Abstract
Lessons Learned.
The maximum tolerated dose of the combination of linsitinib and irinotecan is linsitinib 450 mg daily on days 1–3 every 7 days and irinotecan 125 mg/m2 days 1 and 8 of a 21‐day cycle.
The adverse effects associated with the combination are not significantly increased beyond what is expected of each drug as a single agent.
Multiple negative trials of insulin‐like growth factor‐1 receptor inhibitors performed in unselected patient populations led to the early discontinuation of linistinib development and this trial.
Earlier integration of assessment of potential predictive biomarkers into clinical trials, as was planned in this study, is vital to the development of targeted therapies in oncology.
Background.
This phase I dose‐escalation study was designed to evaluate the safety and tolerability of the combination of irinotecan and insulin‐like growth factor‐1 receptor (IGF‐1R) inhibitor linsitinib in patients with advanced cancer refractory to standard therapy.
Methods.
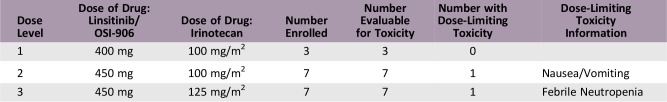
Dose escalation in three specified dose levels was performed according to a standard 3 + 3 design. Dose levels were as follows: (a) linsitinib 400 mg and irinotecan 100 mg/m2, (b) linsitinib 450 mg and irinotecan 100 mg/m2, and (c) linsitinib 450 mg and irinotecan 125 mg/m2. Linisitinib was administered once daily on days 1–3, 8–10, and 15–17, and irinotecan on days 1 and 8. Assessment of a candidate predictive biomarker was planned in all patients, with further evaluation in an expansion cohort of advanced colorectal cancer.
Results.
A total of 17 patients were treated, with 1 patient in both cohort 2 and 3 experiencing dose‐limiting toxicity. Linsitinib 450 mg and irinotecan 125 mg/m2 was the maximum tolerated dose. Sixteen (94%) patients experienced at least one treatment‐related adverse event. Neutropenia was the only grade >3 toxicity (4%). No significant hyperglycemia or QT interval prolongation was noted. No objective responses were observed; 47% (n = 8) had stable disease with median duration of 5.25 months.
Conclusion.
Although the combination was determined safe, the study was halted due to termination of linsitinib development, and biomarker testing was not performed.
Abstract
摘要
背景。本次 I 期剂量递增研究旨在评估标准疗法耐药的晚期癌症患者采用伊立替康和胰岛素样生长因子 1 受体 (IGF‐1R) 抑制剂 Linsitinib 联合治疗的安全性和耐受性。
方法。按照标准的 3 + 3 设计,实施 3 种特定剂量水平的剂量递增。剂量水平如下:(a) Linsitinib 400 mg 和伊立替康 100 mg/m2,(b) Linsitinib 450 mg 和伊立替康 100 mg/m2 以及 (c) Linsitinib 450 mg 和伊立替康 125 mg/m2。Linisitinib 在第 1–3 天、第 8–10 天及第 15–17 天每天给药一次,伊立替康在第 1 天和第 8 天给药。计划在所有患者中对候选的预测性生物标志物进行评估,并对一个扩展的晚期结直肠癌队列实施进一步评估。
结果。一共有 17 名患者接受治疗,在第 2 队列和第 3 队列中分别有 1 名患者出现剂量限制性毒性。Linsitinib 450 mg 和伊立替康 125 mg/m2 为最大耐受剂量。16 名 (94%) 患者至少出现一种与治疗相关的不良反应。中性粒细胞减少症是唯一的 >3 级毒性 (4%)。未发现显著的高血糖症或 QT 间期延长。未观察到客观缓解;在为期 5.25 个月的中位持续时间内,47% (n = 8) 的患者病情稳定。
结论。尽管已经确定这种联合治疗安全无虞,但是,本研究因 Linsitinib 研发终止而停止,未实施生物标记物测试
Discussion
Linsitinib is a potent small‐molecule tyrosine kinase inhibitor of the human IGF‐1R, with a half maximal inhibitory concentration (IC50) of 35 nmol/L, and the homologus insulin receptor, with an IC50 of 75 nmol/L. The drug is selective for these targets [1].
Irinotecan is a topoisomerase I inhibitor that is U.S. Food and Drug Administration approved for the treatment of colorectal cancer with compendia for reimbursement including non‐small cell lung, gastroesophageal, cervical, and ovarian cancers.
The combination of linsitinib and irinotecan was selected for further evaluation based on preclinical data suggesting a synergistic interaction between the drugs [2].
Eligible patients with refractory advanced cancer, and for which irinotecan is in the compendia for reimbursement, were treated with linsitinib, administered by mouth, and irinotecan, by intravenous (IV) infusion, in 21‐day cycles at three dose levels. Once the maximum tolerated dose (MTD) was defined, expansion of this dose level was planned in patients with advanced colorectal cancer. A potential predictive biomarker, the linsitinib integrated classifier score [3], was to be evaluated in this cohort.
A total of 18 patients were enrolled in the trial at a single site. One of seven evaluable patients in the second cohort experienced a dose‐limiting toxicity (DLT) of grade 3 nausea/vomiting requiring hospitalization. A DLT of grade 3 febrile neutropenia/grade 4 neutropenia was documented in one of seven patients treated in cohort 3. Linsitinib 450 mg and irinotecan 125 mg/m2 was determined to be the MTD.
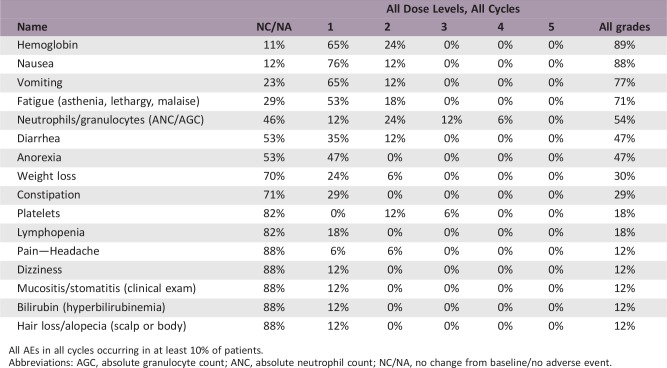
The most common toxicities at least possibly related to treatment and occurring in at least 10% of cycles were nausea, vomiting, fatigue, and anorexia. Hyperglycemia and QTc prolongation were considered adverse events of special interest, although no events above grade 1 severity were documented.
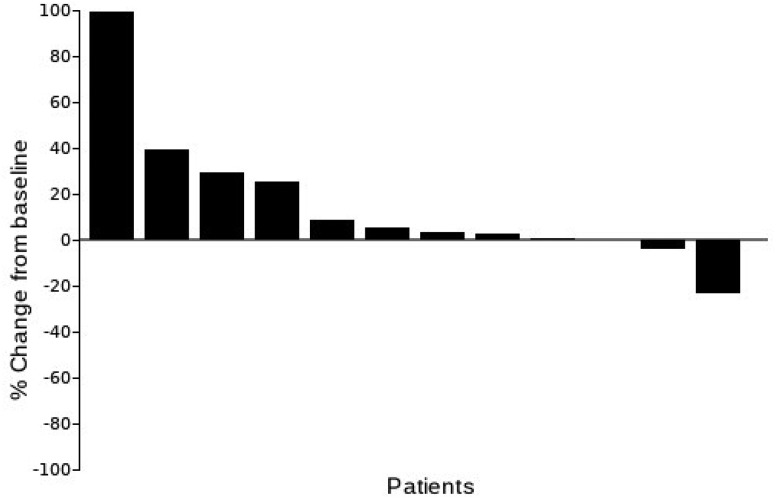
Eight patients (47%) had stable disease. No responses were documented, although one patient with metastatic rectal cancer had a 23% decrease in tumor burden and was treated for 18 cycles. Seven patients (41%) had progressive disease.
Although the combination of linsitinib and irinotecan was determined to be safe at the MTD, the study was halted at this point due to termination of linsitinib development. Thus, the expansion cohort and analysis of the linsitinib integrated classifier and other pharmacodynamic and pharmacokinetic data were not completed.
Trial Information
- Disease
Advanced colorectal, non‐small cell lung, gastroesophageal, cervical, and ovarian cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study – 1
Phase I
- Type of Study – 2
3 + 3
- Primary Endpoint
Maximum tolerated dose
- Primary Endpoint
Safety
- Primary Endpoint
Tolerability
- Secondary Endpoint
Preliminary antitumor activity
- Secondary Endpoint
Correlative endpoint
- Additional Details of Endpoints or Study Design
- An expansion cohort of patients with advanced colorectal cancer who had failed a prior oxaliplatin‐containing regimen was planned at the MTD. These patients were to be assigned to one of two cohorts according to a candidate predictive biomarker—the linsitinib integrated classifier score. The linsitinib integrated classifier is a k‐Top Scoring Pair classifier, developed from gene array data from sensitive and resistant preclinical colorectal cancer (CRC) models, used in combination with IGF‐1R fluorescence in situ hybridization and KRAS mutation status. This classifier was a successful predictor of sensitivity to linsitinib therapy in preclinical patient‐derived CRC xenograft models [3]. Patients in the expansion cohort with a score of 4/5 or above were to be assigned to a single‐agent linsitinib arm, whereas those with lower scores were to receive treatment with single‐agent irinotecan, with linsitinib added to this regimen at the time of progression.
- Investigator's Analysis
Drug tolerable, hints of efficacy
Drug Information
- Drug 1
- Generic/Working Name
Linsitinib/OSI‐906
- Trade Name
- Company Name
OSI Pharmaceuticals
- Drug Type
Small molecule
- Drug Class
Insulin‐like glistItemPairth factors—IGF‐1R and IGF‐2
- Dose
mg per flat dose
- Route
p.o.
- Schedule of Administration
For cycle 1, patients were treated with a single dose of linsitinib on day −3, with further dosing days 2–4, 8–10, and 15–17. Patients received a single‐dose of linisitinb on days 1–3, 8–10, and 15–17 for all additional cycles.
- Drug 2
- Generic/Working Name
Irinotecan
- Trade Name
Camptosar
- Company Name
Pfizer
- Drug Type
Other
- Drug Class
Topoisomerase I
- Dose
mg/m2
- Route
IV
- Schedule of Administration
Day 1 and 8 every 21 days for all treatment cycles.
Dose Escalation Table
Patient Characteristics
- Number of Patients, Male
10
- Number of Patients, Female
8
- Stage
IV
- Age
Median (range): 51 (28–69)
- Number of Prior Systemic Therapies
Median (range): 2 (1–6)
- Performance Status: ECOG
-
0 — 9
1 — 9
2 — 0
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
-
Colon 10
Rectal 4
Esophageal 2
Cervical 1
Ovarian 1
Primary Assessment Method
- Title
Total patient population
- Number of Patients Screened
21
- Number of Patients Enrolled
18
- Number of Patients Evaluable for Toxicity
17
- Number of Patients Evaluated for Efficacy
12
- Evaluation Method
RECIST 1.0
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 8 (53%)
- Response Assessment PD
n = 7 (47%)
- (Median) Duration Assessments Response Duration
12 weeks
- (Median) Duration Assessments Duration of Treatment
6 weeks
Best percentage change from baseline in sum of longest diameters
Adverse Events
All AEs in all cycles occurring in at least 10% of patients.Abbreviations: AGC, absolute granulocyte count; ANC, absolute neutrophil count; NC/NA, no change from baseline/no adverse event.
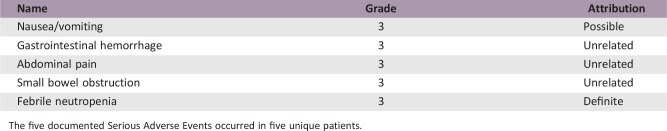
Serious Adverse Events
The five documented Serious Adverse Events occurred in five unique patients.
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Company stopped development
- Investigator's Assessment
Drug tolerable, hints of efficacy
Although this study was discontinued early due to halting of linsitinib development, the dose‐escalation data do provide important safety information regarding this insulin‐like growth factor‐1 receptor (IGF‐1R) inhibitor in combination with irinotecan chemotherapy. In this study, the maximum tolerated dose of linsitinib was 450 mg daily on days 1–3 every 7 days in combination with irinotecan 125 mg/m2 days 1 and 8 of a 21‐day cycle. Overall, this combination was well tolerated across predefined dose levels, with most adverse events (AEs) grade 1–2 in severity.
Hyperglycemia is the primary class‐effect toxicity of IGF‐1R small‐molecule tyrosine kinase inhibitors (TKIs) due to insulin receptor (IR) cross‐targeting at clinically relevant doses [4], [5]. However, such AEs were overall mild in severity in this study, with no events meeting criteria for dose‐limiting toxicity (DLT) in this patient population. It is possible that no significant hyperglycemia was documented in this study because lower doses of linsitinib were used for combination dosing with irinotecan, and patients with baseline glucose elevations were excluded from participation. Elevation in liver function tests has also been documented in phase I studies of linsitinib alone and in combination with everolimus [5], [6], and although grade 3 elevation was observed in one patient on this trial, it was attributed to underlying disease and improved to grade 1 following stenting of a malignant stricture. Although not considered a class effect, QTc prolongation has been a DLT in other studies of linsitinib [4], [5], [7]. In this trial, no grade 3 or greater prolongation of QTc was observed. Unfortunately, due to early discontinuation of this clinical trial, we do not have pharmacokinetic data to further explore its relationship to this toxicity profile.
The early closure of this study and halting of linsitinib development is representative of the fate of IGF‐1R inhibitors in oncology drug development in the last 10 years. Although initially a promising target based on data from various preclinical studies, nearly 40 clinical trials evaluating IGF‐1R monoclonal antibodies, IGF‐1/2‐targeting antibodies, and IGF‐1R/IR small molecule TKIs did not demonstrate a significant clinical benefit in any tumor type [8], [9].
This includes studies evaluating IGF‐1R inhibitors in colorectal cancer, with both single‐agent trials [10] and combination studies with FOLFIRI [11], panitumumab [12], cetuximab/irinotecan [13], and everolimus [6] negative for a significant clinical benefit to patients. However, there were outlier patients across these studies who did achieve partial response or prolonged progression‐free survival on such therapy. It thus remains possible that a subset of colorectal cancer (CRC) patients may still benefit from IGF‐1R inhibitor therapy, although clearly a predictive biomarker is required to select such patients.
An important goal of the expansion cohort of this study was to explore this possibility in patients with advanced CRC; in this case using an integrated classifier to predict response to linsitinib therapy based on k‐Top Scoring Pair in combination with KRAS mutation status and IGF‐1R fluorescence in situ hybridization. Unfortunately, this attempt to identify a predictive biomarker for IGF‐1R targeted therapy came too late in the evaluation of this drug class, and the development of linsitinib was terminated before the classifier was explored in human patients.
Due to discontinuation of development of the majority of IGF‐1R inhibitors, there have been few other efforts to identify a biomarker predictive of activity within or across tumor types. However, a small number of ongoing clinical trials continue to evaluate this target in select tumor types thought to be dependent on IGF‐1R signaling, with the greatest interest in subtypes of sarcoma. Hopefully these and other ongoing studies specifically evaluating potential biomarkers of IGF‐1R inhibitor activity (NCT0271185, NCT02719041, NCT02916394) will lead to the identification of a predictive biomarker that will provide better identification of patients likely to benefit from IGF‐1R inhibition in the broader cancer patient population, as was an initial aim of this clinical trial.
Footnotes
ClinicalTrials.gov Identifier: NCT01016860
Sponsor(s): Stephen Leong
Principal Investigator: Stephen Leong
IRB Approved: Yes
Disclosures
Jennifer R. Diamond: Merck, Bristol‐Meyers Squibb, Bayer, Taiho, Immunomedics, Medimmune, Takeda. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Mulvihill MJ, Cooke A, Rosenfeld‐Franklin M et al. Discovery of OSI‐906: A selective and orally efficacious dual inhibitor of the IGF‐1 receptor and insulin receptor. Future Med Chem 2009;1:1153–1171. [DOI] [PubMed] [Google Scholar]
- 2.Flanigan SA, Pitts TM, Eckhardt SG et al. The insulin‐like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res 2010;16:5436–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitts TM, Tan AC, Kulikowski GN et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: Approach to individualized therapy in early development. Clin Cancer Res 2010;16:3193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puzanov I, Lindsay CR, Goff L et al. A phase I study of continuous oral dosing of OSI‐906, a dual inhibitor of insulin‐like growth factor‐1 and insulin receptors, in patients with advanced solid tumors. Clin Cancer Res 2015;21:701–711. [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Kim ES, Nava‐Parada P et al. Phase I study of intermittent oral dosing of the insulin‐like growth factor‐1 and insulin receptors inhibitor OSI‐906 in patients with advanced solid tumors. Clin Cancer Res 2015;21:693–700. [DOI] [PubMed] [Google Scholar]
- 6.Bendell JC, Jones SF, Hart L et al. A phase Ib study of linsitinib (OSI‐906), a dual inhibitor of IGF‐1R and IR tyrosine kinase, in combination with everolimus as treatment for patients with refractory metastatic colorectal cancer. Invest New Drugs 2015;33:187–193. [DOI] [PubMed] [Google Scholar]
- 7.Fassnacht M, Berruti A, Baudin E et al. Linsitinib (OSI‐906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double‐blind, randomised, phase 3 study. Lancet Oncol 2015;16:426–435. [DOI] [PubMed] [Google Scholar]
- 8.Beckwith H, Yee D. Minireview: Were the IGF signaling inhibitors all bad? Mol Endocrinol 2015;29:1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iams WT, Lovly CM. Molecular pathways: Clinical applications and future direction of insulin‐like growth factor‐1 receptor pathway blockade. Clin Cancer Res 2015;21:4270–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becerra CR, Salazar R, Garcia‐Carbonero R et al. Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: A nonrandomized, open‐label, phase II trial. Cancer Chemother Pharmacol 2014;73:695–702. [DOI] [PubMed] [Google Scholar]
- 11.Cohn AL, Tabernero J, Maurel J et al. A randomized, placebo‐controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second‐line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol 2013;24:1777–1785. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Eng C, Nowara E et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild‐type KRAS metastatic colorectal cancer. Clin Cancer Res 2014;20:4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi T, Muro K, Yoshino T et al. Phase 1 pharmacokinetic study of MK‐0646 (dalotuzumab), an anti‐insulin‐like growth factor‐1 receptor monoclonal antibody, in combination with cetuximab and irinotecan in Japanese patients with advanced colorectal cancer. Cancer Chemother Pharmacol 2013;72:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]