This article highlights a durable response to the immune checkpoint inhibitor nivolumab in a pediatric patient with recurrent/refractory constitutional mismatch repair deficiency‐associated (CMMRD) glioblastoma. Incorporating genomic and/or molecular testing for CMMRD into routine pediatric oncology clinical care can identify a subset of patients likely to benefit from this treatment.
Abstract
Primary brain tumors are a leading cause of cancer‐related morbidity and mortality in children. Glioblastoma (GBM) is a high‐grade astrocytoma that occurs in both children and adults and is associated with a poor prognosis. Despite extensive study in recent years, the clinical management of these tumors has remained largely unchanged, consisting of surgical resection, conventional chemotherapy, and radiotherapy. Although the etiology and genomic drivers in GBM are diverse, constitutional mismatch repair‐deficiency (CMMRD) syndrome is a rare, recessively inherited disease with a predisposition to gliomagenesis. CMMRD results from biallelic mutations in one of the mismatch repair genes including mutL homolog 1 (MLH1), mutS homolog 2 (MSH2), mutS homolog 6 (MSH6), and post‐meiotic segregation increased 2 (PMS2). In this report, we present the case of a 5‐year‐old female with GBM and CMMRD due to an MSH6 homozygous c.1883G>A mutation, who continues to experience an exceptional and durable response (9 months) to the immune checkpoint inhibitor (ICPI) nivolumab. Our patient presented with acute neurologic decline and increased intracranial pressure. Neuroimaging studies revealed a large left frontoparietal mass requiring neurosurgical decompression and resection. Histopathologic analyses resulted in a diagnosis of de novo GBM that was BRAF wild type and negative for programmed death‐ligand 1 protein expression. She received standard‐of‐care treatment with surgery, radiation therapy, and temozolomide; however, the tumor recurred 3 months after the initial diagnosis. Molecular analyses of tumor and blood tissues revealed an MSH6 homozygous c.1883G>A mutation consistent with CMMRD. Given her CMMRD status, she was treated with nivolumab (3 mg/kg doses every 2 weeks for 36 weeks) and showed a 60% reduction in tumor size, improved clinical symptoms, and an ongoing durable response lasting 10 months to date. Our study highlights a durable response to the ICPI nivolumab in a pediatric patient with recurrent/refractory CMMRD‐associated GBM. We show that incorporating genomic and/or molecular testing for CMMRD into routine pediatric oncology clinical care can identify a subset of patients likely to benefit from ICPI.
Key Points.
Constitutional mismatch repair‐deficiency (CMMRD) syndrome, alternatively known as biallelic mismatch repair deficiency syndrome, occurs in subset of pediatric cancer patients, including those with primary brain tumors.
Patients from Arab and other developing countries are predicted to have higher incidence of CMMRD due to high prevalence of consanguinity.
Integration of molecular and/or genomic testing into routine clinical care for pediatric cancer patients is important to identify patients with CMMRD syndrome.
Patient with CMMRD‐associated cancers may show increased responsiveness to immune checkpoint inhibitors.
To the authors' knowledge, this is the first report in the Arab world of a durable response to immune checkpoint inhibitors in a pediatric glioblastoma patient.
Patient Story
A 5‐year‐old girl from the northern region of Saudi Arabia with no prior medical history presented to King Fahad Medical City (KFMC) emergency room in November 2016 with a history of headache, vomiting, left eye strabismus, and lethargy. She had no history of abnormal movement or seizure activity. Her examination was remarkable for mild facial dysmorphism and multiple small café‐au‐lait spots on her trunk. She was small for her age, with both height and weight below the fifth percentile.
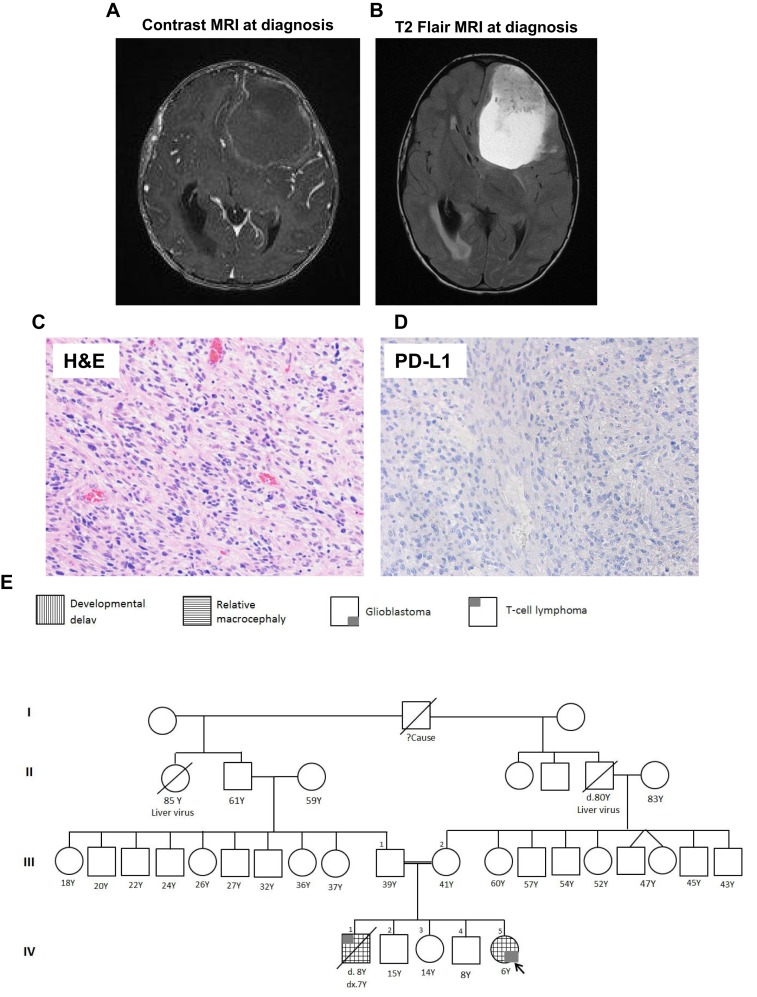
A computed tomography brain scan at presentation showed a large intra‐axial infiltrating mass in the left frontal and parietal lobes, with cystic and hemorrhagic components causing right midline shift and compression of the left lateral ventricle. Diffuse cerebral edema was seen with raised optic discs (papilledema) reflecting the presence of increased intracranial pressure. Magnetic resonance imaging (MRI) of her brain showed a large intra‐axial left frontal predominately cystic complex mass with solid hemorrhagic components causing severe mass effect, right midline shift, and obstructive hydrocephalus (Fig. 1A, 1B). The patient underwent a gross total resection and had an excellent recovery without complications or significant deficit.
Figure 1.
Histological, radiological images of the patients with the family pedigree. MRI with (A) or without (B) contrast showing large intra‐axial left frontal predominately cystic complex mass with solid hemorrhagic components causing a severe mass effect is observed together with a right midline shift and obstructive hydrocephalus. (C): H&E staining demonstrating a densely cellular high‐grade glioma. (D): PD‐L1 immunohistochemistry with Dako 22C3 antibody is negative for immunoreactivity. (E): Four‐generation family pedigree of index patient. Abbreviations: H&E, hematoxylin and eosin; MRI, magnetic resonance imaging; PD‐L1, programmed death‐ligand 1.
Neuropathological analysis of resected tissues revealed a densely cellular and diffusely infiltrating high‐grade glioma (World Health Organization grade 4) with abundant mitoses, microvascular proliferation, and necrosis (Fig. 1C). Although a majority of the tumor cells were characterized by ovoid‐to‐elongated nuclei with moderate nuclear pleomorphism and tapered cell morphology, focal areas were consistent with a primitive neuroectodermal component. Cytological examination of cerebrospinal fluid showed no evidence of malignant cells. Immunohistochemistry for programmed death‐ligand 1 (PD‐L1) protein using the Dako 22C3 antibody showed no expression of PD‐L1 protein (Fig. 1D).
Sequencing analysis of mismatch repair (MMR) genes PMS2, MLH1, MSH2, and MSH6 in tumor and blood from the patient demonstrated the presence of a homozygous c.1883G>A mutation in MSH6 consistent with constitutional mismatch repair‐deficiency (CMMRD) syndrome. Sequencing of tumor DNA showed no evidence of mutations in BRAF.
The patient's family history is notable for parents who are first‐degree relatives and a brother who died at 8 years of age from a T‐cell lymphoma. Genomic testing of her father identified a heterozygous MSH6 c.1883G>A mutation; her mother declined testing. Access to her deceased brother's tumor tissue also demonstrated the presence of a homozygous c.1883G>A mutation in MSH6. The patient and family were referred to genetic counselors and a four‐generation family pedigree was established (Fig. 1E).
An MRI of the patient's brain 1 month postoperatively (December 2016) showed significant tumor recurrence (4 × 3.5 × 2.5 cm) in the left frontal lobe causing mass effect on the left temporal lobe. Whole‐spine MRI during this time (December 2016) demonstrated thin enhancement along the ventral and dorsal surfaces of the conus medullaris, likely representing vascular enhancement. Additionally, the presence of diffuse smooth enhancement along the dorsal roots of cauda equine without any nodularity was noted, which may be reactive due to the recent surgery (supplemental online Fig. 1A).
Following discussion of her case at multidisciplinary neuro‐oncology tumor board, the patient underwent treatment according to the high‐grade glioma KFMC protocol with craniospinal radiation therapy (59.4 Gy) combined with oral temozolomide (TMZ) for 42 days during radiation therapy (90 mg/m2/day, Children's Oncology Group study ACNS0126) [1]. She tolerated radiation therapy very well until it ended in February 2017. After radiation therapy was completed, she started on maintenance cycles of oral TMZ for 5 days (200 mg/m2/day) every 28 days for a planned total of 10 cycles.
In April 2017, a brain MRI showed significant tumor progression, and spinal MRI revealed irregular leptomeningeal enhancement in the lower thoracic cord and cauda equine, raising concerns about craniospinal seeding (supplemental online Fig. 1B). Despite the radiological findings, which were suggestive of an aggressive tumor recurrence, the patient was clinically stable with no neurological symptoms. The possibility of palliative debulking surgery was discussed with the family, but the parents declined additional surgery.
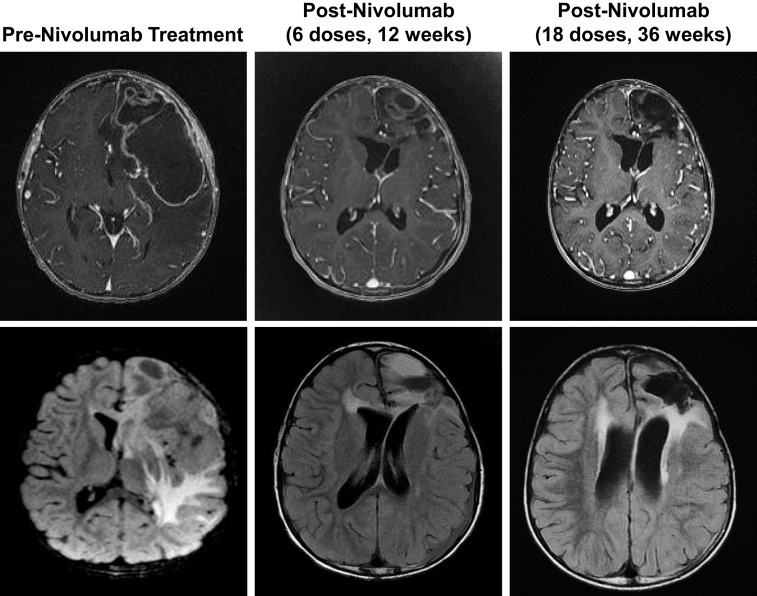
In light of the patient's CMMRD status, maintenance oral TMZ was stopped after three cycles, and she began treatment with nivolumab. Due to reported new onset seizures associated with initiation of nivolumab, levetiracetam was initiated as a prophylactic anticonvulsant. The patient received 18 doses of nivolumab (3 mg/kg, every 2 weeks); MRI evaluation performed after the first 6 and 18 doses of nivolumab showed regression of the tumor, which was associated with improved neurologic status (Fig. 2).
Figure 2.
Brain magnetic resonance imaging (with contrast, top; without, bottom) performed pre‐nivolumab treatment (left) and after 6 (middle) or 18 (right) doses, demonstrating profound tumor reduction.
Molecular Tumor Board
CMMRD, also called biallelic mismatch repair deficiency syndrome (bMMRD), is a rare childhood cancer predisposition syndrome caused by biallelic inactivation of PMS2, MLH1, MSH2, or MSH6. Cancers associated with CMMRD can be grouped into four main classes: hematologic malignancies, central nervous system tumors, colorectal tumors and multiple intestinal polyps, as well as other malignancies, including embryonic tumors and rhabdomyosarcoma. Many patients also show phenotypic features of neurofibromatosis type I, particularly multiple café‐au‐lait macules [2].
Proteins encoded by PMS2, MLH1, MSH2, and MSH6 mismatch repair (MMR) genes are involved in correcting errors that arise during DNA replication. Malignant brain tumors from patients with CMMRD experience diminished replication repair at every cell cycle; therefore, all replicating tumor cells will inevitably accumulate mutations (>600 mutations per cell division), reaching approximately 20,000 exonic mutations in <6 months and resulting in rapid onset of ultra‐hypermutated cancers [3], [4].
Recent studies demonstrated that MMR‐deficient or high microsatellite instability (MSI‐H) tumors respond to treatment with ICPI that target the immune‐checkpoint receptor programmed cell death protein 1 (PD‐1, also known as CD279) [5], [6]. In May 2017, the U.S. Food and Drug Administration (FDA) approved the anti‐PD‐1 therapeutic pembrolizumab for treating any solid tumor demonstrating MSI‐H or MMR‐deficient status.
Glioblastoma is a leading cause of cancer‐related mortality in children. Standard‐of‐care treatment includes surgical resection followed by radiation and chemotherapy (temozolomide); however, tumors frequently recur, requiring new therapeutic options. Immunotherapy using immune checkpoint inhibitors have shown great promise in both adult and pediatric malignancies; however, the need to identify biomarkers that may predict response to ICPI remains ongoing. A prior study reported on two siblings with CMMRD‐associated recurrent multifocal glioblastoma that were treated with the immune checkpoint inhibitor (ICPI) nivolumab and had profound long‐term responses [7].
Genotyping Results and the Interpretation of the Molecular Results
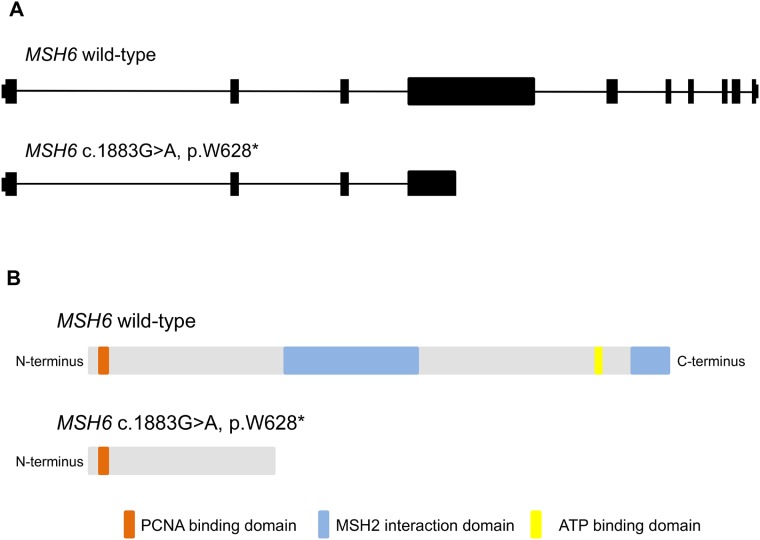
DNA was extracted from the patients’ peripheral blood and formalin‐fixed, paraffin embedded GBM tissues and subjected to clinical sequencing of MMR genes. Briefly, the entire open reading frames of PMS2, MLH1, MSH2, and MSH6 (primers sequences available on request) were amplified from normal (blood) and tumor (GBM) DNA and screened by direct sequencing. Sequencing revealed a homozygous MSH6 c.1883(G>A) mutation with a corresponding protein change of MSH6 (p.W628*) that truncates the protein proximal to the MSH2 interacting domain. Wild‐type MSH6 is composed of 1,361 amino acids, and the mutation detected in this patient occurs within exon four, resulting in <50% of the normal encoded amino acids (Fig. 3).
Figure 3.
Schematic of CMMRD‐MSH6 genetics. Schematic of MSH6 wild‐type genomic (A) and protein (B) structures with corresponding predictions for truncated isoforms resulting from the index patient's MSH6 c.1883G>A, p.W628* mutation.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
In this study, we report for the first time in the Arab world, to the best of our knowledge, successful treatment of a CMMRD‐associated refractory pediatric GBM with an ICPI. By integrating genomic testing into routine clinical care, we identified her CMMRD status and advocated for treatment with an ICPI. This represents a novel integrated approach to managing pediatric cancer patients that can be readily adopted by medical centers in the region and worldwide. We recommend integrating genomic profiling into the management of all pediatric brain tumor patients as these assays often include evaluation of MMR genes, microsatellite instability (MSI) status, and tumor mutational burden. For those patients with a strong family history of cancer, high clinical suspicion for a cancer predisposition syndrome, and/or are positive for a somatic mutation in an MMR gene, we believe these patients should be followed up with dedicated germline testing for MMR alterations with referral to genetic counselors as appropriate.
As an autosomal recessive cancer predisposition syndrome, bMMRD is one of the most devastating cancer syndromes associated with mortality during childhood due to the high rate of malignant gliomas. Recent studies illustrated a high frequency of bMMRD in countries where consanguinity is common but underreported [8]. Early molecular screening for bMMRD patients in these countries will provide opportunities for early cancer surveillance and intervention with ICPI.
Although high PD‐L1 expression in non‐small cell lung cancer (NSCLC) is associated with an increased likelihood of response to ICPI, high tumor mutation burden and/or microsatellite instability is increasingly being used to identify patients likely to benefit from ICPI [9]. Indeed, in our patient, PD‐L1 immunohistochemistry was negative using the Dako 22C3 antibody, the companion diagnostic assay for pembrolizumab in NSCLC. Our findings show that in bMMRD patients, PD‐L1 expression may not reliably predict response to ICPI, and treatment decisions for ICPI in this setting should not be based solely on the PD‐L1 immunohistochemistry results.
Immunotherapy‐associated toxicities include pneumonitis, colitis, hepatitis, hypophysitis, and thyroiditis; however, our patient has not experienced any of these complications [10], [11]. To date, she has tolerated nivolumab therapy very well as an outpatient, and the dosing schedule of every other week provides opportunities for the family to continue care despite living far from the hospital. The optimal duration of immunotherapy is unknown, especially in the pediatric setting. CheckMate‐153 is an ongoing phase IIIb/IV study of nivolumab in metastatic NSCLC and the first randomized trial to evaluate the treatment duration with PD‐1 or PD‐L1 inhibitors. Early results of this study reported that NSCLC patients receiving nivolumab for longer than 1 year showed a marked improvement in outcome compared with those who discontinued treatment at 1 year. Our strategy is to continue nivolumab therapy as long as her overall clinical symptoms remain stable and there are no significant signs of progression or severe toxicity. A second surgical resection may be considered in the future if the tumor becomes more localized.
In this patient, MRI studies were kept to a minimum while she continued to be clinically and neurologically stable. Tumor responses to immunotherapy are different from those seen in traditional chemotherapy, with particular challenges noted for interpreting imaging changes soon after initiation of immunotherapy. The initial flare or enlargement of measurable areas of the tumor or the appearance of new lesions after the start of immunotherapy is known as pseudoprogression [12]. These new lesions may represent small, initially undetected cancer foci prior to starting immunotherapy that became visible after infiltration by activated T cells, causing local inflammation. This radiological tumor flare can be observed clinically as new symptoms of associated cerebral edema. With time, there may be a pattern of stable disease followed by delayed tumor regression that may continue over many subsequent scans [13].
In presenting this case, we hope to underscore the need for integrating molecular data into routine histopathologic analyses to accurately diagnose CMMRD in pediatric patients with malignant brain tumors and provide new rationale for clinical trials evaluating whether ICPI can improve outcomes for patients with CMMRD.
Patient Update
Based on the patient's CMMRD status and recurrence of her GBM following standard‐of‐care with surgery, chemotherapy, and radiotherapy, she began treatment with the ICPI nivolumab. Nine months following the initiation of nivolumab, the patient remains clinically stable, neurologically intact with normal vital signs, intact cranial nerves, the ability to walk independently, and a Glasgow Coma Scale score of 15 out of 15. The patient did not experience any adverse reactions from therapy after 10 months from starting ICPI.
Acknowledgements
The authors gratefully acknowledge King Abdulaziz City for Science and Technology and the Saudi Human Genome Project for the technical support. This study was supported by King Fahad Medical City and Sanad Children's Cancer Support Association.
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
For Further Reading: Adrienne Johnson, Eric Severson, Laurie Gay et al. Comprehensive Genomic Profiling of 282 Pediatric Low‐ and High‐ Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. The Oncologist 2017;22:1478–1490.
Implications for Practice: By providing objective data to support diagnostic, prognostic, and therapeutic decision‐making, comprehensive genomic profiling is necessary for advancing care for pediatric neuro‐oncology patients. This article presents the largest cohort of pediatric low‐ and high‐grade gliomas profiled by next‐generation sequencing. Reportable alterations were detected in 95% of patients, including diagnostically relevant lesions as well as novel oncogenic fusions and mutations. Additionally, tumor mutational burden (TMB) is reported, which identifies a subpopulation of hypermutated glioblastomas that harbor deleterious mutations in DNA repair genes. This provides support for TMB as a potential biomarker to identify patients who may preferentially benefit from immune checkpoint inhibitors.
Author Contributions
Conception/design: Musa AlHarbi, Malak Abedalthagafi
Provision of study material or patients: Latifa AlMubarak, Rasha Aljelaify, Mariam AlSaeed, Amal Almutairi, Fatmah Alqubaishi, M. Emarat Hussain, Ali Abdullah O. Balbaid, Amal Said Marie, Lamia AlSubaie, Saeed AlShieban
Collection and/or assembly of data: Musa AlHarbi, Nahla Ali Mobark, Latifa AlMubarak, Nada AlTassan, Malak Abedalthagafi
Data analysis and interpretation: Latifa AlMubarak, Nada AlTassan, Malak Abedalthagafi,
Manuscript writing: Nahla Ali Mobark, Shakti H. Ramkissoon, Malak Abedalthagafi
Final approval of manuscript: Musa AlHarbi, Nahla Ali Mobark, Latifa AlMubarak, Rasha Aljelaify, Mariam AlSaeed, Amal Almutairi, Fatmah Alqubaishi, M. Emarat Hussain, Ali Abdullah O. Balbaid, Amal Said Marie, Lamia AlSubaie, Saeed AlShieban, Nada alTassan, Shakti H. Ramkissoon, Malak Abedalthagafi
Disclosures
The authors indicated no financial relationships.
References
- 1.Cohen KJ, Pollack IF, Zhou T et al. Temozolomide in the treatment of high‐grade gliomas in children: A report from the children's oncology group. Neuro Oncol 2011;13:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakry D, Aronson M, Durno C et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: Report from the constitutional mismatch repair deficiency consortium. Eur J Cancer 2014;50:987–996. [DOI] [PubMed] [Google Scholar]
- 3.Shlien A, Campbell BB, de Borja R et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra‐hypermutated cancers. Nat Genet 2015;47:257–262. [DOI] [PubMed] [Google Scholar]
- 4.Senovilla L, Galluzzi L, Zitvogel L et al. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol 2013;34:471–481. [DOI] [PubMed] [Google Scholar]
- 5.Le DT, Durham JN, Smith KN et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA 2017;318:1647–1648. [DOI] [PubMed] [Google Scholar]
- 7.Bouffet E, Larouche V, Campbell BB et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. JClin Oncol 2016;34:2206–2211. [DOI] [PubMed] [Google Scholar]
- 8.Amayiri N, Tabori U, Campbell B et al. High frequency of mismatch repair deficiency among pediatric high grade gliomas in Jordan. Int J Cancer 2016;138:380–385. [DOI] [PubMed] [Google Scholar]
- 9.Aguiar PN Jr, De Mello RA, Hall P et al. PD‐L1 expression as a predictive biomarker in advanced non‐small‐cell lung cancer: Updated survival data. Immunotherapy 2017;9:499–506. [DOI] [PubMed] [Google Scholar]
- 10.Abdel‐Wahab N, Alshawa A, Suarez‐Almazor ME. Adverse events in cancer immunotherapy. Adv Exp Med Biol 2017;995:155–174. [DOI] [PubMed] [Google Scholar]
- 11.Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 12.Braschi‐Amirfarzan M, Tirumani SH, Hodi FS Jr et al. Immune‐checkpoint inhibitors in the era of precision medicine: What radiologists should know. Korean J Radiol 2017;18:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada H, Weller M, Huang R et al. Immunotherapy response assessment in neuro‐oncology: A report of the RANO working group. Lancet Oncol 2015;16:e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]