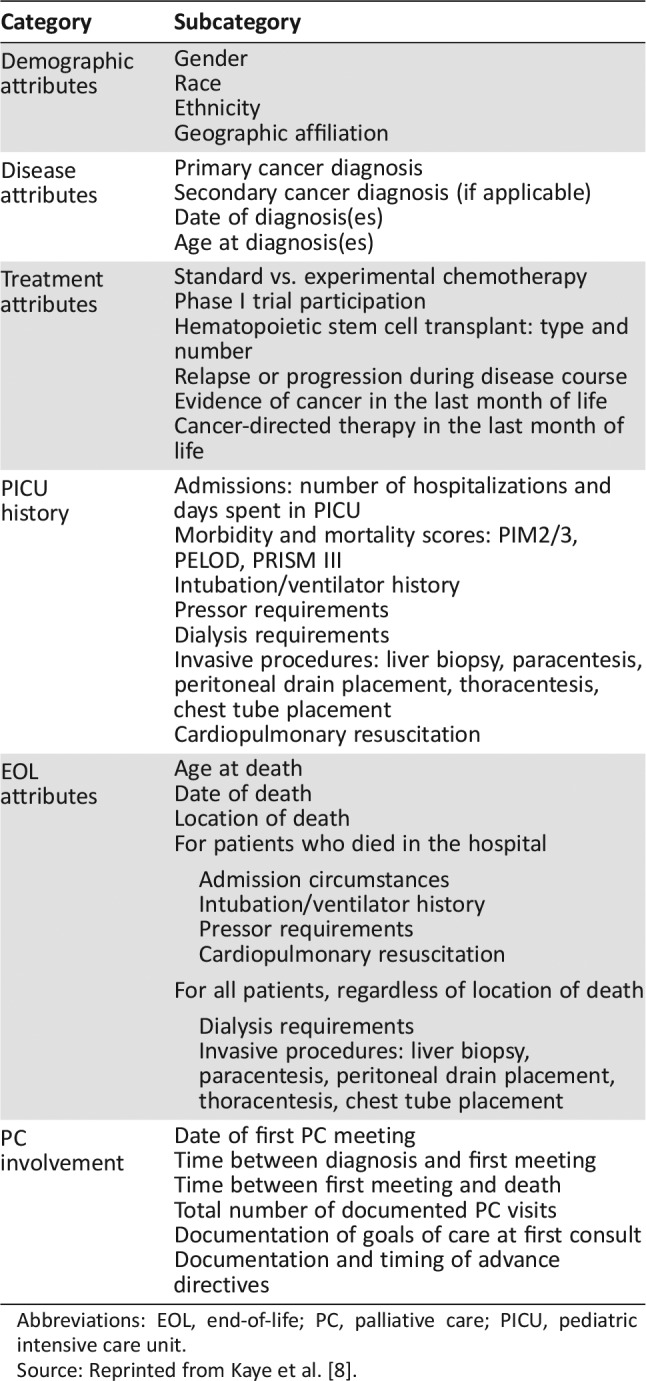
A comprehensive, standardized data abstraction tool was developed by PPO clinicians and researchers (E.C.K., J.N.B.) after a thorough review of the literature [13], [14], [15], [16], [17], [18], [19], [20]; the systematic development of this tool has been previously described [6]. The final standardized abstraction form encompassed 67,308 data cells, with the items targeted for abstraction summarized in Table 1.
Table 1. Pediatric palliative oncology deceased cohort data abstraction items.
Abbreviations: EOL, end‐of‐life; PC, palliative care; PICU, pediatric intensive care unit.
Source: Reprinted from Kaye et al. [8].
The rigorous data extraction and auditing processes have also been described previously [6]. Briefly, six researchers (E.C.K., S.D., C.A.G., J.J., L.J.B., L.‐M.J.) independently abstracted data from the electronic medical record using a standardized tool. Secondary review of paper charts was performed to supplement electronic medical record review. Any questions raised by a team member were discussed in groups of two to four researchers to clarify the abstraction tool and achieve consensus.
Random sampling was performed to obtain a subset of cases to conduct a 10% audit of the entire database; for items relevant only to select subsets of patients, random sampling was altered accordingly to ensure capture of 10% of the subset for auditing. An acceptable threshold for inter‐rater reliability was established a priori at 0.85. All database items met this threshold; however, two items had inter‐rater reliability scores at the lower limit of 0.85. Excluding the cases screened in the prior audit, a subsequent 15% audit was performed on these items, establishing inter‐rater reliability scores of 0.90 for these data.
Statistical Analysis
Demographic, disease, treatment, PC, and EOL characteristics of PPO patients were summarized using descriptive statistics. Multinomial logistic regression was used to determine whether demographic, disease, treatment, PC, and EOL related characteristics of PPO patients were associated with location of death (i.e., PICU, hospital non‐PICU, and home/hospice facility). The effect of categorized number of PICU hospitalizations (0, 1, and ≥2) on location of death could not be computed reliably within the multinomial logistic regression model. Therefore, the number of PICU hospitalizations was used as a continuous variable in the model. Likewise, a small number of patients had more than one transplant; therefore, the number of transplants was used as a continuous variable rather than a categorized variable in the model. Additionally, Fisher's exact test was used to assess the association between categorized number of PICU hospitalizations and location of death. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). A two‐sided significance level of p < .05 was used for all statistical tests.
Results
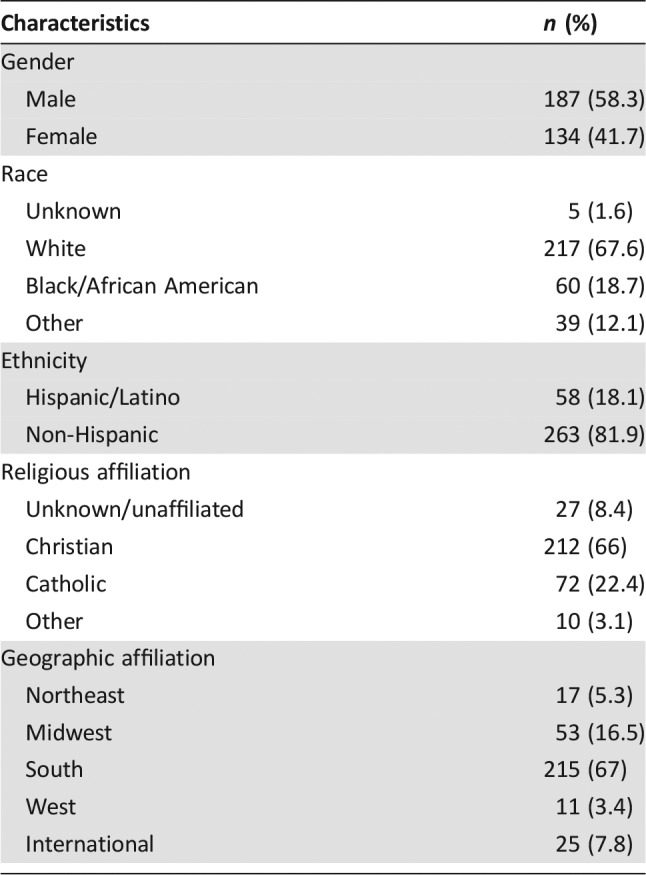
A total of 321 PPO patients died between April 2011 and March 2015 at this large academic cancer center. Demographic characteristics of these patients are summarized in Table 2. Attributes related to disease, treatment, complications, timing of palliative care consultation, and end‐of‐life care for this cohort have been previously described [6].
Table 2. Pediatric palliative oncology patient demographics (n = 321).
The results of multinomial logistic regression models exploring the relationships between PPO patient, disease, treatment, timing of PC involvement, and EOL characteristics and location of death are shown in Tables 3 and 4. Gender, race, treatment plan, enrollment on a phase I protocol, number of PC clinical notes, and age at death were not significantly associated with location of death. Ethnicity, primary cancer diagnosis, history of hematopoietic stem cell transplant, total number of transplants, total number of PICU hospitalizations, receipt of cancer‐directed therapy during the last month of life, time from initial PC consultation to death, hospice involvement, status of advance directives at time of death, and timing between documentation of advance directives and death were all significantly associated with location of death.
Table 3. Multinomial logistic regression modeling of location of death as predicted by demographic, disease, and treatment characteristics.
All models are based on the sample size of 303 unless otherwise noted (18 patients had unknown location of death); reduced sample size is due to missing data. The reference category is the home/hospice setting.
Includes four patients who died in the emergency department.
Only includes the subgroup of patients hospitalized in the PICU.
Abbreviations: CI, confidence interval; HSCT, hematopoietic stem cell transplantation; LMOL, last month of life; OR, odds ratio; PICU, pediatric intensive care unit.
Table 4. Multinomial logistic regression modeling of location of death as predicted by timing of palliative care involvement and end‐of‐life characteristics.
All models are based on the sample size of 303 unless otherwise noted (18 patients had unknown location of death); reduced sample size is due to missing data. The reference category is the home/hospice setting.
Includes four patients who died in the emergency department.
Abbreviations: CI, confidence interval; OR, odds ratio; PC, palliative care; PICU, pediatric intensive care unit.
Hispanic patients had higher odds of dying in the PICU (odds ratio [OR], 4.02; 95% confidence interval [CI], 1.69–9.59; p = .002) and in the hospital ward (OR, 9.27; 95% CI, 4.33–19.85; p < .0001) over the home/hospice setting, compared with non‐Hispanic patients. Patients with leukemia or lymphoma had higher odds of dying in the PICU (OR, 7.42; 95% CI, 3.45–15.97; p < .0001) and in the hospital ward (OR, 6.41; 95% CI, 2.89–14.18; p < .0001) over the home/hospice setting, compared with patients with a primary brain tumor diagnosis. Patients with solid tumors also had higher odds of dying in the hospital ward over the home/hospice setting compared with patients with brain tumors (OR, 2.72; 95% CI, 1.38–5.36; p = .004).
Compared with patients who did not receive a transplant, patients who received at least one transplant had higher odds of dying in the PICU (OR, 4.52; 95% CI, 2.32–8.8; p < .0001) and in the hospital ward (OR, 2.03; 95% CI, 1.05–3.94; p = .036) over the home/hospice setting. A one‐unit increase in the number of transplants received was associated with higher odds of dying in the PICU over the home/hospice setting (OR, 3.75; 95% CI, 2.22–6.35; p < .0001).
Of those patients without any history of PICU hospitalizations, only two died in the PICU. A one unit increase in the number of PICU hospitalizations was associated with higher odds of dying in the PICU (OR, 1.98; 95% CI, 1.55–2.52; p < .0001) and in the hospital ward (OR, 1.31; 95% CI, 1.02–1.67; p = .034) over the home/hospice setting. Patients with one or two or more PICU hospitalizations were more likely to die in the PICU compared with patients who had no PICU hospitalizations (p < .0001).
Patients who received cancer‐directed therapy during the last month of life had higher odds of dying in the PICU (OR, 2.96; 95% CI, 1.51–5.8; p = .002) and in the hospital ward (OR, 1.93; 95% CI, 1.06–3.52; p = .031) over the home/hospice setting, compared with patients who did not receive cancer‐directed therapy during the last month of life. Patients with PC involvement occurring less than 30 days before death had higher odds of dying in the PICU over the home/hospice setting, compared with those with PC involvement occurring 30 days or more before death (OR, 4.7; 95% CI, 2.47–8.97; p < .0001). Compared with patients without hospice involvement, patients with hospice involvement had lower odds of dying in the PICU (OR, 0.02; 95% CI, 0.01–0.06; p < .0001) and in the hospital ward (OR, 0.12; 95% CI, 0.04–0.35; p < .001) over the home/hospice setting.
Lastly, patients with documentation of an advance directive at the time of death had lower odds of dying in the PICU over the home/hospice setting compared with those who did not have an advance directive in place at the time of death (OR, 0.37; 95% CI, 0.15–0.93; p = .033). Compared with patients with an advance directive in place more than 7 days before death, patients with an advance directive in place 7 days or less before death had higher odds of dying in the PICU (OR, 8.31; 95% CI, 3.64–18.98; p < .0001) and in the hospital ward (OR, 2.43; 95% CI, 1.15–5.12; p = .02) over the home/hospice setting.
Discussion
Approximately one in five children with cancer will die [1], and approximately one third to one half of these patients die in the hospital, with a substantial minority dying in an intensive care setting [7], [8]. Even patients enrolled on a palliative care service continue to receive a high burden of intensive therapy and interventions over the course of their illness trajectory, and they often die in inpatient intensive care settings [4], [21]. The location of death for PPO patients has been previously described, with slightly more than half of patients dying at home, a small minority dying in a stand‐alone hospice facility, and more than one third of patients dying in the hospital. Out of those PPO patients who died in the hospital, nearly half were found to die in the PICU [21]. Little is known about which subtypes of PPO patients are most at risk for dying in an intensive care setting. This is the first study to investigate how variables related to patient, disease, treatment, timing of PC involvement, and EOL experiences might predict location of death for this uniquely vulnerable patient population.
Multinomial logistic regression analysis revealed a number of attributes that were not observed to be significantly associated with location of death for PPO patients. Lack of association between phase I trial enrollment and location of death in PPO patients in this study is one such notable negative finding. Clinicians may presume that a patient's (or family's) preference for experimental therapy indicates an immutable desire to pursue intensive therapy until the final breath. These data challenge this assumption, instead demonstrating that patients who enroll on a phase I trial are no more likely to die in high‐acuity settings compared with patients who receive standard therapy. This negative finding corroborates earlier data showing negligible effect of phase I participation on EOL attributes [22], and it suggests that PPO patient enrollment on phase I trials does not preclude subsequent evolution of goals of care. Additionally, we theorize that enrollment on phase I trials for children with cancer may be due less to inherent characteristics or goals of patients and families and due more to trial availability; however, this hypothesis requires further investigation.
Perhaps most compelling among the negative findings is race, which previously has been correlated with increased receipt of aggressive interventions and intensive care use at the EOL in adult patients with cancer [23], [24]. A previous study conducted at this institution 8 years ago likewise showed that race did not affect EOL care planning or resuscitation status for children with cancer, but that study did not query potential disparities related to location of death [25]. To our knowledge, only two other studies have investigated the effect of race on location of death specifically in pediatric patients with cancer, in which black children, adolescents, and young adults were more likely to die in the hospital [26], [27]. The lack of racial disparities identified in the two studies out of this institution may speak to broad financial interventions that level the socioeconomic playing field for all comers at this cancer center; however, further research is needed to explore this hypothesis and better describe racial disparities around EOL care for PPO patients and their families.
In contrast, Hispanic ethnicity significantly increased the odds of PICU death within this large PPO patient population. Ethnic disparities involving location of death for children with cancer have been suggested by two prior studies, in which Hispanic patients were more likely to die in the hospital setting compared with non‐Hispanic patients [26], [27]. Our findings suggest that these disparities may extend across the PPO population as well. However, the extent to which ethnicity truly contributes to location of death and, by extension, receipt of more aggressive interventions at the end of life, remains unknown. In one study, Hispanic pediatric oncology patients enrolled on hospice significantly more often than patients of other races; despite this initial hospice enrollment, however, more than one third of Hispanic patients had withdrawn from hospice by the time of death, and ultimately neither race nor ethnicity were significantly associated with dying on hospice [28]. Given the limited and contradictory body of literature, one cannot yet extrapolate with certainty that Hispanic children with cancer are more likely to die in the PICU. However, the possibility of Hispanic ethnicity as a possible predictor for more aggressive EOL care in PPO patients is potentially concerning and warrants further investigation.
A diagnosis of hematologic malignancy, history and number of transplants, and receipt of cancer‐directed therapy during the last month of life all increased a PPO patient's odds of dying in the PICU. These data corroborate prior findings in the literature, in which hematologic malignancy and history of prior transplant have been associated with greater likelihood of dying in the hospital setting [7], [8], [29]. We hypothesize that the latter two attributes may serve as surrogates for hematologic malignancy, as pediatric patients with hematologic malignancies have been shown to be more likely to undergo transplant and to receive cancer‐directed therapy during the last month of life [4]. Regardless, these findings highlight a particularly vulnerable subset of the PPO population at risk for dying in the PICU, for whom further prospective investigation is needed.
It is important to recognize that cancer‐directed therapy may be provided with palliative intent with the goal of controlling symptoms of end‐stage disease, particularly in the context of children with hematologic malignancies. Recent data indicate that children with cancer who receive “mild” cancer‐directed therapy during the final 12 weeks of life may even experience improved psychological outcomes [30], suggesting that provision of certain disease‐directed therapies may play an important role in mitigating suffering and improving quality of life for certain subsets of patients. Given that patients with hematologic malignancy are more likely to die in high‐acuity settings, it is imperative that clinicians remain vigilant about optimizing symptoms, minimizing interventions that lack clear benefit, and striving to enhance quality of life for these highly vulnerable patients whenever possible. Guidelines for the optimal provision of EOL care for hospitalized children with cancer have been previously published [31].
Increased history of PICU hospitalizations likewise increased a PPO patient's odds of dying in the PICU. To our knowledge, this is the first time this salient finding has been described in the literature. These data are particularly striking because of their immediate relevance to clinical practice, affording pediatric oncologists, intensivists, and palliative care clinicians the opportunity to preemptively identify PPO patients at risk for receiving intensive interventions at the EOL. In the authors’ collective experience, critically ill patients hospitalized in the PICU at the EOL infrequently have the opportunity to shift care locations in order to die in alternative settings. Thus, it is imperative that clinicians initiate advance care planning conversations, including discussion about preferred location of death, significantly prior to the EOL period to allow patients and families a chance to make meaningful and feasible choices about actual location of death. Based on this finding, we advocate that PPO patients with multiple prior PICU hospitalizations receive transparent prognostic information about possible increased risk of PICU death, using this conversation to explore their goals and wishes for EOL care and preferred location of death well ahead of the EOL period.
Conversely, early PC consultation, hospice involvement, and advance directives were all significantly associated with decreased risk of dying in the PICU. Those patients who had documentation of desired resuscitation status occurring a week or less prior to death also had higher odds of dying in the PICU compared with those with earlier advance directive documentation. Although not necessarily causal, these findings suggest that earlier integration of PC principles and resources in the care of children with cancer might affect location of death for this highly vulnerable patient population. However, the fact that early PC referral is a predictor for location of death invariably begs the question: which factors predict early PC referral? Prior subanalysis of these data suggests that hematologic malignancy, cancer‐directed therapy at the end of life, and delayed advance directives documentation are associated with delayed PC involvement in children who died with cancer [32]. Nonetheless, further investigation is needed to better elucidate predictors of delayed PC involvement in this patient population.
Recent studies in the adult population with cancer suggest that delayed referral to palliative care may diminish opportunities to improve EOL care [33], with early PC involvement significantly decreasing the extent of hospitalization during the last 3 months of life and reducing the number of patients who died in the hospital setting [34]. Yet what remains unclear in both adult and pediatric populations with cancer is whether these data reflect goal‐concordant care. Additional research is needed to investigate whether PPO interventions that promote earlier integration of PC and hospice services for children with cancer improve the provision of goal‐concordant care at the EOL, with the ultimate goals of improving quality of life for patients and mitigating decisional regret and complicated grief for bereaved families.
This study has a number of limitations. First, it represents the experience of a single large academic cancer center that provides treatment to patients from across the country and internationally; as such, a potential selection bias exists for higher‐risk patients and families seeking more aggressive therapies. However, the fact that enrollment on a phase I trial was not observed to be associated with location of death for this cohort of patients suggests that those patients and families referred to this cancer center to enroll on an experimental protocol were not, in fact, necessarily seeking more aggressive therapy. Second, retrospective data abstraction is inherently flawed in the context of imperfect documentation. Although incomplete or missing data were minimal in this study, we cannot be certain that missing data occurred randomly. Third, the retrospective cohort design limits our ability to make conclusions about chronology or causality. Rather, this study identifies associations that warrant future study through further prospective investigation. Despite these limitations, this study offers the first retrospective investigation of how patient‐, illness‐, and treatment‐specific variables might affect location of death for children who die from cancer and sets the stage for future work in this important and understudied area.
Conclusion
In the PPO population, neither race nor enrollment on a phase I trial were observed to be associated with location of death. The strongest predictor of dying in the PICU was delayed palliative care involvement, and the strongest protector against PICU death was hospice involvement. Identification of attributes potentially predictive of location of death for PPO patients may inform preemptive clinical delineation of subgroups of patients and families at higher risk for suffering at the EOL and adverse psychosocial outcomes during bereavement, respectively. Further investigation is needed to determine if targeted interventions around earlier integration of palliative care with an emphasis on proactive discussions related to goals of care and preferred location of death may improve provision of goal concordant care and EOL outcomes children with cancer and their families.
Acknowledgments
The authors acknowledge Ms. Deborah Gibson, MA, and Ms. Angela Norris, CRA‐RN, for their assistance with obtaining electronic and paper medical records to facilitate the process of data analysis. This work was sponsored in part by ALSAC.
Footnotes
For Further Reading: Andrew M. Romano, Kristine E. Gade, Gradon Nielsen et al. Early Palliative Care Reduces End‐of‐Life Intensive Care Unit (ICU) Use but Not ICU Course in Patients with Advanced Cancer. The Oncologist 2017;22:318–323.
Implications for Practice: Palliative care has shown clear benefit in quality of life and survival in advanced cancer patients, but less is known about its effect on intensive care. This retrospective cohort study at a university hospital showed that in the last 6 months of life, palliative care significantly reduced intensive care unit (ICU) and hospital admissions, reduced deaths in the hospital, and increased hospice enrollment. It did not, however, change patients' experiences within the ICU, such as number of procedures, code status, length of stay, or disposition. The findings further support that palliative care exerts its benefit before, rather than during, the ICU setting.
Author Contributions
Conception/design: Erica C. Kaye, Jennifer M. Snaman, Deena R. Levine, R. Ray Morrison, Justin N. Baker
Provision of study material or patients: Erica C. Kaye, Deena R. Levine, R. Ray Morrison, Justin N. Baker
Collection and/or assembly of data: Erica C. Kaye, Samantha DeMarsh, Courtney A. Gushue, Jonathan Jerkins, Lindsay Blazin, Liza‐Marie Johnson
Data analysis and interpretation: Erica C. Kaye, Samantha DeMarsh, April Sykes, Zhaohua Lu, Justin N. Baker
Manuscript writing: Erica C. Kaye, April Sykes, Zhaohua Lu, Jennifer M. Snaman, Justin N. Baker
Final approval of manuscript: Erica C. Kaye, Samantha DeMarsh, Courtney A. Gushue, Jonathan Jerkins, April Sykes, Zhaohua Lu, Jennifer M. Snaman, Lindsay Blazin, Liza‐Marie Johnson, Deena R. Levine, R. Ray Morrison, Justin N. Baker
Disclosures
The authors indicated no financial relationships.
References
- 1. Ward E, DeSantis C, Robbins A et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe J, Grier HE, Klar N et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000;342:326–333. [DOI] [PubMed] [Google Scholar]
- 3. Wolfe J, Hammel JF, Edwards KE et al. Easing of suffering in children with cancer at the end of life: Is care changing? J Clin Oncol 2008;26:1717–1723. [DOI] [PubMed] [Google Scholar]
- 4. Snaman JM, Kaye EC, Lu JJ et al. Palliative care involvement is associated with less intensive end‐of‐life care in adolescent and young adult oncology patients. J Palliat Med 2017;20:509–516. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt P, Otto M, Hechler T et al. Did increased availability of pediatric palliative care lead to improved palliative care outcomes in children with cancer? J Palliat Med 2013;16:1034–1039. [DOI] [PubMed] [Google Scholar]
- 6. Kaye EC, Gushue CA, DeMarsh S et al. Illness and end‐of‐life experiences of children with cancer who receive palliative care. Pediatr Blood Cancer 2017;65. doi: 10.1002/pbc.26895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradshaw G, Hinds PS, Lensing S et al. Cancer‐related deaths in children and adolescents. J Palliat Med 2005;8:86–95. [DOI] [PubMed] [Google Scholar]
- 8. Klopfenstein KJ, Hutchison C, Clark C et al. Variables influencing end‐of‐life care in children and adolescents with cancer. J Pediatr Hematol Oncol 2001;23:481–486. [DOI] [PubMed] [Google Scholar]
- 9. Hendrickson K, McCorkle R. A dimensional analysis of the concept: Good death of a child with cancer. J Pediatr Oncol Nurs 2008;25:127–138. [DOI] [PubMed] [Google Scholar]
- 10. Ito Y, Okuyama T, Ito Y et al. Good death for children with cancer: a qualitative study. Jpn J Clin Oncol 2015;45:349–355. [DOI] [PubMed] [Google Scholar]
- 11. Jalmsell L, Onelöv E, Steineck G et al. Hematopoietic stem cell transplantation in children with cancer and the risk of long‐term psychological morbidity in the bereaved parents. Bone Marrow Transplant 2011;46:1063–1070. [DOI] [PubMed] [Google Scholar]
- 12. Rosenberg AR, Baker KS, Syrjala K et al. Systematic review of psychosocial morbidities among bereaved parents of children with cancer. Pediatr Blood Cancer 2012;58:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feudtner C, Kang TI, Hexem KR et al. Pediatric palliative care patients: A prospective multicenter cohort study. Pediatrics 2011;127:1094–1101. [DOI] [PubMed] [Google Scholar]
- 14. Feudtner C, DiGiuseppe DL, Neff JM. Hospital care for children and young adults in the last year of life: A population‐based study. BMC Med 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feudtner C, Feinstein JA, Satchell M et al. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA 2007;297:2725–2732. [DOI] [PubMed] [Google Scholar]
- 16. Gemke RJ, van Vught JA. Scoring systems in pediatric intensive care: PRISM III versus PIM. Intensive Care Med 2002;28:204–207. [DOI] [PubMed] [Google Scholar]
- 17. Gonçalves JP, Severo M, Rocha C et al. Performance of PRISM III and PELOD‐2 scores in a pediatric intensive care unit. Eur J Pediatr 2015;174:1305–1310. [DOI] [PubMed] [Google Scholar]
- 18. Heying R, Schneider DT, Körholz D et al. Efficacy and outcome of intensive care in pediatric oncologic patients. Crit Care Med 2001;29:2276–2280. [DOI] [PubMed] [Google Scholar]
- 19. Dursun O, Hazar V, Karasu GT et al. Prognostic factors in pediatric cancer patients admitted to the pediatric intensive care unit. J Pediatr Hematol Oncol 2009;31:481–484. [DOI] [PubMed] [Google Scholar]
- 20. van Veen A, Karstens A, van der Hoek AC et al. The prognosis of oncologic patients in the pediatric intensive care unit. Intensive Care Med 1996;22:237–241. [DOI] [PubMed] [Google Scholar]
- 21. Kaye E, Gushue C, DeMarsh S et al. Pediatric palliative oncology patients: Demographics, treatment, and end‐of‐life experiences of a vulnerable and under‐studied population (TH340D). J Pain Symptom Manage 2018;55:580–581. [Google Scholar]
- 22. Levine DR, Johnson LM, Mandrell BN et al. Does phase 1 trial enrollment preclude quality end‐of‐life care? Phase 1 trial enrollment and end‐of‐life care characteristics in children with cancer. Cancer 2015;121:1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Check DK, Samuel CA, Rosenstein DL et al. Investigation of racial disparities in early supportive medication use and end‐of‐life care among Medicare beneficiaries with stage IV breast cancer. J Clin Oncol 2016;34:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdollah F, Sammon JD, Majumder K et al. Racial Disparities in end‐of‐life care among patients with prostate cancer: A population‐based study. J Natl Compr Canc Netw 2015;13:1131–1138. [DOI] [PubMed] [Google Scholar]
- 25. Baker JN, Rai S, Liu W et al. Race Does not influence do‐not‐resuscitate status or the number or timing of end‐of‐life care discussions at a pediatric oncology referral center. J Palliat Med 2009;12:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cawkwell PB, Gardner SL, Weitzman M. Persistent racial and ethnic differences in location of death for children with cancer. Pediatr Blood Cancer 2015;62:1403–1408. [DOI] [PubMed] [Google Scholar]
- 27. Rajeshuni N, Johnston EE, Saynina O et al. Disparities in location of death of adolescents and young adults with cancer: A longitudinal, population study in California. Cancer 2017;123:4178–4184. [DOI] [PubMed] [Google Scholar]
- 28. Thienprayoon R, Lee SC, Leonard D et al. Racial and ethnic differences in hospice enrollment among children with cancer. Pediatr Blood Cancer 2013;60:1662–1666. [DOI] [PubMed] [Google Scholar]
- 29. Brock KE, Steineck A, Twist CJ. Trends in end‐of‐life care in pediatric hematology, oncology, and stem cell transplant patients. Pediatr Blood Cancer 2016;63:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolfe J, Orellana L, Ullrich C et al. Symptoms and distress in children with advanced cancer: Prospective patient‐reported outcomes from the PediQUEST Study. J Clin Oncol 2015;33:1928–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson LM, Snaman JM, Cupit MC et al. End‐of‐life care for hospitalized children. Pediatr Clin North Am 2014;61:835–854. [DOI] [PubMed] [Google Scholar]
- 32. Kaye EC, Jerkins J, Gushue CA et al. Predictors of late palliative care referral in children with cancer. J Pain Symptom Manage 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennett MI, Ziegler L, Allsop M et al. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open 2016;6:e012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nieder C, Tollåli T, Haukland E et al. Impact of early palliative interventions on the outcomes of care for patients with non‐small cell lung cancer. Support Care Cancer 2016;24:4385–4391. [DOI] [PubMed] [Google Scholar]