This article describes the results of a survey conducted by the Central European Cooperative Oncology Group to assess the availability of and reimbursement for molecular diagnostic tests and targeted therapies in non‐small cell lung cancer across ten countries in central and southeastern Europe.
Keywords: Non‐small cell lung cancer, Molecular alterations, Precision medicine, Reimbursement, Central and Southeastern Europe
Abstract
This article analyzes the availability of different diagnostic procedures of non‐small cell lung cancer (NSCLC) and the reimbursement landscape of drugs for NSCLC in countries of central and southeastern Europe (CEE).
A survey was conducted by the Central European Cooperative Oncology Group. Results of the survey show that both availability and reimbursement of diagnoses of molecular alterations in NSCLC, the detection of which is essential for therapeutic decisions, varies widely between countries of CEE. Not only is “reflex” testing often substituted by analyses performed only “on demand,” but reimbursement of such assessments varies widely between unavailability and payments by the health care system or even pharmaceutical companies. It was concluded that a structured access to testing and reimbursement should be the aim in order to provide patients with appropriate therapeutic options.
Implications for Practice.
This article provides an overview of the limitations in lung cancer treatment in countries of central and southeastern Europe, as well as the reimbursement status of various lung cancer treatment regimens in these countries, which directly impacts treatment options.
Introduction
Lung cancer is the first cause of cancer‐related mortality, with approximately 1.6 million cancer‐related deaths per year worldwide. The diagnosis is usually made when the disease is already advanced, resulting in a low 5‐year combined survival rate of 17% [1]. This number is expected to improve due to the identification of specific oncogenic alterations and development of targeted therapies, particularly in lung adenocarcinoma, as well as by the introduction of promising immune checkpoint inhibitor treatments. Ongoing efforts—summarized under precision medicine projects—aim at stratifying non‐small cell lung cancer (NSCLC) into subtypes according to genetic mutations followed by the administration of effective targeted therapies to guide disease management in clinical practice. Traditional classifications of NSCLC have largely relied on histology and mainly concerned the differentiation between squamous and nonsquamous NSCLC. However, the complexity of molecular and genetic alterations resulted in an abundance of other variables, fragmenting mainly nonsquamous NSCLC into a broad spectrum of individual subgroups.
The milestone that marked the start of precision medicine was the discovery of activating epidermal growth factor receptor (EGFR) mutations in 2004 followed by observations of an associated therapeutic response to EGFR‐targeting tyrosine kinase inhibitors (TKIs) in lung adenocarcinomas [2]. Subsequent analyses have shown that driver mutations in lung adenocarcinoma not only concern EGFR, but also include anaplastic lymphoma kinase (ALK) rearrangements, Kirsten rat sarcoma viral oncogene (KRAS) mutations, BRAF mutations, as well as other less common genetic alterations, which often may serve as therapeutic targets [3], [4].
Precision medicine in NSCLC is therefore characterized by the appropriate selection of specifically targeted treatments directed at detected molecular alterations. The administration of appropriately targeted treatments in advanced disease resulted in clinically significant benefits, with improved safety and quality of life, as compared with untargeted treatment [5]. Thus, a very close interaction regarding diagnosis and subsequent treatment selection between clinicians and pathologists is a clear and self‐explanatory necessity. Pathologists nowadays not only assess morphology, but also molecular characteristics, and constitute key players within a multidisciplinary team of state‐of‐the‐art NSCLC care.
Current guidelines recommend routine molecular testing for EGFR mutations, ALK rearrangements, and BRAF, ROS1, and RET mutations in patients with advanced nonsquamous NSCLC and in squamous NSCLC presenting with atypical clinical features [6], [7]. These guidelines also recommend having molecular testing completed at the time of confirmed clinical diagnosis of advanced disease or in the case of recurrence [7]. This evidence is based upon our current understanding that platinum doublet chemotherapy—being a historic standard of care for systemic treatment of NSCLC—should be replaced by targeted therapy in the presence of specific druggable gene mutations or genomic alterations [8]. These recommendations imply, however, that the pathologist has direct and unhindered access to appropriate testing techniques, which would be reimbursed by the local health care systems in Europe.
Given the recent progress in the field and the emergence of new targeted therapies with impressively successful therapeutic results, the Central European Cooperative Oncology Group (CECOG) has now convened a group of experts in lung molecular pathology as well as lung cancer specialists from central and southeastern Europe (CEE) to discuss the situation regarding access to molecular testing of NSCLC patients in the region. The aim of the present study was to provide an overview of the availability of molecular testing methods, which are associated with evidence‐based efficacy of targeted therapies in NSCLC encompassing 10 CEE countries (CEECs) and reflecting the situation in 2017. Reimbursement conditions of laboratory testing in these European countries is also described.
Materials and Methods
On December 6, 2016, the CECOG Lung Experts Group, consisting of lung pathologists, pulmonologists, and medical oncologists, held a meeting to discuss conditions of access to diagnosis regarding molecular testing and—consequently—to targeted therapies in NSCLC in CEE countries. The represented CEECs consisted of Austria, Bulgaria, Croatia, Czech Republic, Hungary, Poland, Romania, Serbia, Slovakia, and Slovenia.
Given the complexity and variability of molecular testing approaches, it was unanimously decided that the meeting objective should be addressed through a survey administered in two steps: In the first step, information about the molecular diagnosis procedures (testing process, availability of diagnostic testing, and their local reimbursement) was collected and reflected the current situation in each of the represented countries. The results of this survey are presented in the present article. The second step is currently underway and evaluates the access and reimbursement of targeted therapies in NSCLC based on molecular testing described in step 1.
The survey on availability and reimbursement of molecular testing in NSCLC patients in CEE countries was performed by a 31‐item questionnaire, which referred to the identification of the principal five molecular abnormalities representing the currently recognized predictive markers relevant for therapeutic decisions in NSCLC. These included EGFR mutations, ALK rearrangement, ROS1 mutation, BRAF mutation, and programmed death‐ligand 1 (PD‐L1) expression. Finally, access to liquid biopsy techniques and their reimbursement was also investigated.
The survey was distributed among 10 pathologists to collect country‐specific information regarding the status of molecular diagnostics in the 10 involved CEE countries with regard to the number of new cases of NSCLC per year, the histological type of NSCLC where molecular testing was performed, the testing policy (“reflex” vs. “on demand,” parallel vs. subsequent), turnaround times, the laboratory methodology generally used for each genetic test, and, finally, the extent of reimbursement for testing.
Results
All expert pathologists representing 10 countries of CEE participating in the survey answered all questions. A summary of answers is given in the present report.
Incidence of NSCLC in CEE Countries
The incidence of NSCLC per year varies largely, from approximately 2,500 new cases per year in Bulgaria, Croatia, and Slovakia to a maximum of 18,000 new cases per year in Poland (Table 1). Data reported for Slovenia are based on Cancer Registry of Republic of Slovenia. Hungarian data were provided based on data from National Cancer Registry and the biggest lung cancer centers, and in the rest of the countries, incidence has been estimated based on the experts' feedback due to a lack of national disease registries. The absolute numbers were adjusted per 100,000 inhabitants in order to have an accurate standardized overview at each country level. The mean incidence of NSCLC in the region is 53.4 new cases diagnosed per 100,000 inhabitants per year. Hungary has the highest annual incidence followed by Serbia and Croatia. The lowest annual incidence of NSCLC in the region appears to be in Bulgaria. The annual incidence of NSCLC in absolute numbers and adjusted to 100,000 inhabitants in CEE countries is given in Table 1.
Table 1. Annual incidence in absolute numbers and adjusted to 100,000 inhabitants in countries of central and southeastern Europe.
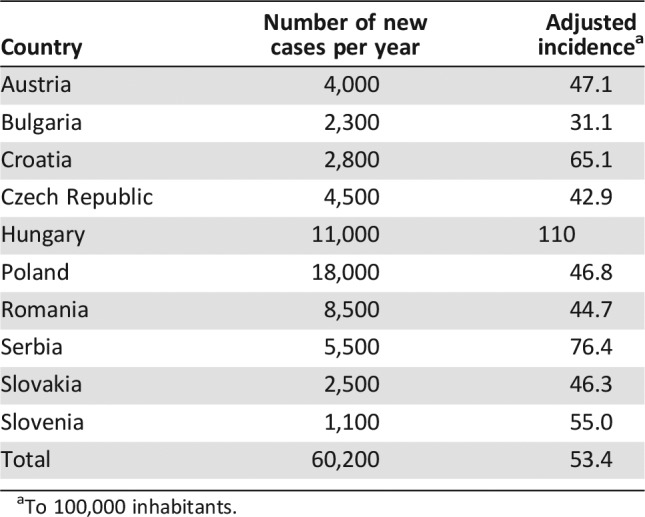
To 100,000 inhabitants.
Availability of Molecular Testing
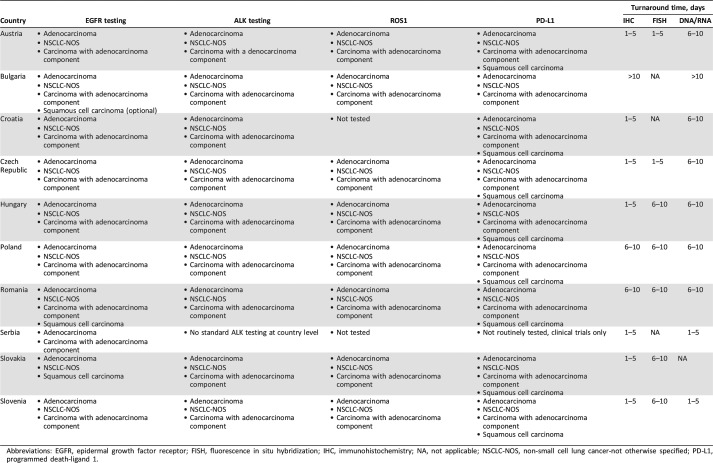
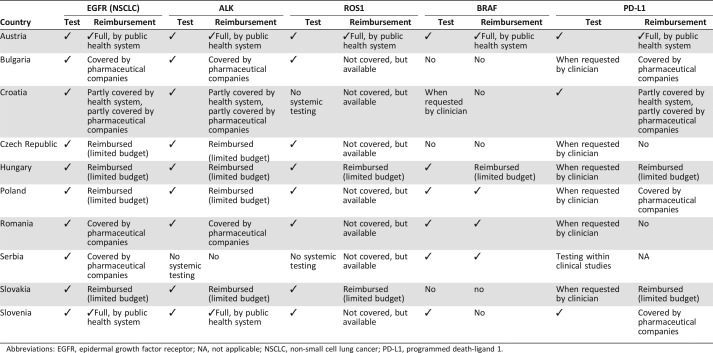
In general, molecular testing is available for this patient population in all CEE countries. Tables 2 and 3 present a detailed overview of the current testing situation including the clinical practice algorithm and reimbursement conditions for each of the five predictive biomarkers. Whereas Table 2 shows the characteristics of molecular testing in CEE countries, Table 3 concentrates on reimbursement procedures.
Table 2. Characteristics of genetic testing in NSCLC in countries of central and southeastern Europe.
Abbreviations: EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NA, not applicable; NSCLC‐NOS, non‐small cell lung cancer‐not otherwise specified; PD‐L1, programmed death‐ligand 1.
Table 3. Availability and reimbursement of testing for EGFR mutation, ALK rearrangement, ROS1 mutation, BRAF mutation, and PD‐L1 overexpression and of liquid biopsies in countries of central and southeastern Europe.
Abbreviations: EGFR, epidermal growth factor receptor; NA, not applicable; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death‐ligand 1.
Molecular Testing and Access in Detail
EGFR Mutations.
Testing Policy.
Genetic testing to identify EGFR oncogenic mutations is available but differs in its provision: It is done by “reflex” in Austria, Slovenia, Croatia, Czech Republic, and Slovakia (for adenocarcinoma and NSCLC‐not otherwise specified) and “on demand” in Bulgaria, Hungary, Poland, Romania, and Serbia. In Austria and Slovakia, it is also performed on demand for squamous cell carcinomas.
Methodology.
A wide range of methods is available for testing. In the majority of countries, multiple methods are used, but usually only one prevails: In 9 of the 10 analyzed CEE countries, the identification of genetic mutation of EGFR is performed predominantly using methods that are reverse transcription polymerase chain reaction (RT‐PCR) based. Methods that are additionally used include next‐generation sequencing (NGS; four countries), Sanger sequencing (three countries), and digital droplet PCR (two countries).
Reimbursement.
Reimbursement of EGFR mutation testing is fully covered by the public health system only in Austria and Slovenia. Although EGFR testing is reimbursed in five additional countries, the allocated budget is limited to a defined countrywide number of tests per year. Therefore, it is unlikely that all patients have access to a reimbursed test. In the remaining four countries (Romania, Bulgaria, Serbia, and Croatia), EGFR testing is supported either completely (Romania, Bulgaria, and Serbia) or partially (Croatia) by the pharmaceutical industry.
ALK Rearrangement.
Testing Policy.
“Reflex” and “on demand” testing is similar to the situation encountered within the context of EGFR mutation testing. The only exception is Serbia, where no routine and systematic ALK testing is currently performed, although it may be accessed upon the patient's wish and by recommendation from the responsible oncologist.
Considering that EGFR and ALK positivity is almost always mutually exclusive [4], a question of the survey referred to parallel testing versus a sequential algorithm typically consisting of EGFR mutation testing being assessed first and—if negative—followed by ALK testing. The answers to the survey showed that in Austria and in the Czech Republic, both parallel and stepwise approaches are used depending on the individual strategy of the respective center. In Romania and Bulgaria, both markers are tested in parallel, whereas in the rest of the countries—except Serbia—tumor samples are tested sequentially, starting with EGFR mutation testing; ALK testing is performed only in the presence of EGFR wild‐type (wt) tumors.
Methodology.
All countries with established testing for ALK use immunohistochemistry (IHC) with clone D5F3, except Bulgaria, where clone 1A4 is used. Fluorescence in situ hybridization (FISH) technology is largely used, except in Croatia and Bulgaria. In some countries, the unequivocal IHC result is considered sufficient to demonstrate ALK abnormalities (Austria, Czech Republic, Croatia, Bulgaria, Hungary, and Slovenia), thus limiting the performance of FISH to IHC‐uncertain/borderline samples only. In other countries, FISH confirmation of an IHC‐positive result is required (Poland and Slovakia). In Romania, FISH is the only method used to determine the ALK status.
Reimbursement.
Reimbursement conditions per country are largely identical to those mentioned for EGFR mutation testing, with Serbia constituting an exception where there are no resources allocated for ALK testing at the country level.
ROS1 Rearrangement.
Testing Policy. As shown in Table 2, ROS1 mutation testing is available in all CEE countries, except Croatia and Serbia. In general, this analysis is performed “on demand” by the request of the oncologist. The test is occasionally performed in Romania and Poland but is routinely assessed as a “reflex” testing in Austria, Slovenia, and Slovakia. Except for few centers in Austria where testing is done in parallel with EGFR and ALK genetic alterations, the general rule in the remaining CEE countries is that ROS1 mutation testing is done in EGFR wt and ALK‐negative cases.
Methodology. In countries where ROS1 mutation is tested, the laboratory method is based on IHC (D4D6 antibody clone) and FISH technologies.
Reimbursement. Reimbursement for ROS1 mutation testing is markedly limited, as compared with EGFR and ALK testing. A full reimbursement is available only in Austria, whereas in Hungary and Slovakia, it is reimbursed with a budget capping.
BRAF Mutations.
Testing Policy. Testing for BRAF mutations is performed in Austria, Croatia, Hungary, Serbia, Slovenia, Poland, and Romania. Three countries (Bulgaria, Czech Republic, and Slovakia) do not perform this analysis in NSCLC.
Methodology. BRAF testing is performed as a part of a lung mutations panel on NGS platforms or by the use of RT‐PCR‐based techniques.
Reimbursement. Testing is reimbursed in Austria, Hungary, Serbia, Poland, and Romania. It is not reimbursed in Slovenia, Croatia, Bulgaria, or Czech Republic.
PD‐L1 Overexpression.
Testing Policy. Testing for PD‐L1 is similar to that of ROS1 mutation: It is available in all CEE countries, except Serbia, where detection of PD‐L1 is reserved for clinical trials. “Reflex” testing for NSCLC is performed only in Austria, Slovenia, and Slovakia. When talking only about adenocarcinoma, the status of PD‐L1 expression is determined in parallel with EGFR and ALK in Austria, Bulgaria, Poland, Romania, and Slovenia, whereas it is done after the acquisition of negative results for EGFR and ALK mutations in Croatia, Czech Republic, and Hungary. In Slovakia, PD‐L1 overexpression is currently tested after EGFR and ALK. However, a change of the protocol is planned at the national level to include this marker into the “parallel testing panel” together with EGFR, ALK, and ROS1.
Methodology. There is a wide spectrum of methods used to detect PD‐L1 expression with various primary antibodies, systems, and protocols. Most centers are using the 22C3 antibody, whereas the clone 28‐8 is used less frequently, and SP142, SP263, and E1L3N are the least used.
Reimbursement. PD‐L1 testing is reimbursed by the public health system in Austria and Hungary. In Slovakia, it is not reimbursed as special analysis but rather as one of the usual IHC tests.
Availability of Liquid Biopsies
Testing Policy.
Molecular analysis of circulating tumor DNA (ctDNA) is routinely performed in five CEE countries (Austria, Croatia, Hungary, Serbia, and Slovenia). In Slovakia, the technique is performed in only two centers, and in the other three countries (Bulgaria, Poland, and Romania), it is not used in routine practice. There is a unique situation in the Czech Republic, as ctDNA testing has been introduced fully validated and is reimbursed. However, due to the lack of reimbursement of treatment with the new generation of TKIs, liquid biopsies are requested by physicians only rarely.
Methodology.
All countries providing liquid biopsies are using either digital droplet PCR, RT‐PCR, or NGS.
Reimbursement.
Liquid biopsies are reimbursed in four CEE countries where they are routinely used (Austria, Croatia, Serbia, and Slovenia) and in the Czech Republic (where they are not used in routine practice). In Hungary, liquid biopsies are partially reimbursed, similar to the other molecular diagnostic tests. In the remaining four countries (Bulgaria, Poland, Romania, and Slovakia), the technique is not reimbursed.
Discussion
Our survey results constitute the first comprehensive report from CEE countries with data about availability, reimbursement, and access to diagnostic tests of specific predictive markers in NSCLC, including EGFR mutations, ALK and ROS‐1 rearrangement, BRAF mutations, PD‐L1 expression, and liquid biopsies.
The European Society for Medical Oncology's (ESMO) 2020 vision statement recognized the importance of availability and affordability of high‐quality care to everyone as a main obstacle to progress in cancer management [9]. The ESMO studies have clearly demonstrated the inequity of access to newly developed anticancer therapies between economically more‐ and less‐developed countries in Europe, as well as in low‐income countries outside of Europe [10], [11]. However, an important prerequisite for the clinical applicability of precision medicine is not only the identification and awareness of predictive markers for the optimal selection of patients for targeted treatment by pathologists and clinicians, but also the presence of an appropriate infrastructure. The latter refers to the availability of sophisticated equipment in pathology laboratories, training of personnel, and the presence of standard operating procedures, which would define the necessary steps required for fast, successful, and reliable testing. Finally, an important requirement is the existence of a framework to ensure the quality of testing. This includes validation of individual methods, adequate internal control mechanisms, participation in external quality assurance programs, and regular meetings where the results are discussed within multidisciplinary tumor boards. Only correct and reliable results of molecular pathologic analyses can lead the way to the optimal use of targeted treatment by medical oncologists or pulmonologists in the treatment of advanced NSCLC.
The current article forms the basis of our endeavor to analyze currently available diagnostic possibilities in 10 countries from CEE, as the availability of testing is the decisive step that results in the administration of targeted therapies. Thus, limitations in access to diagnostic procedures might ultimately lead to restrictions of therapeutic means. As the latter aspect represents a major problem per se and exceeds in its magnitude and importance the scope of the present article, registration and reimbursement issues for targeted drugs in this particular part of Europe will be the subject of a subsequent article (manuscript in preparation).
Thus, the present survey provides a representative impression of strategies regarding molecular testing and their methodologies and reimbursement in the included CEE countries, which constitute a large European region with a high incidence of lung cancer. This is of particular interest, as in this particular geographic region, health care spending (with some rare exceptions) is relatively low [12], the introduction of innovative treatments is frequently protracted due to impediments in reimbursement, and, finally, mortality from cancer tends to be higher than in Western European countries.
The results of our survey clearly show that in countries where testing for predictive markers for the state‐of‐the‐art treatment of NSCLC is reimbursed by the public health system, these procedures are routinely performed in all diagnosed patients. This constitutes a desirable aim. However, patients with NSCLC from countries where appropriate molecular testing is insufficiently or not at all reimbursed are facing serious difficulties regarding access to testing and as a result may not benefit from the full implementation of the concept of precision medicine. The support for testing from alternative sources, such as the pharmaceutical industry, is no doubt partly compensating for the lack of public health system support, but it is not a viable or long‐term solution, as it is hampered by a series of ethical issues and conflicts of interest. Thus, these practices should be abolished and replaced by reimbursement of testing by the public health system.
It is worth mentioning that the situation in testing for mutations of EGFR and ALK shows an improvement in CEE countries, as compared with the situation encountered in 2013 [13]. However, “reflex” testing is still not being routinely performed in all countries, as it would be recommended by current guidelines, which aim at the most direct access to targeted care based on the results of appropriate diagnostic procedures [6].
We showed here that the average turnaround time for genetic testing ranges between 5 and 10 days, depending on the technology used to identify the specific oncogenic alteration. This is clinically relevant, as in the case of “reflex” testing, this might be considered a reasonable time frame. Conversely, in the case of testing of molecular predictive markers “on demand,” this time interval represents an additional burden and leads to potential delays in initiating the most adequate targeted treatment. Liquid biopsies, with their rapid turnover time, may play an important part in this very context in the near future, provided that this approach will reach a comparable sensitivity to that of testing of mutations in tumor tissue.
Conclusion
Considering the major importance of molecular testing in defining the treatment algorithm of NSCLC patients with advanced disease and an associated survival benefit from targeted therapies, the results of our survey translate into a call to action with the aim of structuring and aligning diagnostic strategies and reimbursement processes at the regional level. Thus, access to precision medicine would go a step further from planning to implementation in routine clinical practice with the objectives of improving the lives of patients with lung cancer and, subsequently, reducing mortality.
Acknowledgments
The work of A.R. (Fingerland Department of Pathology of Charles University) was supported by research grant CZ.02.1.01/0.0/0.0/16_013/0001674 (Ministry of Education, Youth and Sports, Czech Republic). CECOG is grateful to AstraZeneca for a grant making this work and survey possible.
Author Contributions
Conception/design: Ales Ryska, Rares Buiga, Albena Fakirova, Izidor Kern, Włodzimierz Olszewski, Lukas Plank, Sven Seiwerth, Erika Toth, Eri Zivka, Christiane Thallinger, Christoph Zielinski, Luka Brcic
Collection and/or assembly of data: Ales Ryska, Rares Buiga, Albena Fakirova, Izidor Kern, Włodzimierz Olszewski, Lukas Plank, Sven Seiwerth, Erika Toth, Eri Zivka, Christiane Thallinger, Christoph Zielinski, Luka Brcic
Data analysis and interpretation: Ales Ryska, Rares Buiga, Albena Fakirova, Izidor Kern, Włodzimierz Olszewski, Lukas Plank, Sven Seiwerth, Erika Toth, Eri Zivka, Christiane Thallinger, Christoph Zielinski, Luka Brcic
Manuscript writing: Ales Ryska, Christoph Zielinski, Luka Brcic
Final approval of manuscript: Ales Ryska, Rares Buiga, Albena Fakirova, Izidor Kern, Włodzimierz Olszewski, Lukas Plank, Sven Seiwerth, Erika Toth, Eri Zivka, Christiane Thallinger, Christoph Zielinski, Luka Brcic
Disclosures
Luka Brcic: AstraZeneca (RF), Merck Sharp & Dohme, Pfizer, Roche (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Han Y, Li J. Sample types applied for molecular diagnosis of therapeutic management of advanced non‐small cell lung cancer in the precision medicine. Clin Chem Lab Med 2017;55:1817–1833. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014,311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shea M, Costa DB, Rangachari D. Management of advanced non‐small cell lung cancers with known mutations or rearrangements: Latest evidence and treatment approaches. Ther Adv Respir Dis 2016;10:113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr KM, Bubendorf L, Edelman MJ et al. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non‐small‐cell lung cancer. Ann Oncol 2014;25:1681–1690. [DOI] [PubMed] [Google Scholar]
- 6.Novello S, Barlesi F, Califano R et al. Metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27(suppl 5):v1–v27. [DOI] [PubMed] [Google Scholar]
- 7.Rekhtman N, Leighl NB, Somerfield MR. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Pncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Oncol Pract 2015;11:135–136. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Popat S, Reinmuth N et al. Metastatic non‐small‐cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii27–iii39. [DOI] [PubMed] [Google Scholar]
- 9.ESMO 2020 Vision. Available at http://www.esmo.org/content/download/68849/1233986/file/ESMO‐2020‐vision‐brochure.pdf. Accessed March 10, 2018.
- 10.Cherny N, Sullivan R, Torode J et al. ESMO European Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol 2016;27:1423–1443. [DOI] [PubMed] [Google Scholar]
- 11.Cherny NI, Sullivan R, Torode J et al. ESMO International Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in countries outside of Europe. Ann Oncol 2017;28:2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrdoljak E, Bodoky G, Jassem J et al. Cancer control in central and eastern Europe: Current situation and recommendations for improvement. The Oncologist 2016;21:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryska A, Berzinec P, Brcic L et al. NSCLC molecular testing in central and eastern European countries. BMC Cancer 2018;18:269. [DOI] [PMC free article] [PubMed] [Google Scholar]